1
Third stage
Medicine
Lec-1
د
.
عبدالحق
1/1/2014
Water Balance in Human Body
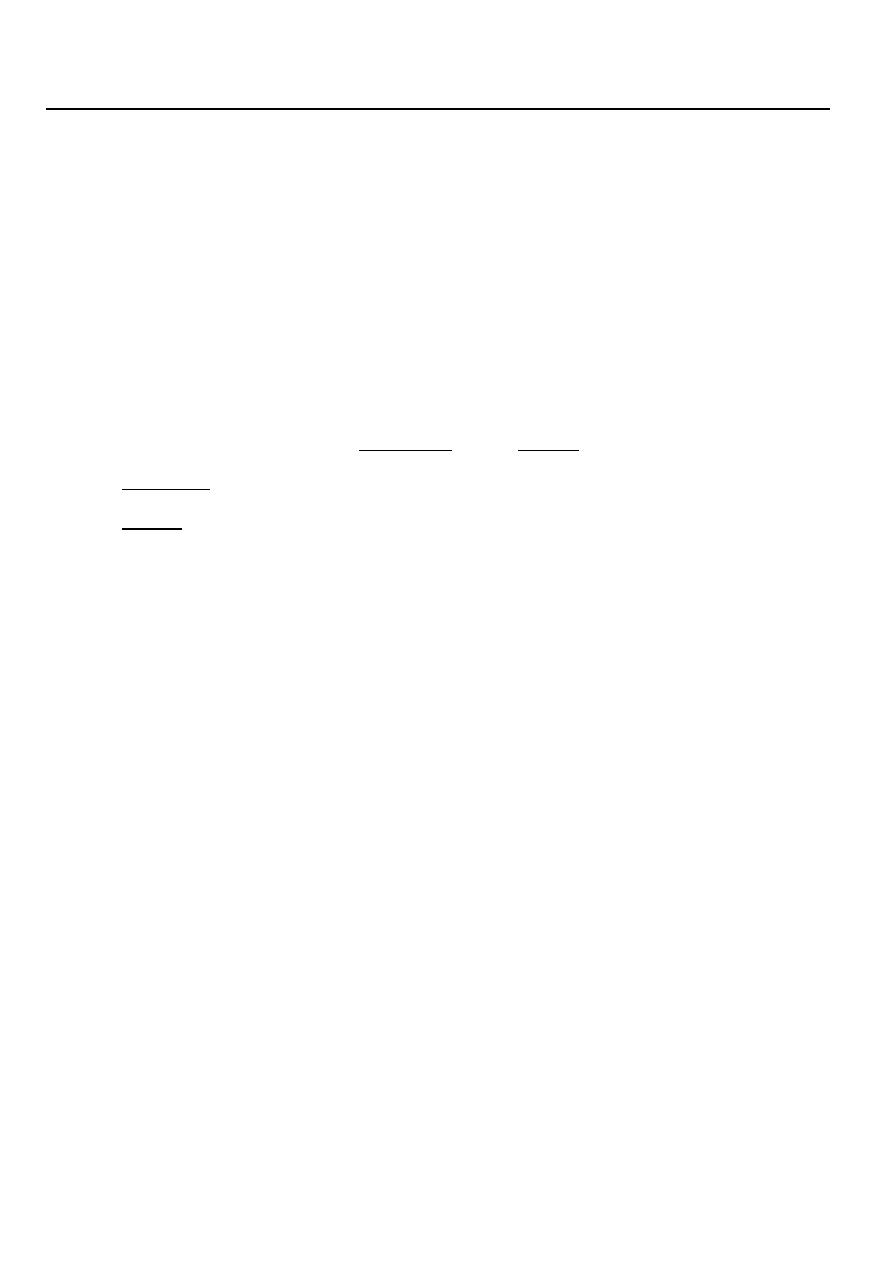
Body Fluid Compartments
In lean adults, body fluids constitute 55% of female and 60% of male total body mass
– Intracellular fluid (ICF) :
• About 2/3 of body fluid
– Extracellular fluid (ECF) :
– 1/3 of body fluid which include Interstitial fluid & Plasma
• Interstitial fluid between cell is 80% of ECF
• Plasma in blood is 20% of ECF
• Also includes lymph, cerebrospinal fluid, synovial fluid, aqueous humor, vitreous
body, endolymph,, pleural, pericardial, and peritoneal fluids
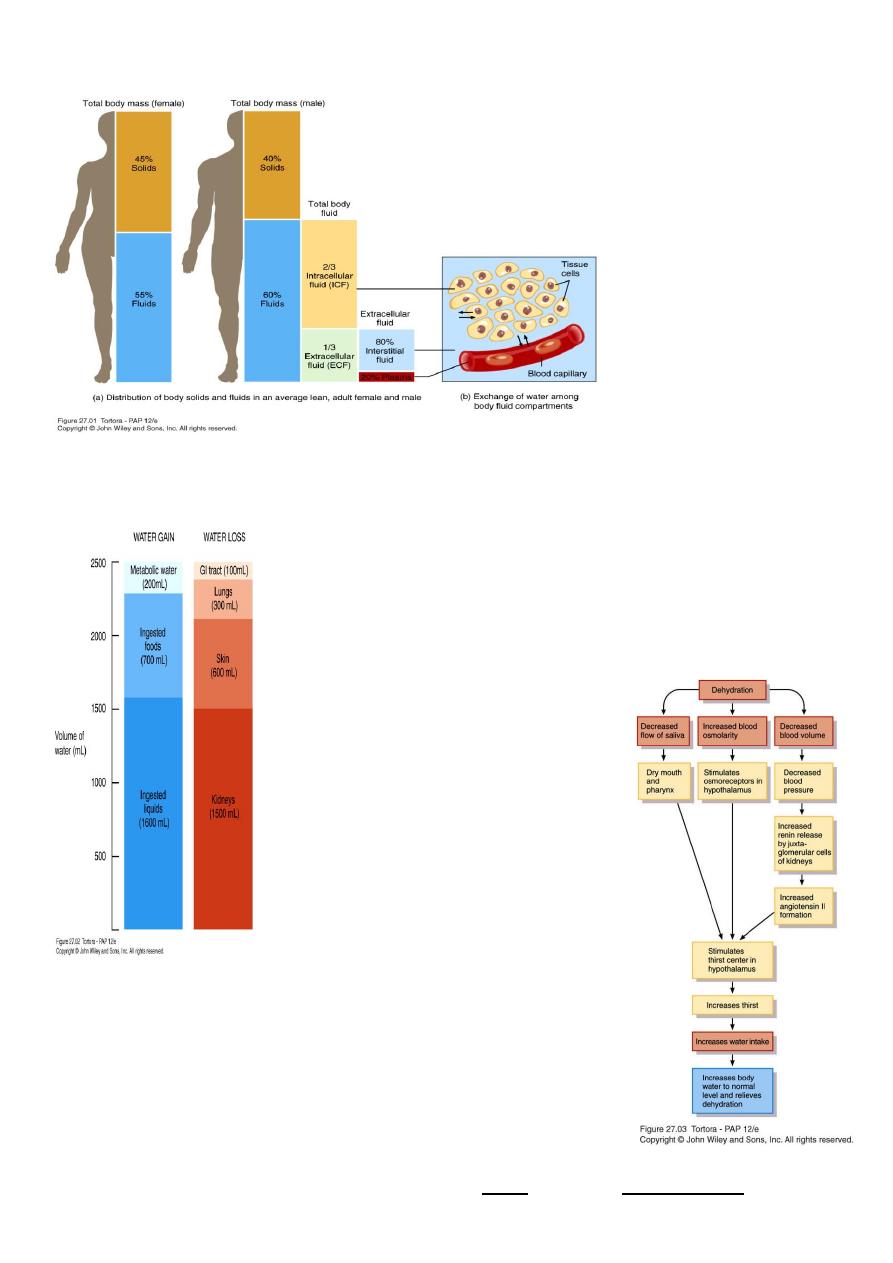
Sources of Body Water Gain and Loss
* Fluid balance related to electrolyte balance
– * Kidneys excrete excess water through dilute urine or excess electrolytes through
concentrated urine
Body can gain water by
– Ingestion of liquids and moist foods (2300mL/day)
– Metabolic synthesis of water during cellular respiration (200mL/day)
Body loses water through
– Kidneys (1500mL/day)
– Evaporation from skin (600mL/day)
– Exhalation from lungs (300mL/day)
– Feces (100mL/day)
2
Body Fluid Compartments
Daily Water Gain and Loss
Regulation of body water gain
– Mainly by volume of water intake
– Dehydration – when water loss is greater than gain
1.Decrease in volume,
2.increase in osmolarity of body fluids Stimulates thirst center in hypothalamus

3
Regulation of water and solute loss
– Extent of urinary salt loss is the main factor that determines body fluid volume
– Main factor that determines body fluid osmolarity is extent of urinary water loss
3 hormones regulate renal Na
+
and Cl
-
reabsorption
Angiotensin II and aldosterone promote urinary Na
+
and Cl
-
reabsorption of (and water by
osmosis) when dehydrated
Atrial natriuretic peptide (ANP) promotes natriuresis, excretion of Na
+
and Cl
-
followed by
water excretion .
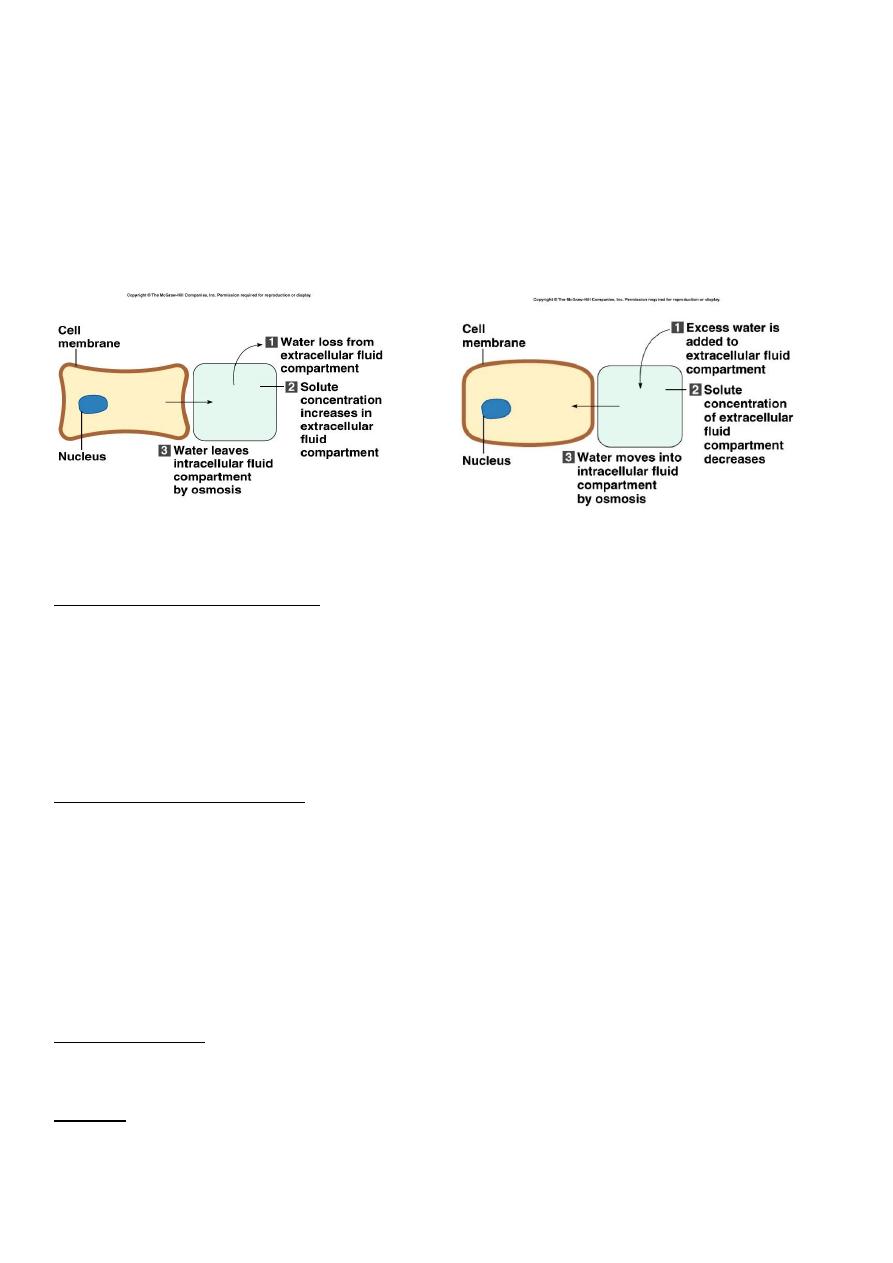
Movement of water between compartments
– Normally, cells neither shrink or swell because intracellular and interstitial fluids have
the same osmolarity
Increasing osmolarity (tonicity) of interstitial fluid draws water out of cells and cells shrink
Decreasing osmolarity of interstitial fluid causes cells to swell
– Changes in osmolarity most often result from changes in Na
+
concentration
– Water excess & intoxication – drinking water faster than the kidneys can excrete it
• Can lead to convulsions, coma or even death
Osmosis
is the primary method of water movement into and out of body fluid
compartments.
4
Osmosis is the net movement of water molecules through a selectively permeable
membrane from an area of high water concentration to an area of lower water
concentration.
The concentration of solutes determines the direction of water movement.
Most solutes in the body are electrolytes – inorganic compounds which dissociate into ions
in solution.
“Where sodium goes, water follows.”
Solutes : dissolved particles
Electrolytes – charged particles
Cations – positively charged ions
Na
+
, K
+
, Ca
++
, H
+
Anions – negatively charged ions
Cl
-
, HCO
3
-
, PO
4
3-
Non-electrolytes - Uncharged
Proteins, urea, glucose, O
2
, CO
2
ICF differs considerably from ECF
ECF : most abundant cations are Na
+
followed by Ca
++
ECF anions : Cl
-
, HCO3
-
Sodium-functions
Impulse transmission, muscle contraction, fluid and electrolyte balance
Chloride
Regulating osmotic pressure, forming HCl in gastric acid
5
ICF : most abundant cation is K
+
followed by Mg
++
ICF anions : negatively charged proteins and phosphates (HPO
4
2-
) ICF contain more protein
than plasma
Potassium - function
Resting membrane potential ,
action potentials of nerves and muscles
Maintain intracellular volume
Regulation of pH
Controlled by aldosterone
• Na
+
/K
+
pumps play major role in keeping K
+
high inside cells and Na
+
high outside cell
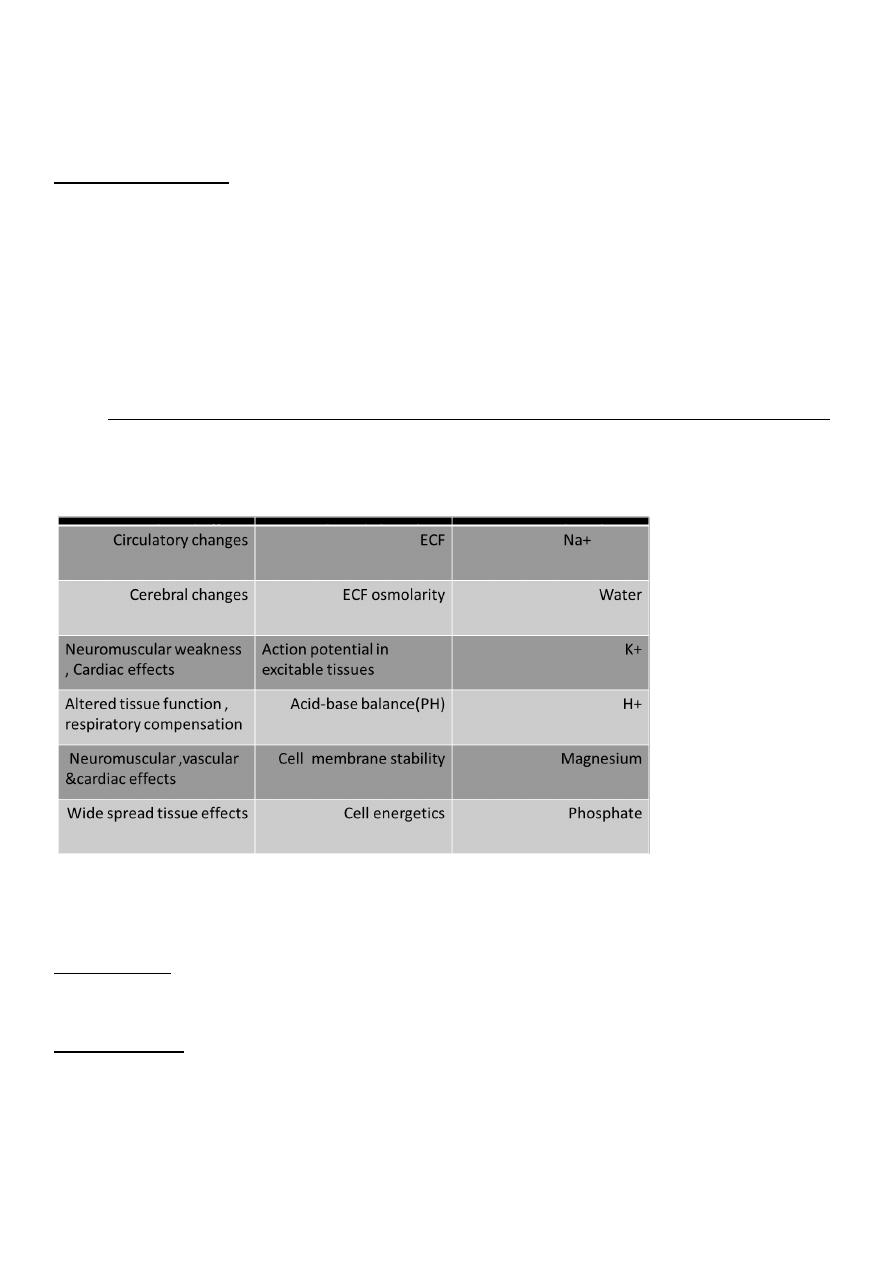
Manifestation of disorders of water , electrolytes and acid-base status
How to interpret electrolyte , urea and creatinine results
Na+ (sodium)
• Largely reflects reciprocal changes in body water content
K+ (potassium)
• May reflect K shifts in and out of cells
• Low levels usually mean excessive losses (gastrointestinal or renal)
• High levels usually mean renal dysfunction

6
Cl− (chloride)
• Generally changes in parallel with plasma Na
• Low in metabolic alkalosis • High in some forms of metabolic acidosis
HCO3− (bicarbonate)
• Abnormal in acid–base disorders
Urea
• Increased with a fall in glomerular filtration rate (GFR),reduced renal perfusion or urine
flow rate, and in high protein intake or catabolic states
Creatinine
• Increased with a fall in GFR, in individuals with high musclemass, and with some drug
