
1
Third stage
Medicine
Lec-5
د
.
زين
العابدين
1/1/2014
Autoimmune Diseases
“Autoimmunity and Immunity-Mediated Disorders”
Autoimmunity
Autoimmunity is the failure of self-tolerance; and is a specific immunological reaction
against self-structures (i.e., the immune system mistakenly attacks and destroys healthy
body tissue “Horror Autotoxicus”).
Tolerance
Tolerance is the state of specific immunological unresponsiveness, i.e., the absence of
specific immune response to a particular antigen in a fully immunocompetent person.
Tolerance is a mechanism which the immune system can learn.
Mechanisms of tolerance
Tolerance is divided into:
1. Central
2. Peripheral
Tolerized cells are:
1. T-cells
2. B-cells
3. Phagocytic cells:
A. Central tolerance
Clones of B- and T-cells reactive to self-antigens are deleted in the bone marrow and the
thymus respectively by a process called negative selection which explain the basis of the
previously called “ Clonal Deletion theory”.
2
B. Peripheral tolerance
There are different mechanisms to deal with escaped self-reactive B- and T- cells after
leaving the bone marrow and thymus in order to control their response by:
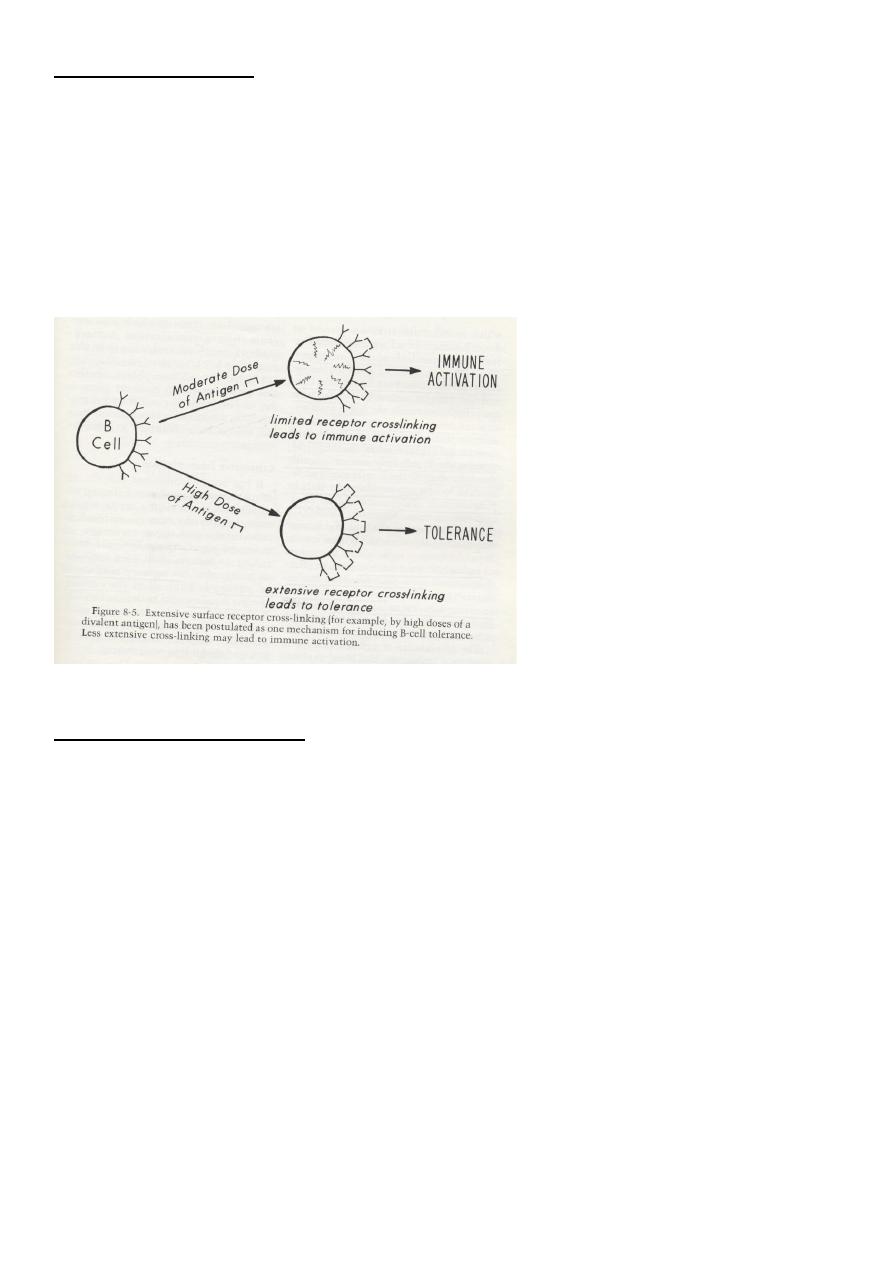
1. Excessive cross-linking of B-cells receptors for antigens (clonal anergy/ignorance).
2. Inadequate co-stimulatory signals, e.g., CD28-CD80 (clonal anergy/ignorance)
3. Role of T-reg cells and their cytokines (TGF-beta, IL10)
4. Idiotype-Anti-idiotype network (immune network theory)
C. Phagocytic Cells Tolerance
Antigens or immune complexes (blocking factors) which cannot be cleared up (persist) from
the body result in tolerance.
Promotion or break of tolerance
Antigens could be immunogenic or tolerogenic, depending on different factors such as dose
and route of administration.
Immunogenic antigens lead to the stimulation and proliferation of lymphocytes (refer to
the lecture of Antigens/Immunogens).
Tolerogenic antigens lead to apoptosis or a state of anergy of lymphocyte response. There
are different mechanisms that contribute in promotion of tolerance by:
1. High doses of antigens leading to excessive cross-linking of B-cell antigen receptors.
2. Failure of clearance of the antigen or immune complexes by the RES (persistence of
antigens/phagocytic cells tolerance)

3
3. Low levels of co-stimulatory signals
4. Intravenous or oral administration of the antigens.
5. Sequestered antigens, e.g., sperms extravasations, lens
6. Molecular mimicry (cross-reactivity), e.g., rheumatic fever carditis due to molecular
mimicry between group A beta-haemolytic streptococcal epitopes and cardiac myosin
epitopes.
7. Infective agents may provide excessive T-cell stimulation, tissue destruction and
antigenic modification (e.g., bacterial antigens may act as adjuvants, or tissue cross-
reactivity (e.g., reactive arthritis to gonococcal infection, or enteropathic arthritis to certain
bacterial infection as Campylobactor, Salmonella). This includes modified antigens by
viruses, chemicals, or drugs.
8. Superantigens that activate numerous T-cell clones.
9. Age and gender, e.g. female preponderance to SLE with DR4, and male preponderance to
ankylosing spondylitis "AS" (B27)
10. Life style (smoking in Goodpasture's syndrome) and stress
11. Complement deficiencies (C1q, C2, and C3) are associated with SLE-like autoimmune
diseases due to inhibition of clearance of immune complexes and apoptotic cell bodies.
Classification of autoimmune diseases
I. Organ-specific autoimmune diseases
- Insulin-dependent DM (type-I)
- Rheumatoid arthritis

4
- Grave's disease
- Goodpasture's syndrome
- Myasthenia gravis
- Multiple sclerosis
- Pernicious anaemia
- Autoimmune haemolytic anaemia
- Sjogren’s syndrome
- Dermatomyositis
II. Systemic autoimmune diseases
- SLE
- Systemic sclerosis
- Mixed connective tissue disease
Aetiology of Autoimmune Diseases
I. Release of sequestrated antigens: Certain tissues, e.g., eye lens, sperms, CNS are
immunologically at “privileged sites” (inaccessible to the immune system).
II. Infectious microbes may produce peptide antigens that are similar to and cross react
with, self antigens, this is called “molecular mimicry” or “Idiotype Cross-Reaction”
Example one: Antibodies formed against M protein of Streptococcus pyogenes cross react
with cardiac myosin leading to rheumatic fever.
Example two: Rubella and diabetes type I
Example three: Certain bacterial infections (Campylobacter, Salmonella, Shigella, N.
gonorrhoeae) and reactive arthritis.
III. Alteration of self antigens or appearance of new antigens under the effect of chemicals,
viral infections or drugs such as anaesthetic agent halothane in causing hepatic necrosis by
autoantibodies and activated T-cells
IV. Decrease in suppressor functions of T cells (which play a role in maintenance of
tolerance) or defects in immune regulatory mechanisms.
V. T-Cell Bypass - A normal immune system requires the activation of B-cells by T-cells
before the former can produce antibodies in large quantities.
VI. Genetic predisposition
1. There is a familial incidence of autoimmune diseases. Certain diseases run in families,
e.g., SLE, RA
2. There is a concordance rate of susceptibility to development of diseases; example in
type-I DM 30%-50% concordance in monozygotic twins.
5
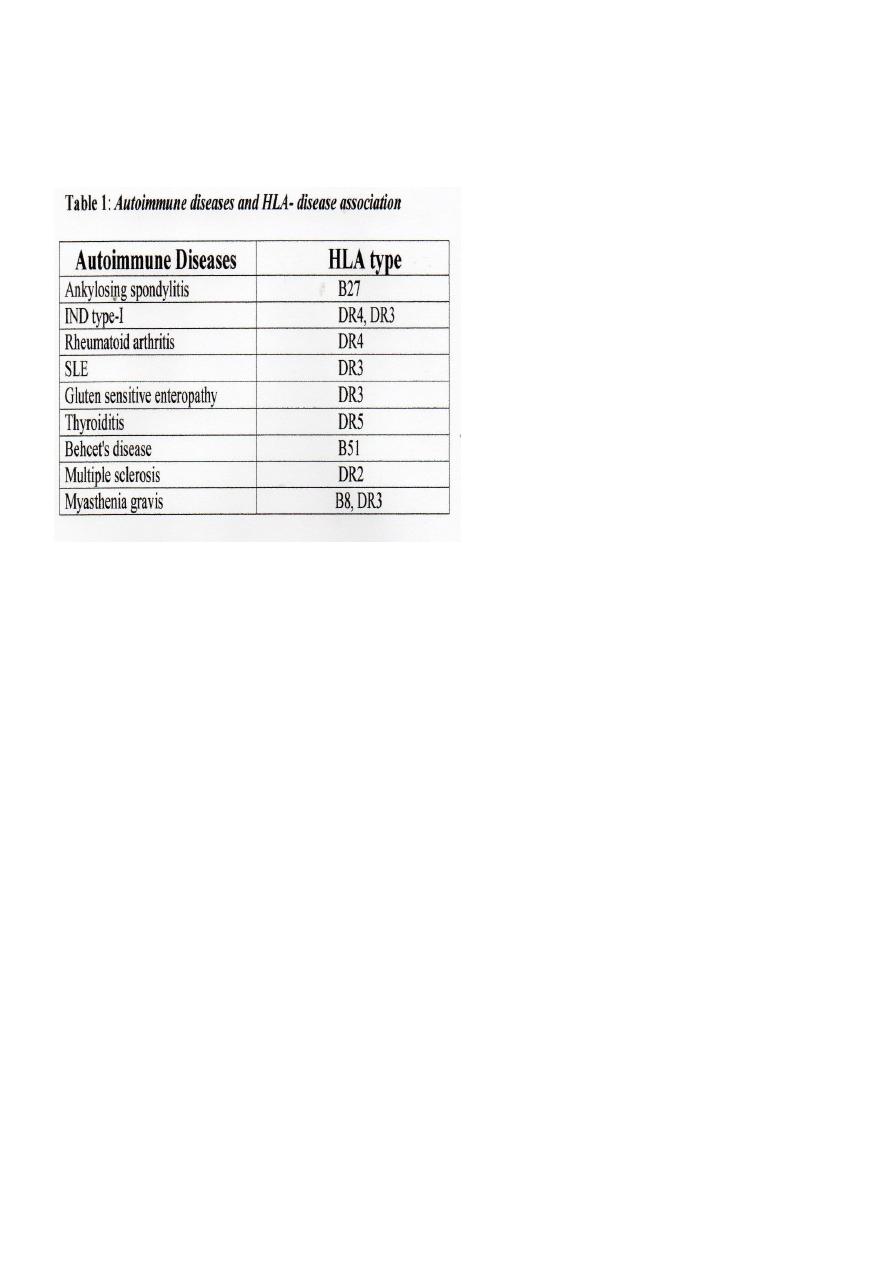
3. Most of autoimmune diseases appear to be associated with certain HLA antigens/ genes;
each with relative risk (see Table 1).
4. Females are more prone to develop autoimmune diseases than males with the exception
for ankylosing spondylitis (for unclear reasons).
Mechanisms of Tissue Damage in Autoimmune Diseases
1. Cytotoxic reactions (type II) as occurs in autoimmune haemolytic anaemias, Hashimoto’s
thyroiditis, and Grave’s disease
2. Immune complex deposition (type III) in blood vessel walls, skin, joints, glomeruli, or
generalized, e.g., SLE and rheumatoid arthritis.
3. DHS (type IV) by T cells as in ulcerative colitis, coeliac disease, Gillian-Bare syndrome
(occurs with influenza virus and Campylobacter infection).
Symptoms of autoimmune diseases
Symptoms of an autoimmune disease vary based on the disease and location of the
abnormal immune response. Symptoms that often occur with autoimmune diseases include
fatigue, malaise, and fever. Symptoms of autoimmune disorders can come and go. When
symptoms get worse, it is called a flare-up. Most autoimmune diseases are
, but many
can be controlled with treatment.
Laboratory Diagnosis of Autoimmune Diseases
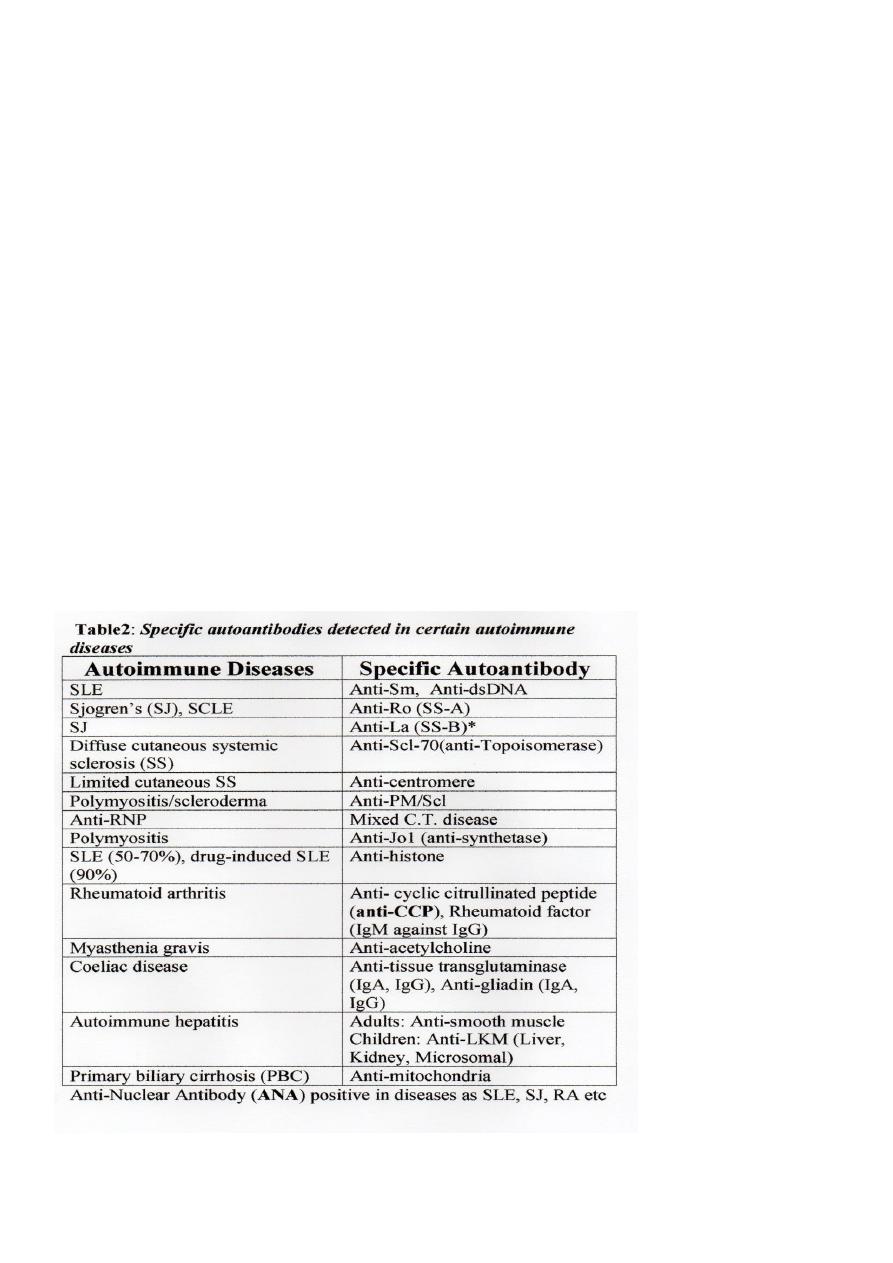
1. Autoantibodies – see the Table
2. Total immunoglobulin concentration may be increased
6
3. Cryoglobulins are antibodies directed against other immunoglobulins that form immune
complexes which precipitate in cold.
- Type I: Monoclonal IgM paraprotein with no particular specificity, e.g. lymphoproliferative
diseases especially Waldonstrom’ macroglobulinaemia; normal complement level
- Type II: Monoclonal IgM agains IgG constant region, e.g. hepatitis C or B; decreased C4
- Type II: Polyclonal IgM or IgG against IgG constant region, e.g. hepatitis C or B, SLE, RA;
decreased C4
4. Complement levels may be decreased in particular C3 and C4.
Low serum level in active SLE, low in synovial fluid in active RA.
5. Immune complexes may be detected in serum. Biopsy from
organs may also show immune complex deposition or lymphocyte infiltration. Tissue
immune complexes and their pattern of staining is usually detected by
immunofluorescence staining, e.g., SLE glomerulonephritis, or RA (lumpy-bumpy pattern on
glomerular basement membrane).
6. HLA typing- See Table 1.
7. General laboratory tests such as CBC, ESR, and CRP…
7
Management of autoimmune diseases
The right treatment of autoimmune disease depends on several factors including the type
of disease, how severe it is, and any accompanying symptoms. The goals of treatment are
to relieve symptoms, preserve organ function, and target disease mechanisms. Multi-
discplinary specialists in this field of treatment include rheumatologists (e.g., arthritis,
scleroderma, SLE), endocrinologists (diabetes, thyroid diseases), and neurologists (MS, MG),
haematologist (pernicious or haemolytic anaemias ), gastroenterologist (Coeliac disease),
dermatologist (pemphigus/pemphigoid)
1. Anti-inflammatory drugs, e.g., NSAIDs (e.g., diclofenac “voltaren”, ibuprofen),
corticosteroids, or hydroxychloroquine
2. Immunosuppressive agents, e.g., azathioprine, cyclosporine, methotrexate,
cyclophosphamide. Side effects of medications used to suppress the immune system can be
severe, such as infections that can be hard to control.
3. Biological agents- see lecture 3/General Medicine
4. Plasmapheresis (plasma exchange therapy) to remove the offending immune complexes
and antibodies may be conducted.
5. Some patients may need supplements to replace a hormone or vitamin that the body is
lacking. Examples include thyroid supplements, vitamins such as B12, or insulin injections.
6. Surgery sometimes is required to correct or preserve functions.
1. Clonal Anergy/Clonal Ignorance theory
Occurs when:
- T-cells recognize antigens without adequate levels of the “co-stimulatory signals” between
B (APC) and T-cells as a second signal, e.g., CD28-CD80 (Remember that the first signal is
between TCR –Ag and HLA combination).
- B-cells with excessive cross linking of surface receptors by high doses (high zone tolerance)

8
2. Idiotype-Antiidiotype Network theory
Disturbance of the balance on B cells between help and suppression is mediated by CD4
subpopulation.
3. Molecular Mimicry
Similarity between invading antigens and self antigens results in tolerance to the foreign
(invading) antigen.
4. T-reg cell (suppressor population) theory
Block or reduction of inhibitory cytokine production by T-reg cells (e.g. IL-10, or TGF-beta)
leading to uncontrolled B-cells antibody response.
