
DISEASES OF THE
FEMALE GENITAL
TRACT
Part two

Endometrial Hyperplasia
This is an exaggerated endometrial proliferation
induced by sufficiently prolonged excess of
estrogen relative to progestin.
The severity of hyperplasia is classified according
to two parameters
a. architectural crowding of the glands &
b. cytologic atypia.
Accordingly there are three categories
1. Simple hyperplasia
2. Complex hyperplasia
3. Atypical hyperplasia

These three categories represent a spectrum based
on
the level and duration of the estrogen excess.
The risk of developing carcinoma depends on the
severity of the hyperplastic changes and associated
cellular atypia.
Simple hyperplasia carries a
negligible risk
, while a
woman with
atypical hyperplasia has a 20% risk of
developing endometrial carcinoma
.
Thus When atypical hyperplasia is discovered, it
must be carefully evaluated for the presence of
cancer and must be monitored by repeated
endometrial biopsy.

Any
estrogen excess
may lead to hyperplasia.
Potential contributors include
1. Failure of ovulation, e.g. around the menopause
2. Prolonged administration of estrogenic steroids
3. Estrogen-producing ovarian lesions such as
a. polycystic ovaries (Stein-Leventhal syndrome)
4. Obesity, because adipose tissue processes steroid
precursors into estrogens.

• A 45-year-old female has had menorrhagia
for the past 3 months. An endometrial
biopsy shows a rather mild degree of
endometrial hyperplasia. Which of the
following conditions most likely helped to
produce the findings in this case?
1-Ovarian mature cystic teratoma
2-Chronic endometritis
3-Failure of ovulation
4-Pregnancy
5-Use of oral contraceptives

Gross features
In
simple hyperplasia
the endometrium is
diffusely thickened .
In
complex & atypical hyperplasia
there is
usually
focal thickening
of the
endometrium.

Simple hyperplasia without atypia, also known as cystic
or mild hyperplasia, is characterized by glands of
various sizes and irregular shapes with cystic dilatation.
There is a mild increase in the gland-to-stroma ratio.
The epithelial growth pattern and cytology are similar to
those of proliferative endometrium, . These lesions
uncommonly progress to adenocarcinoma
approximately 1%)
Simple hyperplasia with atypia is uncommon.
Architecturally it has the appearance of simple
hyperplasia, but there is cytologic atypia within the
glandular epithelial cells, as defined by loss of polarity,
vesicular nuclei, and prominent nucleoli.
Approximately 8% of such lesions progress to
carcinoma.

Complex hyperplasia without atypia shows an increase in the
number and size of endometrial glands, marked gland
crowding, and branching of glands. the glands may be
crowded back-to-back with little intervening stroma .
However, the glands remain distinct and the epithelial cells
remain cytologically normal. This class of lesions has about a
3% progression to carcinoma,
Complex hyperplasia with atypia has considerable
morphologic overlap with well-differentiated endometrioid
adenocarcinoma and an accurate distinction between
complex hyperplasia with atypia and cancer may not be
possible without hysterectomy It has been found that
approximately 23% to 48% of women with a diagnosis of
complex hyperplasia with atypia have carcinoma when a
hysterectomy is performed shortly after the endometrial
biopsy or curettage.
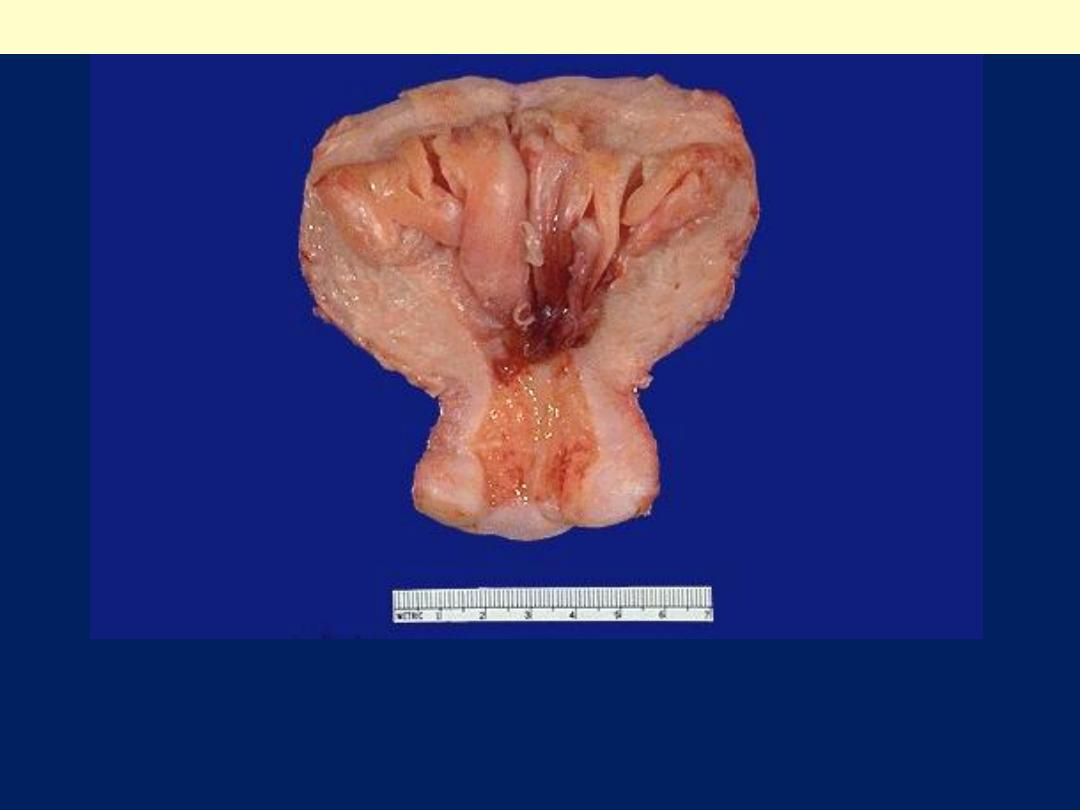
The prominent thickened folds of endometrium in this uterus
(opened to reveal the endometrial cavity) are an example of
hyperplasia.
Diffuse endometrial hyperplasia
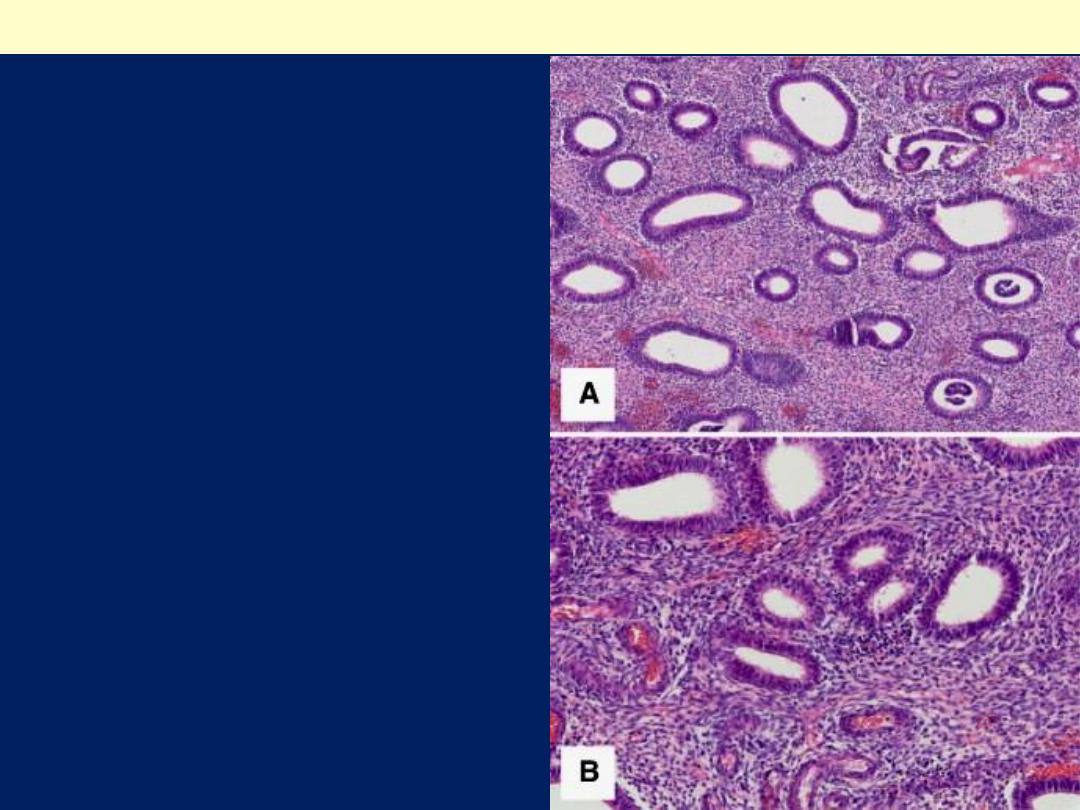
Closely packed
endometrial glands with
irregular distribution,
separated by abundant
endometrial stroma (A).
Glands are lined by
proliferative-type
endometrial epithelium
without atypia (B).
Complex endometrial hyperplasia without atypia
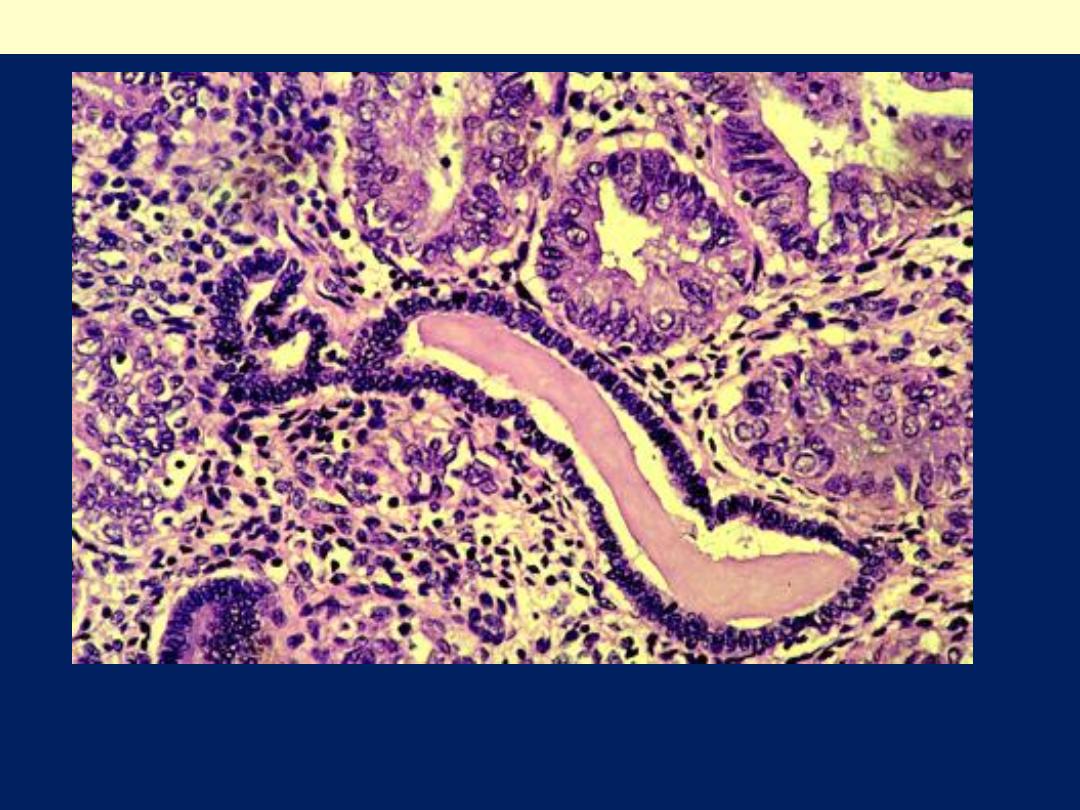
Nuclear and cytoplasmic differences are present between
atypical glands (upper Rt.) and residual normal endometrial
glands (center).
Atypical endometrial hyperplasia

Tumors of the Endometrium and
Myometrium
The most common neoplasms of the body of
the uterus are
1. Endometrial polyps,
2. Smooth muscle tumors.
3. Endometrial carcinomas.
All tend to produce
bleeding
from the uterus
as the earliest manifestation.
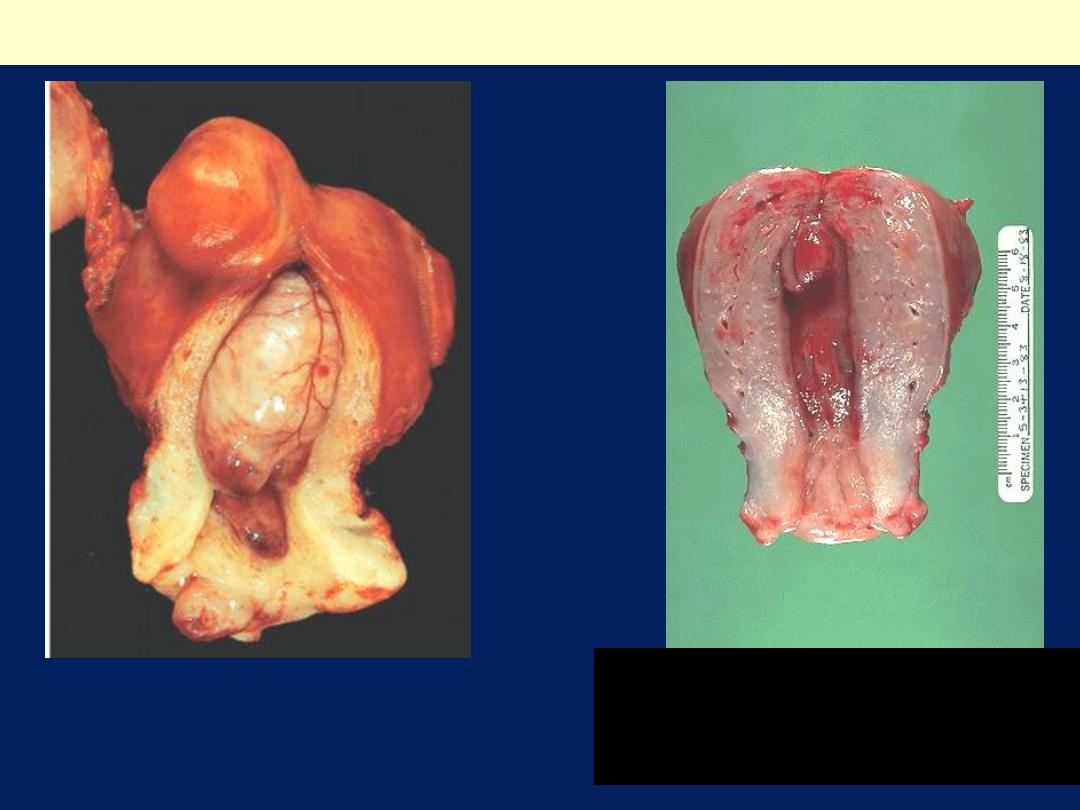
Endometrial polyp
Huge endometrial polyp filling the
endometrial cavity. There is also a smaller
endocervical polyp and a subserosal
leiomyoma.
The uterus has been opened anteriorly
through cervix and into the endometrial cavity.
High in the fundus and projecting into the
endometrial cavity is a small endometrial
polyp. Such benign polyps may cause uterine
bleeding.

•
Endometrial polyps are exophytic masses of
variable size that project into the endometrial
cavity. They may be single or multiple and are
usually sessile, measuring from 0.5 to 3 cm in
diameter, but are occasionally large and
pedunculated. Polyps may be asymptomatic or
may cause abnormal bleeding (intramenstrual, or
postmenopausal) if they ulcerate or undergo
necrosis. Most commonly the glands within polyps
are hyperplastic or atrophic, but they can
occasionally demonstrate secretory changes
(functional polyps) . Atrophic polyps, which largely
occur in postmenopausal women, most likely
represent atrophy of a hyperplastic polyp. Rarely,
adenocarcinomas arise within endometrial polyps
.
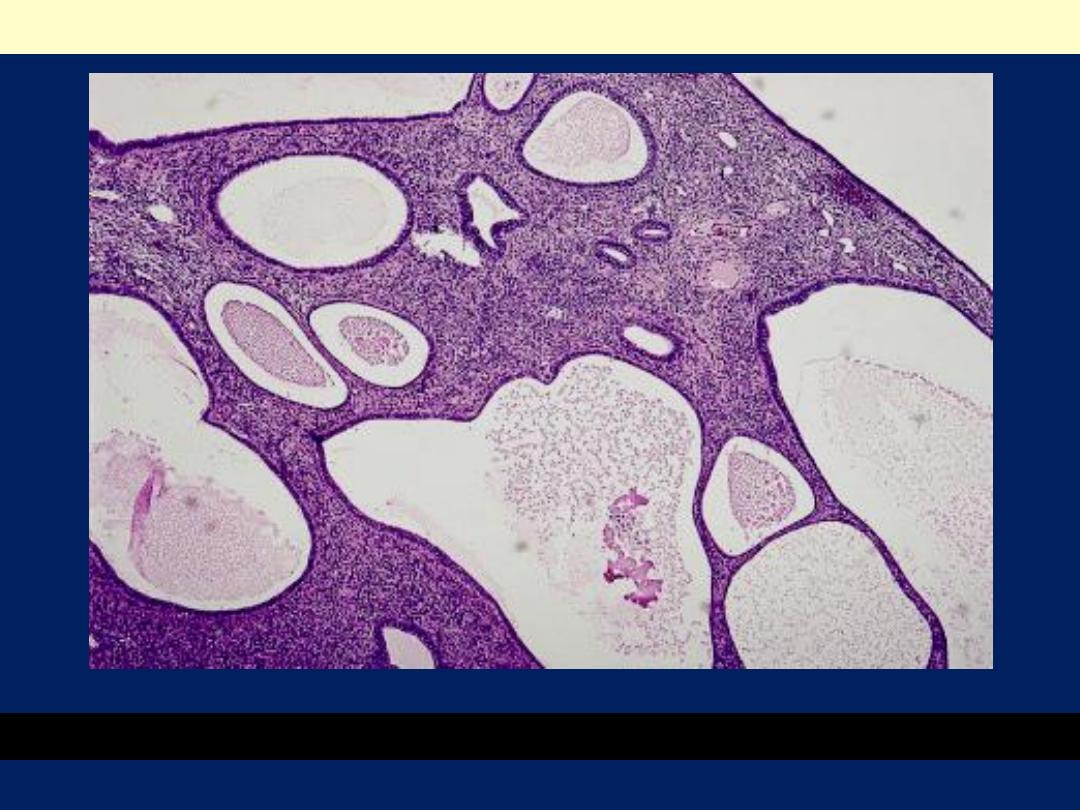
Cystically dilated glands and a fibrous stroma with thick-walled vessels.
Endometrial polyp LP mic

Leiomyomas
Are
benign tumors that arise from the smooth
muscle cells in the myometrium.
Because of their firmness they are also called
fibroids
.
They are
the most common benign tumor in
females
and are found in 30% to 50% of
women during reproductive life.
Estrogens and possibly oral contraceptives
stimulate their growth
; conversely, they
shrink postmenopausally.

Gross features
They are sharply circumscribed, firm gray-white masses
with a characteristic
whorled
cut surface.
They may occur
singly
, but are
often multiple tumors
scattered within the uterus, ranging in size from small
seedlings to massive neoplasms that dwarf the size of
the uterus.
Some are embedded within the myometrium
(
intramural
),
whereas others may lie directly beneath
the endometrium
(
submucosal
)
or directly beneath the
serosa
(
subserosal
).
Larger neoplasms may show foci of ischemic necrosis
with areas of hemorrhage and cystic softening (red
degeneration)
After menopause they may become densely
collagenous and even calcified
.
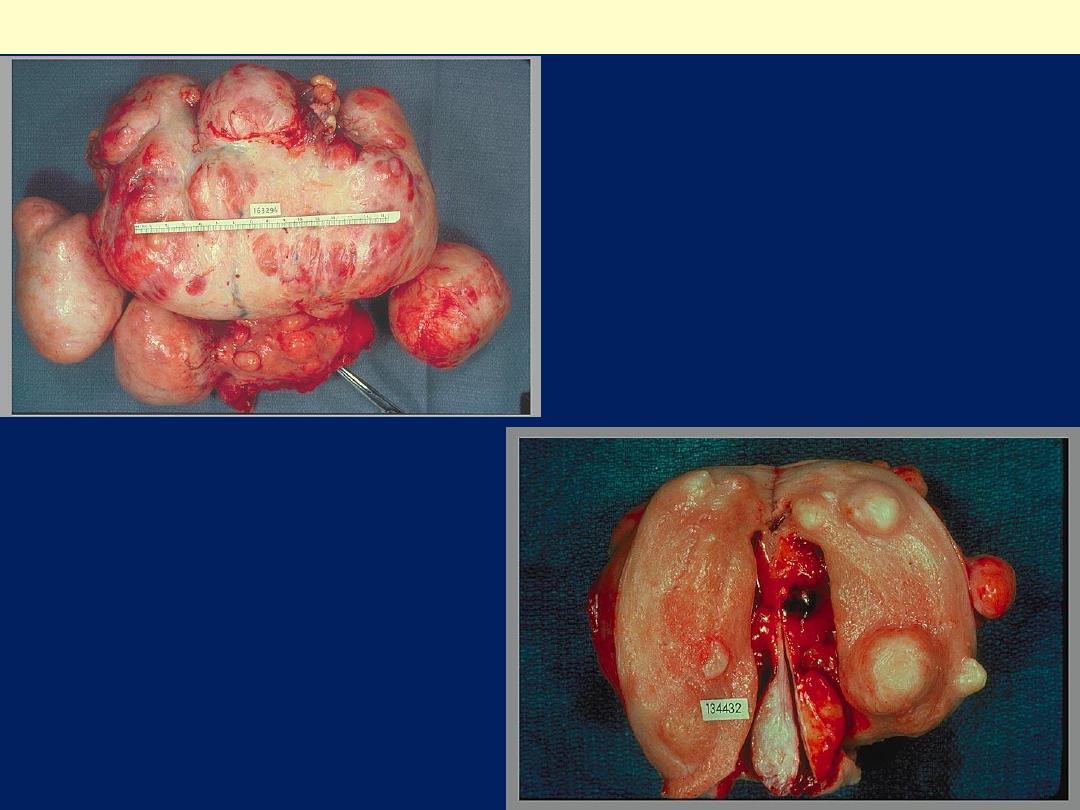
Markedly distorted
uterine corpus with
multiple irregular
masses protruding
from its surface.
These are subserosal
leiomyomata.
Leiomyomas uteri
The uterus has been opened
revealing several large masses
that protrude from under the
endometrial mucosa
(submucosal). There are also
intramural and subserosal
examples. One of the
submucosal tumor is in the form
of pedunculated polyp that has
protruded through the cervix
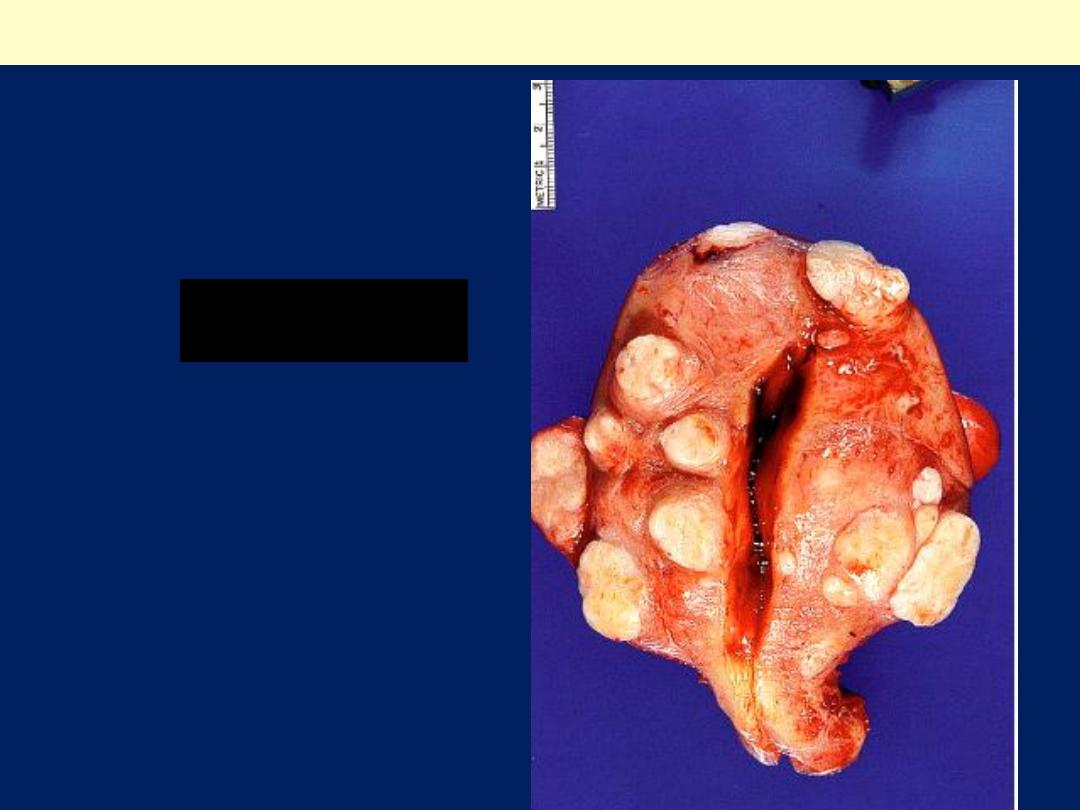
Leiomyoma uterus gross
Multiple uterine
leiomyomas
.
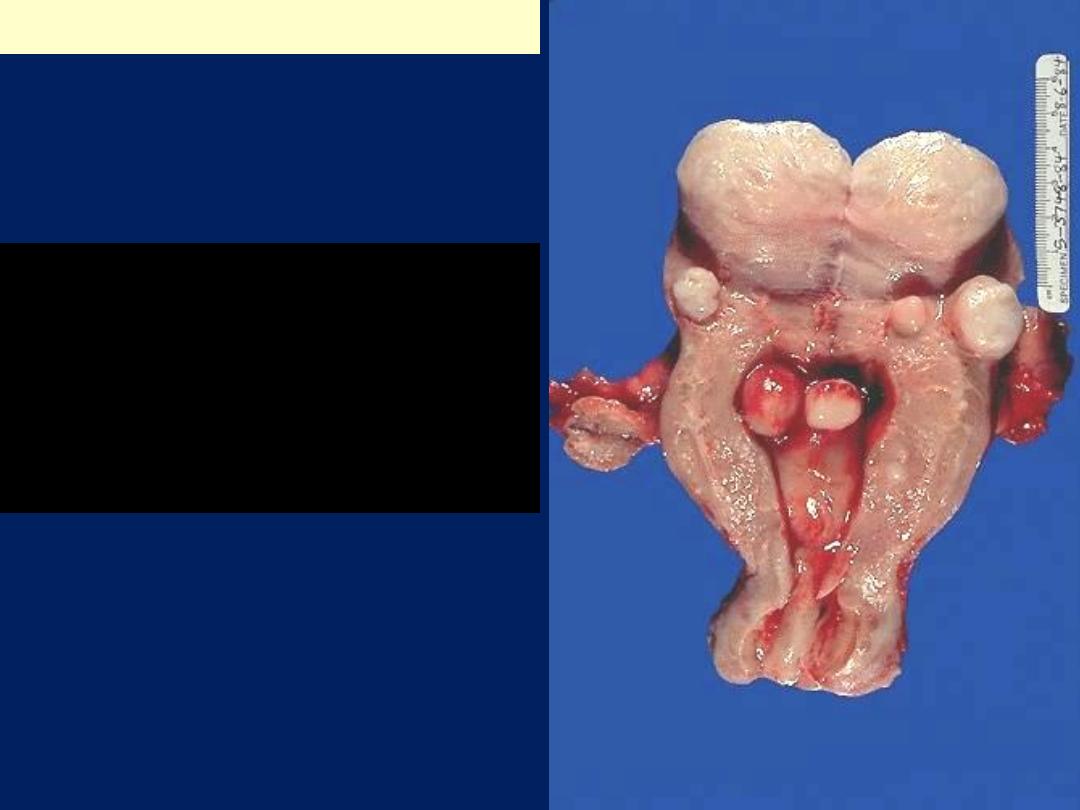
Smooth muscle tumors of the
uterus are often multiple.
Seen here are submucosal,
intramural, and subserosal
leiomyomata of
the uterus.
Uterus, leiomyomata
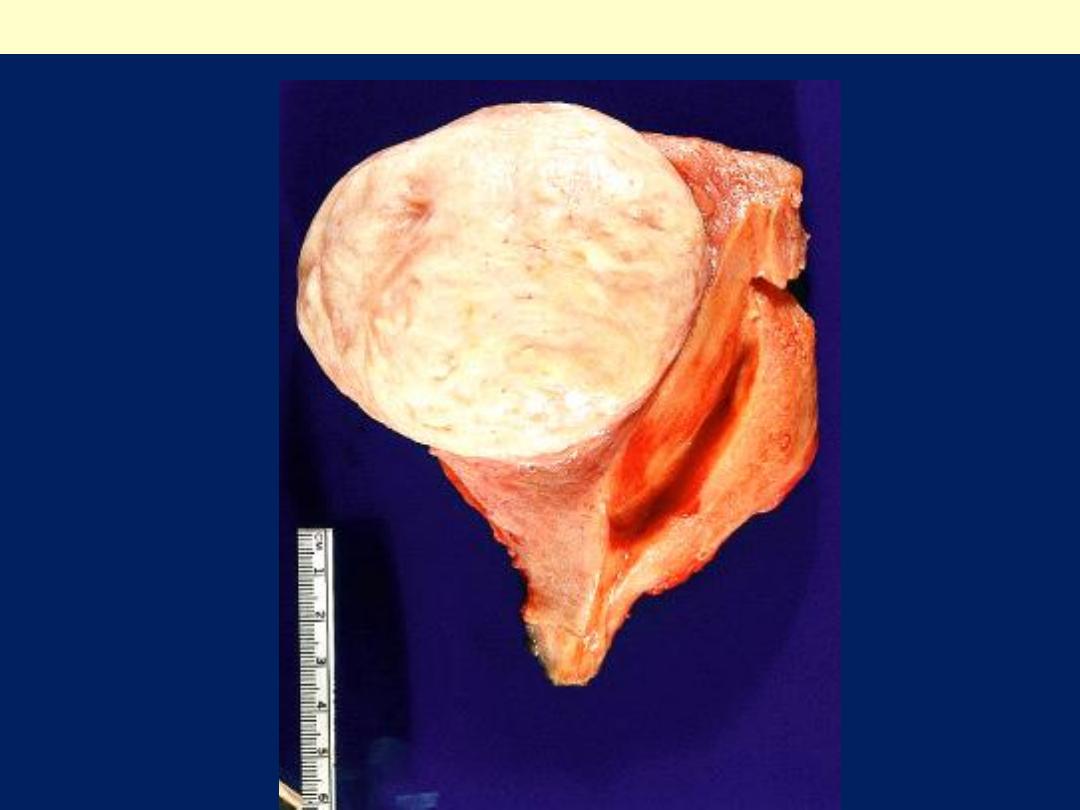
Leiomyoma uterus gross

Microscopic features
There are
whorling bundles of smooth muscle cells
.
Foci of fibrosis, calcification, ischemic necrosis,
cystic degeneration, and hemorrhage may be
present.
Leiomyomas of the uterus may be entirely
asymptomatic and be discovered only on routine
pelvic examination or imaging studies.
The most frequent manifestation, when present, is
menorrhagia
.
Large masses in the pelvic region may become
palpable or may produce a
dragging sensation
.
Benign leiomyomas rarely transform into sarcomas.
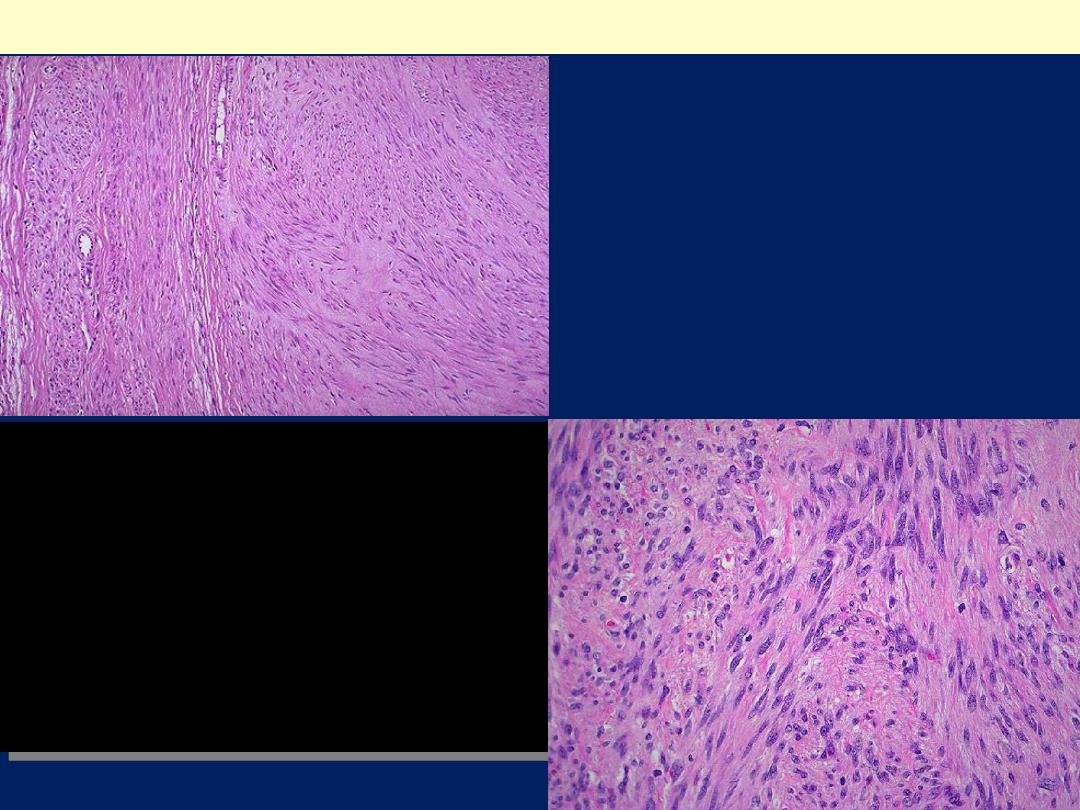
Here is the microscopic appearance
of a benign leiomyoma. Normal
myometrium is at the left, and the
neoplasm is well-differentiated so
that the leiomyoma at the right
hardly appears different. Bundles of
smooth muscle are interlacing in the
tumor mass.
Leiomyoma uteri
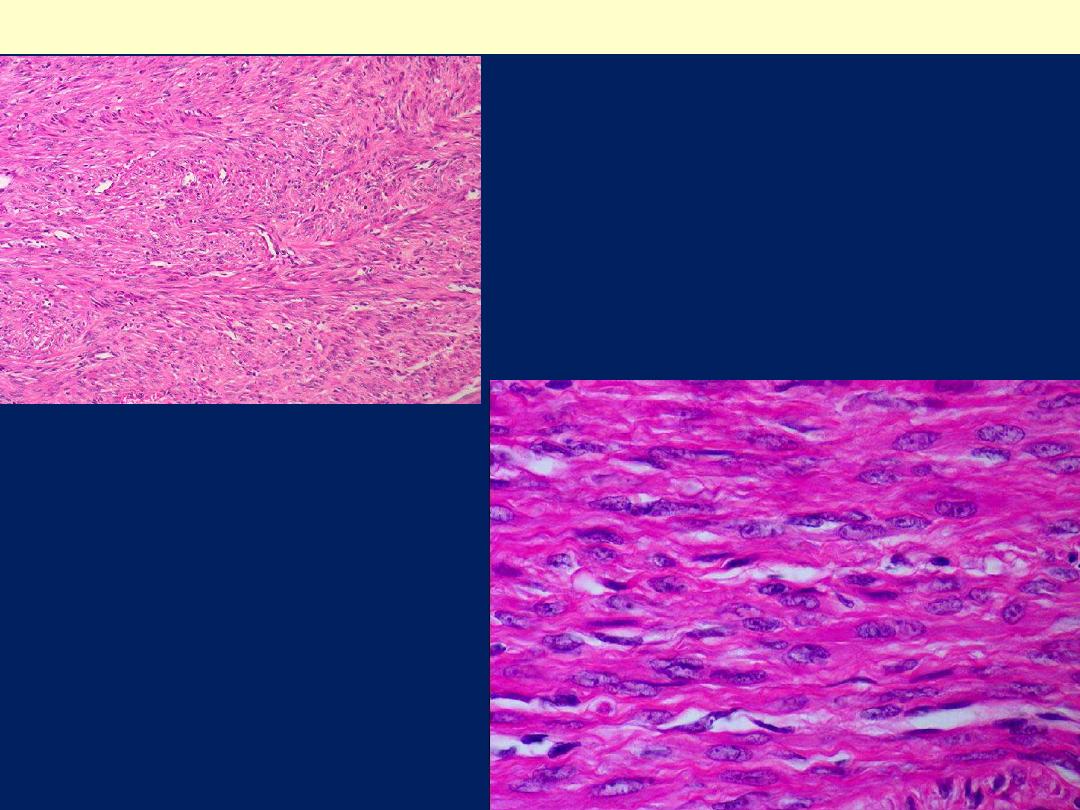
Leiomyoma uteri
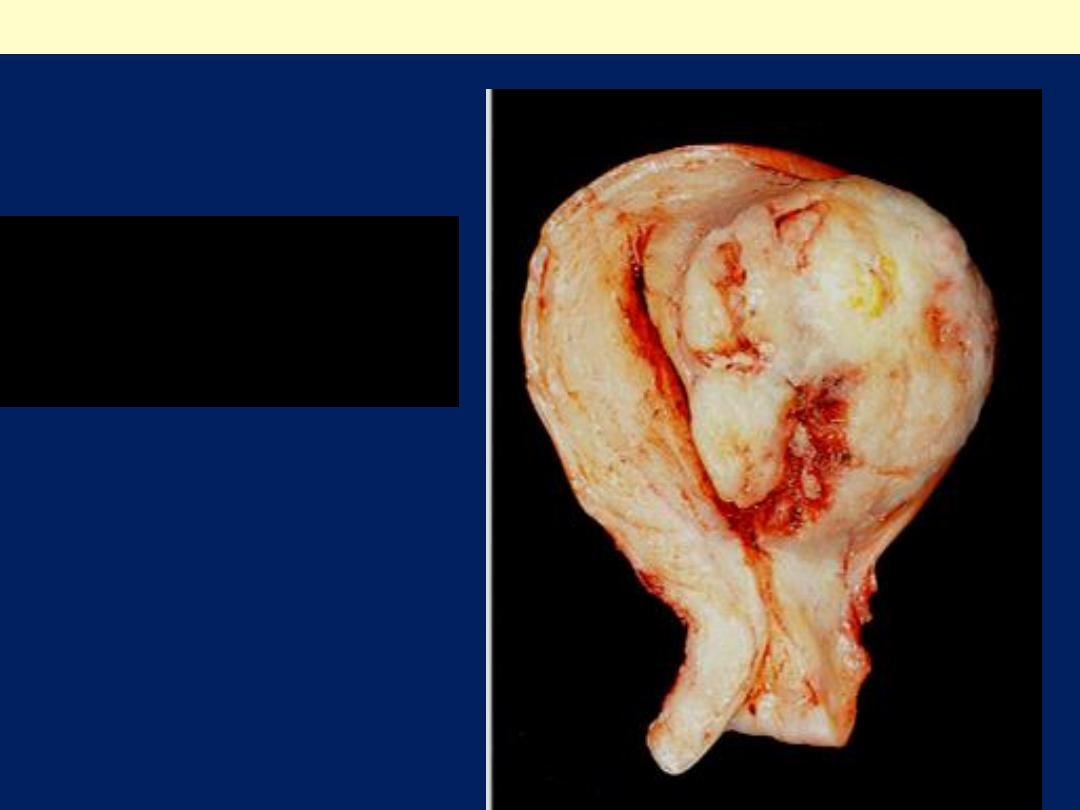
Leiomyosarcoma uterus gross
The tumor forms a large ,
irregular intramural and
submucous mass. There are
foci of hemorrhage (red) and
necrosis( yellow).
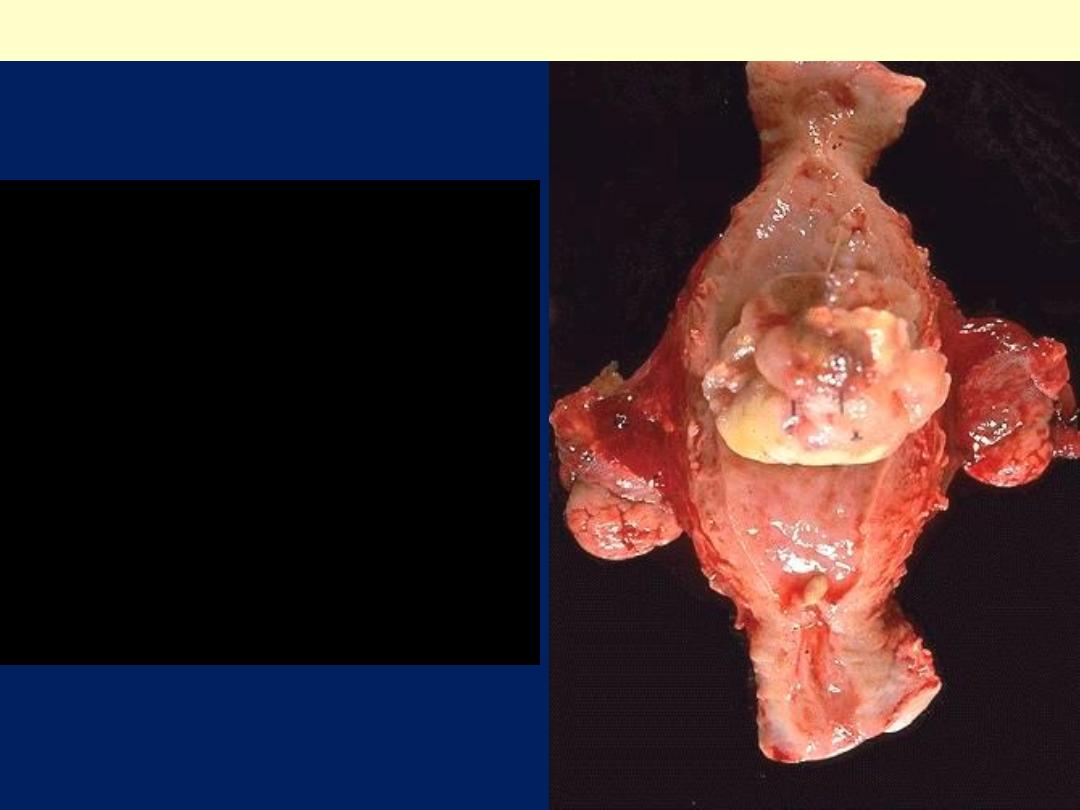
This is a leiomyosarcoma
protruding from myometrium
into the endometrial cavity of
this uterus that has been
opened sagittally so that the
halves of the cervix appear at
up and down. Fallopian tubes
and ovaries project from both
sides. The irregular nature of
this mass suggests that is not
just an ordinary leiomyoma.
Uterus, leiomyosarcoma, Gross

Leiomyosarcomas
Typically arise de novo from the
mesenchymal
cells of
the myometrium.
They are
almost always solitary tumors
, in
contradistinction to the frequently multiple leiomyomas.
Gross features
The tumor is typically bulky
It infiltrates the uterine wall.
Sometimes it projects into the endometrial cavity.
They are frequently soft, hemorrhagic, and necrotic.

Microscopic features
They show a wide range of differentiation,
from those that closely resemble leiomyoma
to wildly anaplastic tumors.
The diagnostic features of leiomyosarcoma
include
tumor necrosis
,
cytologic atypia
, and
mitotic activity
.
Sometimes increased mitotic activity is seen
in benign smooth muscle tumors in young
women,
thus assessment of all three features
is needed to make a diagnosis of malignancy
Recurrence
after removal is common with
these cancers, and many metastasize,
typically to the lungs.
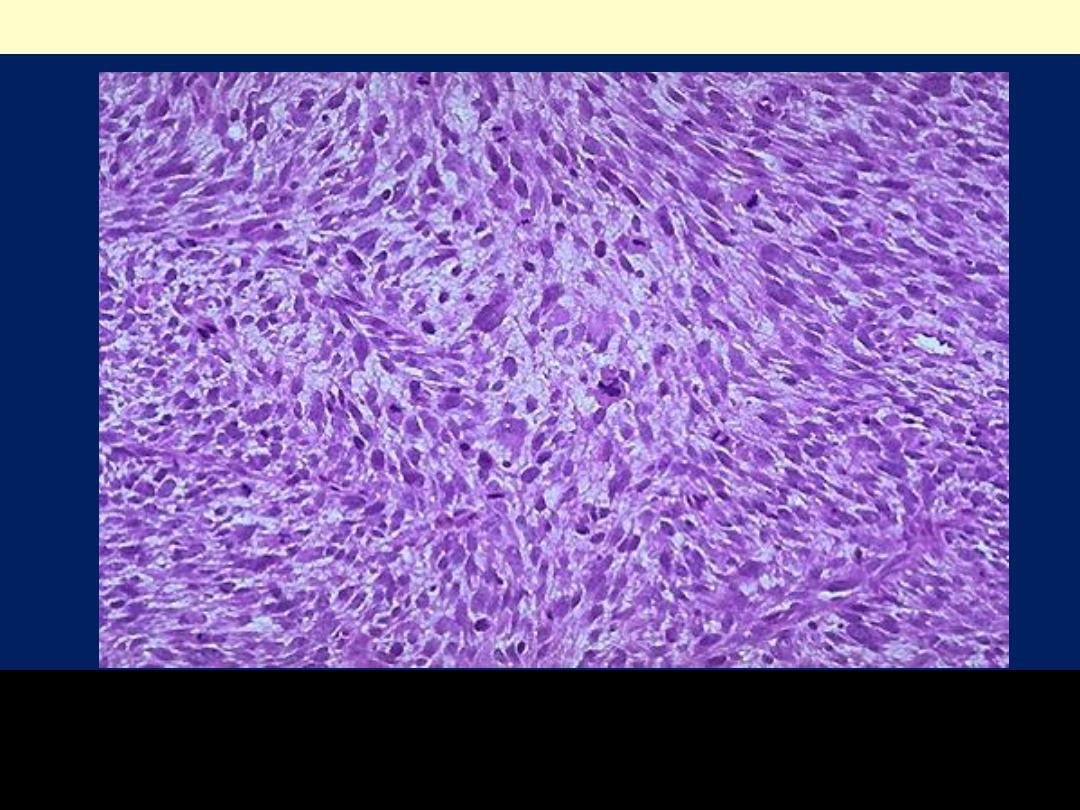
The tumor is much more cellular and the cells have much
more pleomorphism and hyperchromatism than the benign
leiomyoma. An irregular mitosis is seen in the center.
Leiomyosarcoma
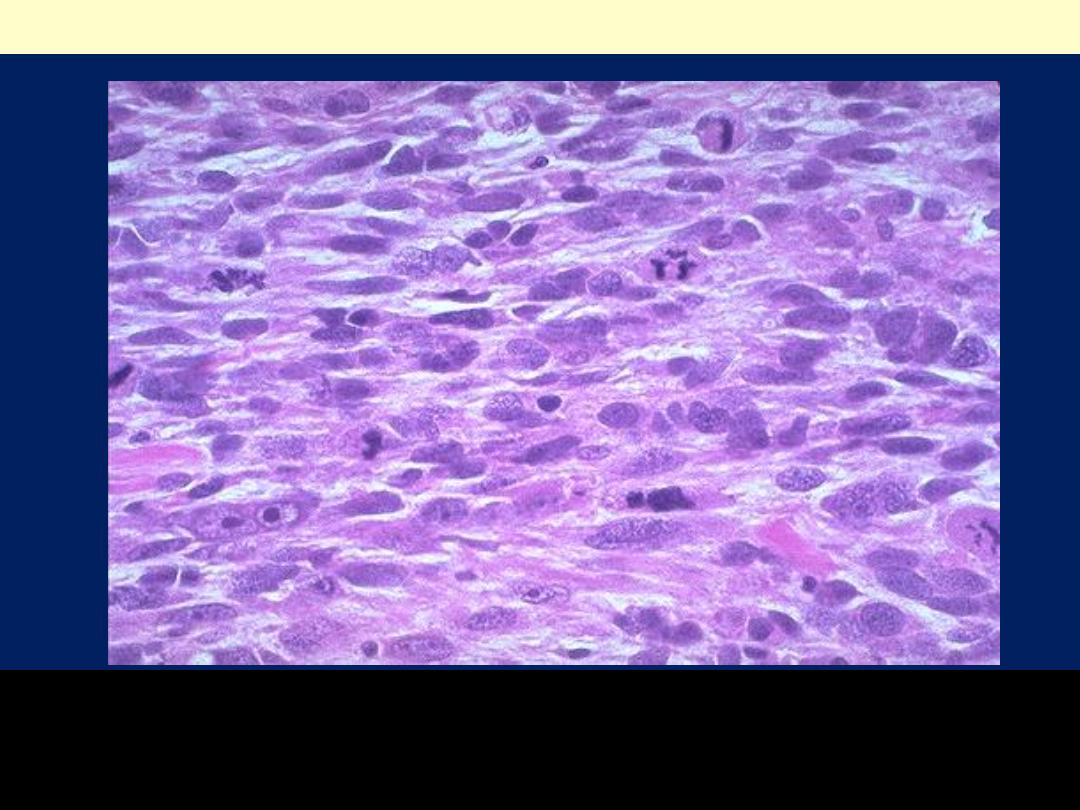
As with sarcomas in general, leiomyosarcomas have spindle
cells. Several mitoses are seen here, just in this one high
power field.
Leiomyosarcoma

Endometrial Carcinoma (EMC)
After the dramatic drop in the incidence of
cervical carcinoma, EMC is currently the most
frequent cancer occurring in the female genital
tract.
Epidemiology and Pathogenesis
EMC appears most frequently
around the age of
60 years.
There are
two clinico-pathological settings in
which endometrial carcinomas arise:
1. In perimenopausal women with estrogen
excess; these are of endometrioid type
2. In older women with endometrial atrophy; these
are of serous type.

Well-defined risk factors for endometrioid
carcinoma include
a.
Obesity: associated with increased synthesis of
estrogens in fat depots
b. Diabetes
c. Hypertension
d. Infertility: women tend to be nulliparous, often
with anovulatory cycles.
e-prolonged estrogen replacement therapy and
estrogen-secreting ovarian tumors increase the
risk of this form of cancer.
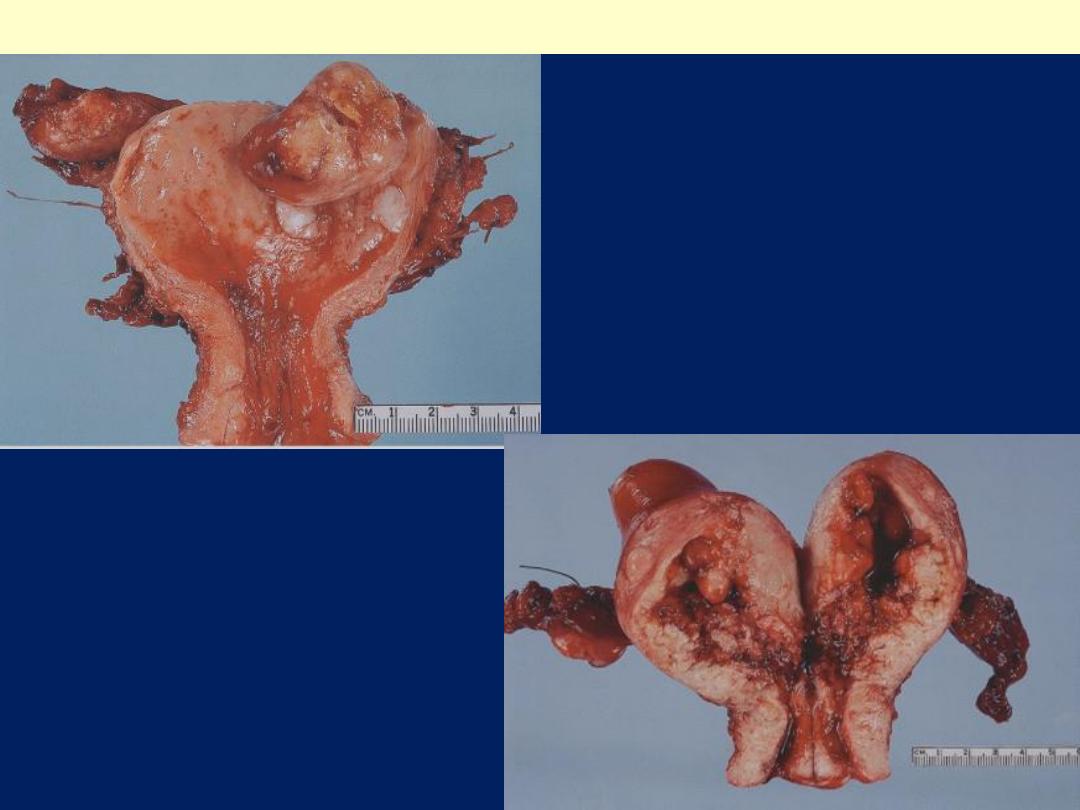
The tumor shown is
polypoid (exophytic)
Endometrial adenocarcinoma
The tumor shown is
extensive, nodular & highly
infiltrating.
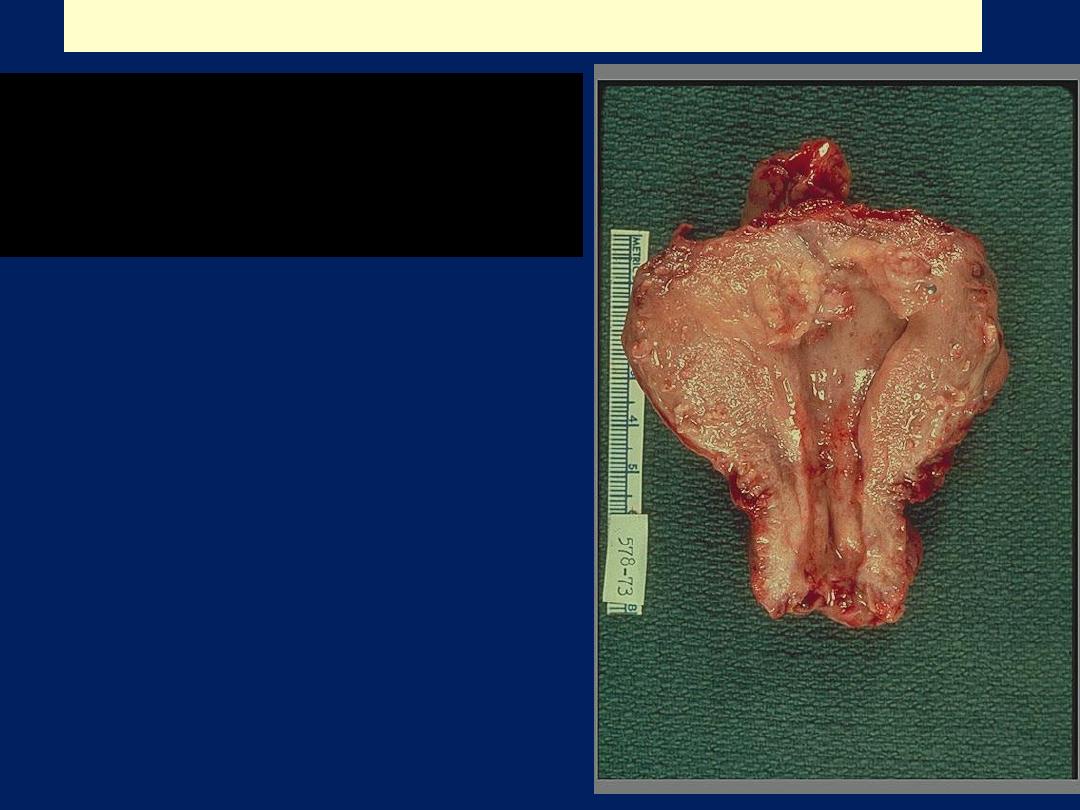
Endometrial adenocarcinoma
A mass with foci of hemorrhage
within the uterine corpus
consistent with a fungating
endometrial adenocarcinoma
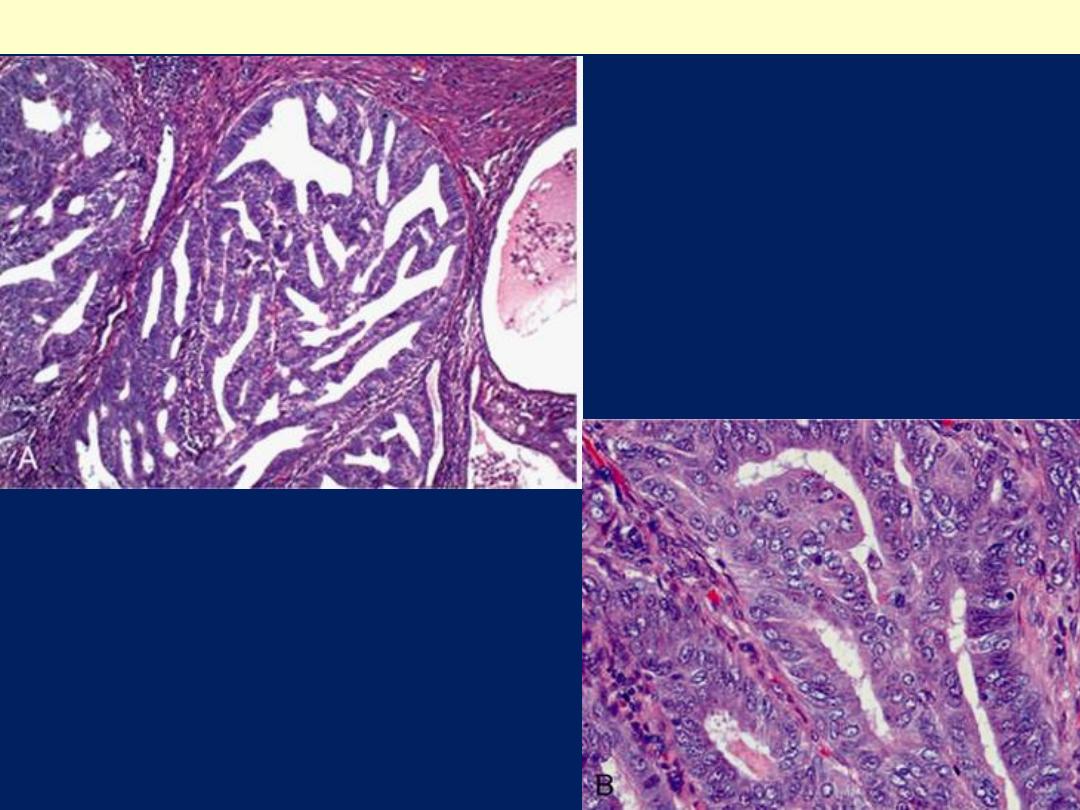
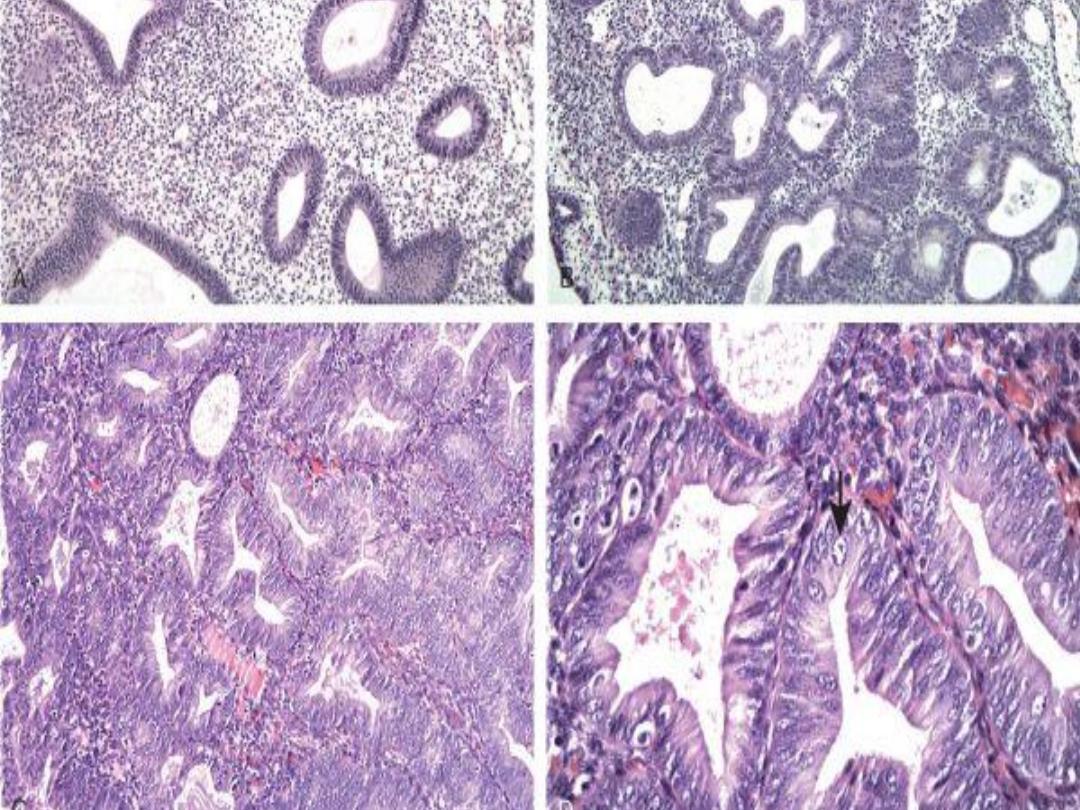
A, Endometrioid type,
infiltrating myometrium
and displaying cribriform
architecture. B, Higher
magnification reveals loss
of polarity and nuclear
atypia.
Endometrioid adenocarcinoma
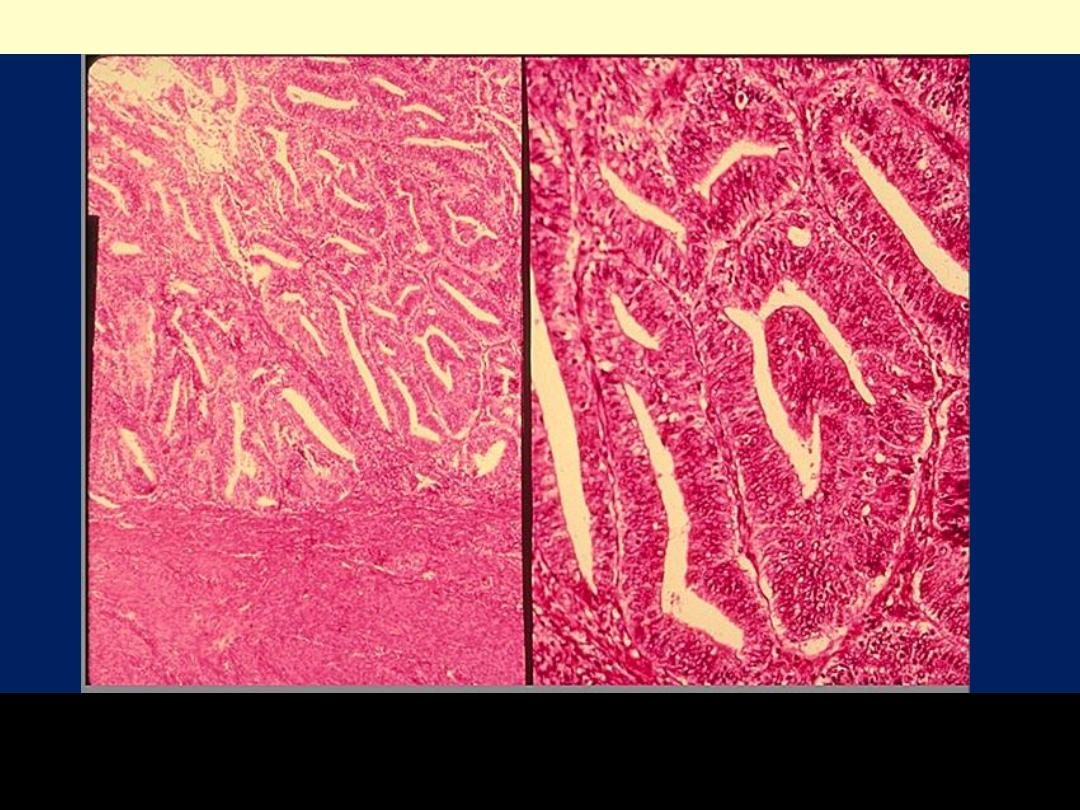
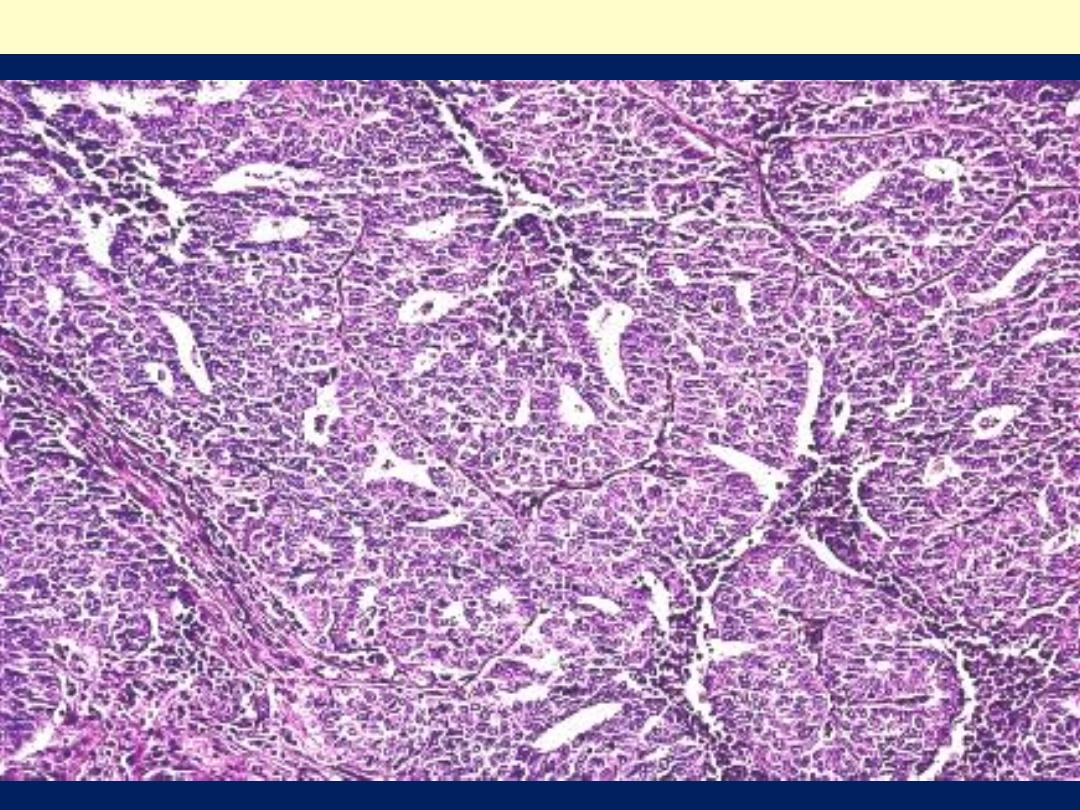
The endometrial glands are crowded, in a back-to-back arrangement;
these glands are very complex with a gland within gland pattern. The
lining consists of cells with pseudostratification and disorganization
Endometrioid carcinoma
Moderately-differentiated endometrioid adenocarcinoma
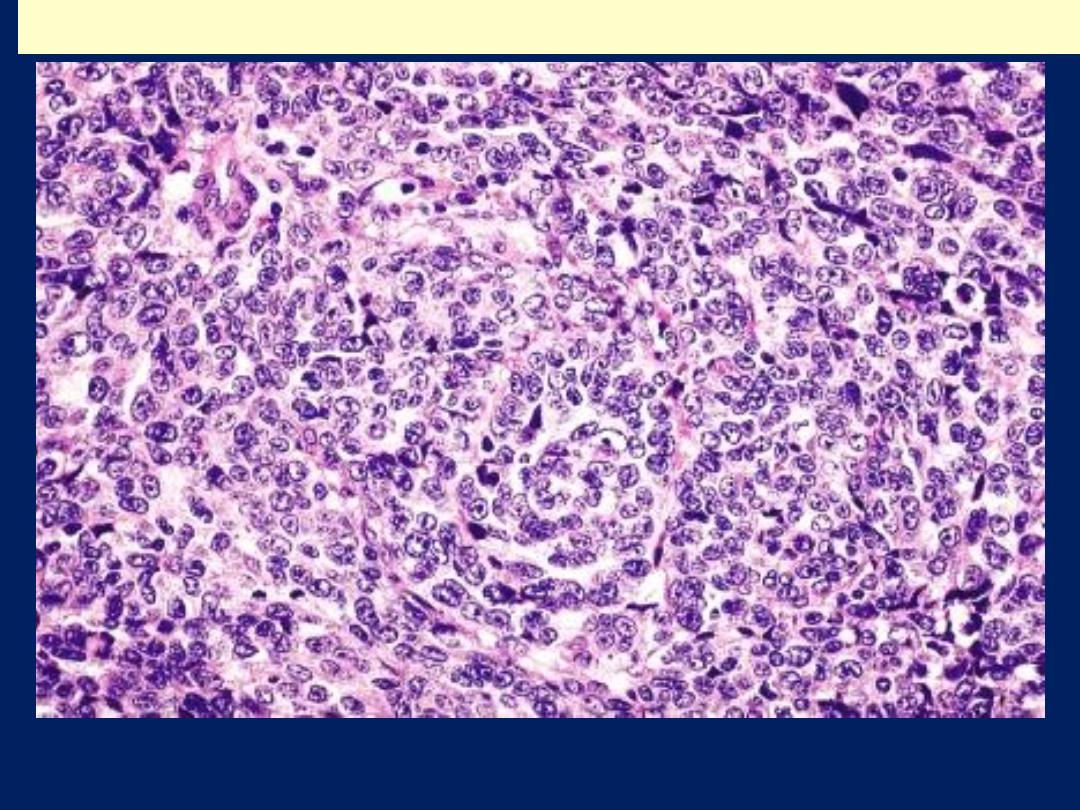
Poorly-differentiated endometrioid adenocarcinoma
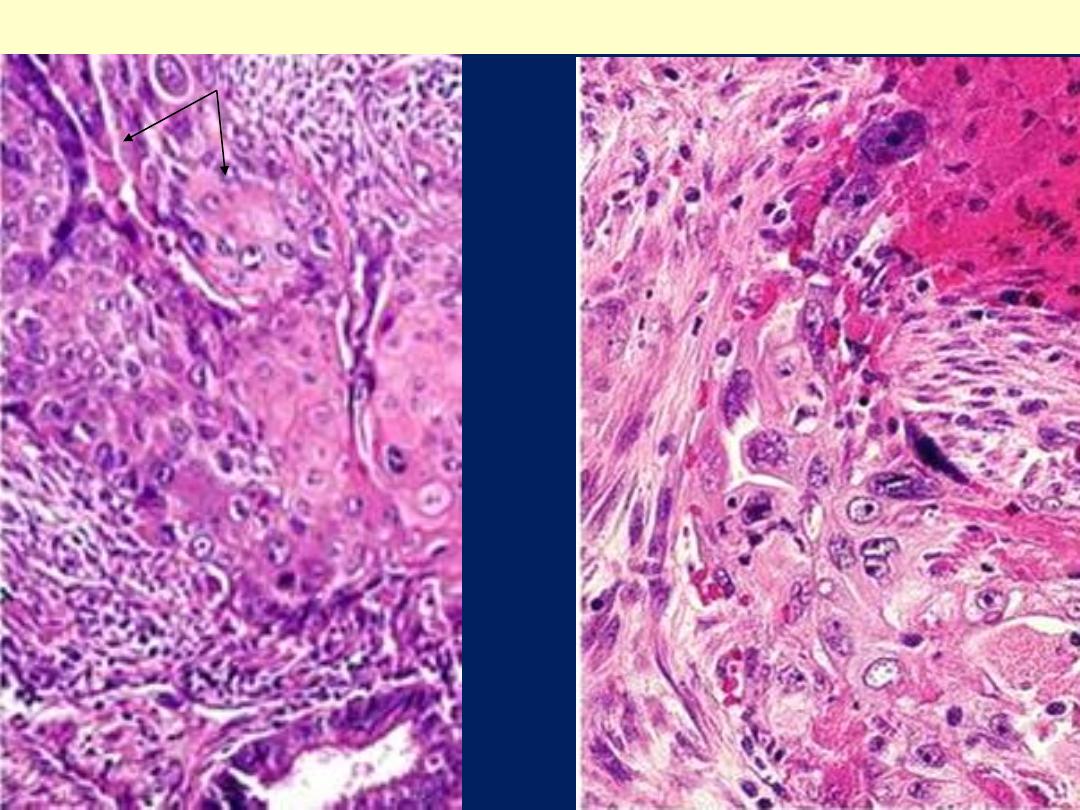
Adenosquamous carcinoma
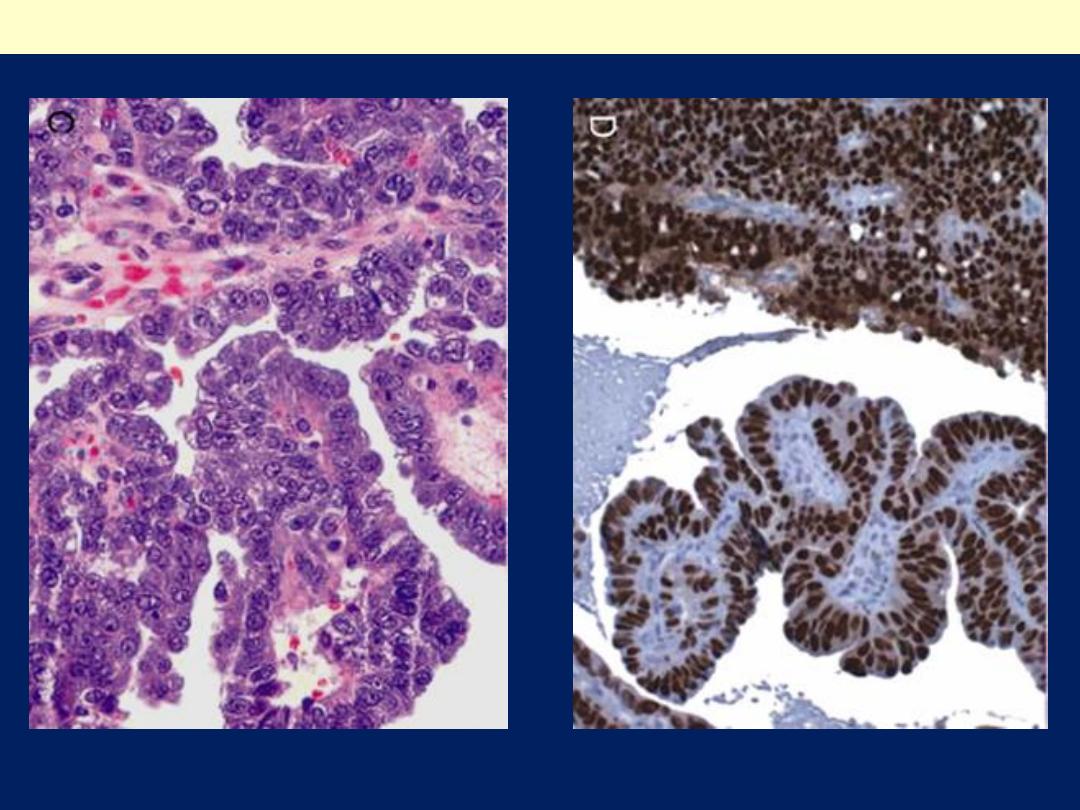
C, displaying formation of papillae and marked cytologic atypia. D, Immunohistochemical
stain for p53 reveals accumulation of mutant p53 in serous carcinoma.
Serous carcinoma of the endometrium
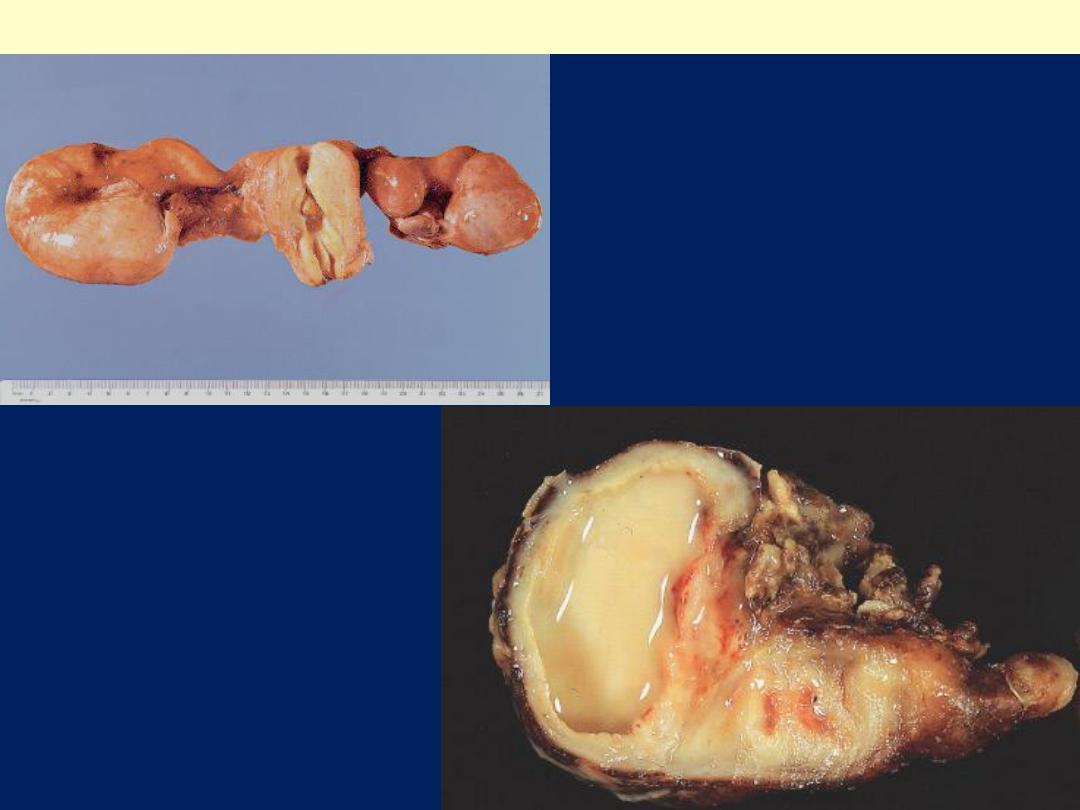
Pyosalpinx
Outer aspect of pyosalpinx.
Cut surface of pyosalpinx; the tube
is distended with yellow pus
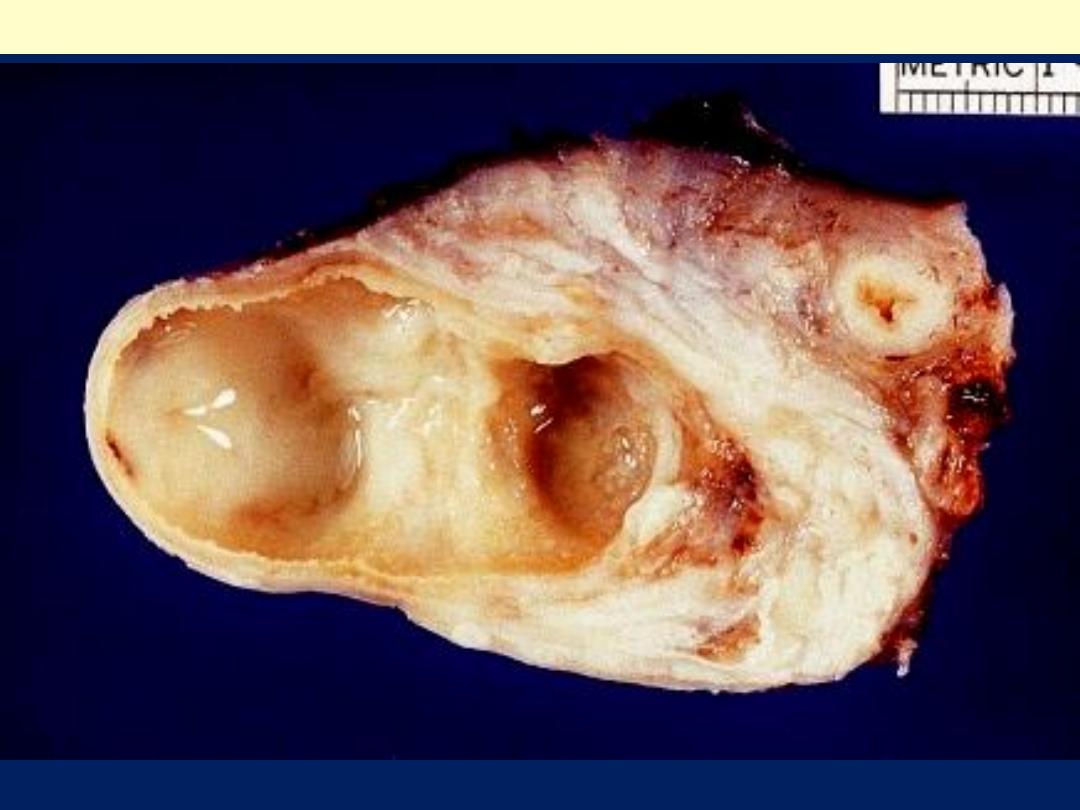
Tubo-ovarian abscess
Fusion of fallopian tube and ovary into a tubo-ovarian abscess.
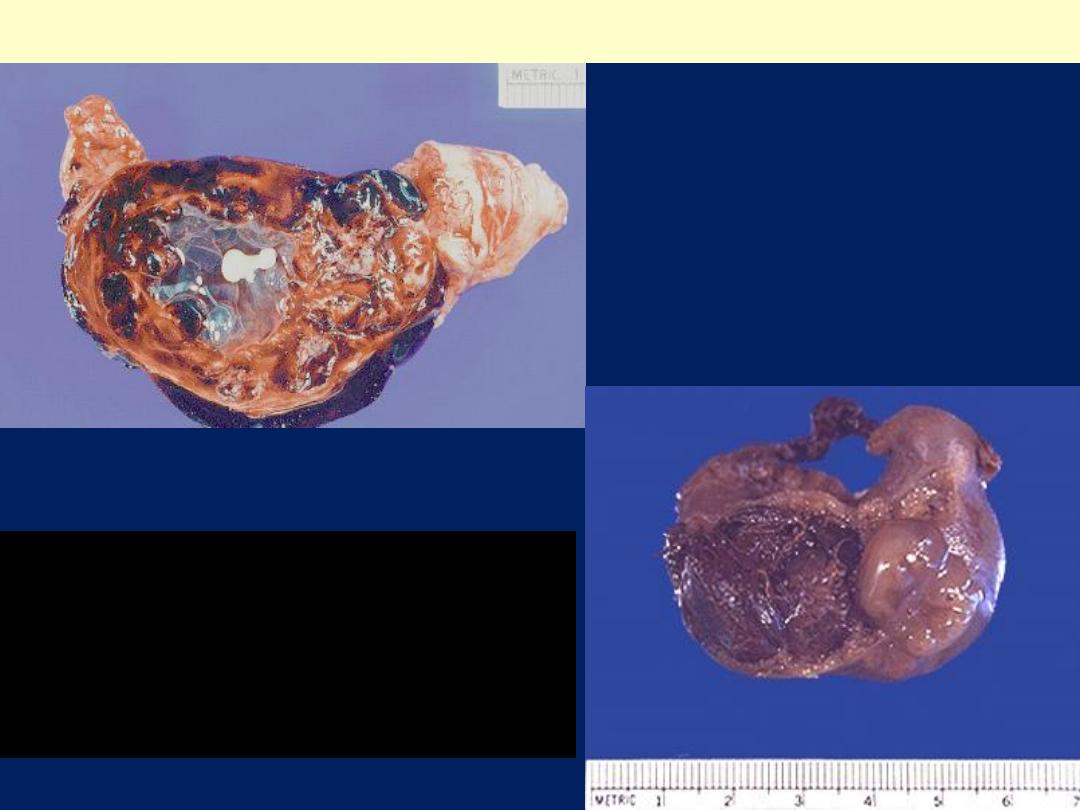
Hematosalpinx: tubal gestation
Ruptured tubal pregnancy
with marked hemorrhage
(hematosalpinx). The tiny
embryo is identifiable in
the center of the clot.
This is a ruptured tubal ectopic
pregnancy. Note the twin
fetuses at the lower right
adjacent to the blood clot at the
left
*
.
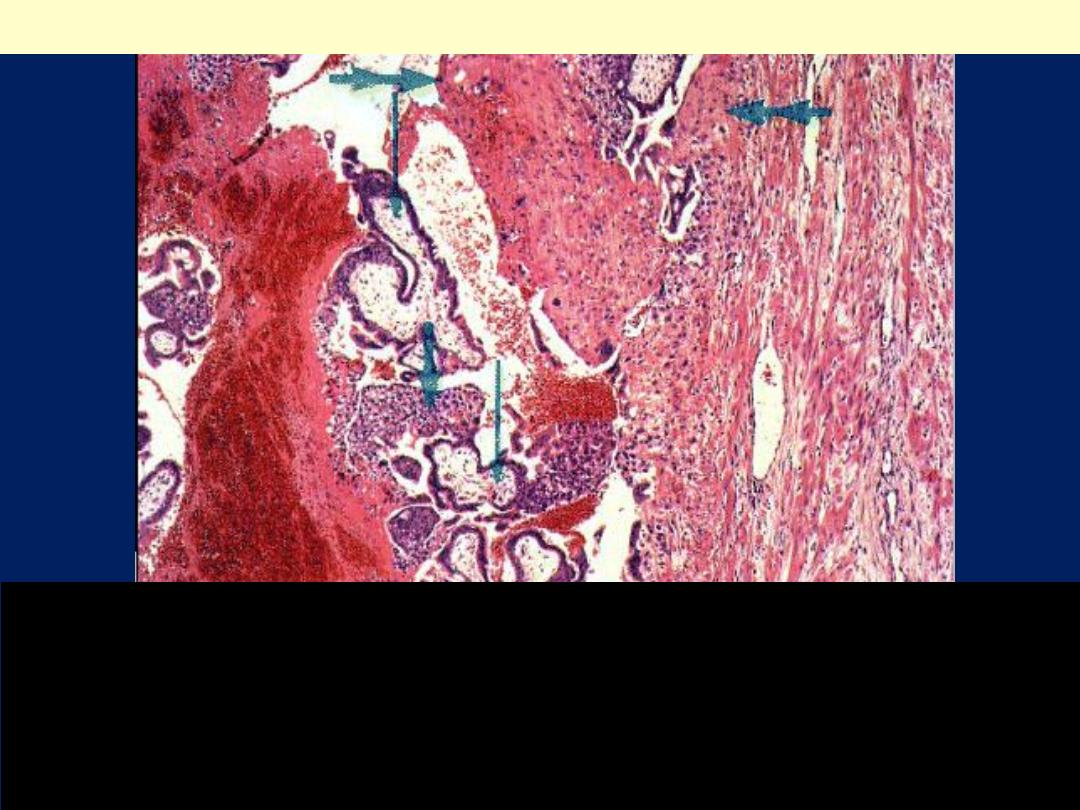
A positive pregnancy test (presence of human chorionic
gonadotropin), ultrasound, and culdocentesis with presence
of blood are helpful in making the diagnosis of ectopic
pregnancy. Note the presence of chorionic villi &
hemorrhage within the tubal lumen
Tubal pregnancy

Gestational Trophoblastic Tumors
These are divided into four morphologic
categories:
1. Hydatidiform mole
a. Complete
b. Incomplete
2. Invasive mole
3. Choriocarcinoma.
4. Placental site trophoblastic tumor

All the three produce
human chorionic gonadotropin
(hCG),
which can be detected in the circulating blood and
urine, at much higher titers .
The titers are progressively rising from hydatidiform mole
to invasive mole to choriocarcinoma.
In addition to aiding diagnosis, the fall or rise in the level of
the hormone in the blood or urine can be used to monitor
the effectiveness of treatment.

1. Hydatidiform Mole:
•
Complete and Partial
•
The typical hydatidiform mole is a large mass of
hydropic swollen chorionic villi, appearing
grossly as grapelike structures.
A. The complete hydatidiform mole
does not
permit embryogenesis and thus does not
contain fetal parts.
and the chorionic epithelial cells are diploid (46,
XX or, uncommonly, 46, XY).

B. The partial hydatidiform mole
permits early embryogenesis , has some
normal chorionic villi, and is almost always
triploid (e.g., 69, XXY).
The two patterns result from
abnormal
fertilization
;.
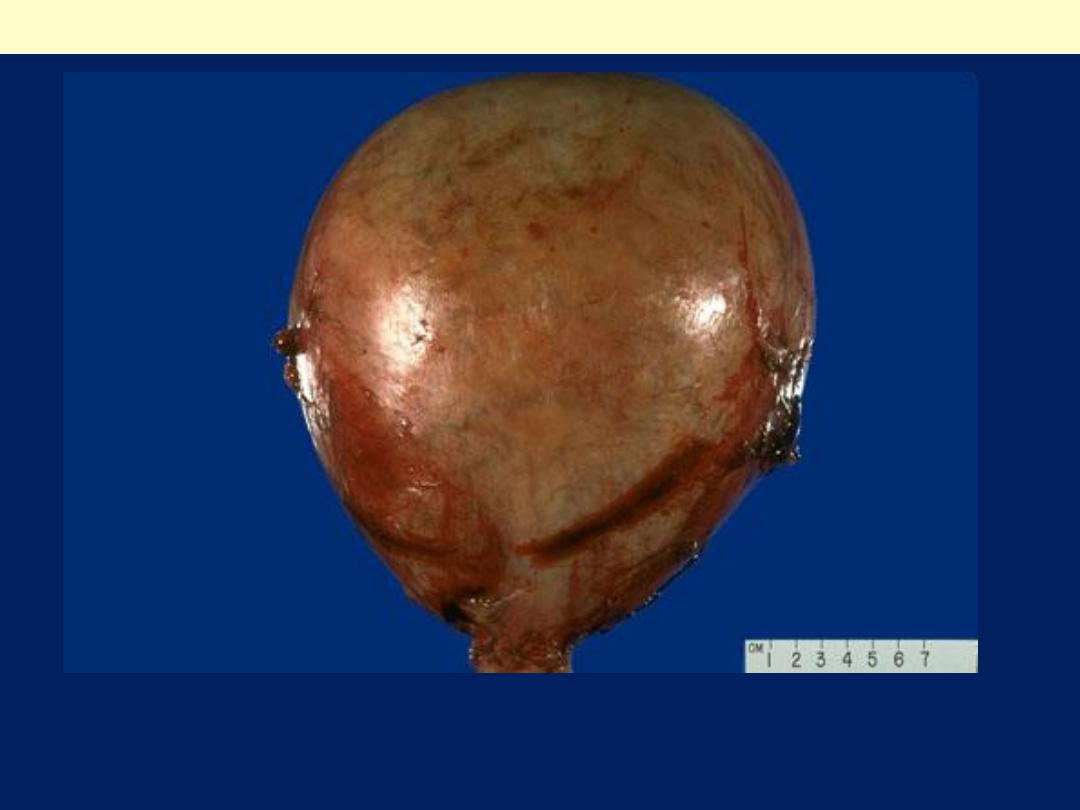
This is only a second trimester pregnancy, but note how large for dates
the uterus is because of a molar pregnancy. An ultrasound in this case
revealed no fetus, only a "snowstorm" effect.
Complete hydatidiform mole
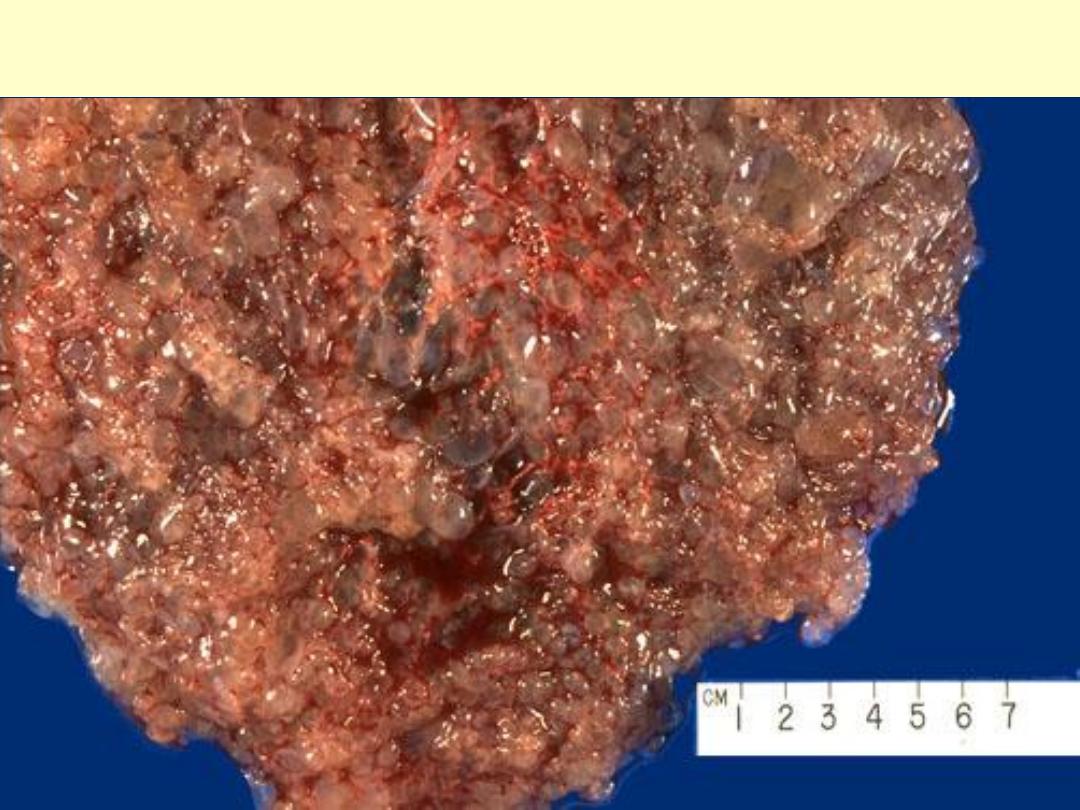
Complete hydatidiform mole:A mass of tissue with grape-like
swollen villi.

The grape-like villi of a hydatidiform mole are seen here suspended in saline. With molar
pregnancy, the uterus is large for dates, but no fetus is present. HCG levels are markedly
elevated. Patients with a hydatidiform mole are often large for dates and have hyperemesis
gravidarum more frequently. Patients may present with bleeding, and may pass some of the
grape-like villi.
Complete hydatidiform mole
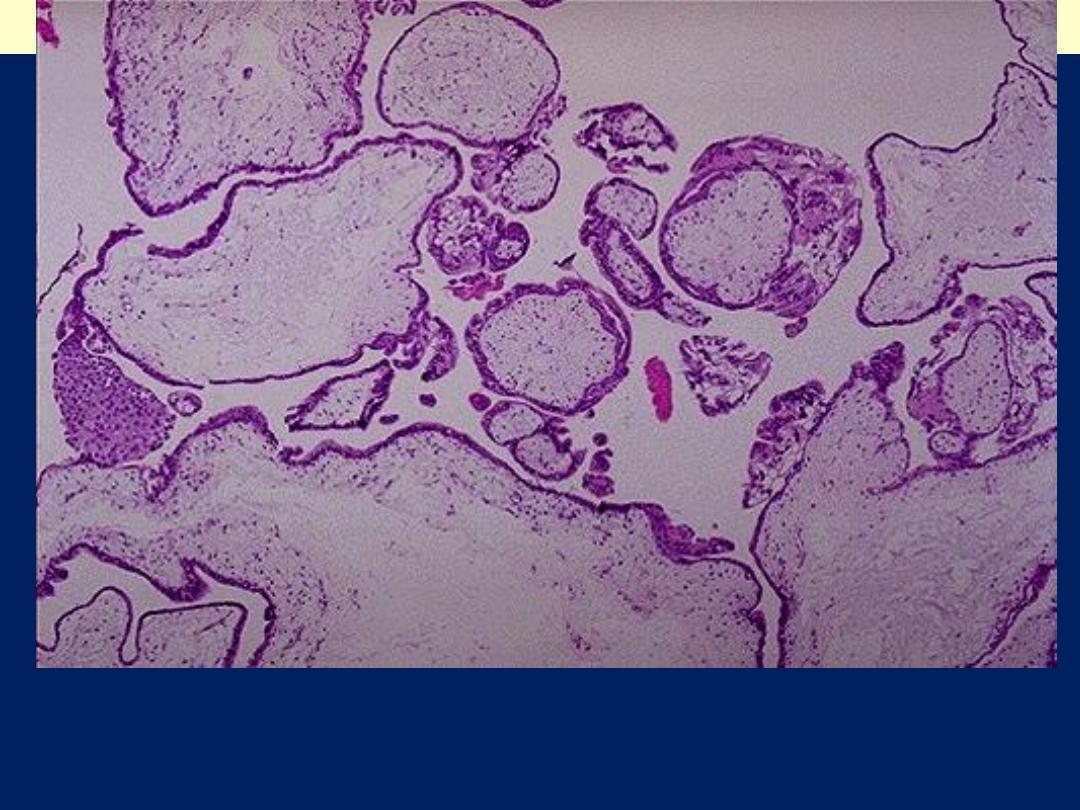
Complete H. mole: Note the large avascular villi and areas of
trophoblastic proliferation. An ultrasound confirms the diagnosis before
currettage is done to evacuate the molar tissue seen here.
Complete hydatidiform mole
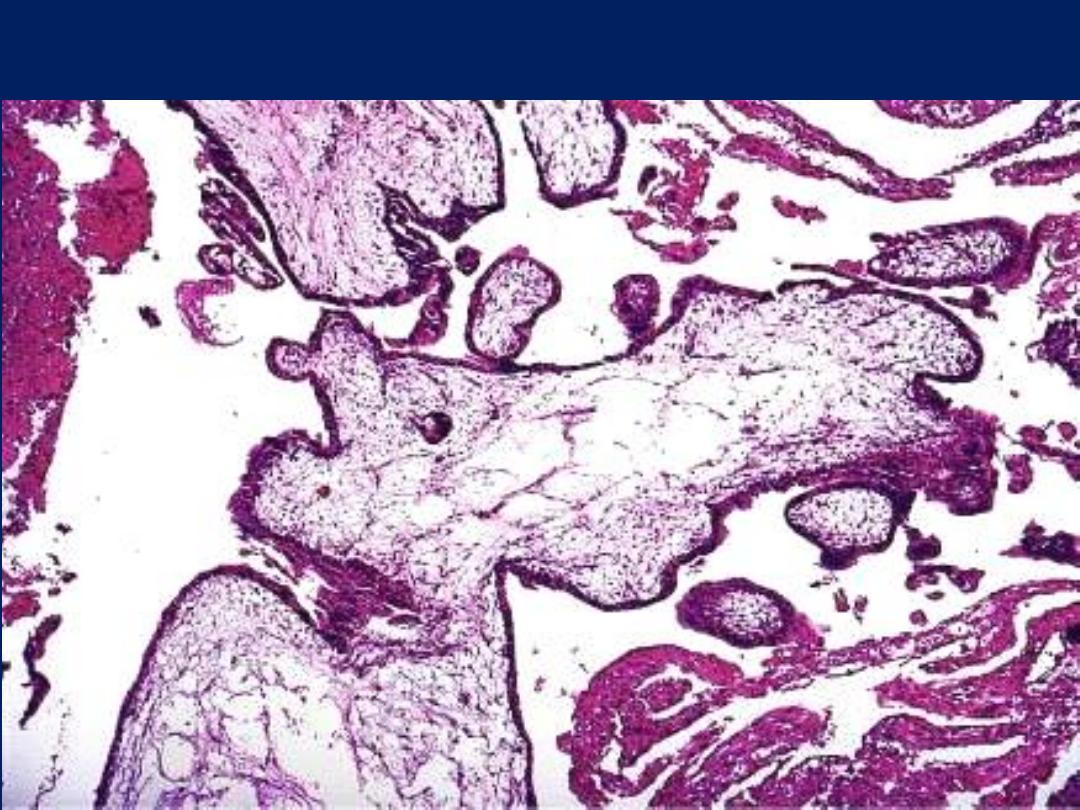
Partial mole showing scalloping of villi and isolated trophoblastic cells
embedded in the stroma.
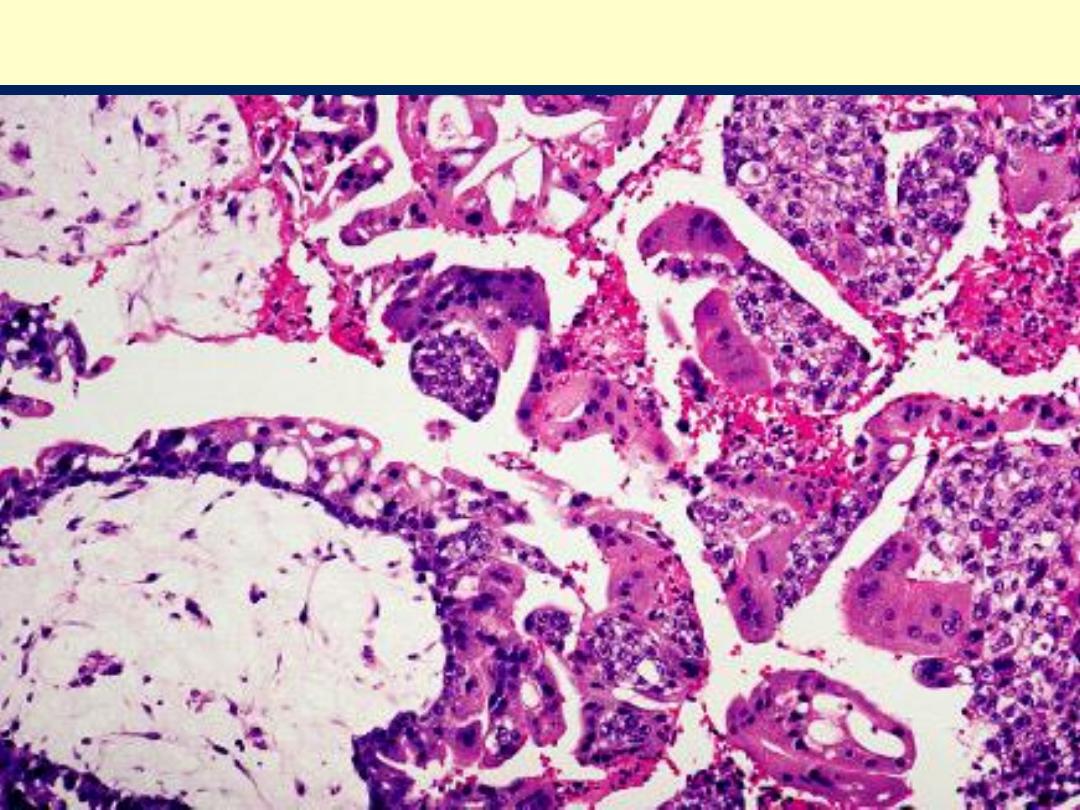
Complete H. mole showing large villi with stromal edema and
marked trophoblastic proliferation.
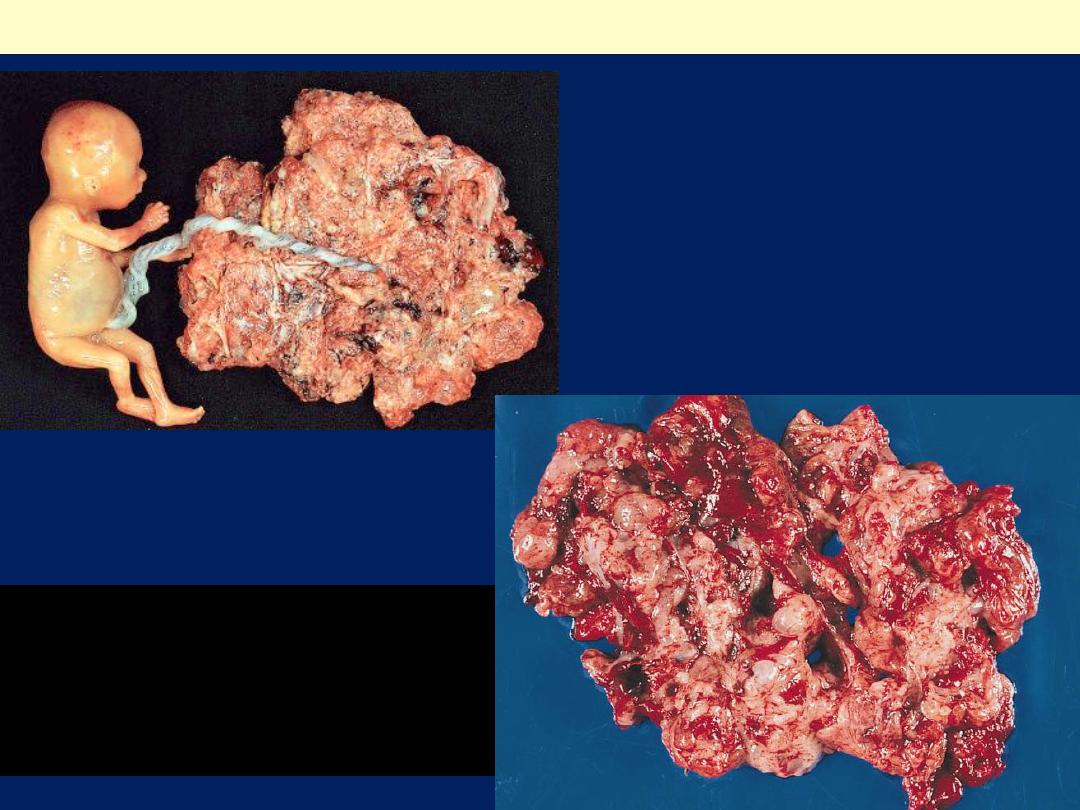
Partial mole with attached fetus.
The diagnosis was confirmed by
biopsy and flow cytometry. The
fetus showed no abnormality and
was connected to the mole by a
normal umbilical cord.
Partial Hydatidiform mole
Gross appearance of partial
mole. The hydropic degeneration
of the villi is not as pronounced
as in the classical complete
mole.
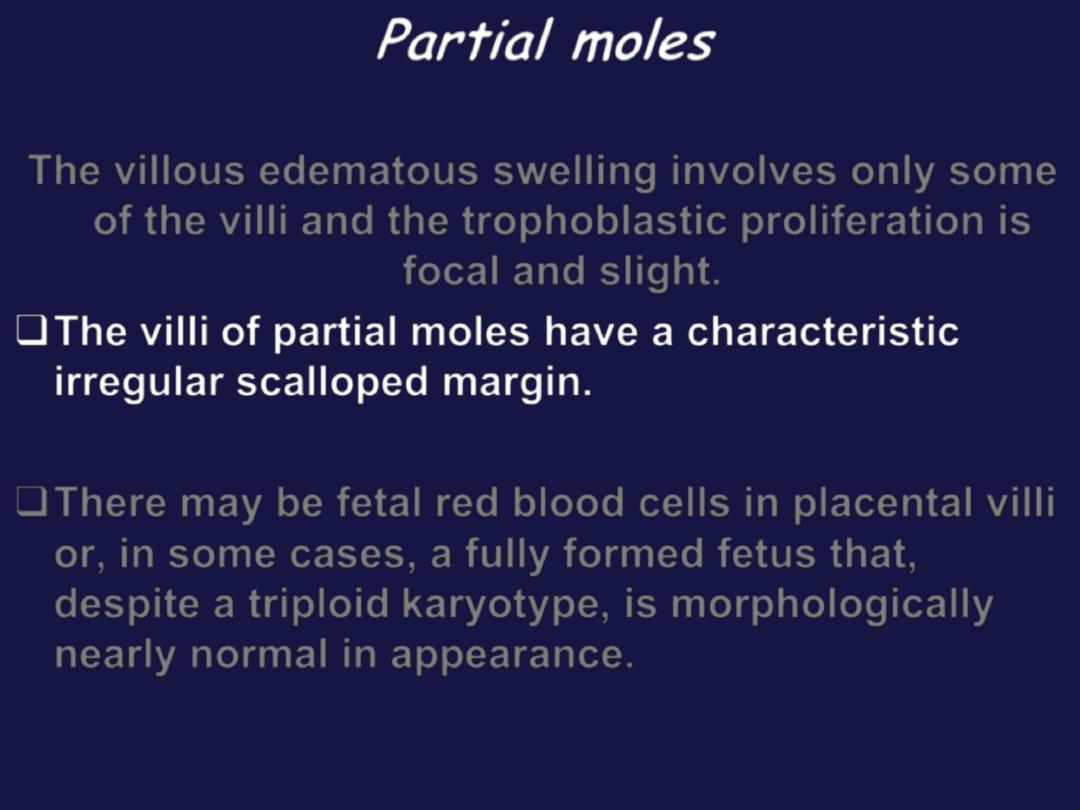
Partial moles
The villous edematous swelling involves only some
of the villi and the trophoblastic proliferation is
focal and slight.
The villi of partial moles have a characteristic
irregular scalloped margin.
There may be fetal red blood cells in placental villi
or, in some cases, a fully formed fetus that,
despite a triploid karyotype, is morphologically
nearly normal in appearance.
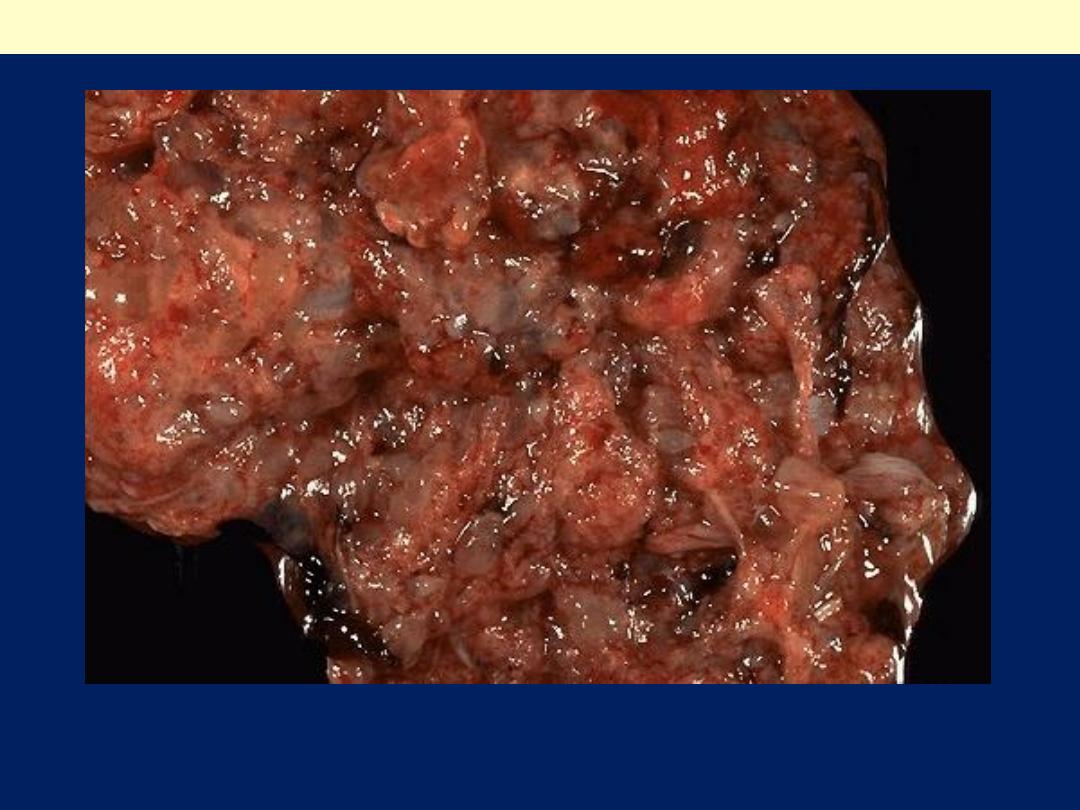
This is a partial mole that occurs when two sperms fertilize a single ovum. The result is
triploidy (69 XXY). Only some of the villi are grape- like, and a fetus can be present, but
rarely survives past 15 weeks.
Partial hydatidiform mole
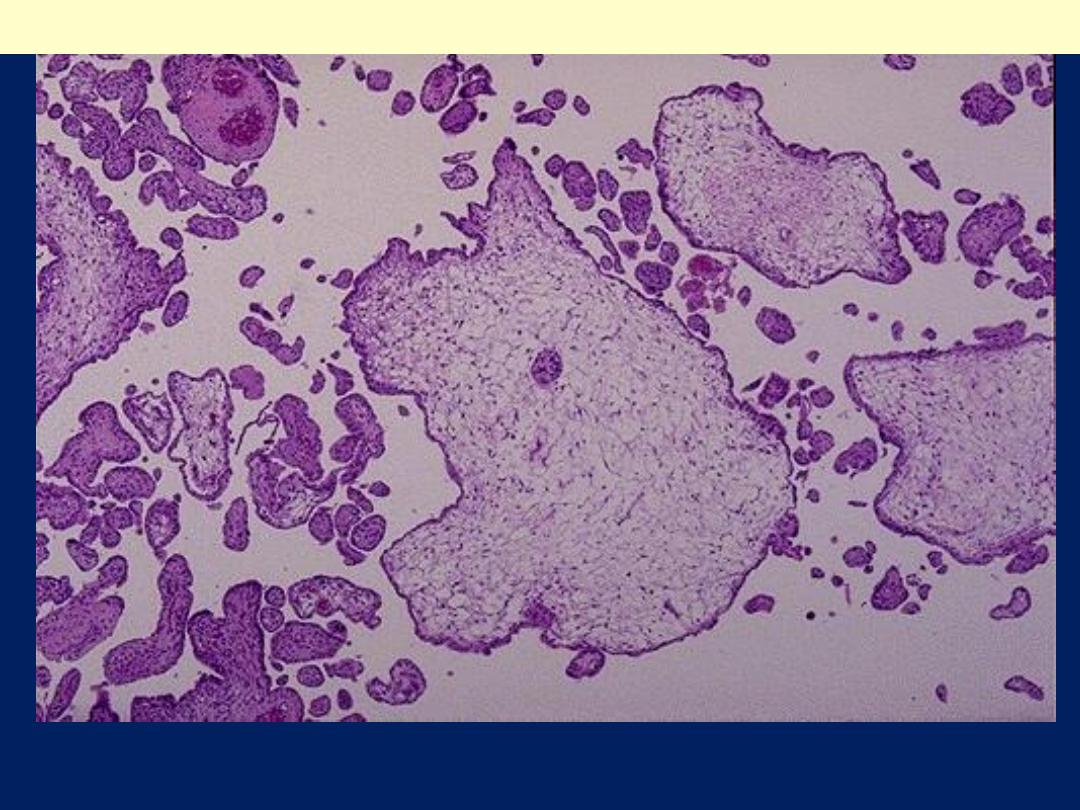
In partial moles, some villi (as seen here at the lower left) appear normal,
whereas others are swollen. There is minimal trophoblastic proliferation.
Partial hydatidiform mole

2.
Invasive Mole
is a complete mole that is more invasive locally but
do not have the metastatic potential of a
choriocarcinoma.
An invasive mole retains hydropic villi, which
penetrate the uterine wall deeply, possibly causing
rupture and sometimes life-threatening hemorrhage.
Local spread to the broad ligament and vagina may
also occur.
Microscopically,
the epithelium of the villi is
hyperplastic and atypical.
Invasive mole
.
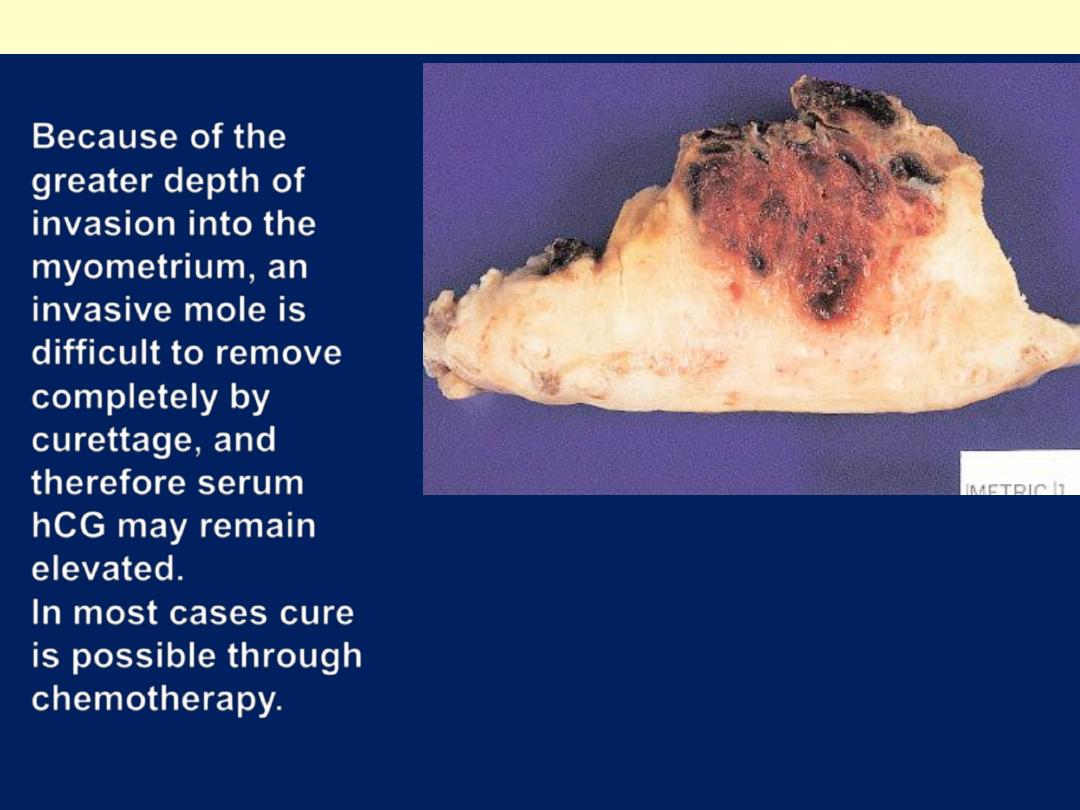
Because of the
greater depth of
invasion into the
myometrium, an
invasive mole is
difficult to remove
completely by
curettage, and
therefore serum
hCG may remain
elevated.
In most cases cure
is possible through
chemotherapy.
Gross appearance
of invasive mole
A hemorrhagic mass
has permeated
half of the thickness
of the myometrial wall
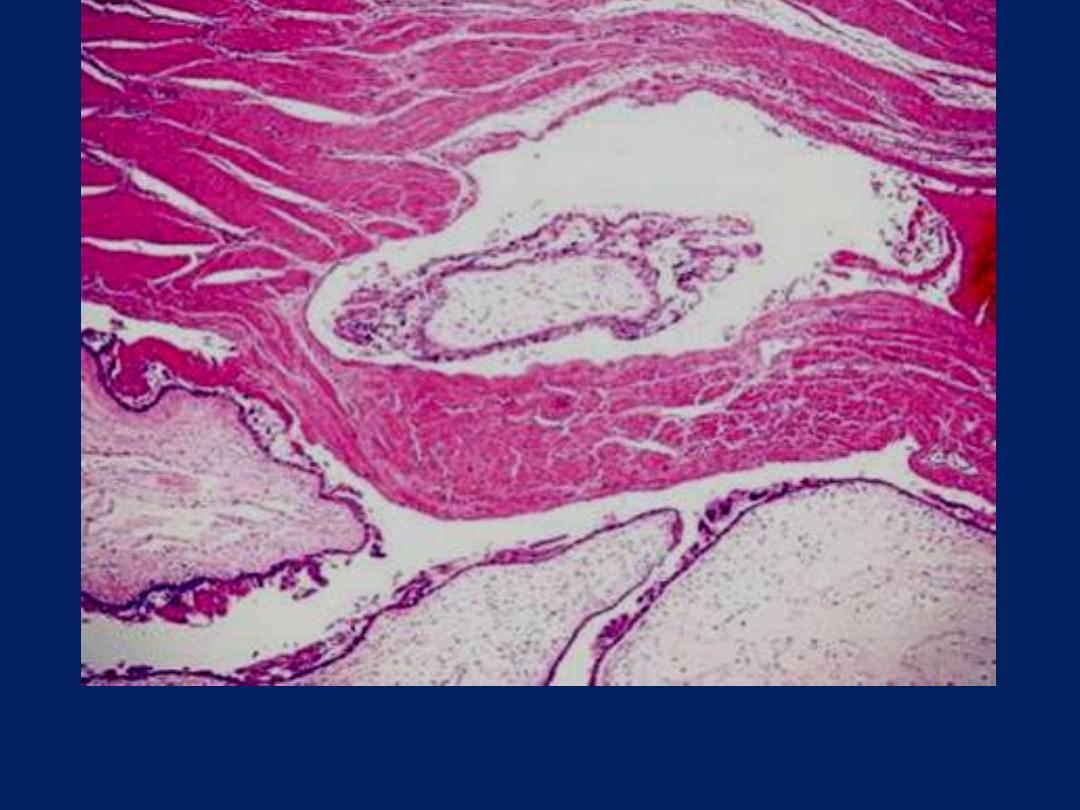
Innasive mole: Hydropic villi covered by proliferating trophoblast are
seen permeating the myometrium in this invasive mole.

3. Choriocarcinoma
is a very aggressive malignant tumor arises
either from gestational chorionic epithelium or,
less frequently, from totipotential cells within
the gonads or elsewhere.
The risk is somewhat greater before age 20 and
is significantly elevated after age 40.
Approximately 50% of choriocarcinomas
complicate complete hyaditidiform moles;
about 25% arise after an abortion, and most of
the remainder follow what had been a normal
pregnancy.

Most cases are discovered by the
appearance of a bloody or brownish
discharge accompanied by a rising titer of
hCG, particularly the β-subunit, in blood and
urine, and the absence of marked uterine
enlargement, such as would be anticipated
with a mole.
In general, the titers are much higher than
those associated with a mole.
Choriocarcinoma- uterus
Gross features
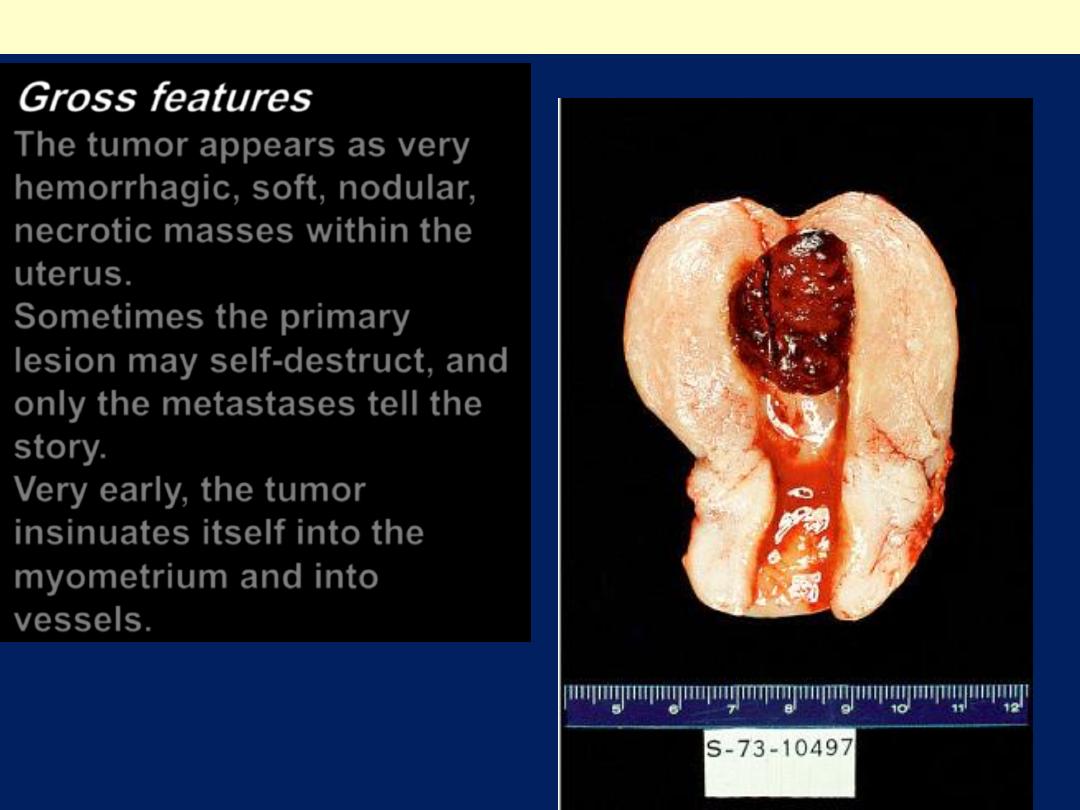
The tumor appears as very
hemorrhagic, soft, nodular,
necrotic masses within the
uterus.
Sometimes the primary
lesion may self-destruct, and
only the metastases tell the
story.
Very early, the tumor
insinuates itself into the
myometrium and into
vessels.
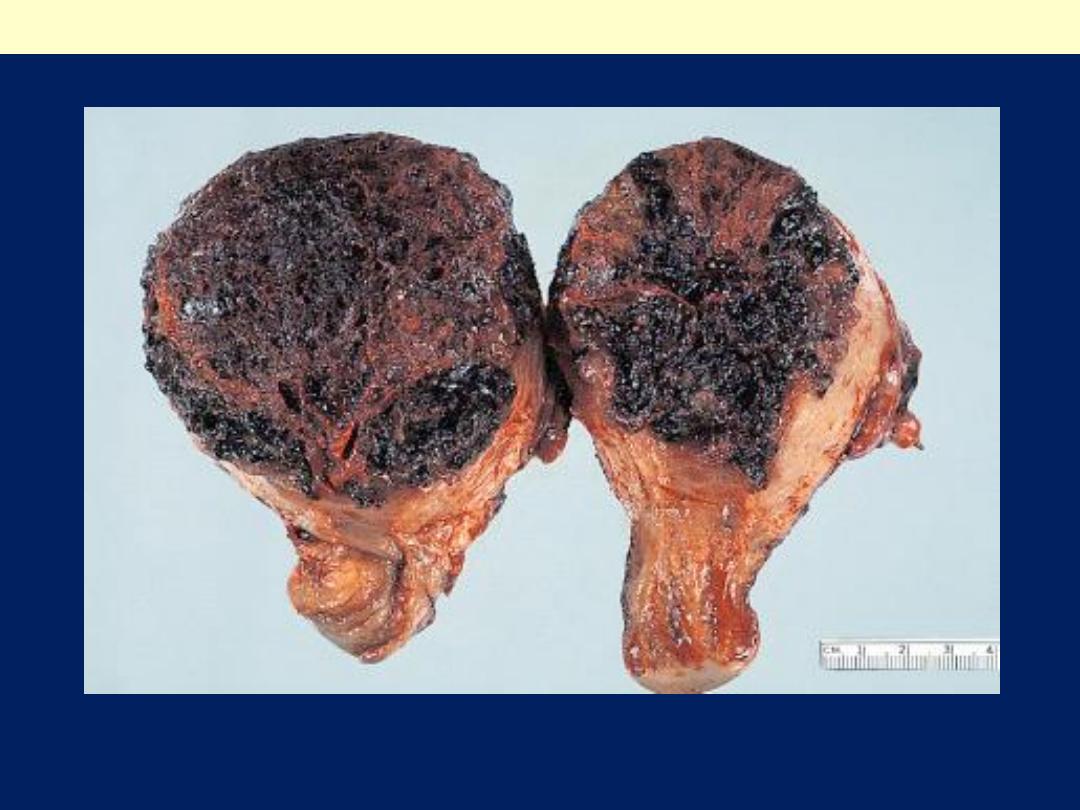
Uterine choriocarcinoma showing typical highly hemorrhagic appearance.
Choriocarcinoma uterus
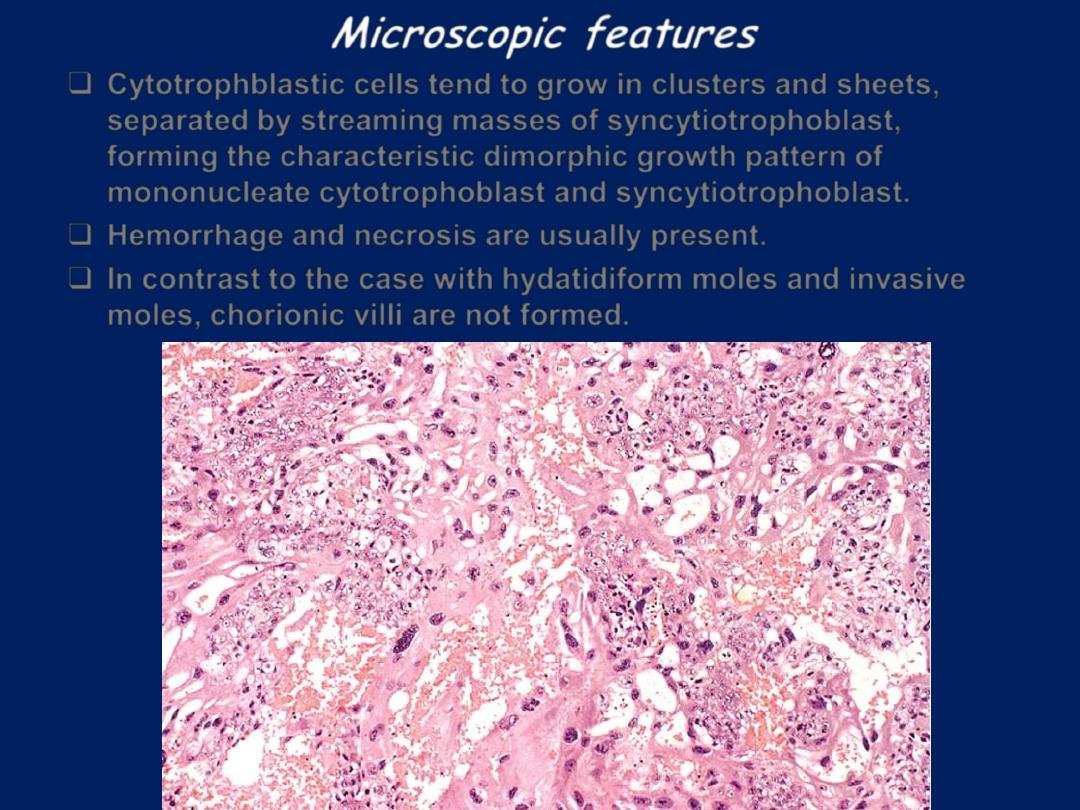
Microscopic features
Cytotrophblastic cells tend to grow in clusters and sheets,
separated by streaming masses of syncytiotrophoblast,
forming the characteristic dimorphic growth pattern of
mononucleate cytotrophoblast and syncytiotrophoblast.
Hemorrhage and necrosis are usually present.
In contrast to the case with hydatidiform moles and invasive
moles, chorionic villi are not formed.
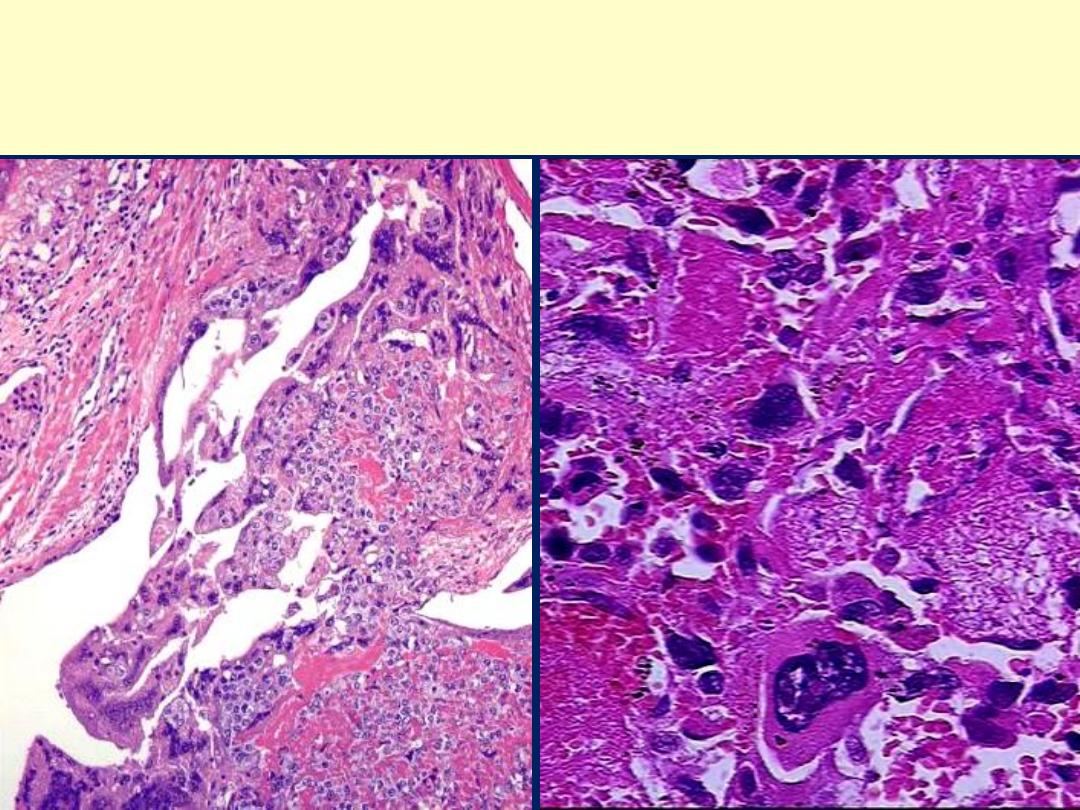
Choriocarcinoma uterus:There is an intimate admixture of malignant
syncytiotrophoblast and cytotrophoblast in choriocarcinoma. No
chorionic villi are seen
