
Viral Hepatitis
Dr. Nadia Aziz
C.A.B.C.M.
Department of community medicine

Objectives
1- Define types of viral hepatitis & their clinical
features.
2- Identify the causative agents & the
occurrence of the disease.
3- Identify the controlling measures.

I. Viral Hepatitis A
ICD-10 B15
Identification
Infection occurs
in childhood asymptomatically
or
mild
illness
Onset of illness
in adults
is usually
abrupt
with
fever
,
malaise
,
anorexia
,
nausea
and
abdominal discomfort
, followed within a few days
by
jaundice
.

Viral Hepatitis A
In general,
severity increases with age
, but
complete recovery without sequelae or
recurrences is the rule.
Case fatality
normally low, 0.1%–0.3%, it can reach 1.8% for adults
over 50
, persons with
chronic liver disease
have an
elevated risk of death from fulminant hepatitis A.

Viral Hepatitis A
Diagnosis
Demonstration of
IgM antibodies
against hepatitis
A virus. IgM anti-HAV becomes detectable 5–10
days after exposure.
Specific antibodies
, detected by
EIA
(enzyme
immunoassay), also establishes the diagnosis.

Viral Hepatitis A
Infectious agent
Hepatitis A virus (HAV), (
RNA
virus)
Mode of transmission
Person-to-person by the
fecal-oral route

Viral Hepatitis A
Occurrence
Worldwide, geographic areas can be characterized by
high
,
intermediate
, or
low
levels of endemicity.
Outbreaks due to food contaminated by
food
handlers
& common source
outbreaks have been related to
contaminated water
.

Viral Hepatitis A
Disease transmission
is most frequent
among
household
and
sexual contacts
of acute
cases, in
day care centers
with diapered children,
travellers
to countries where the disease is
endemic, among
injecting drug users
and among
homosexual

Viral Hepatitis A
Incubation period
Average
28–30
days (range 15–50 days).
Period of communicability
Maximum infectivity occurs during the latter
half of
incubation
and continues for a few days
after onset
of jaundice
Homologous immunity after infection probably lasts
for life
.

Viral Hepatitis A
Methods of control
A. Preventive measures
:
1)
Educate
the public about good sanitation and
personal hygiene
2) Provide
proper water treatment
and
distribution systems and sewage disposal.

Viral Hepatitis A
3) There are 4
inactivated
vaccines
and they are
not licensed
for use in
children under 1
.
These vaccines are
safe
,
immunogenic
and
efficacious.

Viral Hepatitis A
Vaccines
Protection may begin as soon as 14–21 days
after a
single dose
of vaccine
A
second dose
is felt to be necessary for
long-term
protection.
The use of hepatitis A vaccine is most successful when
vaccination is started
early in the course of the outbreak

Viral Hepatitis A
Close personal contacts (e.g. household, sexual
contact of hepatitis A patients)
A patients should be given
postexposure
prophylaxis
with
IG
within
2 weeks of last
exposure
, preferably simulta-neously with
hepatitis A vaccine
given at a separate injection
site.

Viral Hepatitis A
B. Control of patient, contacts and the immediate
environment
Isolation:
enteric precautions
during the first 2 weeks of
illness, but no more than 1 week after onset of
jaundice

Viral Hepatitis A
Immunization of contacts:
Active immunization
with
vaccine
should be given as
soon as possible, but no later than 2 weeks after
exposure.
Passive immunization
with
IG
(IM) should be given as
soon as possible after exposure, but also within 2
weeks.

II. VIRAL HEPATITIS B
ICD-10 B16
Identification
A small proportion of acute hepatitis B virus
(HBV) infections may be clinically recognized,
less than 10%
of children and
30% – 50% of
adults
, show icteric disease.

VIRAL HEPATITIS B
Clinical symptoms
The onset is usually insidious,
with
anorexia
, vague
abdominal discomfort
,
nausea
and
vomiting
, sometimes
arthralgia
and rash, often progressing to
jaundice
.
Fever
may be absent or mild.

VIRAL HEPATITIS B
Severity ranges from
inapparent
cases detectable
only by liver function tests to
fulminating, fatal
cases of acute hepatic necrosis.
The case-fatality rate is about
1%
, higher in those
over 40
.
Fulminant HBV infection also occurs in
pregnancy
and among
newborns of infected mothers
.

VIRAL HEPATITIS B
After acute HBV infection, the
risk of developing
chronic infection varies inversely with age
,
chronic
HBV infection occurs among about
90%
of
infants
infected at birth,
20%–50%
of
children
infected from 1 to 5 years,
and
1%–10%
of
older children and adults
.

VIRAL HEPATITIS B
Chronic HBV infection is common in persons
with
immunodeficiency
.
Persons with chronic infection
may or may not
have a history of clinical hepatitis
.

VIRAL HEPATITIS B
An estimated
15%–25%
of persons with chronic
HBV infection will die prematurely of either
cirrhosis
or
hepatocellular carcinoma
.
HBV may be the cause of up to
80% of all cases of
hepatocellular carcinoma
worldwide.

VIRAL HEPATITIS B
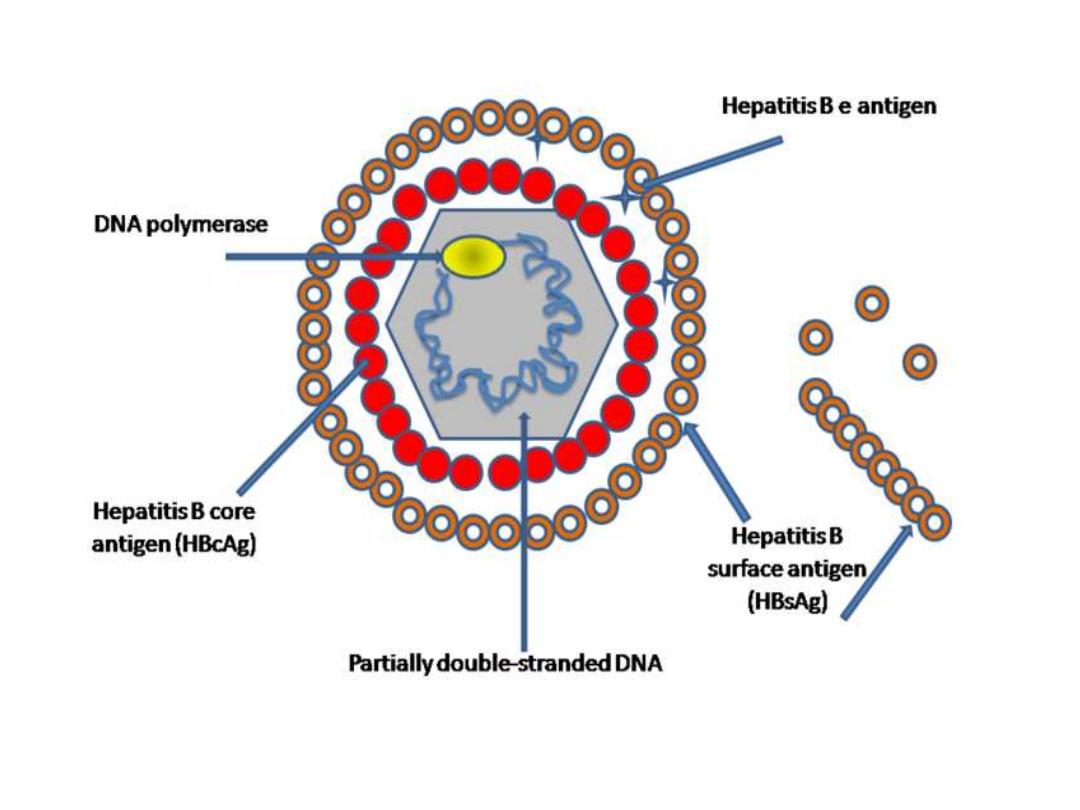
Infectious agent
Hepatitis B virus (HBV), partially double-
stranded
DNA
virus composed of core (
HBcAg
),
surrounded by an outer lipoprotein
coat containing the surface antigen (
HBsAg
).

VIRAL HEPATITIS B
Diagnosis
1) hepatitis B surface antigen (
HBsAg
) and
antibody to HBsAg (
anti-HBs
).
2) hepatitis B core antigen (
HBcAg
) and antibody
to HBcAg (
anti-HBc
).
3) hepatitis B e antigen (
HBeAg
) and antibody to
HBeAg (
anti-HBe
).

VIRAL HEPATITIS B
Diagnosis
Demonstration of
anti-HBc
in serum indicates
HBV
infection
, current or past, high titres of
IgM anti-HBc occur during acute infection.

VIRAL HEPATITIS B
Diagnosis
HbsAg
is present in serum during
acute infections
and persists in chronic infections
.
The presence of HBsAg indicates that the person
is potentially infectious.
The presence of
HBeAg
is associated with
relatively
high infectivity
.

VIRAL HEPATITIS B
Occurrence
Worldwide
WHO estimates that more than
2 billion
persons
have been infected with HBV (including 350
million chronically infected).
most infections occur during
infancy

VIRAL HEPATITIS B
High-risk groups
: including
injecting
drug users
, heterosexuals with
multiple
partners
,
homosexual
, household contacts and
sex partners
of HBV-infected persons,
health care
and public safety workers
who
have exposure to blood in the
workplace,
hemodialysis
patients

VIRAL HEPATITIS B
Mode of transmission
blood and blood products, saliva, cerebrospinal
fluid, peritoneal, pleural, pericardial and synovial
fluid, amniotic fluid, semen and vaginal secretions
Transmission occurs by
percutaneous
(IV, IM, SC,
intradermal) and
permucosal
exposure to
infective body fluids.

VIRAL HEPATITIS B
Incubation period
Usually 45–180 days, average
60–90
days.
Period of communicability
Infectivity starts
weeks before the onset
of first
symptoms and remain infective
through the acute clinical
course
of the disease.
Susceptibility
is general.

VIRAL HEPATITIS B
Methods of control
A. Preventive measures
:
1)Two types of effective hepatitis B
vaccines
have
been licensed and shown to be
safe
and
highly
protective.
Routine infant immunization eliminates transmission
(chronic infections are acquired among young
children).

VIRAL HEPATITIS B
Vaccines
licensed administered in
3 IM doses:
for infants, the first dose is given at
birth
with
subsequent doses
1 to 2
and
6
to
18
months
later.

VIRAL HEPATITIS B
Vaccine
infants
born to
HBsAg positive women
, should
receive
HBIG
Pregnancy is
not
a contraindication for
receiving hepatitis B vaccine.

VIRAL HEPATITIS B
2) In blood banks, all donated blood should be
tested for HBsAg
by sensitive tests.
Maintain
surveillance
for all cases of post
transfusion hepatitis.

VIRAL HEPATITIS B
B. Control of patient, contacts and the immediate
environment:
1) Report to local health authority:
Class 2
2)
Isolation
: Universal precautions to prevent
exposures to blood and body fluids.

VIRAL HEPATITIS B
3) Immunization of contacts:
for post exposure prophylaxis include
HBIG
and
hepatitis B
vaccine
.

VIRAL HEPATITIS B
For
previously unimmunized
persons exposed to
blood from an HBsAg positive source,
a single dose of
HBIG
should be given at least
within 24 hours
of high-risk needle stick
exposure, and the
hepatitis B vaccine
series
should be started.

VIRAL HEPATITIS B
4) Specific treatment:
No specific
treatment
available for acute hepatitis B.
Alpha interferon
,
lamivudine
and
adefovir
have
been licensed for treatment of chronic hepatitis B.

III. VIRAL HEPATITIS C
ICD-10 B17.1
Identification
Onset is usually
insidious
, with
anorexia
, vague
abdominal discomfort
,
nausea
and
vomiting
,
progression to
jaundice less frequent than with
hepatitis B.

VIRAL HEPATITIS C
Although initial infection may be
asymptomatic
(more than
90%
of cases) or
mild
, a high
percentage (
50%–80%
)
develop a chronic
infection.
Of chronically infected persons, about
half
will eventually develop
cirrhosis
or
cancer of the
liver

VIRAL HEPATITIS C
Diagnosis
Tests detecting antibody to the hepatitis C virus
(anti-HCV) include:
Enzyme immunoassay (
EIA
) and the
Recombinant
immunoblot assay.
These tests do not distinguish between acute,
chronic, or resolved infection.

VIRAL HEPATITIS C
Acute or chronic HCV infection should be
confirmed by a sensitive HCV
RNA assay.
Infectious agent
The hepatitis C virus is an enveloped
RNA
virus

VIRAL HEPATITIS C
Occurrence:
Worldwide.
WHO estimates that some 130–170 million
people are chronically infected with HCV.
HCV is one of the most common global causes
of
chronic hepatitis
,
cirrhosis
, and
liver cancer
.

VIRAL HEPATITIS C
Mode of transmission
HCV is primarily transmitted
parenterally
.
Sexual
and
mother-to-child
have been
documented but less frequent
than the parenteral route.

VIRAL HEPATITIS C
Incubation period
Ranges from 2 weeks to 6 months; commonly
6–9
weeks.
Susceptibility
Susceptibility is general. The degree of immunity
following infection is not known.

VIRAL HEPATITIS C
Methods of control
A. Preventive measures
:
Prophylactic IG is not effective.
B.
Control of patient, contacts and the immediate
environment:
Combination therapy of
ribavirin
and
slow-
release interferons

IV. DELTA HEPATITIS
ICD-10 B17.0
Identification
Signs and symptoms
resembling those of hepatitis B
,
always associated with a
coexistent hepatitis B virus
(HBV) infection
.
HDV is
unable to infect a cell by itself
and requires
co-infection with the HBV
to undergo a complete
replication cycle.

DELTA HEPATITIS
Infectious agent
HDV is a virus-like particle consisting of a coat
of
HBsAg
and a unique internal antigen, the
delta antigen, a single- stranded
RNA

V. VIRAL HEPATITIS E
ICD-10 B17.2
Identification
Clinical course
similar to hepatitis A
The case-fatality rate is similar to hepatitis A
except in pregnant women
, where it may reach
20%
among those infected during the
third
trimester
of pregnancy.

VIRAL HEPATITIS E
Infectious agent
The hepatitis E virus (HEV), single-stranded
RNA
virus.
Outbreaks often occur as
waterborne
epidemics

Thank you
&
Good Luck
