
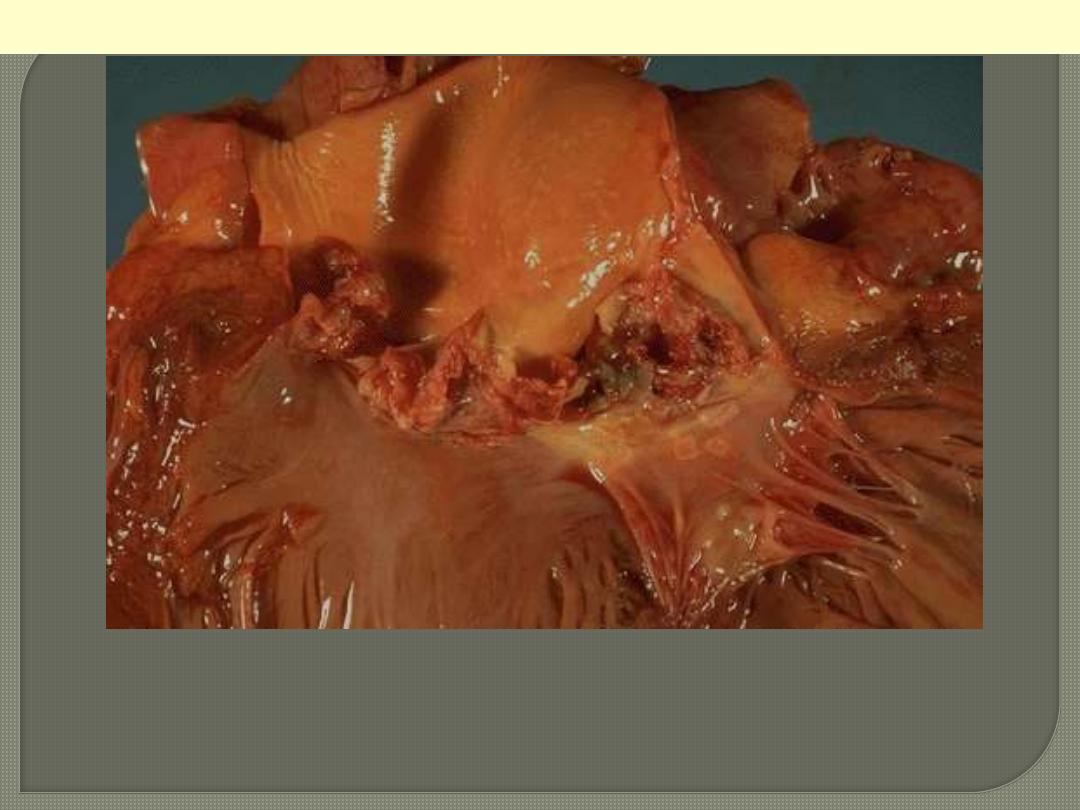
The more virulent bacteria cause the acute form that can lead to
serious destruction, as shown here in the aortic valve. Irregular
reddish tan vegetations overlie valve cusps that are being destroyed.
Acute bacterial endocarditis
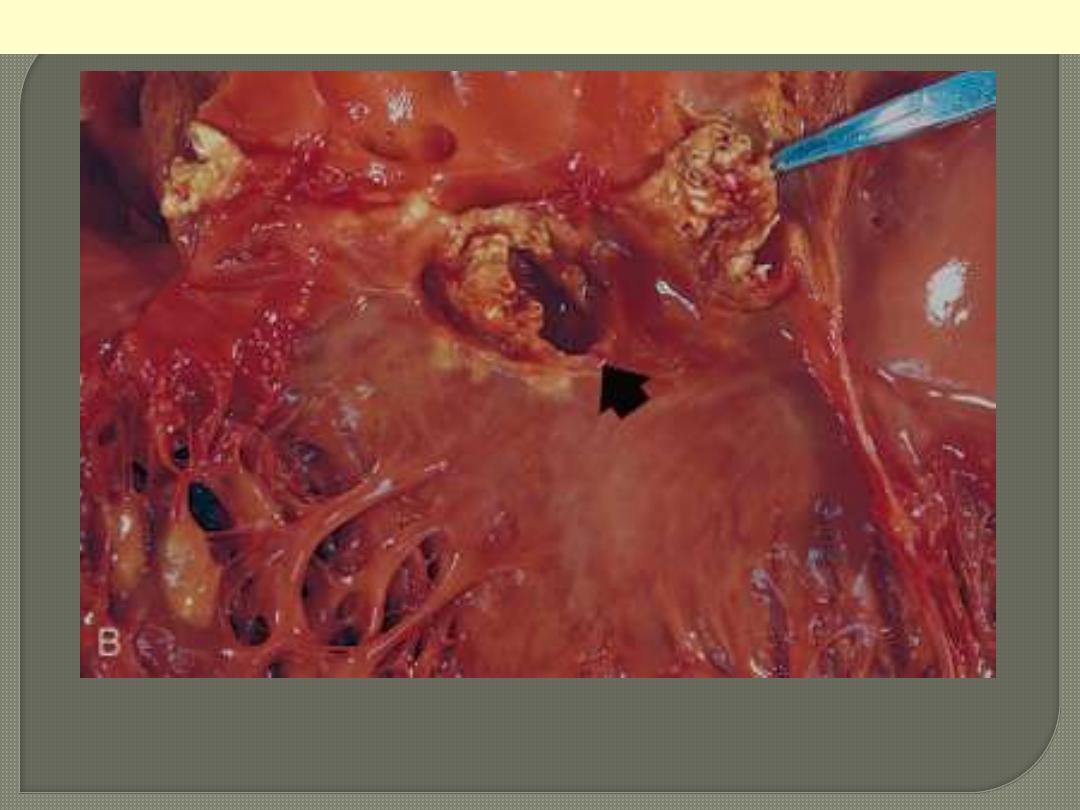
The disease is caused by Staphylococcus aureus with extensive
cuspal destruction and ring abscess (arrow).
Acute endocarditis of congenitally bicuspid aortic valve
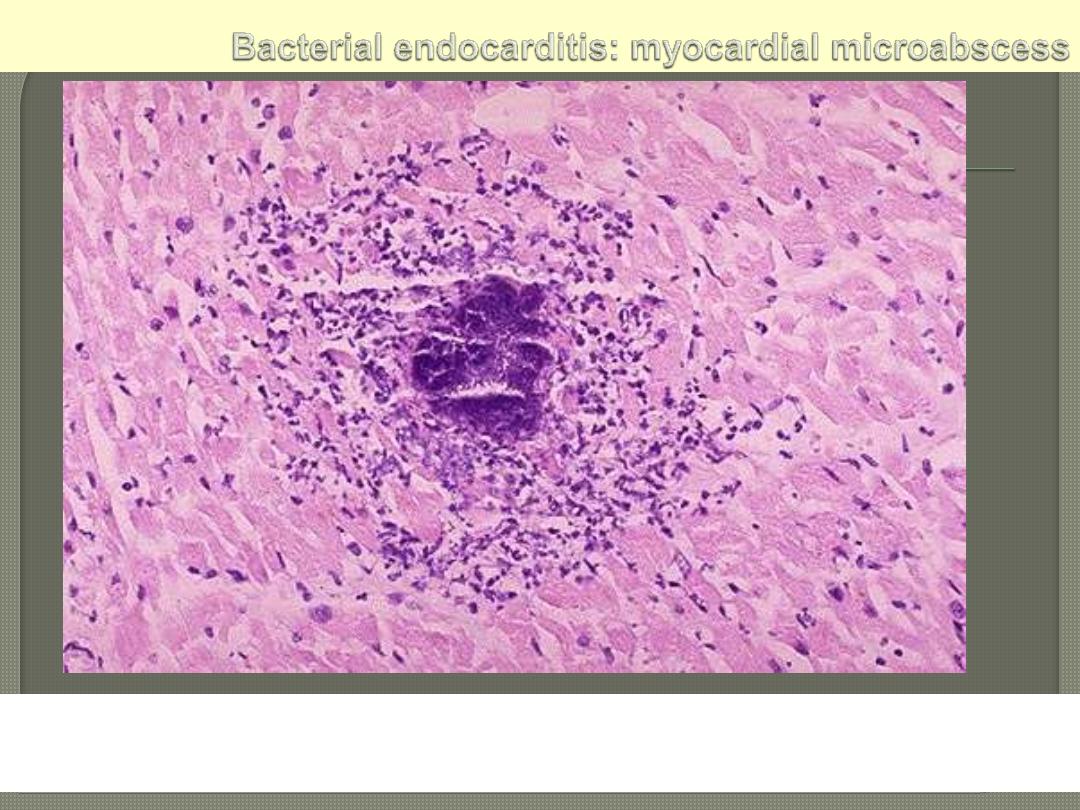
The center consists of blue bacterial colonies and is surrounded by
acute inflammatory cells.
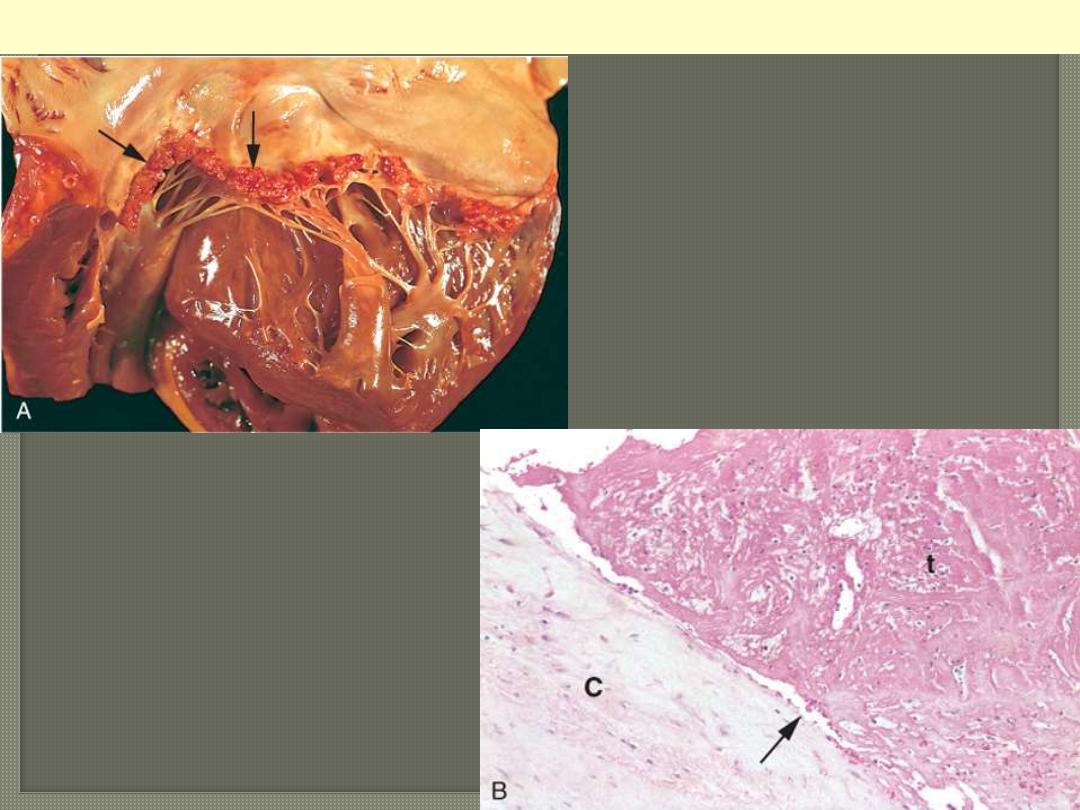
A, Nearly complete row of
thrombotic vegetations along the line
of closure of the mitral valve leaflets
(arrows). B, Photomicrograph of
nonbacterial thrombotic
endocarditis, showing bland
thrombus, with virtually no
inflammation in the valve cusp (c) or
the thrombotic deposit (t). The
thrombus is only loosely attached to
the cusp (arrow).
Nonbacterial thrombotic endocarditis

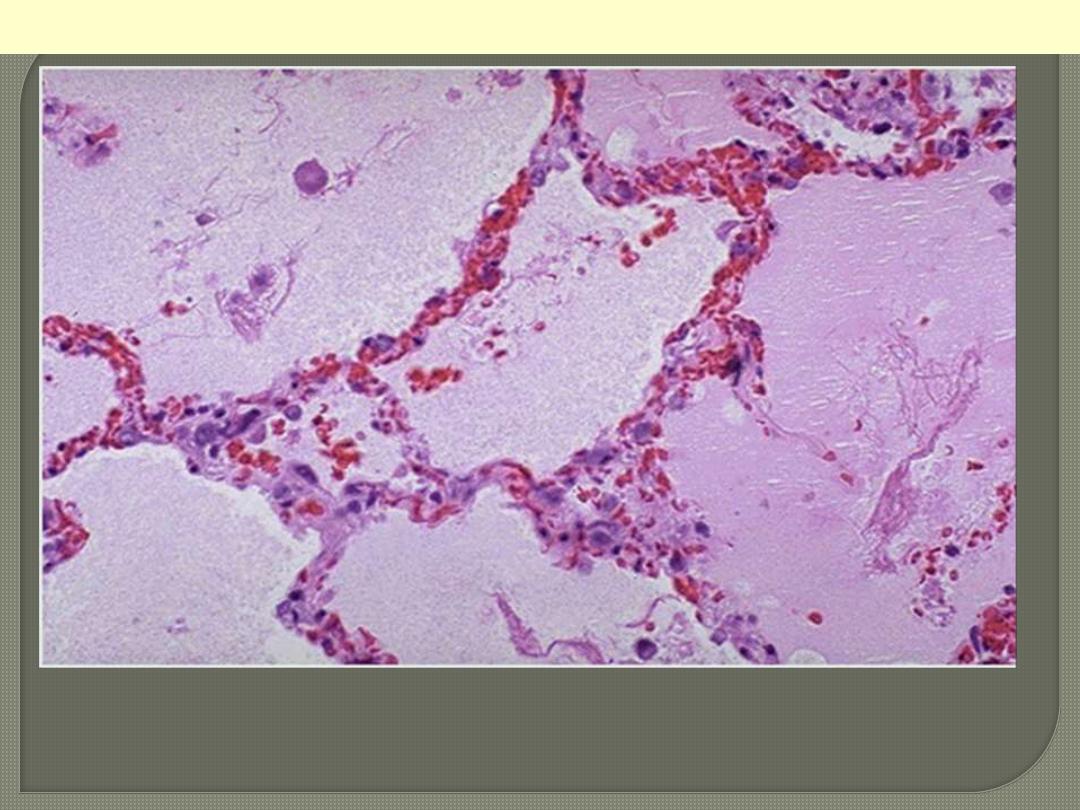
Note the prominent congested septal capillaries and the faint
staining edema fluid filling alveolar spaces
Pulmonary edema; a case of Lt heart failure
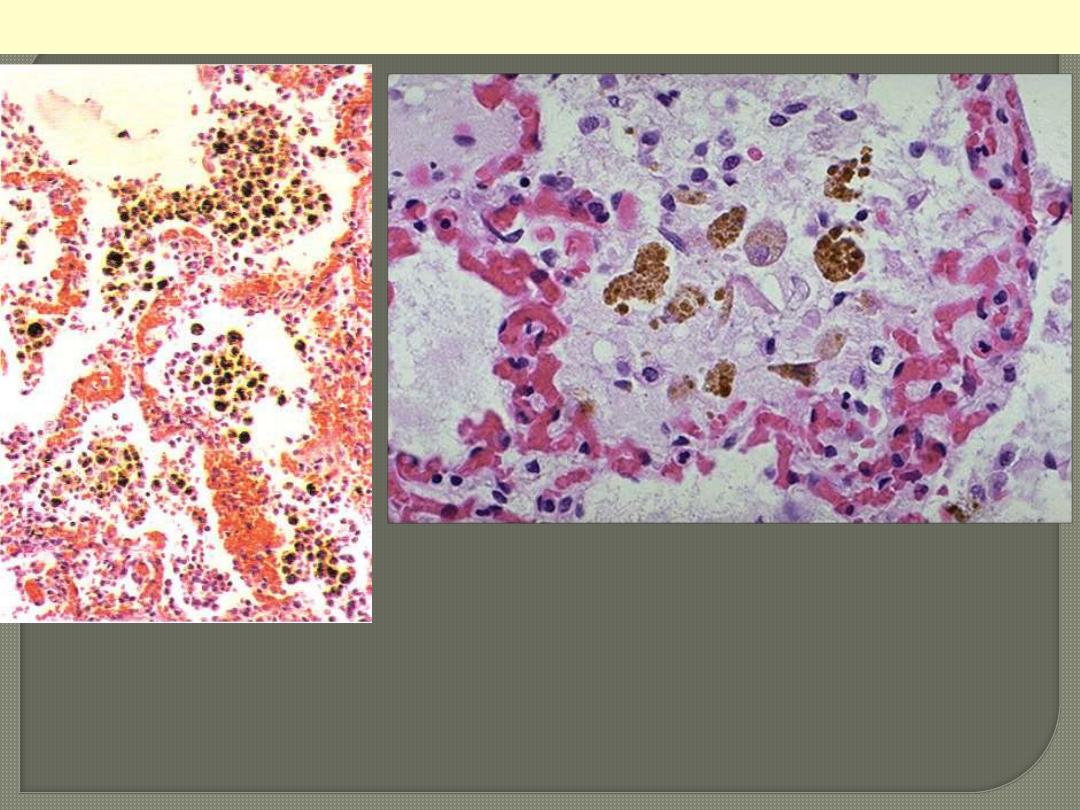
Chronic venous congestion (CVC) lung: low power (Lt) and high Power (Rt)
views. Note: 1. engorgement of septal capillaries 2. hemosiderin-laden
macrophages (the hemosiderin granules within cytoplasm of macrophages
appear brownish)
CVC lung

cut surface shows mottled
appearance; the dark areas
represent congestions around
central veins. This altration
has been likened to the cut
surface of a nutmeg “nutmeg
liver”.
Chronic venous (passive) congestion liver
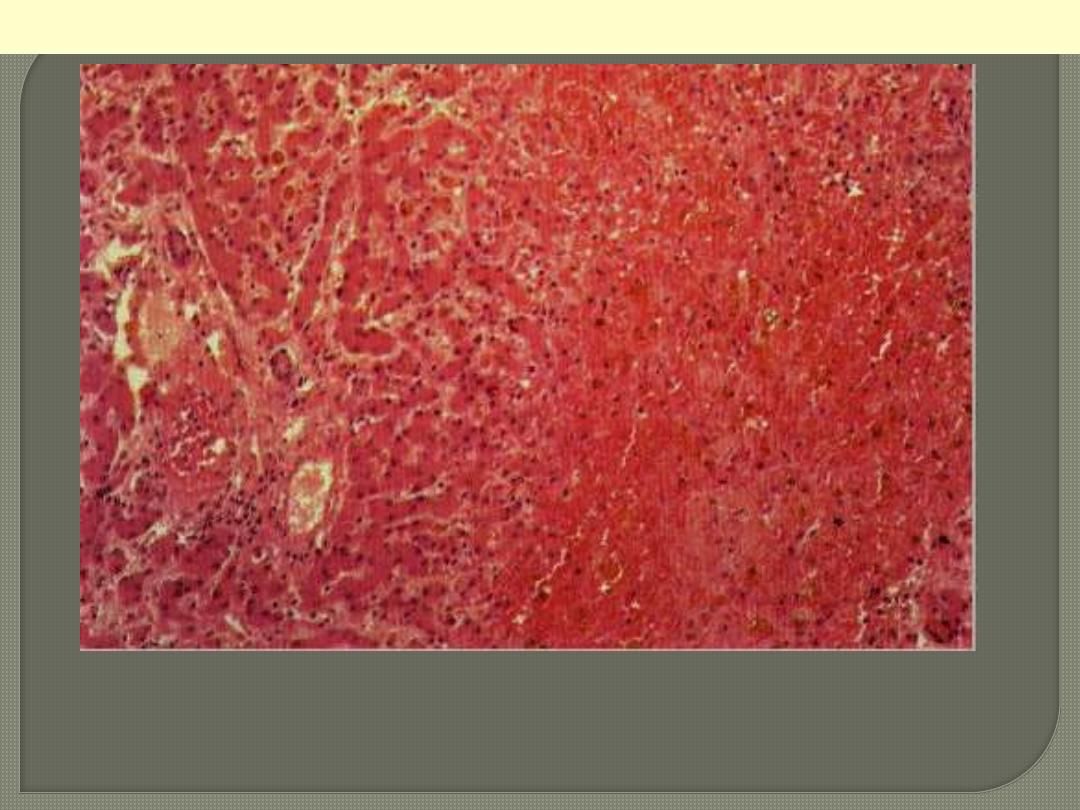
The centrilobular area shows accumulation of blood with damage to the
centrilobular liver parenchyma. The portal tract and surrounding periportal
parenchyma, however, are relatively spared.
CVC liver
Portal & periportal zone
Centrilobular zone

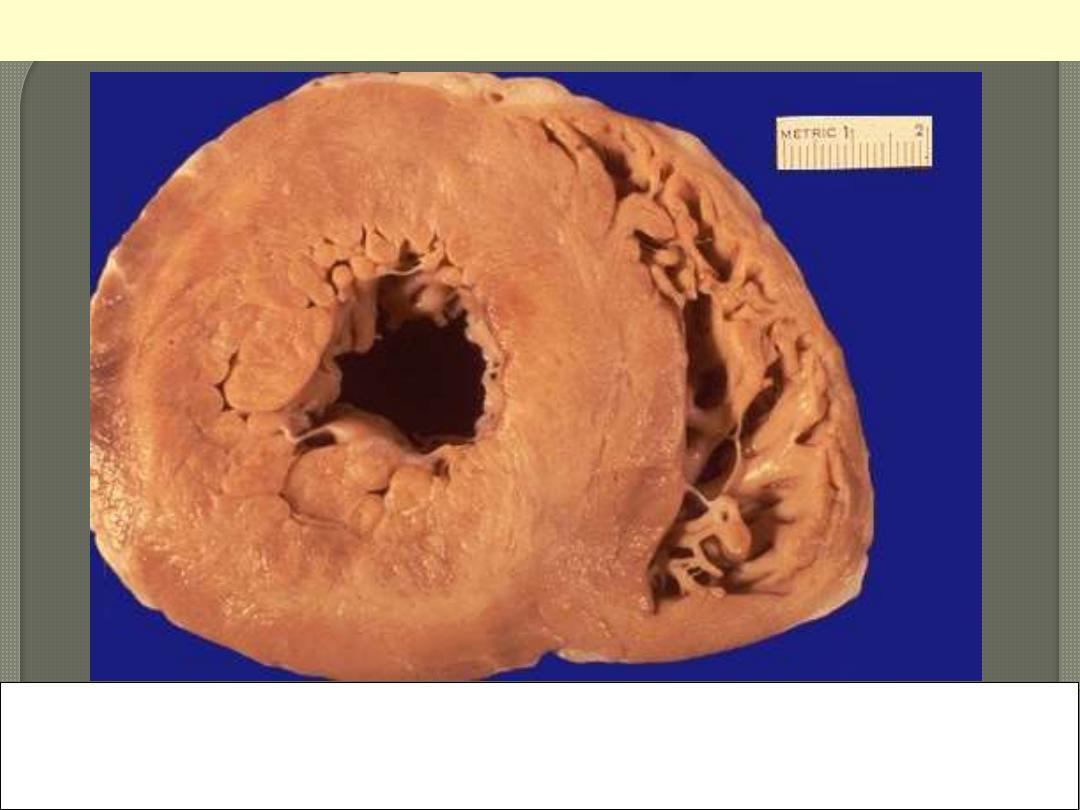
This is a cross section of the heart with marked concentric thickening of the Lt ventricular wall. Note
the corresponding decrease in the ventricular cavity. The LV (Lt) is over 2 cm in thickness (N: 1-1.5
cm). The wt of the heart consequently increase to over the normal of 350 g. some times up to 800 g.
Systemic hypertension is a common cause.
Concentric Hypertrophy of Lt V
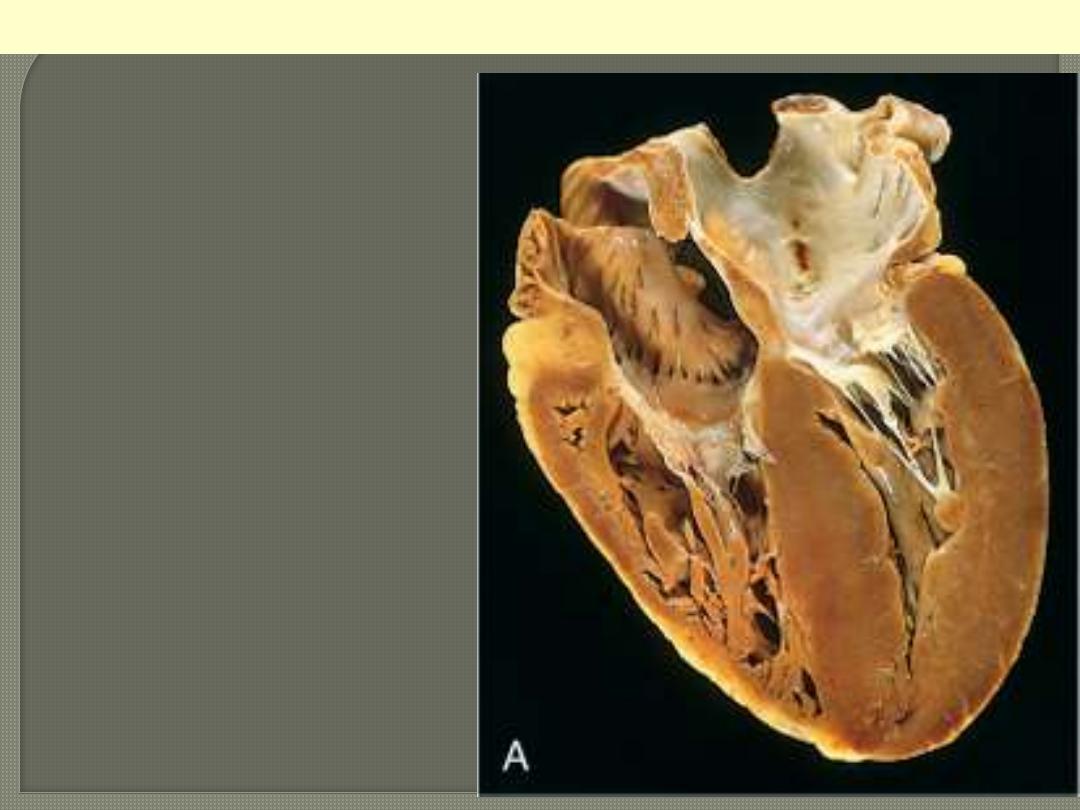
Pressure hypertrophy due to left ventricular
outflow obstruction. The left ventricle is to
your right in this apical four-chamber view
of the heart.
Left ventricular hypertrophy
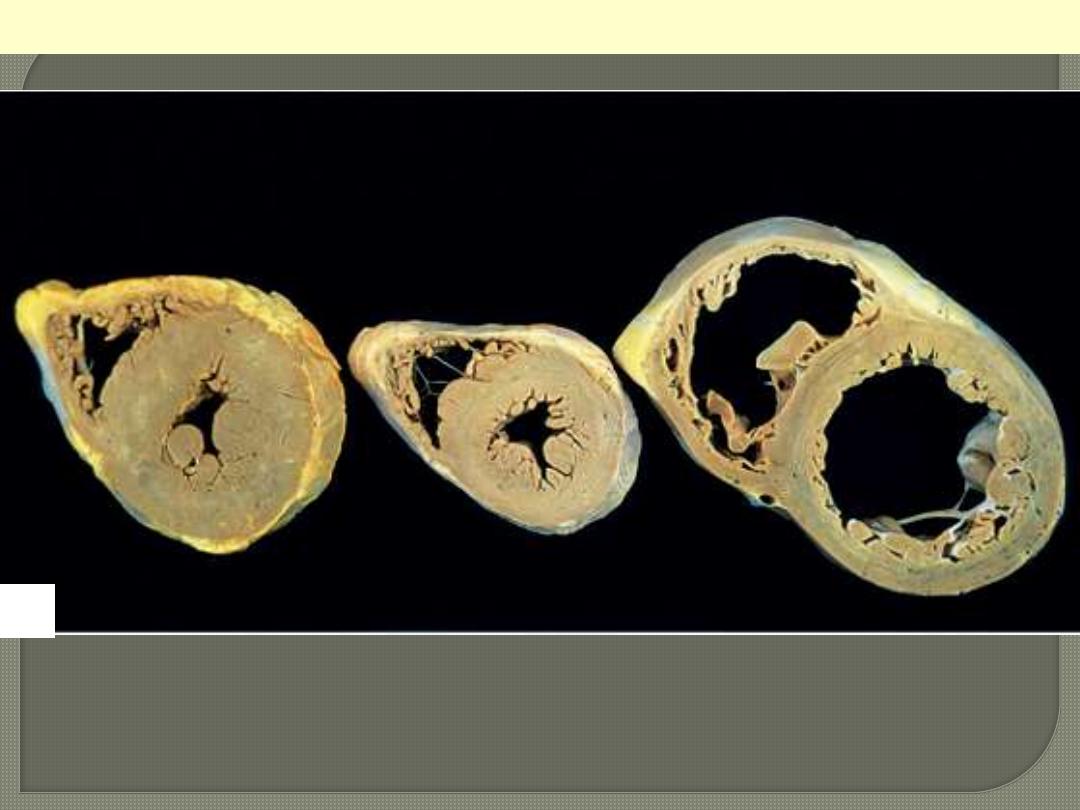
Altered cardiac configuration in LVH without and with dilation, viewed in transverse heart sections.
Compared with a normal heart (center), the pressure-hypertrophied hearts (left) have increased mass
and a thick left ventricular wall, but the hypertrophied and dilated heart (right) has increased mass
but a normal wall thickness.
Lt ventricular hypertrophy Vs normal & hypertrophy with dilation

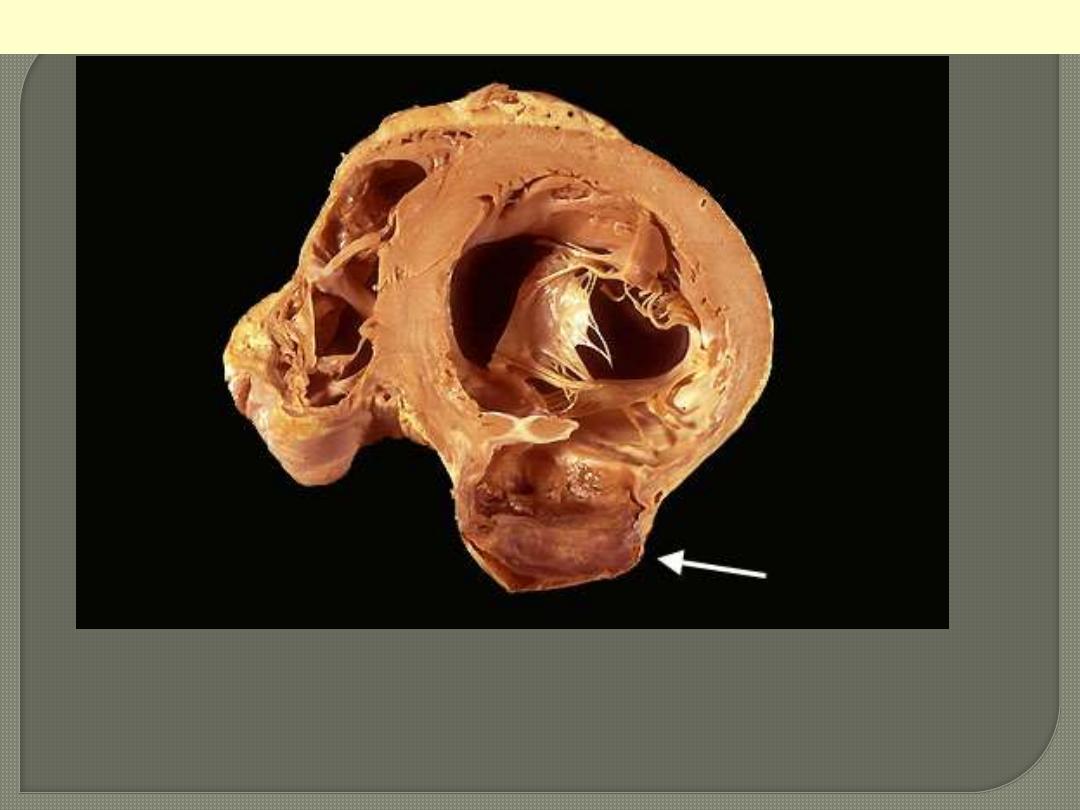
A cross section through the heart reveals a ventricular aneurysm with a
very thin wall at the arrow. Note how the aneurysm bulges out. The
stasis in this aneurysm allows mural thrombus, which is present here, to
form within the aneurysm.
LV aneurysm complicating MI
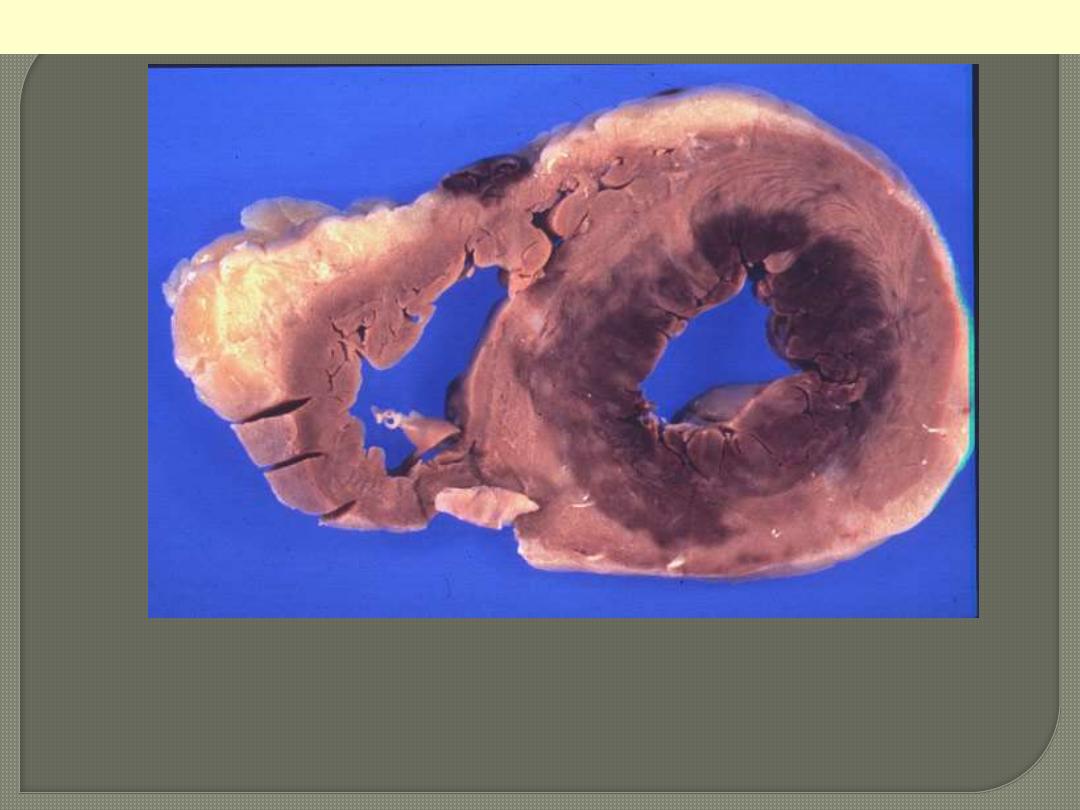
This infarct is limited to the inner third to one half of the LV wall.
The red-blue cyanotic discoloration is totally encircling the Lt V
inner wall.
Subendocardial myocardial infarction
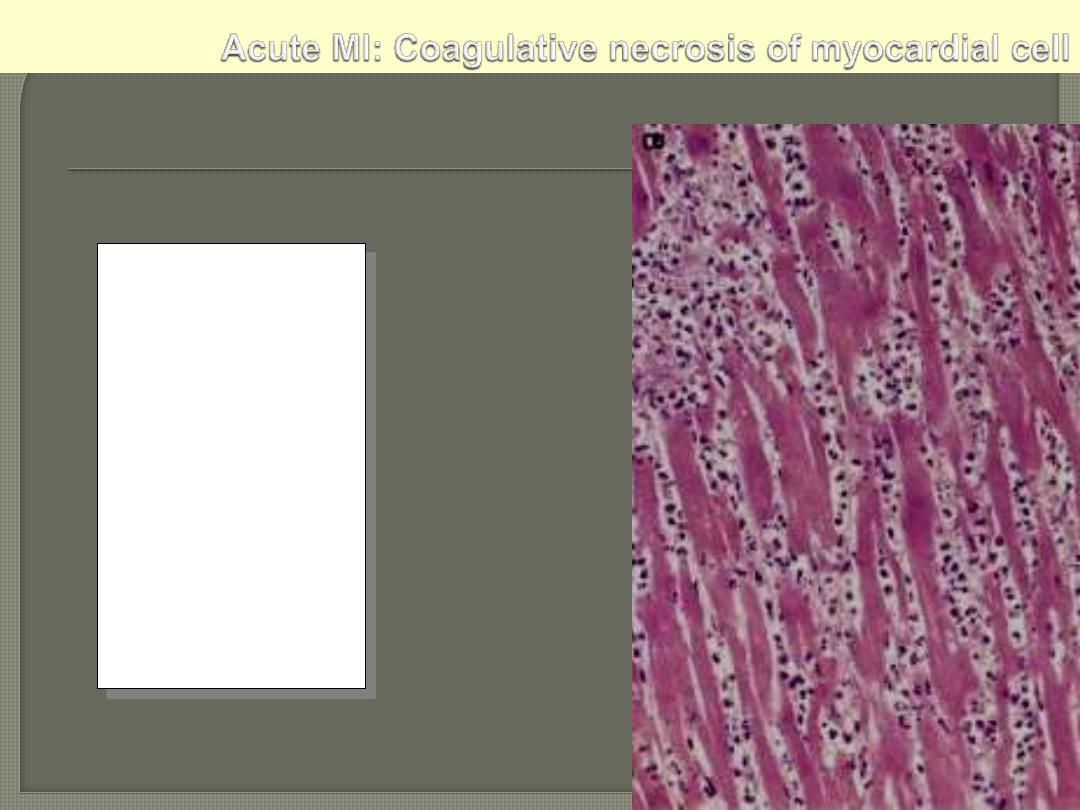
This myocardial
infarction is about
3 to
4 days old
. There is an
extensive acute
inflammatory cell
infiltrate and the
myocardial fibers are
so necrotic that the
outlines of them are
only barely visible.
The cytoplasm is rather
homogeneous, deeply
eosinophilic, devoid of
cross striation and
there are no nuclei.
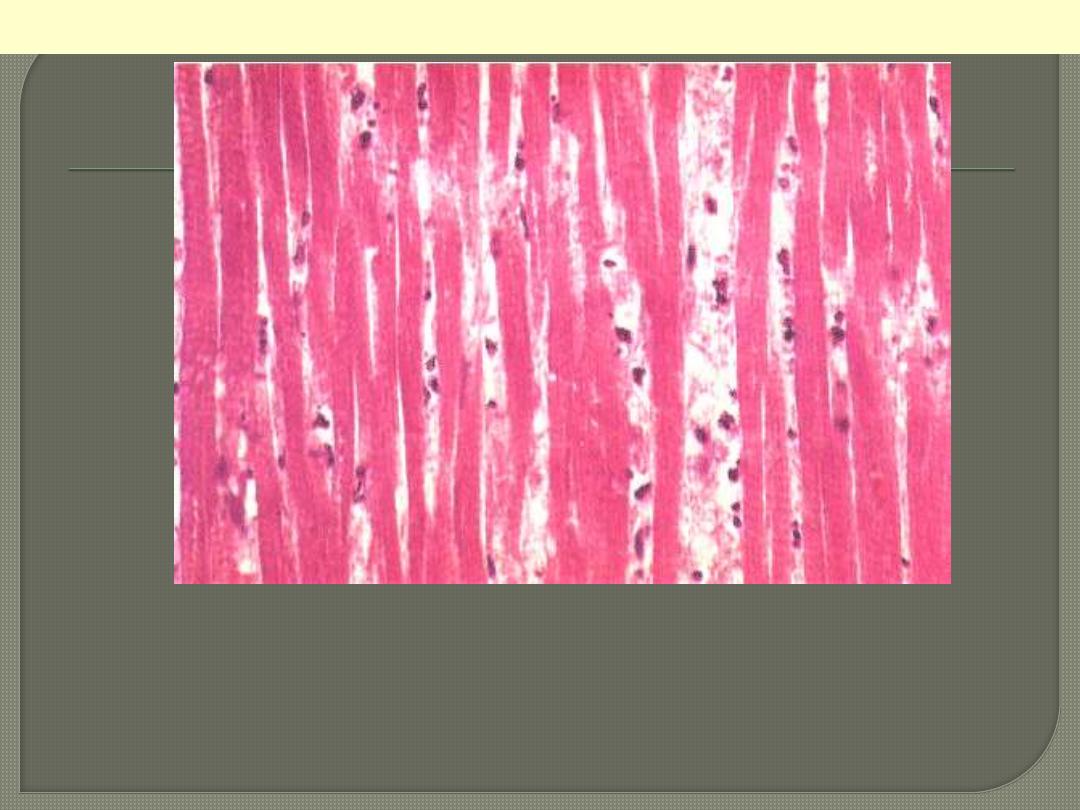
Nnecrotic muscle fibres of the infarct at higher magnification. The nuclei
having disappeared by karyolysis. The fibres are more deeply eosinophilic
than normal fibres. The striations are still detectable focally. In the
interstitial tissue there are some nuclear fragments, macrophages which
have migrated into the dead muscle.
Myocardial Infarction: Coagulative Necrosis
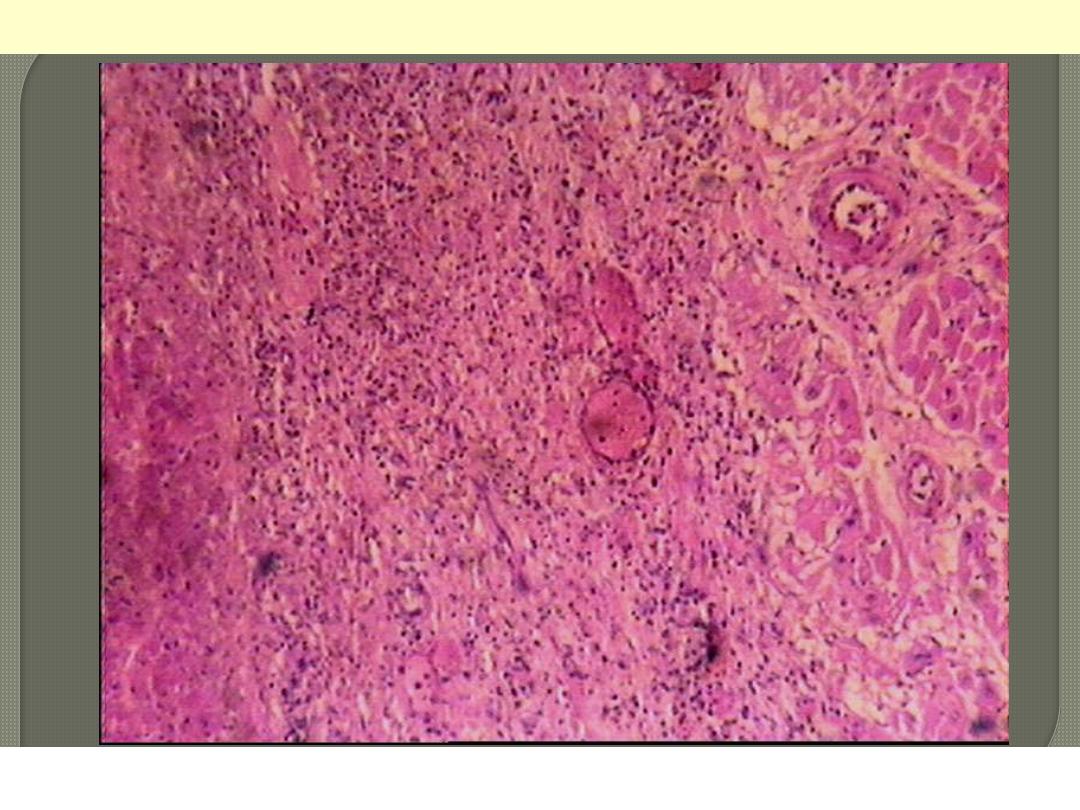
Necrosis
Normal
Line of demarcation
Myocardial infarction-line of demarcation
By 10 to 14 days from the onset the infarct is rimmed by a hyperemic zone of highly vascularized
granulation tissue (line of demarcation)
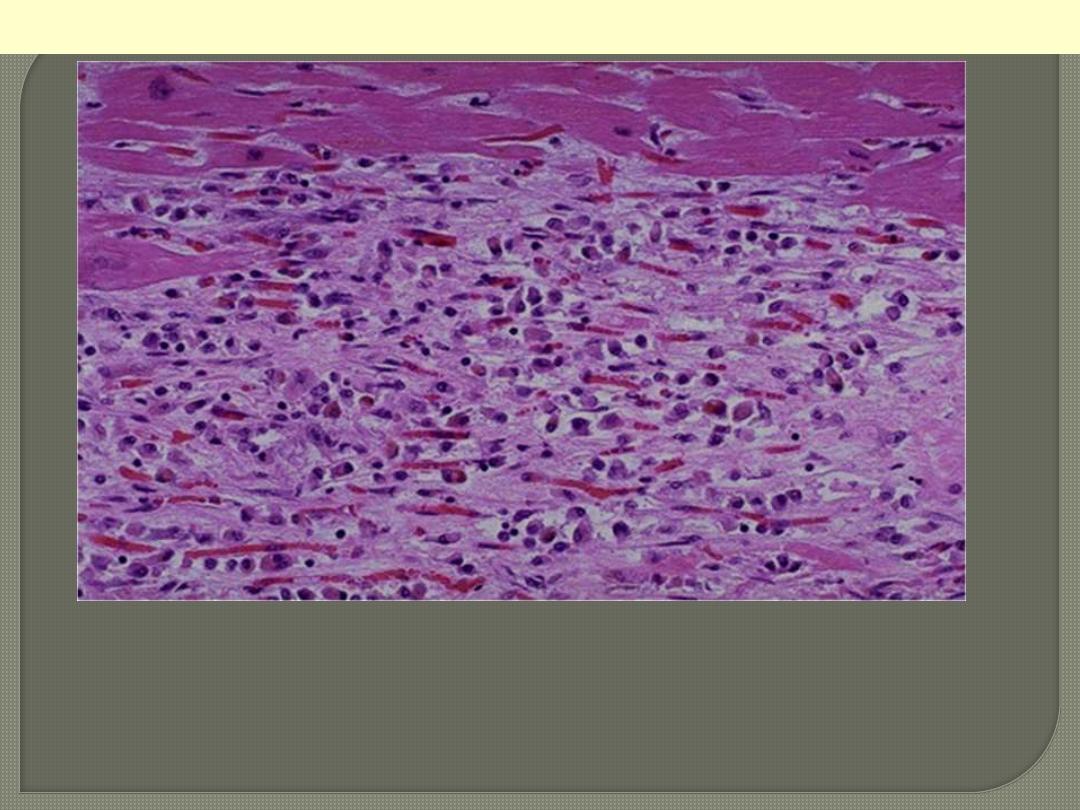
This is a myocardial infarction of 1 to 2 weeks in age. Note that there
are remaining normal myocardial fibers at the top. Below these
fibers are many macrophages along with numerous capillaries and
fibroblasts with deposition of collagen.
Myocardial Infarction: Coagulative Necrosis

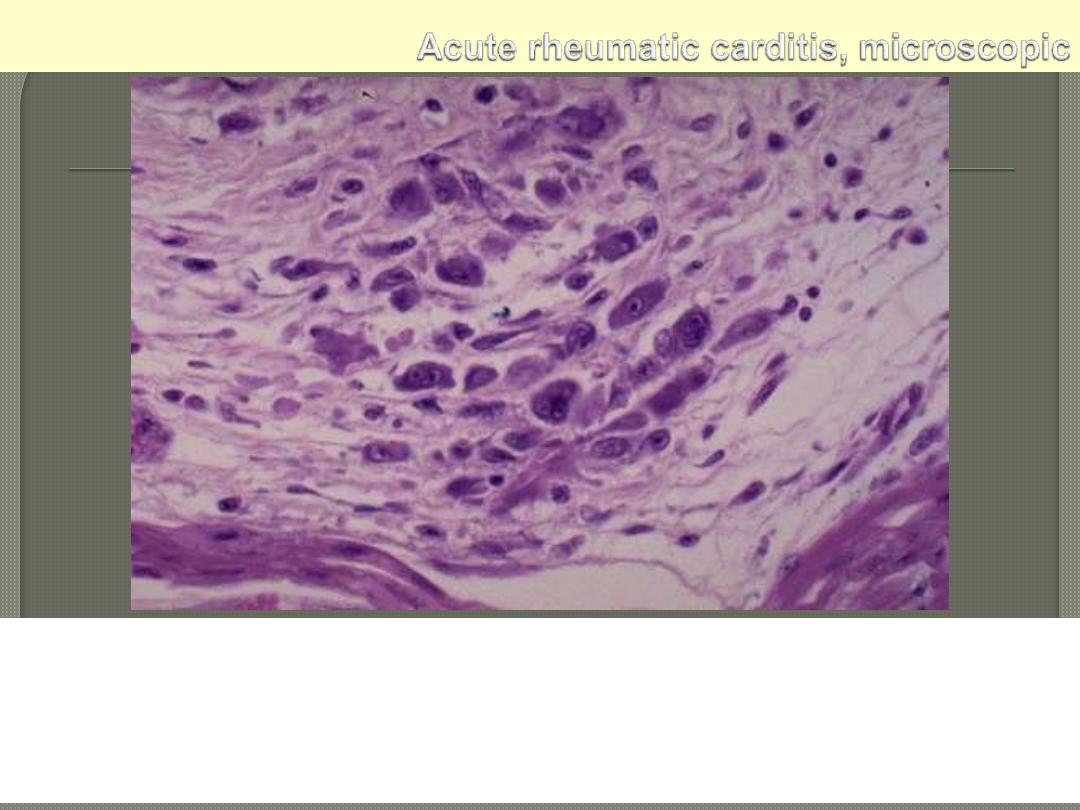
Aschoff nodule. Aschoff giant cell. Several appear here as large cells
with two or more nuclei that have prominent nucleoli. Scattered
inflammatory cells accompany them and can be mononuclears or
occasionally neutrophils.
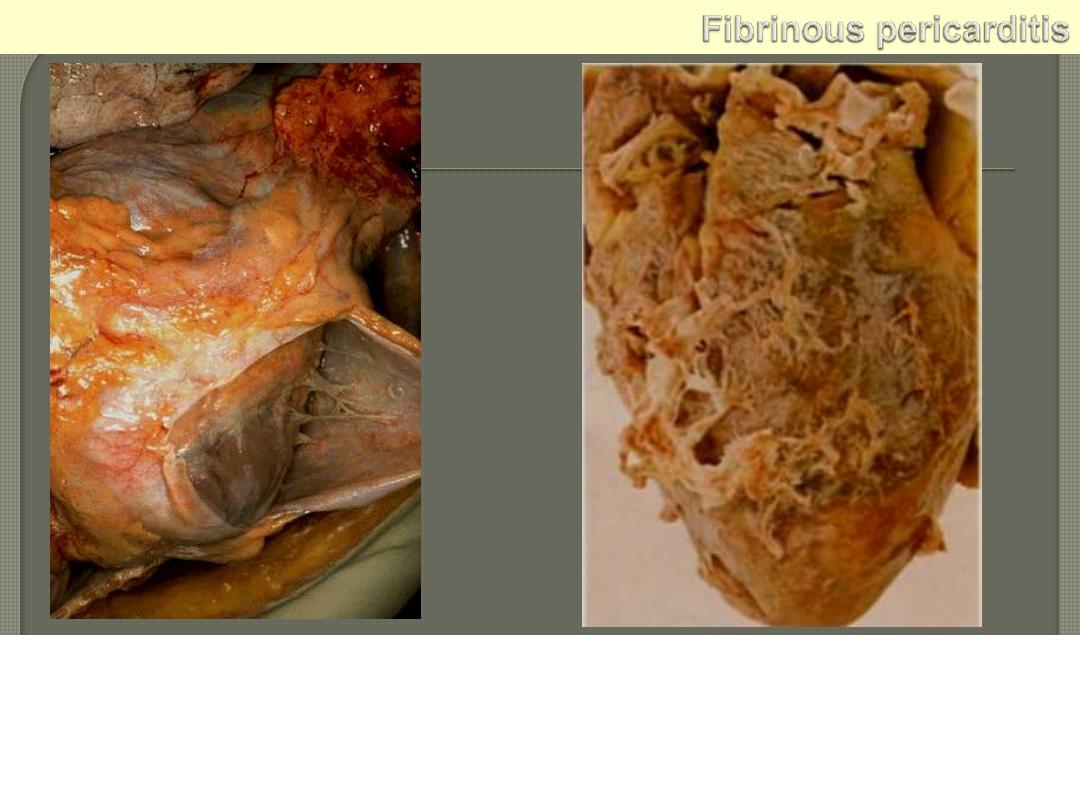
Lt. The pericardium has been opened to reveal the surface of the
heart. There are thin strands of fibrinous exudate that extend from
the parietal to visceral pericardium.. This is typical for a fibrinous
pericarditis. Rt. More florid example of fibrinous pericarditis.
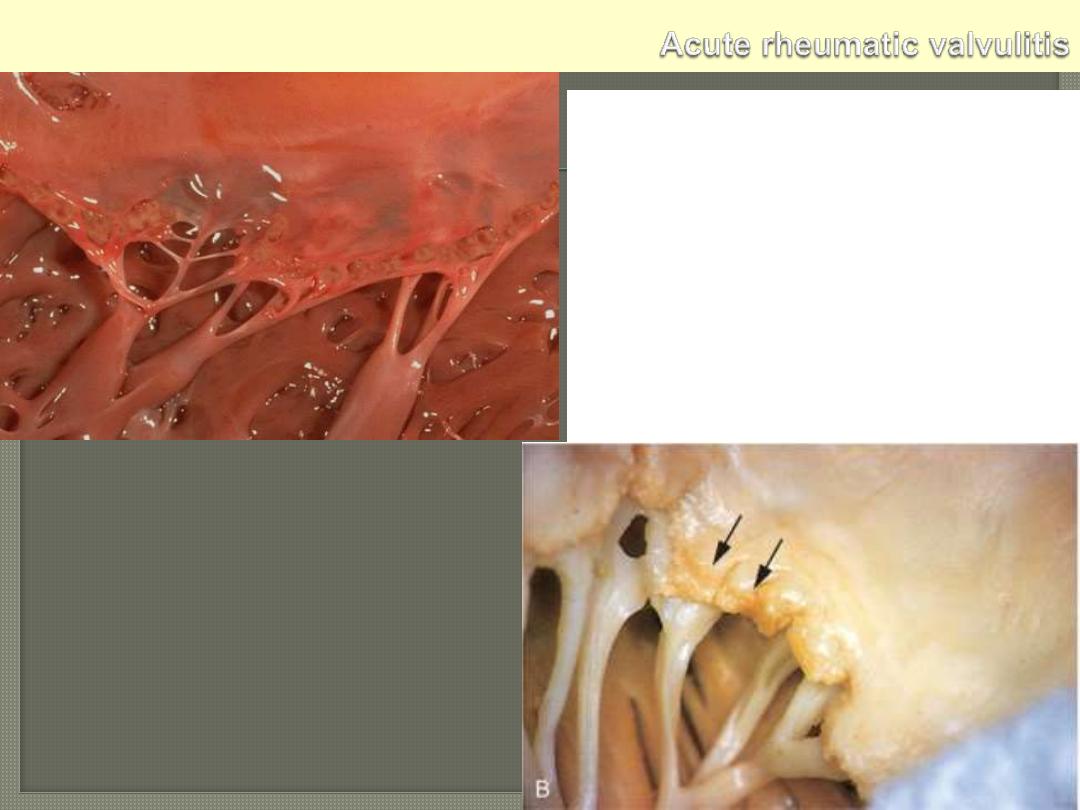
Small verrucous vegetations seen along the
closure line of this mitral valve. These warty
vegetations average only a few millimeters and
form along the line of valve closure over areas
of endocardial inflammation. The lower photo.
display Acute rheumatic mitral valvulitis
superimposed on chronic rheumatic heart
disease. Small vegetations (verrucae) are visible
along the line of closure of the mitral valve
leaflet (arrows). Previous episodes of rheumatic
valvulitis have caused fibrous thickening and
fusion of the chordae tendineae.
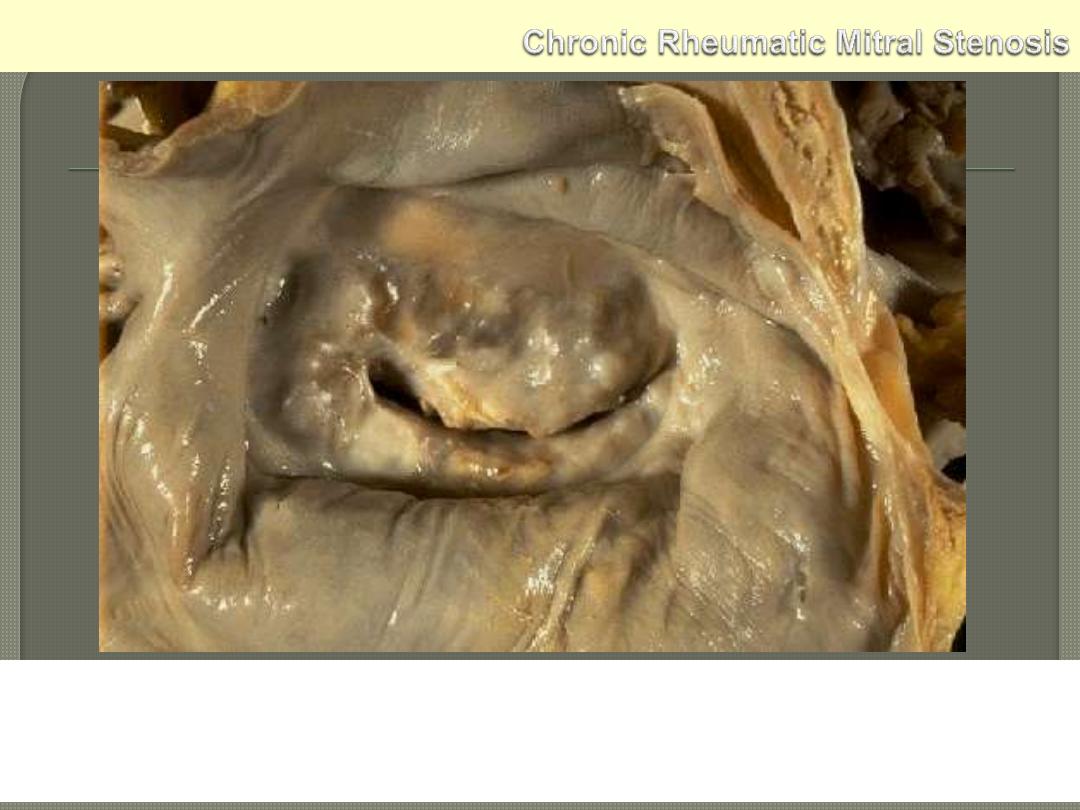
The mitral valve as seen from above in the left atrium. The mitral
valve demonstrates the typical "fish mouth" shape with chronic
rheumatic scarring.
