
Blood vessels

Aneurysms
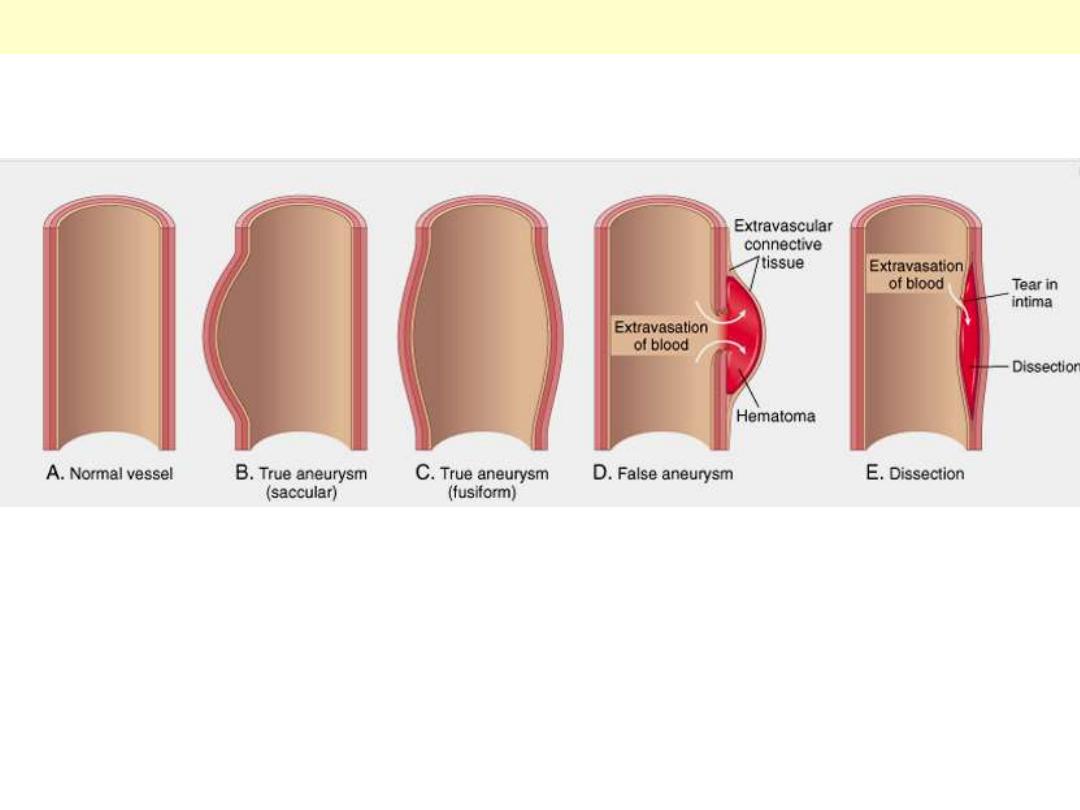
A. Normal vessel. B, True aneurysm, saccular type. The wall focally bulges outward and may be
attenuated but is otherwise intact. C, True aneurysm, fusiform type. There is circumferential dilation
of the vessel, without rupture. D, False aneurysm. The wall is ruptured, and there is a collection of
blood (hematoma) that is bounded externally by adherent extravascular tissues. E, Dissection. Blood
has entered (dissected) the wall of the vessel and separated the layers. Although this is shown as
occurring through a tear in the lumen, dissections can also occur by rupture of the vessels of the vaso
vasorum within the media.
Morphological types of aneurysms
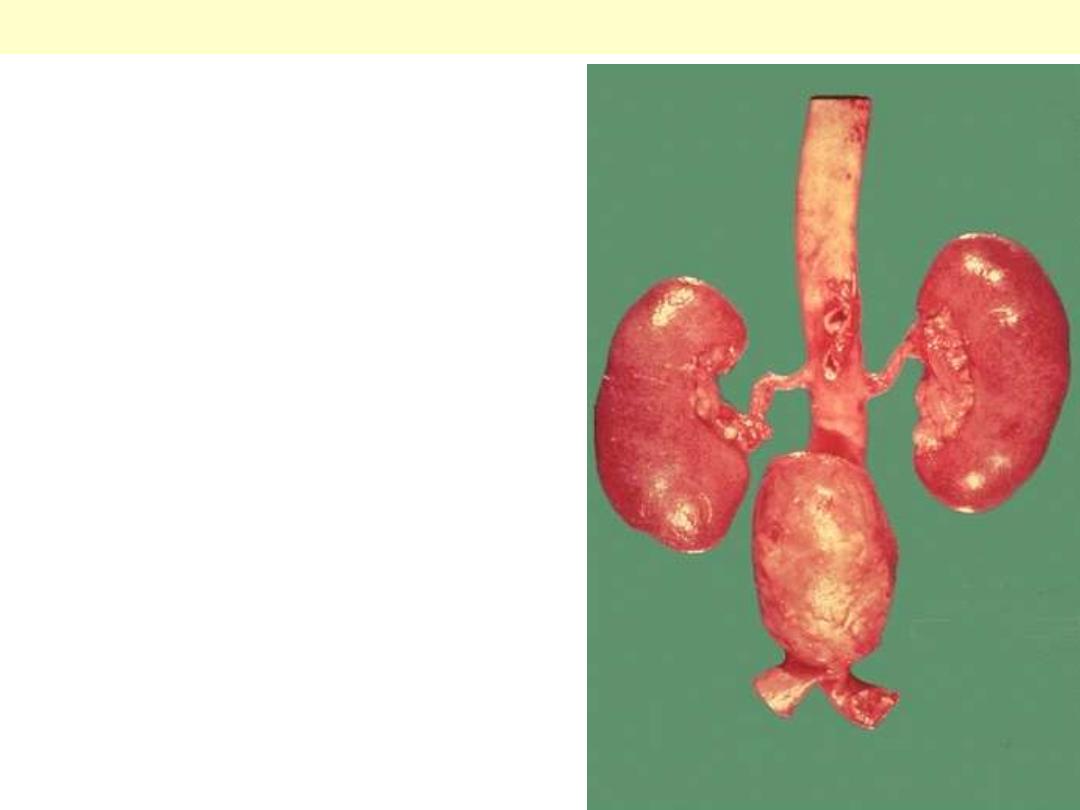
A large "bulge" appears just above the aortic
bifurcation. Such aneurysms are prone to rupture
when they reach about 6 to 7 cm in size. They may be
felt on physical examination as a pulsatile mass in the
abdomen. Most such aneurysms are located below the
renal arteries so that surgical resection can be
performed with placement of a dacron graft.
Atherosclerotic aneurysm of the abdominal aorta
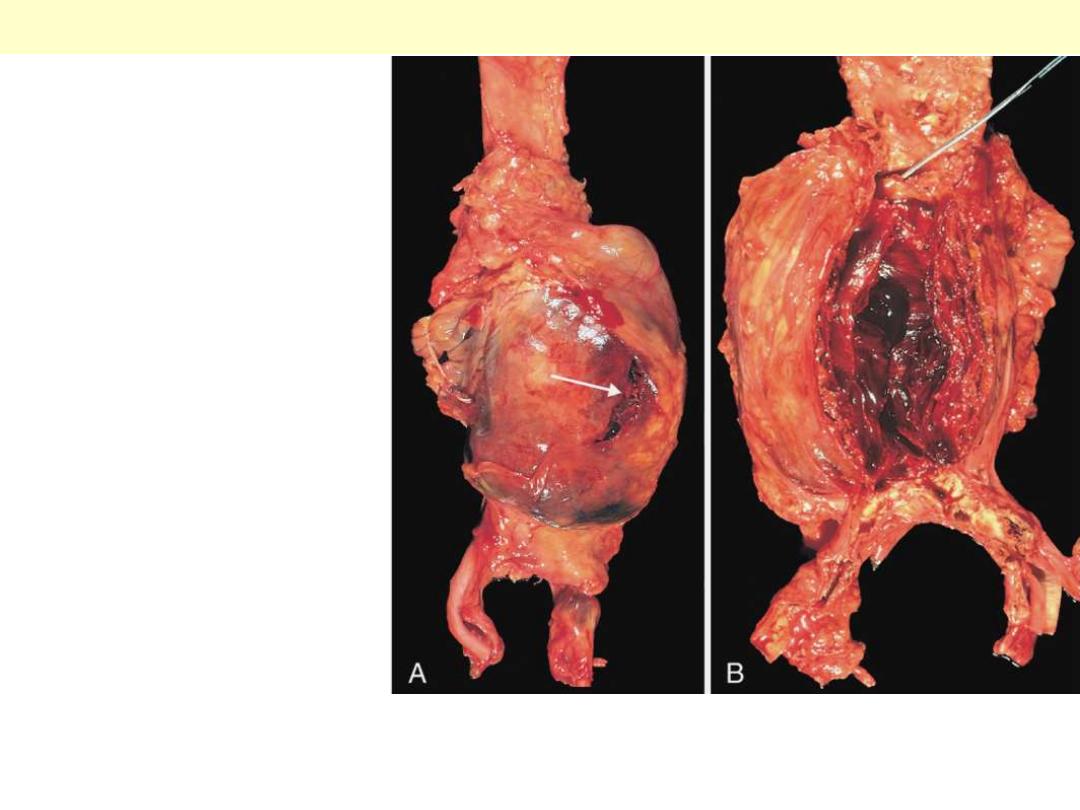
A, External view, gross
photograph of a large aortic
aneurysm that ruptured (arrow).
B, Opened view, with the location
of the rupture tract indicated by
a probe. The wall of the
aneurysm is exceedingly thin, and
the lumen is filled by a large
quantity of layered but largely
unorganized thrombus.
Abdominal aortic aneurysm
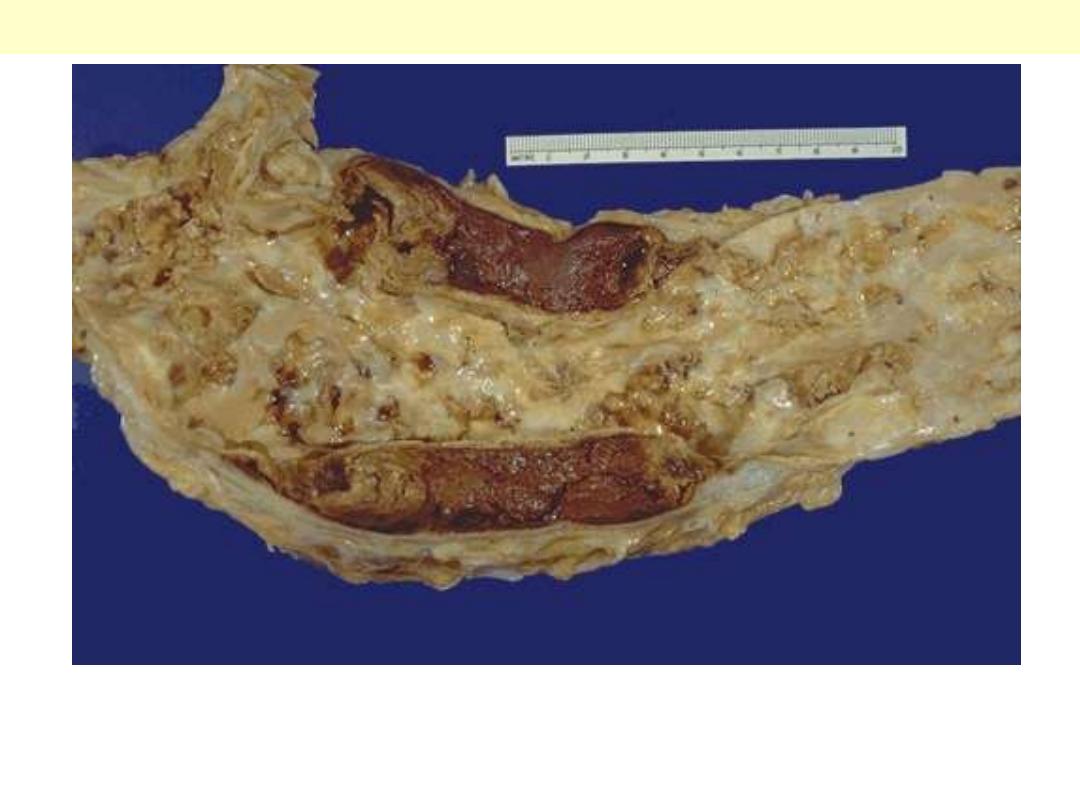
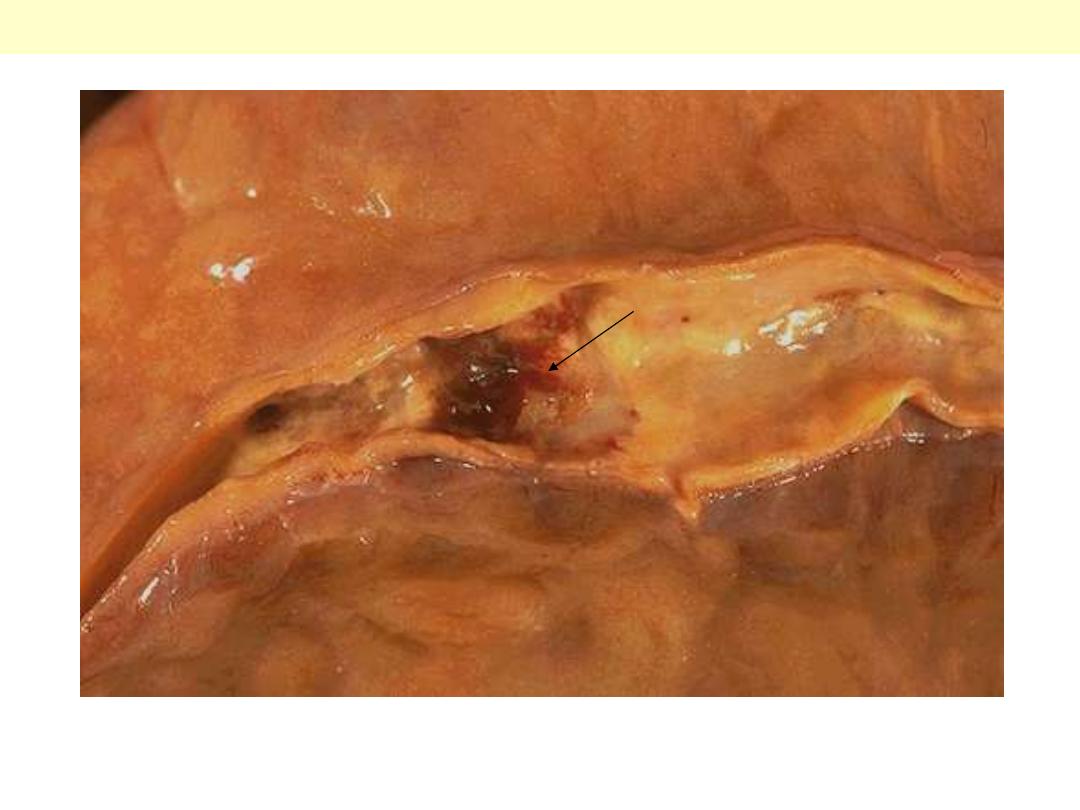
This aorta has been opened longitudinally to reveal an area of fairly limited dissection that is
organizing. The red-brown hematoma can be seen in on both sides of the section as it extends around
the aorta. The dissection creates a "double lumen" to the aorta. This aorta shows in addition severe
atherosclerosis.
Aortic dissection
This aortic dissection occurred just above the
aortic root in a patient with Marfan's syndrome.
The tear extends across the aorta.
Hemopericardium with tamponade occurred
within minutes of this event.
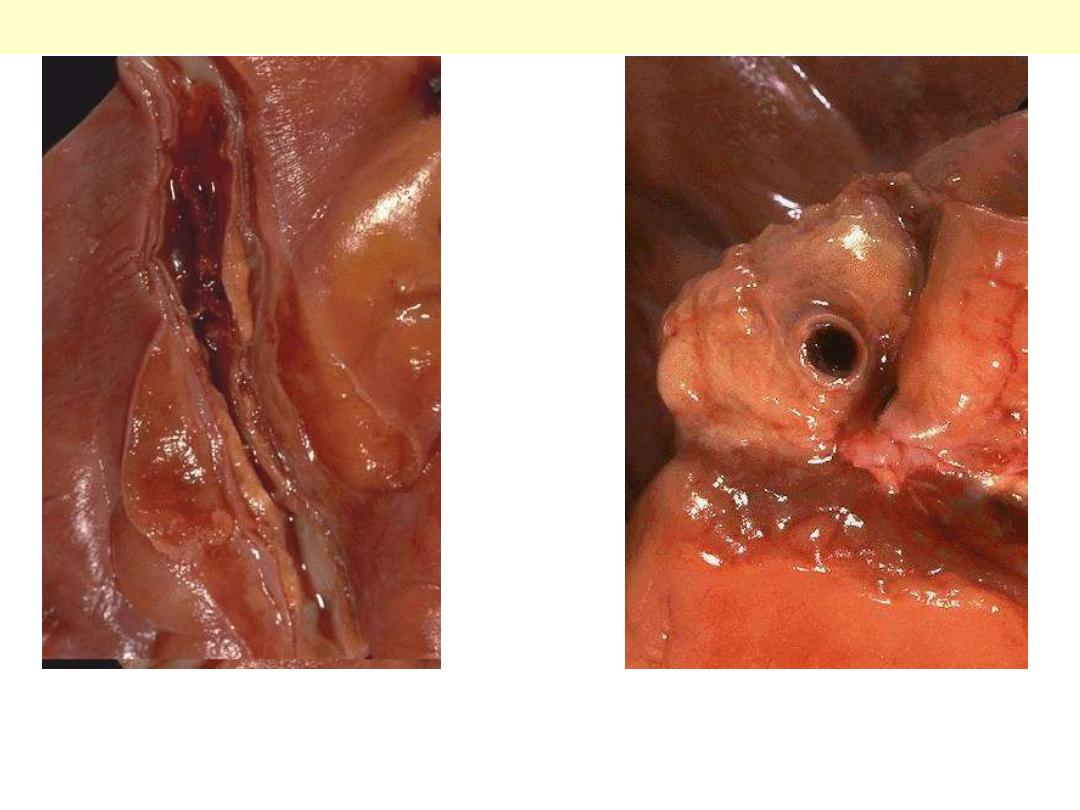
Aortic dissection
an opened aorta with proximal dissection originating
from a small, oblique intimal tear (identified by the
probe), allowing blood to enter the media and create
an intramural hematoma (narrow arrows). Note that
the intimal tear has occurred in a region largely free
of atherosclerotic plaque and that propagation of the
intramural hematoma is arrested at a site more
distally where atherosclerosis begins (broad arrow).
Aortic dissection
The dissection goes into the muscular wall creating an aorta with double lumina.
Aortic dissection
Original lumen
Dissection

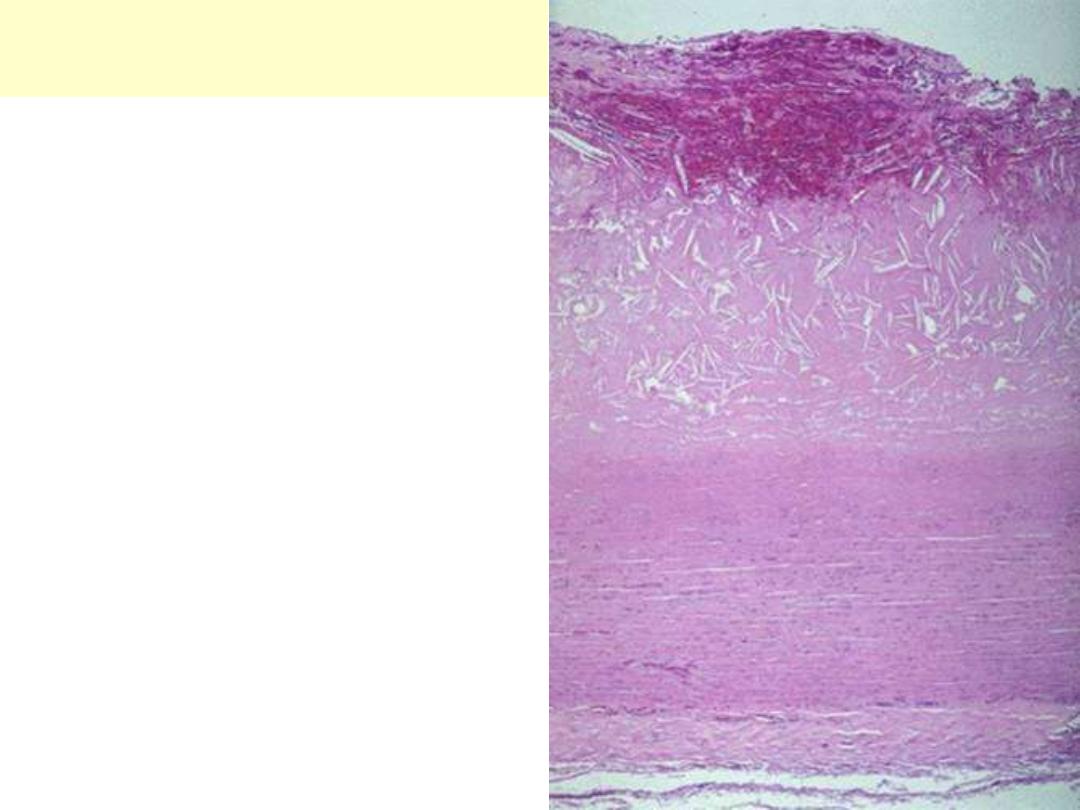
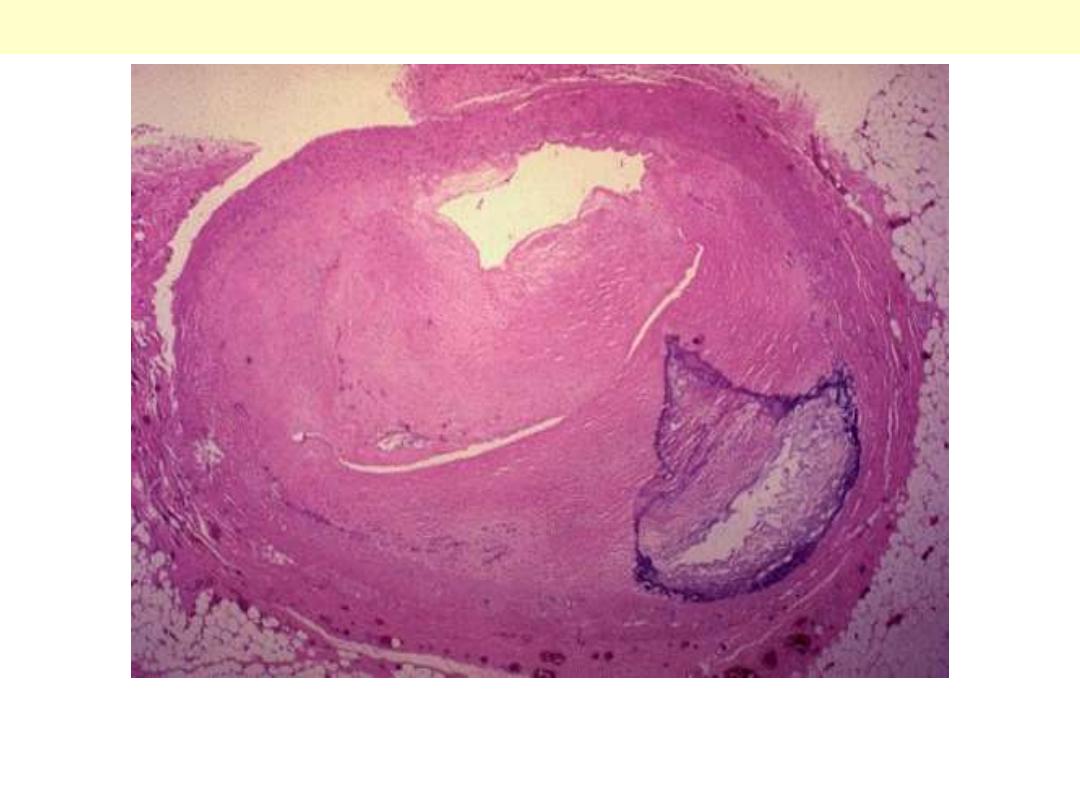
Aortic dissection: medial cystic degeneration
This special stain highlight the elastic fibers of the aortic wall. There is extensive fragmentation &
destruction of the fibers associated with several coalescent cystic areas within the wall.
The right carotid artery is compressed by blood dissecting upward from a tear with aortic dissection.
Blood may also dissect to coronary arteries. Thus patients with aortic dissection may have symptoms of
severe chest pain (for distal dissection) or may present with findings that suggest a stroke (with carotid
dissection) or myocardial ischemia (with coronary dissection).
Extension of aortic dissection

The aortic root is widened here and the
commissures of the aortic valve cusps are
pulled apart. The aorta above this shows
peculiar wrinkling that is typical for
syphilitic aortitis. The widening of the root
can cause aortic insufficiency and also
aneurysmal dilation of the ascending
aorta. Such dilation may also be seen with
Marfan's syndrome, but the intima would
not show the wrinkling.
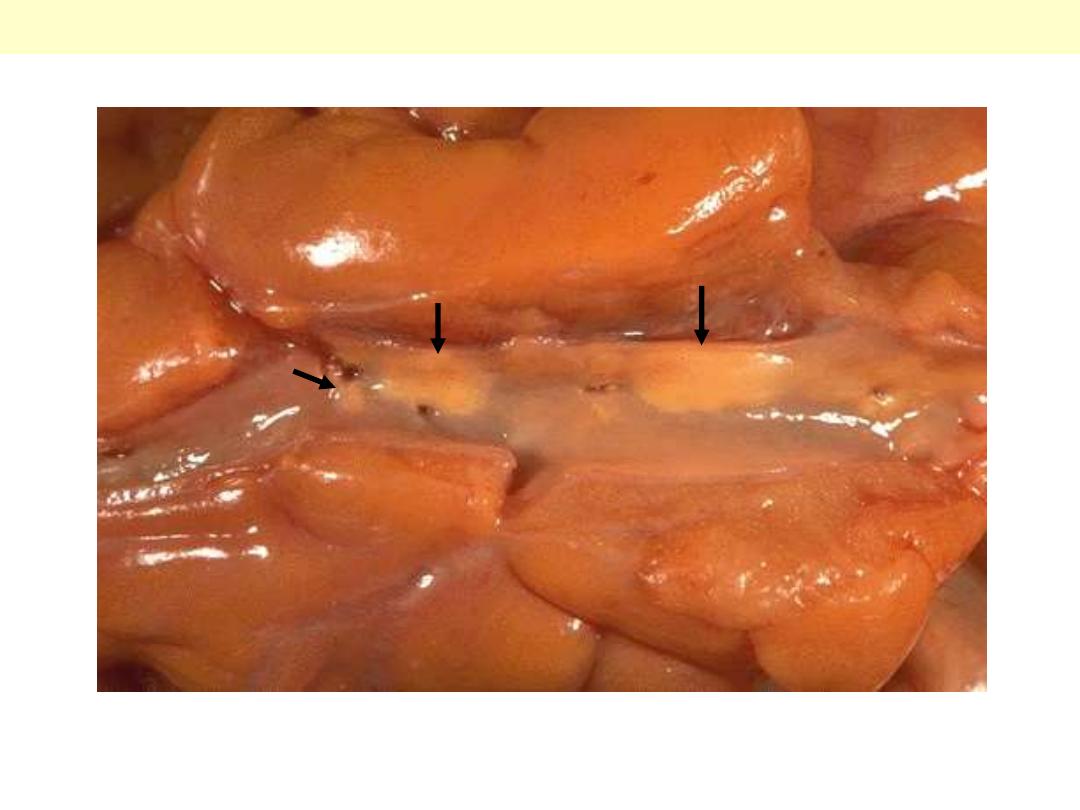
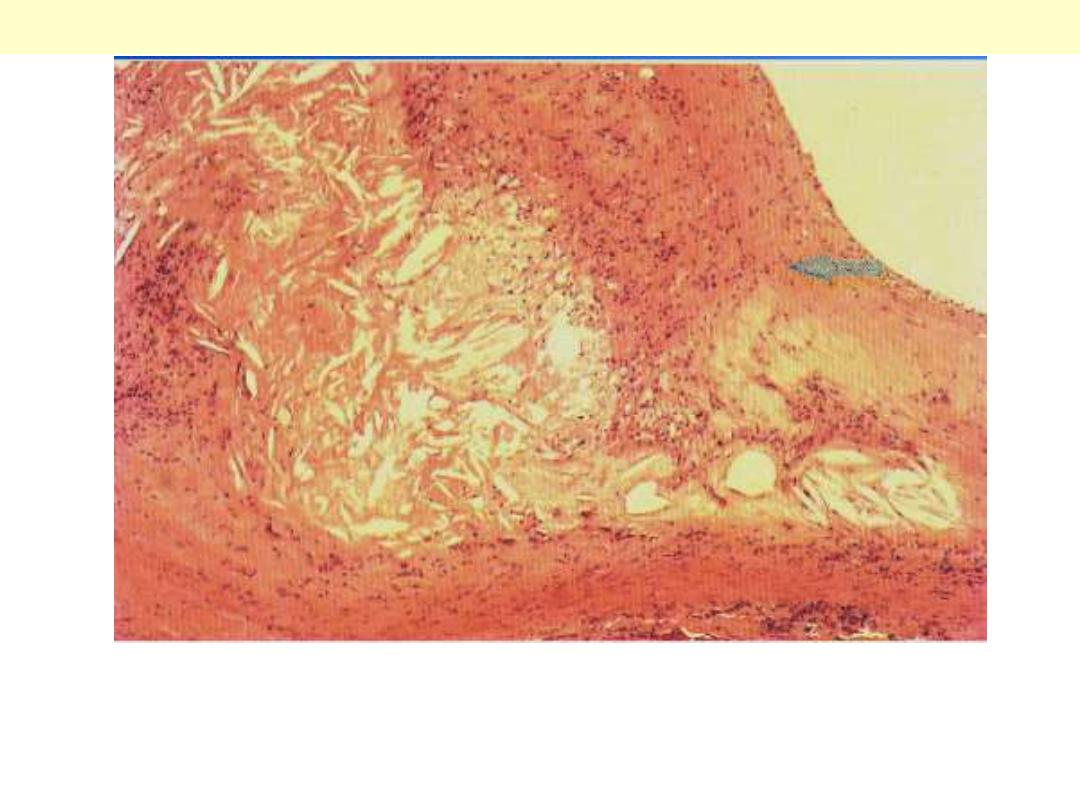
Syphilitic aneurysm
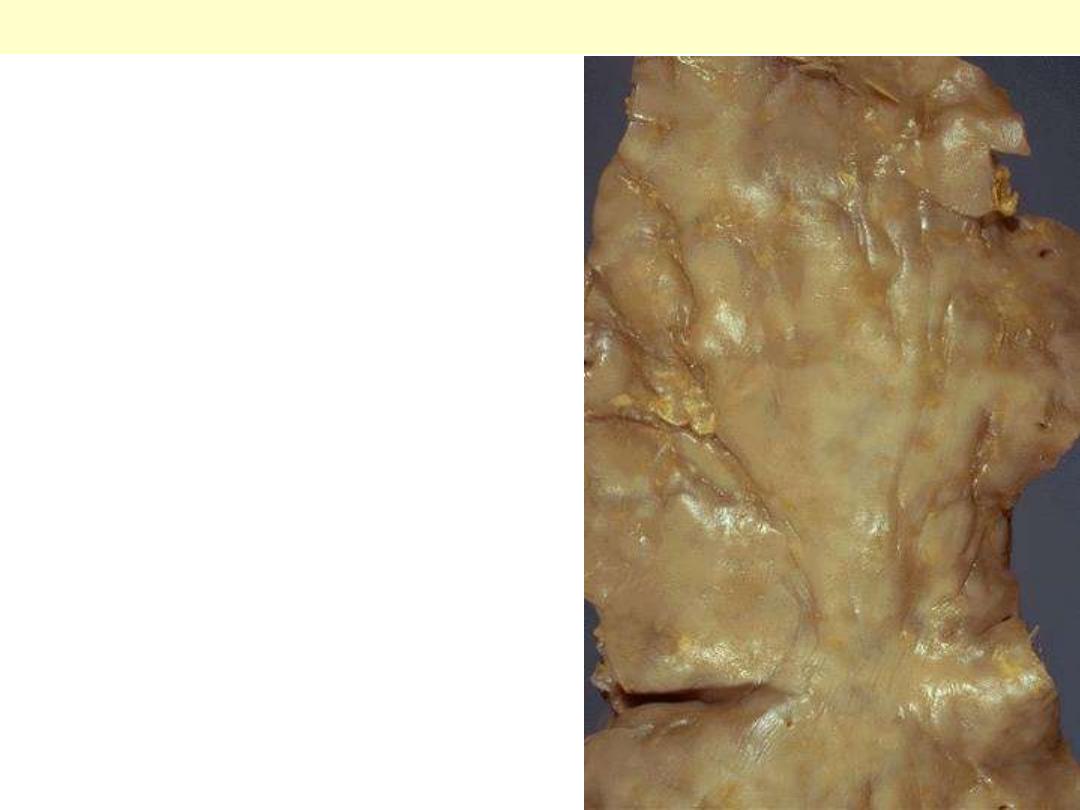
The surface of the aorta shows wrinkling or "tree-
barking" that is typical for syphilitic aortitis. The
aortitis involves the vasa vasora and leads to focal
medial loss that produces the wrinkling.
Syphilitic aneurysm
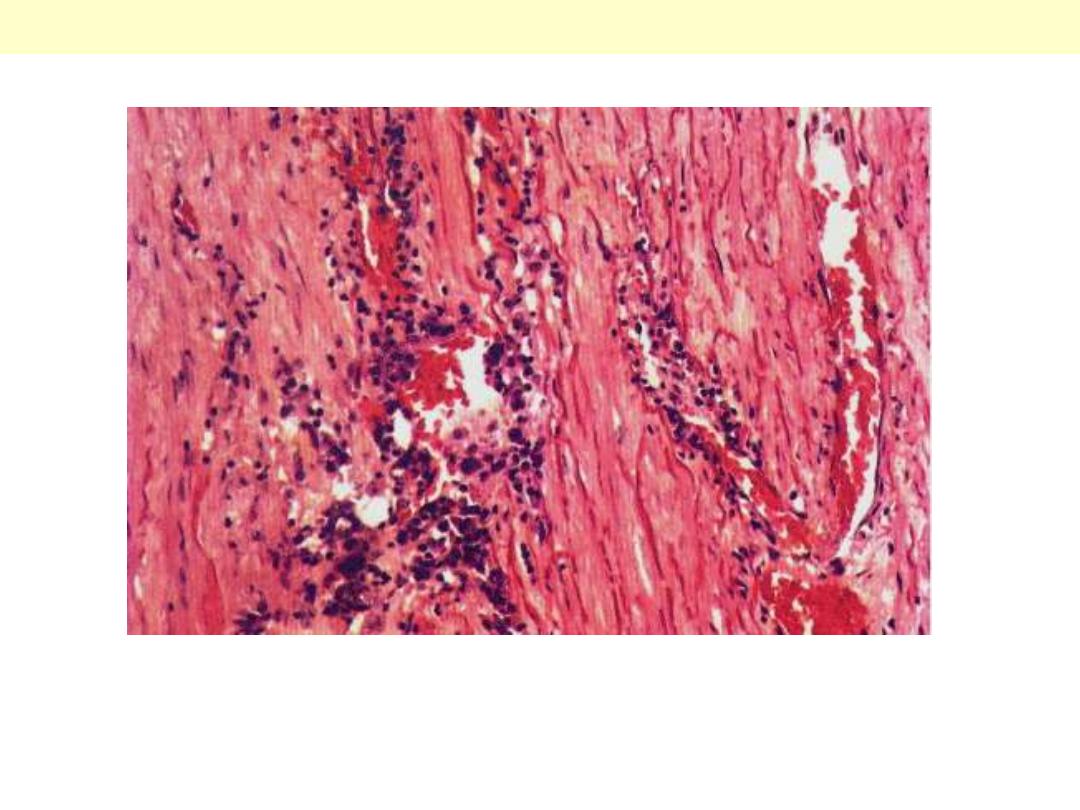
A case of syphilitic aortitis showing lymphoplasmacytic infiltration of the vasa vasorum within the
aortic adventitia.
Syphilitic aortitis
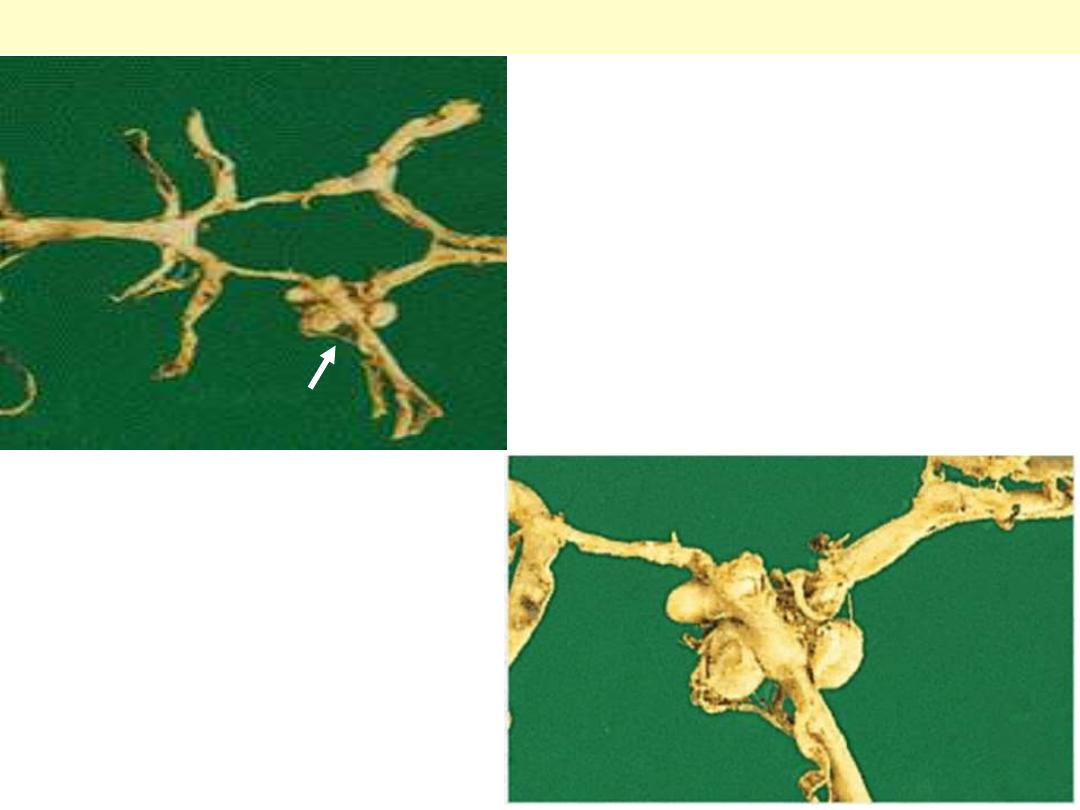
Circle of Willis with anterior, middle and posterior
cerebral arteries linked by communicating vessels.
Berry aneurysms are seen arising where the internal
carotid bifurcates into middle and anterior cerebral
arteries (arrow).
Berry aneurysms
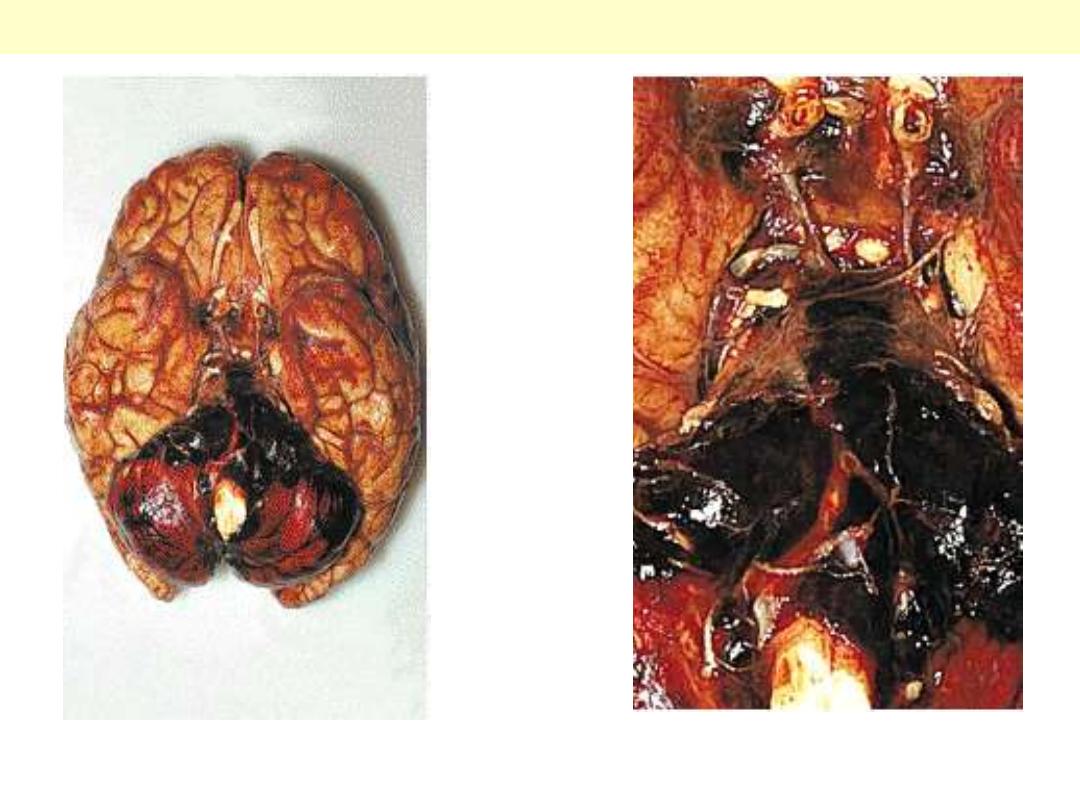
Blood is present in the sub-arachnoid space over the cerebellum. in this case the aneurysm was arising
at the tip of the basilar artery.
Ruptured Berry aneurysm with subarachnoid Hge

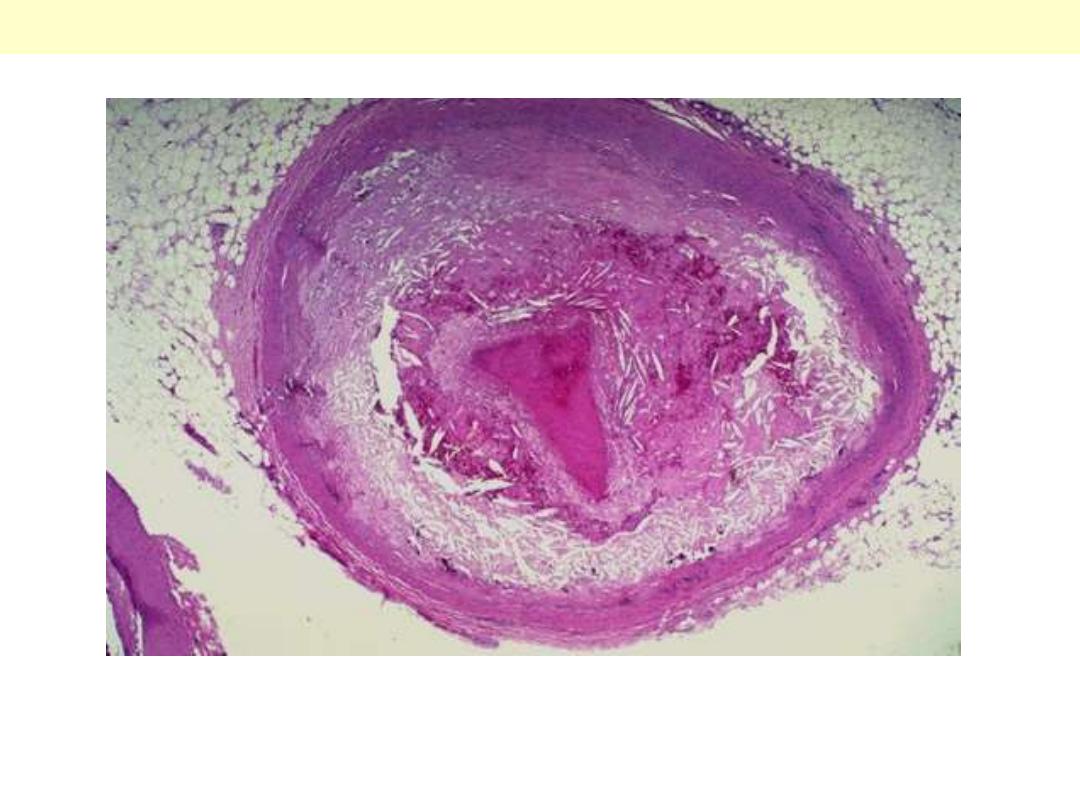
Arteriosclerosis
A coronary artery has been opened longitudinally. The coronary extends from left to right across the
middle of the picture and is surrounded by epicardial fat. This coronary shows only mild
atherosclerosis, with only an occasional yellow-tan lipid plaques (arrows) and no narrowing.
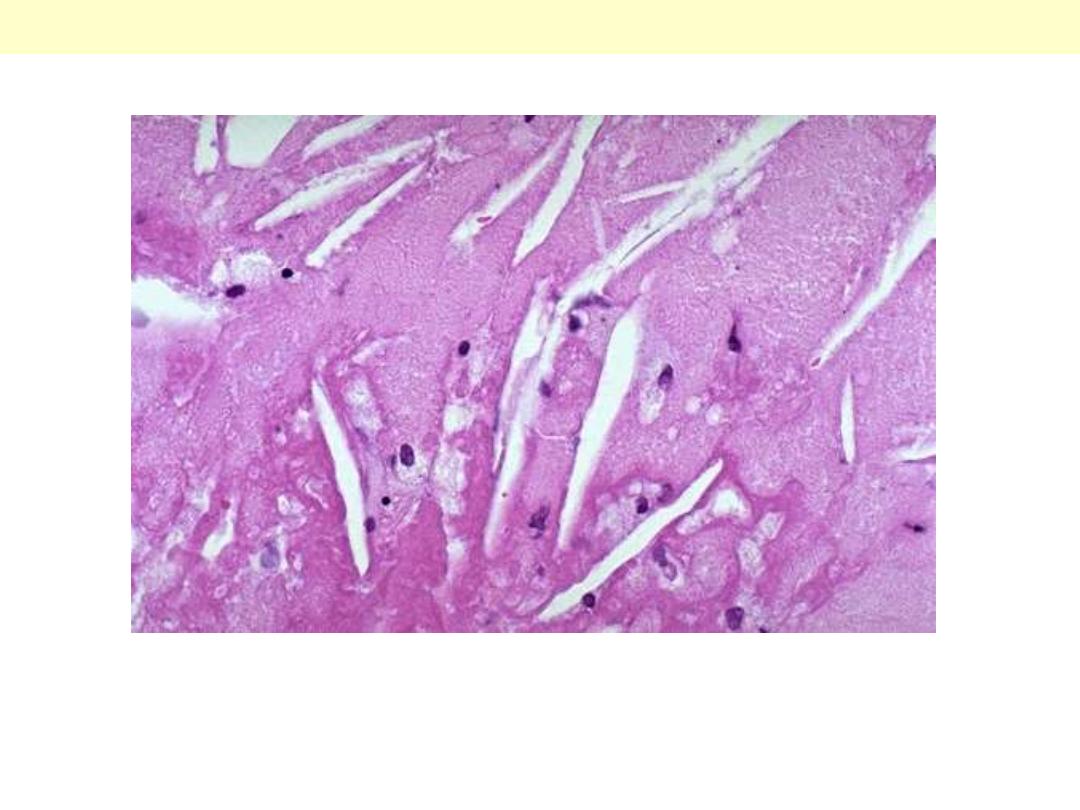
Mild degree of coronary athersclerosis
The lumen of the artery is at the top right corner, and the band of smooth muscle at the bottom is the
atrophic media. The intima is enormously thickened, by the presence deep in it (centre and left) of
amorphous material containing large numbers of cholesterol crystals (the unstained clefts). There are
many foamy (lipid-filled) macrophages and chronic inflammatory cells in this zone and also in the
thick layer of dense fibrous tissue layer (arrow) which separates it from the lumen.
Coronary artery atheromatous plaque: (HE) medium power
Media
This microscopic cross section of the aorta shows
a large luminal atheroma. Cholesterol clefts are
numerous in this atheroma. The surface shows
intraplaque hemorrhage.
Aorta: atheromatous plaque with
hemorrhage. (HE) low power
This high magnification of an atheroma shows numerous foam cells (arrows) and an occasional
cholesterol cleft. A few dark blue inflammatory cells are scattered within the atheroma.
Atheromatous plaque. (HE) High power
Media
Atheromatous plaque. (HE) High power
This is a high magnification of an atheroma with foam cells and cholesterol clefts.
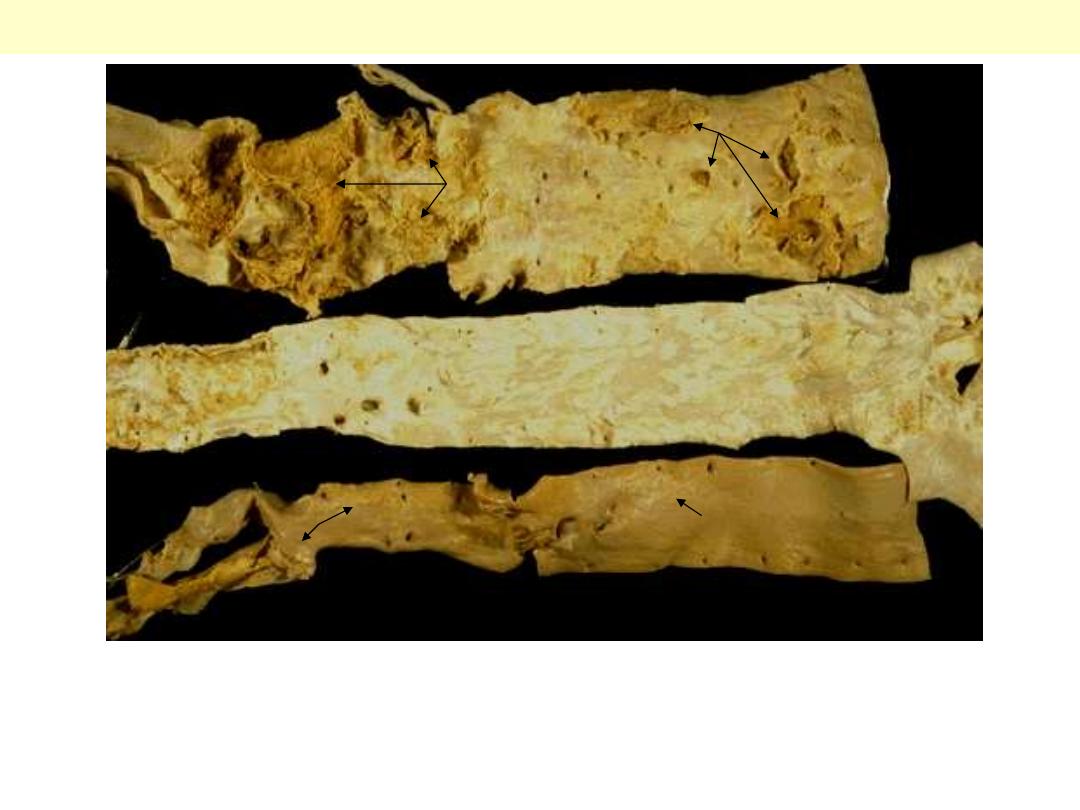
These three aortas demonstrate mild, moderate, and severe atherosclerosis from bottom to top. At the
bottom, the mild atherosclerosis shows only scattered lipid plaques (arrows). The aorta in the middle
shows many more larger whitish plaques. The severe atherosclerosis in the aorta at the top shows
extensive ulceration in the plaques (arrows).
Atherosclerosis aorta
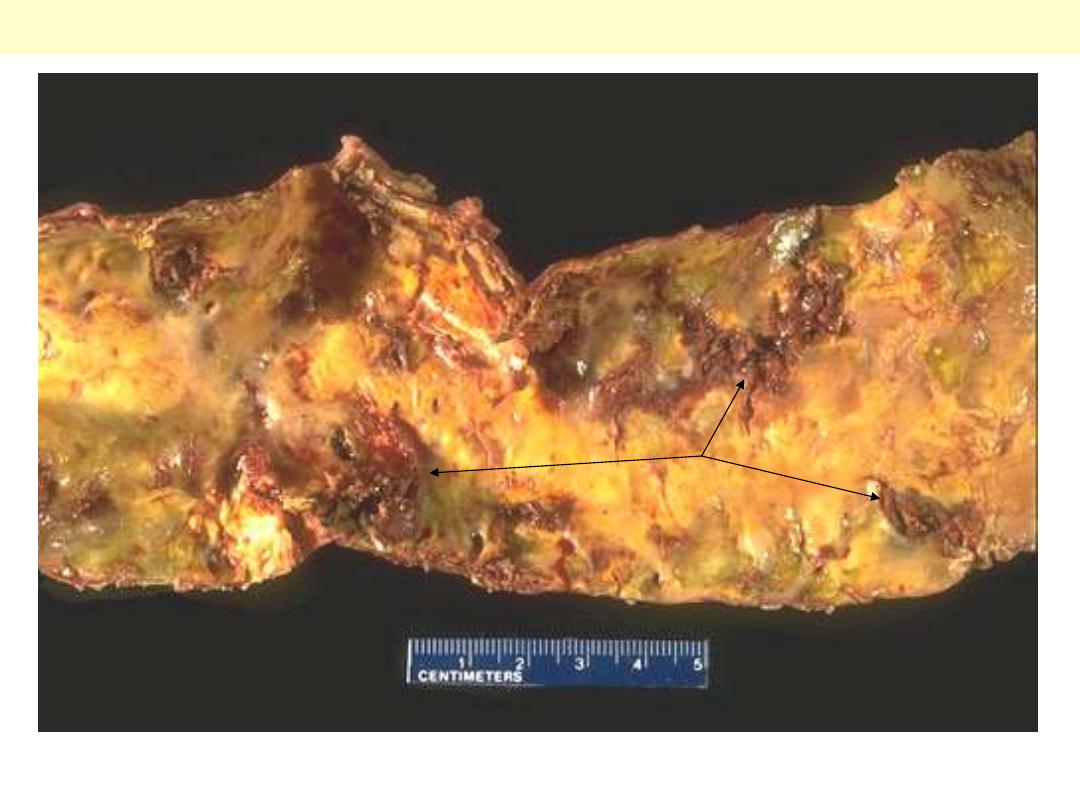
This is severe atherosclerosis of the aorta in which the atheromatous plaques have undergone
ulceration along with formation of overlying mural thrombus (arrows).
Atherosclerosis aorta: ulcerations with superadded thrombosis
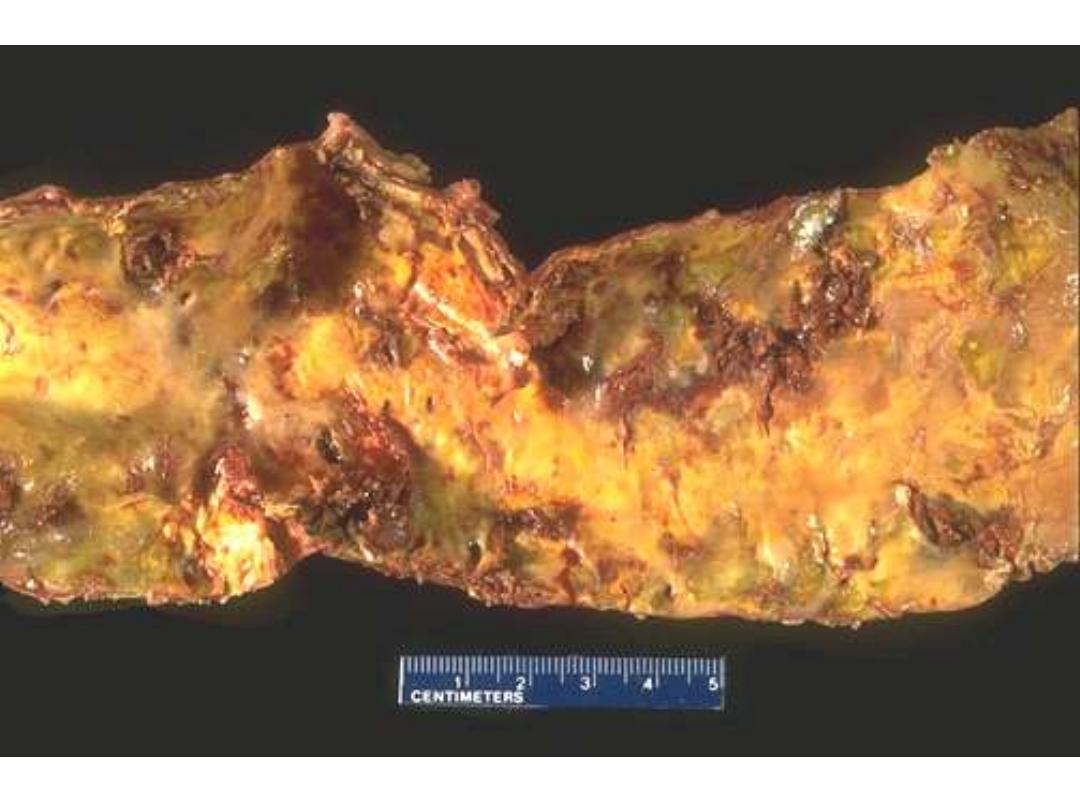
This is coronary atherosclerosis with the complication of hemorrhage into atheromatous plaque
(arrow). Such hemorrhage acutely may narrow the arterial lumen.
Coronary atherosclerosis: plaque hemorrhage
There is a severe degree of narrowing in this coronary artery. It is "complex" in that there is a large
area of calcification on the lower right, which appears bluish on this H&E stain. Complex atheroma
have calcification, thrombosis, or hemorrhage. Such calcification would make coronary angioplasty
difficult.
Stenosing coronary atheroma with calcification
Lt. the anterior descending coronary (opened longitudinally) to show severe stenosing atherosclerosis
with superimposed reddish thrombosis with its propagating portion filling the minimally narrowed
proximal portion. Lt. A closer view showing the artery cross section, which totally occluded by the
reddish thrombus. the dark red thrombus is apparent in the lumen of the coronary.
Coronary atherosclerosis with superimposed thrombosis
There is a pink to red recent thrombosis in this narrowed coronary artery. The open, needle-like spaces
in the atheromatous plaque are cholesterol clefts.
Coronary atherosclerosis with superimposed occlusive thrombosis
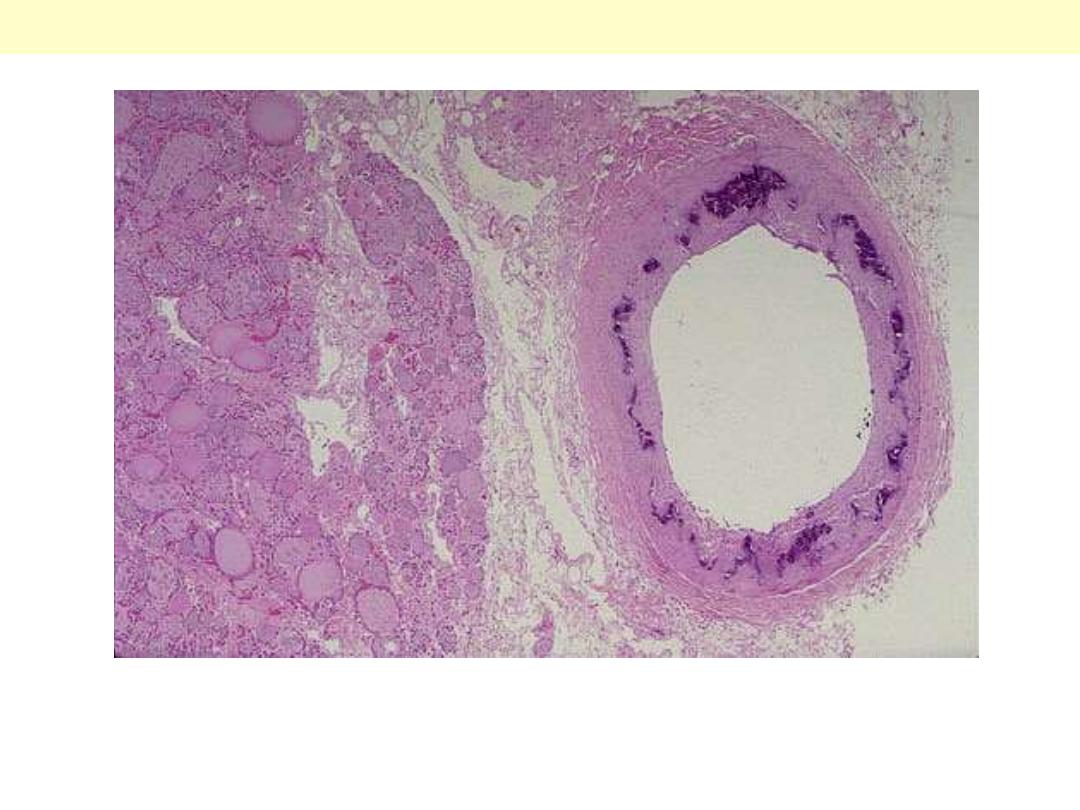
Ring-like calcification (blue color) seen affecting the media of this artery to the right of thyroid tissue
at the left; there is no luminal narrowing. This finding occurs most often in the elderly and is of no
clinical significance, other than that the calcified arteries may be visualized on radiographs, and you
need to know what is represented.
Monckeberg's medial calcific sclerosis thyroid
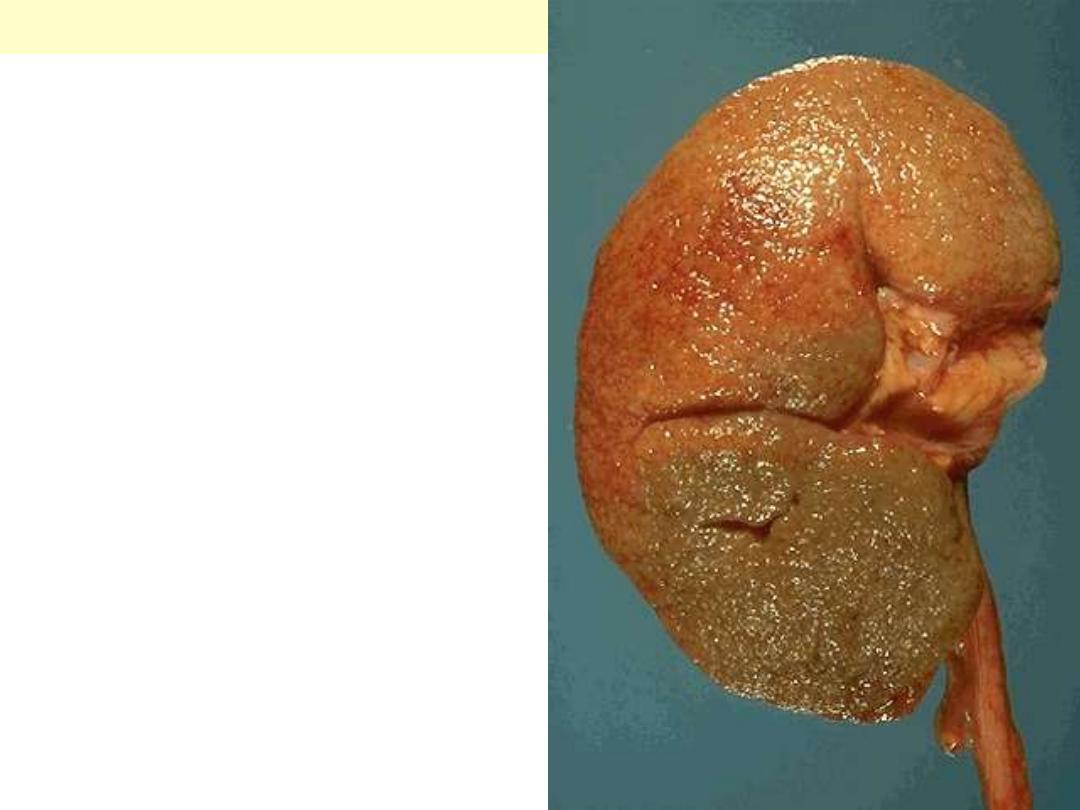
The smaller arterioles in the kidney have become
thickened and narrowed. This leads to patchy
ischemic atrophy with focal loss of parenchyma that
gives the surface of the kidney the characteristic
granular appearance as seen here.
Benign nephrosclerosis
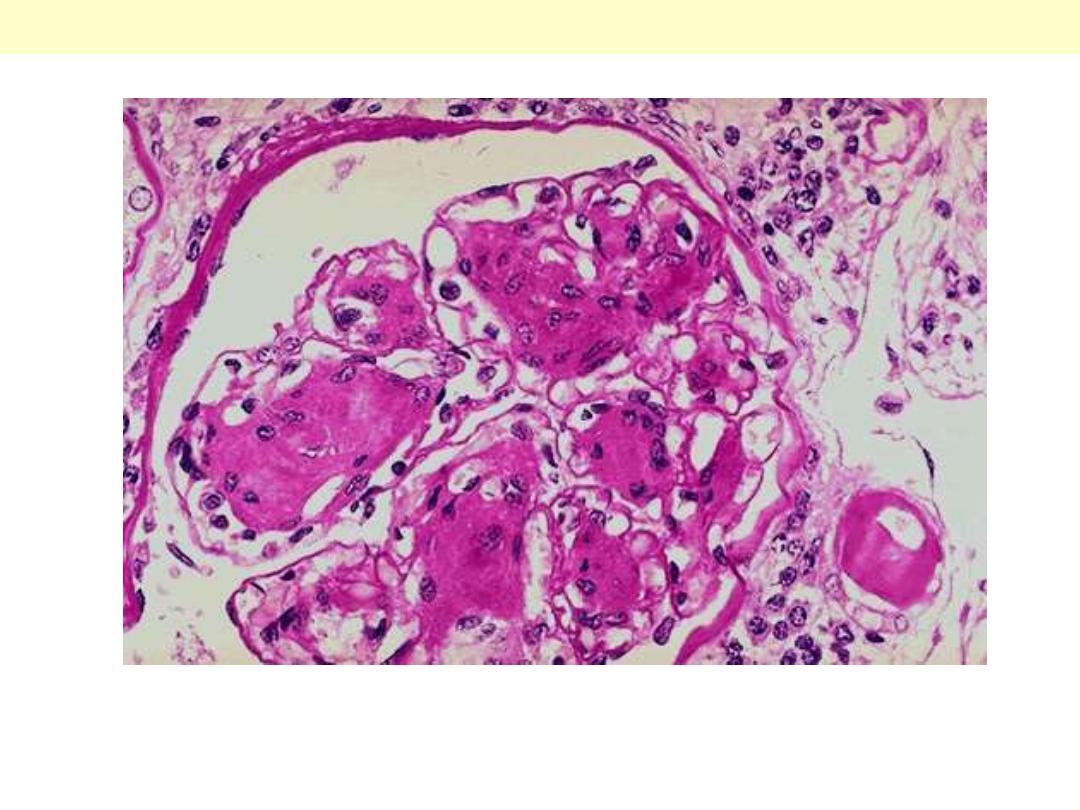
Arteriolosclerosis is typically seen in the kidneys. One form, called hyaline arteriolosclerosis, is
demonstrated by the markedly thickened arteriole to the lower right of this glomerulus with PAS stain.
Hyaline arteriolosclerosis is seen in the elderly, but more advanced lesions are seen in persons with
diabetes mellitus and/or with hypertension.
Hyaline arterioloscelrosis Kidney
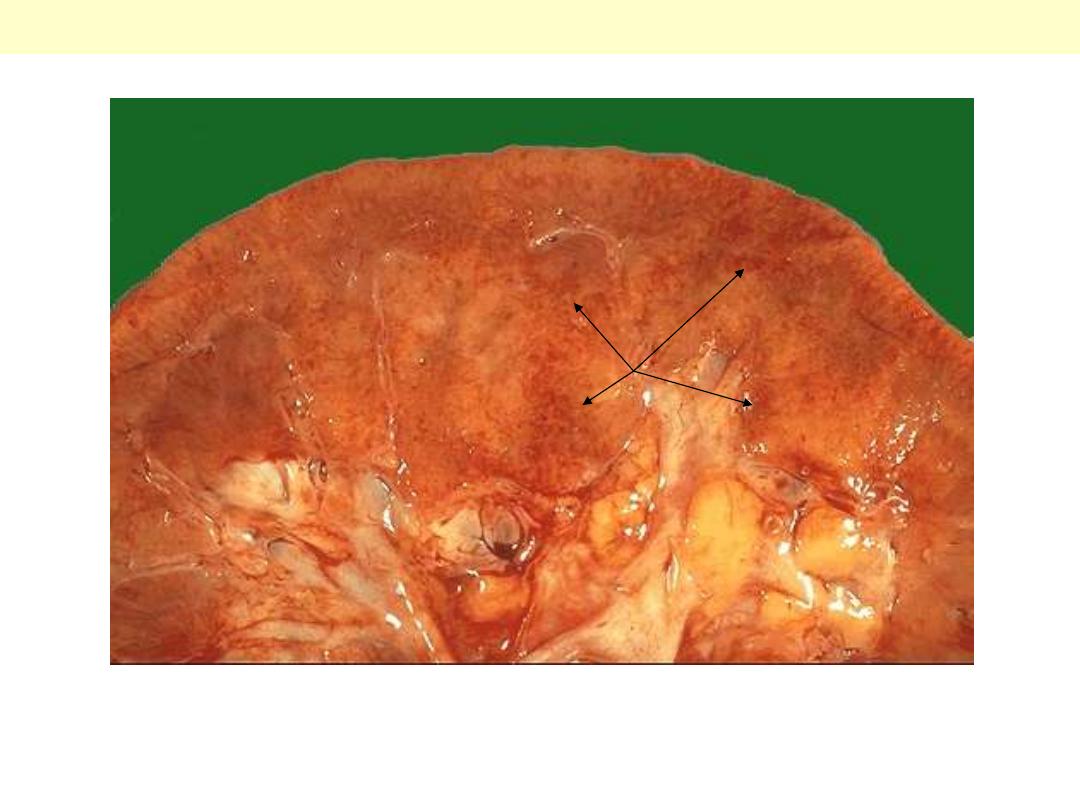
In malignant nephrosclerosis, the kidney demonstrates focal small hemorrhages. This is due to an
accelerated phase of hypertension in which blood pressures are very high (such as 300/150 mm Hg).
Malignant neophrosclerosis
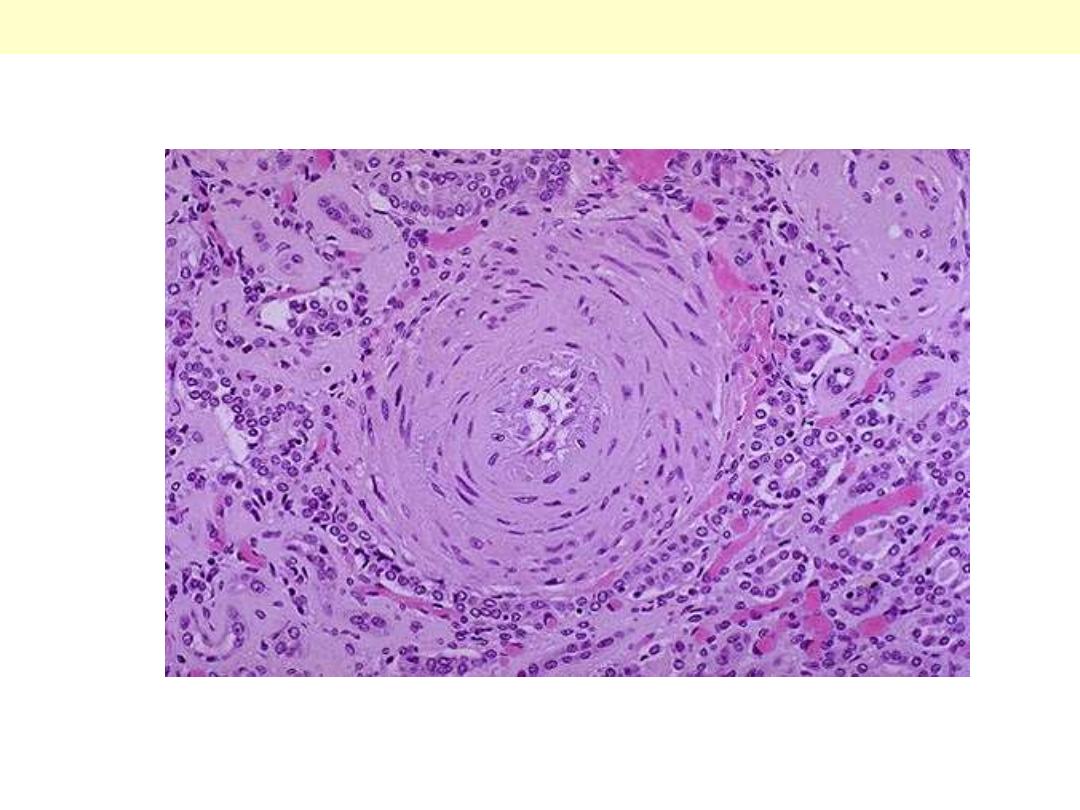
Onion-skin concentric, laminated thickening of the arteriolar wall with progressive narrowing of the
lumen.
Hyperplastic arterilosclerosis
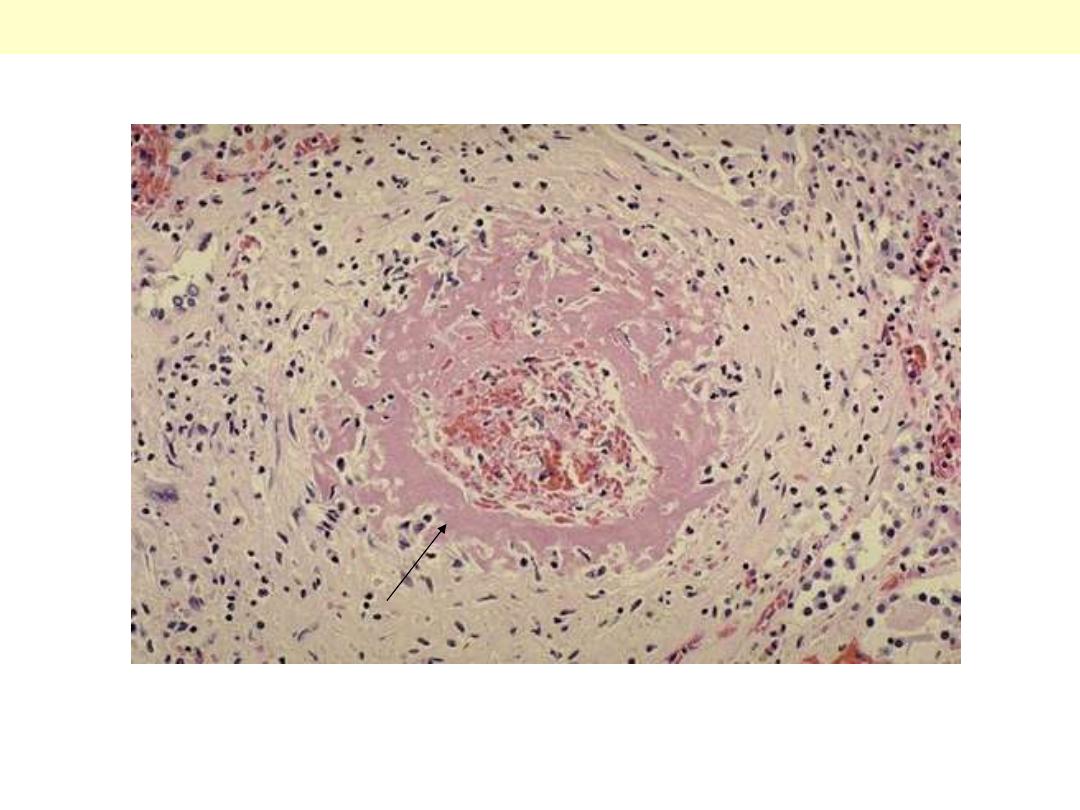
One complication of hyperplastic arteriolosclerosis with malignant hypertension is fibrinoid necrosis,
as seen here in a renal arteriole. Rupture of the affected arterioles lead to grossly visible minute
hemorrhages.
Hyperplastic arterilosclerosis with fibrinoid necrosis

AV - Malformations
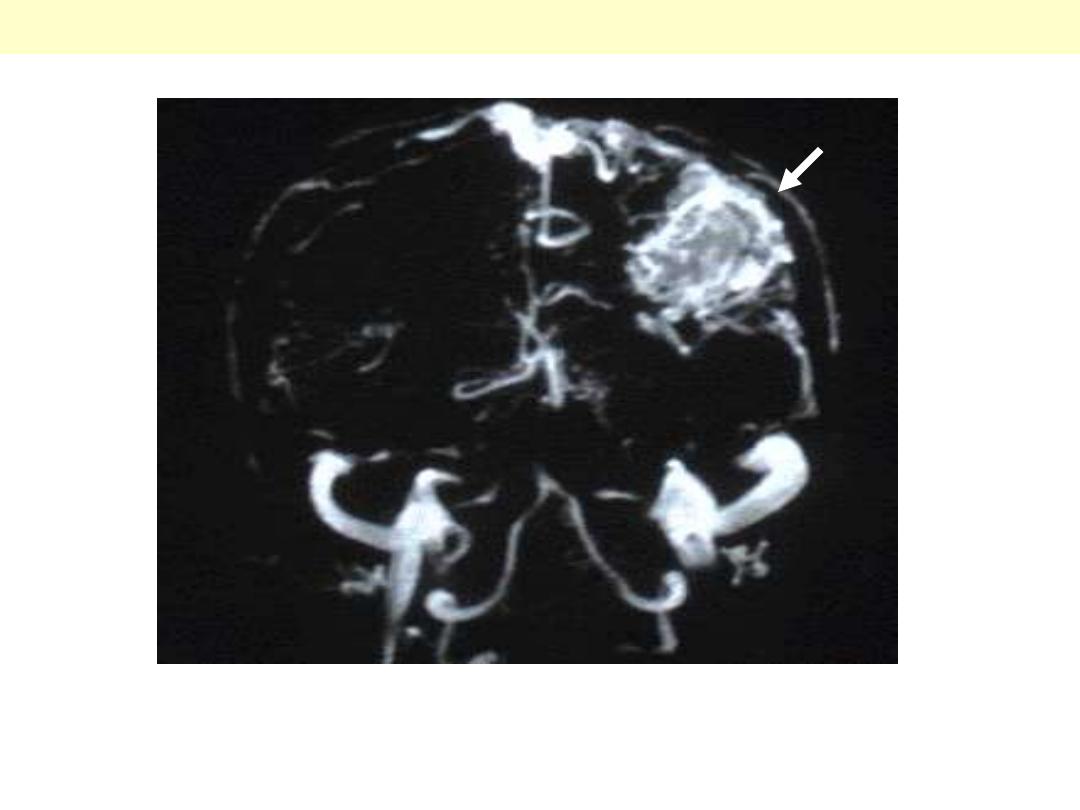
There is a large abnormal mass of vessels in the parietal lobe (arrow). Such abnormal vessels are prone
to bleeding.
AVM (MRI of brain)
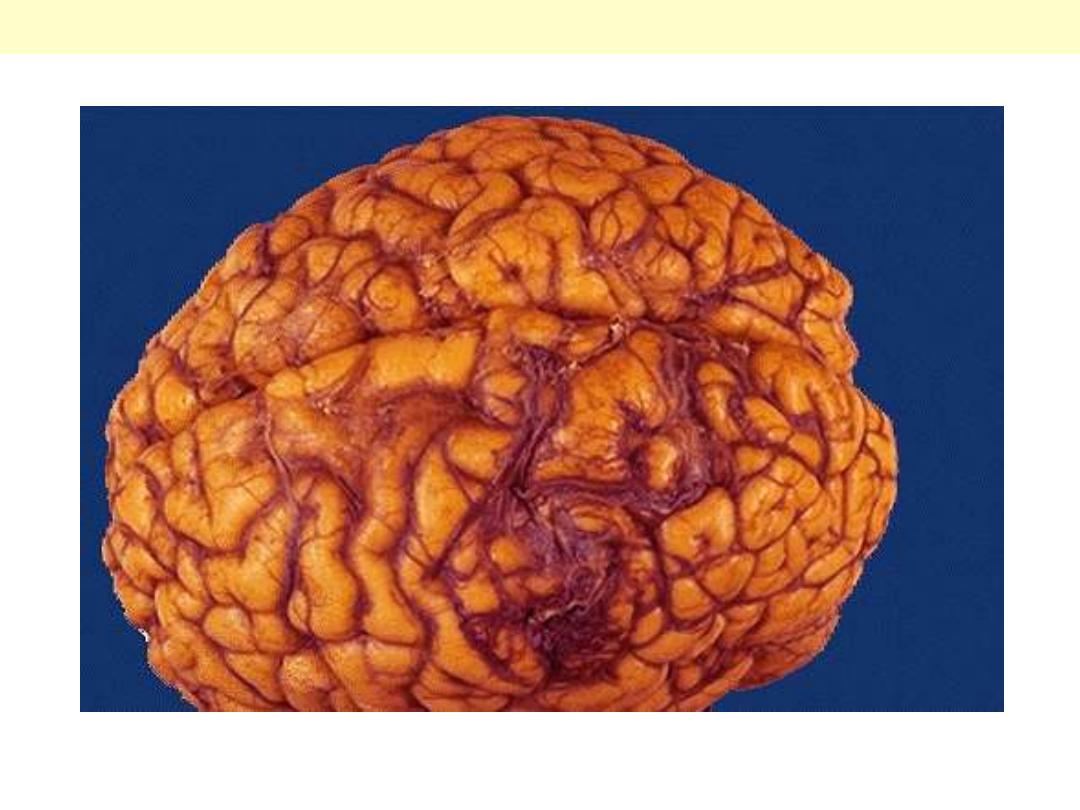
There is a mass of irregular, tortuous vessels over the left posterior parietal region. This is one cause for
hemorrhage, particularly in persons aged 10 to 30 years.
AVM Brain
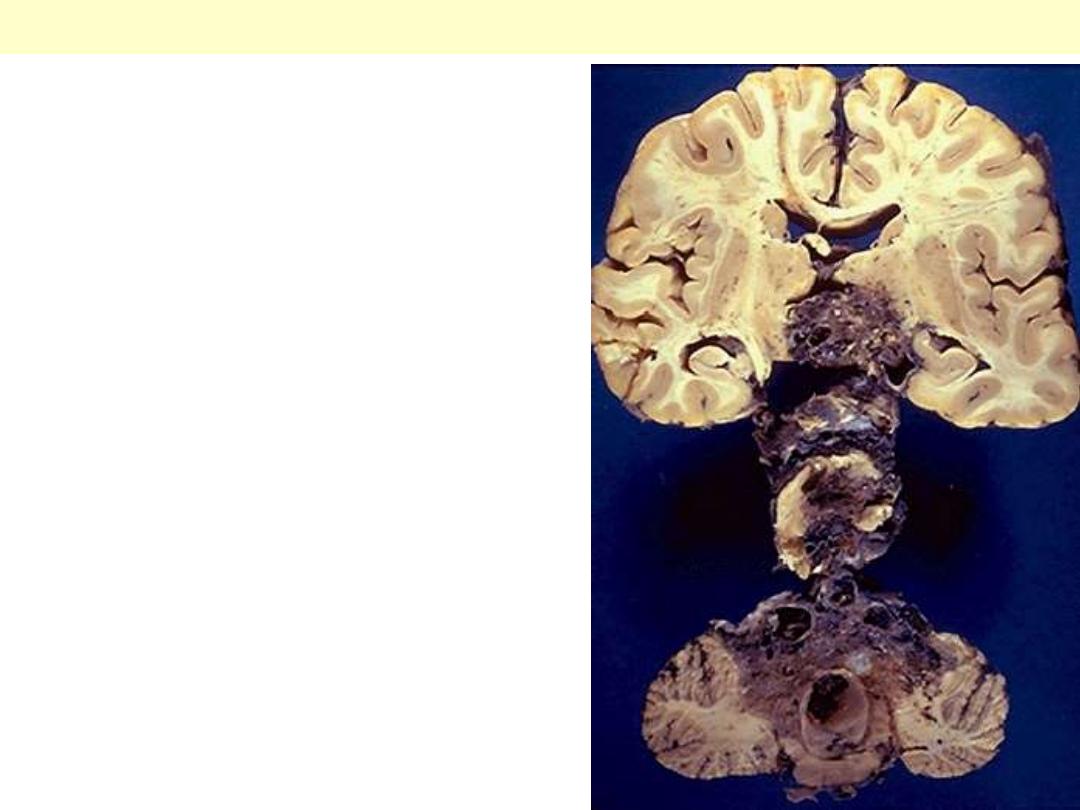
Serial sections reveal a large vascular malformation
involving much of the brainstem. Such lesions,
however, are most common in the cerebral
hemispheres.
AVM Brainstem

Varicose veins
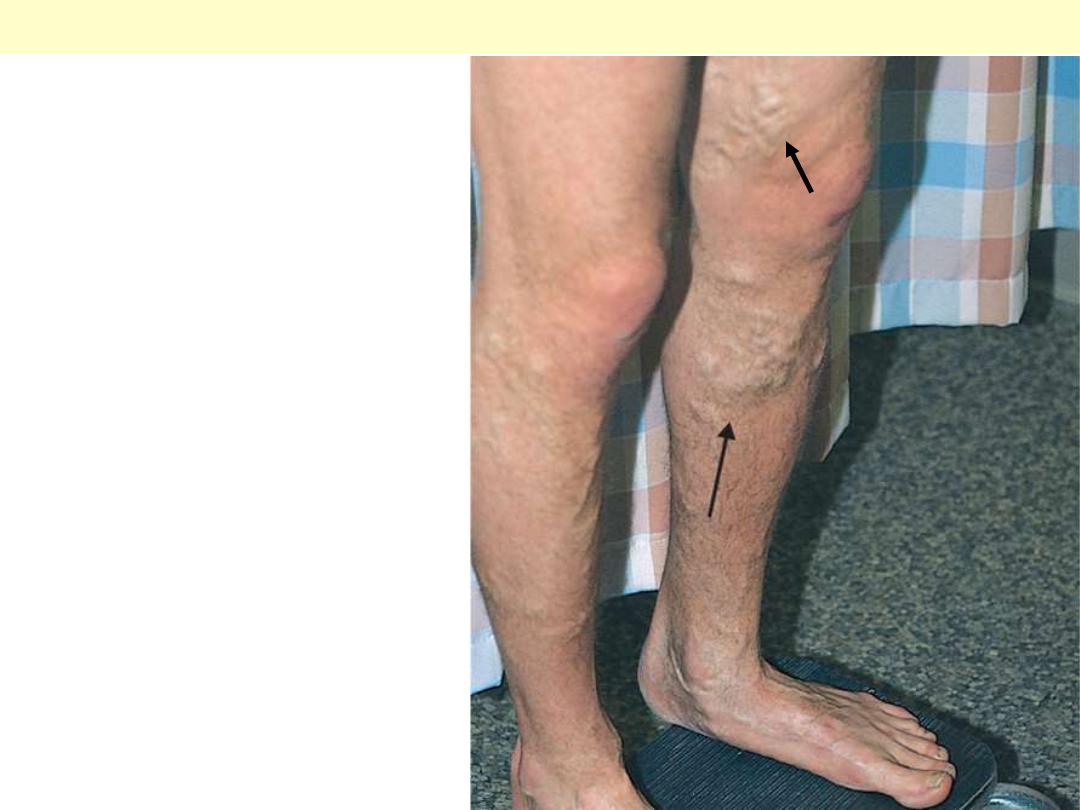
Note the prominently dilated & tortuous
veins below & above the knee
Varicose veins of the leg

Vascular tumors
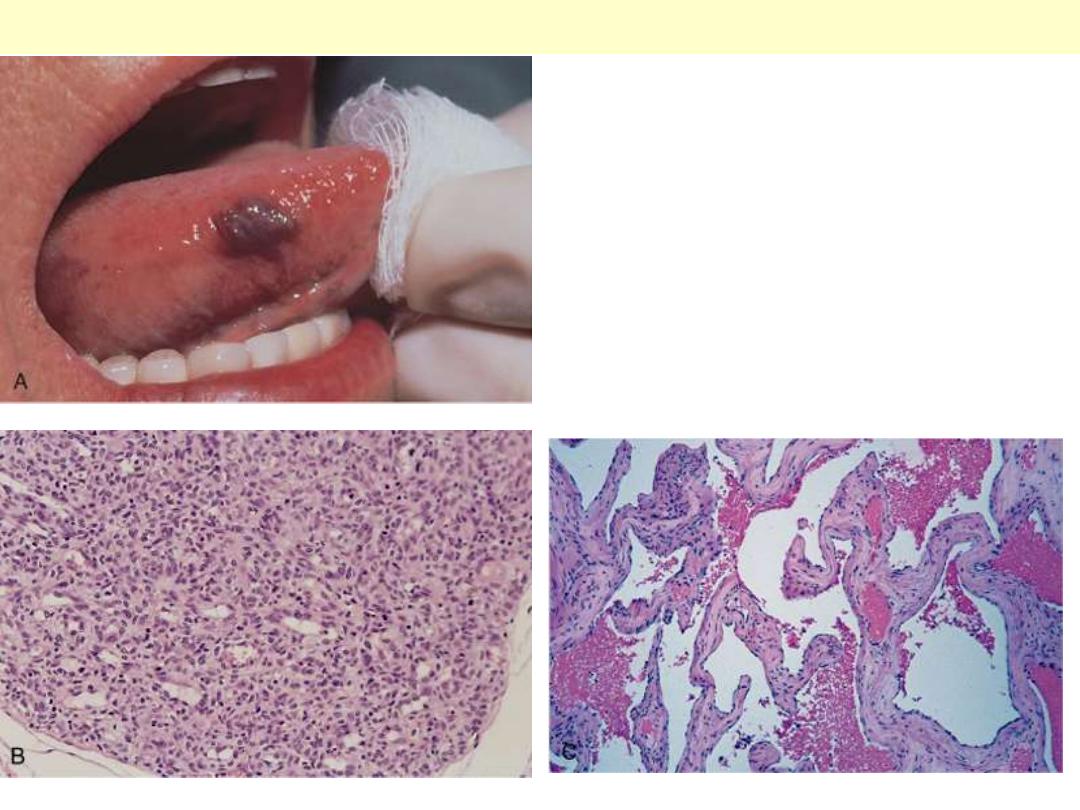
A, Hemangioma of the tongue. B, Histology of
juvenile capillary hemangioma. C, Histology of
cavernous hemangioma
Hemangioma
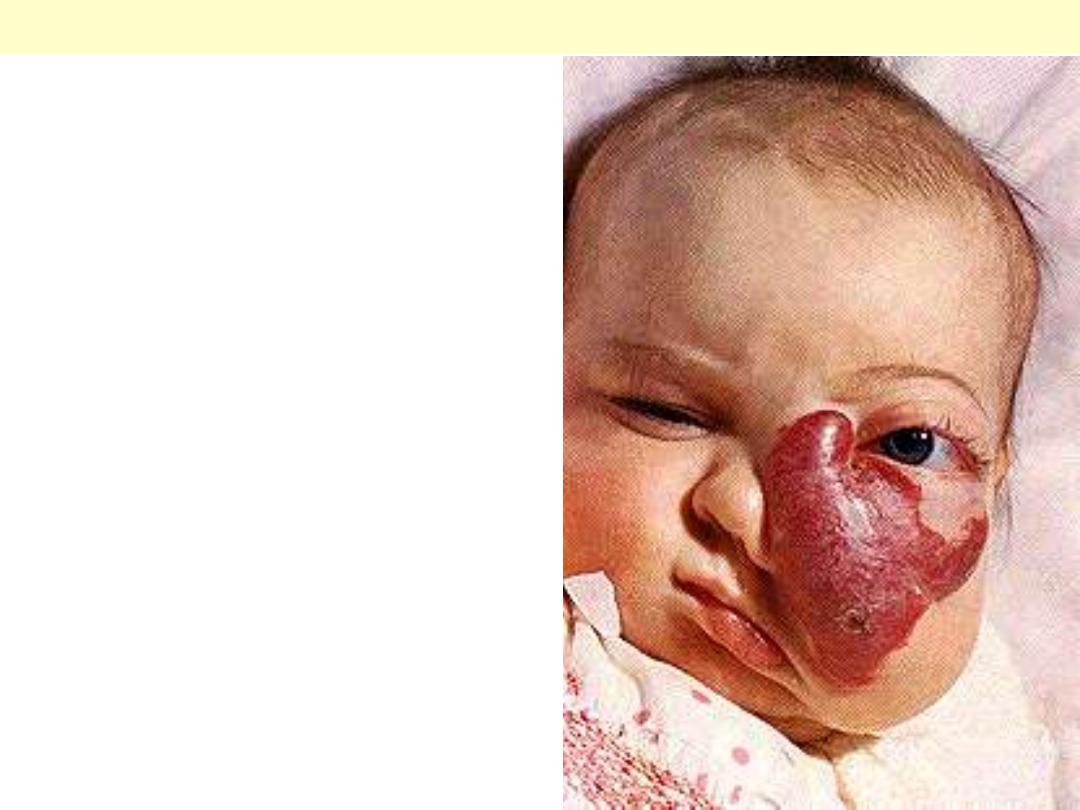
Female infant shows a massive lesion distorting the
nose and cheek.
Disfiguring hemangioma of the face
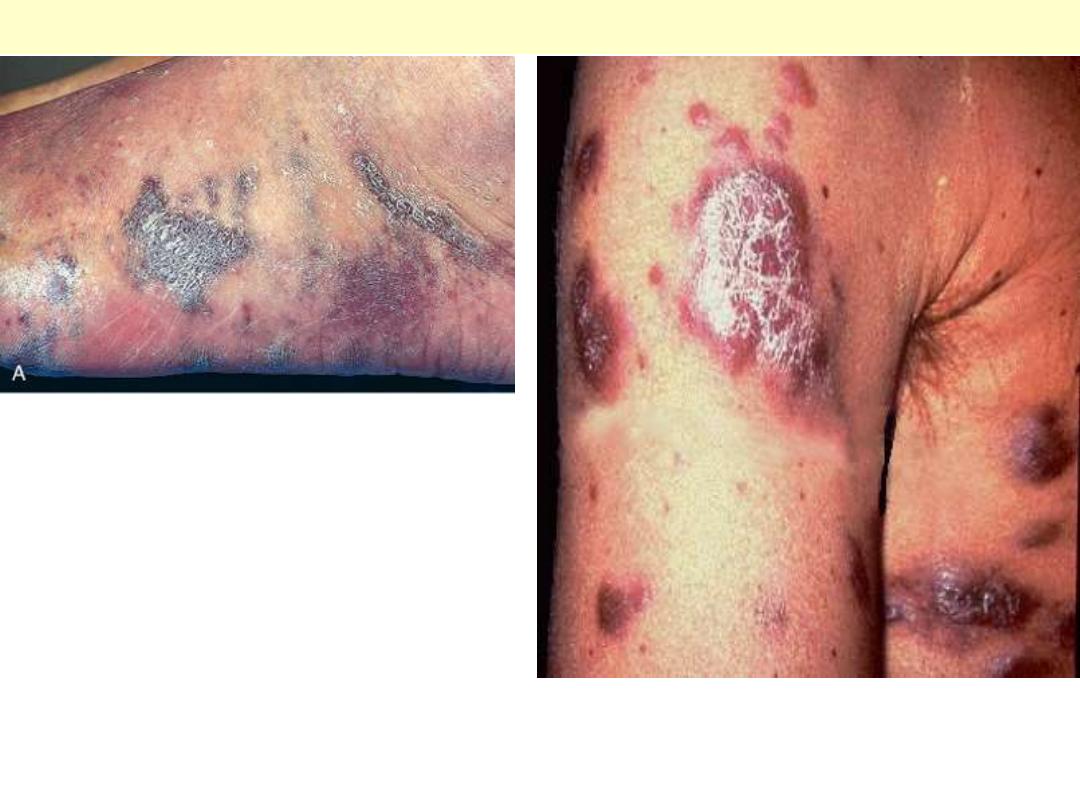
Rt. Gross photograph, illustrating coalescent red-purple patches and plaques of the skin of foot.
Lt. in the nodular stage, the lesions become nodular, larger, and more numerous.
Kaposi sarcoma

Vasculitits
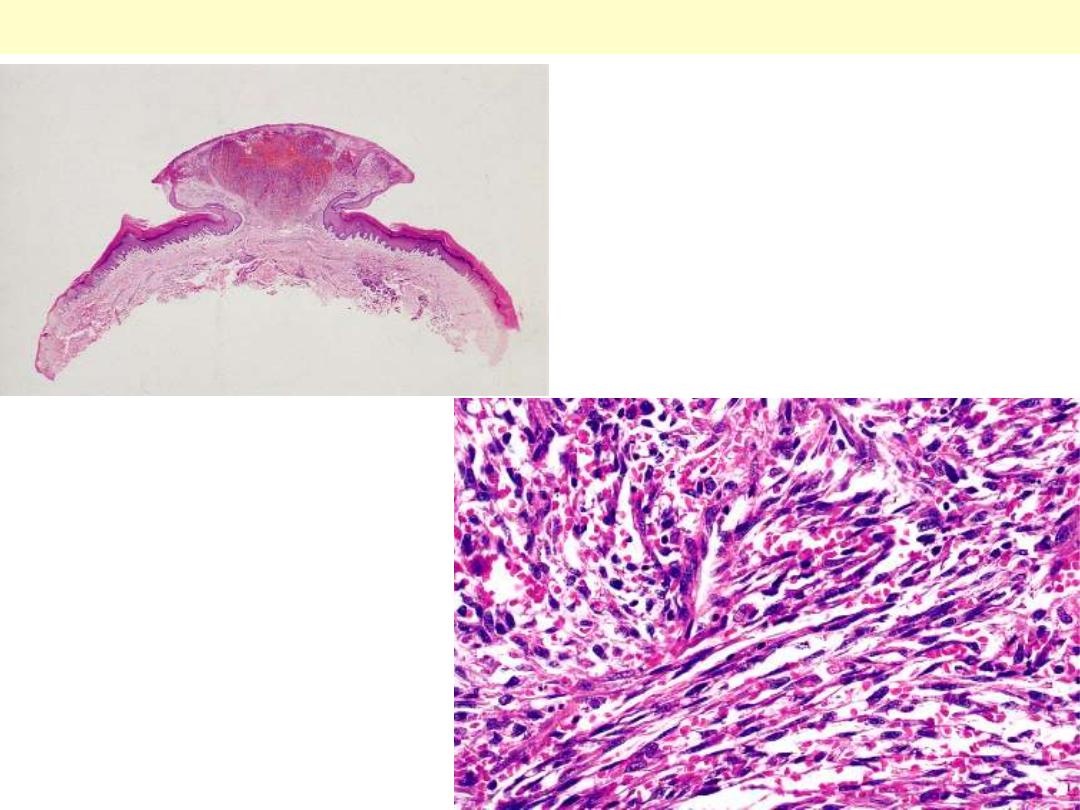
Low-power view of a lesion of Kaposi's sarcoma
having a prominent nodular shape.
Microscopic appearance of Kaposi’s
sarcoma. Elongated spindle cells
showing minimal atypia are separated
by slits containing red blood cells.
Kaposi sarcoma
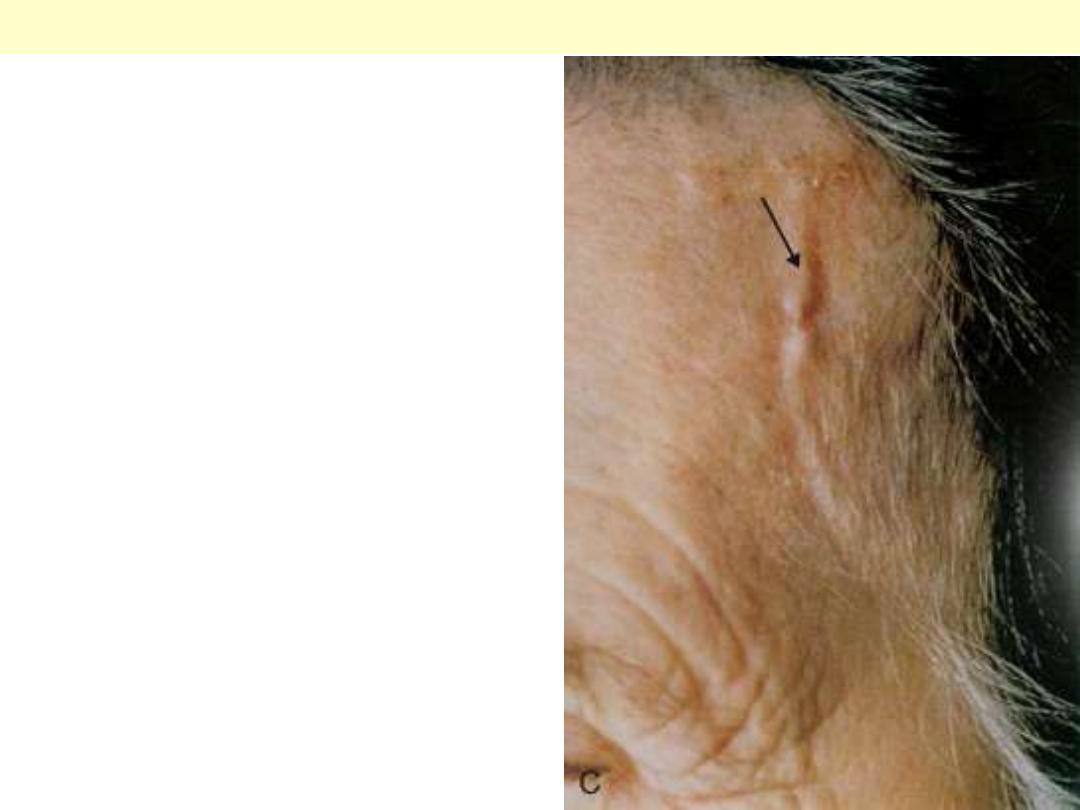
Artery of a patient with temporal arteritis showing
a thickened, nodular, & prominent segment of a
vessel on the surface of head (arrow).
Temporal arteritis
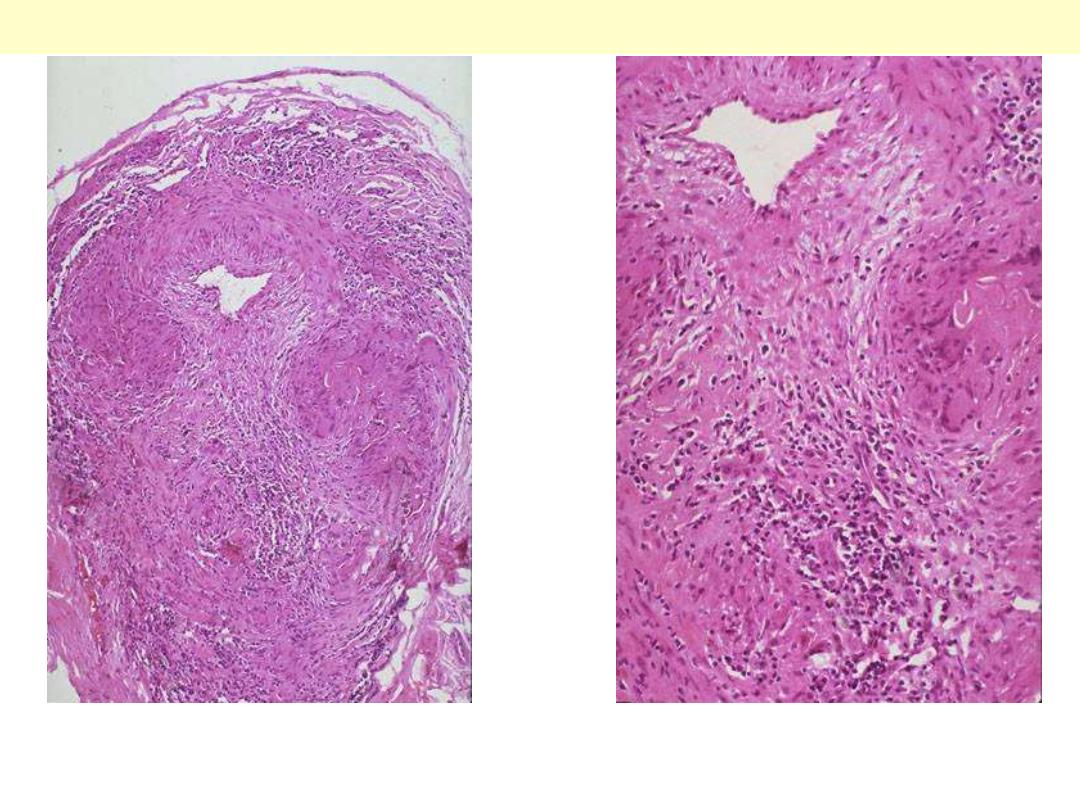
Giant cell (temporal) arteritis
Temporal arteritis is one manifestation of giant cell arteritis, which can affect mainly branches of
external carotid artery. There is thickening of the wall by granulomatous inflammation (with several
multinucleated giant cells) of the media. The lumen is markedly narrowed.
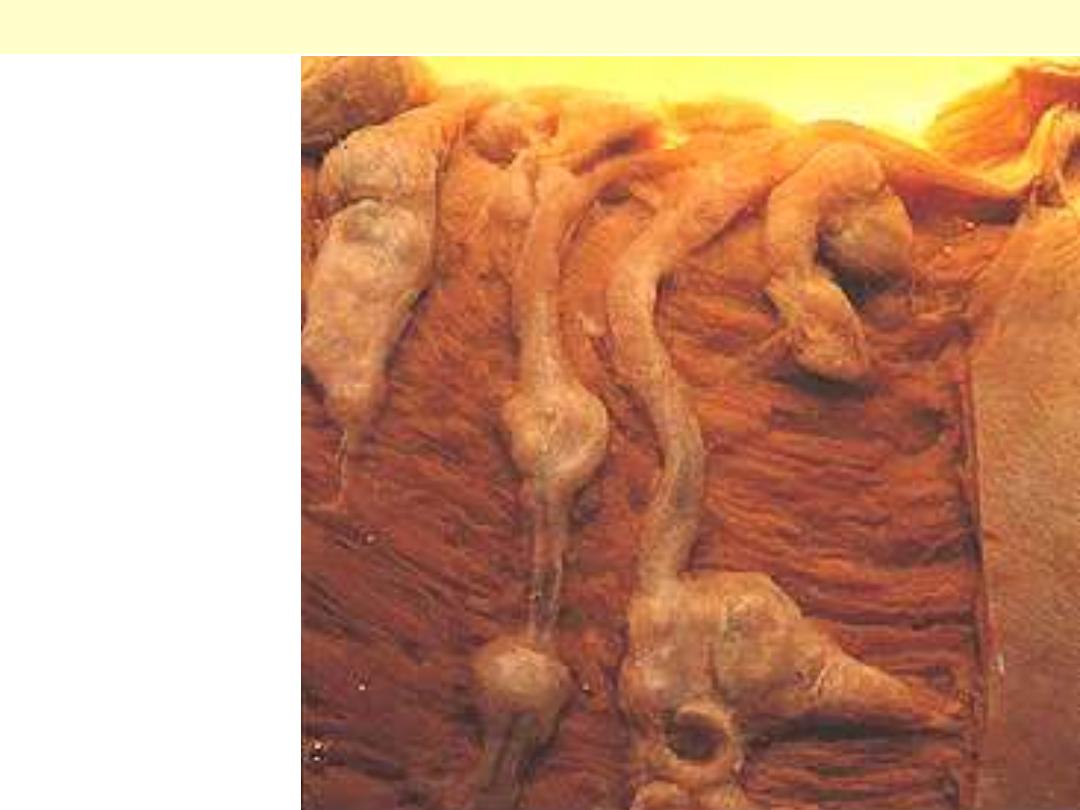
PAN coronaries prominent
aneurysmal dilatations
PAN of the coronary artery branches
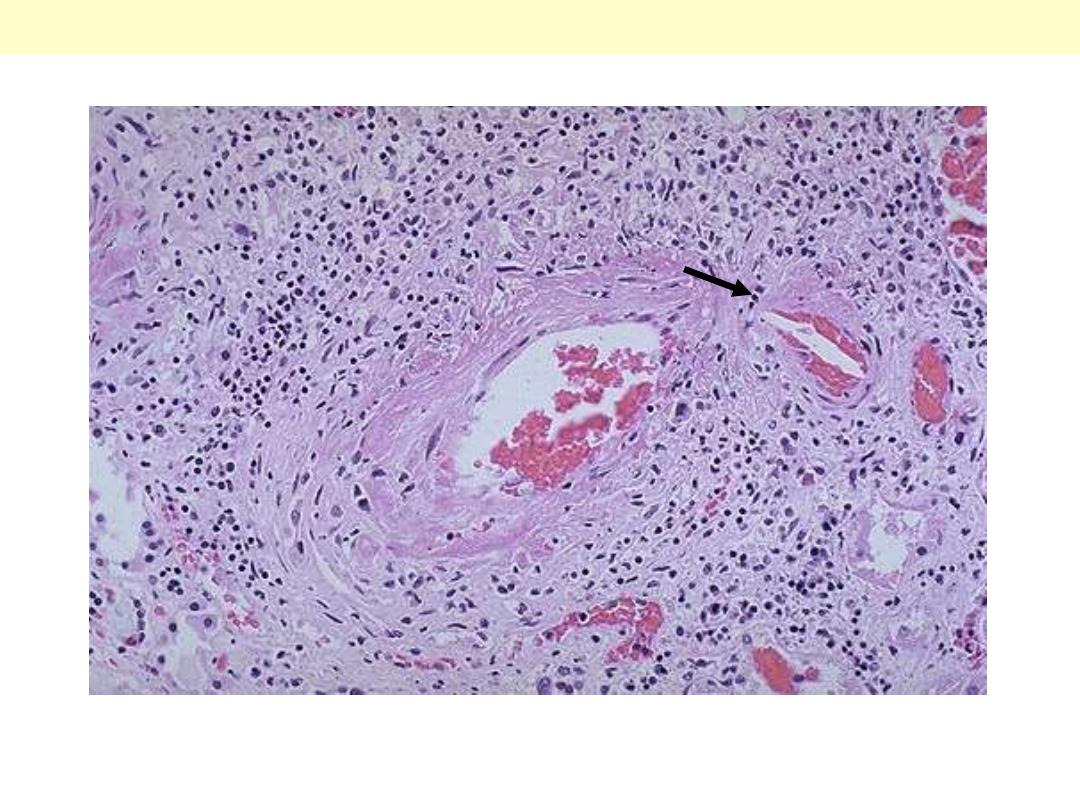
PAN of the a renal arterial branch
There are inflammatory cell infiltration scattered in and around the vessel. The wall shows fibrinoid
necrosis with aneurysmal dilatation (arrow). The ANCA serology is usually positive.
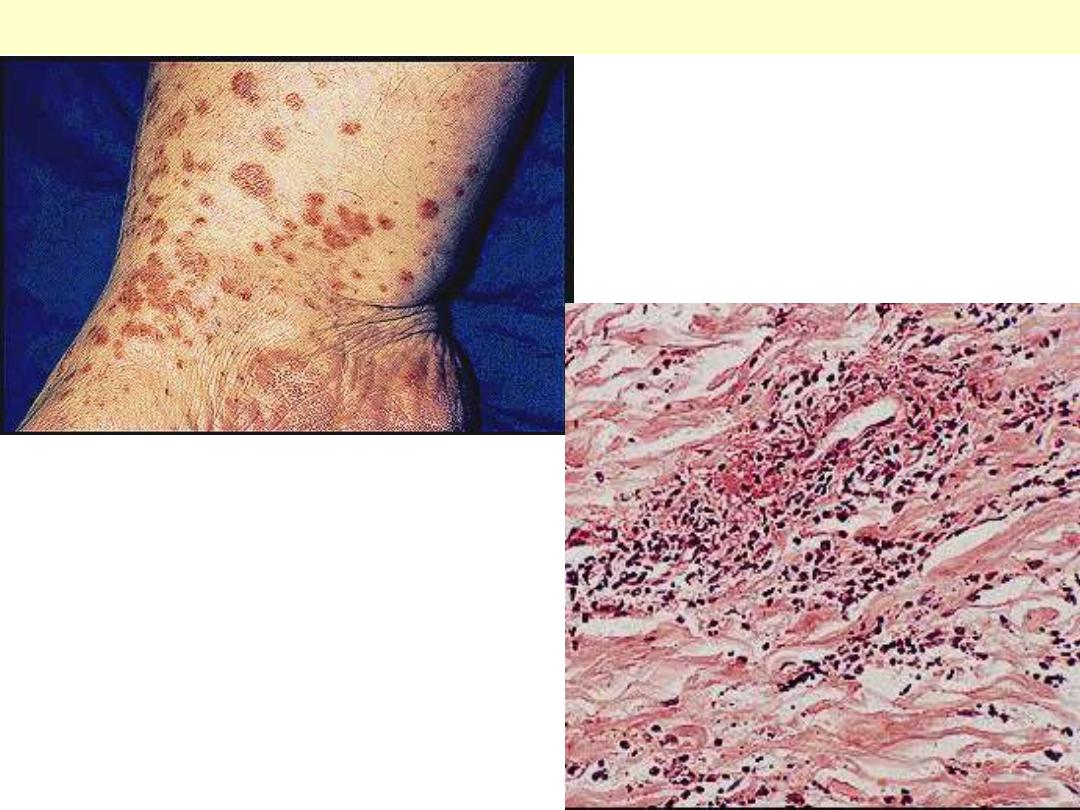
Leucocytoclastic vasculitis (allergic vasculitis):
typical erythematous maculopapular lesins are
present on the medial aspect of the ankle.
Leucocytoclastic vasculitis
This venule shows inflammation, fibrinoid necrosis
and there is marked leucocytoclasis
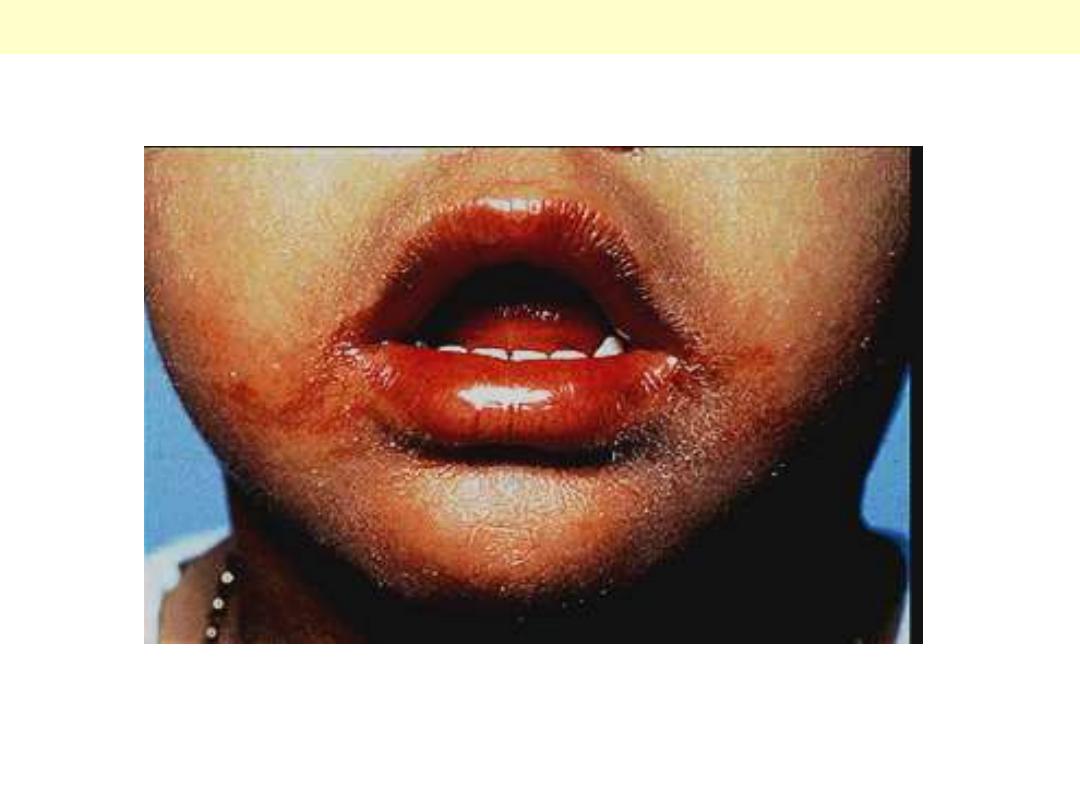
The lips are erythematous and swollen; angular cheilitis is evident.
Kawasaki disease (Mucocutaneous lymph node syndrome)
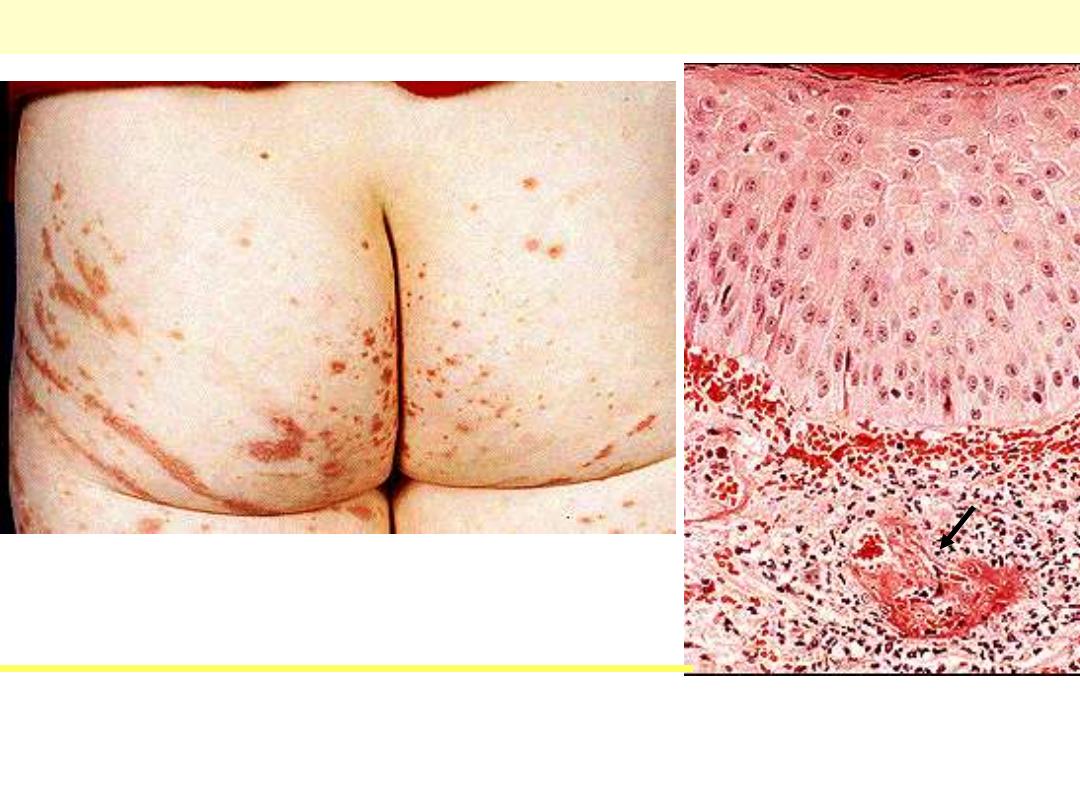
Henoch-Schonlein purpura
Lt. Palpable purpura in the classical distribution on the buttocks and
thighs. Rt. small venule showing fibrinoid necrosis with related
inflammation
Lt. Palpable purpura in the classical
distribution on the buttocks and thighs.

Heart

Cardiomyopathies
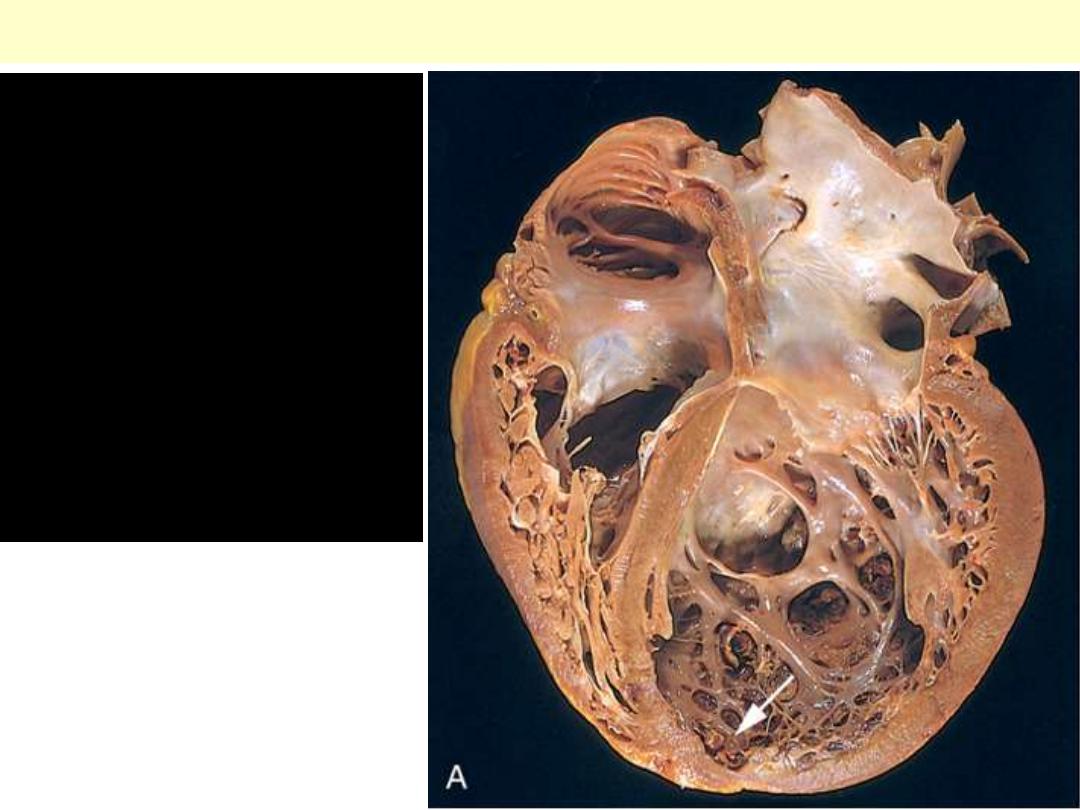
Dilated (Congestive) cardiomyopathy
Dilated
cardiomyopathy
(DCM). Four-
chamber dilatation
and hypertrophy are
evident. There is a
small mural thrombus
(arrow) at the apex of
the left ventricle.
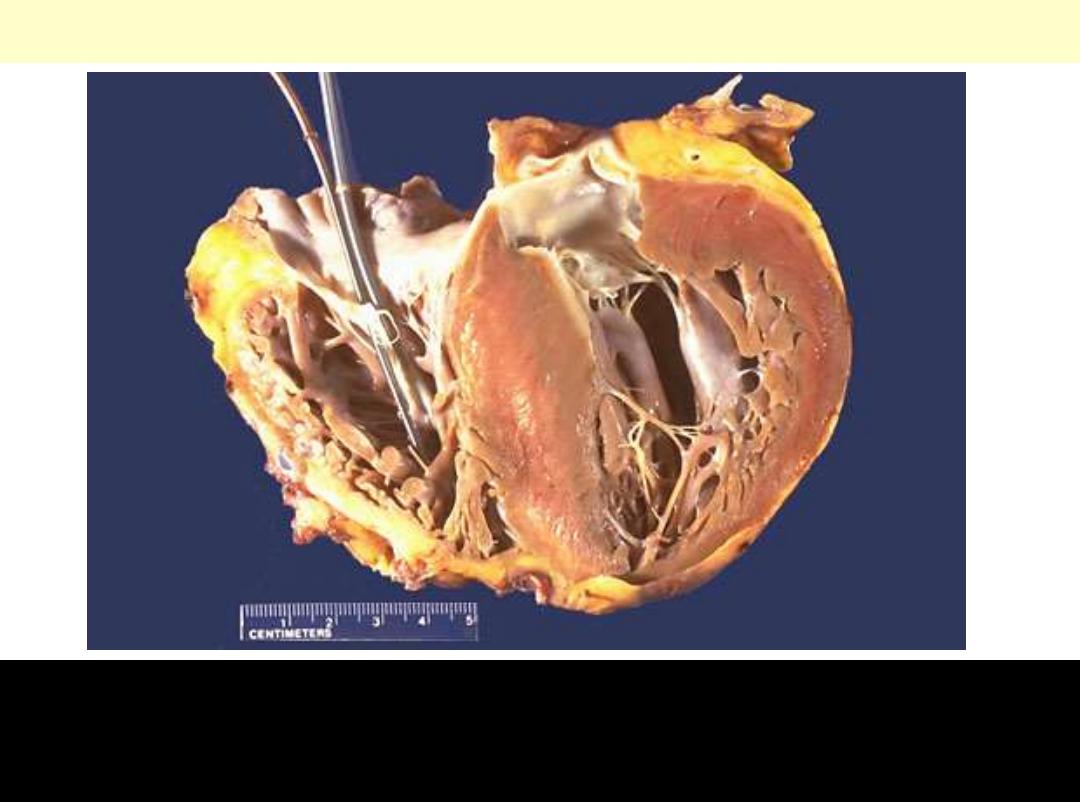
Hypertrophic cardiomyopathy
There is marked left ventricular hypertrophy, with asymmetric
bulging of a very large interventricular septum into the left
ventricular chamber.
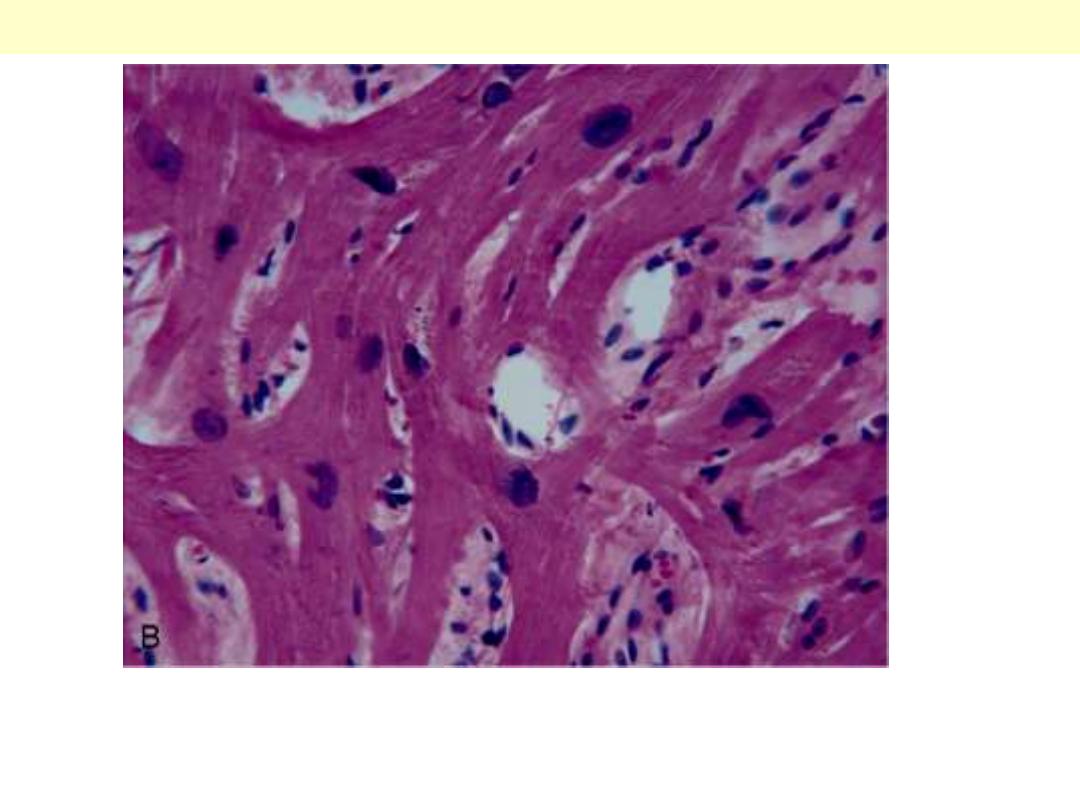
Microscopic appearance demonstrating disarray, extreme
hypertrophy, and characteristic branching of myocytes.
Hypertrophic CMP

Congenital HD
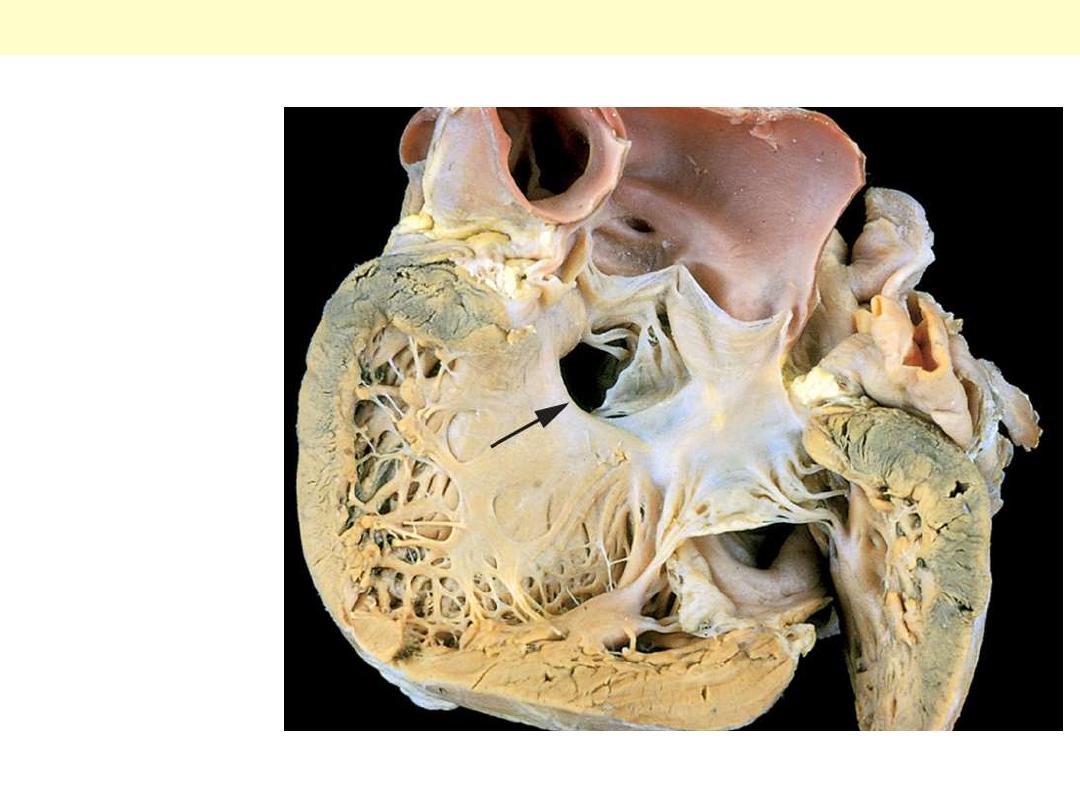
Dissection
exposing the Lt.
ventricle. There
is incomplete
closure of the
ventricular
septum (arrow)
allows free
communication
and L to R
shunt
Ventricular Septal Defect (VSD)
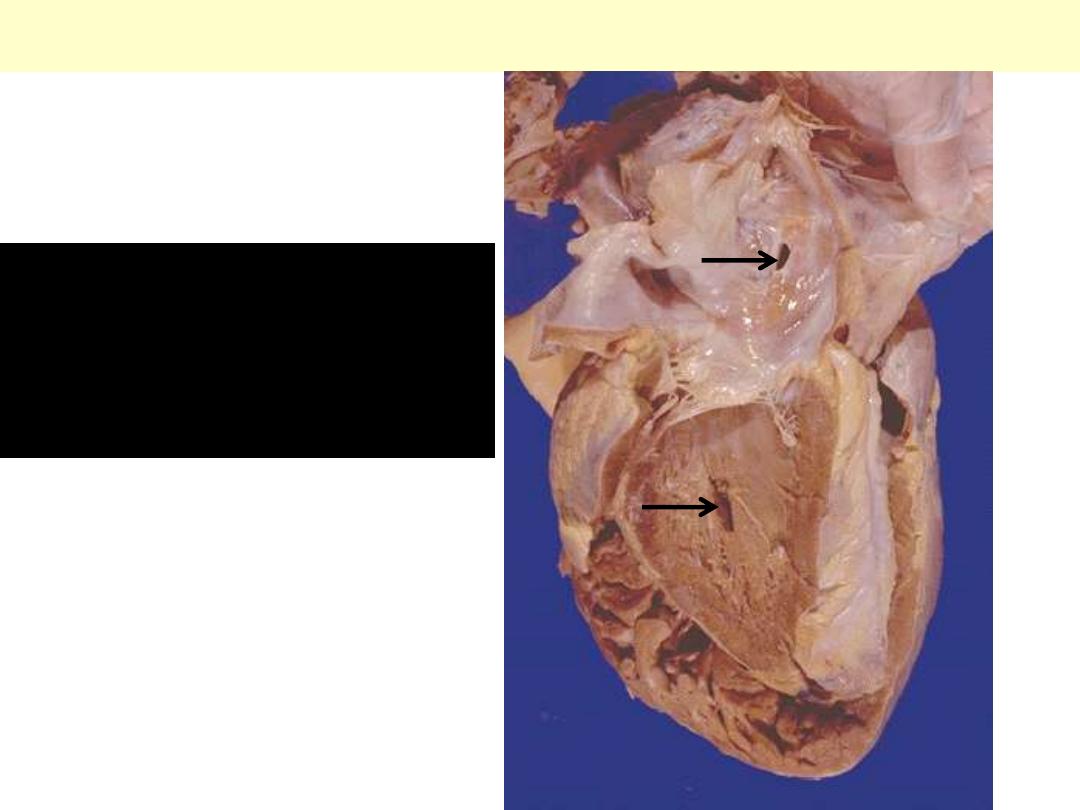
Heart ASD & VSD
A heart with both an
ASD and a VSD. The
heart is opened on the left
side.
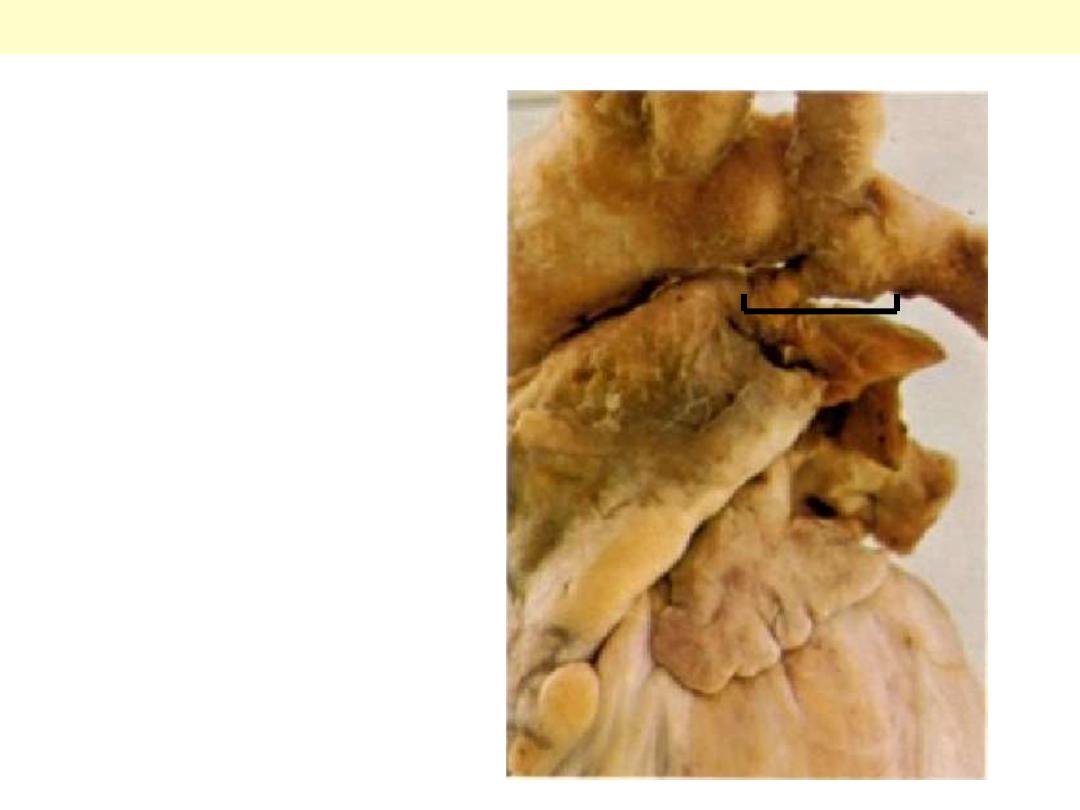
The aortic arch at the top
and pulmonary trunk
below. A short thick
ductus joins the aortic
arch with the Lt.
pulmonary artery. Any
ductus arteriosus that is
patent after the age of
three months is
abnormal.
Patent ductus arteriosus
Pulmonary
trunk
Aortic arch
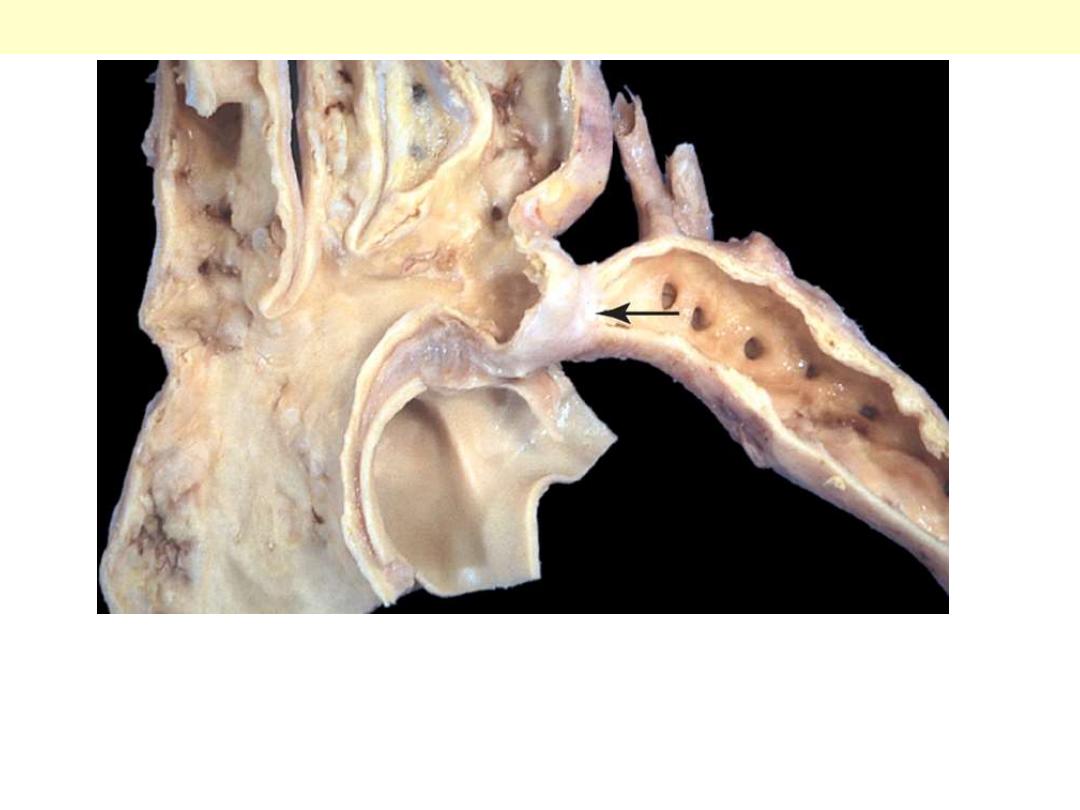
The area of coarctation is visible here as a segmental narrowing of the aorta
(arrow). Such lesions typically present later in life than do preductal coarctations.
Note the dilated ascending aorta and major branch vessels proximal to the
coarctation. A large amount of blood reaches the lower extremities via dilated,
tortuous collateral channels.
Coarctation of the aorta: postductal type
Coarctation of aorta postductal form
This portion of aorta was resected from
a patient with a coarctation. The aorta
narrows postductally here to about a 3
mm opening.
The aorta is opened longitudinally here to
reveal a coarctation.

Endocarditis – Infective + Non-
infective
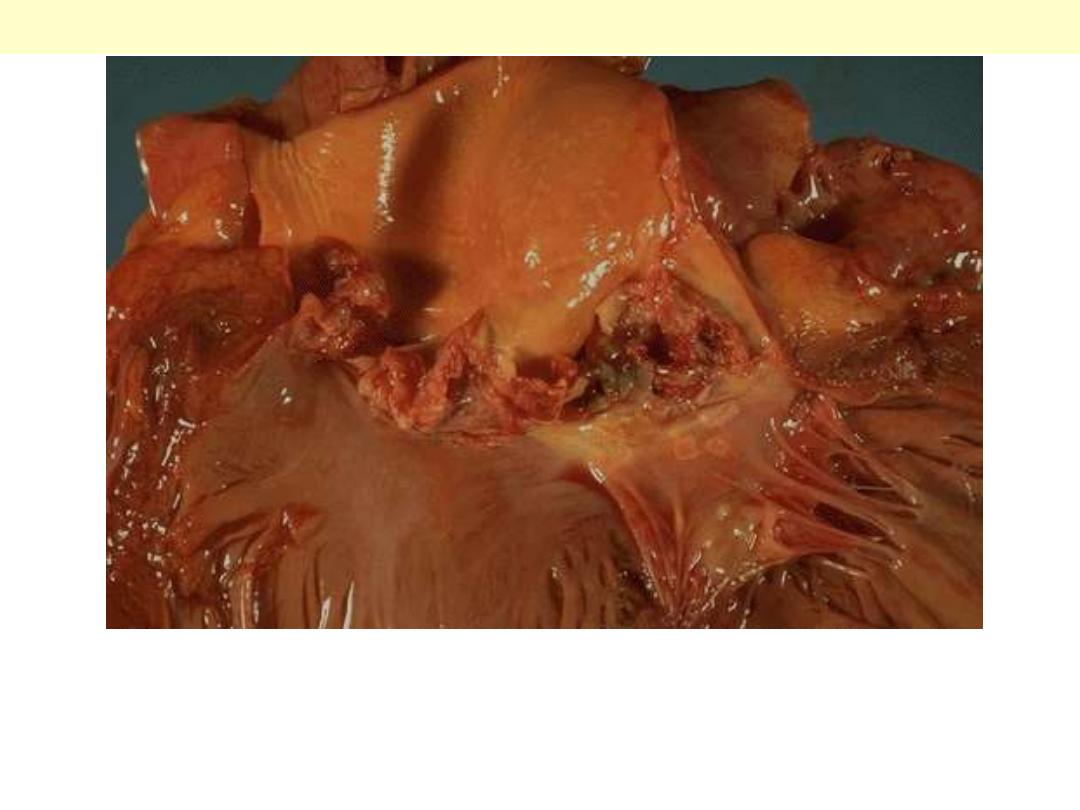
The more virulent bacteria cause the acute form that can lead to
serious destruction, as shown here in the aortic valve. Irregular
reddish tan vegetations overlie valve cusps that are being destroyed.
Acute bacterial endocarditis
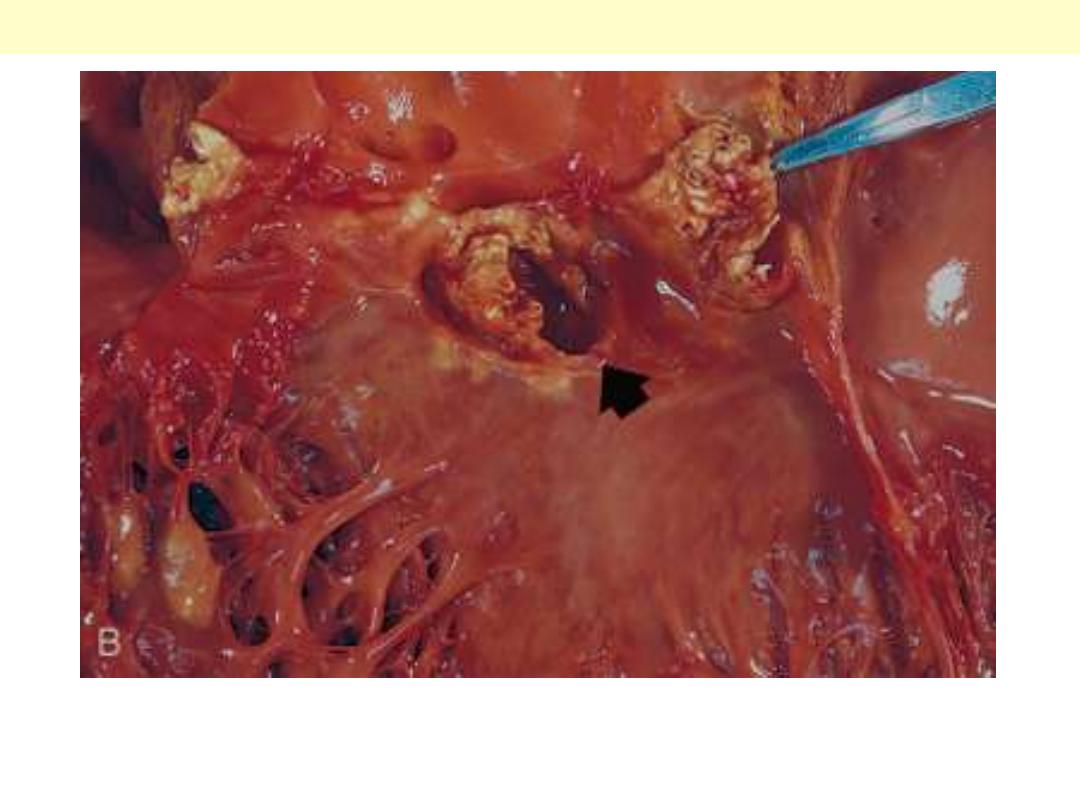
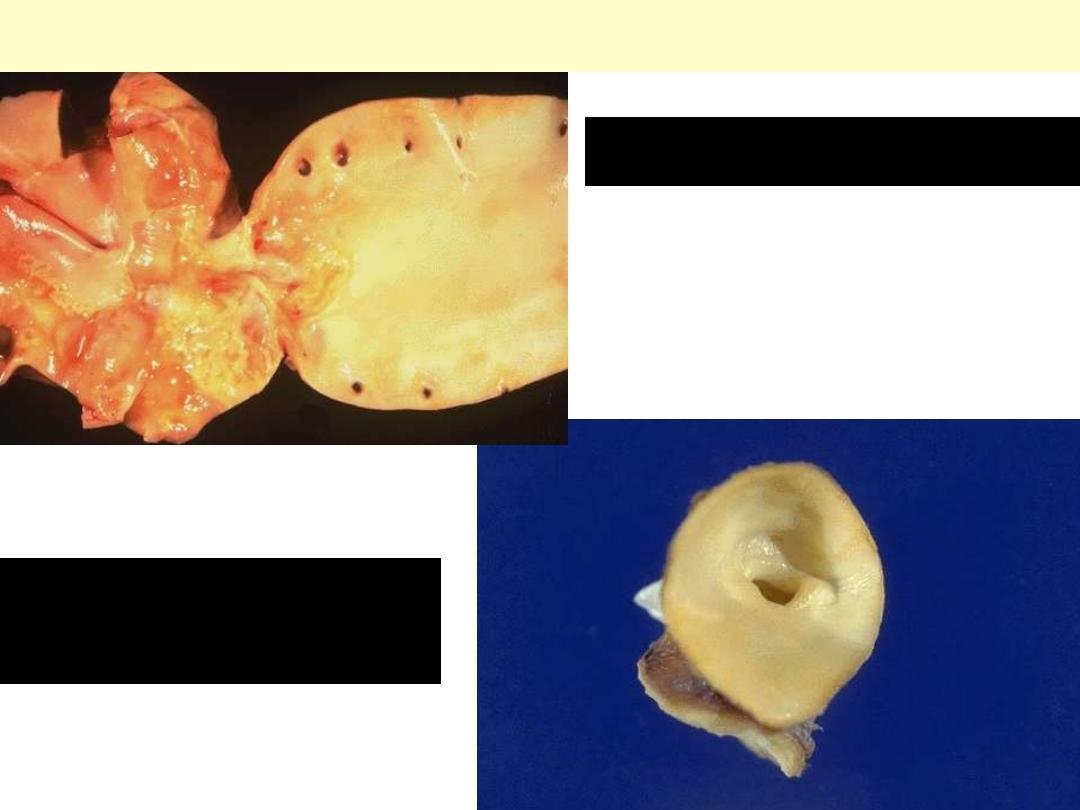
The disease is caused by Staphylococcus aureus with extensive
cuspal destruction and ring abscess (arrow).
Acute endocarditis of congenitally bicuspid aortic valve
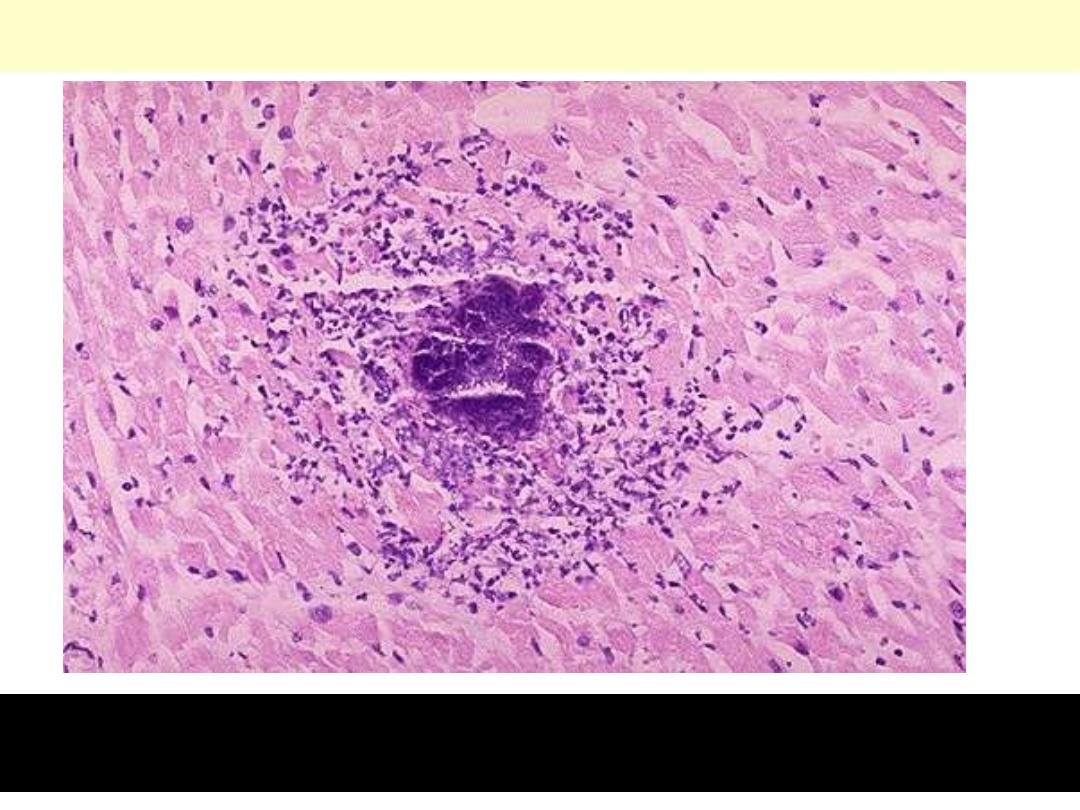
Bacterial endocarditis: myocardial microabscess
The center consists of blue bacterial colonies and is surrounded by
acute inflammatory cells.
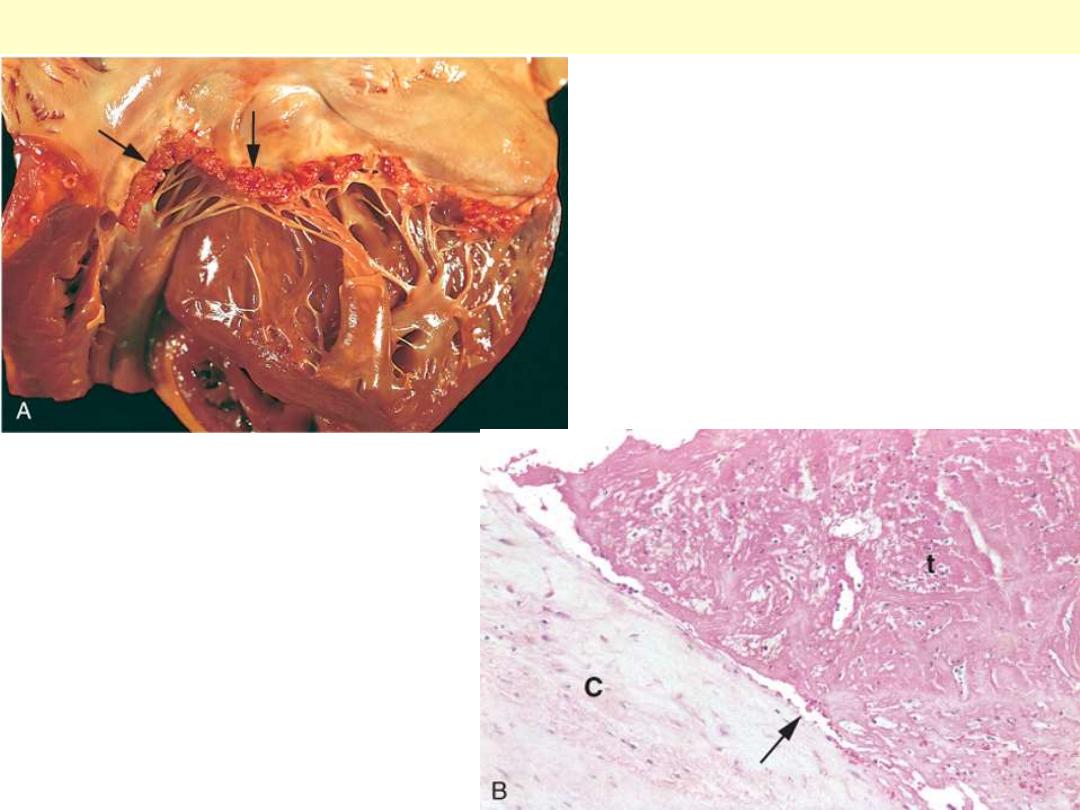
A, Nearly complete row of
thrombotic vegetations along the line
of closure of the mitral valve leaflets
(arrows). B, Photomicrograph of
nonbacterial thrombotic
endocarditis, showing bland
thrombus, with virtually no
inflammation in the valve cusp (c) or
the thrombotic deposit (t). The
thrombus is only loosely attached to
the cusp (arrow).
Nonbacterial thrombotic endocarditis
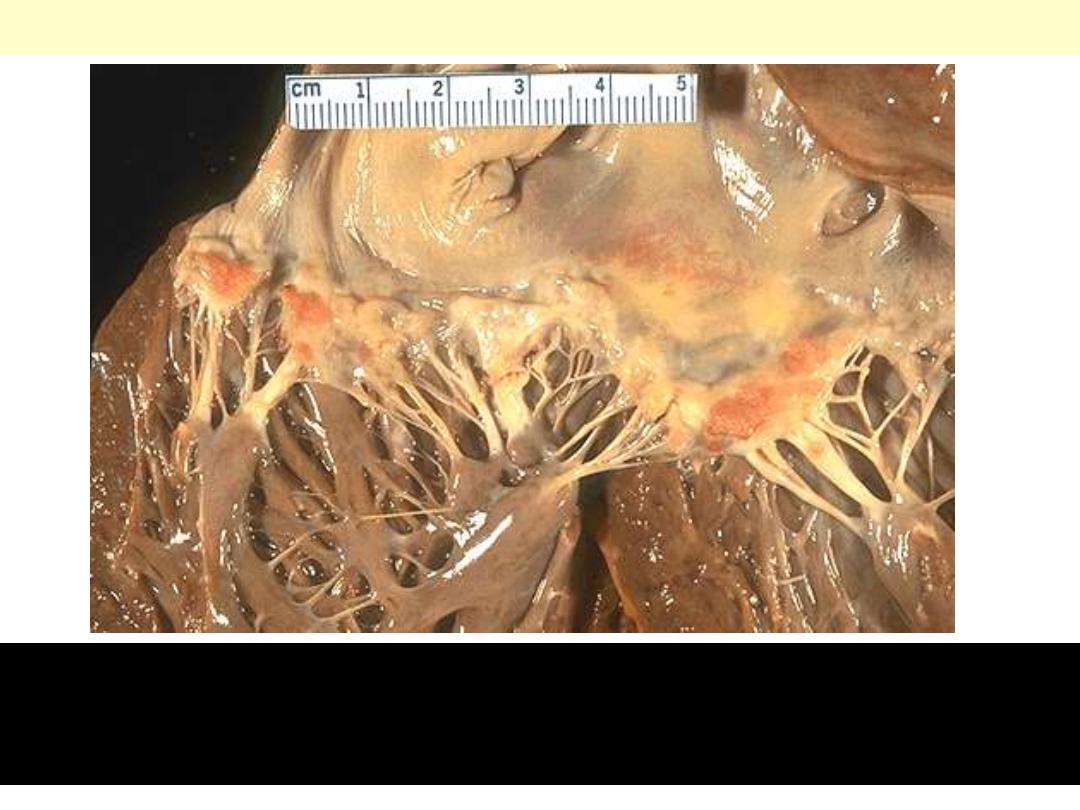
Libman-Sacks endocarditis
Flat, pale tan, spreading vegetations over the mitral valve surface
and even on the chordae tendineae. This patient has systemic lupus
erythematosus (consistent with Libman-Sacks endocarditis).

Heart failure - Effects
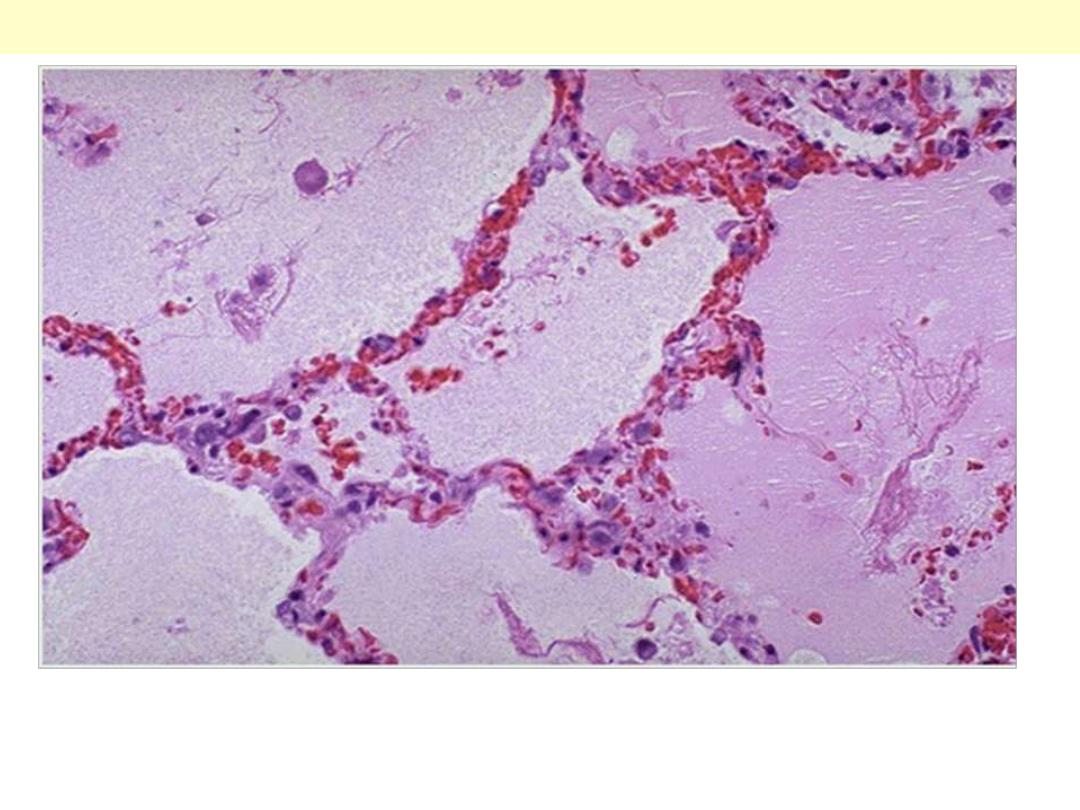
Note the prominent congested septal capillaries and the faint
staining edema fluid filling alveolar spaces
Pulmonary edema; a case of Lt heart failure
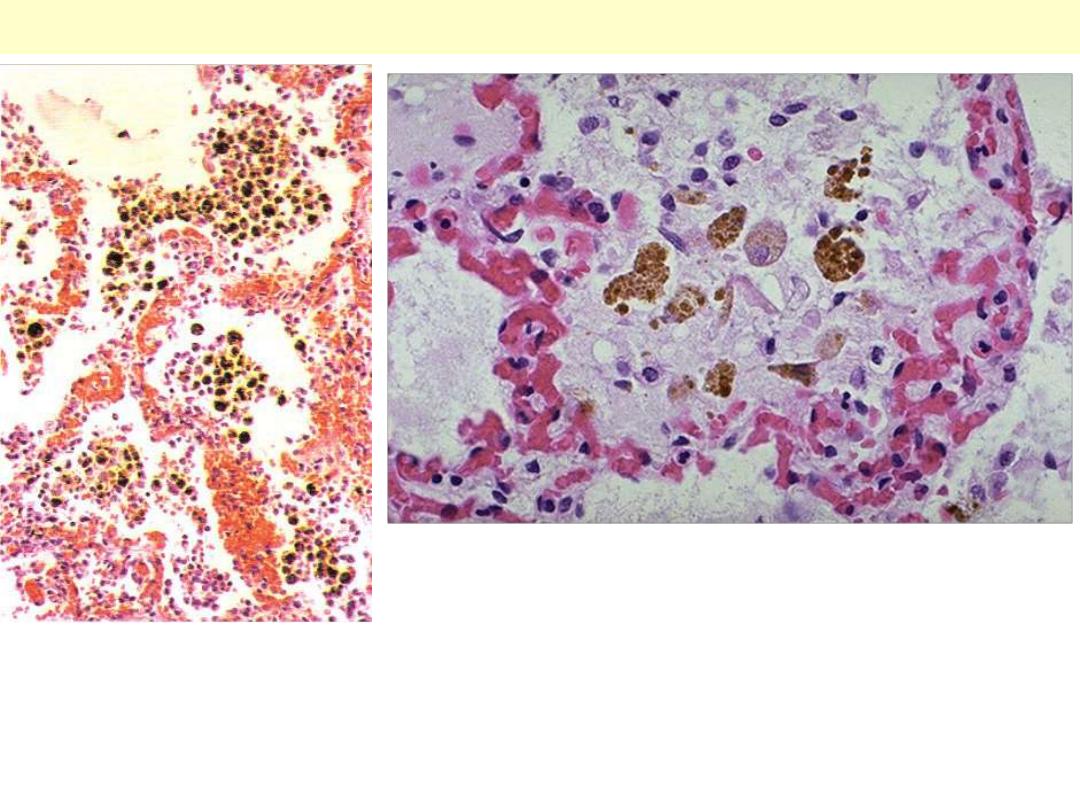
Chronic venous congestion (CVC) lung: low power (Lt) and high Power (Rt)
views. Note: 1. engorgement of septal capillaries 2. hemosiderin-laden
macrophages (the hemosiderin granules within cytoplasm of macrophages
appear brownish)
CVC lung

cut surface shows mottled
appearance; the dark areas
represent congestions around
central veins. This altration
has been likened to the cut
surface of a nutmeg “nutmeg
liver”.
Chronic venous (passive) congestion liver
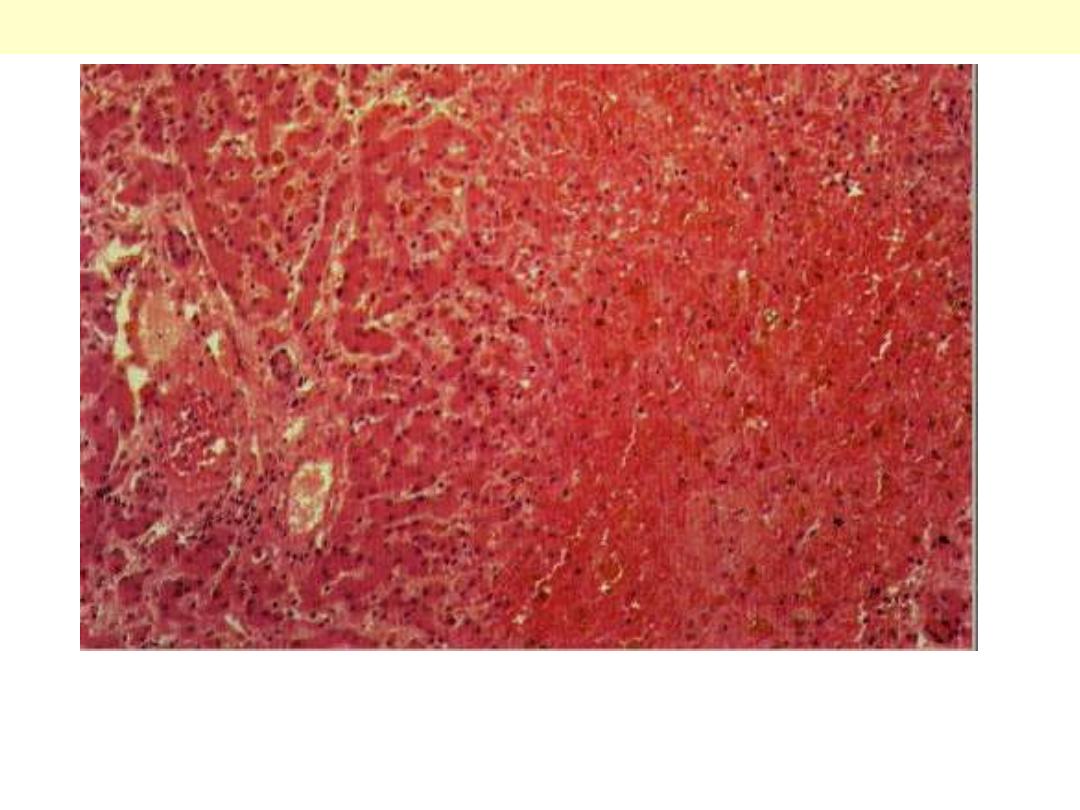
The centrilobular area shows accumulation of blood with damage to the
centrilobular liver parenchyma. The portal tract and surrounding periportal
parenchyma, however, are relatively spared.
CVC liver
Portal & periportal zone
Centrilobular zone
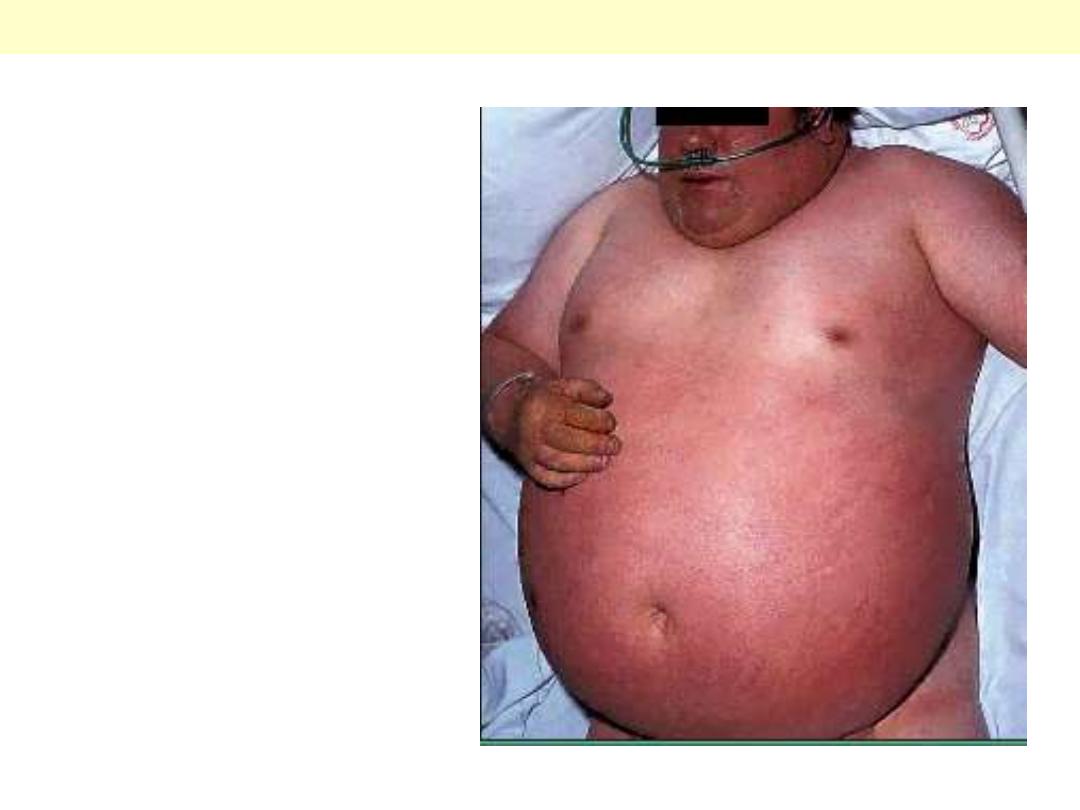
Rt sided heart failure secondary to
lung disease (note cyanosis)
Ascites
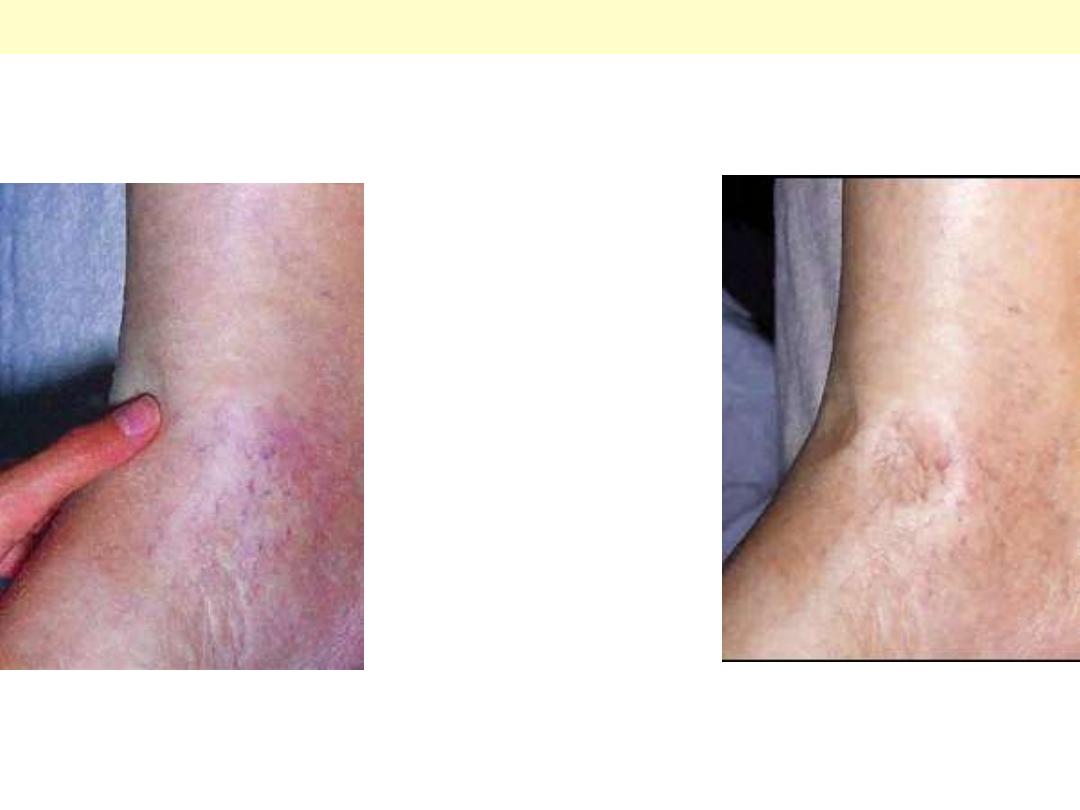
Pitting edema of Rt sided heart failure (ankle region)

Hypertensive HD
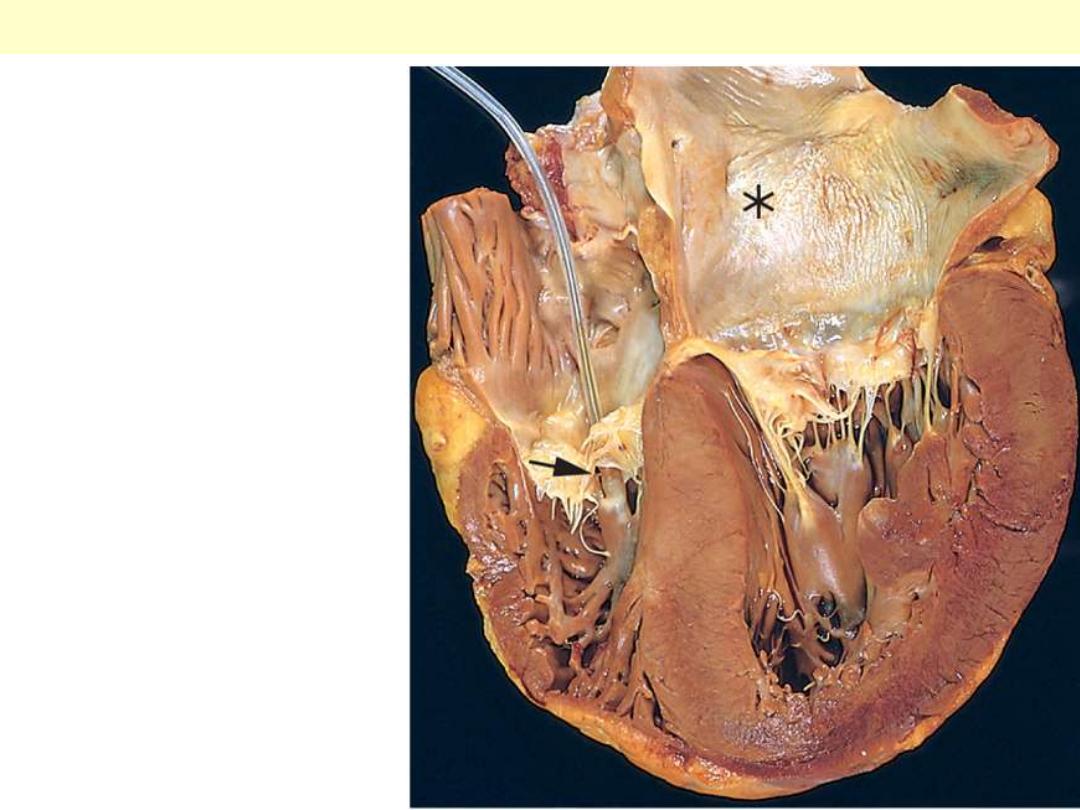
Hypertensive heart disease
with marked concentric
thickening of the left
ventricular wall causing
reduction in lumen size.
The left ventricle is on the
right in this apical four-
chamber view of the heart.
A pacemaker is
incidentally present in the
right ventricle (arrow).
Note also the left atrial
dilation (asterisk) due to
relative stiffening of the
left ventricle causing
impaired diastolic
relaxation and subsequent
atrial volume overload.
Hypertensive LVH with Lt atrial dilation
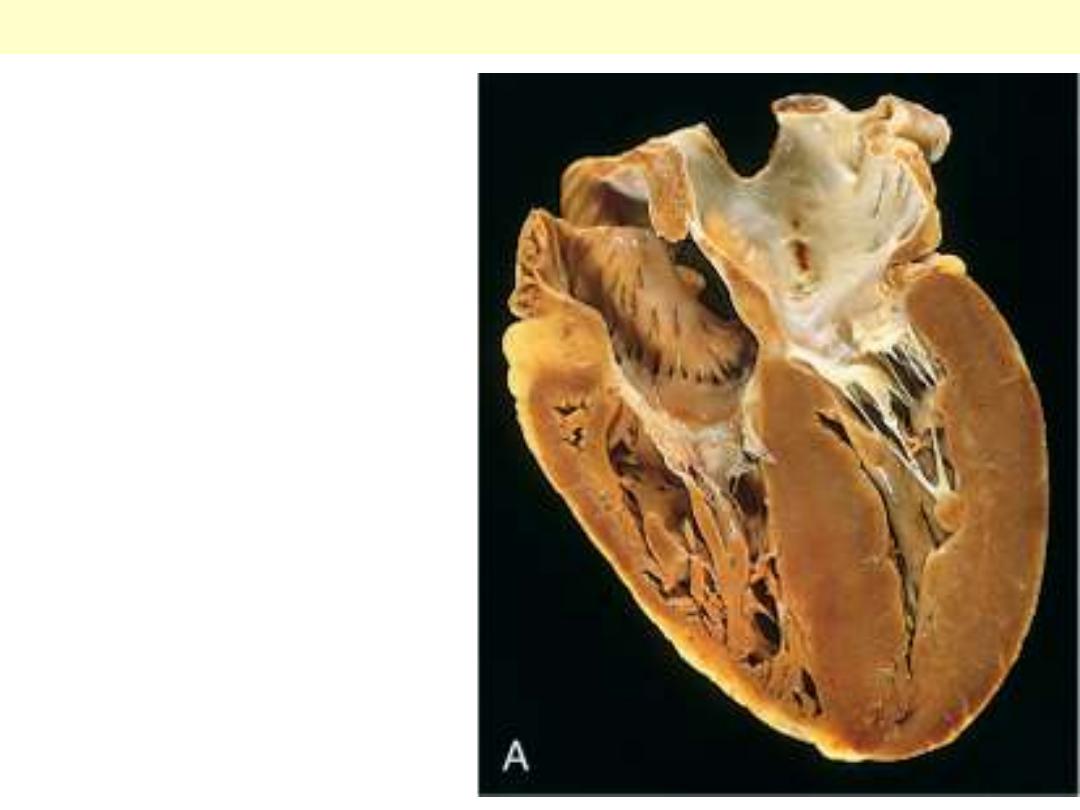
Chronic cor
pulmonale:
characterized by a
markedly dilated and
hypertrophied right
ventricle, with
thickened free wall
and hypertrophied
trabeculae (apical
four-chamber view of
heart, right ventricle
on left). The shape of
the left ventricle (to
the right) has been
distorted by the right
ventricular
enlargement.
Rt ventricular hypertrophy with dilation

Heart - Hypertrophy
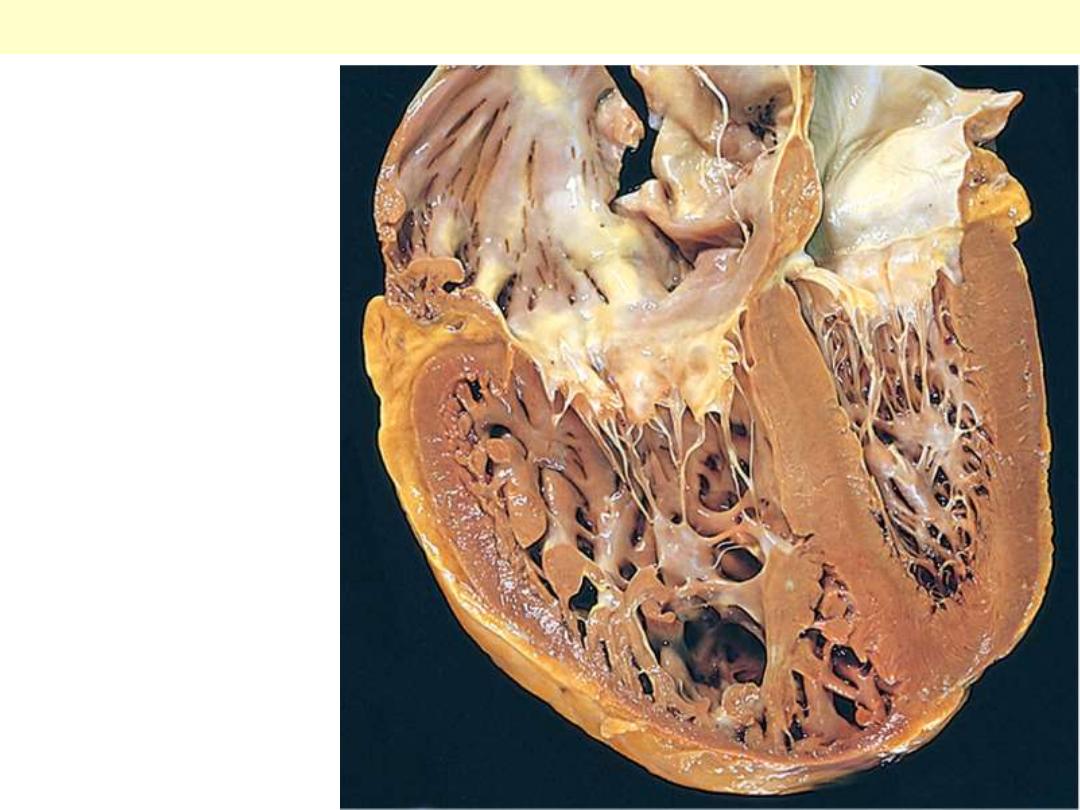
This is a cross section of the heart with marked concentric thickening of the Lt ventricular wall. Note
the corresponding decrease in the ventricular cavity. The LV (Lt) is over 2 cm in thickness (N: 1-1.5
cm). The wt of the heart consequently increase to over the normal of 350 g. some times up to 800 g.
Systemic hypertension is a common cause.
Concentric Hypertrophy of Lt V
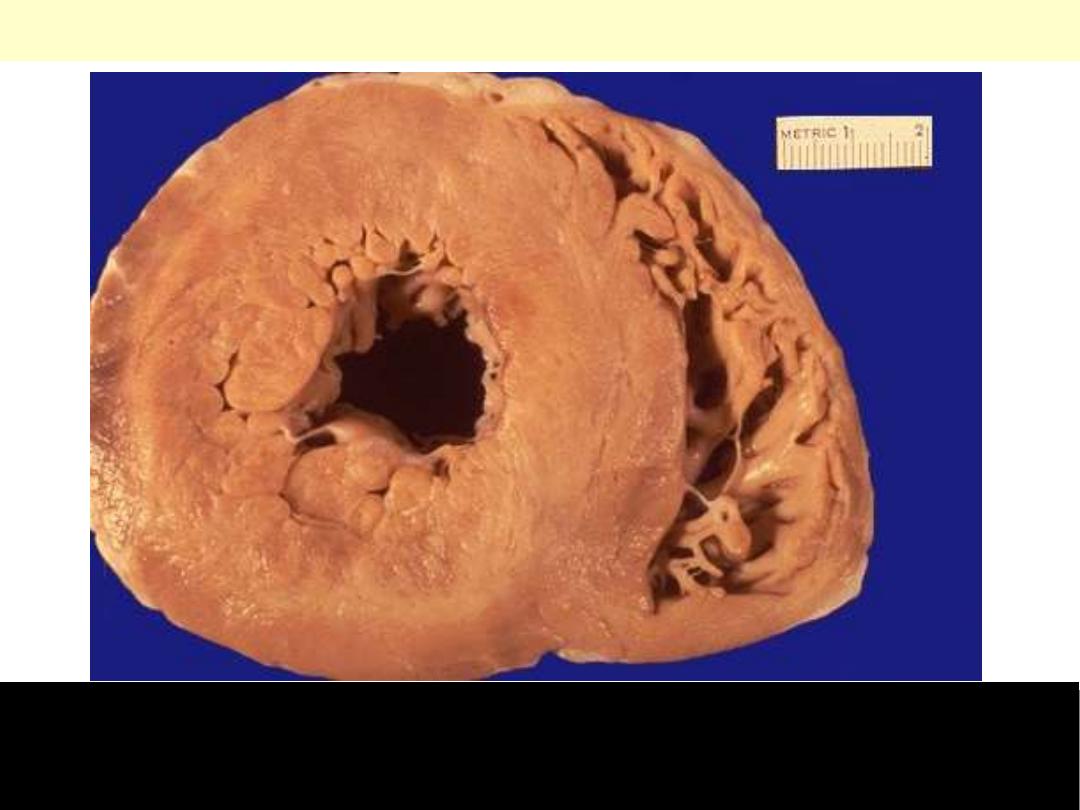
Pressure hypertrophy due to left ventricular
outflow obstruction. The left ventricle is to
your right in this apical four-chamber view
of the heart.
Left ventricular hypertrophy
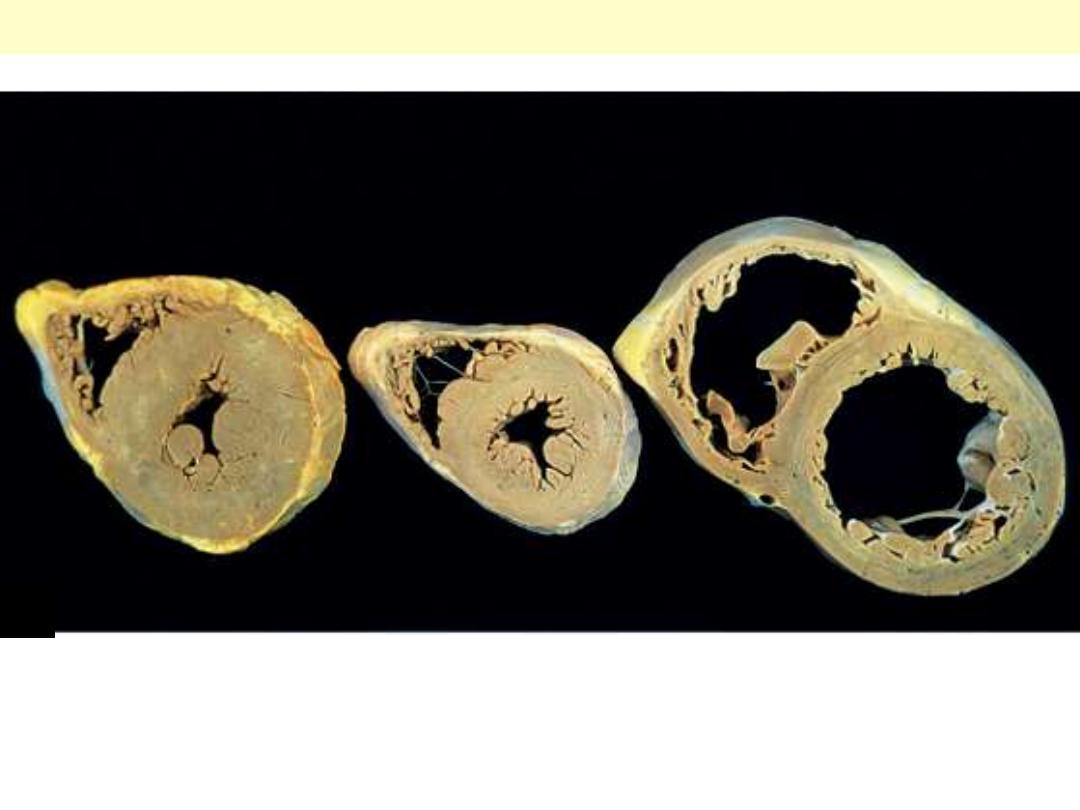
Altered cardiac configuration in LVH without and with dilation, viewed in transverse heart sections.
Compared with a normal heart (center), the pressure-hypertrophied hearts (left) have increased mass
and a thick left ventricular wall, but the hypertrophied and dilated heart (right) has increased mass
but a normal wall thickness.
Lt ventricular hypertrophy Vs normal & hypertrophy with dilation

Ischemic HD
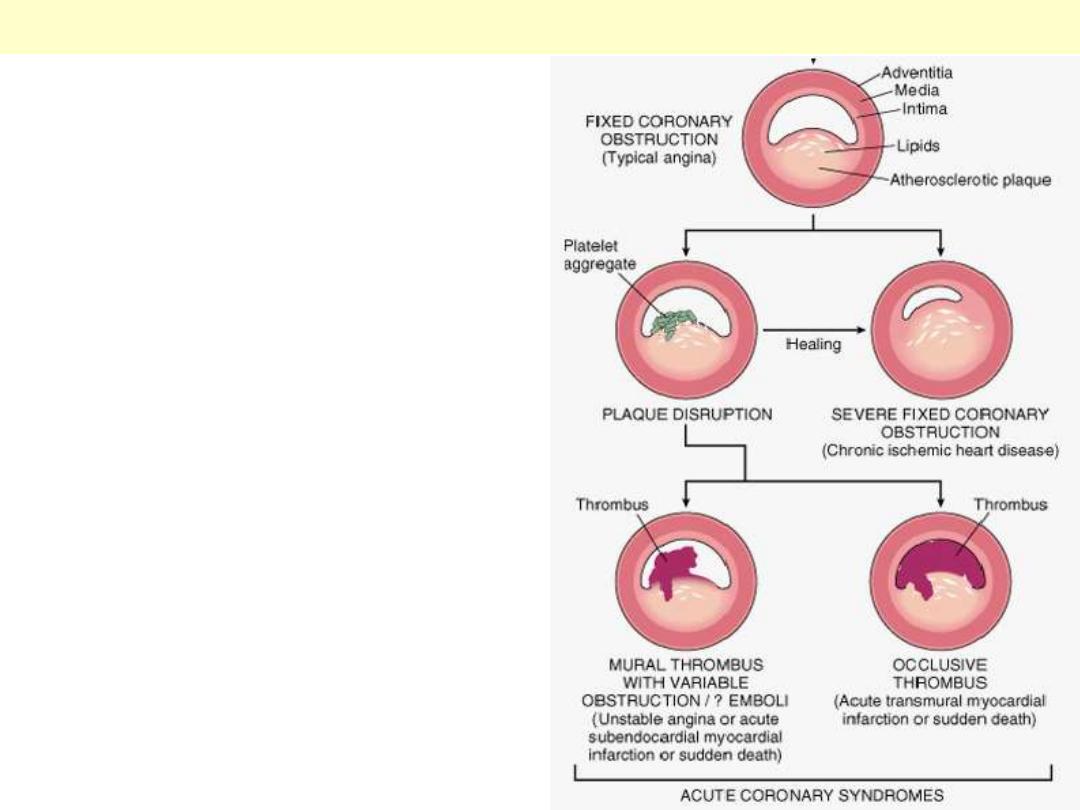
Beginning with stable chronic plaque, responsible
for typical angina, and leading to the various
acute coronary syndromes.
Sequential progression of coronary artery lesion
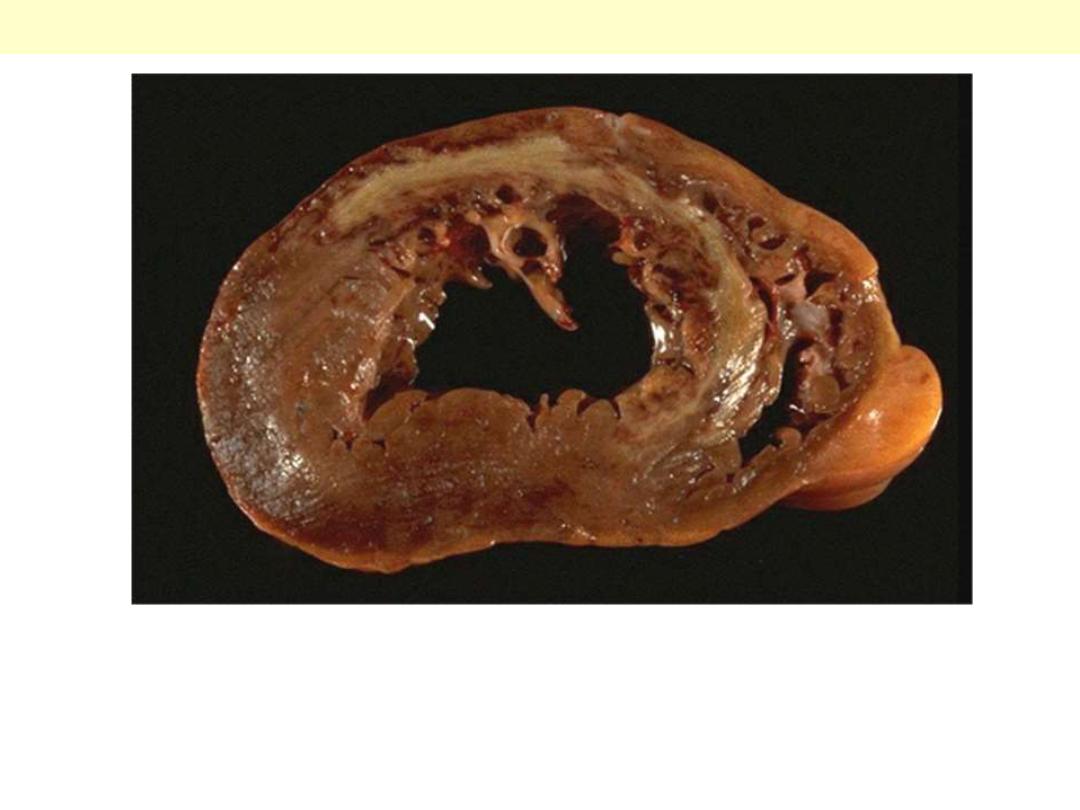
This cross section reveals a large myocardial infarction involving the
anterior left ventricular wall and septum. The color of the infarct is
whitish-yellow. This is an example of pale (anemic) infarction.
MI involving LV anterior free wall and septum
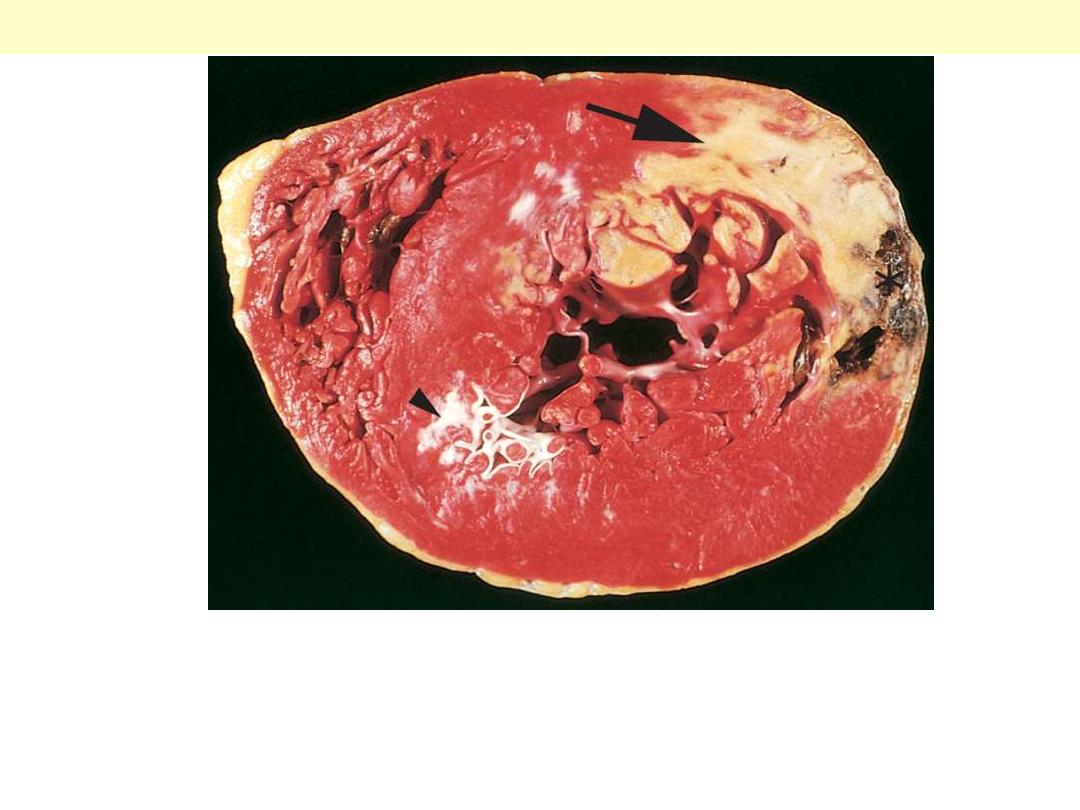
There is yellowish discoloration of the myocardium. Note the anterior scar
(arrowhead), indicative of old infarct. The myocardial hemorrhage at the Lt.
ventricular lateral wall (asterisk) is due to ventricular rupture and was the acute
cause of death in this patient (specimen is oriented with the posterior wall at the
top).
Acute myocardial infarct of the posterolateral left ventricle
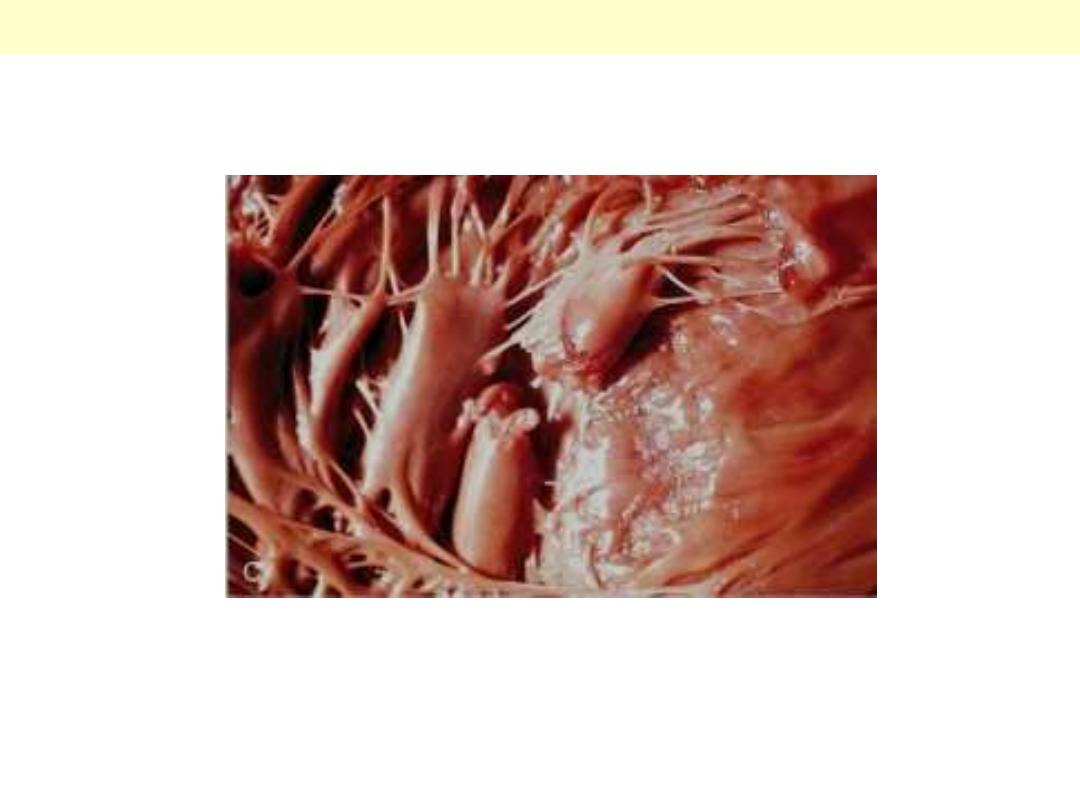
Complete rupture of a necrotic papillary muscle.
MI papillary muscle rupture
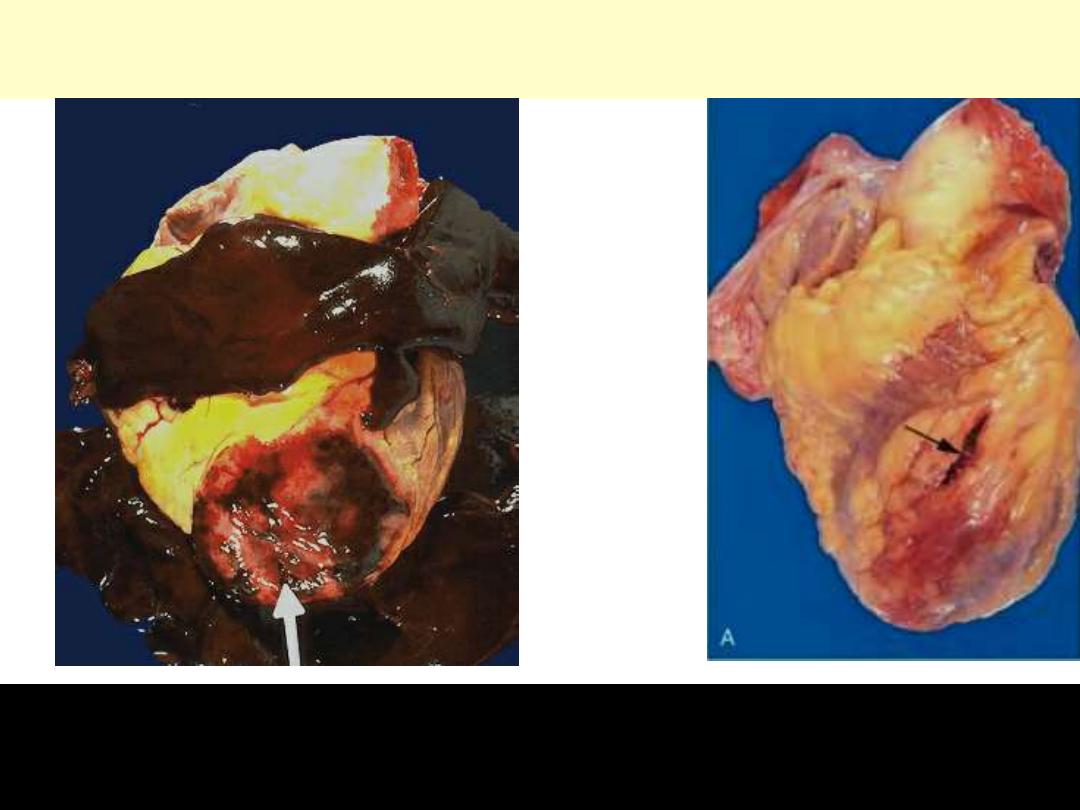
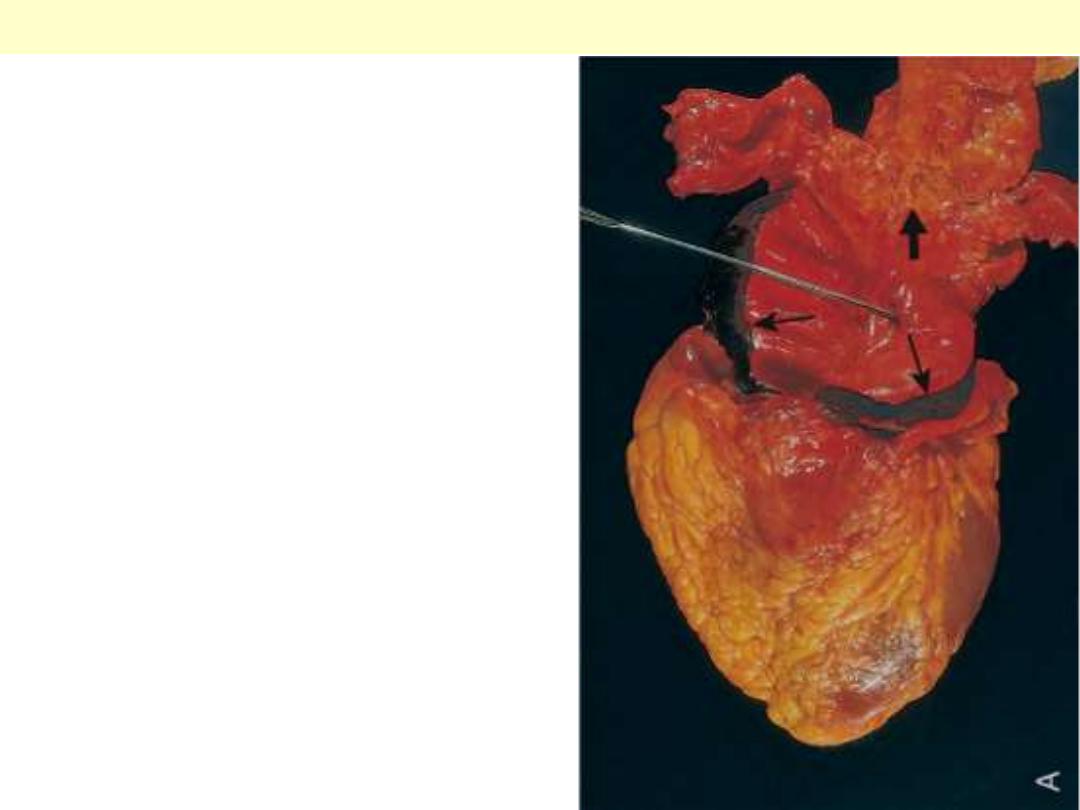
Transmural myocardial infarction with rupture and hemopericardium
This is most likely to occur in the first week between 4 to 5 days following the initial event, when the
myocardium is the softest. The arrow marks the point of rupture in this anterior myocardial infarction
of the left ventricular free wall. Note the dark red blood clot forming the hemopericardium. The
hemopericardium can lead to tamponade.
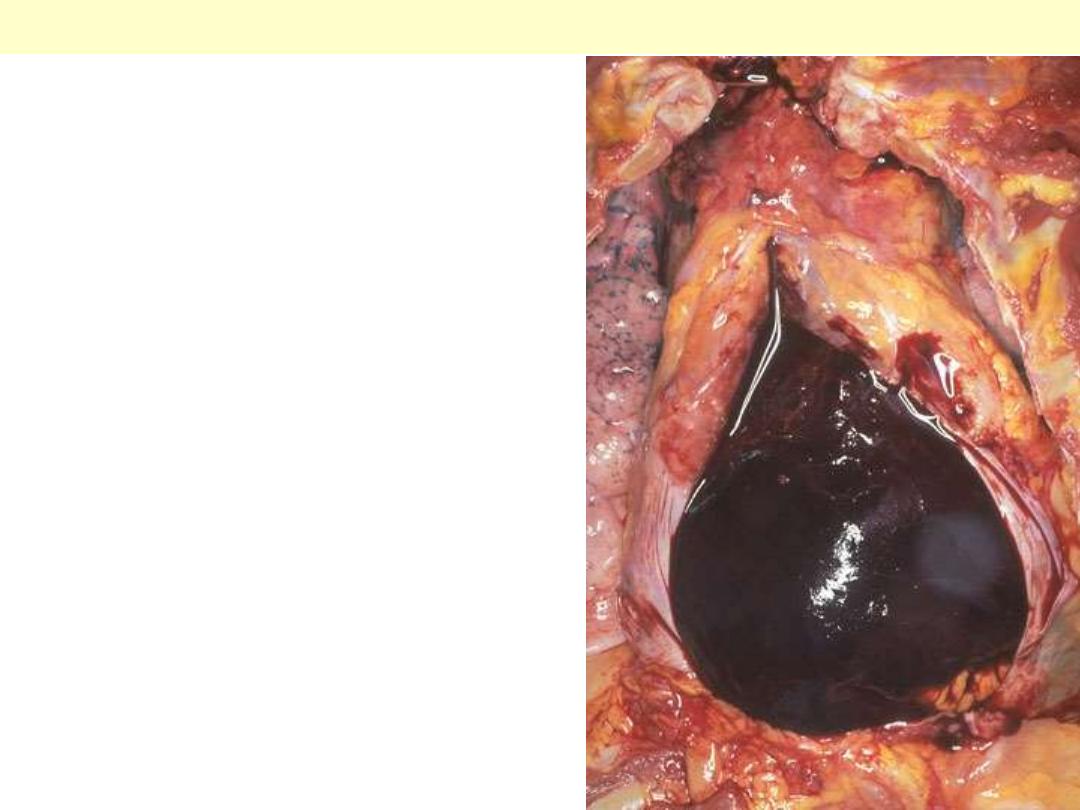
The pericardial sac is opened to display
hemo pericardium. Such a massive
amount of hemorrhage can lead to
cardiac tamponade. This may result
from rupture of the heart as a
complication of myocardial infarction or
an aortic dissection, when blood dissects
through the media proximally.
Hemopericardium
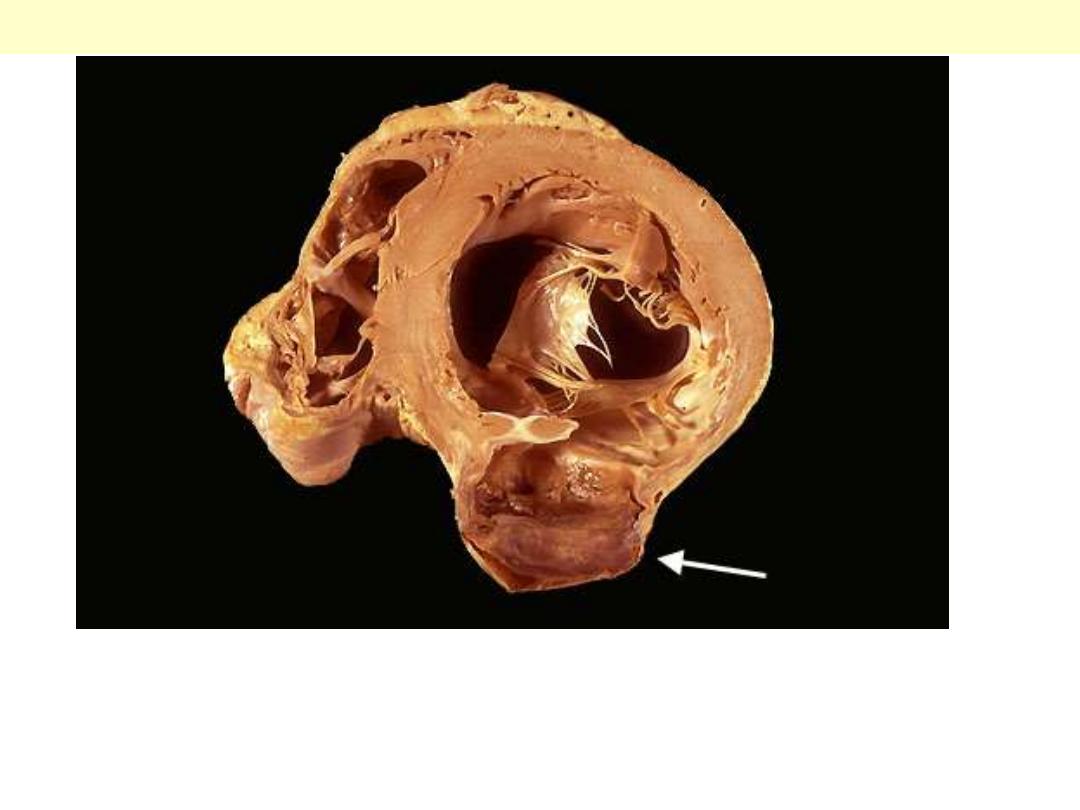
A cross section through the heart reveals a ventricular aneurysm with a
very thin wall at the arrow. Note how the aneurysm bulges out. The
stasis in this aneurysm allows mural thrombus, which is present here, to
form within the aneurysm.
LV aneurysm complicating MI
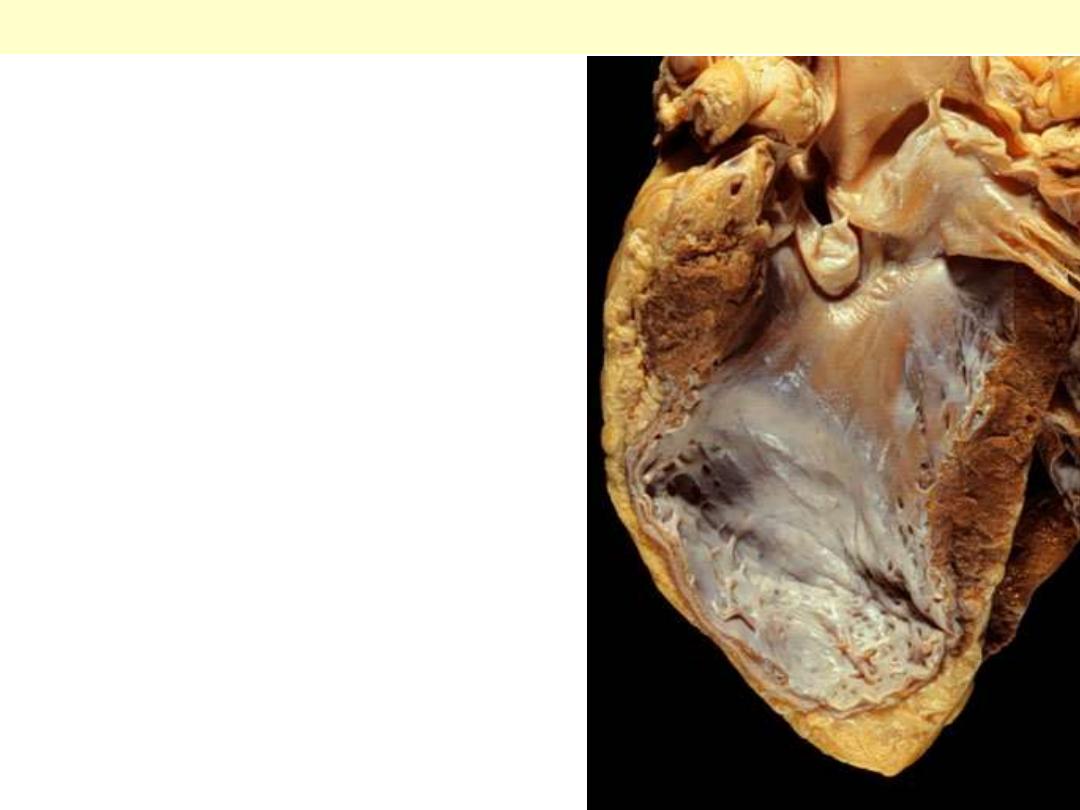
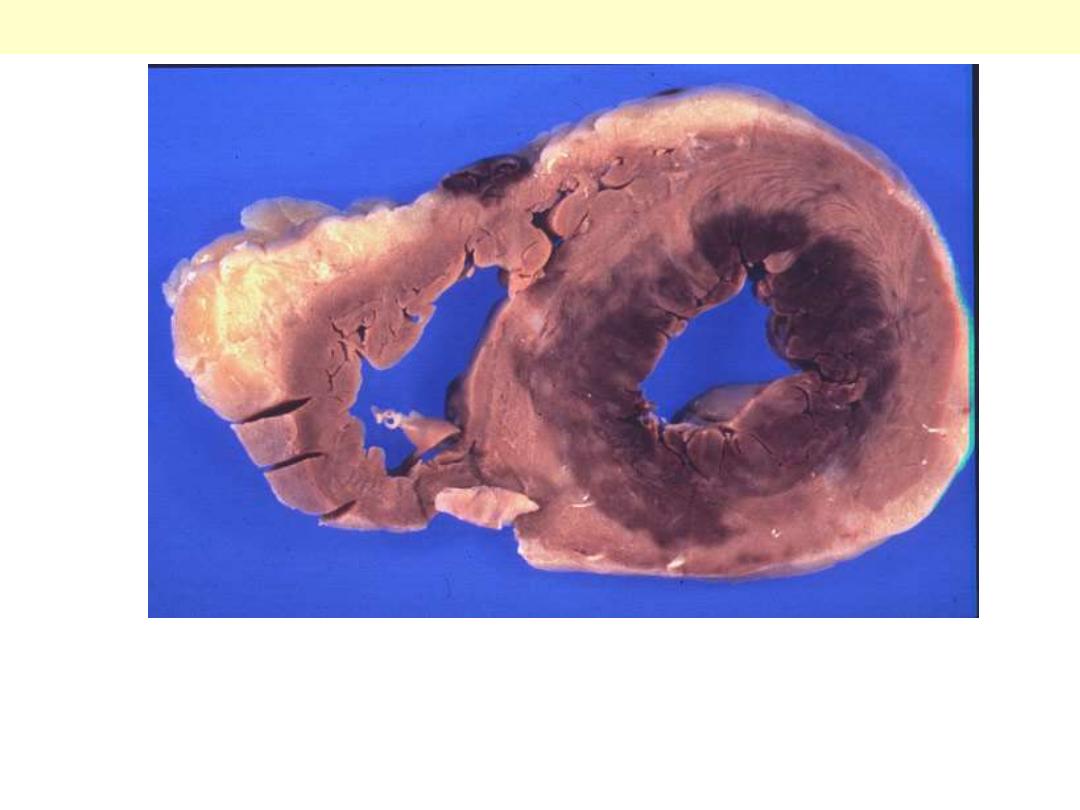
There has been a previous
extensive transmural myocardial
infarction involving the free wall
of the left ventricle. Note that the
thickness of the myocardial wall is
normal superiorly, but inferiorly
is only a thin fibrous wall. The
infarction was so extensive that,
after healing, the ventricular wall
was replaced by a thin band of
collagen, forming an aneurysm.
Such an aneurysm represents
non-contractile tissue that reduces
stroke volume and strains the
remaining myocardium.
Lt ventricular aneurysm complicating healed MI
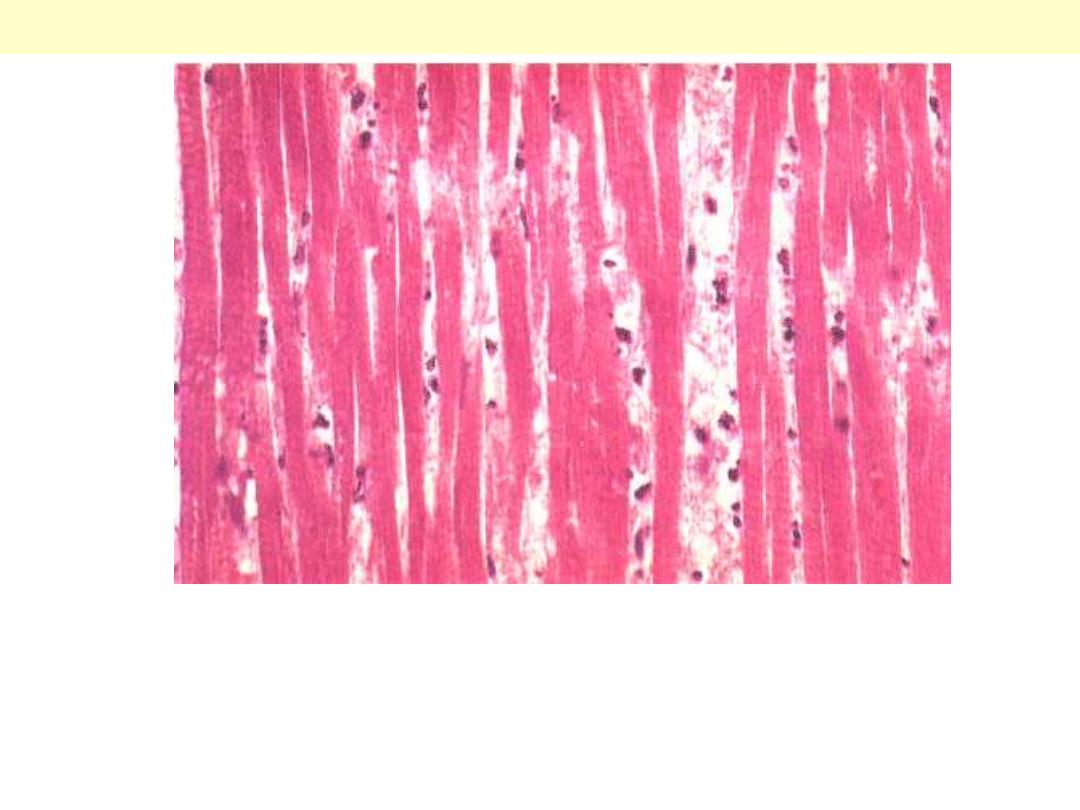
This infarct is limited to the inner third to one half of the LV wall.
The red-blue cyanotic discoloration is totally encircling the Lt V
inner wall.
Subendocardial myocardial infarction

Acute MI: Coagulative necrosis of myocardial cell
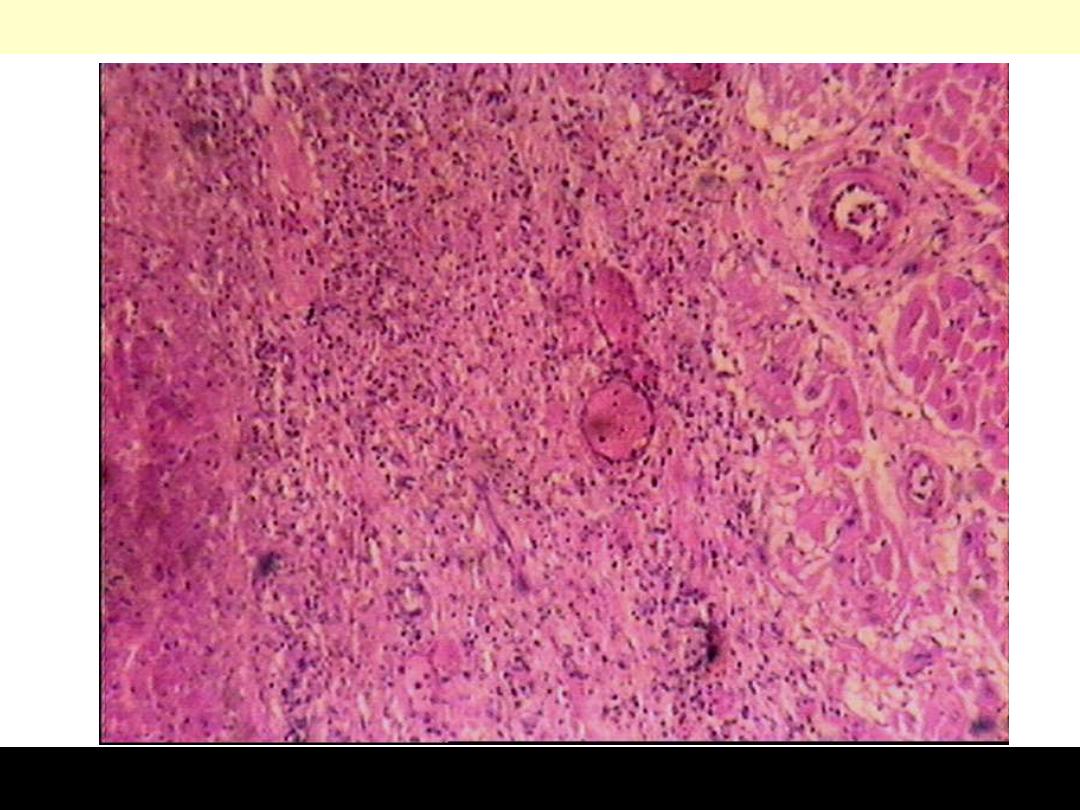
This myocardial
infarction is about 3 to
4 days old. There is an
extensive acute
inflammatory cell
infiltrate and the
myocardial fibers are
so necrotic that the
outlines of them are
only barely visible.
The cytoplasm is rather
homogeneous, deeply
eosinophilic, devoid of
cross striation and
there are no nuclei.
Nnecrotic muscle fibres of the infarct at higher magnification. The nuclei
having disappeared by karyolysis. The fibres are more deeply eosinophilic
than normal fibres. The striations are still detectable focally. In the
interstitial tissue there are some nuclear fragments, macrophages which
have migrated into the dead muscle.
Myocardial Infarction: Coagulative Necrosis
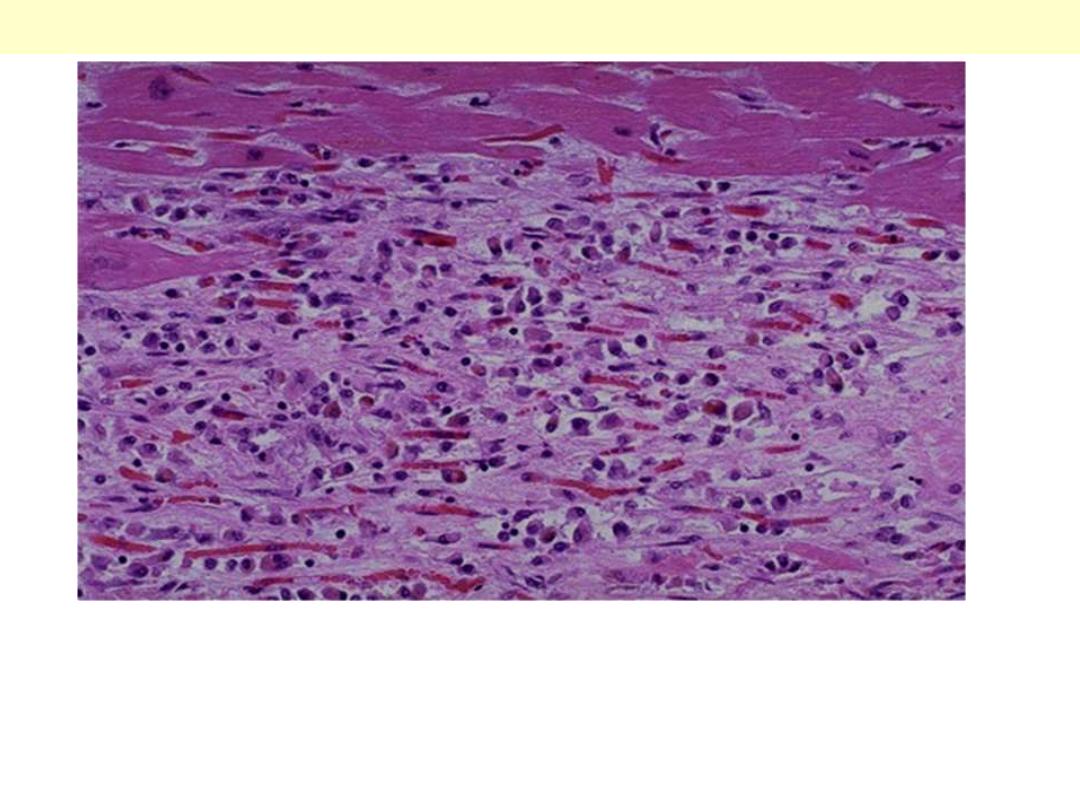
Necrosis
Normal
Line of demarcation
Myocardial infarction-line of demarcation
By 10 to 14 days from the onset the infarct is rimmed by a hyperemic zone of highly vascularized
granulation tissue (line of demarcation)
This is a myocardial infarction of 1 to 2 weeks in age. Note that there
are remaining normal myocardial fibers at the top. Below these
fibers are many macrophages along with numerous capillaries and
fibroblasts with deposition of collagen.
Myocardial Infarction: Coagulative Necrosis

Pericarditis
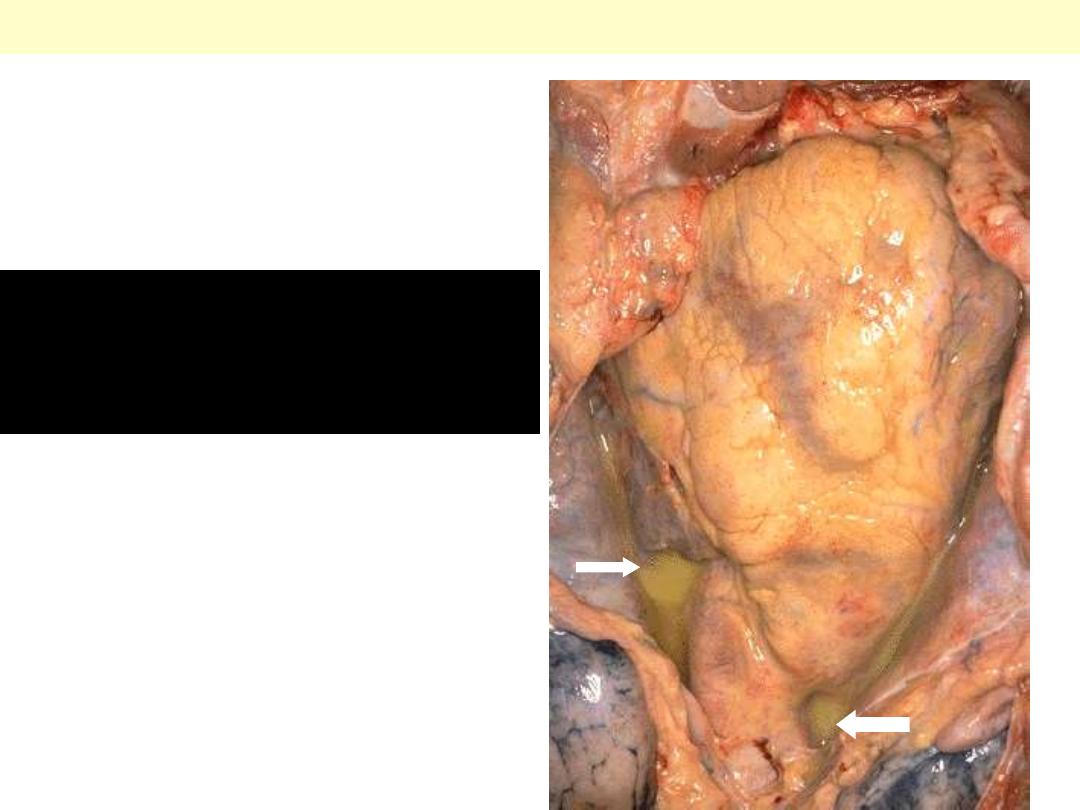
Purulent pericarditis
There is a yellowish exudate
(pus) that has pooled in the
lower pericardial sac.
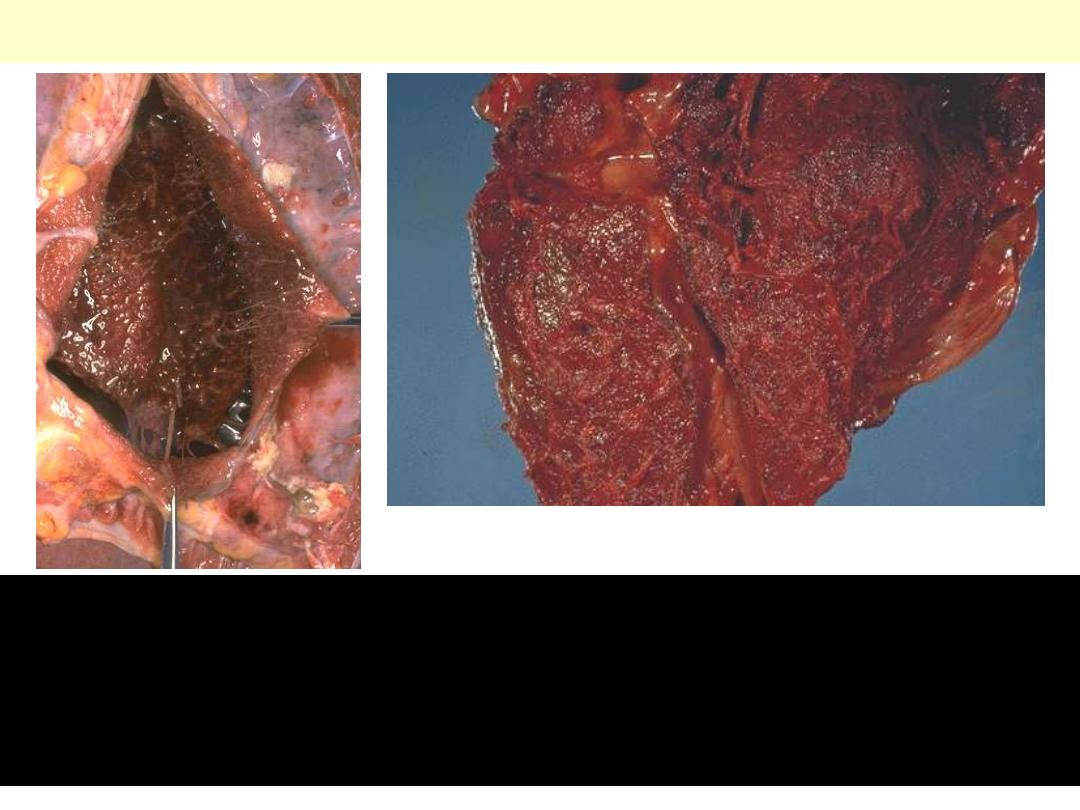
Hemorrhagic pericarditis
There is not only fibrin, but also hemorrhage. It is really just fibrinous
pericarditis with hemorrhage. Without inflammation, blood in the
pericardial sac would be called “hemopericardium". The surface of the
heart shows a roughened and red appearance. Hemorrhagic pericarditis is
most likely to occur with metastatic tumor and with tuberculosis.

Rheumatic HD
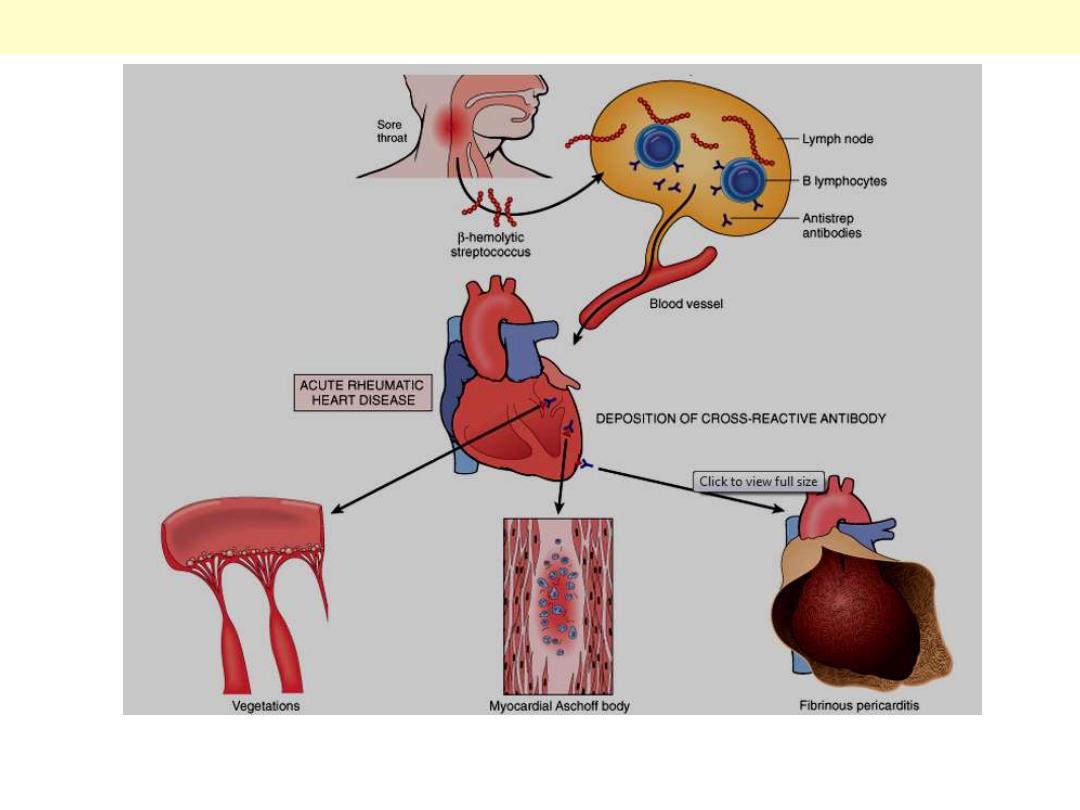
Acute rheumatic fever causes changes in the endocardium, myocardium, and epicardium. Chronic
rheumatic heart disease is almost always caused by deformity of the heart valves, particularly the
mitral and aortic valves.
Pathogenesis and key morphologic changes of acute RHD
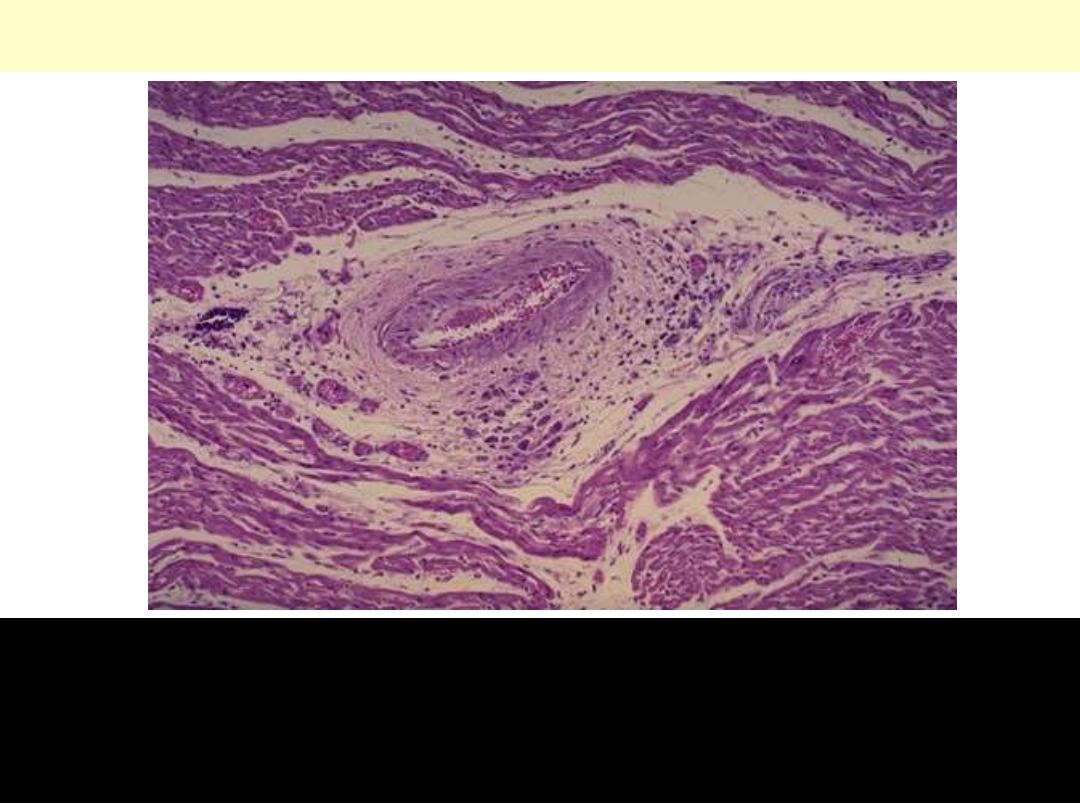
Acute rheumatic carditis
Acute rheumatic carditis is marked by a peculiar form of
granulomatous inflammation with so-called "Aschoff nodules" seen
best in myocardium. These are centered in interstitium around
vessels as shown here.
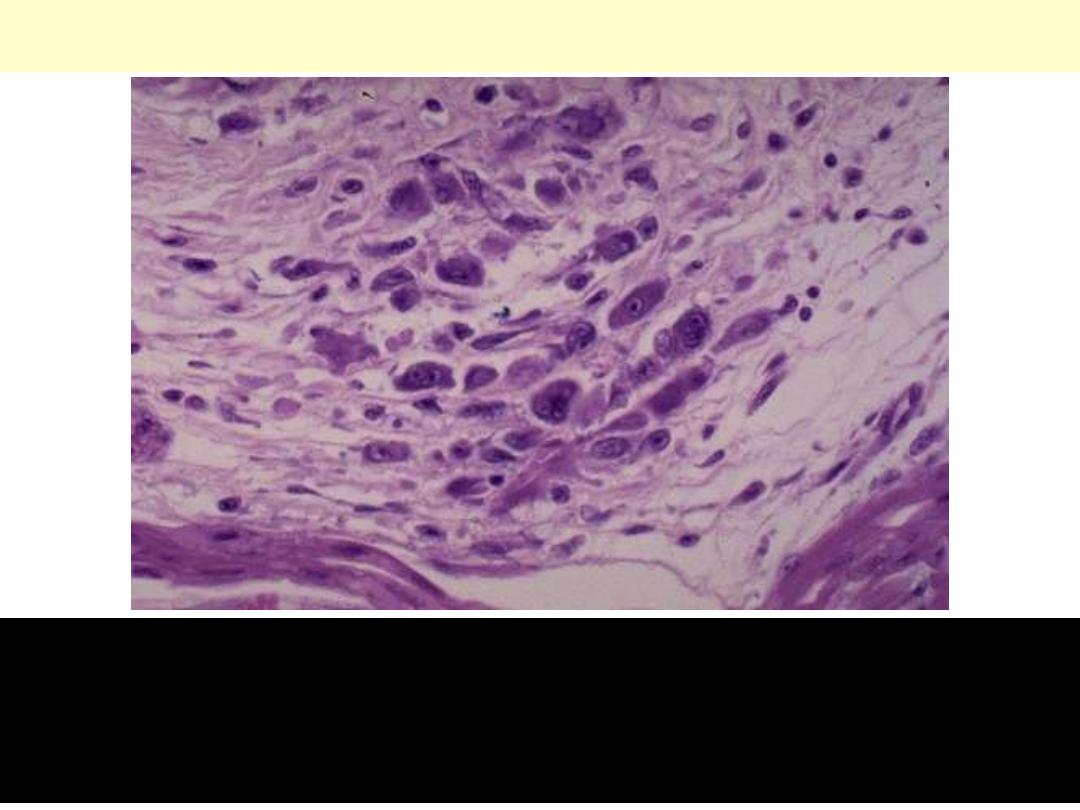
Acute rheumatic carditis, microscopic
Aschoff nodule. Aschoff giant cell. Several appear here as large cells
with two or more nuclei that have prominent nucleoli. Scattered
inflammatory cells accompany them and can be mononuclears or
occasionally neutrophils.
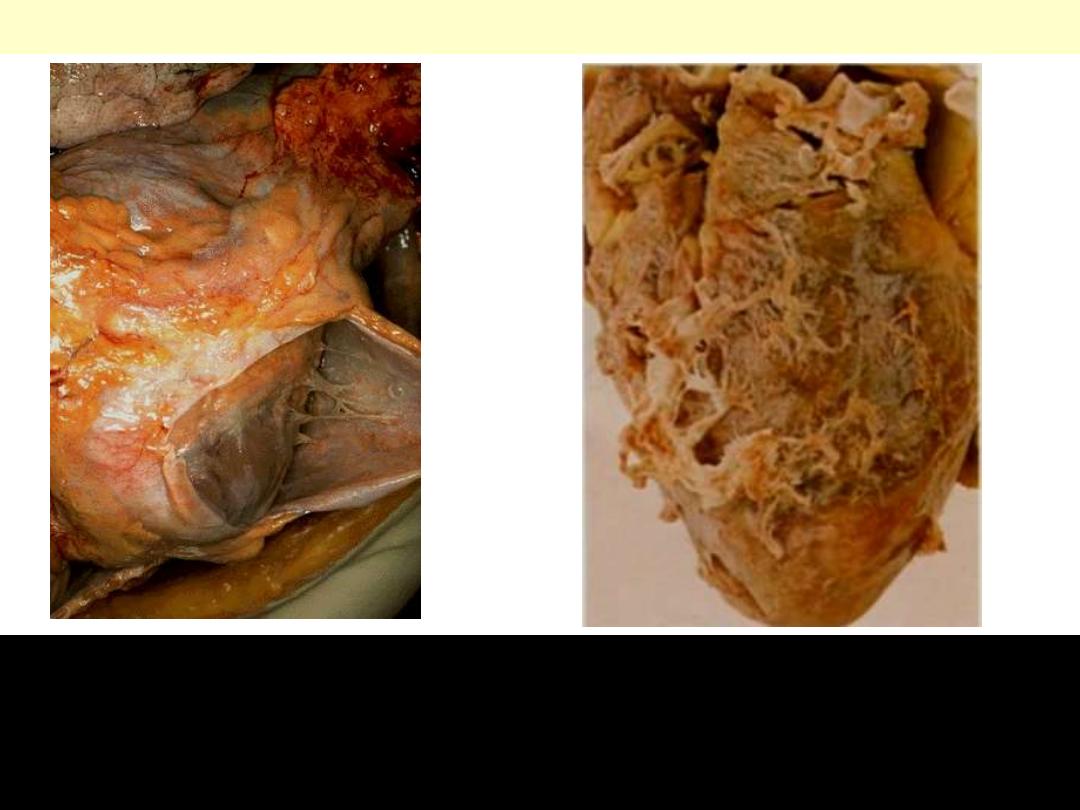
Fibrinous pericarditis
Lt. The pericardium has been opened to reveal the surface of the
heart. There are thin strands of fibrinous exudate that extend from
the parietal to visceral pericardium.. This is typical for a fibrinous
pericarditis. Rt. More florid example of fibrinous pericarditis.
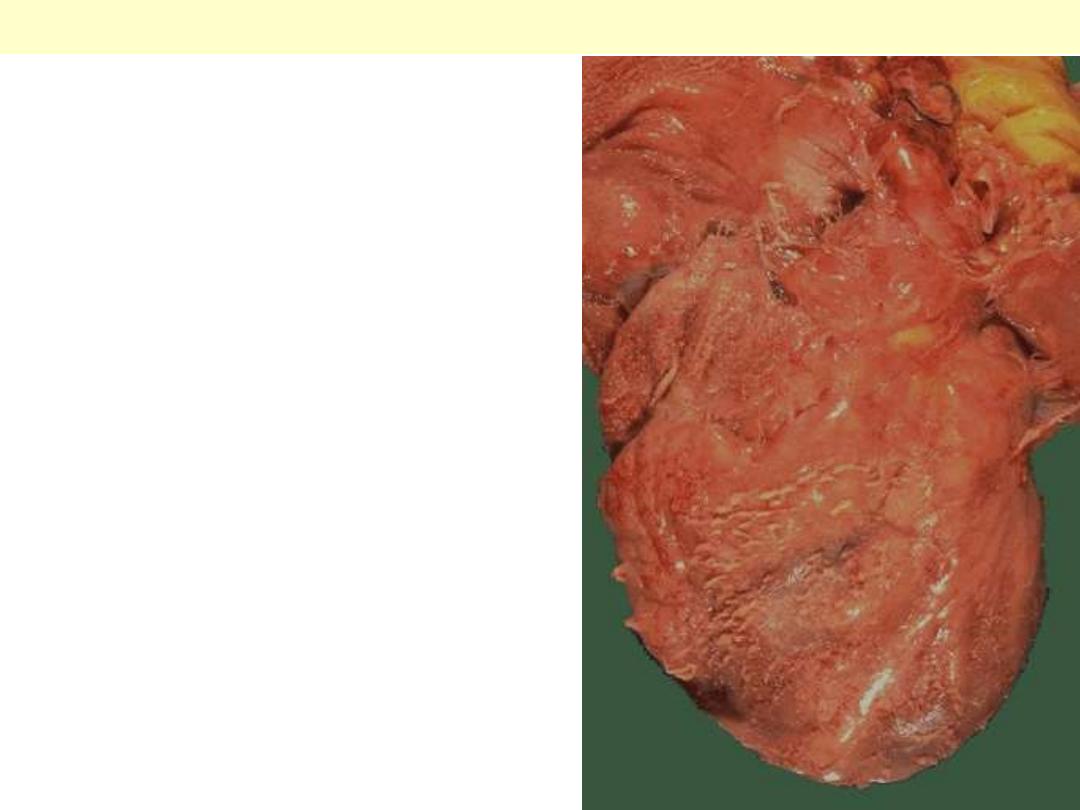
The epicardial surface of the
heart shows a shaggy fibrinous
exudate. This is another example
of fibrinous pericarditis. This
appearance has often been called
a "bread and butter"
pericarditis, but you would have
to drop your buttered bread on
the carpet to really get this effect.
The fibrin often results in the
finding on physical examination
of a "friction rub" as the strands
of fibrin on epicardium and
pericardium rub against each
other.
Fibrinous pericarditis
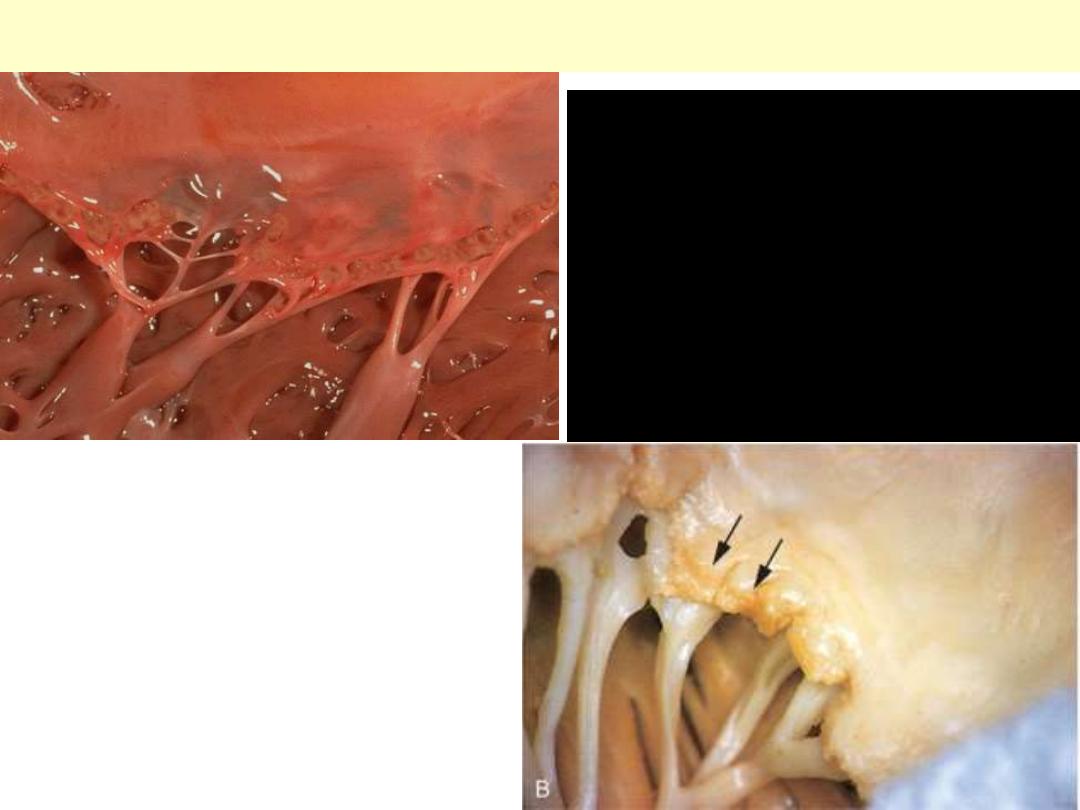
Acute rheumatic valvulitis
Small verrucous vegetations seen along the
closure line of this mitral valve. These warty
vegetations average only a few millimeters and
form along the line of valve closure over areas
of endocardial inflammation. The lower photo.
display Acute rheumatic mitral valvulitis
superimposed on chronic rheumatic heart
disease. Small vegetations (verrucae) are visible
along the line of closure of the mitral valve
leaflet (arrows). Previous episodes of rheumatic
valvulitis have caused fibrous thickening and
fusion of the chordae tendineae.
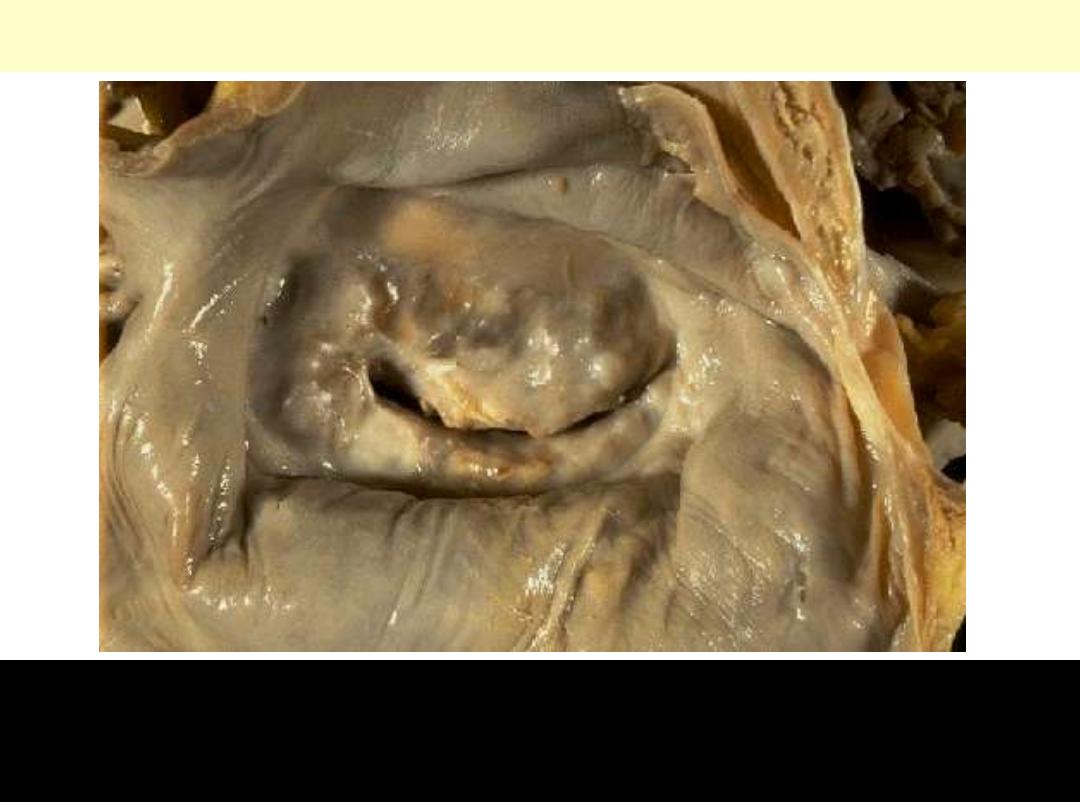
Chronic Rheumatic Mitral Stenosis
The mitral valve as seen from above in the left atrium. The mitral
valve demonstrates the typical "fish mouth" shape with chronic
rheumatic scarring.

Tumors
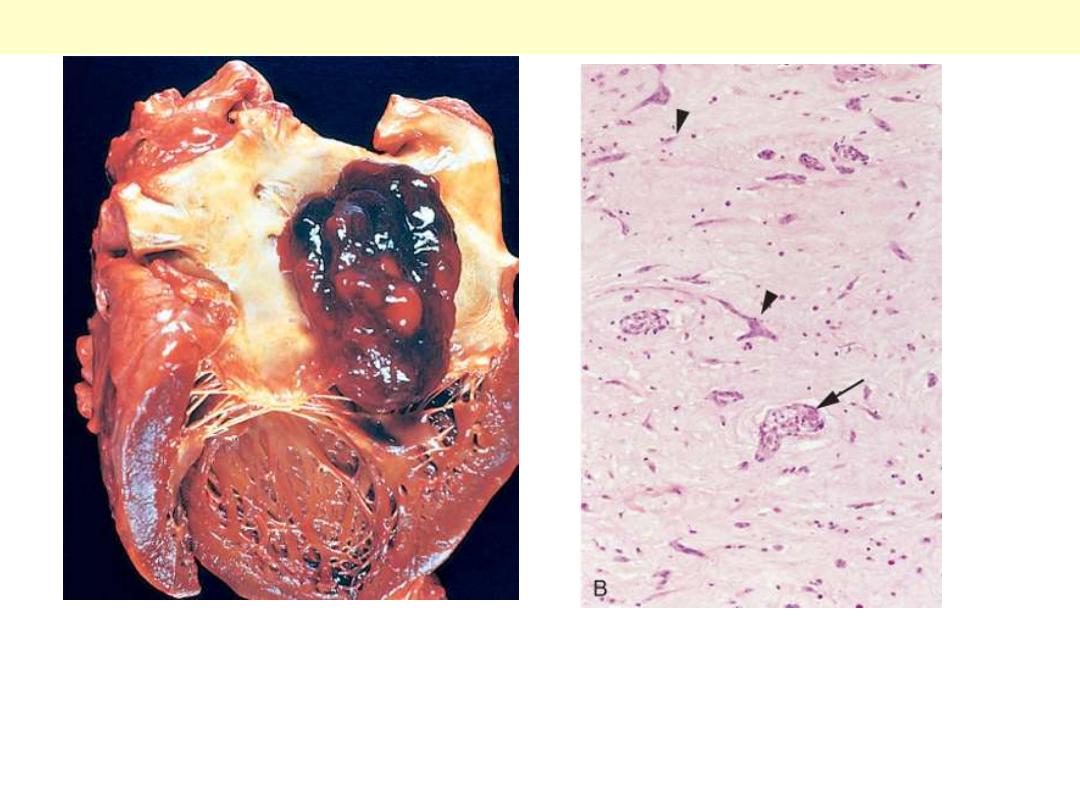
A, Gross photograph showing large pedunculated lesion arising from the region of the fossa
ovalis and extending into the mitral valve orifice. B, Microscopic appearance, with
abundant amorphous extracellular matrix in which are scattered collections of
multinucleated myxoma cells (arrowheads) in various groupings, including abnormal
vascular formations (arrow).
Left Atrial Myxoma
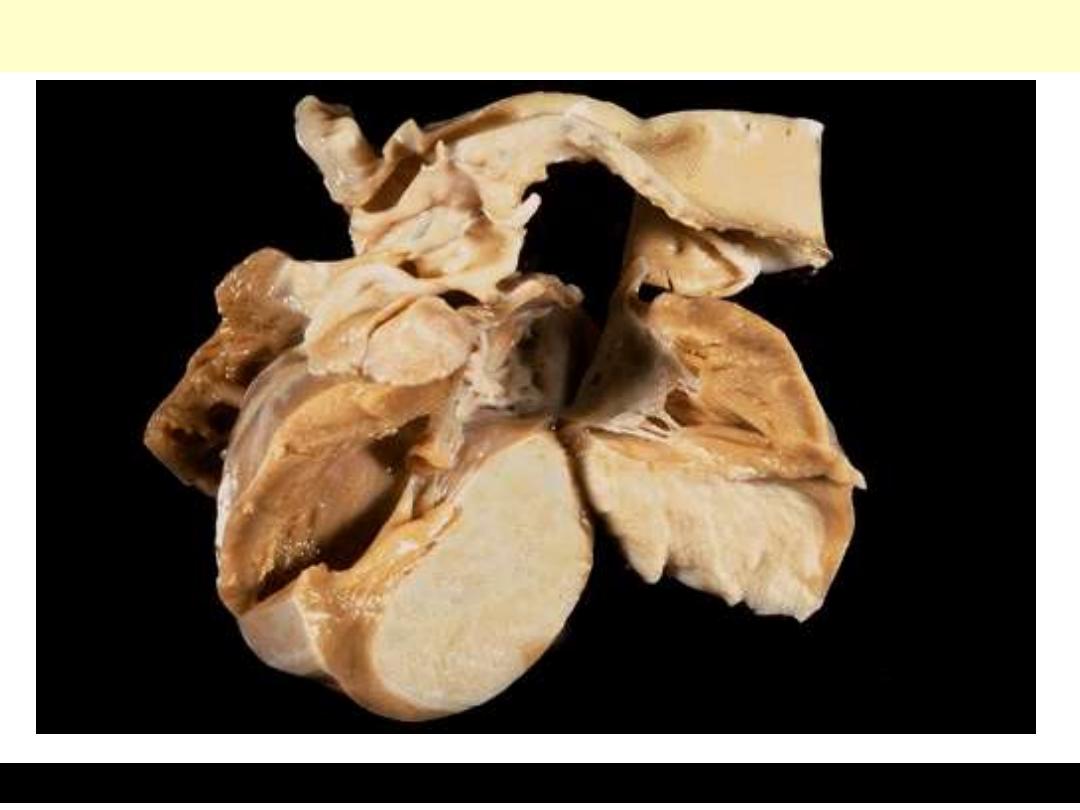
Cardiac rhabdomyoma
A large firm, white tumor mass is filling much of the left ventricle.

Valvular HD
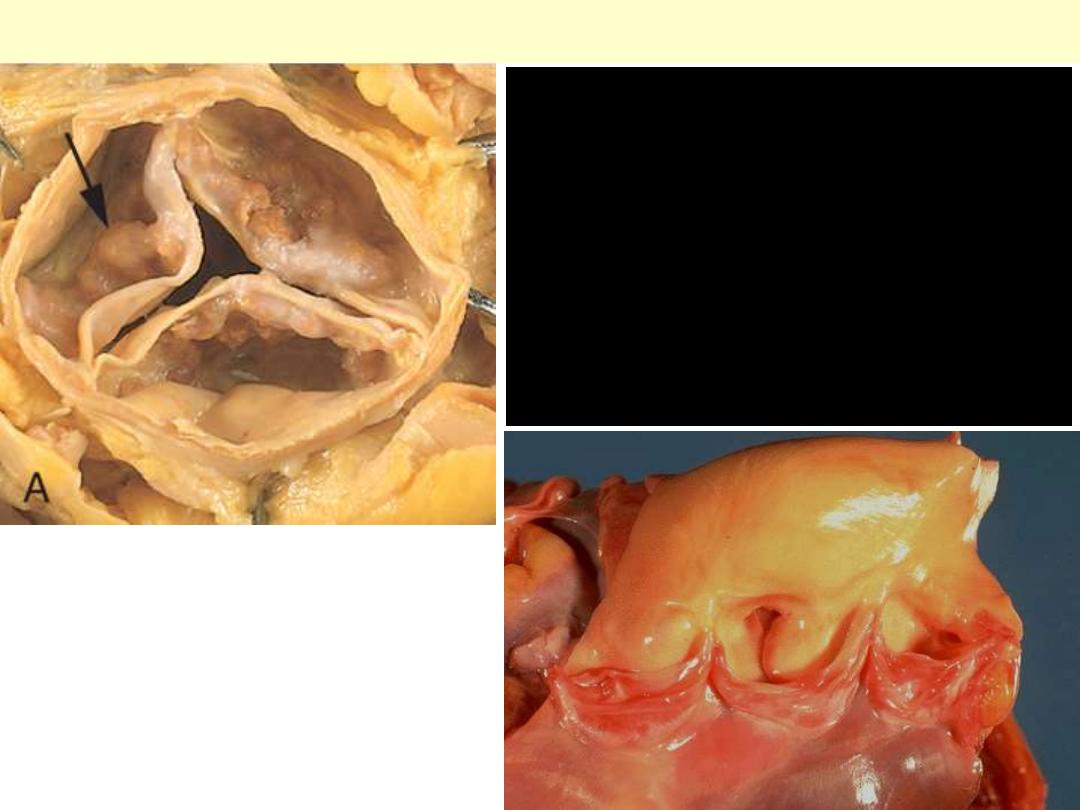
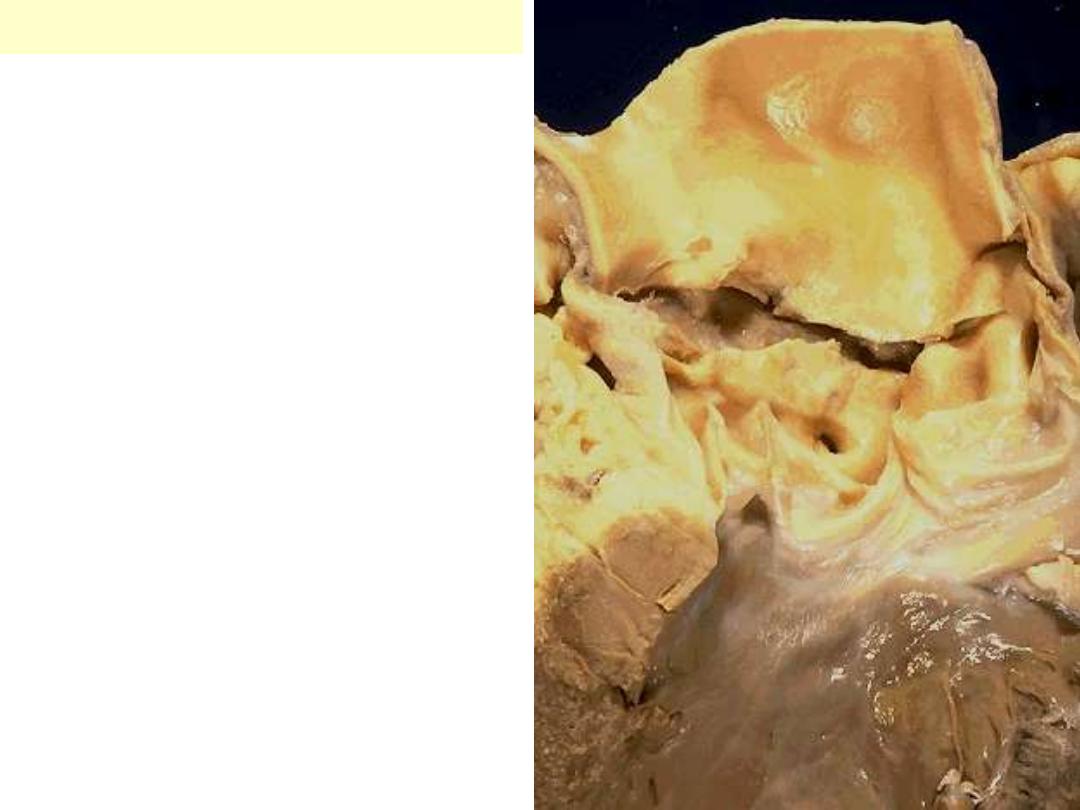
Senile calcific aortic stenosis
An aortic valve need not be
bicuspid to calcify. Sometimes in
older adults, a normal tricuspid
aortic valve will undergo
calcification, a so-called "senile
calcific aortic stenosis." Nodules of
calcification are seen on the cusps
here.
Normal
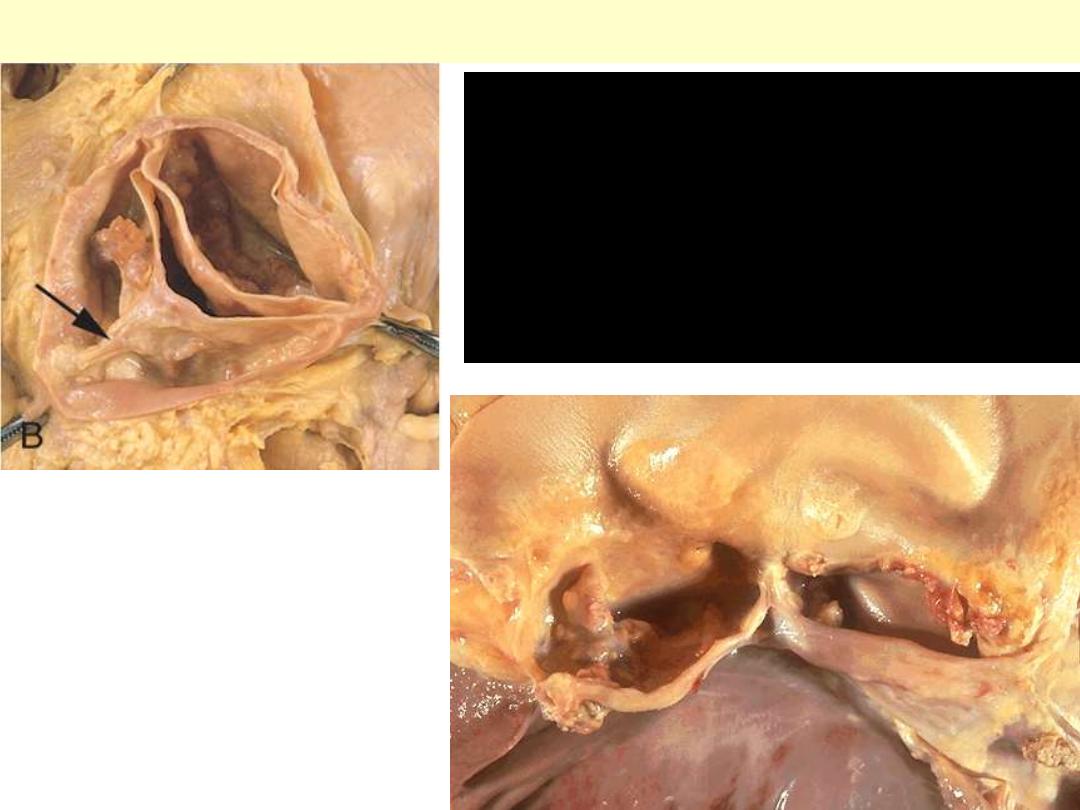
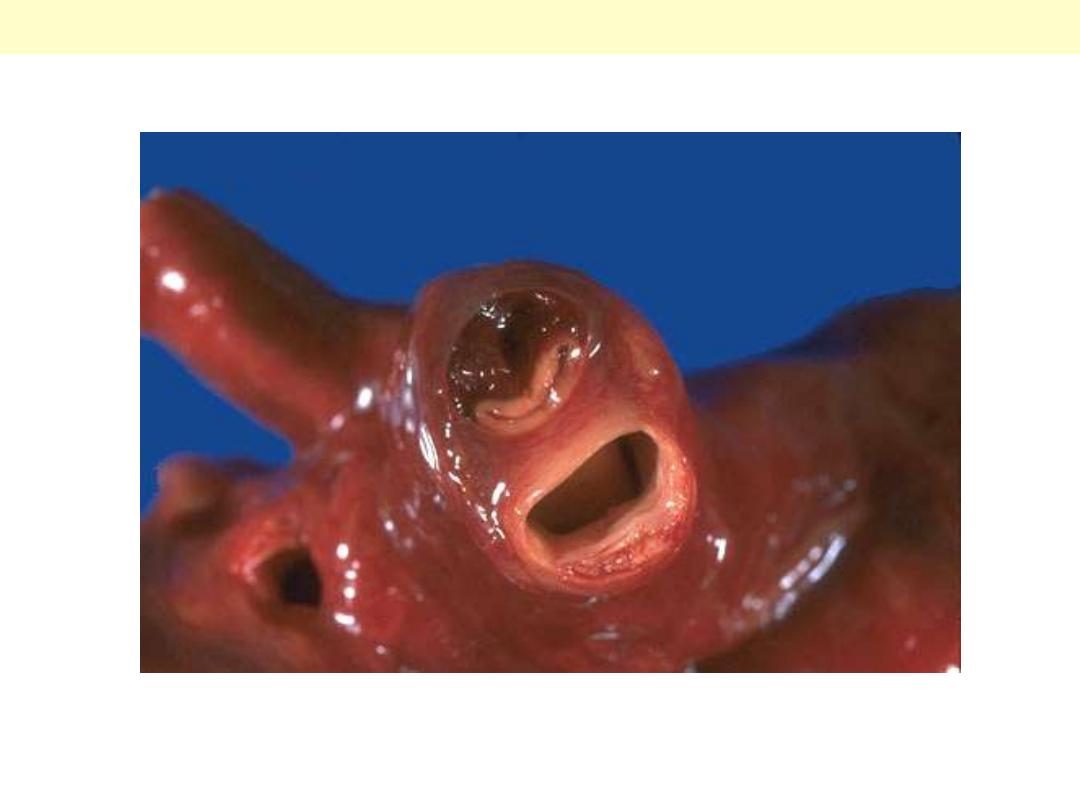
Calcific bicuspid aortic valve
Congenital bicuspid aortic valve. Most
bicuspid valves are prone to calcification.
The dense white nodules of calcification
are present on either valve surface. The
valve here has been opened with the
aortic outflow above and the left
ventricular myocardium below.
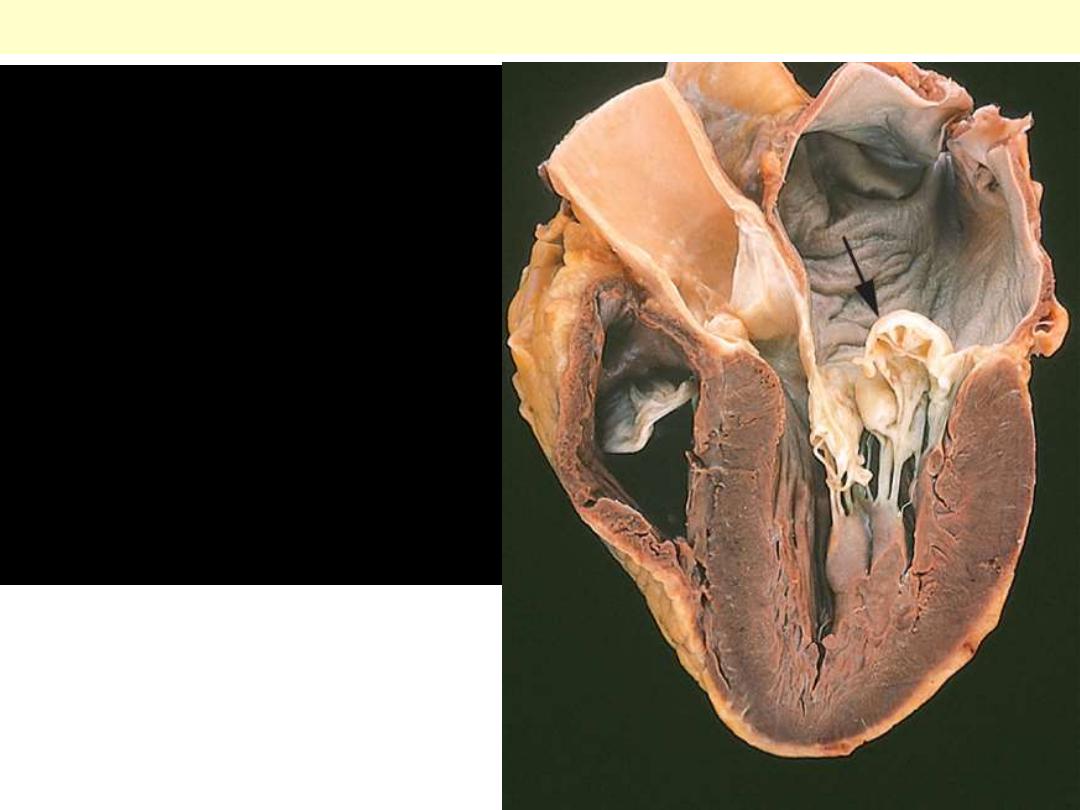
Floppy mitral valve
Myxomatous degeneration
of the mitral valve. Long
axis of left ventricle
demonstrating hooding
with prolapse of the
posterior mitral leaflet
into the left atrium
(arrow). The left ventricle
is on the right in this
apical four-chamber view.
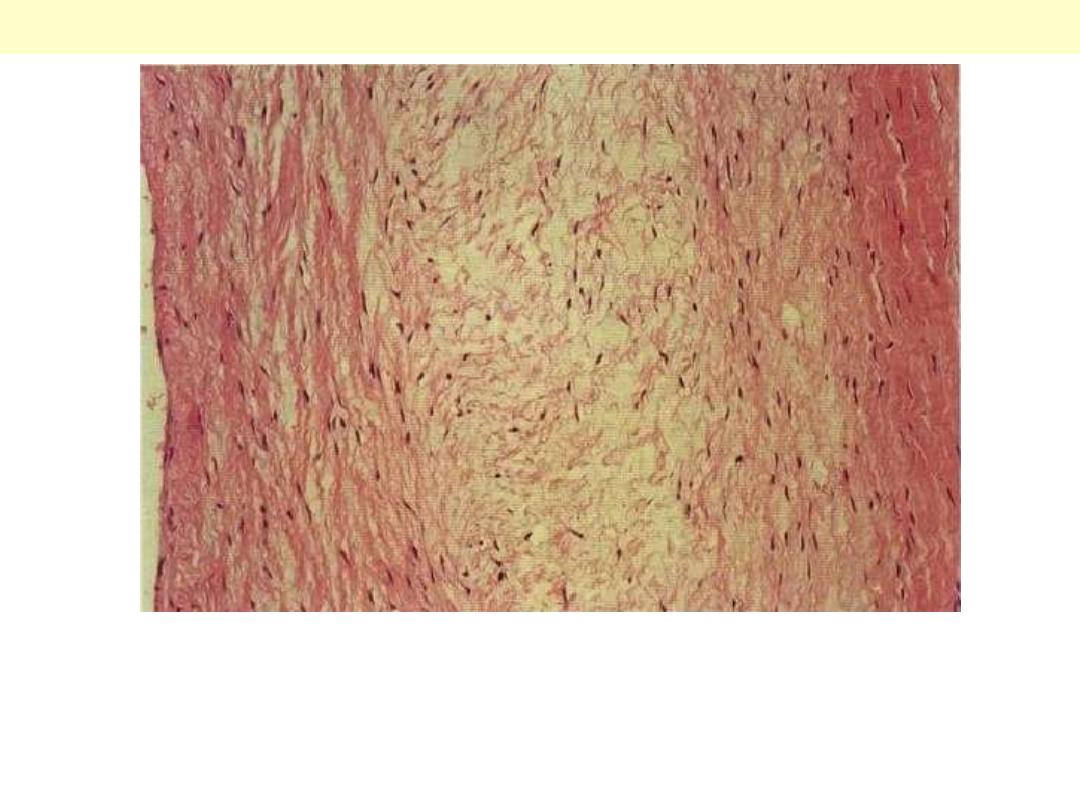
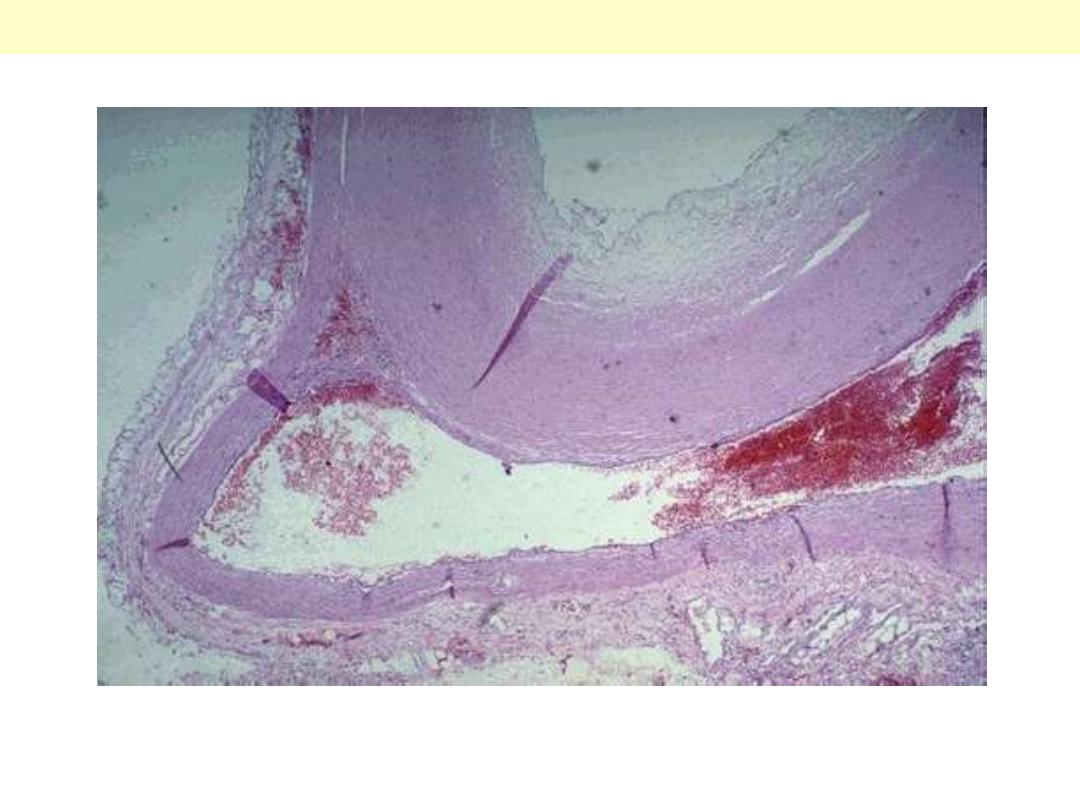
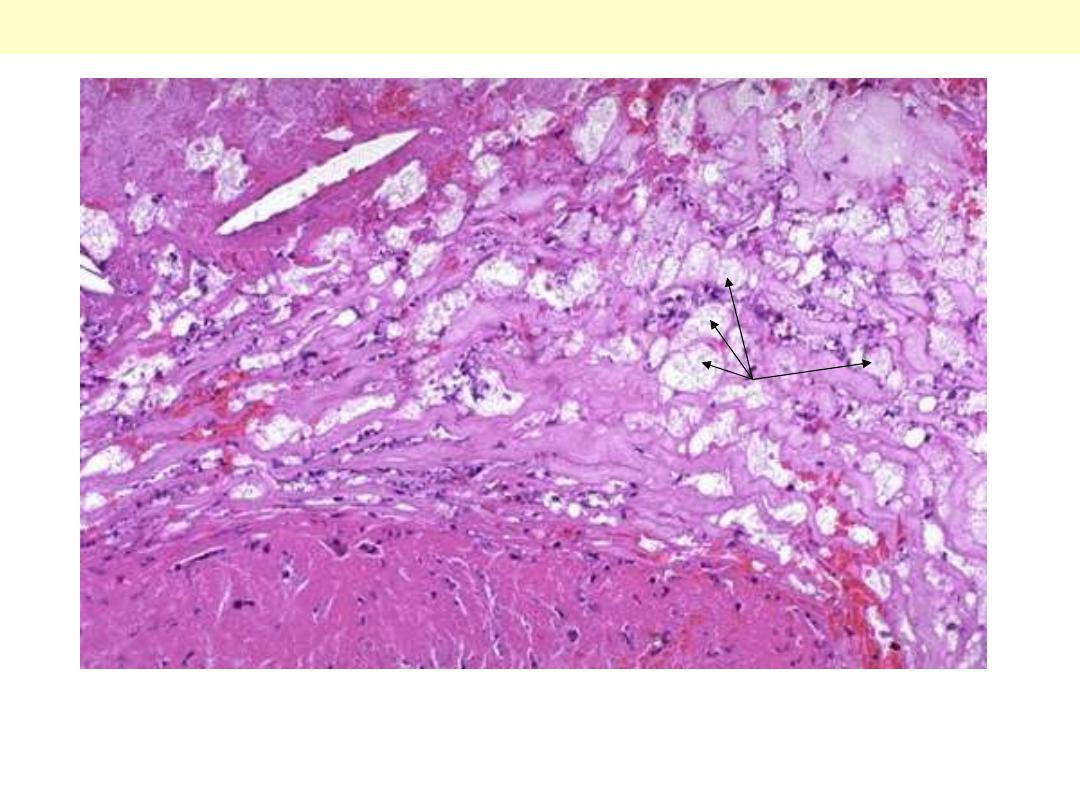
The essential histological change is attenuation of the fibrosa layer of
the valve accompanied by focally marked thickening of the
spongiosa layer with deposition of mucoid (myxomatous) material
(center)
Floppy mitral valve
