
part III

OBJECTIVES:
By the end of these series of lecture on respiratory system pathology the students
would be able to:
1- define the pathogenesis and the pathological features of the most common tumor
like and tumor (benign and malignant) conditions of the upper respiratory tract
including: allergic nasal polyp, angiofibroma, nasopharyngeal carcinoma, laryngeal
tumors including: vocal cord nodule, laryngeal papilloma and laryngeal carcinoma.
2- describe atelectasis of the lung and its classification.
3- define adult respiratory distress syndrome and its causes and pathological features.
4- define (the pathogenesis and the pathological features) and list obstructive
pulmonary disease: asthma, emphysema, chronic bronchitis and broncheactasis.
5- describe the pathogenesis, types and pathological features of diffuse interstitial
lung diseases.
6- define and list the granulomatous lung diseases.
7- identify the pathogenesis and pathological features of pulmonary infections.
8- outline the pathogenesis and the pathological features of pulmonary tuberculosis.
9- list and classify the most important lung tumors describing the pathogenesis and
pathological features, with clinical presentations including paraneoplastic syndrome
descriptions.
10- define diseases of the pluera and list the most common neoplasms of the pleura.


Tuberculosis
Tuberculosis is by far the most important of the chronic penumonia; it causes 6%
of all deaths worldwide. Tuberculosis is
"a communicable chronic granulomatous
disease caused by Mycobacterium tuberculosis".
It usually involves the lungs but
may affect any organ or tissue. Tuberculosis thrives wherever there is poverty,
crowding, and chronic debilitating illness; elderly, with their weakened defenses,
are also susceptible.
Certain disease states also increase the risk:
1. Diabetes mellitus
2. Hodgkin lymphoma
3. Chronic lung disease (particularly silicosis)
4. Chronic renal failure
5. Malnutrition & Alcoholism
6. Immunosuppression including HIV infection.

Most of these predisposing conditions are related to
impairment of T cell-mediated immunity against the
Mycobacteria.
The latter are slender rods that are acid fast, thus stained
positively with ZN stain. M. tuberculosis hominis is responsible
for most cases of tuberculosis.
Oropharyngeal and intestinal tuberculosis contracted by
drinking milk contaminated with Mycobacterium bovis is now
rare in developed nations.
Other mycobacteria, particularly
M. avium-intracellulare,
are
much less virulent than M. tuberculosis and rarely cause
disease. However, it
complicates up to 30% of patients with
AIDS.
Pathogenesis
Primary TB
In the previously unexposed immunocompetent individual
, the source of the
organism is
exogenous
; this leads to the development of cell mediated
immunity; primarily mediated by
T
H
1 cells,
which stimulate macrophages to
kill bacteria but this is associated simultaneously with the development of
destructive tissue hypersensitivity in the form of caseation necrosis.
The virulent organisms once inside macrophages impair effective
phagolysosomal digestion, which in turn leads to
unrestricted mycobacterial
proliferation
. Thus, the
earliest phase of primary tuberculosis is characterized
by bacillary proliferation within alveolar macrophages, with resulting
bacteremia and seeding of multiple sites
.
Nevertheless, most persons at this
stage are asymptomatic; only about 5% of the infected develop significant
disease.
The activated macrophages release a variety of mediators including secretion
of
TNF
, which is responsible for recruitment of monocytes, which in turn
undergo activation and differentiation into the
"epithelioid histiocytes" that
characterize the granulomatous response.
About
3 weeks
are needed for the development of the
hypersensitivity reaction
.

Pathological features of primary TB
The inhaled bacilli are embedded in the distal airspaces of the lower part of
the upper lobe or the upper part of the lower lobe, usually close to the pleura.
As sensitization develops, a
bout1 cm area of gray-white inflammatory
consolidation develops (the Ghon focus). The center of this focus undergoes
caseous necrosis.
Tubercle bacilli, either free or within phagocytes, drain to the regional nodes,
which also often caseate.
This combination of Ghon focus and nodal
involvement is referred to as the Ghon complex
.
During the first few weeks, there is also lymphatic and hematogenous
dissemination to other parts of the body.
In approximately
95%
of cases, development of cell-mediated immunity
controls the infection. Hence,
the Ghon complex undergoes progressive
fibrosis, often followed by radiologically detectable calcification, and, despite
seeding of other organs, no lesions develop.

Microscopically,
there is the characteristic granulomatous
inflammatory reaction that forms both caseating
and noncaseating tubercles.
Individual tubercles are microscopic; it is only
when multiple granulomas coalesce that they
become macroscopically visible.
The granulomas are usually enclosed within a
fibroblastic rim with lymphocytes.
Multinucleate giant cells are present in the
granulomas.
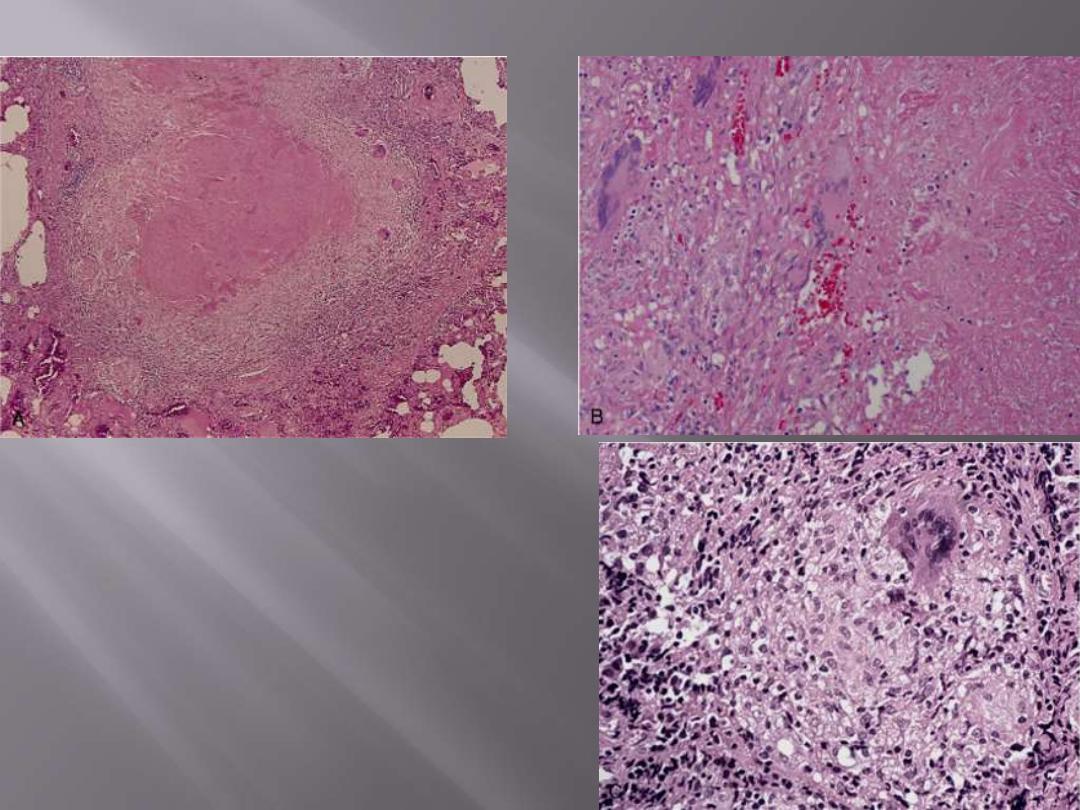
A characteristic tubercle at low magnification (A) and
in detail (B) illustrates central granular caseation
(right) that is surrounded by epithelioid and
multinucleated giant cells (left). This is the usual
response seen in individuals who have developed cell-
mediated immunity to the organism. C, Occasionally,
even in immunocompetent individuals, tubercular
granulomas may not show central caseation; hence,
irrespective of the presence or absence of caseous
necrosis, special stains for acid-fast organisms must
be performed when granulomas are present in
histologic sections.
The morphologic spectrum of tuberculosis
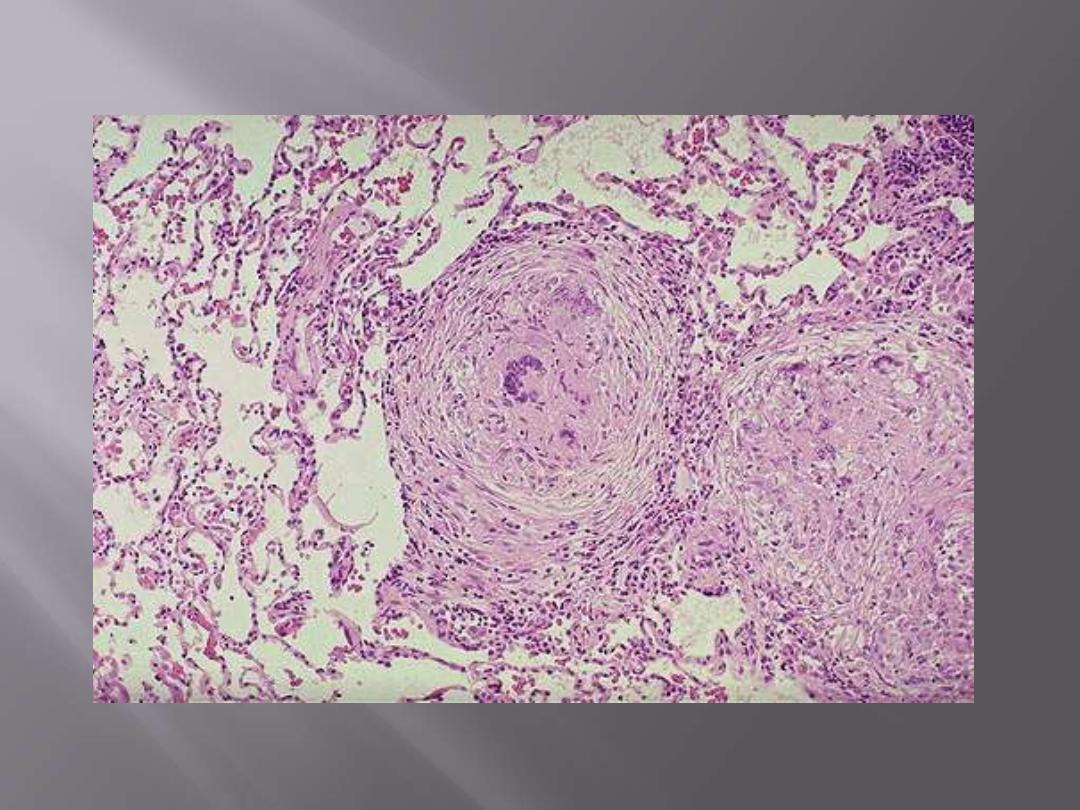
Multiple noncaseating epithelioid granuloma of TB
Note that the granulomas are enclosed within a fibroblastic rim with lymphocytes.
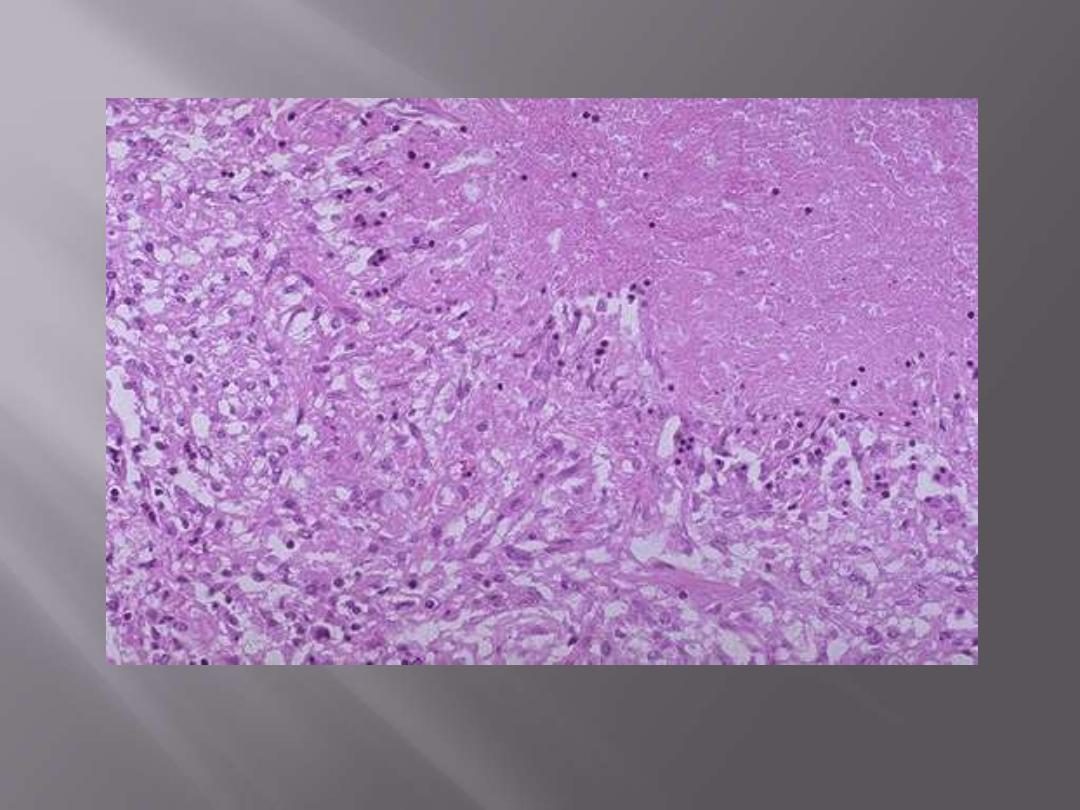
Microscopically, caseous necrosis is characterized by acellular pink areas of necrosis, as seen here at
the upper right, surrounded by a granulomatous inflammatory process.
Caseating epithelioid granuloma of TB
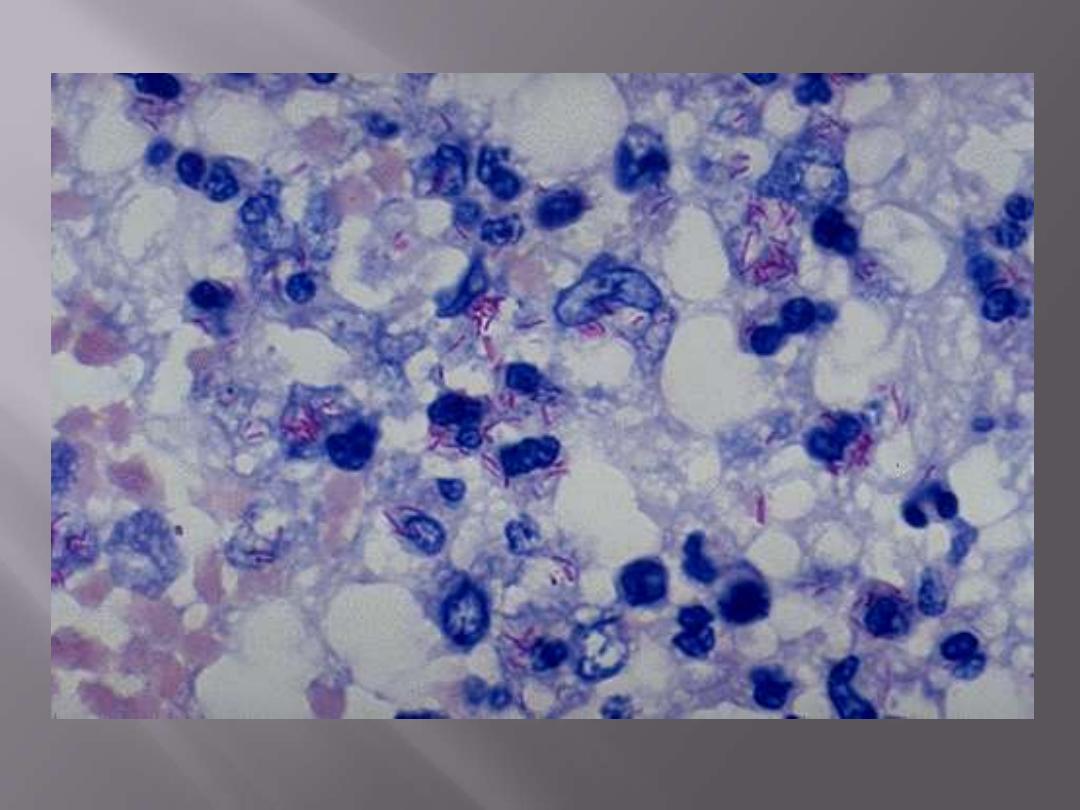
Mycobacterium tuberculosis: acid-fast stain
Note the red rods--hence the terminology acid fast bacilli (AFB)
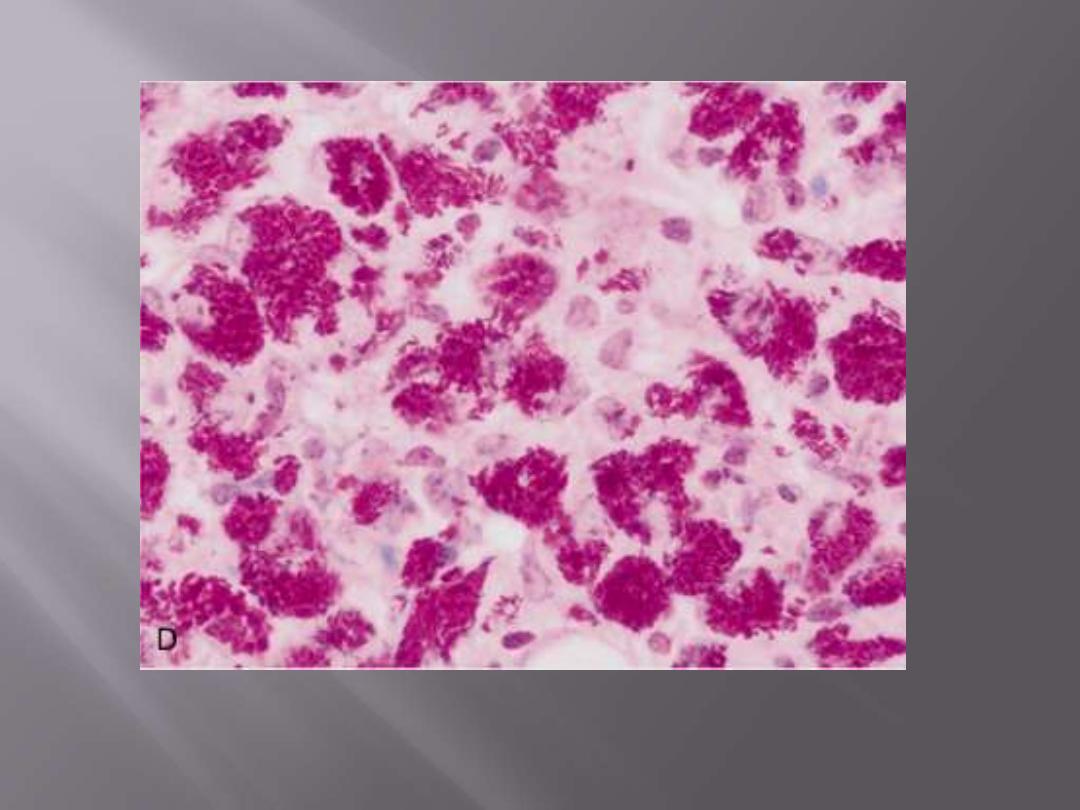
In immunosuppressed individuals, tuberculosis may not elicit a granulomatous response ("nonreactive
tuberculosis"); instead, sheets of foamy histiocytes are seen, packed with mycobacteria that are
demonstrable with acid-fast stains.
Mycobacterium tuberculosis: acid-fast stain
The chief potential harmful outcomes of primary
tuberculosis are
1. Induction of destructive tissue hypersensitivity, which is more damaging on
subsequent infection
(secondary TB)
2. Healed foci of scarring may harbor viable bacilli for years, and thus be a
potential nidus for reactivation
when host defenses are compromised
3. The disease progresses relentlessly into
progressive primary tuberculosis
(uncommon). This occurs in
immunocompromised individuals e.g. AIDS
patients or in those with nonspecific impairment of host defenses
(malnourished children or elderly).
Immunosuppression results in the
absence of a tissue hypersensitivity reactio
n and thus there are no granulomas
but only sheets of foamy histiocytes packed with the bacilli
(nonreactive
tuberculosis).
Progressive primary tuberculosis often resembles acute bacterial pneumonia,
with lower and middle lobe consolidation, hilar lymphadenopathy, and
pleural effusion; cavitation is rare.
Lympho-hematogenous dissemination
may
result in the development of
tuberculous meningitis and miliary tuberculosis.

Secondary Tuberculosis (Postprimary) (Reactivation Tuberculosis)
previously sensitized host.
Pathogenesis
Reactivation of the dormant primary infection (as in nonendemic, low-
prevalence areas) or re-exposure to the bacilli in a previously sensitized host
(as in endemic areas) results in rapid recruitment of defensive reactions but
also tissue necrosis (caseation). This occurs when the protection (immunity)
offered by the primary infection is weakened.
only less than 5% with primary disease subsequently develop secondary
tuberculosis.
Secondary pulmonary tuberculosis is classically localized to the apex of one or
both upper lobes. This may relate to high oxygen tension in the apices
.
Because of the preexistence of hypersensitivity, the bacilli excite a marked
tissue response that tends to wall off the focus.
As a result of this localization,
the regional lymph nodes involvement is less prominent than they are in
primary tuberculosis.
Cavitation occurs readily in the secondary form
, & is almost inevitable in
neglected cases. As a result erosion of airways occurs; this converts the patient
into a source of infection to others; he now raises sputum containing bacilli.

Gross features of secondary TB
The initial lesion is usually a small focus of consolidation,
less than 2 cm in diameter, near the apical pleura. Such
foci are sharply circumscribed, firm, and gray-white to
yellow areas that have a variable amount of central
caseation and peripheral fibrosis. This, if neglected
progresses to cavitations.
Microscopic features
The active lesions show characteristic coalescent tubercles
with central caseation. TB bacilli can be demonstrated by
specific staining methods.
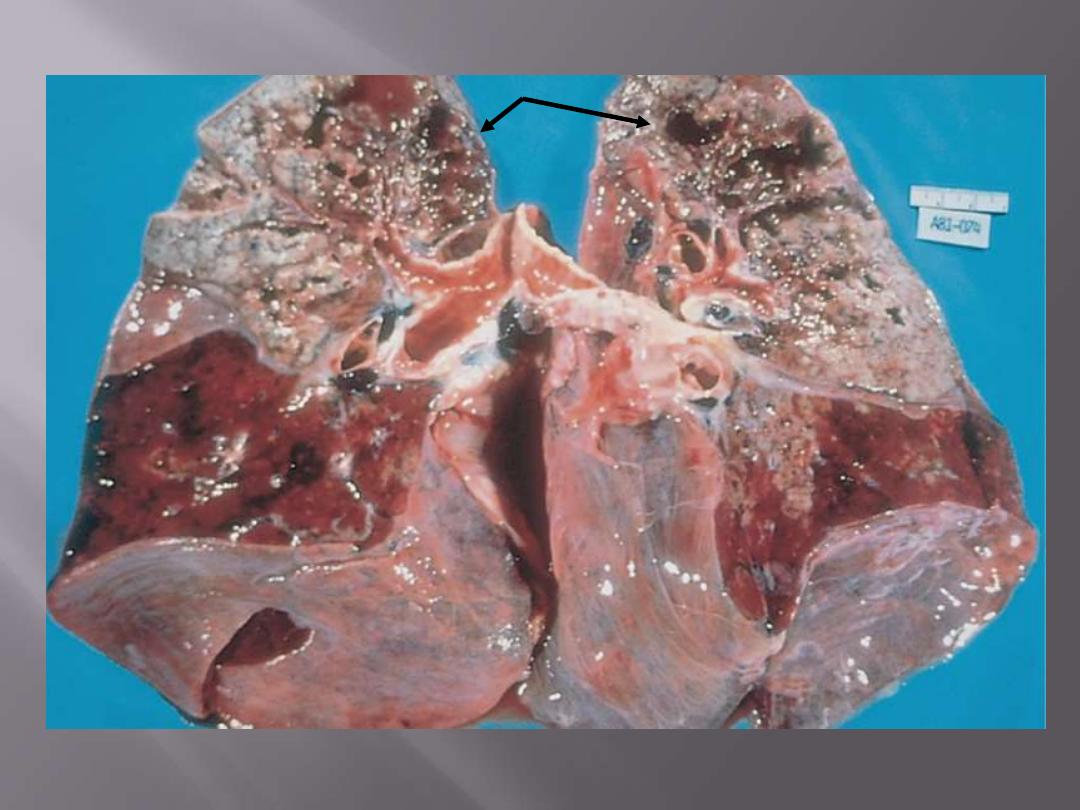
The upper parts of both lungs show multiple areas of softening and cavitation (arrows)
Secondary pulmonary tuberculosis

Progression of secondary TB
In favorable cases, the initial localized apical parenchymal damage undergoes
progressive healing by fibrosis & eventually represented by fibrocalcific scars.
This happy outcome occurs either spontaneously or after therapy.
Alternatively, the disease may progress and extend along several different
pathways:
A. Progressive pulmonary tuberculosis:
the apical lesion enlarges with
expansion of the area of caseation. Erosion into a bronchus evacuates the
caseous center, creating a ragged, irregular cavity lined by caseous material ;
whereas erosion of blood vessels results in hemoptysis. The pleural cavity is
always involved and serous pleural effusions, tuberculous empyema, or fibrous
obliteration may develop.
B. Miliary pulmonary disease
occurs when organisms drain through lymphatics
into the lymphatic ducts, which empty into the venous return to the right side
of the heart and thence into the pulmonary arteries. Individual lesions are
either microscopic or small (2-mm) foci of yellow-white; these scatter diffusely
through the lungs (miliary is derived from the resemblance of these foci to millet
seeds).
Miliary lesions may expand and coalesce to yield almost total
consolidation of large regions or even whole lobes of the lung.
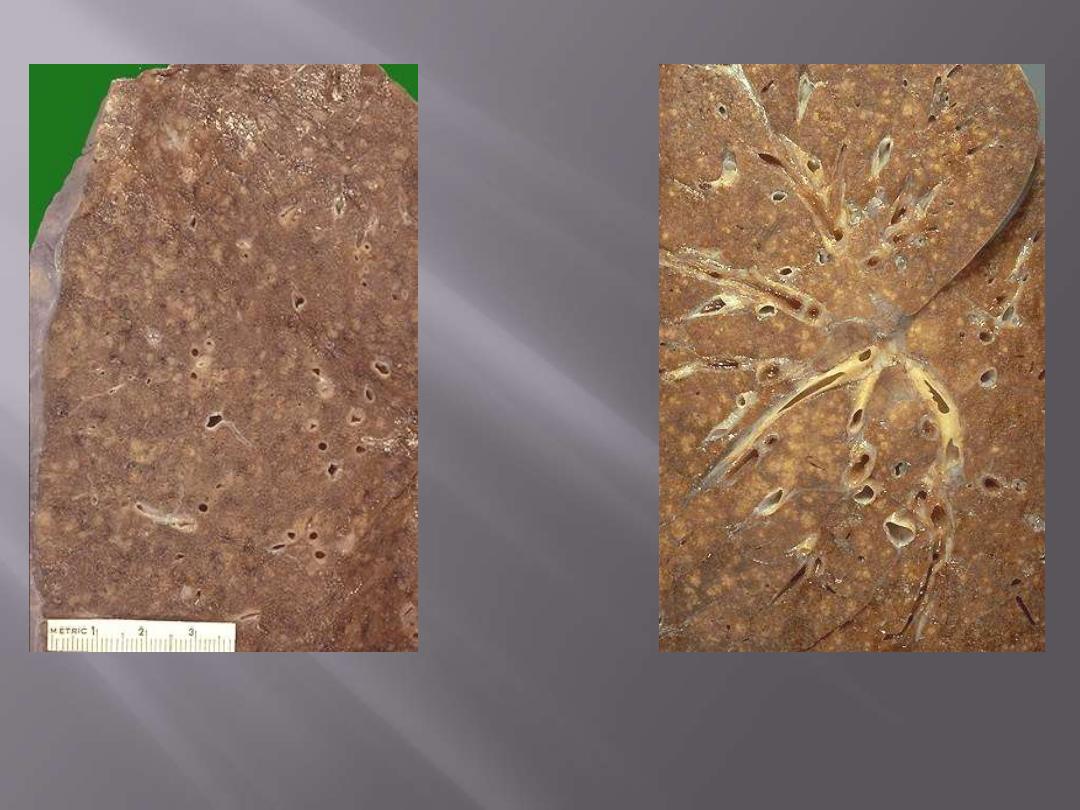
Lt. multitude of small (2 to 4 mm), yellow-white foci ; these scatter diffusely through the lungs. The
miliary pattern gets its name from the resemblance of the granulomas to millet seeds.
Rt. A zoom in appearance; the miliary pattern is seen throughout the lung.
Miliary pulmonary TB

C. Endobronchial, endotracheal,
and laryngeal tuberculosis may develop
when infective material is spread either through lymphatic channels or from
expectorated infectious material. The mucosal lining may be studded with
minute granulomatous lesions.
D. Systemic miliary tuberculosis
occurs when infective foci in the lungs
invade the pulmonary venous return to the heart; the organisms
subsequently disseminate through the systemic arterial system. Almost every
organ in the body may be seeded. The appearances are similar to miliary
pulmonary disease. Miliary tuberculosis is most prominent in the liver, bone
marrow, spleen , adrenals, meninges, kidneys, fallopian tubes, and
epididymis.
E. Isolated-organ tuberculosis
may appear in any one of the organs or tissues
seeded
hematogenously
and may be the presenting manifestation of
tuberculosis. Organs typically involved include the
meninges (tuberculous
meningitis), kidneys (renal tuberculosis), adrenals (formerly an important
cause of Addison disease), bones (tuberculous osteomyelitis), and fallopian
tubes (tuberculous salpingitis).
When the vertebrae are affected, the disease
is referred to as
Pott disease
. Paraspinal "cold" abscesses in persons with
this disorder may track along the tissue planes to present as an abdominal
or pelvic mass.
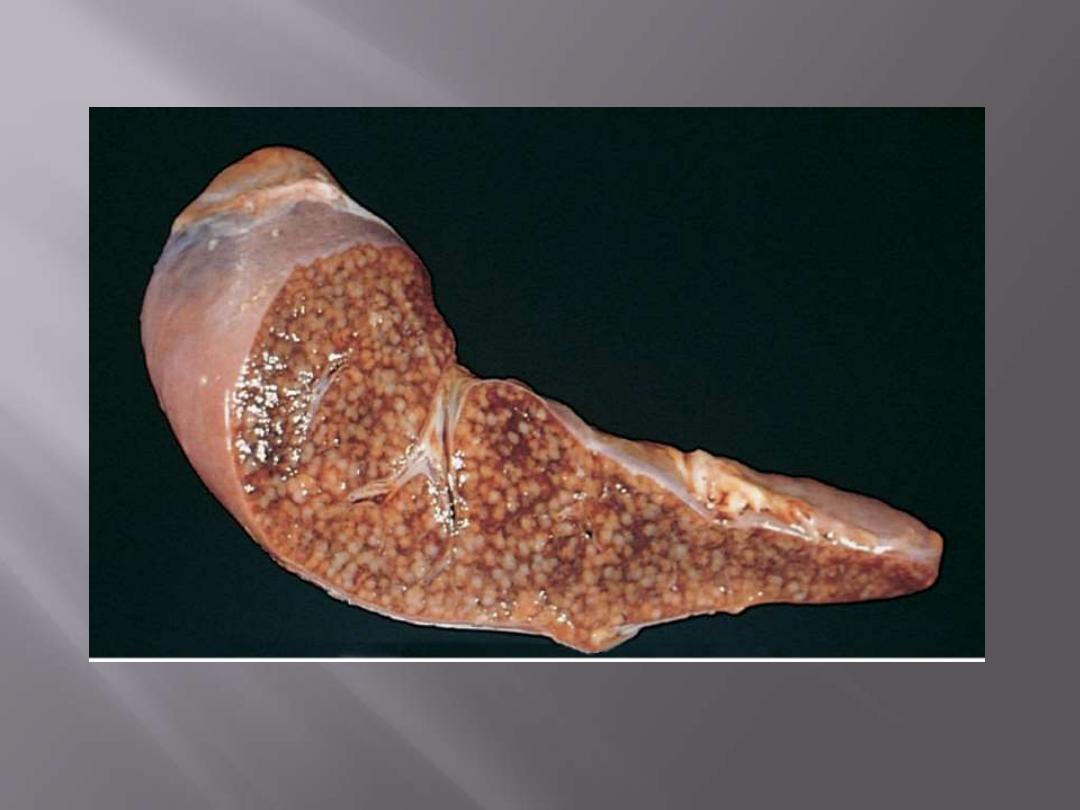
The cut surface shows numerous gray-white granulomas.
Miliary tuberculosis of the spleen

F. Tuberculous Lymphadenitis
is the most frequent form of
extrapulmonary tuberculosis, usually occurring in the cervical
region ("scrofula"). It tends to be unifocal, and most individuals
do not have evidence of ongoing extranodal disease.
H. Intestinal tuberculosis
was fairly common as a primary focus
of tuberculosis contracted by the drinking of contaminated milk.
In developed countries today, intestinal tuberculosis is more
often a
complication of protracted advanced secondary
tuberculosis,
secondary to the swallowing of coughed-up
infective material. Typically, the organisms are trapped in
mucosal lymphoid aggregates of the small and large bowel,
which then undergo inflammatory enlargement with ulceration
of the overlying mucosa,
particularly in the ileum.

The diagnosis of pulmonary disease
is based in part on the
history and on physical and radiographic findings of consolidation or
cavitation in the apices of the lungs. However,
tubercle bacilli must be
identified to establish the diagnosis.
The most common method for diagnosis of tuberculosis remains
demonstration of acid-fast organisms in sputum by special stains e.g. acid-
fast stain
; most protocols require at least two sputum examinations before
labeling the case as sputum negative.
Conventional cultures for mycobacteria require up to 10 weeks.
PCR amplification
of M. tuberculosis DNA allows for even greater rapidity
of diagnosis and is currently approved for use. PCR assays can detect as
few as 10 organisms in clinical specimens, compared with greater than
10,000 organisms required for smear positivity. However, culture remains
the gold standard because it also allows testing of drug susceptibility.
Prognosis
is generally favorable if infections are localized to the lungs,
but it worsens significantly when the disease occurs in the setting of
aged,
debilitated, or immunosuppressed persons, who are at high risk for
developing miliary tuberculosis, and in those with multi-drug resistant-TB.
Amyloidosis may appear in persistent cases.

Nontuberculous Mycobacterial Disease
is mostly in the form of chronic but clinically localized
pulmonary disease in immunocompetent individuals.
Strains implicated most frequently include M. avium-
intracellulare.
Nontuberculous mycobacteriosis may present as upper
lobe cavitary disease, mimicking tuberculosis, especially in
individuals with a long-standing history of smoking or
alcoholism.
The presence of concomitant chronic pulmonary disease
e.g. COPD, is an important risk factor.
In immunosuppressed individuals (primarily, HIV-positive
patients), M. avium-intracellulare presents as disseminated
disease, associated with systemic symptoms.

LUNG TUMORS
Bronchial carcinomas
constitute
95%
of primary lung
tumors; the remaining
5%
includes
bronchial carcinoids,
sarcomas, lymphomas
, and a few benign lesions.
Pulmonary Hamartoma
is the most common benign lesion;
it is rounded
small (3-4 cm)
discrete mass that often displayed as "coin" lesion on chest
radiographs.
They consist mainly of mature cartilage with a scattered of
bronchial glands that are often admixed with fat, fibrous
tissue, and blood vessels in varying proportions.
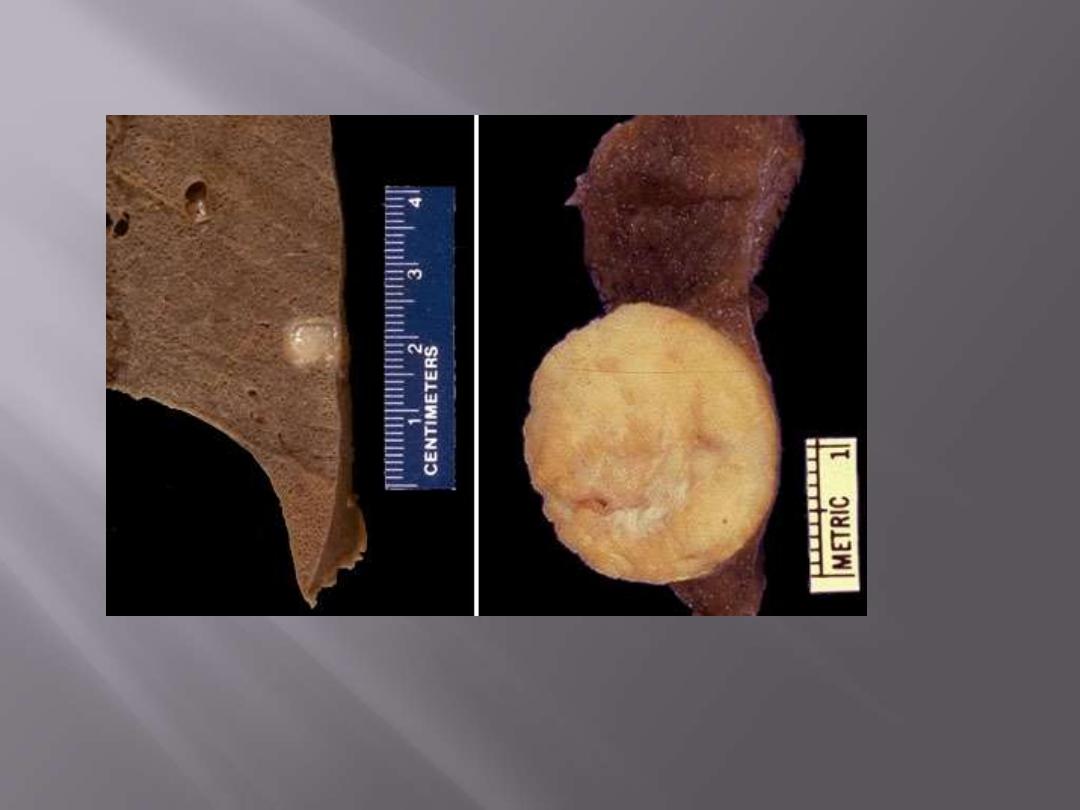
Here are two examples of a benign lung neoplasm known as a pulmonary hamartoma. These
uncommon lesions appear on chest radiograph as a "coin lesion" that has a differential diagnosis of
granuloma and localized malignant neoplasm. They are firm and discreet and often have calcifications
in them that also appear on radiography. Most are small (less than 2 cm).
Pulmonary hamartoma
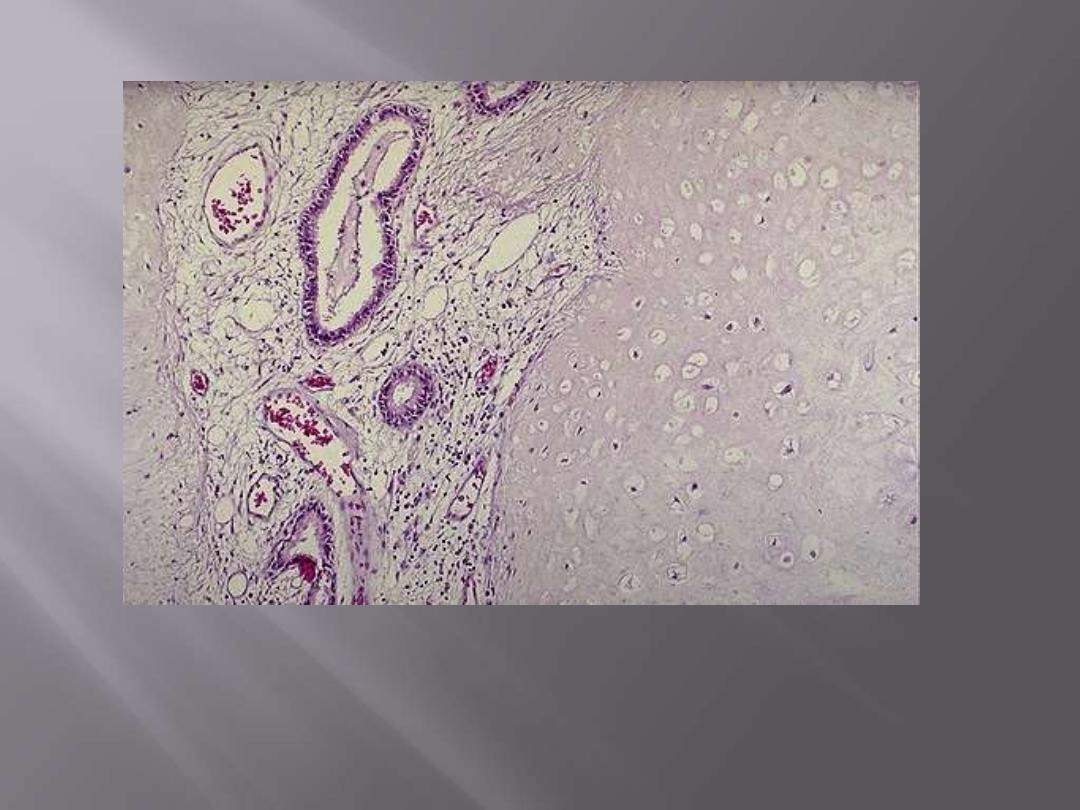
The pulmonary hamartoma is seen microscopically to be composed mostly of benign cartilage
on the right that is jumbled with a fibrovascular stroma and scattered bronchial glands on the
left. A hamartoma is a neoplasm in an organ that is composed of tissue elements normally found
at that site, but growing in a haphazard mass.
Pulmonary hamartoma

Carcinomas
Carcinoma of the lung is the commonest cause of cancer-
related deaths in industrialized countries.
The rate of increase among males is slowing down, but it
continues to accelerate among females; it has overrun breast
cancer as a cause of death since 1987.
This is undoubtedly related to the strong relationship of
cigarette smoking and lung cancer.
Most patients are in the age group of
50-60 years.
The prognosis of lung cancer is very poor: the 5-year survival
rate for all stages combined is about 15%.

Squamous cell carcinoma ( 25-40%).
Adenocarcinoma (25-40%)
Small cell carcinoma (20-25%)
Large cell carcinoma (10-15%)

There are four major histologic types of lung carcinomas
1. Squamous cell carcinoma
2. Adenocarcinoma
3. Small-cell carcinoma
4. Large-cell carcinoma.
Adenocarcinomas are the most common primary tumors arising in women,
in lifetime nonsmokers, and in persons younger than 45 years.
For therapeutic purposes, carcinomas of the lung are divided into two groups:
small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC).
The
latter category includes
squamous cell, adenocarcinomas, and large-cell
carcinomas.
The reason for this division is that virtually
all SCLCs have metastasized by
the time of diagnosis
and hence are
not curable by surgery
. Therefore, they
are best treated by
chemotherapy, with or without radiation.
NSCLCs usually respond poorly to chemotherapy and are better treated by
surgery.
These two groups show genetic differences.
SCLCs are characterized by a high frequency of
RB gene mutations
, while
the
p16 gene
is commonly inactivated in NSCLCs.

Etiology and Pathogenesis
It seems that large areas of the respiratory mucosa have undergone
mutation after exposure to carcinogens
("field effect").
On this fertile soil, those cells that accumulate additional mutations
ultimately develop into cancer.
The role of Cigarette smoking
is the main agent responsible for the genetic changes that give rise to lung
cancers.
About
90%
of lung cancers occur in active smokers (or those who stopped
recently).
The increased risk is
60 times
greater among habitual heavy smokers (two
packs a day for 20 years) compared with nonsmokers.
Women have a higher susceptibility to carcinogens in tobacco than men.
Although cessation of smoking decreases the risk of developing lung cancer
over time, genetic changes that predate lung cancer can persist for many
years in the bronchial epithelium of ex-smokers.

Passive smoking
(proximity to cigarette smokers)
increases the risk to twice that of nonsmokers.
There is a linear correlation between the intensity
of smoking and the appearance of squamous
metaplasia that progresses to squamous dysplasia
and carcinoma in situ,
before culminating in
invasive cancer.
Squamous and small-cell carcinomas show the
strongest association with tobacco exposure.

The role of occupation-related environmental agents;
These may act
alone
or
synergistically
with smoking to be
pathogenitically related to some lung cancers, for e.g.
radioactive ores;
dusts containing arsenic, chromium, uranium, nickel, vinyl chloride, and
mustard gas.
Exposure to
asbestos increases the risk of lung cancer 5 times in
nonsmokers.
However, heavy smoking with asbestos exposure increases
the risk to
50 times.
The role of hereditary (genetic) factors
:
not all persons exposed to tobacco smoke develop cancer.
It seems that the effect of carcinogens is modulated by hereditary
(genetic) factors.
Many procarcinogens require metabolic activation via the
P-450 enzyme
system for conversion into carcinogens.
Evidences support this scenario in that persons with specific genetic
abnormalities of P-450 genes have an increased capacity to metabolize
procarcinogens derived from cigarette smoke and thus sustain the
greatest risk of developing lung cancer.
The role of bronchioalveolar stem cells (BASCs) in
the development of peripheral adenocarcinoma:
the
"cell of origin"
for peripheral adenocarcinomas is the
BASCs.
Following peripheral lung injury, the multipotent BASCs
undergo expansion to replenish the normal cell types
found in this location, thereby facilitating epithelial
regeneration.
BASCs are considered the tumor initiating cells i.e. the
first to acquire the somatic
K-RAS mutation
that enables
their daughter cells to
escape normal "checkpoint"
mechanisms and result in pulmonary adenocarcinomas.

Gross Features
Carcinomas of the lung begin as small mucosal lesions that are usually firm
and gray-white. Further enlargement result in either intraluminal masses,
or invasion of the bronchial wall to form large bulky masses pushing into
adjacent lung parenchyma.
Obstruction of the bronchial lumen often
produces distal atelectasis and infection.
Some tumors tend to a
rise centrally near the hilum
i.e. in major brochi,
these are exemplified by
squamous cell & small cell carcinomas
.
Adenocarcinomas
may occur
centrally but are usually more peripherally
located,
many arising in relation to peripheral lung scars
("scar
carcinomas").
Large tumors may undergo cavitation; this is caused by central necrosis.
These tumors may extend to the pleura, invade the pleural cavity and chest
wall, and spread to adjacent intrathoracic structures.
Metastasis occurs first to
peribronchial, hilar and mediastinal lymph nodes
More distant spread can occur via the
lymphatics or the hematogenous
route.
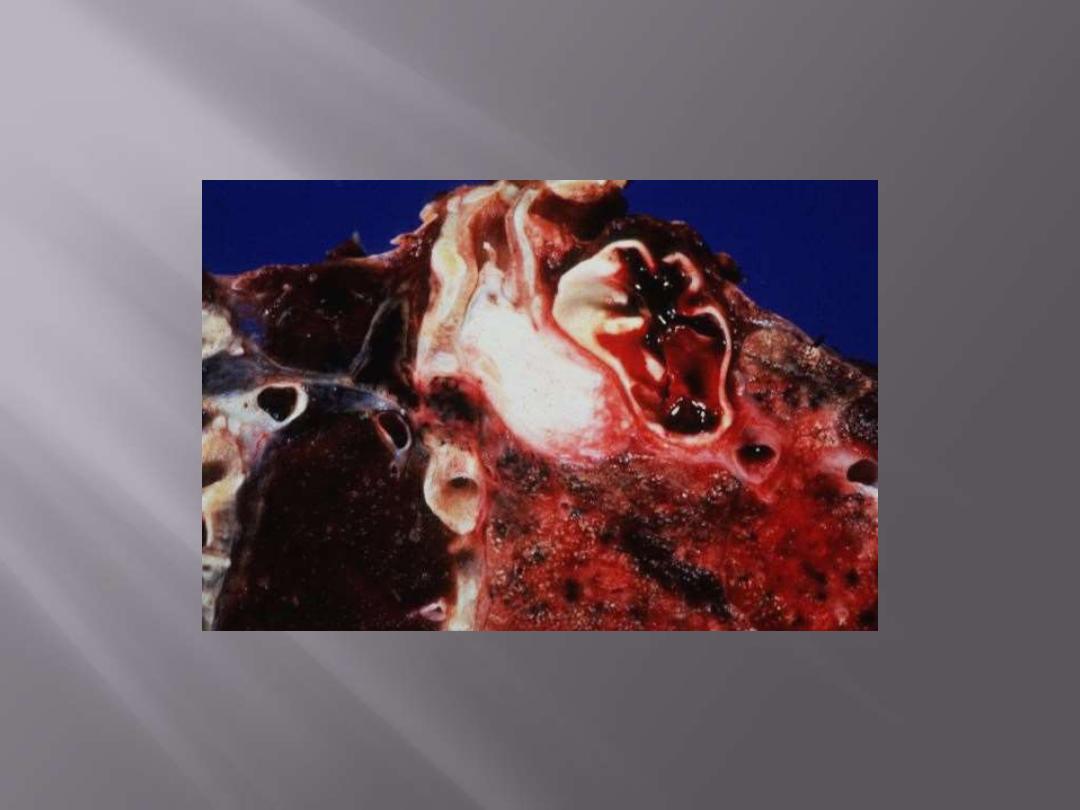
Squamous cell carcinoma: Note whitish endobronchial obstructive mass.
Early bronchial carcinoma
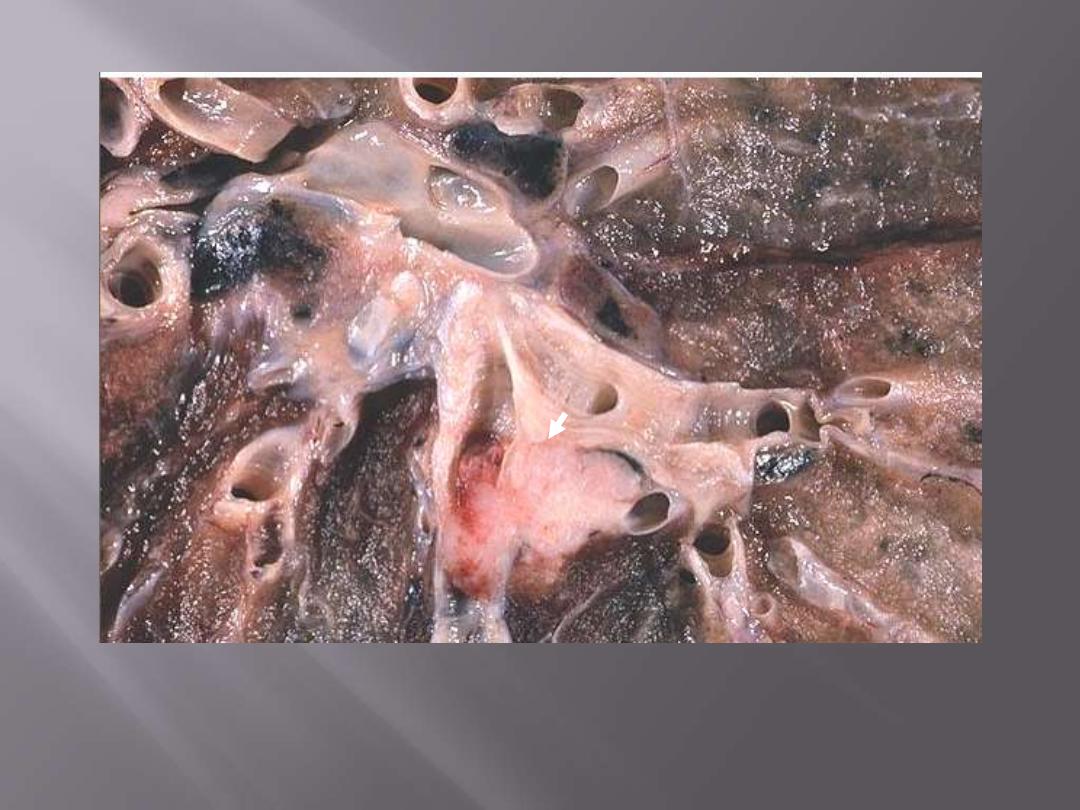
This is a fairly small carcinoma which has arisen in a bronchus blue (arrow), and then invaded into
surrounding lung tissue; tumor obstructing bronchus
Early bronchial carcinoma
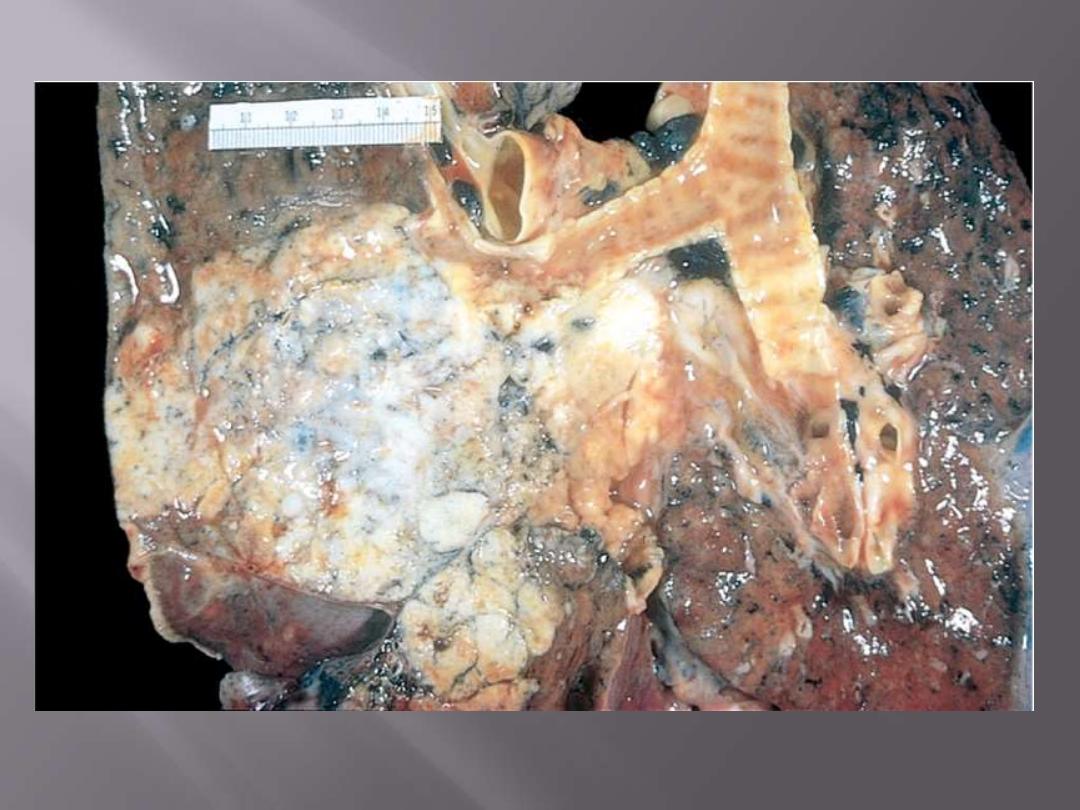
The tumor usually begin as central (hilar) masses and grow contiguously into the peripheral
parenchyma and adjacent pleura
Squamous cell carcinomas
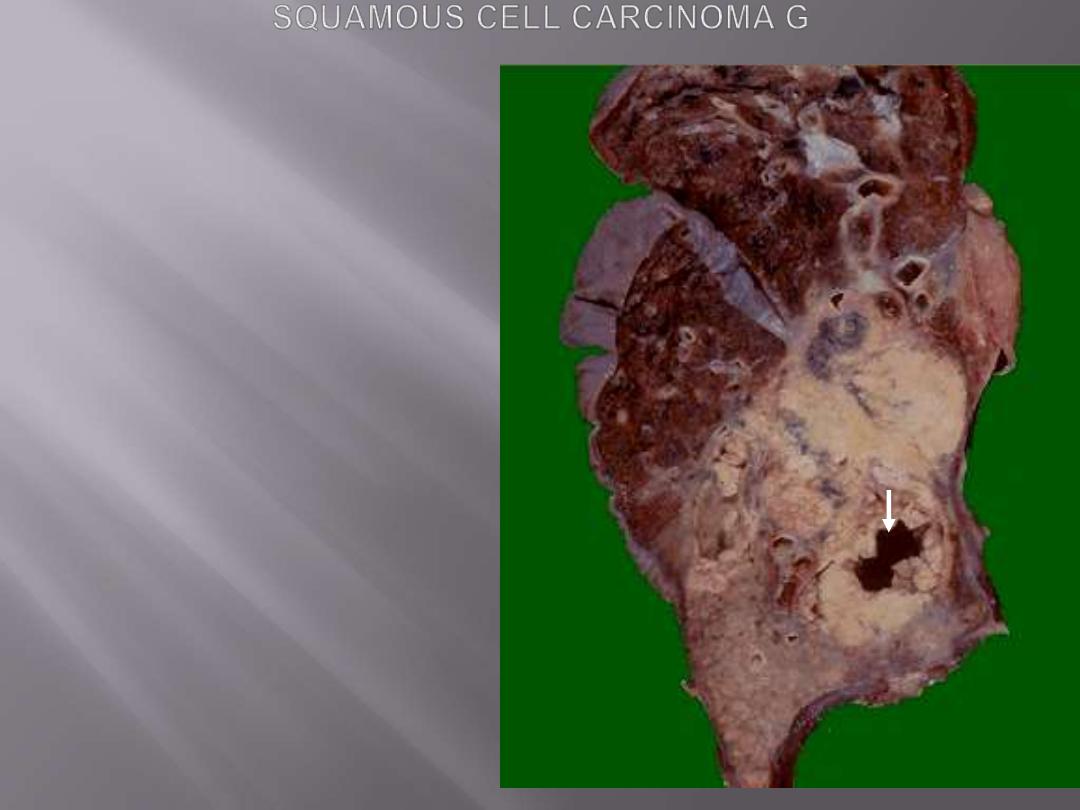
This is a large squamous cell carcinoma in
which a portion of the tumor demonstrates
central cavitation (arrow), probably because
the tumor outgrew its blood supply.
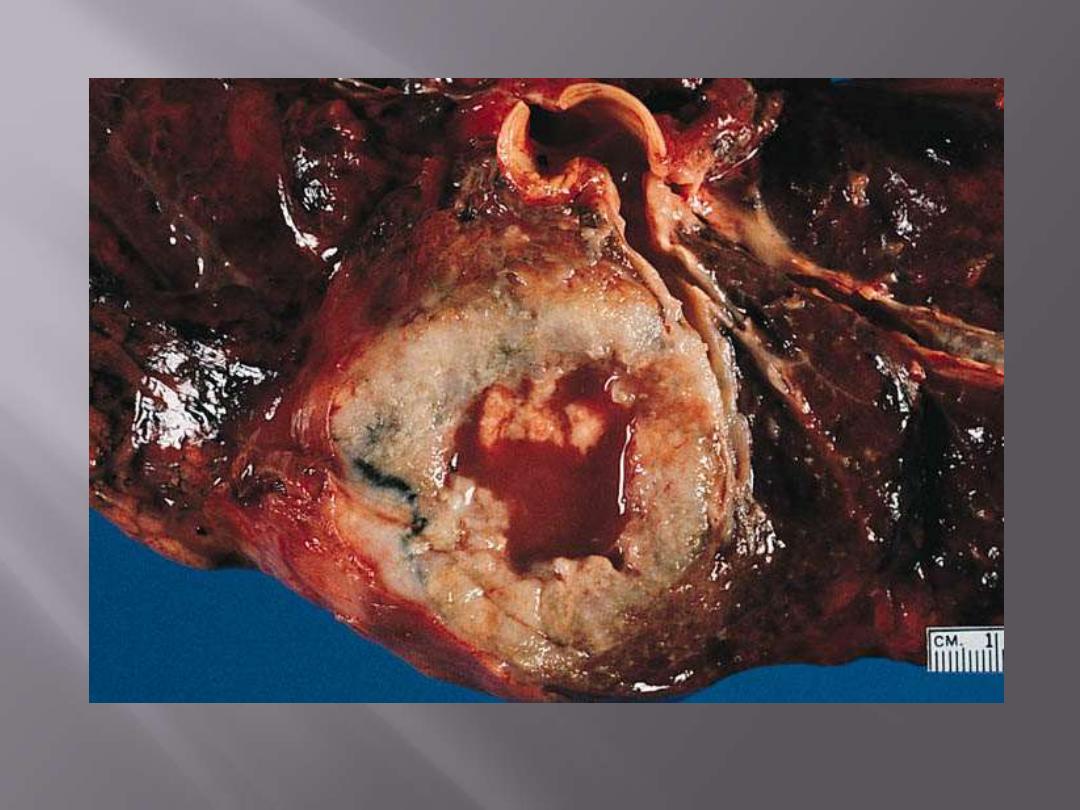
Squamous cell carcinoma showing large central area of necrosis and cystic change.
Squamous cell carcinoma
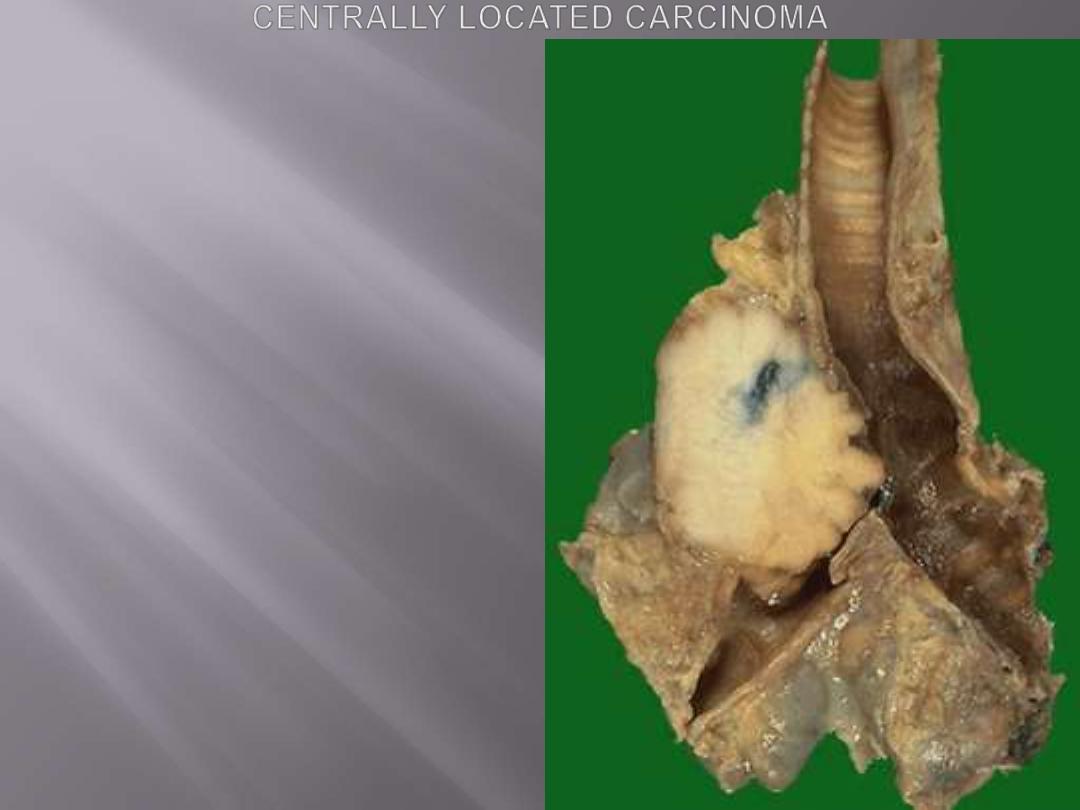
This pale white tumor is obstructing the right main
bronchus. a squamous cell carcinoma of the lung that is
arising centrally in the lung (as most squamous cell
carcinomas do). Microscopy shows squamous cell
carcinoma.

Microscopic features
Squamous cell carcinomas
are often preceded for years by
squamous metaplasia
or dysplasia in the bronchial epithelium
, which then
transforms to
carcinoma in situ,
a phase that may last
for several years.
These tumors range from
well-differentiated showing
keratin pearls and intercellular bridges to poorly
differentiated neoplasms having only minimal
residual squamous cell features.
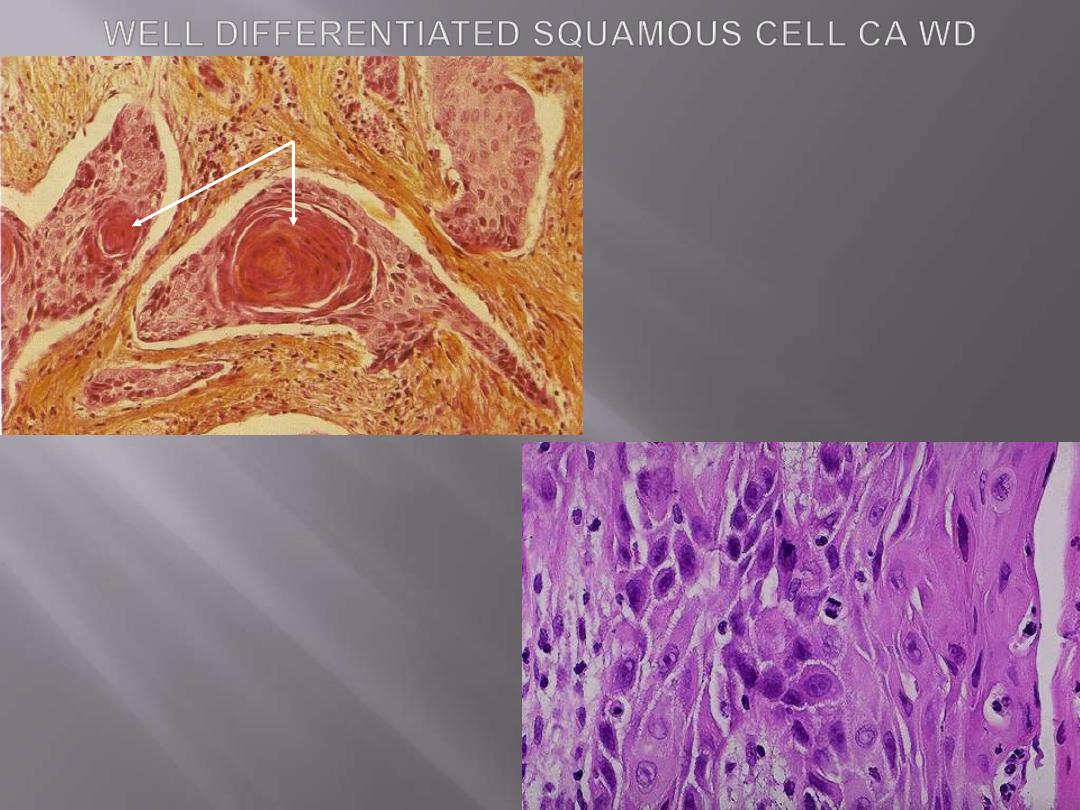
Above: nests of squamous cells with keratin
production (arrow).
Below: the pink cytoplasm with distinct cell
borders and intercellular bridges
characteristic for a squamous cell
carcinoma are seen here at high
magnification. Such features are seen in
well-differentiated tumors (those that more
closely mimic the cell of origin).

Adenocarcinomas
assume a variety of forms, including
gland
forming (acinar), papillary, and solid types.
The solid variant often requires demonstration
of
intracellular mucin
production by special
stains to establish its adenocarcinomatous
nature.
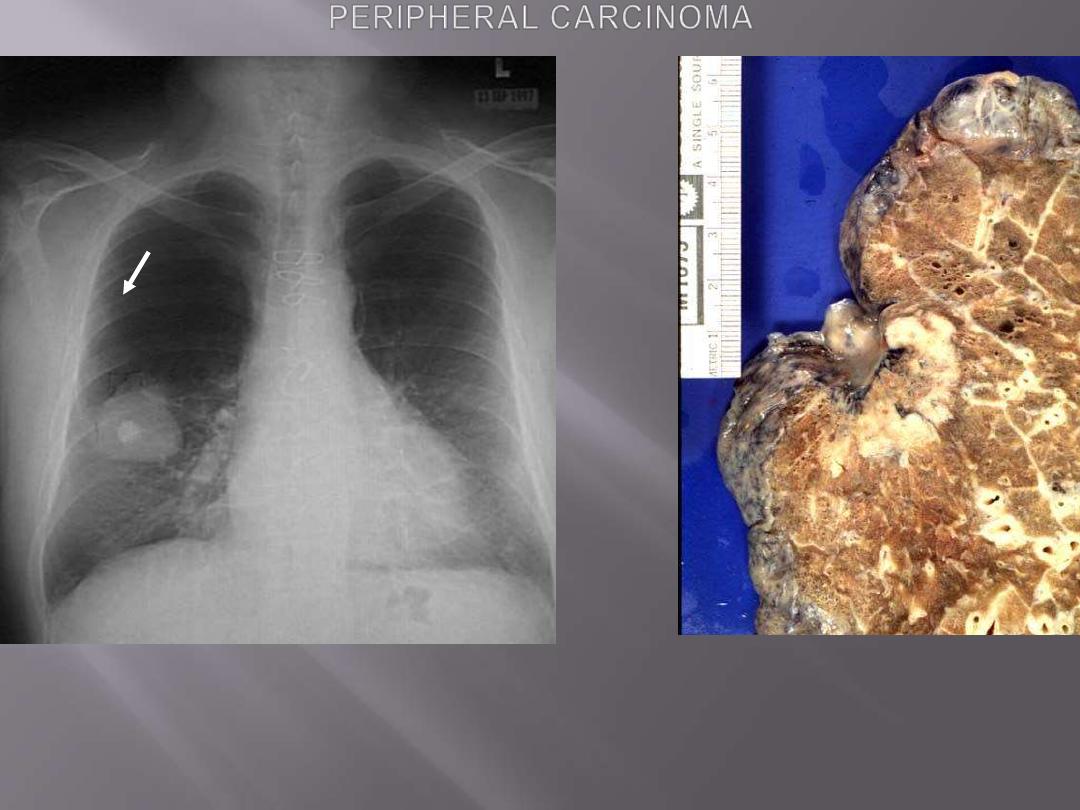
Rt. This chest radiograph demonstrates a large 5 cm diameter adenocarcinoma of the right lower lobe.
The bright opacity in the middle of the mass is a calcified granuloma.
Lt. Adenocarcinoma: Peripheral mass located immediately under visceral pleura, with pleural
retraction.
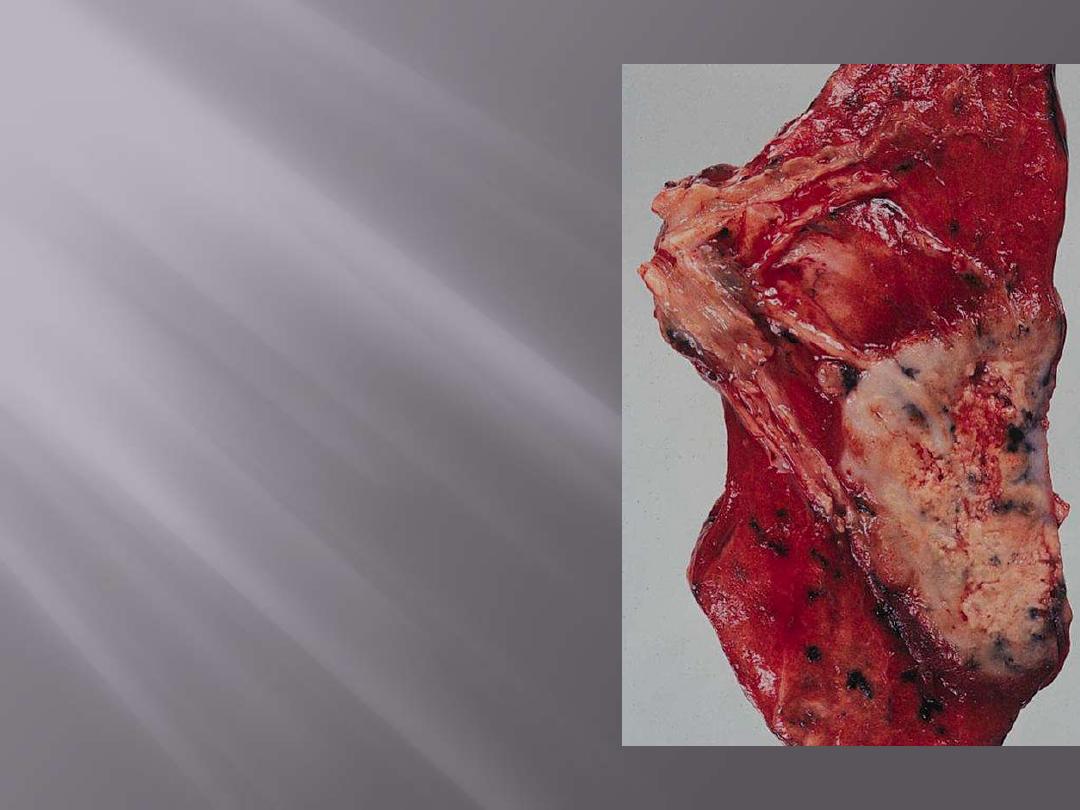
Adenocarcinoma. The tumor is peripherally located and
has extended to the pleura.
Peripheral carcinoma
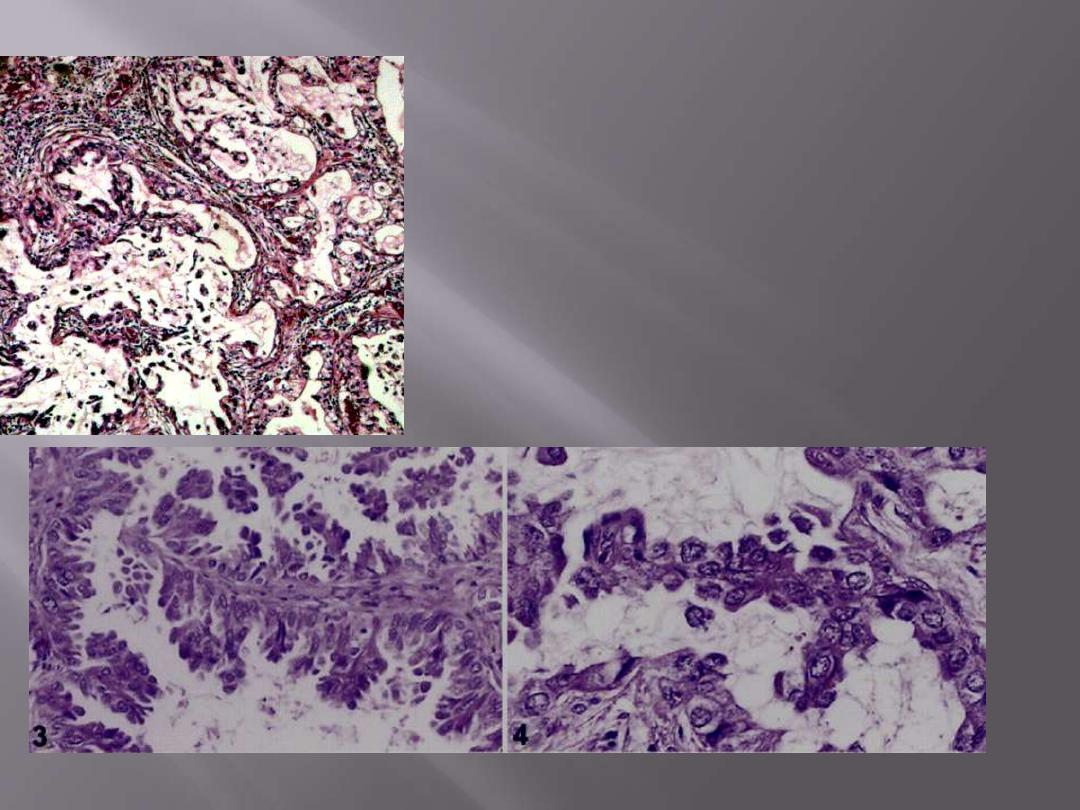
Malignant epithelial cells forming glandular
structures with mucin secretion.
Pulmonary adenocarcinoma
papillary and micropapillary structures

Bronchioloalveolar carcinomas (BACs)
are a subtype of adenocarcinomas.
They involve
peripheral parts of the lung, either as a
single nodule or, more often, as multiple diffuse
nodules that may coalesce to produce pneumonia-
like consolidation.
The key feature of BACs is their growth
along
preexisting structures and preservation of alveolar
architecture.
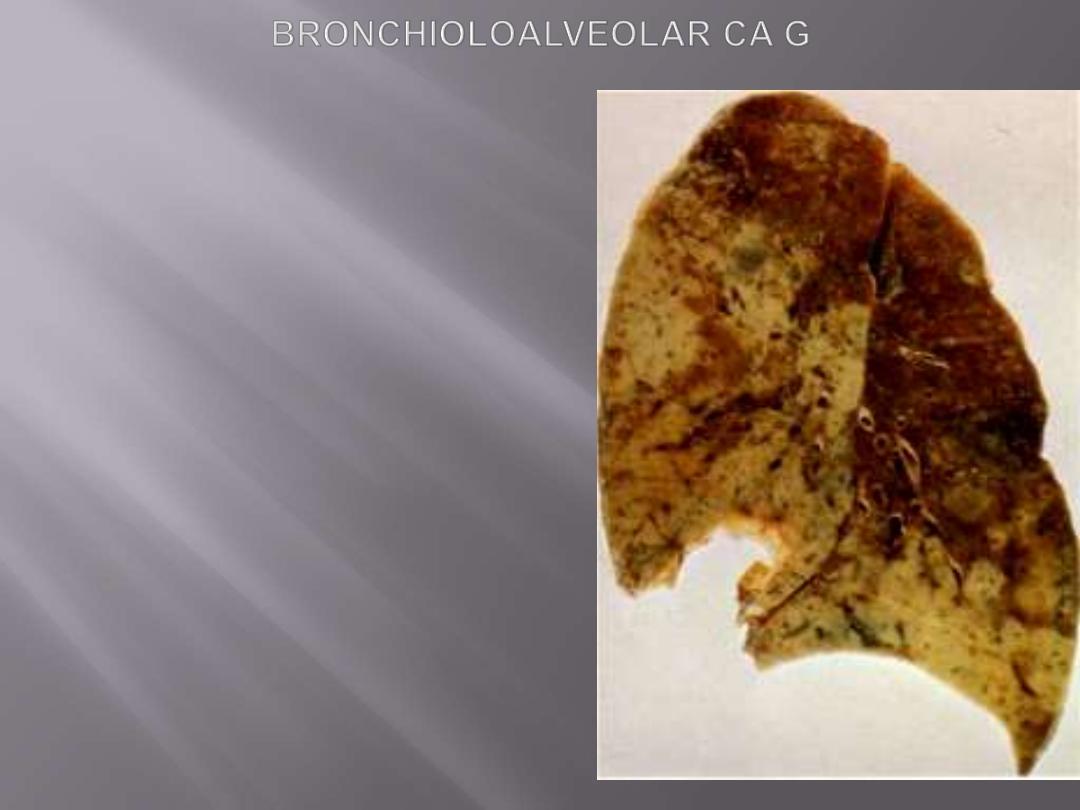
Greyish-white tumor involves 2/3 of left upper lobe and
1/3 of the lower lobe. The color and distribution suggest
pneumonic consolidation. Secondary tumors can
peoduce the same appearance.
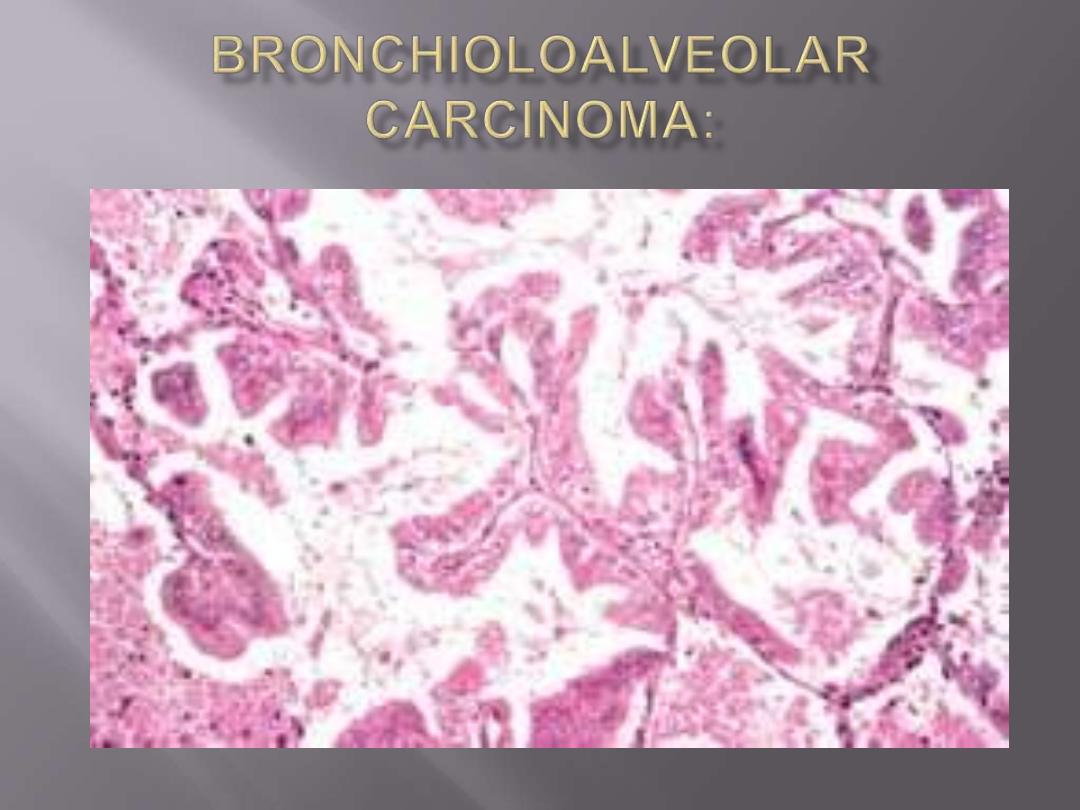
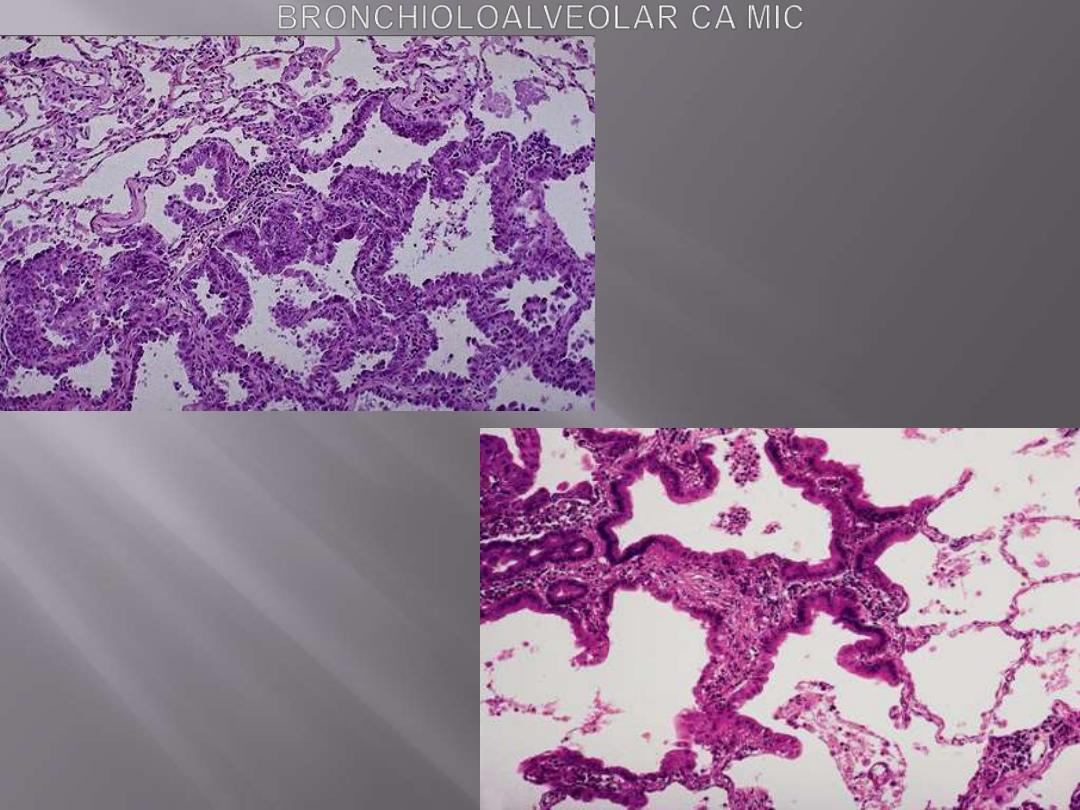
The tumor is composed of columnar cells
that proliferate along the framework of
alveolar septae. The cells are well-
differentiated.

Large-cell carcinomas
undifferentiated malignant epithelial tumors that lack
the cytologic features of glandular or squamous
differentiation
.
The cells have large nuclei, prominent nucleoli.
Large-cell
carcinomas
probably
represent
undifferentiated examples of squamous cell or
adenocarcinomas at the light microscopic level.
Ultrastructurally, however, minimal glandular or
squamous differentiation is common.
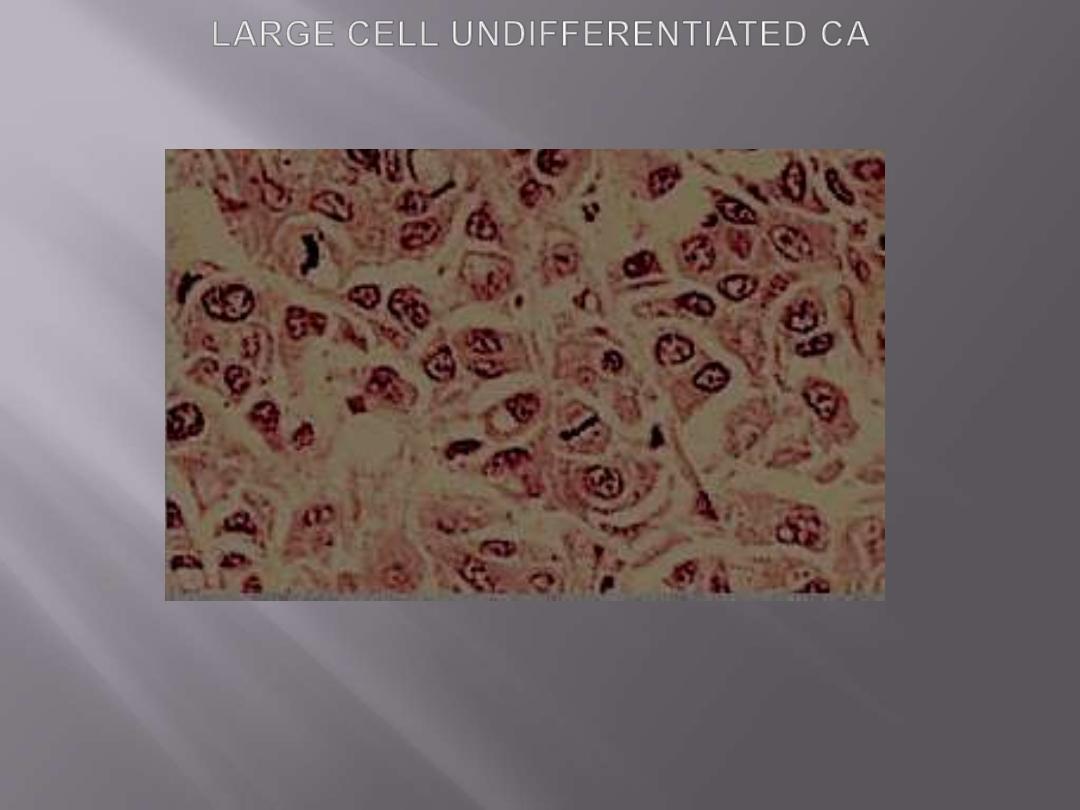
Undifferentiated large cell carcinoma. Tumor is formed by large cells growing in solid nests without
evidence of glandular or squamous differentiation.

Small-cell lung carcinomas
are composed of small tumor cells with a round to
fusiform nuclei with finely granular chromatin, and
scant cytoplasm.
Mitotic
figures are frequently seen.
Despite the term of
"small,"
the neoplastic cells are
usually
twice the size of resting lymphocytes.
Necrosis
is invariably present and may be extensive.
These tumors are derived from
neuroendocrine cells
of the lung, and hence they express a variety of
neuroendocrine markers in addition to many
polypeptide
hormones
that
may
result
in
paraneoplastic syndromes.
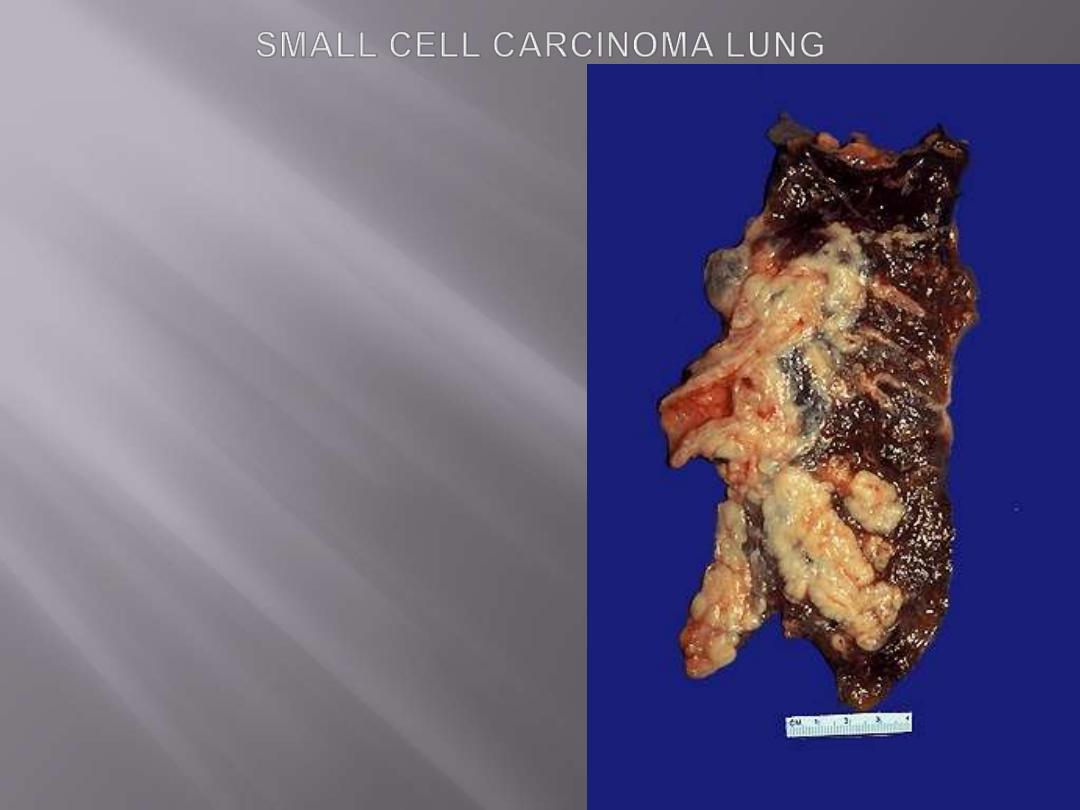
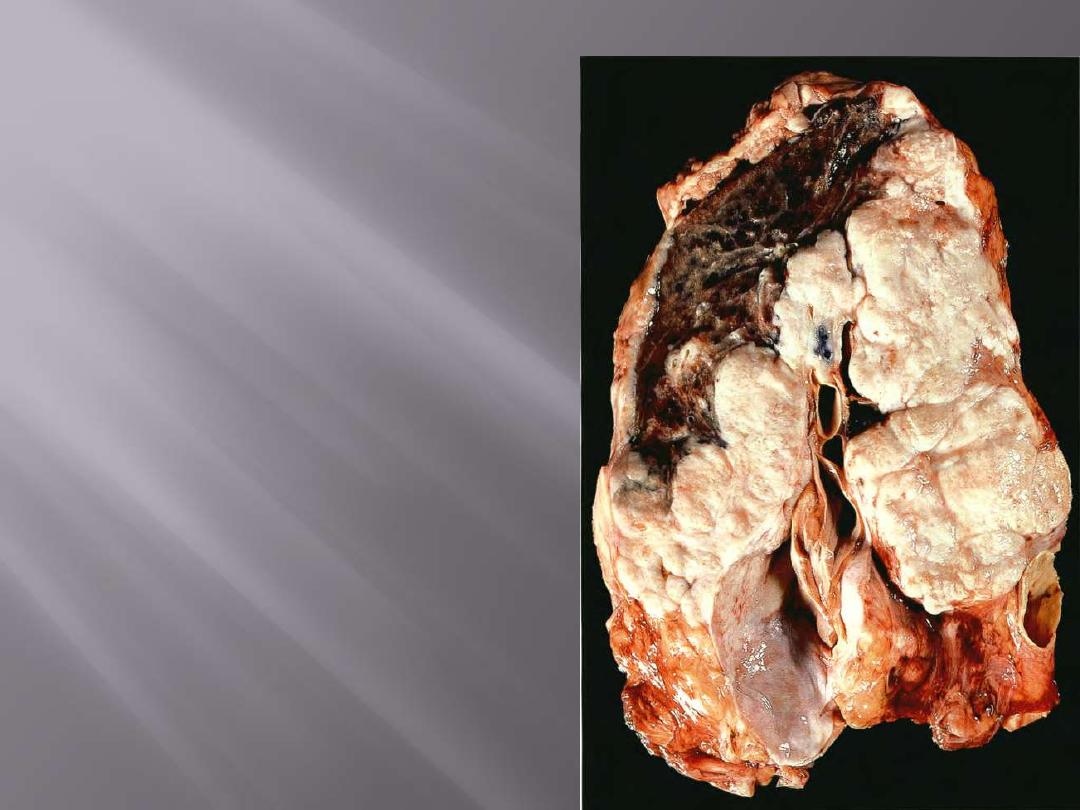
Arising centrally in this lung and spreading
extensively is a small cell anaplastic (oat cell)
carcinoma. The cut surface of this tumor has a
soft, lobulated, white to tan appearance. The
tumor seen here has caused obstruction of the
main bronchus to left lung so that the distal
lung is collapsed. Oat cell carcinomas are very
aggressive and often metastasize widely before
the primary tumor mass in the lung reaches a
large size.
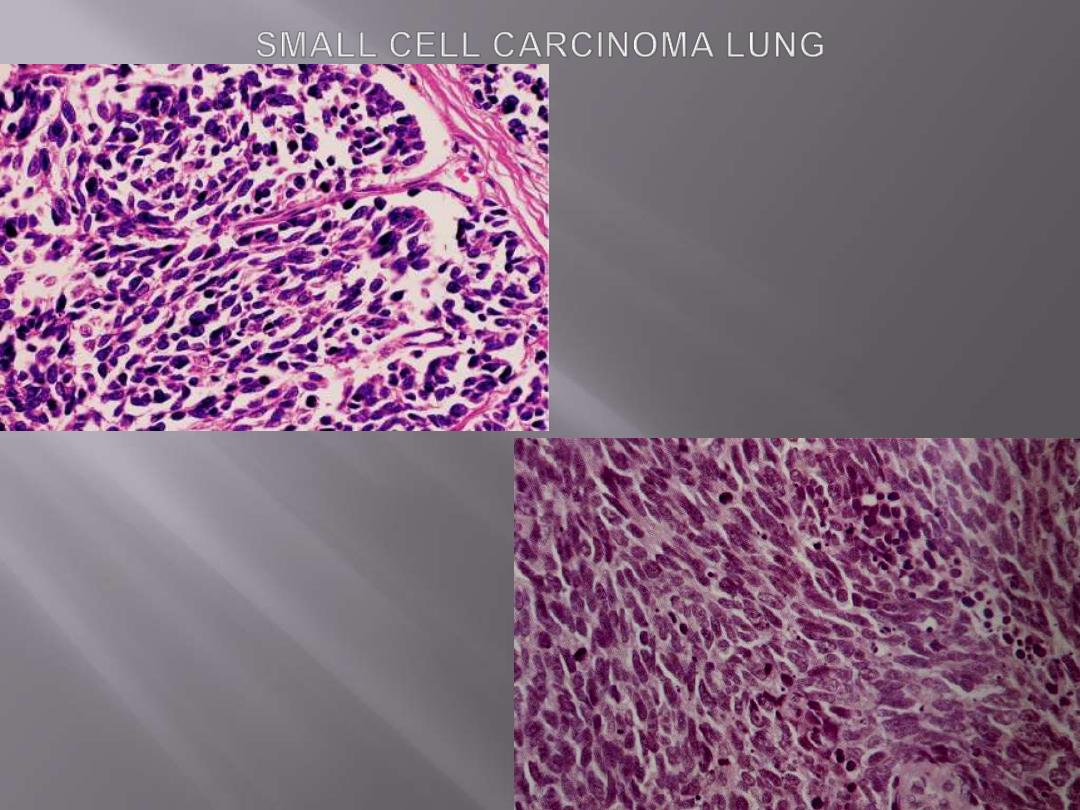
The tumor is composed of small cells with darkly
staining oval to spindle nuclei and extremely scanty
cytoplasm.

Combined tumors:
a minority of bronchogenic carcinomas reveal
more than one line of differentiation, sometimes
several, suggesting that all are derived from a
multipotential progenitor cell.

All subtypes of lung cancer show involvement of successive
chains of nodes about the
carina, in the mediastinum, and
in the neck (scalene nodes) and clavicular regions and,
sooner or later, distant metastases.
Involvement of the
left supraclavicular node (Virchow node)
is particularly characteristic and sometimes calls attention
to an occult primary tumor.
These cancers, when advanced, often extend into the
pericardial or pleural spaces, leading to inflammation and
effusions.
They may compress or infiltrate the superior vena cava to
cause either venous congestion or the full-blown
vena caval
syndrome.

Apical neoplasms
may invade the brachial or cervical
sympathetic plexus to cause severe pain in the distribution
of the ulnar nerve or to produce
Horner syndrome
(ipsilateral enophthalmos, ptosis, miosis, and anhidrosis).
Such apical neoplasms are sometimes called
Pancoast
tumors,
and the combination of clinical findings is known as
Pancoast syndrome.
Pancoast tumor is often accompanied by destruction of the
first and second ribs and sometimes thoracic vertebrae.
As with other cancers, tumor-node-metastasis (TNM)
categories have been established to indicate the size and
spread of the primary neoplasm.

Course & prognosis
Carcinomas of the lung are silent lesions that more often than not have
spread beyond curable resection at the time of diagnosis.
Too often, the tumor presents with symptoms related to metastatic
spread to the brain (mental or neurologic changes), liver
(hepatomegaly), or bones (pain).
Overall, NSCLCs have a better prognosis than SCLCs.
NSCLCs
(squamous cell carcinomas or adenocarcinomas) are
detected before metastasis or local spread, cure is possible by
lobectomy or pneumonectomy.
SCLCs
, on the other hand, is almost always have spread by the time of
the diagnosis, even if the primary tumor appears small and localized.
Thus, surgical resection is not a practical treatment. They are very
sensitive to chemotherapy but invariably recur. Median survival even
with treatment is 1 year.

Paraneoplastic syndromes
Up to
10%
of all patients with lung cancer develop clinically overt
paraneoplastic syndromes. These include
1. Hypercalcemia
caused by secretion of a parathyroid hormone-related
peptide.
2. Cushing syndrome
(from increased production of ACTH);
3. Inappropriate secretion of ADH
4. Neuromuscular syndromes,
including a myasthenic syndrome,
peripheral neuropathy, and polymyositis
5. Clubbing of the fingers and hypertrophic pulmonary
osteoarthropathy
6. Hematologic manifestations,
including migratory thrombophlebitis,
nonbacterial endocarditis, and disseminated intravascular coagulation.
Hypercalcemia is most often encountered with squamous cell carcinomas, the
hematologic syndromes with adenocarcinomas. The remaining syndromes are
much more common with small-cell neoplasms, but exceptions occur.

Bronchial Carcinoids
are thought to arise from the
Kulchitsky cells
(neuroendocrine cells) of the
bronchial mucosa and represent about
5% of all pulmonary neoplasms.
The mean age of occurrence is
40 years.
Ultrastructurally,
the neoplastic cells contain cytoplasmic dense-core
neurosecretory granules and may secrete hormonally active polypeptides.
In contrast to the more ominous small-cell carcinomas, carcinoids are often
resectable and curable.
Gross features
Most bronchial carcinoids originate in main bronchi and grow in one of two
patterns
1. An Obstructing spherical, intraluminal mass
2. A mucosal plaque penetrating the bronchial wall to fan out in the peribronchial
tissue.
Some tumors, (about 10%) metastasize to hilar nodes but distant metastasis is
rare.

Microscopic features
They are composed of nests of uniform cells that have regular round
nuclei with
"salt-and pepper"
chromatin, absent or rare mitoses
A subset designated atypical carcinoid that differ from the typical by
1. Having higher mitotic rate, cytologic pleomorphism , and focal necrosis
2. Having a higher incidence of lymph node and distant metastasis.
Typical carcinoid, atypical carcinoid, and small-cell
carcinoma can be considered to represent a continuum
of increasing histologic aggressiveness and malignant
potential
within
the
spectrum
of
pulmonary
neuroendocrine neoplasms.
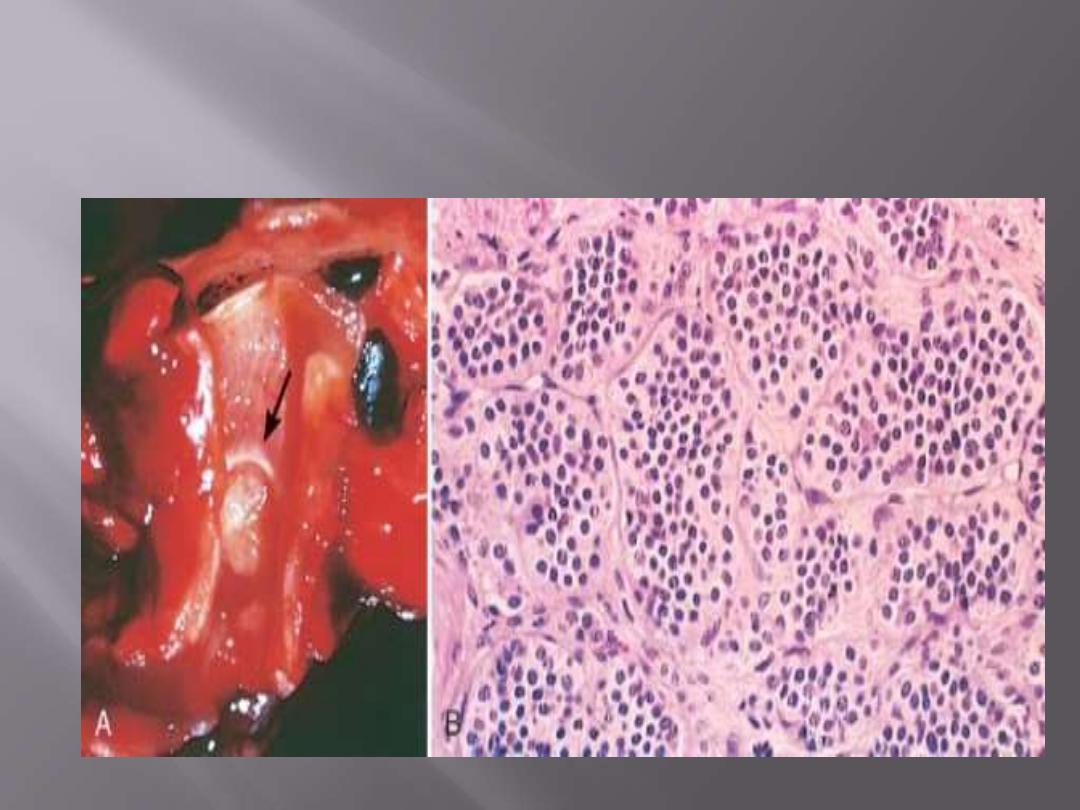
A, Bronchial Carcinoid Tumor: Yellow intrabronchial mass (lower) has resulted in obstructive
bronchiectasis
B, Histologic appearance demonstrating trabecular arrangement of small, rounded, uniform cells.
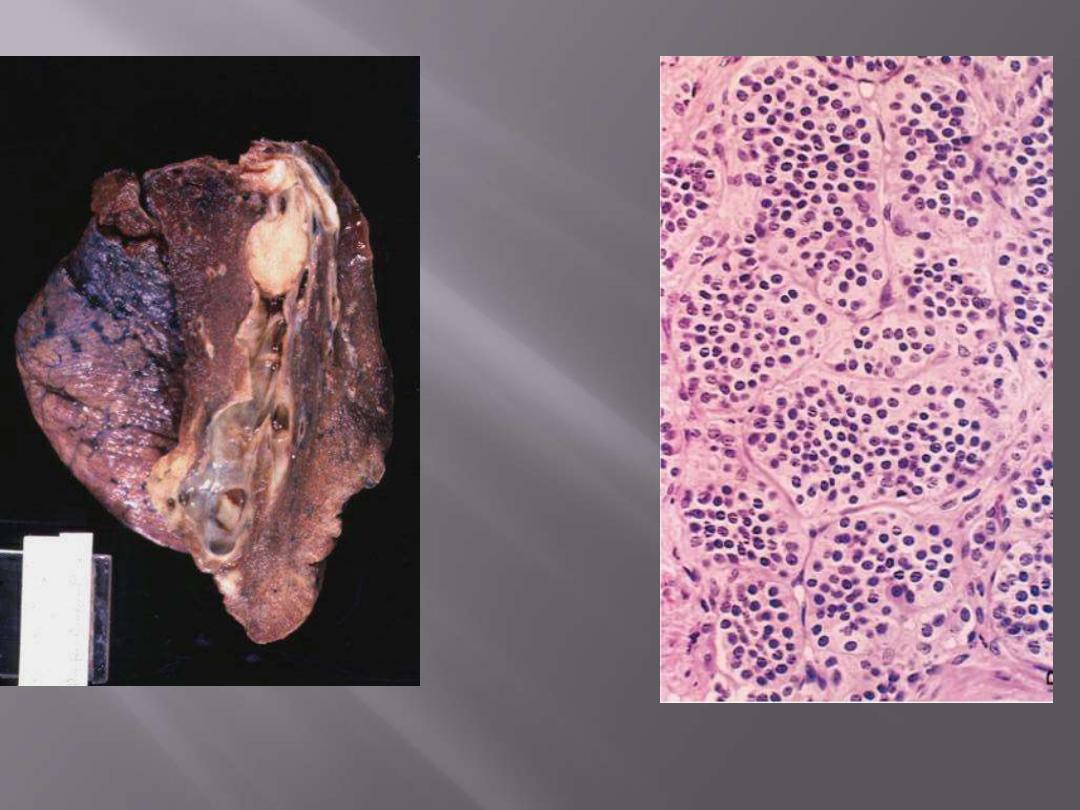
Bronchial carcinoid
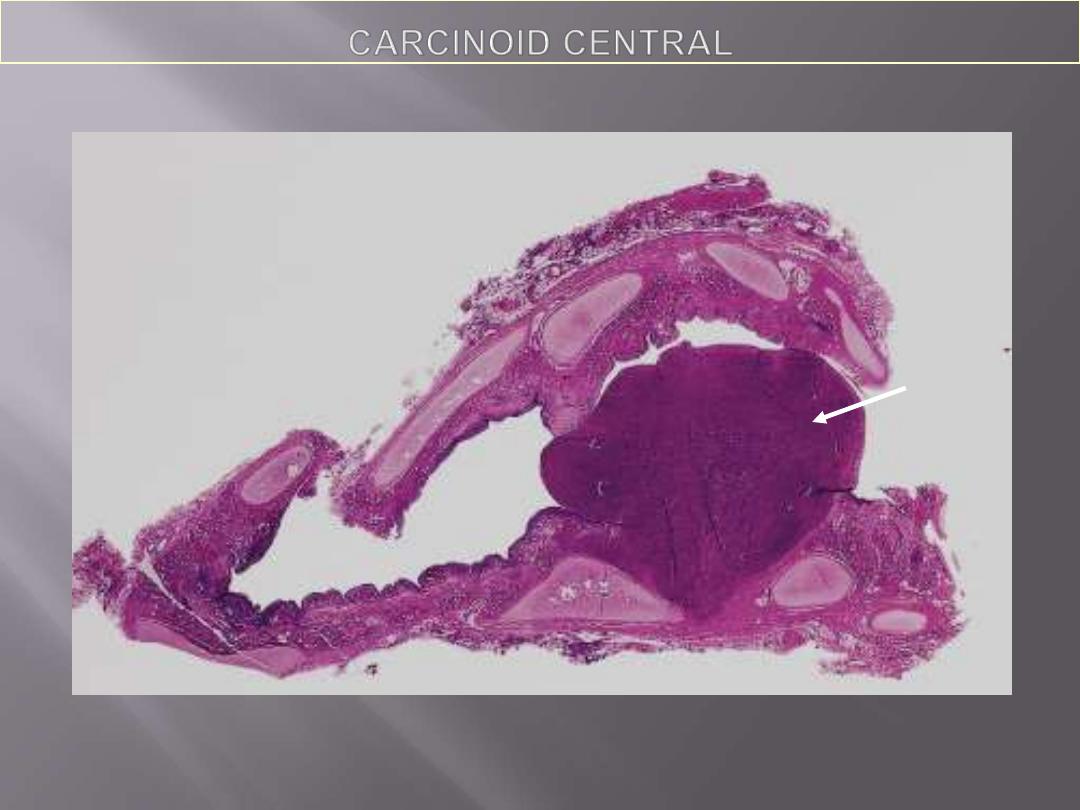
Whole mount of carcinoid tumor showing polypoid endobronchial growth.
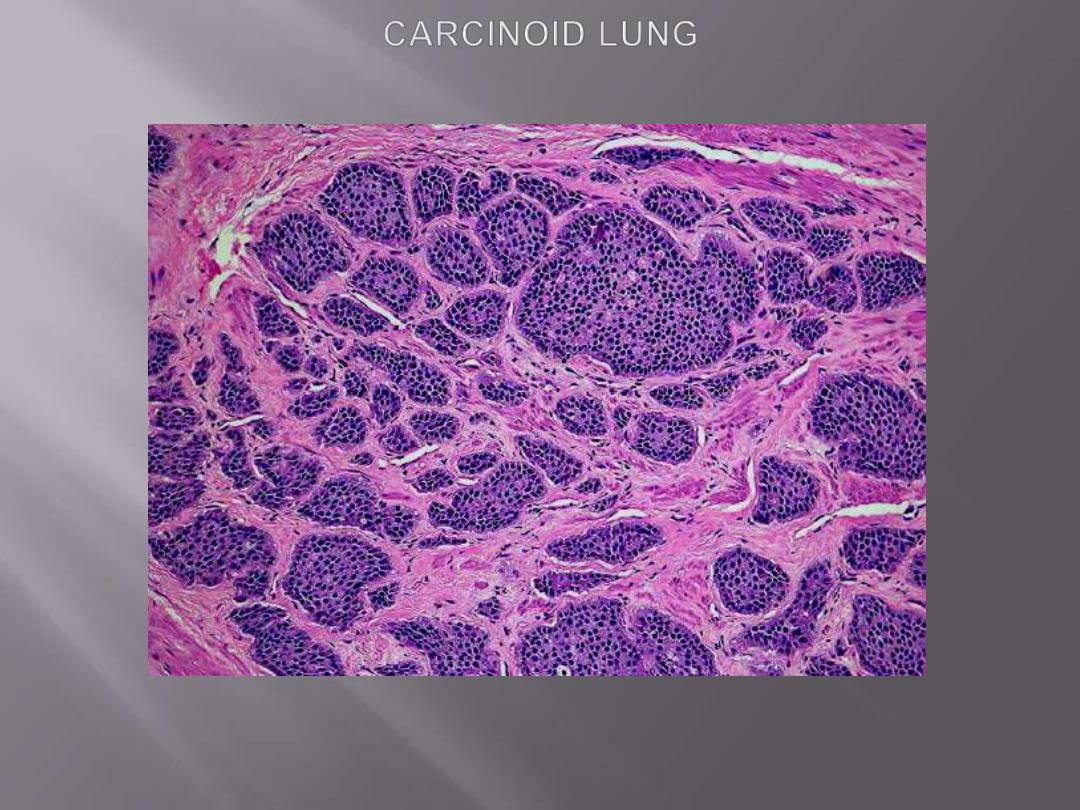
The tumor is composed of nests of uniform cells that have regular round nuclei & absent or rare
mitoses
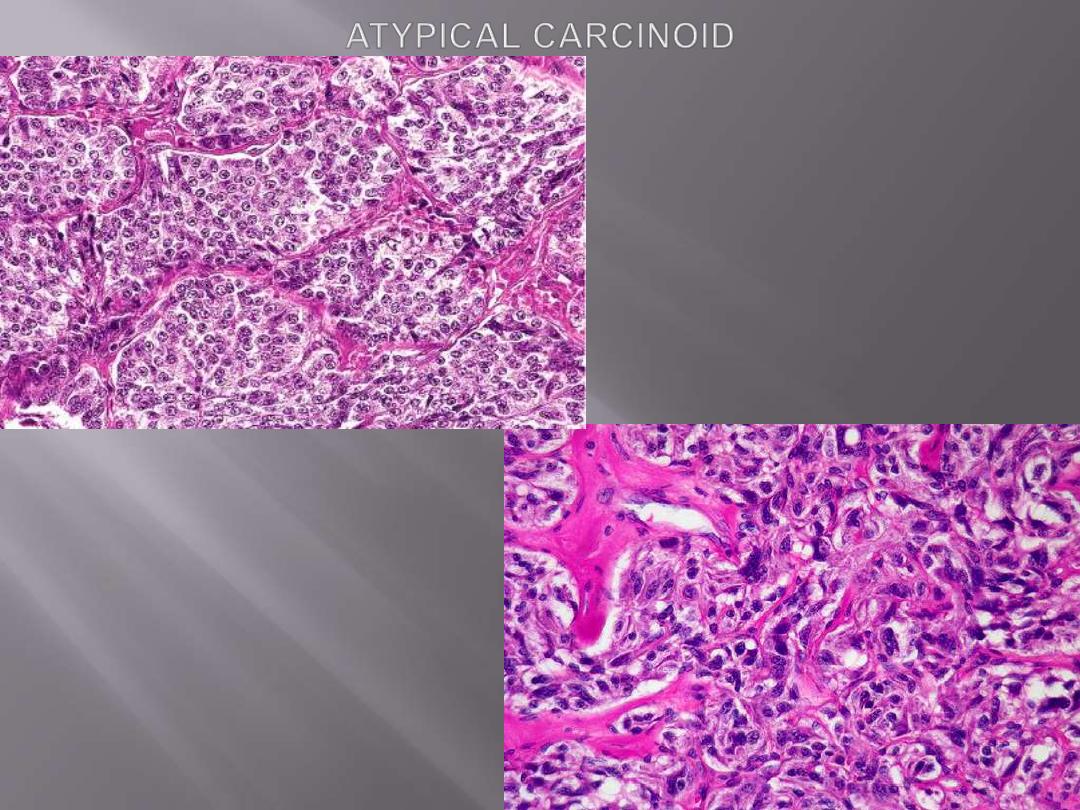
There is typical nesting of marked
pleomorphic cells. mitotic activity, and
necrosis are also present elsewhere.

Course & prognosis
Most clinical features are related to their intraluminal
growth (cough, hemoptysis, and recurrent bronchial and
pulmonary infections).
Some are asymptomatic and discovered by chance on
imaging studies. Only rarely do they induce the
carcinoid syndrome,
characterized by intermittent
attacks of diarrhea, flushing, and cyanosis.
The reported
10-year
survival rates for typical
carcinoids are
85%
, but these drop to
35%
for atypical
carcinoids.
Only 5% of patients with SCLC are alive at 10 years.

THE PLEURA
Pleural Effusion
refers to the presence of fluid in the pleural space. The fluid
can be either a transudate or an exudate. A transudate is termed
hydrothorax
, which
is mostly due to CHF. An exudates is characterized by protein content of 3.0 gm/dl
or more and, often, inflammatory cells. The four
principal causes of pleural
exudate are
1. Microbial invasion; either direct extension of a pulmonary infection or blood-
borne seeding (suppurative exudates or empyema)
2. Cancer (lung carcinoma, metastatic cancers to the lung or pleural
mesothelioma
3. Pulmonary infarction
4. Viral pleuritis
Less common causes include SLE, rheumatoid arthritis, uremia.
Cancer should be suspected as the underlying cause of an exudative effusion in
any patient older than the age of 40, particularly when there is no febrile
illness, no pain, and a negative tuberculin test result. These effusions
characteristically are large and frequently bloody (hemorrhagic). Cytologic
examination may reveal malignant and inflammatory cells.

Pneumothorax
refers to air in the pleural sac. It may occur in young,
healthy adults, usually men without any known pulmonary disease
(spontaneous pneumothorax),
or as a result of some thoracic (e.g. a fractured
rib) or lung disorder (secondary pneumothorax).
Secondary pneumothorax
occurs with rupture of any pulmonary lesion
situated close to the pleural surface that allows inspired air to gain access to the
pleural cavity. Such pulmonary lesions include
emphysema, lung abscess,
tuberculosis, carcinoma, etc. Supportive mechanical ventilation with high
pressure may also trigger secondary pneumothorax.
A serious complication may occur through a ball-valve leak that creates a
tension pneumothorax.
This shifts the mediastinum, which may be
followed by impairment of the pulmonary circulation that may be fatal.
With prolonged collapse, the lung becomes vulnerable to infection, as does the
pleural cavity.
Empyema
is
thus
an
important
complication
of
pneumothorax
(pyopneumothorax).

Hemothorax
is the collection of whole blood (in contrast to
bloody effusion) in the pleural cavity, is a complication of a
ruptured intrathoracic aortic aneurysm that is almost always
fatal.
Chylothorax
is a pleural collection of a milky lymphatic
fluid containing microglobules of lipid. It implies obstruction
of the major lymph ducts, usually by an intrathoracic cancer
(e.g., a primary or secondary mediastinal neoplasm, such as
a lymphoma).

Malignant Mesothelioma
is a rare cancer of mesothelial cells, usually arising in the
parietal or visceral pleura.
It also occurs, much less commonly, in the peritoneum and
pericardium.
In the USA approximately 50% of individuals with this
cancer have a history of exposure to
asbestos.
The latent period for developing malignant mesotheliomas
is long
(25 to 40 years)
after initial asbestos exposure.
The combination of cigarette smoking and asbestos
exposure greatly increases the risk of bronchogenic
carcinoma, but it does not increase the risk of developing
malignant mesotheliomas.

Gross features
These tumors begin in a localized area but in the
course of time spread widely.
At autopsy, the affected lung is typically
ensheathed by a yellow-white, firm layer of tumor
that obliterates the pleural space
The neoplasm may directly invade the thoracic
wall or the subpleural lung tissue.
M/67. This vertical slice of the right lung shows the
manner in which a pleural mesothelioma causes
thickening of the pleura and encases the whole
lung and mediastinum.
Malignant mesothelioma pleura
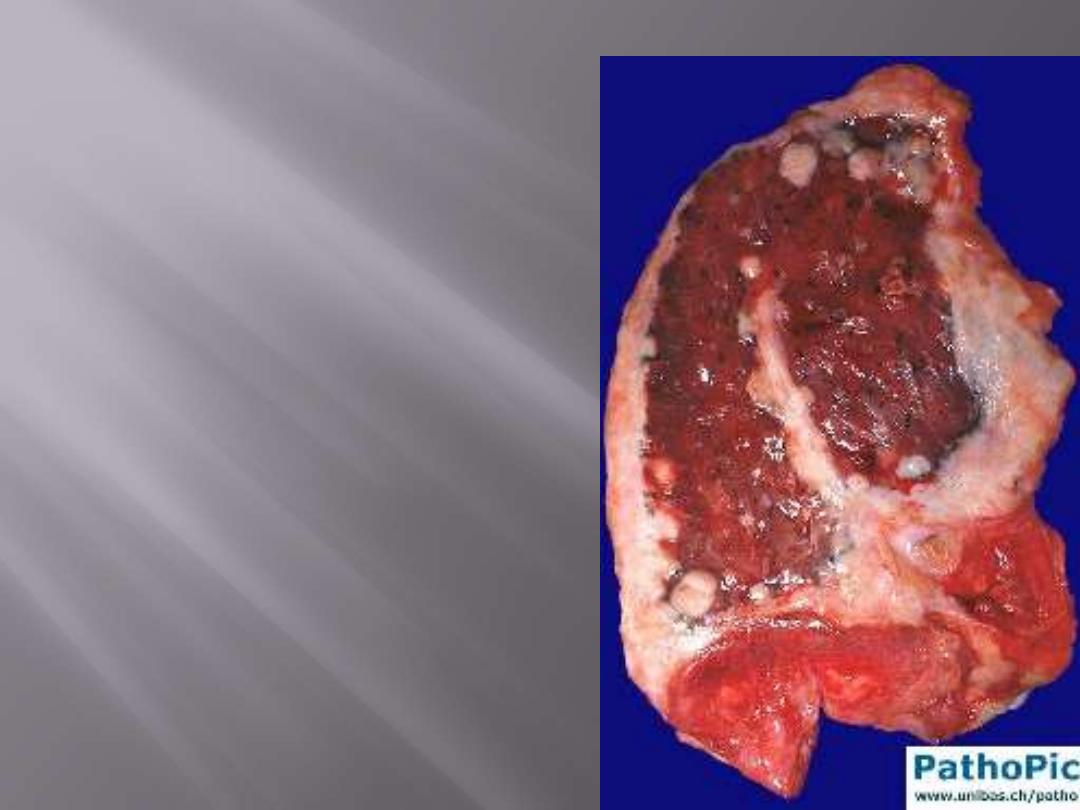
Malignant mesothelioma
Thick, firm, white, pleural tumor that ensheaths the
lung. Nodular invasion into the lung parenchyma is also
evident.
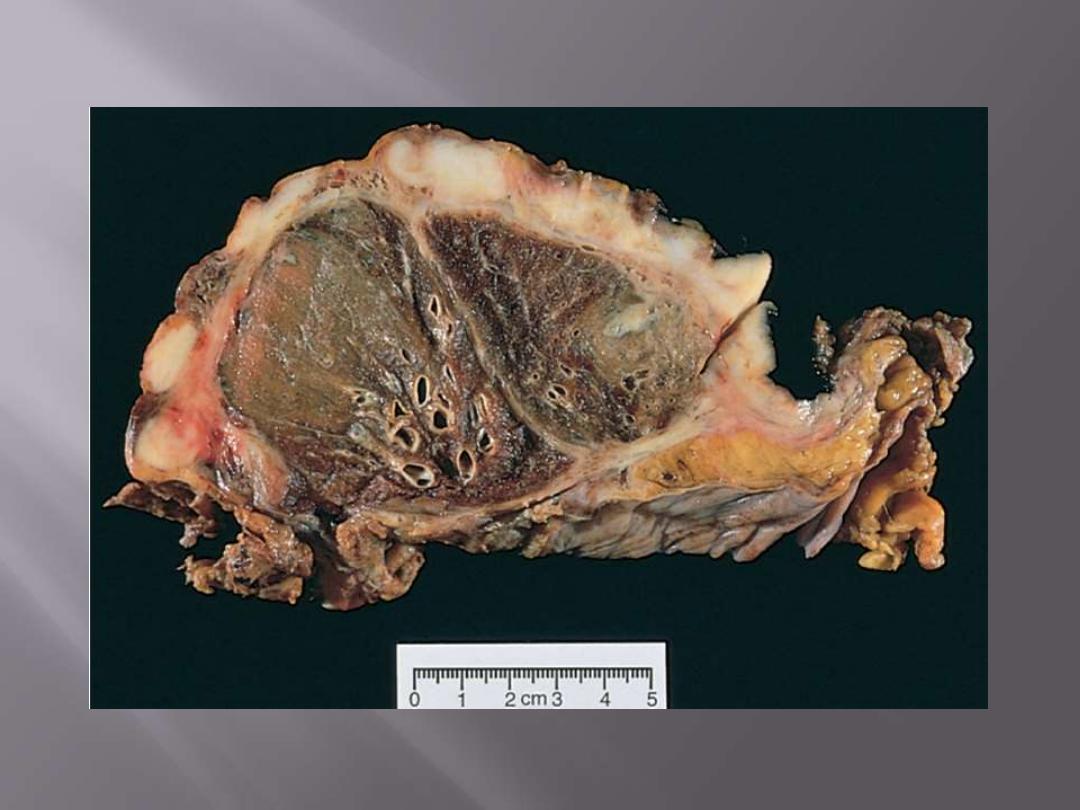
Note the thick, firm, white, pleural tumor that ensheaths this bisected lung.
Malignant mesothelioma

Microscopic features
Mesotheliomas conform to one of three patterns:
1. Epithelial,
in which cuboidal cells line tubular and
microcystic spaces, into which small papillary buds
project.
2. Sarcomatoid,
in which spindled cells grow in sheets
3. Biphasic,
having both sarcomatoid and epithelial
areas.
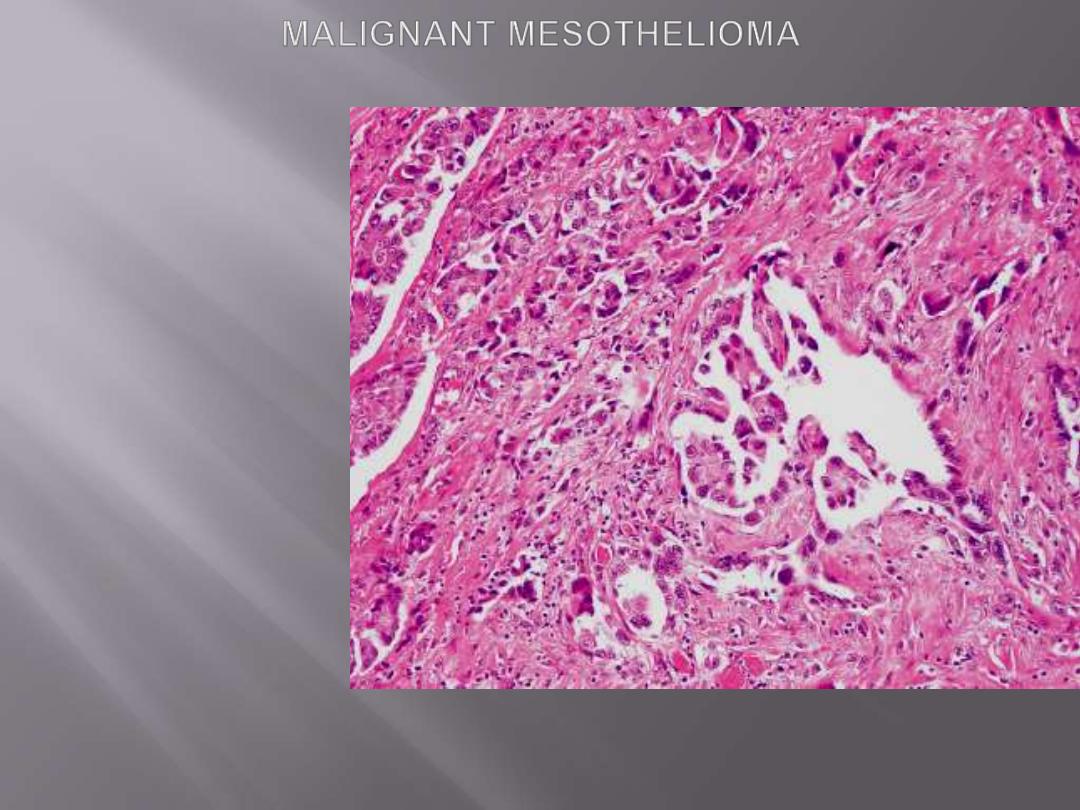
Malignant mesothelioma with papillary formations and desmoplastic stromal reaction.

