
Diffuse Interstitial
(Infiltrative, Restrictive) Lung Diseases:
Diffuse chronic involvement of
pulmonary connective tissue (mainly
the delicate interstitium) between the
alveoli.
Identified by reduced total lung vital
capacity.
Result in scarring and destruction of
the lung
End stage or honeycomb lung

Pathogenesis:
accumulation of inflammatory and
immune effector cells within the
alveolar wall and spaces (alveolitis)
early in the disease which leads to
distorted alveolar structure and
mediator release.
mediators injure parenchyma and
initiate fibrosis.

They include:
Idiopathic Pulmonary fibrosis
Collagen disorders
Pneumoconiosis.
Sarcoidosis

Idiopathic
Pulmonary
Fibrosis
(IPF)
(cryptogenic fibrosing alveolitis)
is characterized histologically by diffuse interstitial fibrosis,
which in advanced cases results in severe hypoxemia and
cyanosis.
Males (usually over 60 years) are more often affected.
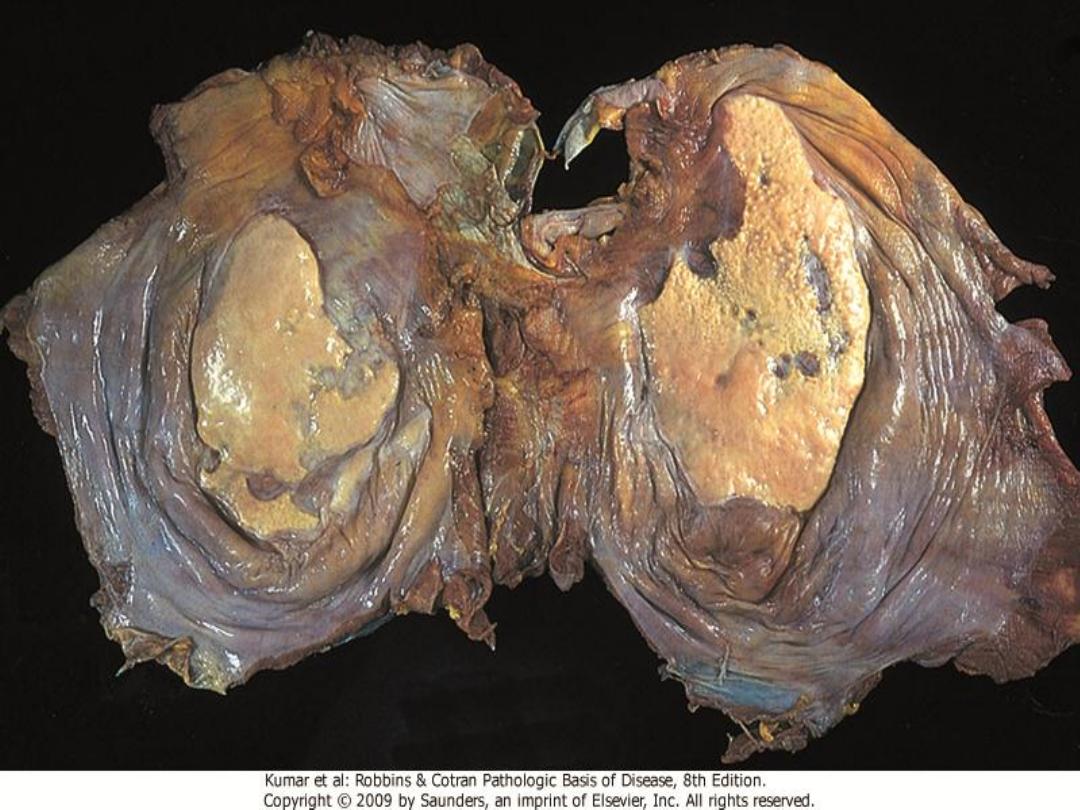
Grossly,
the pleural surfaces of the lung have cobblestone
appearance because of the retraction of scars along the
interlobular septa.
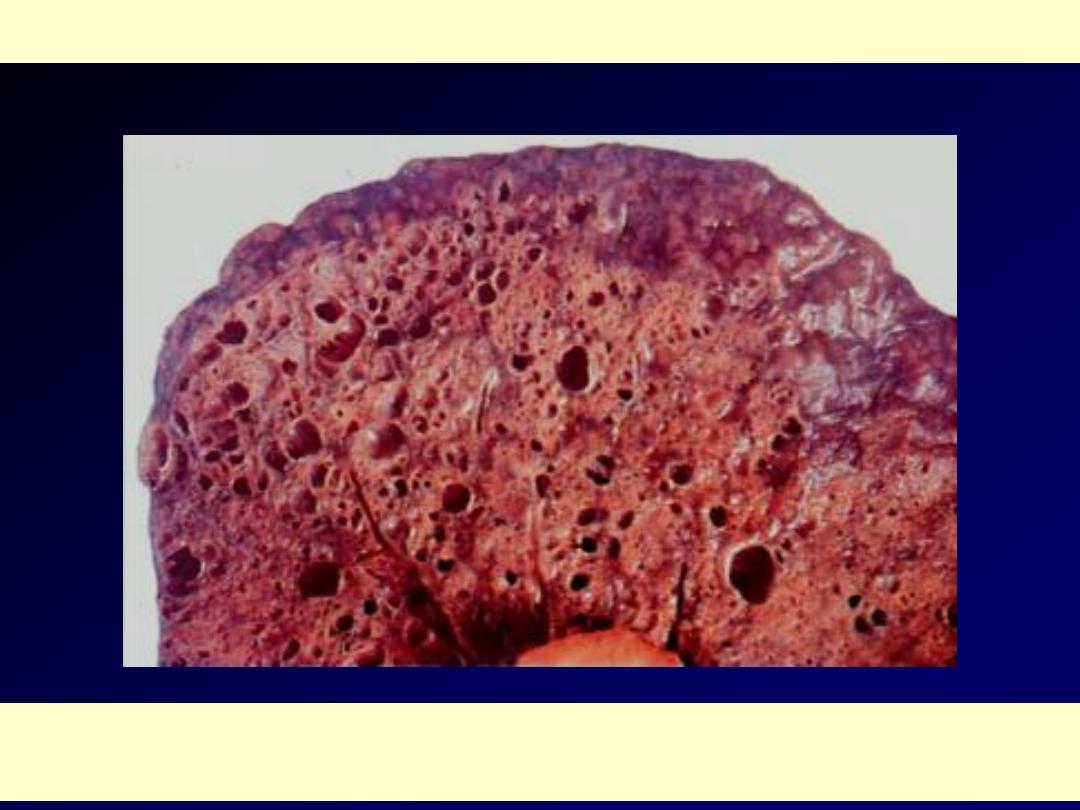
The histologic hallmark is patchy
interstitial fibrosis, which varies in intensity
The dense fibrosis causes collapse of alveolar walls and
formation of cystic spaces lined by hyperplastic type II
pneumocytes (honeycomb fibrosis)
The interstitial inflammation is usually patchy and consists
of lymphocytes. Secondary pulmonary hypertensive changes
are often present.
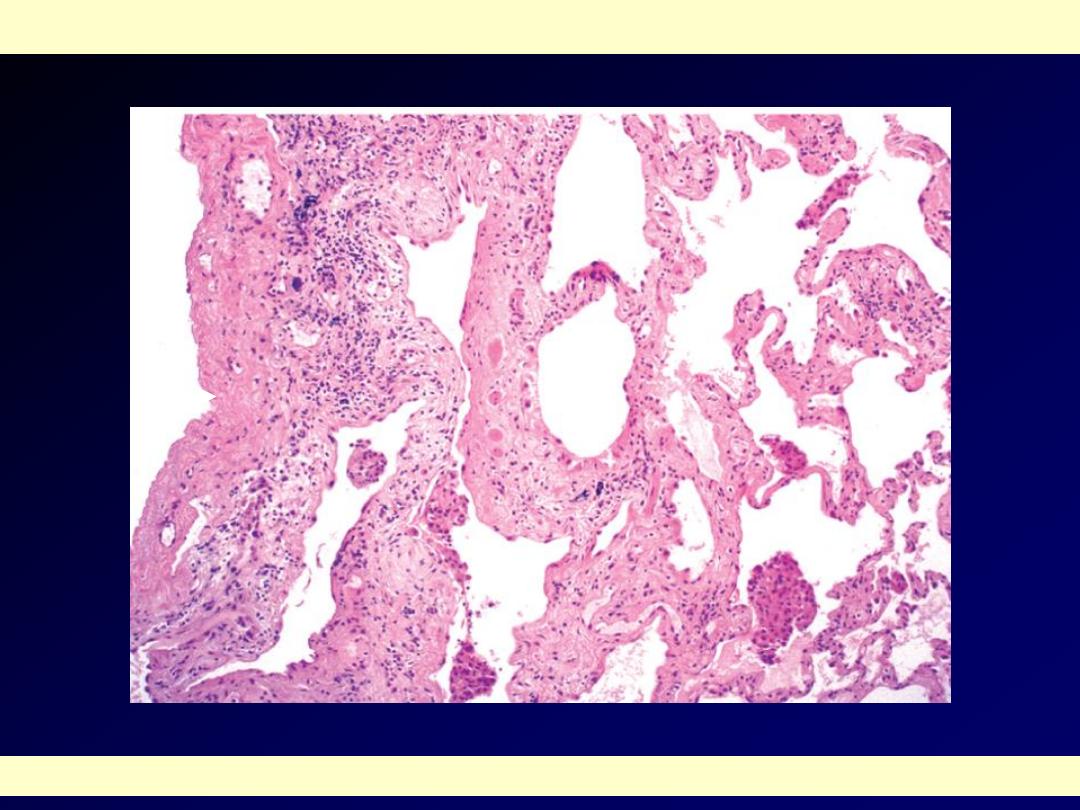
The fibrosis, which varies in intensity, is more pronounced in the subpleural region (arrow)
Idiopathic pulmonary fibrosis

Pulmonary Involvement in Collagen
Vascular Diseases
Many collagen vascular diseases (e.g., SLE,
rheumatoid arthritis, systemic sclerosis) are
associated with pulmonary manifestations.
The histologic changes are in part similar to that
of IPF, vascular sclerosis, organizing pneumonia,
and bronchiolitis.
Pleural involvement may also be present.
Pulmonary involvement in these diseases is usually
associated with a poor prognosis.
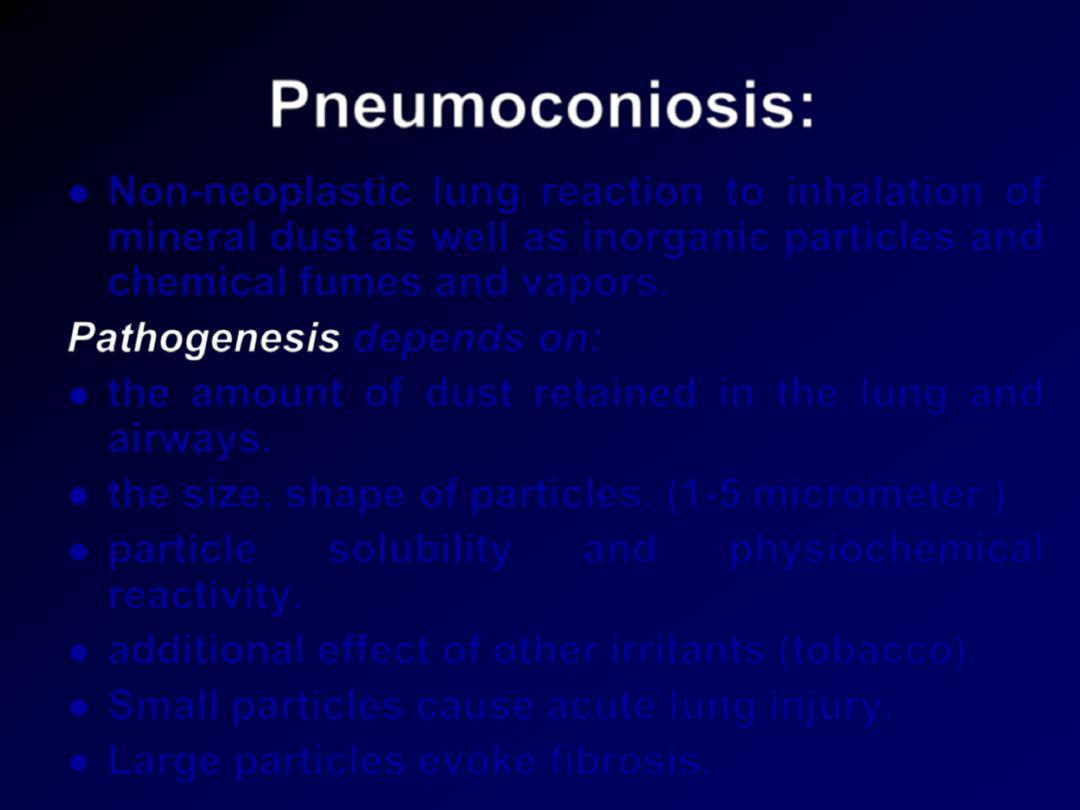
Pneumoconiosis:
Non-neoplastic lung reaction to inhalation of
mineral dust as well as inorganic particles and
chemical fumes and vapors.
Pathogenesis
depends on:
the amount of dust retained in the lung and
airways.
the size, shape of particles. (1-5 micrometer )
particle
solubility
and
physiochemical
reactivity.
additional effect of other irritants (tobacco).
Small particles cause acute lung injury.
Large particles evoke fibrosis.

1. Coal Workers' Pneumoconiosis
The spectrum of lung findings in coal workers
includes
a. Asymptomatic anthracosis,
in which
pigment accumulates
without cellular reaction
. It is
also commonly seen in all urban dwellers and
tobacco smokers. Inhaled carbon pigment is
engulfed by alveolar or interstitial macrophages,
which then accumulate in the connective tissue along
the lymphatics, including the pleural lymphatics, or
in lymph nodes.

b. Simple coal workers' pneumoconiosis
is
characterized by nodules that consist of dust-laden
macrophages with small delicate network of collagen
fibers.
c. Progressive massive fibrosis
develops in 10% of those with the above; it occurs
through the coalescence of the fibrotic nodules.
The fibrosis is extensive and lung function is
impaired
with
subsequent
pulmonary
hypertension, and corpulmonale.
There is no increased frequency of bronchogenic
carcinoma (cf. silicosis & asbestosis).
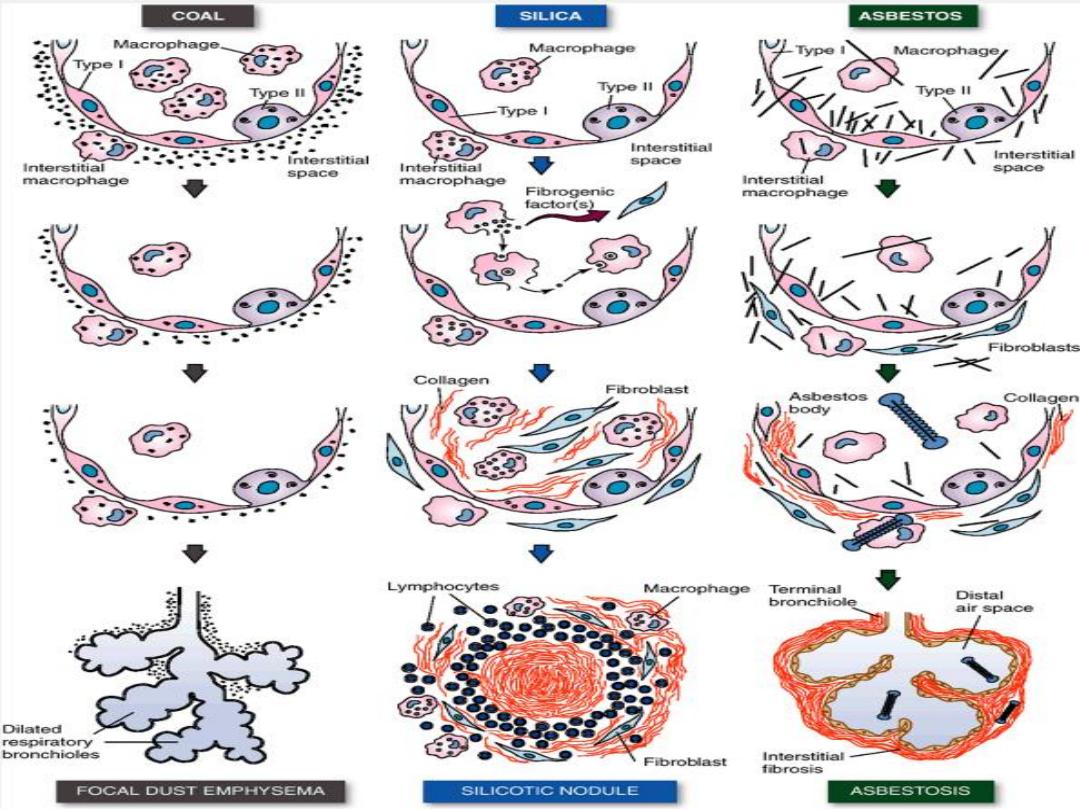
2. Silicosis
is the most common chronic occupational disease in the world. It is
caused by
inhalation of silica crystals mostly quartz
, usually in
occupational settings.
The condition is characterized by
the formation of silicotic
nodules,
at first tiny, discrete, pale or black (when mixed with
carbon) involving the
upper zones of the lungs
.
Microscopically
, the silicotic nodule consists of concentrically
arranged hyalinized collagen fibers surrounding an amorphous
center. Polarized microscopy reveals weakly birefringent silica
particles, primarily in the center of the nodules. With progression,
the individual nodules
coalesce into large collagenous scars
, and
eventually progressive
massive fibrosis
. Silicosis is associated with
an increased susceptibility to
tuberculosis
presumably due
depression of cell-mediated immunity.
Silica from occupational
sources is carcinogenic in humans. However, this subject
continues to be controversial.

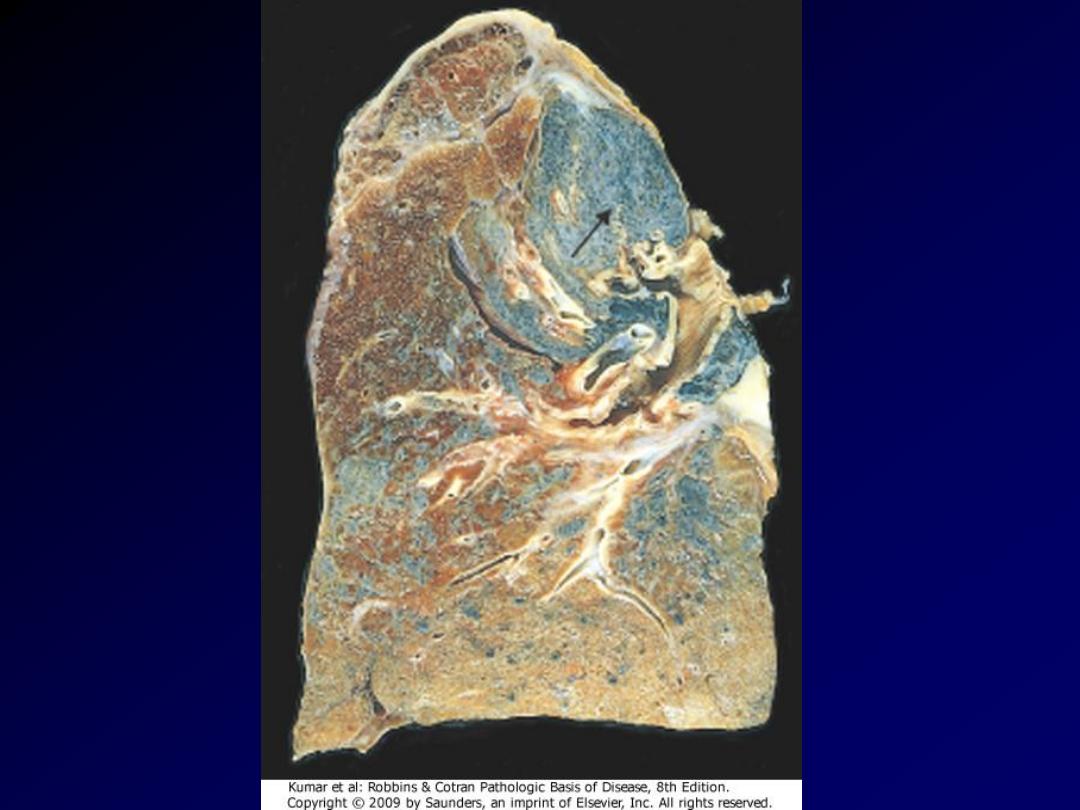
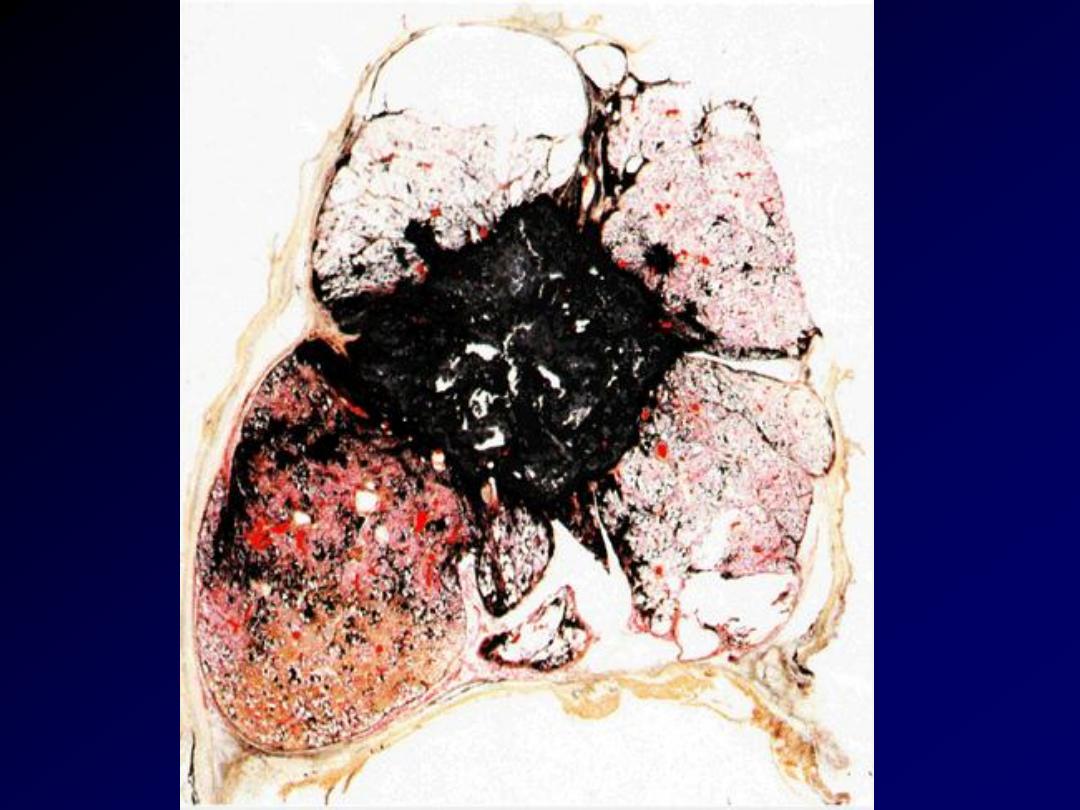
M/69. Multiple silicotic nodules can be seen
under the pleura. This man had worked as a
miner for most of his life.
Silicosis lung
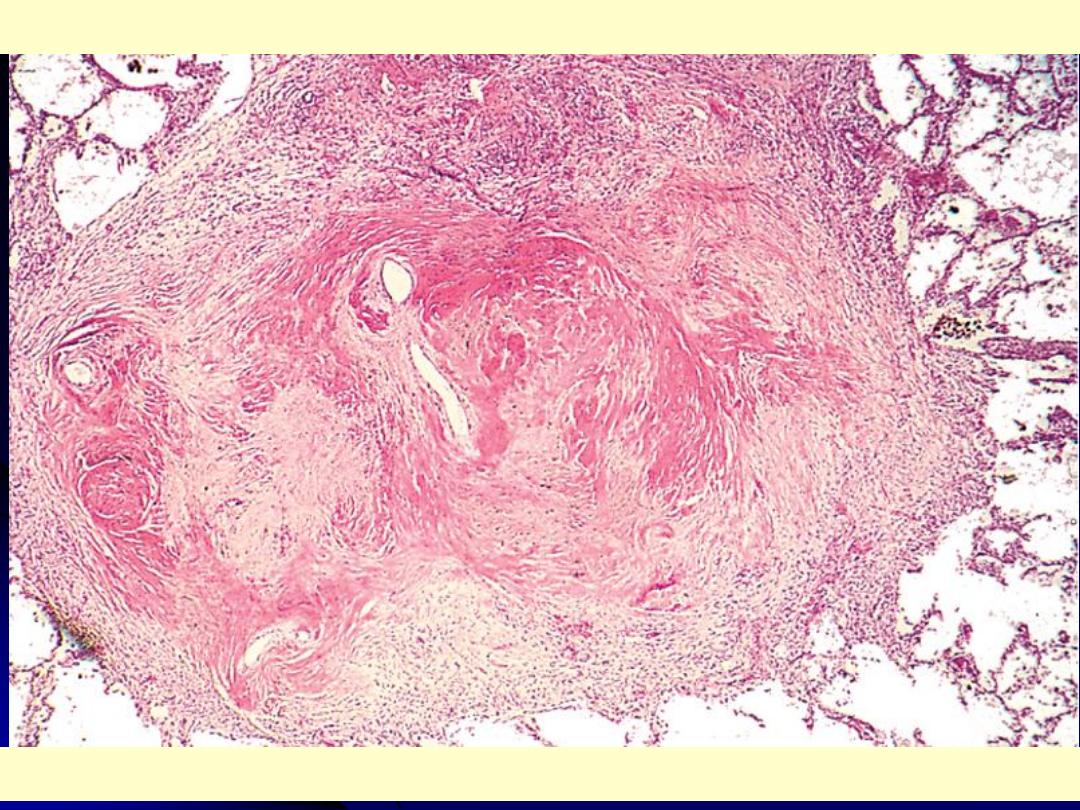
Several coalescent collagenous silicotic nodules.
Silicosis lung

3. Asbestosis and Asbestos-Related Diseases
Asbestos is a family of silicate crystals with a fibrous spatial arrangement.
Occupational exposure to asbestos is associated with
1. Interstitial pulmonary fibrosis (asbestosis)
2.
Localized
fibrous
pleural plaques
3. Pleural effusions
4. Bronchogenic
carcinoma
5. Malignant mesotheliomas (pleural, peritoneal) 6. Laryngeal carcinoma
An increased incidence of asbestos-related cancers is noted in family
members of asbestos workers.
Asbestosis
signifies
diffuse
pulmonary
interstitial
fibrosis
&
characteristically shows the presence of asbestos bodies, which are seen as
golden brown, beaded rods.
They consist of asbestos fibers coated with an iron-protein material.
In contrast to coal workers pneumoconiosis and silicosis, asbestosis begins
in the lower lobes and subpleural regions, but the entire lungs become
affected as fibrosis progresses. Simultaneously, the visceral pleura
undergoes fibrous thickening.
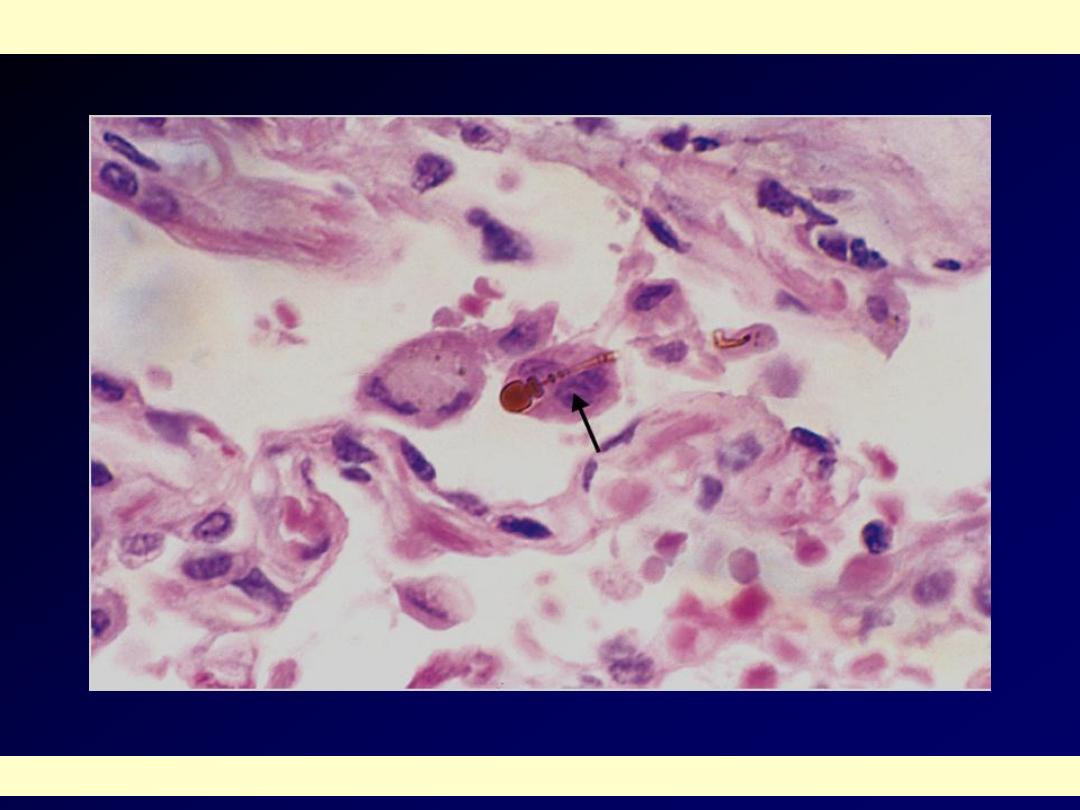
High-power detail of an asbestos body, revealing the typical beading and knobbed ends (arrow).
Asbestos body

Pleural plaques
are the most common manifestation of
asbestos exposure and are well-circumscribed patches of
dense collagen that develop most frequently
on the parietal
pleura and over the domes of the diaphragm.
The risk of bronchogenic carcinoma is increased about five
times for asbestos workers.
The risk for
mesotheliomas
, normally a very rare tumor, is
more than
1000 times greater
.
Both pleural and peritoneal mesotheliomas have an
association with asbestos exposure.
Concomitant cigarette smoking greatly increases the risk
of bronchogenic carcinoma but not that of mesothelioma.
The carcinoma & mesothelioma associated with asbestos
exposure have a particularly poor prognosis.
HONEYCOMB LUNG
The lung has a honeycomb appearance grossly. There is prominent interstitial fibrosis. Etiologies
include DIP, UIP, interstitial pneumonitis associated with collagen vascular diseases, asbestosis,
berylliosis, sarcoidosis, LIP, DAD, recurrent aspiration, allergic alveolitis, and idiopathic.

Granulomatous Diseases

Sarcoidosis:
It is a systemic disease of unknown
cause in which there are non-caseating
granulomas in many tissues and
organs. There is bilateral hilar
lymphadenopathy and lung
involvement in 90% of cases.

Pathogenesis:
immunological factors: accumulation of
CD4 T cells, increased T- cell derived
(TH1) cytokines resulting in T-cell
expansion and macrophage activation
and granuloma formation.
Genetic
familial and racial clustering
HLA (A1 and B8)
Environment (mycobacterium)

Morphology:
Non-
caseating granuloma, Langhan’s
and foreign body giant cells.
Chronicity leads to formation of fibrous
rim around granuloma or replacement by
hyaline scar.
Schaumann bodies: laminated
concretions (calcium and proteins).
Stellate inclusions asteroid bodies within
giant cells
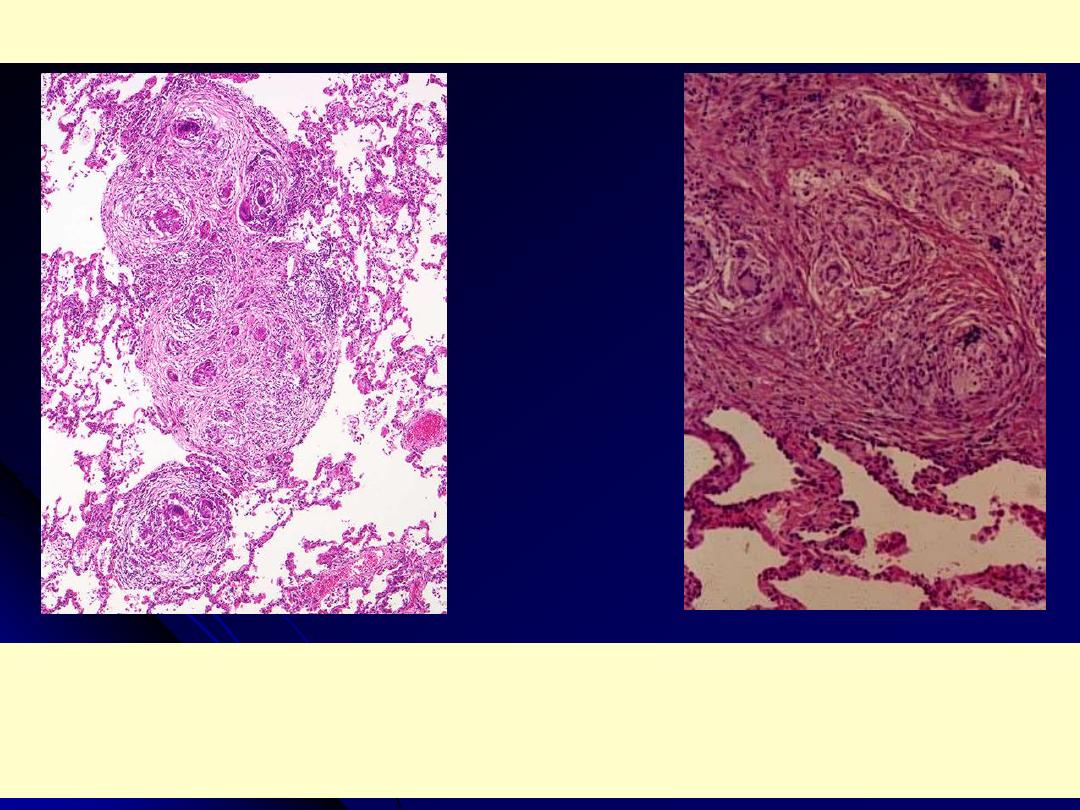
Sarcoidosis lung
Rt. Low poer: non caseating granulomas are present in the lung parenchyma. The inciting agent for
this granulomatous disease is unknown - speculation ranges from an unidentified microorganism to
tree pollen!. Sarcoidosis typically presents with non-caseating granulomas.
Lt. Medium power: there is interstitial non-caseating granulomatous inflammation. Giant cells and
histiocytes form nodular aggregates without necrosis
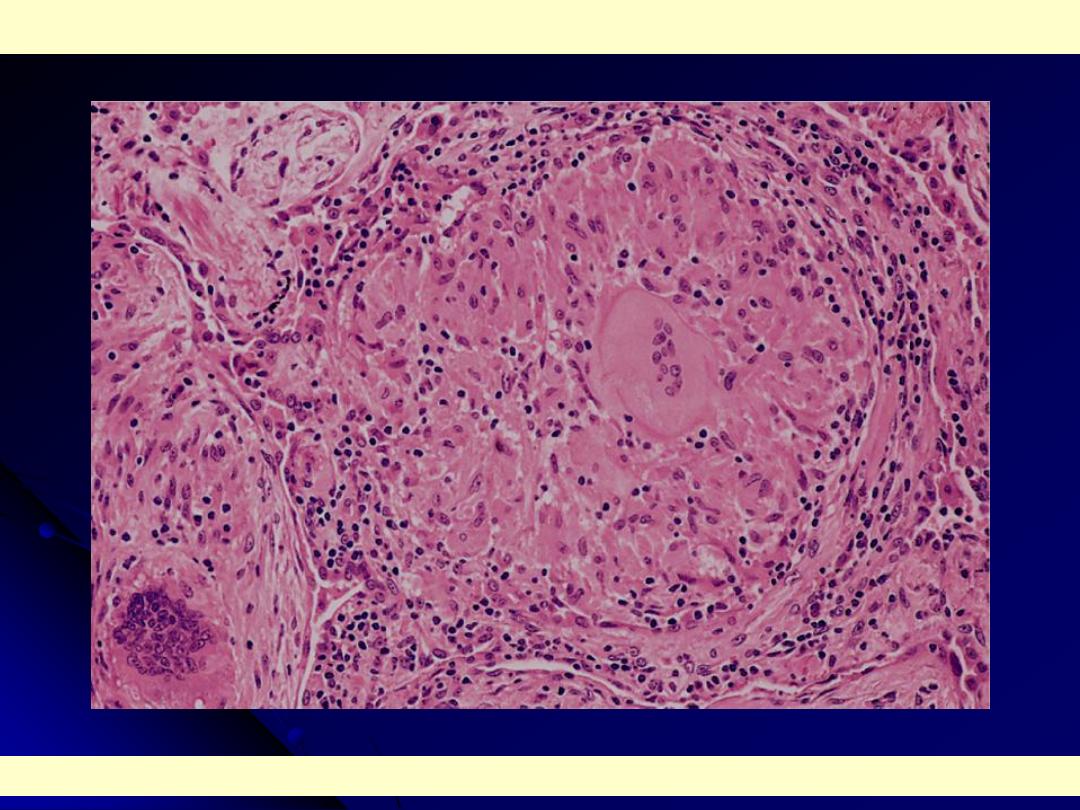
High power: characteristic sarcoid noncaseating granulomas in lung with many giant cells.
Sarcoidosis
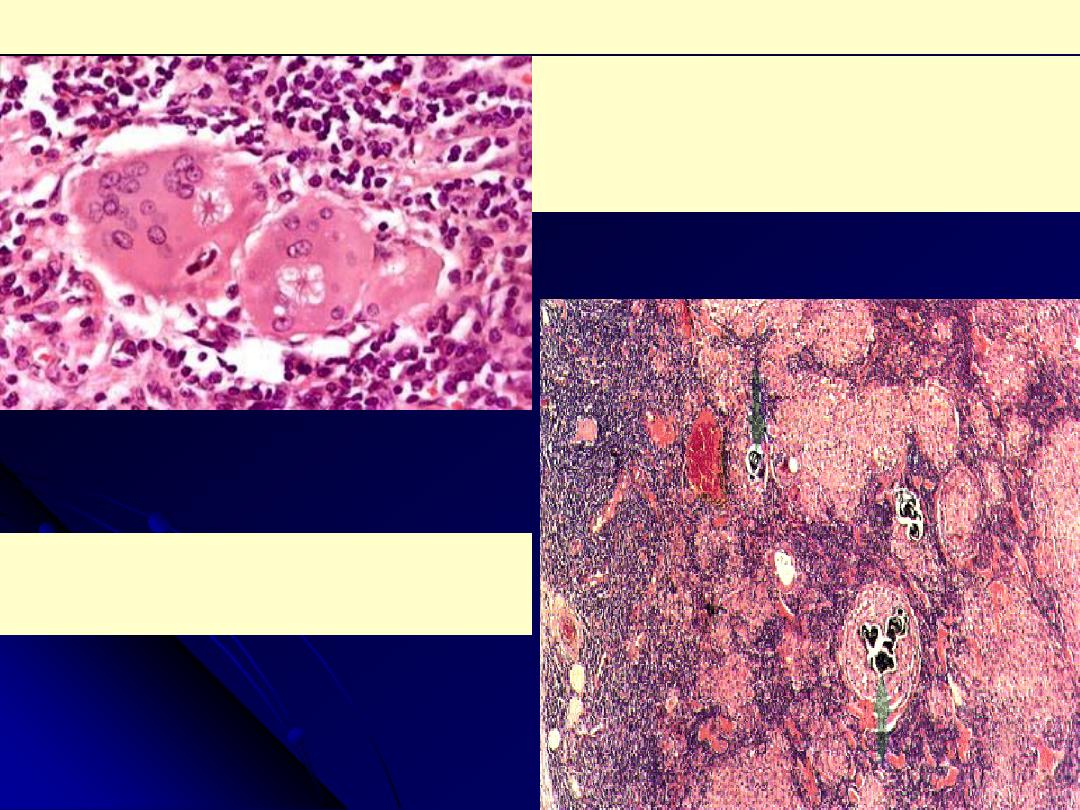
Each of the two multinucleated giant cells shown
here has an asteroid body with surrounding
vacuoles in the cytoplasm. The basophilic body in
the giant cell to the left may be an early, small
Schaumann body.
Sarcoidosis lymph node
Multiple noncaseating epithelioid
granulomas There is no caseation, but
some contain calcified laminated
Schaumann bodies (arrows).

Pulmonary Infections
(Pneumonia)
can result whenever defense mechanisms
are impaired or whenever the general
resistance of the host is lowered.
loss or suppression of cough reflex (coma,
anesthesia, chest pain, NM disorders).
Injury to the mucociliary apparatus
(cigarette, hot or corrosive gases, viral,
genetic).

Pulmonary Infections
(Pneumonia)
Interference with phagocytic or bactericidal
action of alveolar macrophages (alcohol,
tobacco, anoxia, oxygen intoxication).
Pulmonary congestion and edema.
Accumulation of secretion ( C.F. and bronchial
obstruction).
Defects in innate immunity (neutrophils and
complements) and humeral immunity leading
to increased infection with pyogenic
organisms.

Pulmonary Infections
(Pneumonia)
Hematogenous spread.
Hospitalized patients (nosocomial
infections).

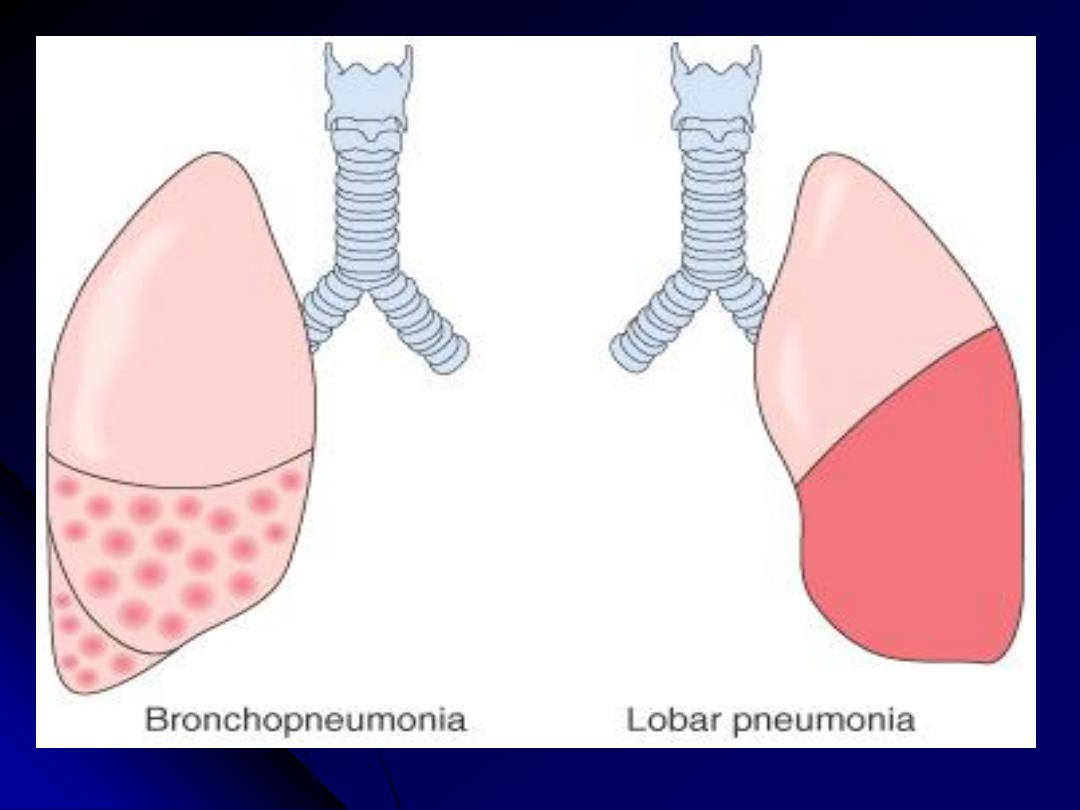
Acute bacterial pneumonias
can present as one of two anatomic
(and radiographic) patterns
1. Bronchopneumonia
showing a patchy distribution of inflammation that
generally involves more than one lobe. The initial infection is of the bronchi and
bronchioles with extension into the adjacent alveoli.
2. Lobar pneumonia
, which is by contrast, affecting the contiguous airspaces of
part or all of a lobe; these are homogeneously filled with an exudate that can be
visualized on radiographs as a lobar or segmental consolidation
Streptococcus pneumoniae
is responsible for more than 90% of lobar
pneumonias. The anatomic distinction between lobar pneumonia and
bronchopneumonia is often become blurred because (a) many organisms can
produce either of the two patterns of distribution and (b) confluent
bronchopneumonia can be hard to distinguish radiologically from lobar
pneumonia.
Classifying pneumonias by the setting in which they arise considerably narrows
the list of suspected pathogens and hence help choosing the suitable empirical
antibiotic for treatment.
Pneumonia can arise in seven distinct clinical
settings ("pneumonia syndromes"), and the causative pathogens
are reasonably specific to each category.

Community Acquired Acute
Pneumonia (CAAP):
bacterial or viral.
bacterial pneumonia follows an upper
respiratory tract viral infection.
bacterial invasion lead to filling of alveoli
with inflammatory exudates (consolidation
or solidification).

Predisposing conditions:
Extremes of age
Chronic diseases (e.g. CHF, COPD, &
DM).
Congenital or acquired
immunodeficiency.
Decreased or absent splenic function.

Streptococcal Pneumonia:
Most common cause of CAAP.
Gram stained sputum reveals neutrophils
containing gram positive lancet shaped
diplococci.
Blood (culture more specific less
sensitive).
Penicillin treatment
Resistant strains
Pneumococcal vaccines containing
capsular polysaccharide.

Hemophilus influenzae Pneumonia
Life threatening acute lower respiratory
tract infection and meningitis in children
In adults, it causes CAAP.
Maroxella catarrhalis
: In elderly.
Klebsiella Pneumoniae
: in malnourished
and alcoholics.
Pseudomonas aeroginosa
: Nosocomial,
cystic fibrosis and neutropenic patients.
Legionella peumophila
: In cardiac, renal,
immunologic, hematological diseases and
organ transplant.

Staphylococcus aureus:
Secondary bacterial pneumonia in children
and adults following viral respiratory
illness.
Drug abusers
Abscess and empyema.
Nosocomial pneumonia

Morphology of pneumonia:
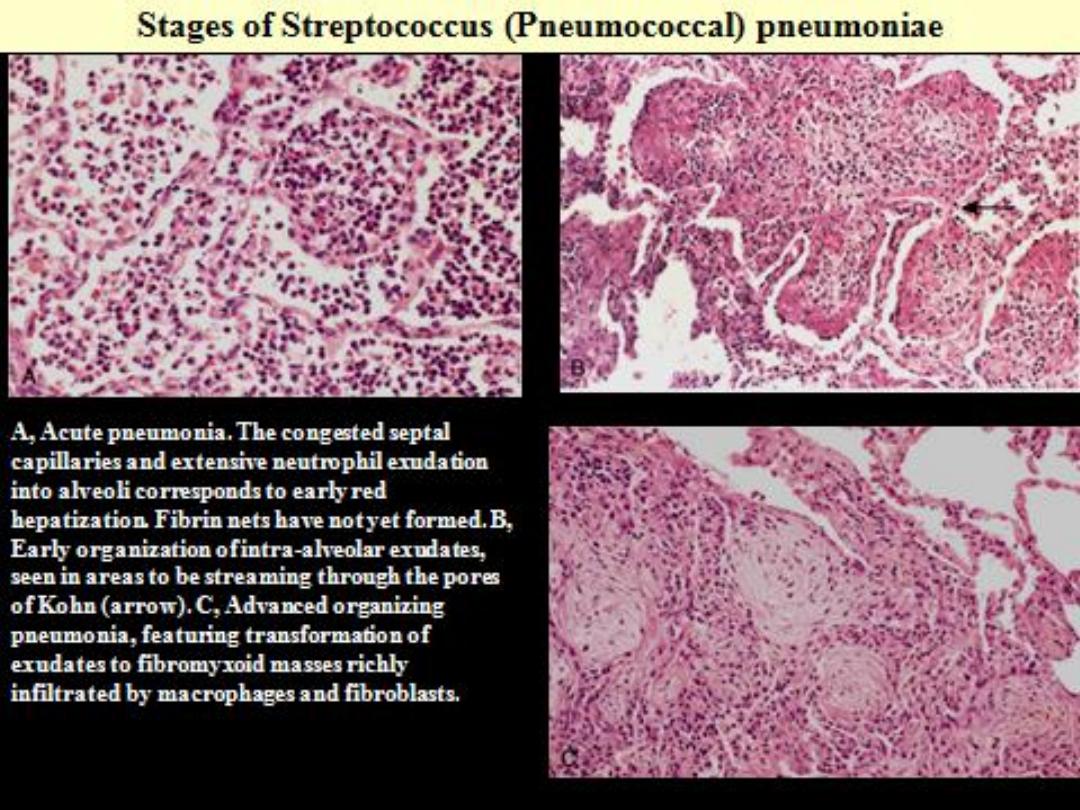
Lobar Pneumonia
: Four stages
Congestion
: Vascular engorgement, intra
alveolar fluid with few neutrophils.
Red hepatization
: massive confluent
exudation of RBCs, neutrophils and fibrin (liver
like).
Gray hepatization
: disintegration of RBCs,
persistence of fibrinosuppurative exudates.
Resolution
: enzymatic digestion of exudate
leading to resorption or it is coughed out or
ingested by macrophages or organized by
fibroblasts.

Morphology of pneumonia:
Bronchopneumonia
:
The consolidation is patchy through one
lobe, multilobar, bilateral and basal
(secretions gravitate into lower lobes).
Suppurative neutrophil rich exudates fills
bronchioles, bronchi and adjacent
alveoli.

Bronchopneumonia
Lobar Pneumonia
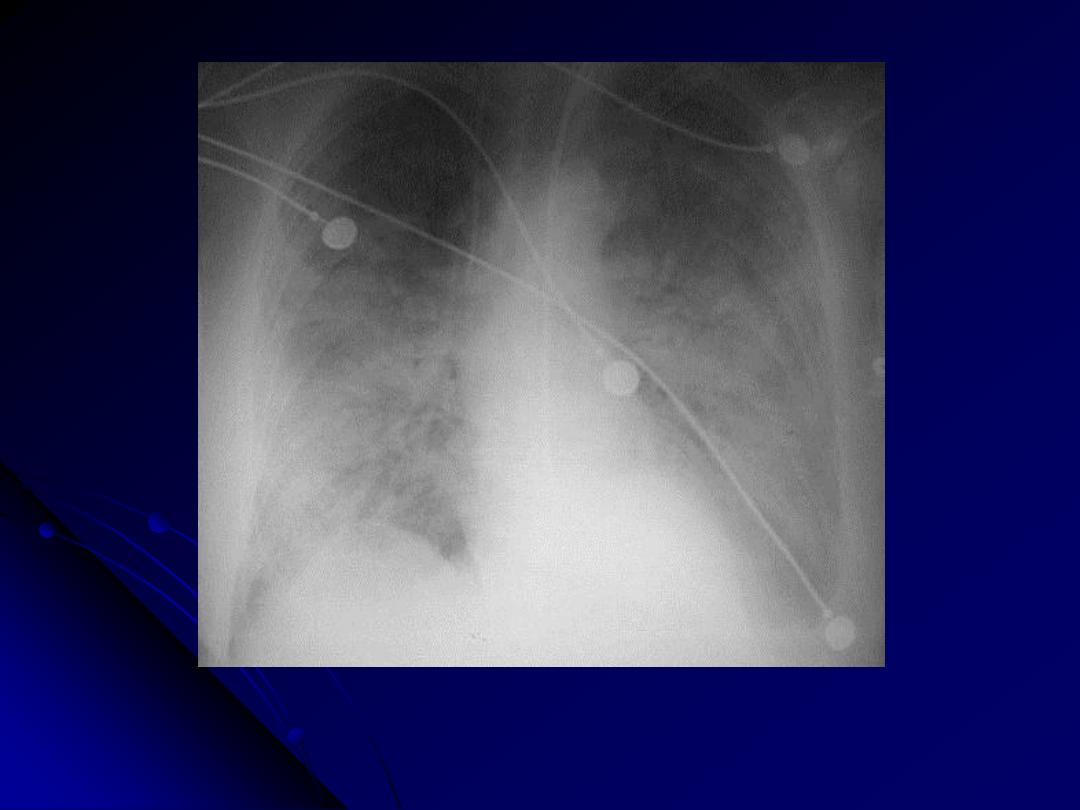
Patchy infiltrates consistent with a bronchopneumonia from a bacterial infection. Typical
organisms include Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas
aeruginosa, Hemophilus influenzae, Klebsiella pneumoniae, among others.
CXR: bronchopneumonia
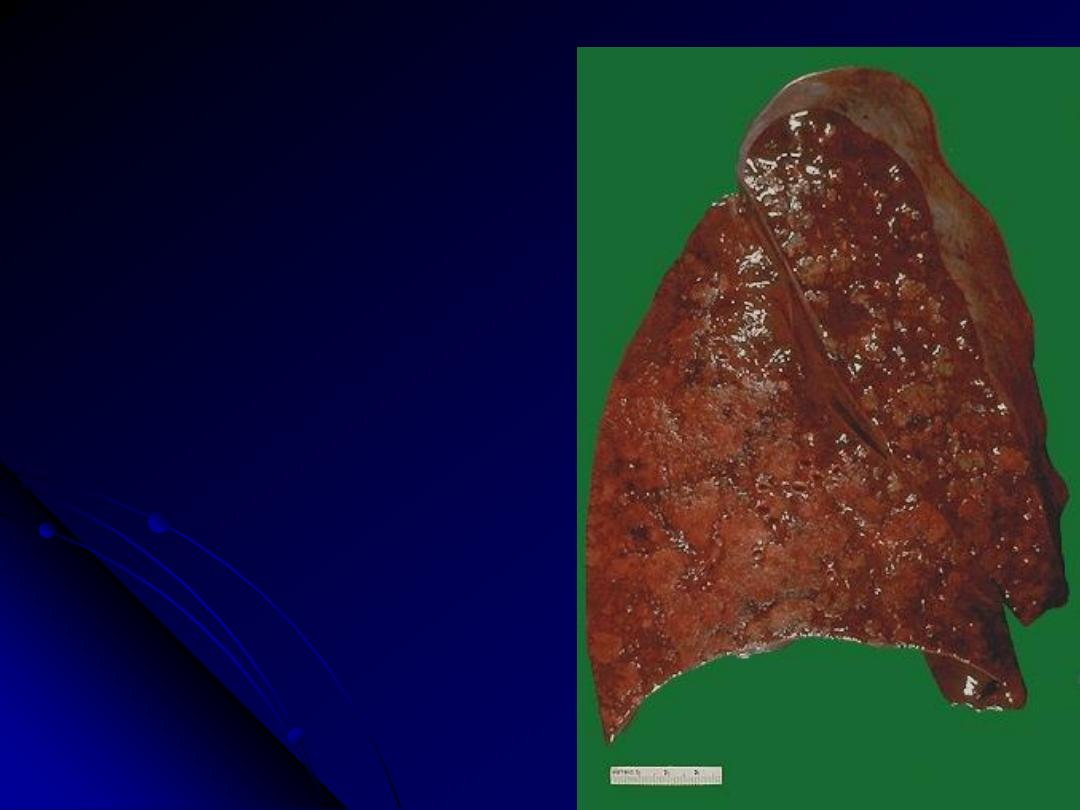
The cut surface of this lung demonstrates the
typical appearance of a
bronchopneumonia with areas of tan-
yellow consolidation. Remaining lung is
dark red because of marked pulmonary
congestion.
Bronchopneumonia (lobular pneumonia) is
characterized by patchy areas of pulmonary
consolidation. These areas become almost
confluent in the left lower lobe on the
bottom left of the photograph. The areas
of consolidation are firmer than the
surrounding lung.
Bronchopneumonia
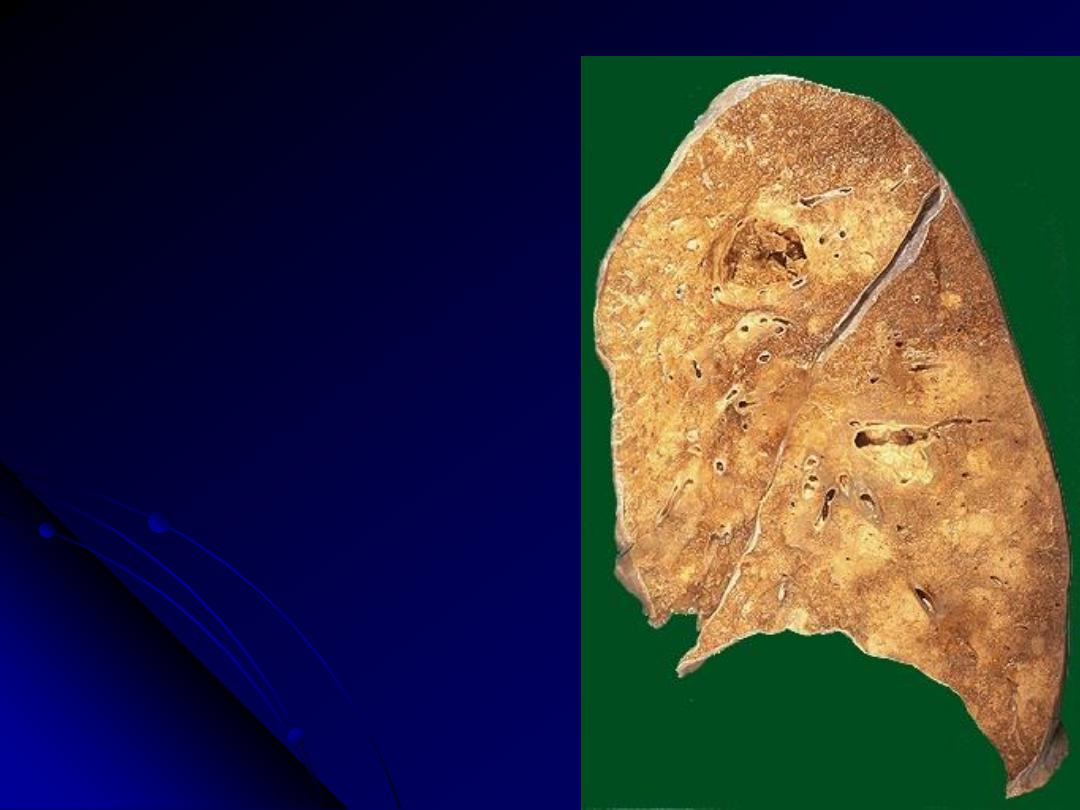
Two lung abscesses, one in the upper
lobe and one in the lower lobe of this left
lung. An abscess is a complication of
severe pneumonia, most typically from
virulent organisms such as S. aureus.
Abscesses complicating bronchopneumonia
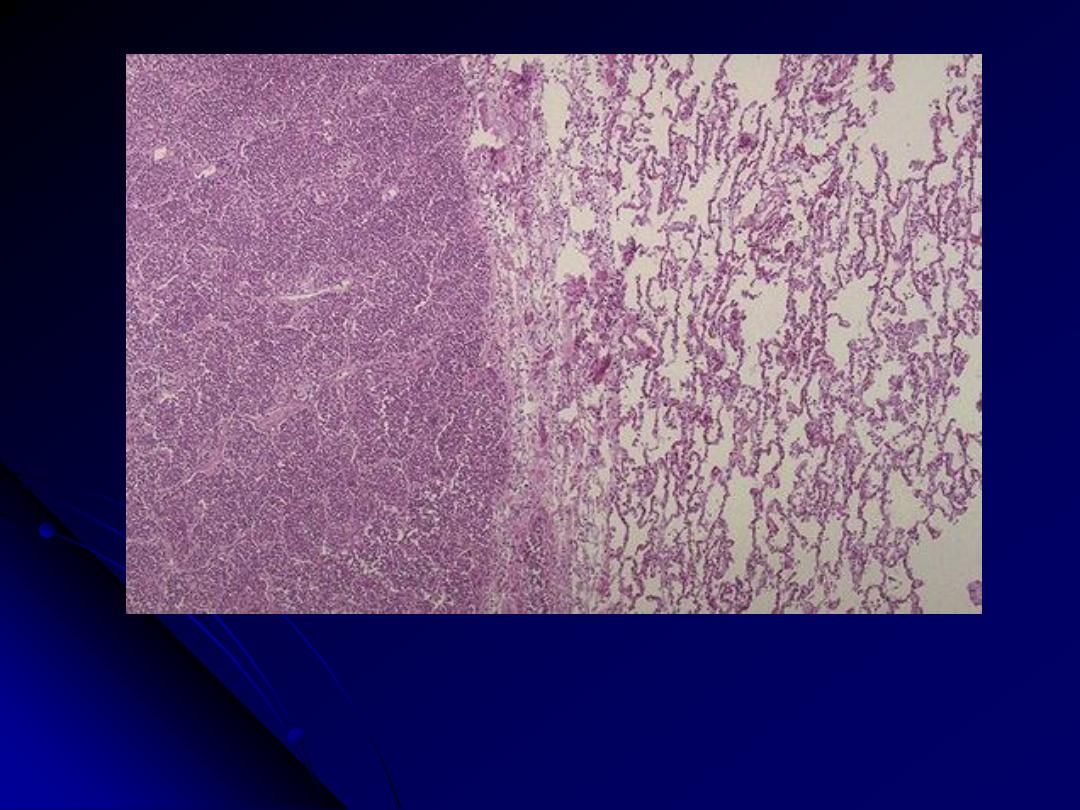
At the left the alveoli are filled with a neutrophilic exudate that corresponds to the areas of
consolidation seen grossly with the bronchopneumonia. This contrasts with the aerated
lung on the right of this photomicrograph.
Bronchopneumonia
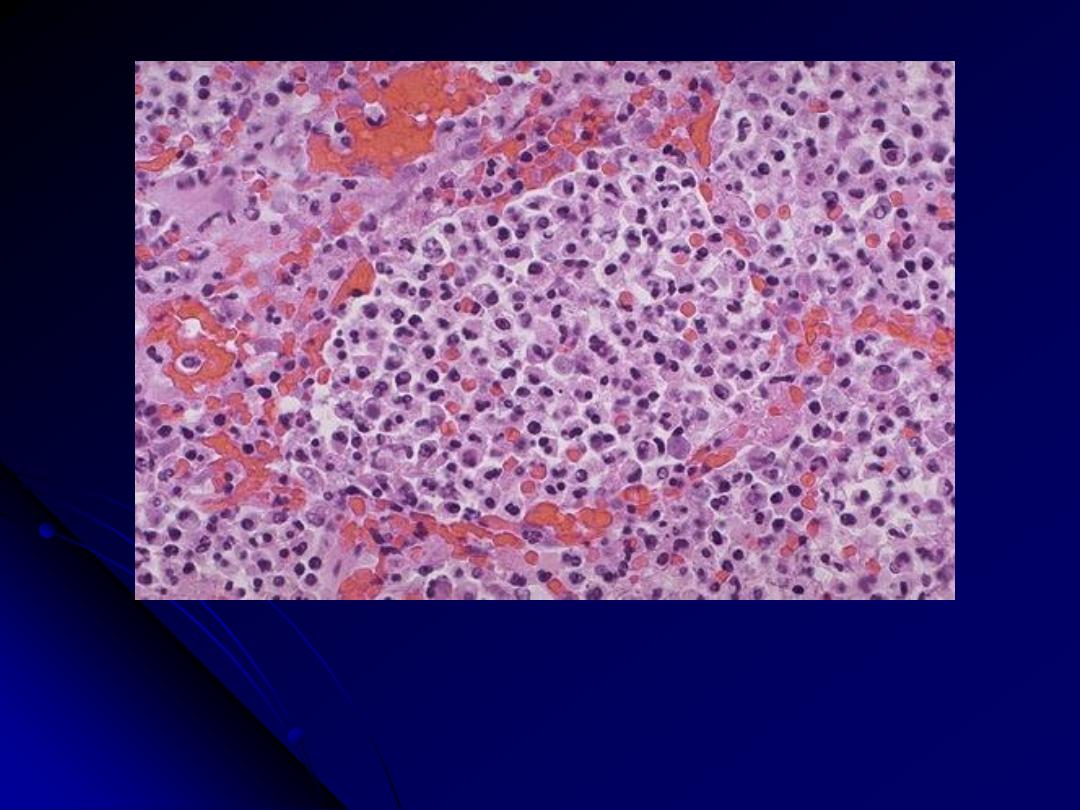
At high magnification, the alveolar exudate of mainly neutrophils is seen. The
surrounding alveolar walls have capillaries that are dilated and filled with
RBC's. Such an exudative process is typical for bacterial infection. This
exudate gives rise to the productive cough of purulent yellow sputum seen
with bacterial pneumonias.
Bronchopneumonia

Complications of Pneumonia:
Abscess formation
Empyema
Organization
Bactremic dissemination

Community Acquired
Atypical
Pneumonia
:
Acute febrile respiratory disease.
Patchy inflammatory changes confined to
alveolar septa and pulmonary interstitium.
Moderate amount of sputum, no physical
findings.
Attachment of organisms to upper
respiratory tract epithelium leading to
necrosis and inflammatory response.

Pathologic features of atypical pneumonias
Regardless of cause, the morphologic patterns are similar.
The process may be patchy, or it may involve whole lobes bilaterally or
unilaterally.
Grossly,
there are red-blue, congested areas.
Microscopically
, the inflammatory reaction is largely confined within the
alveolar walls, which are widened by edema & mononuclear
inflammatory infiltrate of lymphocytes, histiocytes, and, occasionally,
plasma cells.
In contrast to bacterial pneumonias
, alveolar spaces are free of cellular
exudates.
In severe cases ARDS may develop.
Identifying the causative agent can be difficult. Tests for Mycoplasma
antigens and polymerase chain reaction (PCR) testing for Mycoplasma
DNA are available. Patients with community-acquired pneumonia for
which a bacterial agent seems unlikely are treated with a macrolide
antibiotic effective against Mycoplasma and Chlamydia pneumoniae,
because these are the most common treatable pathogens.

Influenza Infections
The influenza virus is RNA virus, bound by a
nucleoprotein
that determines the virus type
(A, B, or C)
.
The spherical surface of the virus is a lipid bilayer containing
the viral
hemagglutinin and neuraminidase
, which determine
the
subtype
(e.g., H1N1, H3N2, etc.).
Host antibodies to the hemagglutinin and neuraminidase
prevent and ameliorate, respectively, future infection with the
influenza virus.
The type A viruses
are the major cause of pandemic and
epidemic influenza infections.
Epidemics of influenza occur through
mutations
of the
hemagglutinin and neuraminidase antigens that allow the virus
to escape most host antibodies
(antigenic drift).

Pandemics
, which last longer and are more widespread
than epidemics, may occur when both the hemagglutinin
and neuraminidase are replaced through recombination of
RNA segments with those of animal viruses, making all
animals susceptible to the new influenza virus
(antigenic
shift).
Commercially
available
influenza
vaccines
provide
reasonable protection against the disease, especially in
vulnerable infants and elderly individuals.
A particular subtype of
avian influenza ("bird flu," H5N1)
has caused massive outbreaks in domesticated poultry in
parts of
Southeast Asia
in the last few years; this strain is
particularly dangerous, since it has the potential to "jump"
to humans and thereby cause an unprecedented, worldwide
influenza pandemic.

The 2009 outbreak of
influenza A virus subtype H1N1
is an
epidemic of a new strain of influenza virus identified in April
2009, commonly referred to as
"Swine flu."
It is thought to be a
mutation of four known strains of influenza A virus subtype
H1N1: one
endemic in humans, one endemic in birds, and two
endemic in pigs (swine).
The signs of infection with swine flu are similar to influenza.
People at higher risk of serious complications include people age
65 years and older, children younger than 5 years old, pregnant
women, people of any age with chronic medical conditions (such
as asthma, diabetes, or heart disease), and people who are
immunosuppressed.
Transmission is through Sneezes or coughs, and contaminated
objects (touching something with flu viruses on it and then
touching your mouth or nose).
Influenza viruses are not known to be transmissible to people
through eating processed pork or other food products derived
from pigs."

Severe Acute Respiratory Syndrome (SARS)
This first appeared in the
end of 2002 in China
, and
subsequently spread to several neighboring countries
(Hong Kong, Taiwan etc,), where large outbreaks also
occurred.
Between 2002 and 2003, when the outbreak ended, over
8,000 cases and about 750 deaths had been ascribed to
SARS.
The cause is a previously undiscovered
coronavirus
(SARS-CoV),
which has the ability to infect the lower
respiratory tract and induce viremia.
The lungs of patients dying of SARS, usually shows
ARDS changes with multinucleated giant cells.

3. Nosocomial Pneumonia (hospital-acquired)
defined as
"pulmonary infections acquired in the course of a hospital stay".
They are common in hospitalized persons with severe illness, immune
suppression, or prolonged antibiotic therapy. Those on mechanical
ventilation are also susceptible; infections acquired in this setting are
designated
ventilator-associated pneumonia
. Gram-negative rods and
S. aureus are the most common offenders.
4. Aspiration Pneumonia
occurs in markedly debilitated patients
or those who aspirate gastric contents either while unconscious (e.g.,
after a stroke) or during repeated vomiting. The resultant pneumonia is
partly chemical
, resulting from the extremely irritating effects of the
gastric acid, and
partly bacterial
. Recent studies implicate
aerobes (S.
pneumoniae, S. aureus, H. influenzae, and Pseudomonas aeruginosa)
more commonly than anaerobes (such as Bacteroides).
This type of
pneumonia is often necrotizing with a fulminant clinical course. In
those who survive,
absces
s formation is a common complication
5. Necrotizing pneumonia & Lung Abscess
Lung Abscess
refers to "a localized area of suppurative necrosis
within the pulmonary parenchyma, resulting in the formation of one
or more large cavities".
Necrotizing pneumonia
often coexists or evolves into lung abscess,
making the distinction between the two somewhat subjective.
The causative organism may be introduced into the lung by any of
the following mechanisms:
a. Aspiration of infective material
from carious teeth or infected
sinuses or tonsils, as during oral surgery, anesthesia, coma, or
alcoholic intoxication and in debilitated patients with depressed
cough reflexes.
b. Aspiration of gastric contents,
usually accompanied by infectious
organisms from the oropharynx.
to two-thirds of cases.

c. As a complication of necrotizing bacterial pneumonias
,
particularly those caused by S. aureus, Streptococcus
pyogenes, K. pneumoniae, Pseudomonas spp. etc.
d. Mycotic infections and bronchiectasis
e. Bronchial obstruction,
particularly with bronchogenic
carcinoma.
f. within a necrotic portion of a tumor
g. Septic embolism
, from septic thrombophlebitis or from
infective endocarditis of the right side of the heart.
h. hematogenous spread
of bacteria in disseminated
pyogenic infection as with staphylococcal bacteremia.
Anaerobic bacteria are present in almost all lung abscesses,
sometimes in vast numbers, and they are the exclusive
isolates in one-third to two-thirds of cases.

Microscopic features
There is suppurative liquefactive necrosis
Depending on the chronicity, the above may be surrounded by variably
thickened fibrous tissue and mononuclear infiltration by variable
amounts of (lymphocytes, plasma cells, macrophages).
Complications
1. Rupture into the pleural cavity producing bronchopleural fistulas, the
consequence of which is pneumothorax or empyema.
2. Embolization of septic material to the brain, gives rise to meningitis or
brain abscess.
3. Secondary amyloidosis may develop in chronic cases
Course & prognosis
The manifestations of a lung abscess are similar to those of bronchiectasis
(productive cough of copious, foul sputum).
Abscesses occur in up to15% of
persons with bronchogenic carcinoma;
thus, when a lung abscess is
suspected in an older person, underlying carcinoma must be considered.
Overall, the mortality rate is in the range of 10%.

Pathological Features
Abscesses vary in diameter from
very small lesions to large
cavities of 5 cm or more.
The localization and number depend on the mode of
development. Pulmonary abscesses resulting from
aspiration
are
much more common on
the right side
(more vertical airways),
and are mostly
single
. In this location, they tend to occur in the
posterior segment of the upper lobe and in the apical segments of
the lower lobe.
Abscesses that develop in the
course of pneumonia or
bronchiectasis are commonly multiple, basal, and diffusely
scattered.
Septic
emboli
and
abscesses
arising
from
hematogenous seeding are commonly multiple and may affect
any region of the lungs.
As the focus of suppuration enlarges, it usually ruptures into
airways. Thus, the contained exudate may be partially drained,
producing an air-fluid level on radiographic examination.

6. Chronic Pneumonia
is mostly a localized lesion in an
immunocompetent person
, with or without regional lymph node involvement.
There is typically
granulomatous inflammation
, which may be due to bacteria
(e.g., M. tuberculosis) or fungi. In the
immunocompromised,
there is usually
systemic dissemination of the causative organism, accompanied by widespread
disease.
Tuberculosis
Tuberculosis is by far the most important of the chronic penumonia; it causes
6% of all deaths worldwide. Tuberculosis is
"a communicable chronic
granulomatous disease caused by Mycobacterium tuberculosis".
It usually involves
the lungs but may affect any organ or tissue. Tuberculosis thrives wherever there
is poverty, crowding, and chronic debilitating illness; elderly, with their
weakened defenses, are also susceptible. Certain disease states also increase the
risk:
1. Diabetes mellitus
2. Hodgkin lymphoma
3. Chronic lung disease (particularly silicosis)
4. Chronic renal failure
5. Malnutrition & Alcoholism
6. Immunosuppression including HIV infection.
7. Pneumonia in the Immunocompromised Host
The appearance of a pulmonary infiltrate and signs of infection (e.g.,
fever) are some of the most common and serious complications in
immunocompromised persons whose immune and defense systems are
suppressed by disease, immunosuppression for organ transplants,
malignancy,
or
irradiation.
A
wide
variety
of
opportunistic
microorganisms, many of which rarely cause infection in normal hosts,
can cause these pneumonias.
Examples of pulmonary opportunistic pathogens include
1. Bacteria (P. aeruginosa, Mycobacterium spp., etc)
2. Viruses (cytomegalo and herpesvirus viruses)
3. Fungi (P. jiroveci, Candida spp., Aspergillus spp., and
Cryptococcus neoformans).

Pneumocystis Pneumonia
P. jiroveci (formerly P. carinii), an opportunistic infectious agent long
considered to be a protozoan, is now believed to be more closely related to
fungi.
Virtually all persons are exposed to Pneumocystis during early childhood,
but in most the infection remains latent.
Reactivation and clinical disease occurs almost exclusively in those who are
immunocompromised
(very commonly in AIDS patients but also in the
severely malnourished infants).
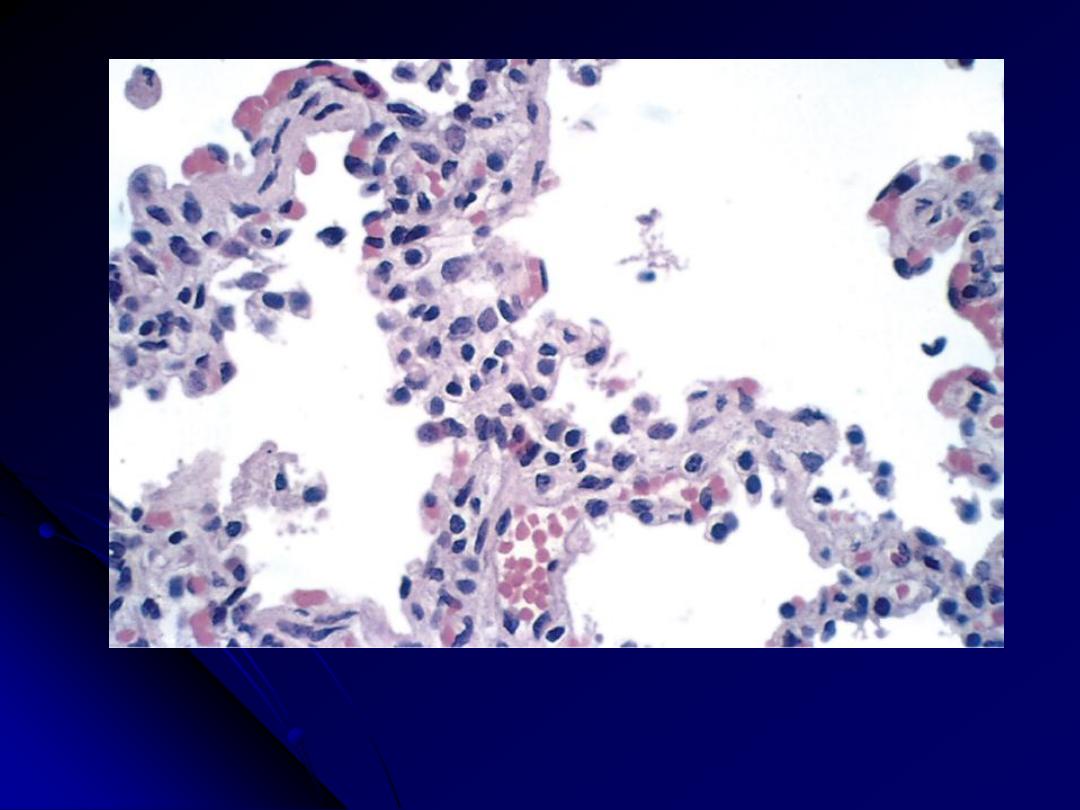
Pneumocystis produce an
interstitial pneumonitis with a characteristic
intra-alveolar foamy, pink-staining exudate with H&E stains ("cotton
candy" exudate).
Silver stains of tissue sections reveal
cup-shaped cyst in the alveolar
exudates.
The most sensitive and effective method of diagnosis is to identify the
organism in bronchoalveolar lavage fluids or in a transbronchial biopsy
specimen.
Immunofluorescence antibody kits and PCR-based assays have also become
available for use on clinical specimens
.
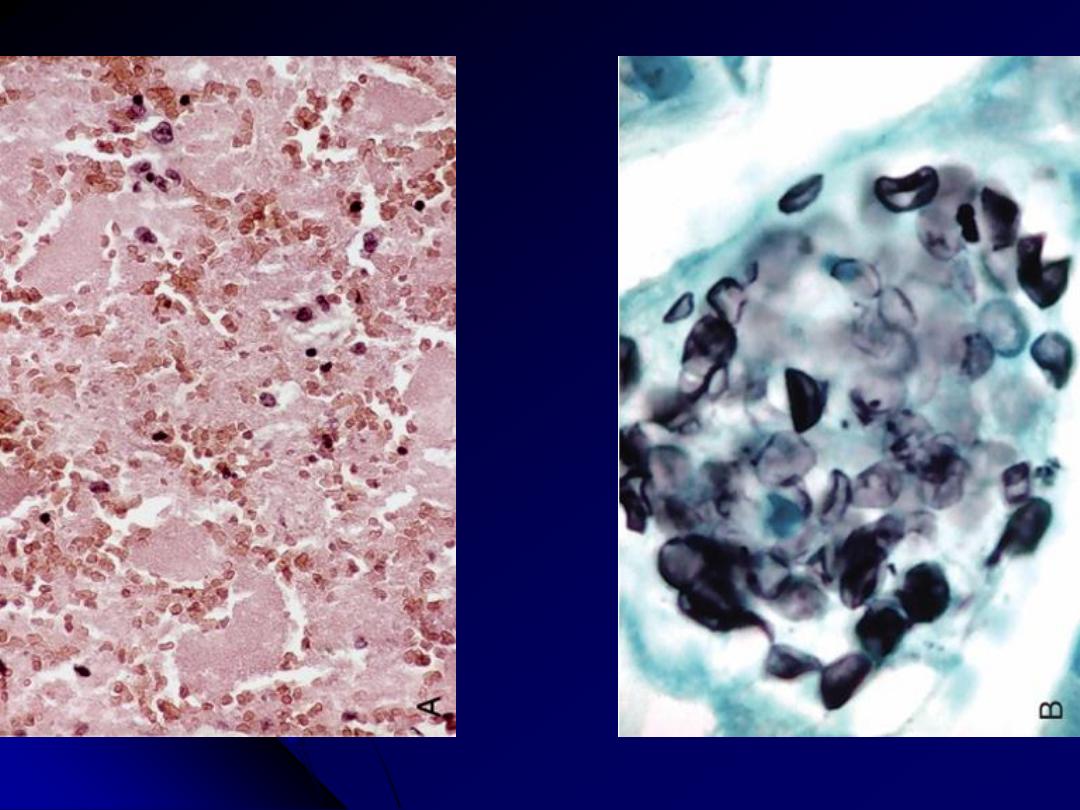
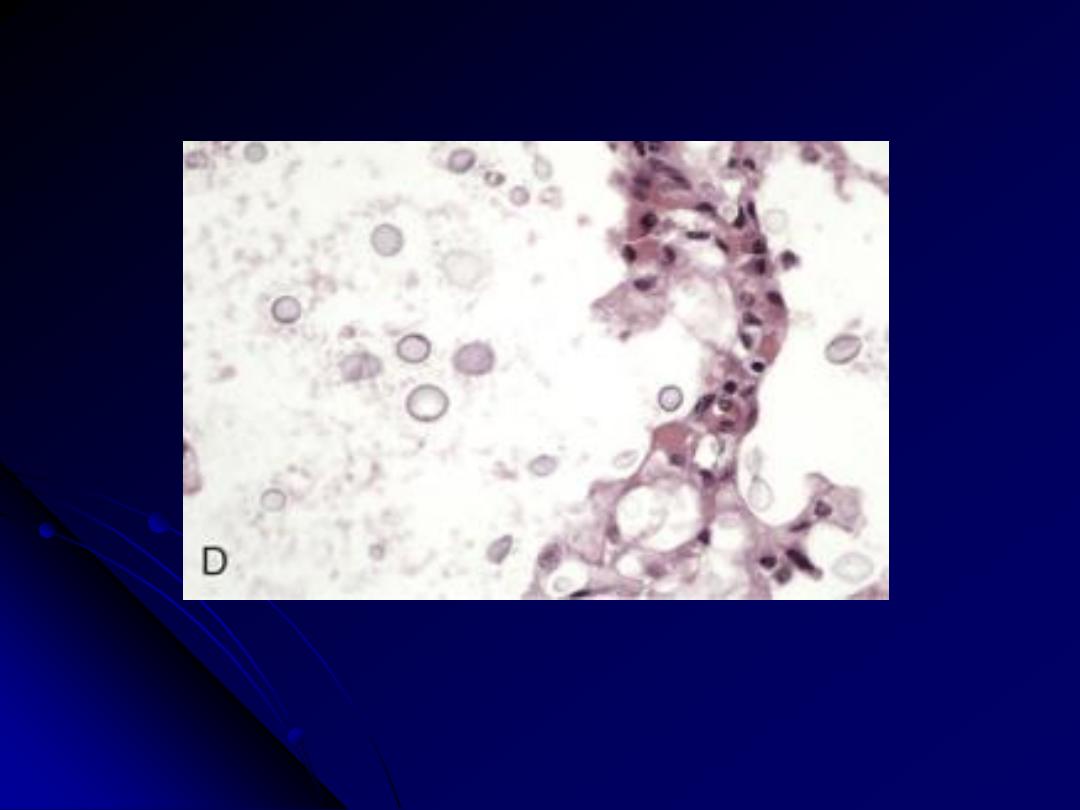
A, The alveoli are filled with a characteristic foamy "cotton candy" exudate. B, Silver stain
demonstrates cup-shaped cysts (5-8 μm in diameter) within the exudate.
Pneumocystis pneumonia
Opportunistic Fungal Infection
1. Candidiasis
aCndida al
bicans is the most frequent disease-causing fungus.
It is a normal inhabitant of the oral cavity.
Systemic candidiasis (with associated pneumonia) is a disease
that is restricted to immunocompromised patients.
In tissue sections, C. albicans demonstrates yeastlike forms,
pseudohyphae, and true hyphae.
The organisms may be visible with routine hematoxylin and
eosin stains, but a variety of special "fungal" stains
(methenamine-silver, periodic acid-Schiff) are commonly used
to highlight the pathogens.
Candida pneumonia
usually presents radiographically as
bilateral
nodular
infiltrates,
resembling
Pneumocystis
pneumonia.
The diagnosis of candidiasis is made by observing the characteristic pseudohyphae and budding yeasts
in tissue sections or exudates. (silver stain)
Candidiasis

2. Cryptococcosis
is caused by
C. neoformans
& is almost exclusively
presents
as
an
opportunistic
infection
in
immunocompromised hosts, particularly those with
AIDS or hematolymphoid malignancies.
The fungus, 5- to 10-μm yeast, has a thick, gelatinous
capsule, which is invaluable for the diagnosis.
The capsular antigen is the substrate for latex
agglutination assay, which is positive in more than
95% of patients infected with the organism.
Human
cryptococcosis
usually
manifests
as
pulmonary, central nervous system, or disseminated
disease.
Cryptococcosis of the lung in a patient with AIDS. The yeast forms are somewhat variable in size;
unlike in Candida, pseudohyphae are not seen.
Cryptococcosis
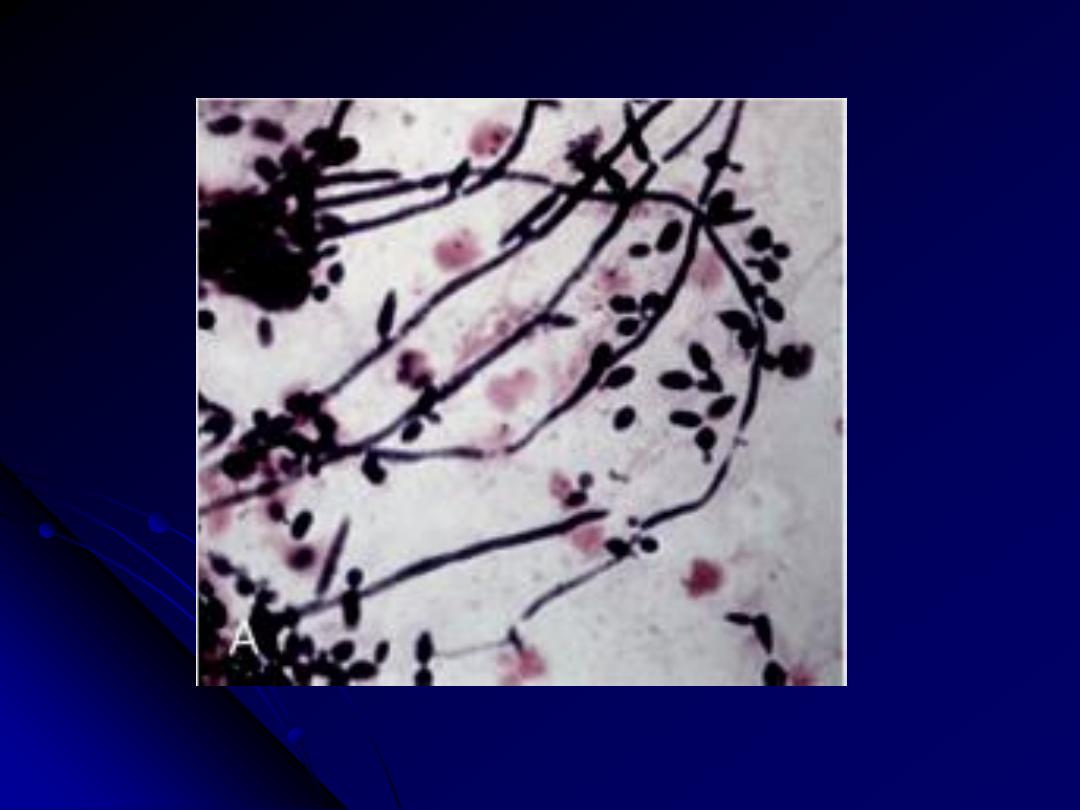
3. The Opportunistic Molds: Mucormycosis and
invasive aspergillosis
are
uncommon
infections
almost
always
limited
to
immunocompromised hosts.
The hyphae of Mucormycosis are nonseptate and branch at right
angles; in contrast, the hyphae of Aspergillus species are septate and
branch at more acute angles
Pulmonary mucormysosis can cause cavitary lung lesions or may
present radiologically with diffuse "miliary" involvement.
Invasive aspergillosis
is associated with necrotizing pneumonia, the
fungus tends to invade blood vessels, and thus systemic dissemination,
especially to the brain, is often a fatal complication.
Allergic pulmonary aspergillosis
occurs in patients with asthma who
develop an exacerbation of symptoms due to reaction to the fungus
growing in the bronchi.
Aspergilloma ("fungus ball")
occurs by colonization of preexisting
pulmonary cavities (e.g., ectatic bronchi or lung cysts, post-tuberculous
cavitary lesions) by the fungus.
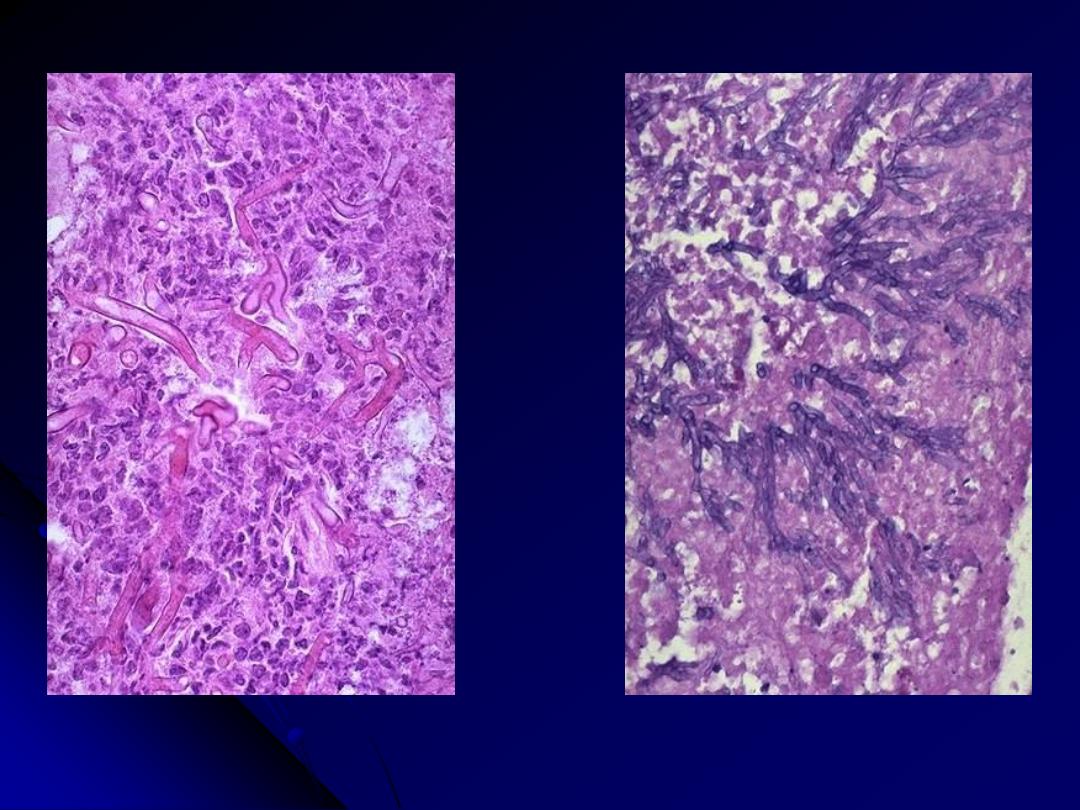
Rt. Mucor produces broad, short, non-septate hyphae that branch at right angles.
Lt. The hyphae of Aspergillus are septate and branch at more acute angles
Mucormycosis Vs Aspergillosis
