

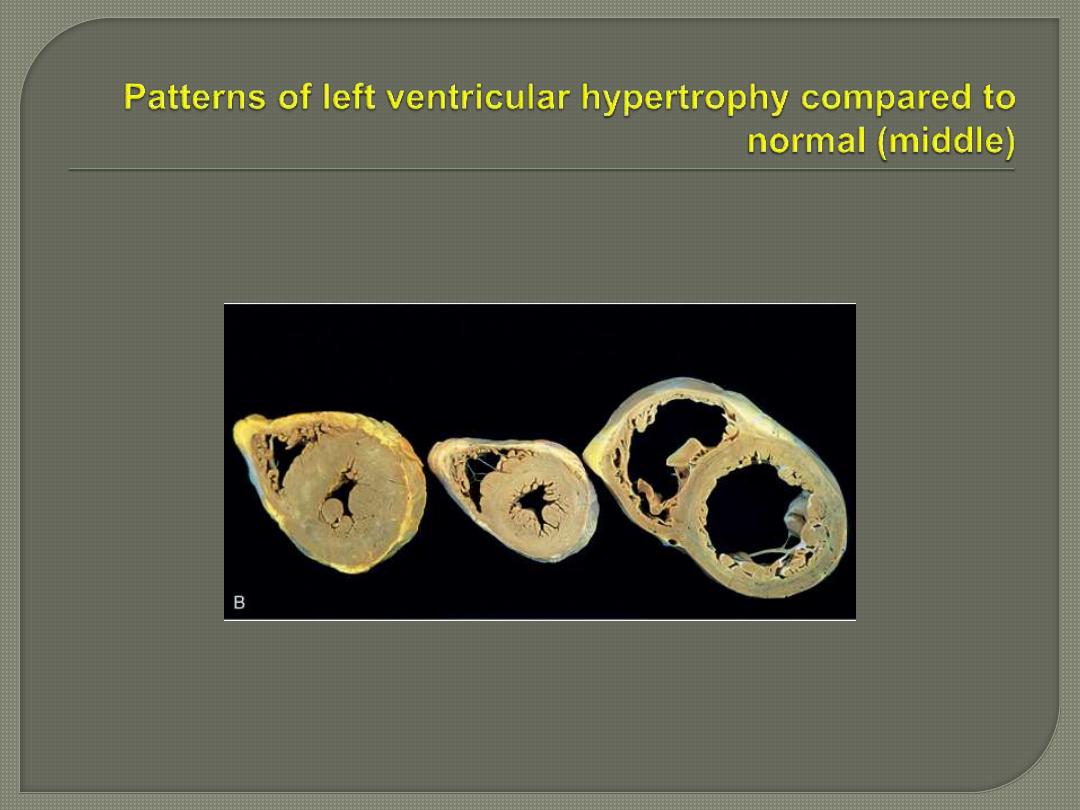

In pressure-overload hypertrophy there is
concentric hypertrophy of the left ventricle.
This hypertrophy may reduce the cavity
diameter i.e. restrict diastolic filling.
In contrast, in volume-overload hypertrophy
there is also dilation that increases the size
of the ventricular cavity
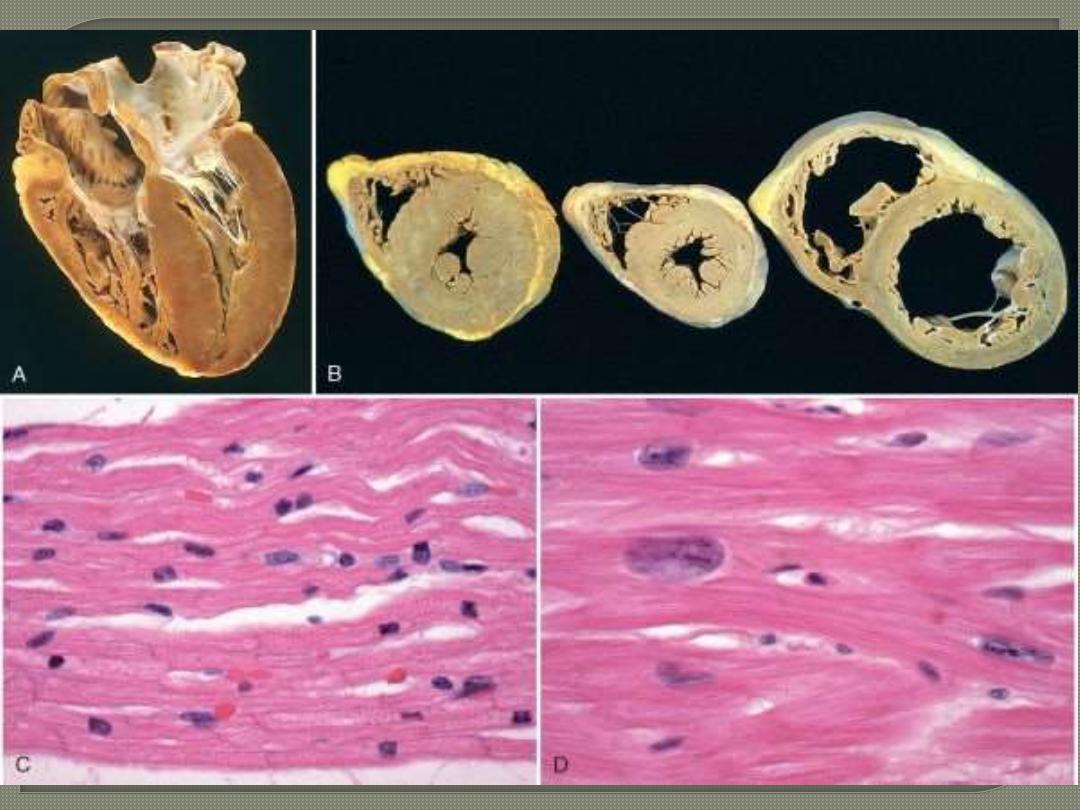
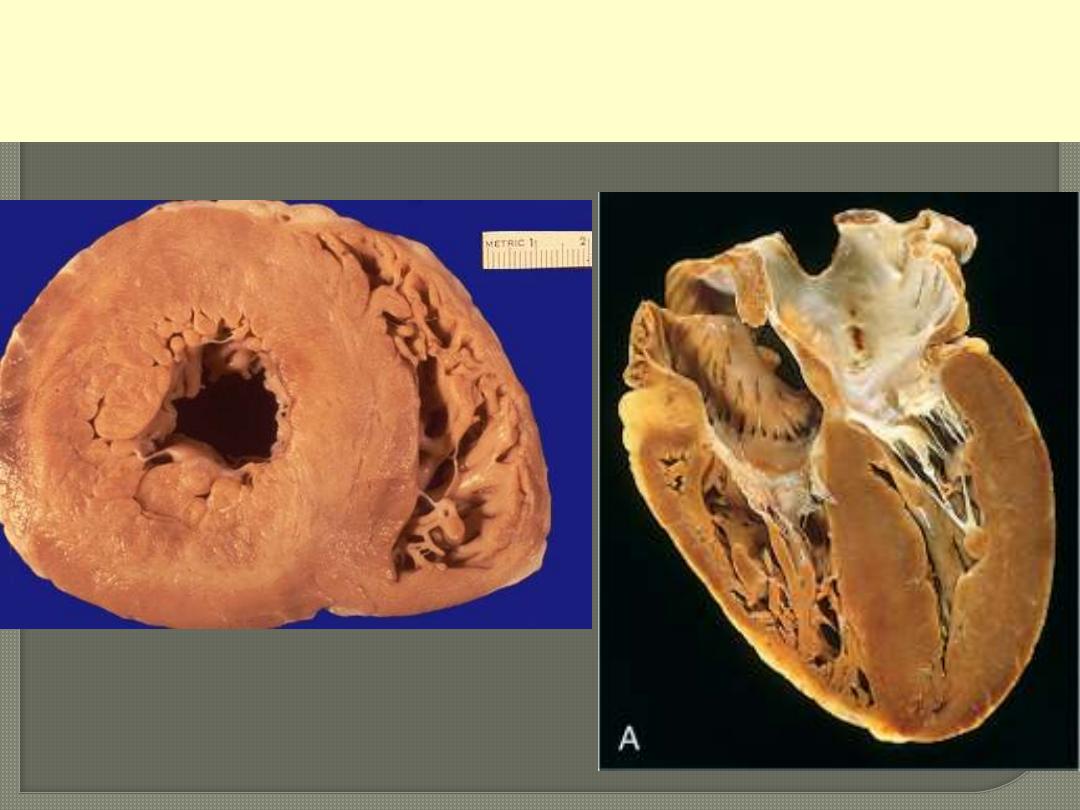
A post-mortem heart from a case with long standing aortic stenosis.
The photos below represent a cross section & longitudinal four
chamber-revealing section. Shows marked hypratrophy.

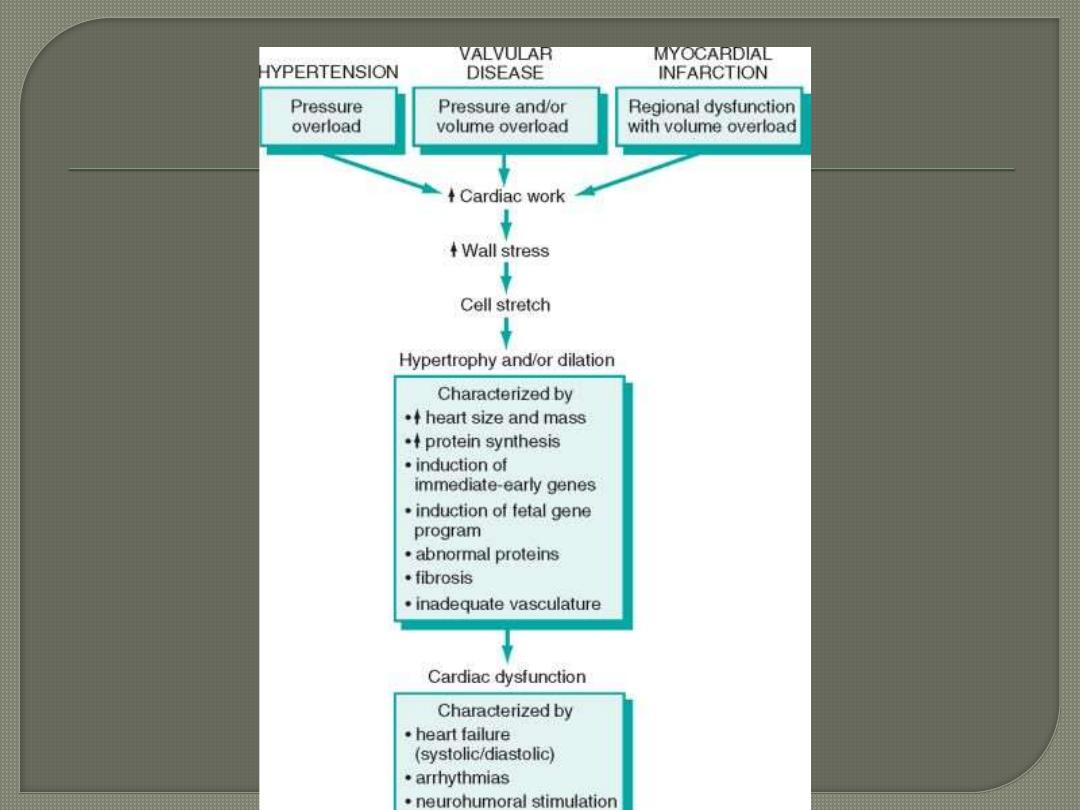
Hypertrophied heart needs increased oxygen
consumption
sustained cardiac hypertrophy often
progresses to cardiac failure.
physiologic hypertrophy that is induced by
regular tough exercise is rather an extension
of normal growth and has minimal or no
harmful effect.

Congestive Heart Failure is the end point of many
cardiac diseases.
The failing heart is unable to pump sufficient blood to
meet the requirements of the body.
Inadequate cardiac output (forward failure) means that
the failing ventricle can no longer pump the whole
blood delivered to it by the venous circulation. Thus,
there is an associated increase in venous pressure &
congestion of the venous circulation (backward
failure). So other organs are eventually affected by
some combination of forward and backward failure.

Excluded from HF definition are
conditions in which inadequate cardiac
output is not due to cardiac
abnormality e.g. shock states including
blood loss

Heart failure is a common eventual outcome of
many forms of heart disease.
Left-sided and right-sided failure can occur
independently. Nevertheless, because the
CVS is a closed circuit, failure of one side
(particularly the left side) often produces
excessive strain on the other side,
terminating in global heart failure.

Causes of left-sided cardiac failure include
1.
IHD (the most common)
2.
Systemic hypertension (the next most
common)
3. Mitral or aortic valve disease
4. Primary diseases of the myocardium
(cardiomyopathies

Pathophysiological effects of Left-sided heart
failure
The clinical effects of LHF primarily result
from chronic pulmonary venous congestion
and the consequences of diminished cardiac
output i.e. multi-organ ischemia.

The morphological changes in left-HF are
divided into cardiac & extracardiac.
Cardiac changes: in addition to the causative
disease (e.g. myocardial infarction, valvular
deformity, etc.), there is usually LVH and
often dilation. The latter often leads to
secondary enlargement of the left atrium with
resultant atrial fibrillation that may lead to
further reduction of the stroke volume and
blood stasis with possible thrombus
formation (particularly in the atrial
appendage and the risk of systemic
embolization.
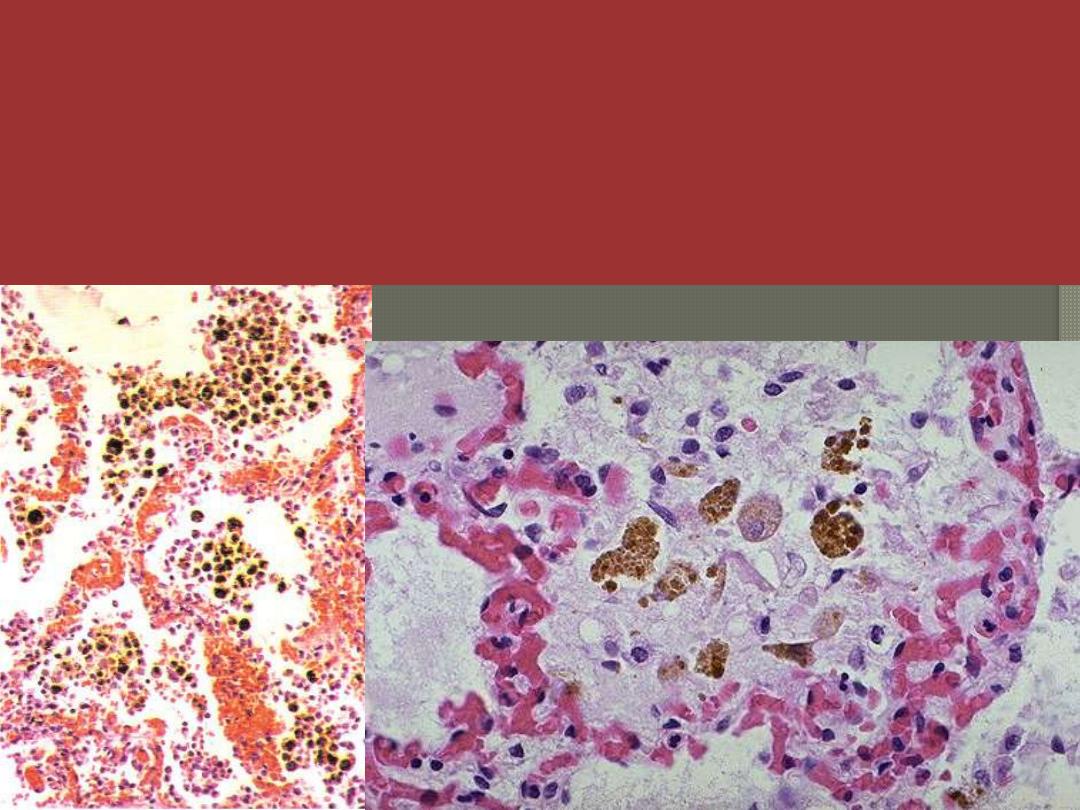
Chronic venous congestion (CVC) lung: low power (Lt) and high Power (Rt) views. Note: 1.
engorgement of septal capillaries 2. hemosiderin-laden macrophages (the hemosiderin granules
within cytoplasm of macrophages appear brownish)
• Changes in the lungs due to chronic venous congestion
grossly as (Brown induration).
Microscopically there is congestion of venules and capillaries,
edematous widening of alveolar septa, accumulation of edema
fluid in the alveolar spaces, heart failure cells and later increased
interstitial fibrosis
.
CVC lung

The Kidneys suffer a reduction in perfusion
due to reduced cardiac output, which
activates the renin-angiotensin-aldosterone
system, inducing retention of salt and water
with consequent expansion of the
interstitial fluid and blood volumes.
If the perfusion deficit of the kidney
becomes severe, impaired excretion of
nitrogenous products may cause azotemia.

Brain changes
Hypoxic encephalopathy may occur in
far-advanced LHF, causing irritability
and restlessness, which may progress
to coma.

Causes of right-sided heart failure include
1- Left ventricular failure (the most common);
it is due to its associated pulmonary
congestion with elevation of pulmonary
arterial pressure.
2. Intrinsic diseases of the lung parenchyma
and/or pulmonary vasculature (cor
pulmonale)
3. Right sided valve diseases
4. Congenital heart diseases.

Right-sided heart failure (RHF)
Chronic pulmonary hypertension (secondary
to LHF, or pulmonary disease ) predispose
to RVH and often dilation which are confined
to the right ventricle and atrium in pure RHF.
The major morphologic and clinical effects
of pure right-sided heart failure differ from
those of left-sided heart failure in that
Pulmonary congestion is minimal
Engorgement of the systemic and portal
venous systems is prominent.

1-CVC of liver
2-Congestive splenomegaly and Ascites can occur
due to increased portal venous congestion.
3-Chronic bowel wall congestion and edema
interferes absorption of nutrients.
4-Kidneys: congestion of the kidneys is more marked
with RHF than with LHF, leading to greater fluid
retention, peripheral edema, and more pronounced
azotemia.
5-Brain: venous congestion and hypoxic
encephalopathy.

1-CVC-LIVER (Nutmeg liver)
Morphological changes of
RHF ,
The Liver is usually increased
in size and weight (congestive
hepatomegaly),
cut section that displays
nutmeg appearance.
Sometimes when LHF is also
present, the severe central
hypoxia produces
centrilobular necrosis (central
hemorrhagic necrosis). With
long-standing severe RHF,
the central areas can become
fibrotic, creating cardiac
fibrosis.
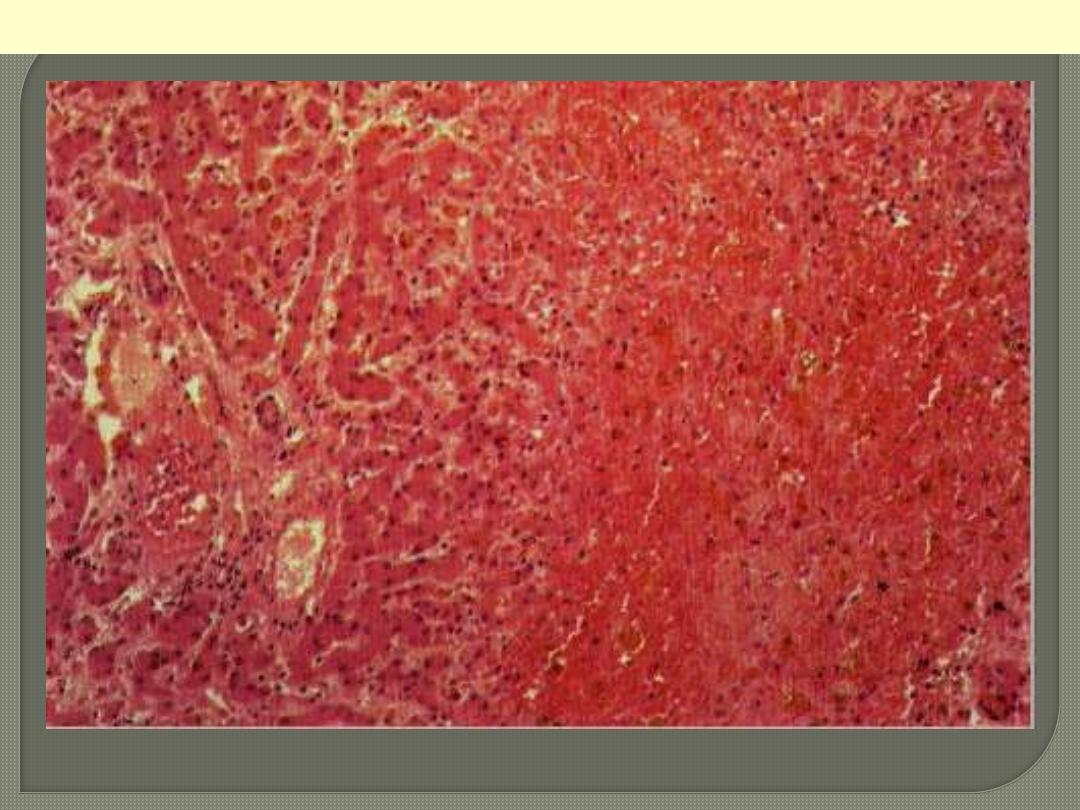
The centrilobular area shows accumulation of blood with damage to the centrilobular liver
parenchyma. The portal tract and surrounding periportal parenchyma, however, are relatively spared.
CVC liver
Portal & periportal zone
Centrilobular zone

Pleural and Pericardial Spaces: accumulation of fluid
(effusion) in the pleural space .
Thus, while pulmonary edema indicates left-sided
heart failure, pleural effusions accompany right-
sided heart failure. Pleural effusions can cause partial
collapse of the corresponding lung.
Subcutaneous peripheral edema of the dependent
portions, especially ankle and pretibial soft tissues,
is a hallmark of RH. In chronically bedridden
patients, the edema may be primarily presacral.
Generalized massive edema (anasarca) may also
occur in severe, advanced cases
.

Heart disease, especially ischemic, is the
predominant cause of disability and death. It
accounts for about 40% of all postnatal deaths; this
is twice the number of deaths caused by all forms of
cancer combined.
Five categories of disease account for nearly all
cardiac mortality
Congenital heart disease
Ischemic heart disease (IHD)
Hypertensive heart disease
Valvular heart disease (Rheumatic, etc.)
Cardiomyopathies (non-ischemic)

ISCHEMIC HEART DISEASE
IHD resulting from an imbalance
between the supply and demand of
the heart for oxygenated blood.
four ischemic syndromes may result
1- Angina pectoris
2- Myocardial infarction
3- Chronic ischemic heart disease
4- Sudden cardiac death, which may be
superimposed on any of the above
three.

The heart may suffer a deficiency of oxygen supply in
the following circumstances
1. Reduction in coronary blood flow (90% of the cases)
Atherosclerosis (the main cause)
Coronary artery spasm
Hemodynamic derangement (as in shock and HF)
Non-atherosclerotic coronary diseases (e.g. arteritis)
2. Increased demand
as in tachycardia, ventricular hypertrophy
3. Reduced oxygen carrying capacity of the blood
a- Anemia
b-Advanced lung diseases
c-Carbon monoxide poisoning
d- Cigarette smoking
e- Cyanotic congenital heart diseases

The role of coronary atherosclerosis:
Over 90% of patients with IHD have
advanced coronary atherosclerosis.
This is defined as having one or more
stenotic lesions causing at least 75%
reduction of the cross sectional area of
at least one of the major coronary
arteries.

The role of platelets:
Rupture of an atheromatous plaque
exposes subendothelial collagen, which
is thrombogenic causing platelet
adherence, activation, release reactions
resulting in the production of a large
pool of activated platelets within the
coronary system.
The aggregated platelets may lead to
Occlusive thrombosis
The activated platelets liberate
vasoactive products that include
thromboxane A2, histamine, and
serotonin, which contribute to a
possible coronary vasospasm.

long-term use of small doses of aspirin
have resulted in a reduction in death
from IHD due to inhibition of synthesis
of thromboxane A2, a potent aggregator
of platelets and a vasoconstrictor.
Diets rich in fish with their
polyunsaturated omega-3 fatty acids
substantially reduced platelet
aggregation.

The role of vasospasm:
Vasospasm of large atheromatous
epicardial arteries has been documented
angiographically in some patients with
angina or MI.
This may contribute to rupture or fissuring
of plaques leading to thrombosis and
platelet aggregation.
Platelets activation could initiate or
aggravate coronary artery spasm through
their products e.g. thromboxane A2.


The acute ischemic coronary syndromes include:
Unstable angina
Acute MI (transmural or subendocardial)
Sudden cardiac death
Slowly developing occlusions may stimulate
collateral vessels over time, which protect against
myocardial ischemia and infarction even with an
eventual high-grade stenosis.
Although only a single major coronary artery may be
affected by the stenosis, two or all three coronaries
(LAD, LCX, and RCA) are often involved.
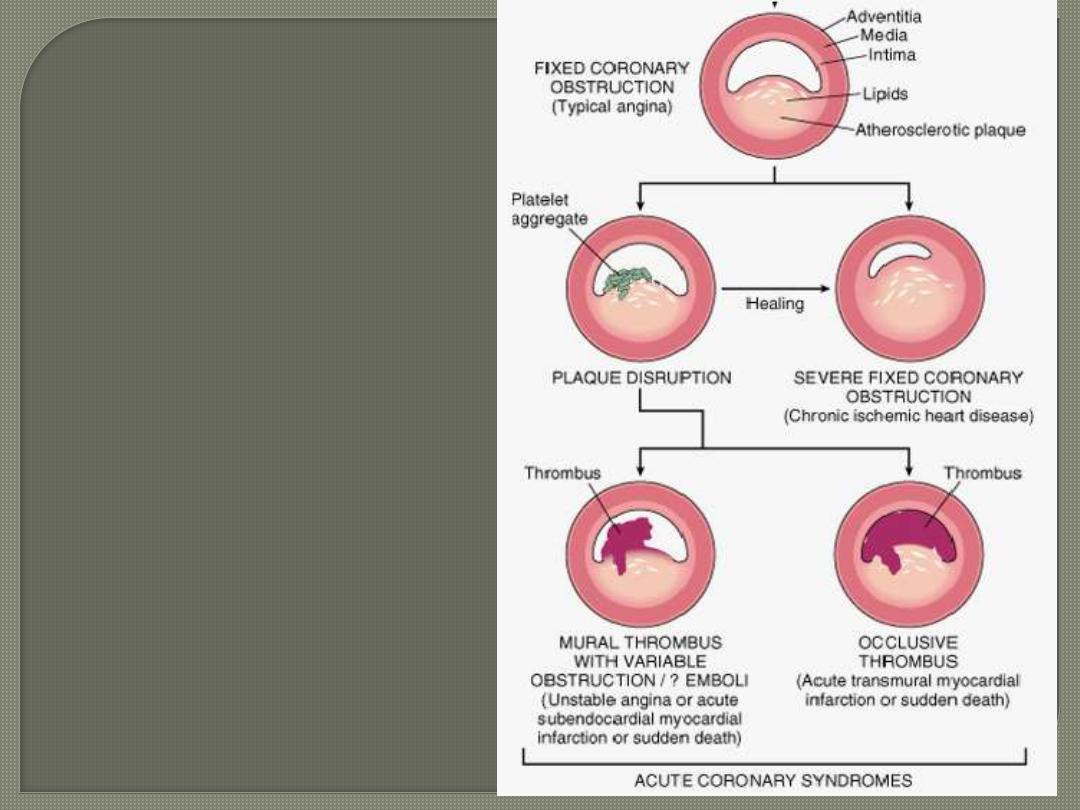
Sequencial progression of
Coronary artery lesion
. In acute transmural MI, the
usual event is an occlusive
thrombus superimposed on a
disrupted but partially stenotic
atheromatous plaque.
In contrast, with unstable angina,
acute subendocardial infarction,
or sudden cardiac death, the
luminal obstruction by
thrombosis is usually
incomplete.
Sudden cardiac death can be the
result of regional myocardial
ischemia that induces a fatal
ventricular arrhythmia (e.g.
ventricular fibrillation).

Angina pectoris is characterized by
paroxysmal, usually recurrent attacks of
substernal or chest discomfort or pain
caused by transient myocardial ischemia.
This ischemia is not sufficient enough to
cause infarction.
1- Stable (typical) angina is the most common
form that is caused by reduction of coronary
perfusion to a critical level by chronic fixed
stenosing atherosclerosis of 75% or greater
of the original lumen. causes symptomatic
ischemia whenever there is increased cardiac
workload, such as that produced by physical
activity, emotional excitement, etc. It is
usually relieved by rest or nitroglycerin.

2- Unstable angina is characterized by
progressively increased frequency and more
prolonged attacks of angina.
It is induced by disruption of an atherosclerotic
plaque with superimposed thrombosis and
possibly embolization to a more distal vessels
and/or vasospasm ,cause a severe reduction of
the arterial lumen by 90%.
Unstable angina lies intermediate between stable
angina on the one hand and MI on the other.

Myocardial infarction is the leading cause of death in
many countries. Over 50% of these fatalities occur
before the patient reaches the hospital
Morphologically MI are divided into two types
1-Transmural infarction: the vast majority of these
(90%) are caused by an occlusive coronary thrombus
overlying an ulcerated or fissured stenotic atheroma.
It seems that behind every acute MI a dynamic
interaction has occurred among several or all of the
following:
•
severe coronary atherosclerosis
•
acute atheromatous change (fissuring, ulceration,
etc.)
•
Platelete activation
•
superimposed thrombosis
•
vasospasm

2-Subendocardial myocardial infarction
The subendocardium is most
vulnerable region to any reduction in
coronary blood flow.
Almost always there is advanced
coronary atherosclerosis and then
thrombosis
There is a suspicion that a thrombus
often initiates the process, but is then
spontaneously lysed. In support of this
hypothesis is the beneficial effect of
fibrinolytic treatment of patients with
recently developed subendocardial
infarcts.

Sequence of gross changes.
1-Myocardial infarcts less than12 hours old
are usually inapparent on gross examination.
2-By 18-24 hours, the lesion displays pallor
or red-blue cyanotic discoloration (due to
stagnated, trapped blood)
3-Thereafter the infarct becomes
progressively a more sharply defined, yellow,
softened area

4-By the end of the first week it is rimmed
by a hyperemic, narrow zone of highly
vascularized connective tissue (line of
demarcation)
5-Over the succeeding weeks, the
necrotic muscles are progressively
replaced by granulation tissue.
6- Scarring is well advanced by the end of
six weeks, but the time required for total
replacement depends on the size of the
original infarct.

This cross section reveals a large myocardial infarction involving the anterior left ventricular wall and
septum. The color of the infarct is whitish-yellow. This is an example of pale (anemic) infarction.
MI involving LV anterior free wall and septum
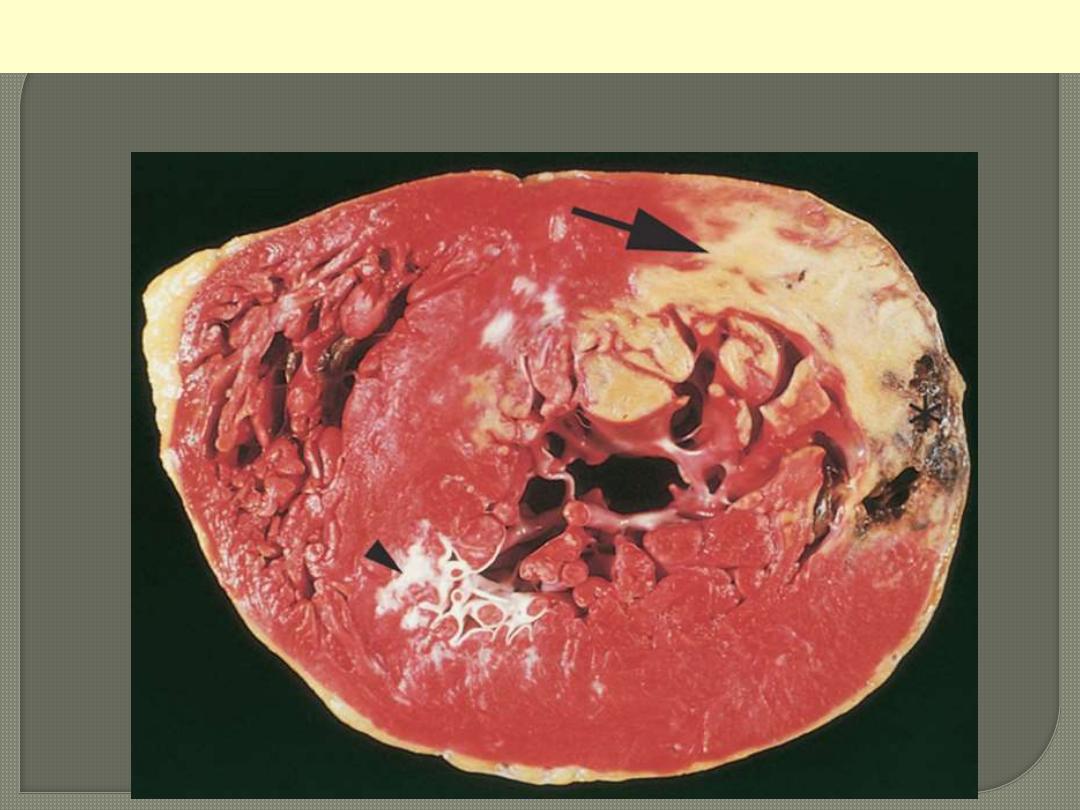
A cross section of a post-mortem heart; posterior wall above.
Describe

1-Typically the myocardial cells show
coagulative necrosis. This is not detectable for
the first 4 to 8 hours.
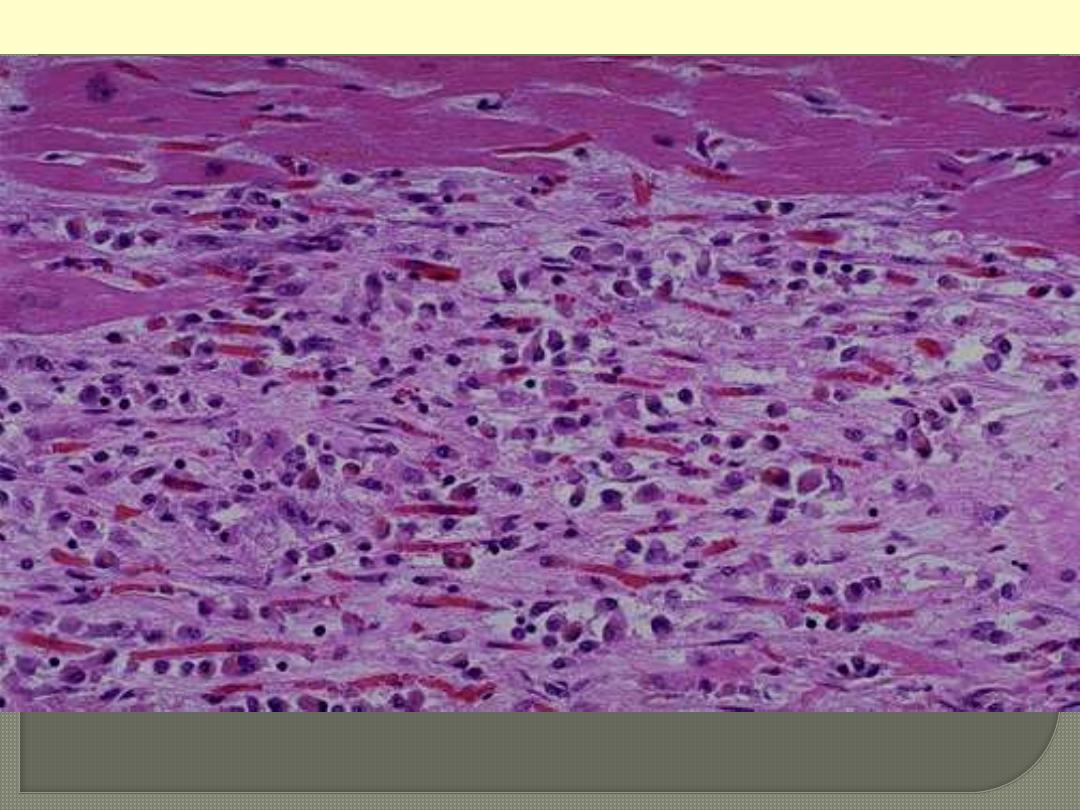
2-The necrotic area is invaded by acute
inflammatory cells & later by macrophages.
3-Gradual replacement of by granulation tissue
froms a line of demarcation.
4-Then healing by fibrosis.
Once an MI is completely healed, it is
impossible to distinguish its age.
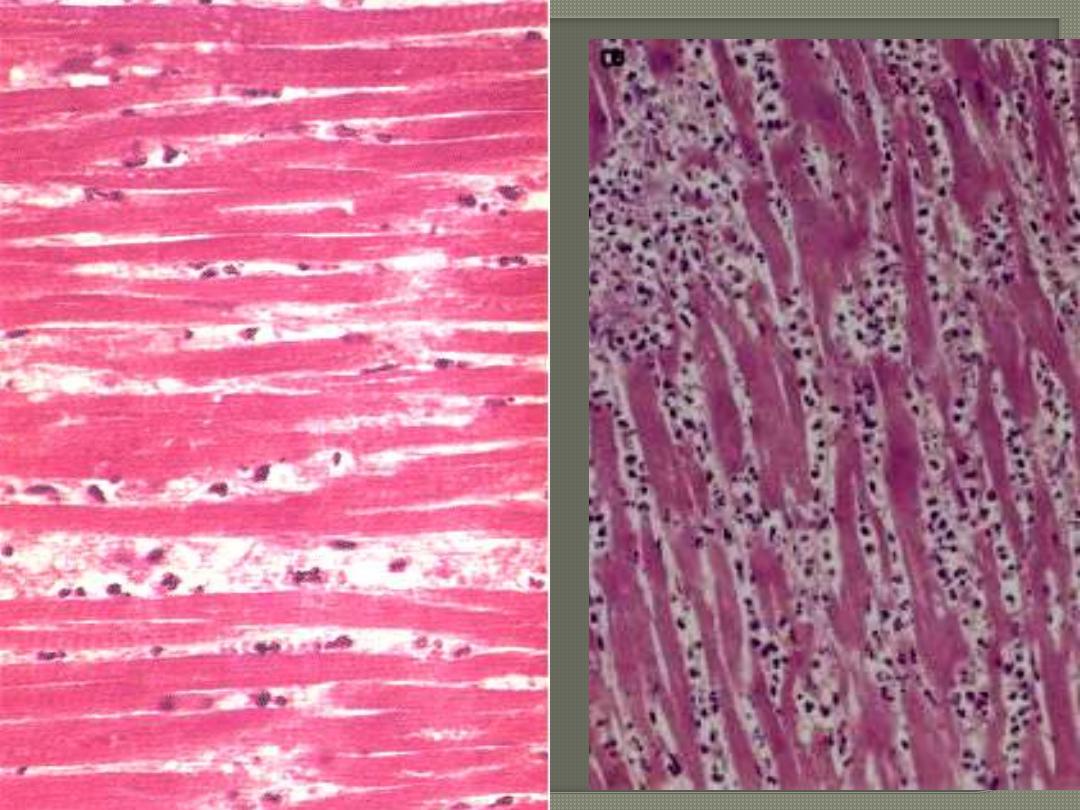
This is a myocardial infarction of 1 to 2 weeks in age. Note that there are remaining normal myocardial
fibers at the top. Below these fibers are many macrophages along with numerous capillaries and
fibroblasts with deposition of collagen.
MI


Complications of Myocardial Infarction
patients with acute MI sustain one or more of the
following complications
1.
Heart failure, which is proportional to the size of the
infarct.
2.
2. Arrhythmias; due to conduction disturbances and
myocardial irritability following MI. Responsible for up
to 50% of deaths that occur within 1 hr of onset of MI;
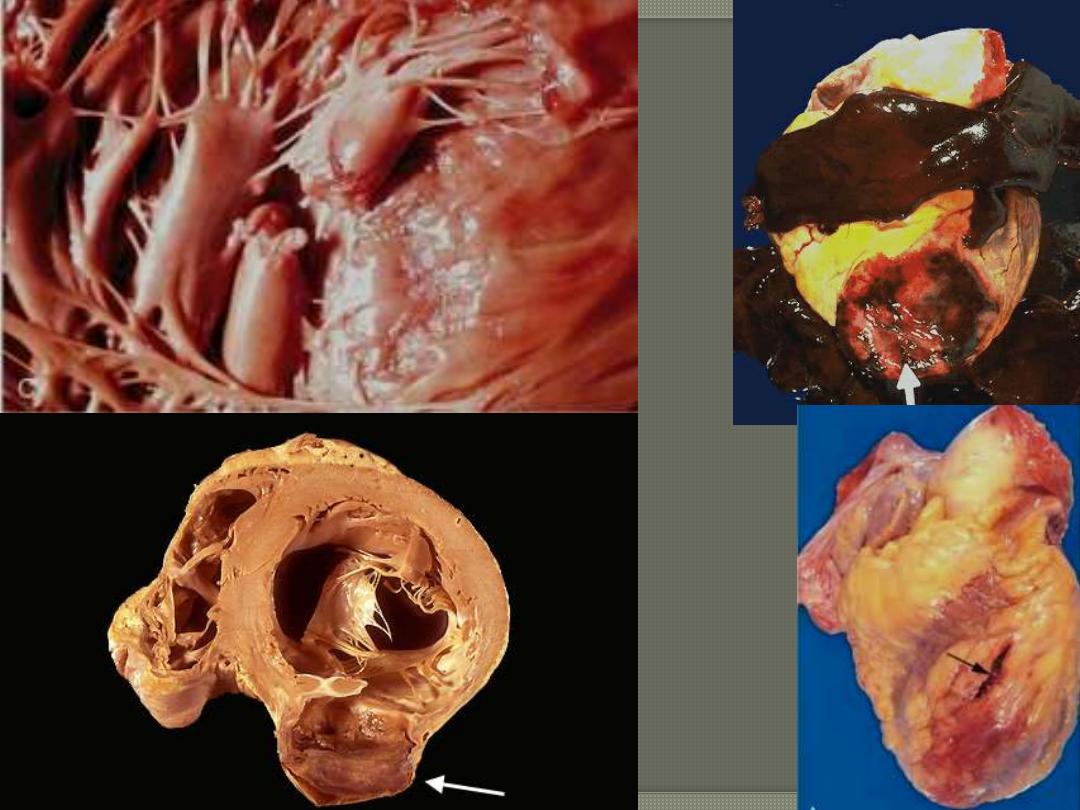
3. Myocardial rupture occurs in up to 5% of patients and is
the result of weakening of necrotic and subsequently
inflamed myocardium and include
a. Rupture of the ventricular free wall;
b. Rupture of the ventricular septum,
c. Rupture of the papillary muscles, resulting in acute
severe mitral regurgitation.
4. Pericarditis: a fibrinous pericarditis can occur
but usually resolve
5. Infarct expansion: owing to the weakening of
necrotic muscle, there may be disproportionate
stretching, thinning, and dilation of the infarct
region (especially with anteroseptal infarcts),
which is often associated with mural thrombus.
6.
Mural thrombus;occurs due to locally deficient
contractility (causing stasis) and endocardial damage
that exposes the subendocardial thrombogenic zone
with eventual thrombus formation that could act as a
potential embolus.

7. DVT and pulmonary embolism
8. Progressive late heart failure.
9- Ventricular aneurysm of the ventricular wall that is
bounded by a healed fibrotic myocardium, which
paradoxically bulges during systole.

Chronic ischemic heart disease
(ischemic cardiomyopathy)
By definition this is a "progressive heart
failure that complicates ischemic myocardial
damage".
CIHD is characterized by the development of
severe, progressive heart failure, sometimes
punctuated by episodes of angina or MI.
Arrhythmias are common.

Sudden cardiac death
Is defined as "unexpected death from cardiac
causes early after symptom onset (usually
within 1 hour) or without the onset of
symptoms".
SCD is a complication and often the first
clinical manifestation of IHD.


HYPERTENSIVE HEART DISEASE
This refers to the adaptive response on the
part of the heart to the increased pressure
overload induced by hypertension.
The left-sided (Systemic) HHD may lead to
1- Even mild hypertension if prolonged can
lead to LVH.
2- cardiac dilation, congestive heart failure or
sudden death.
3- atrial fibrillation (owing to left atrial
enlargement).
Effective control of hypertension can
prevent or lead to regression of cardiac
hypertrophy and its associated risks.
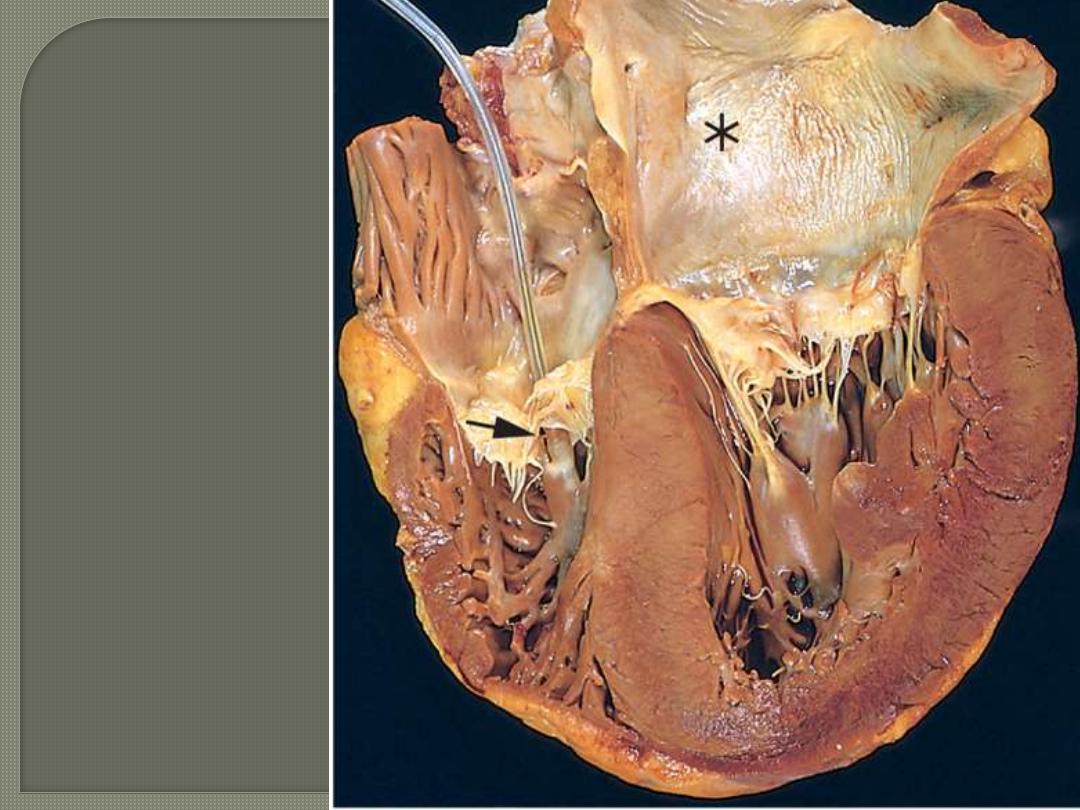
Left ventricular
Hypertrophy And
left atrial dilation in
Systemic hypertension

Right-sided (pulmonary) HHD (Cor pulmonale)
Pulmonary hypertension leads to RVH, dilation
and eventually RHF.
Pulmonary hypertension is caused by
disorders of the lungs or pulmonary
vasculature.
Cor pulmonale may be acute or chronic.
Acute cor pulmonale can follow massive
pulmonary embolism.
Chronic cor pulmonale usually implies right
ventricular hypertrophy (and dilatation)
secondary to prolonged pressure overload
caused by obstruction of the pulmonary
arteries or arterioles
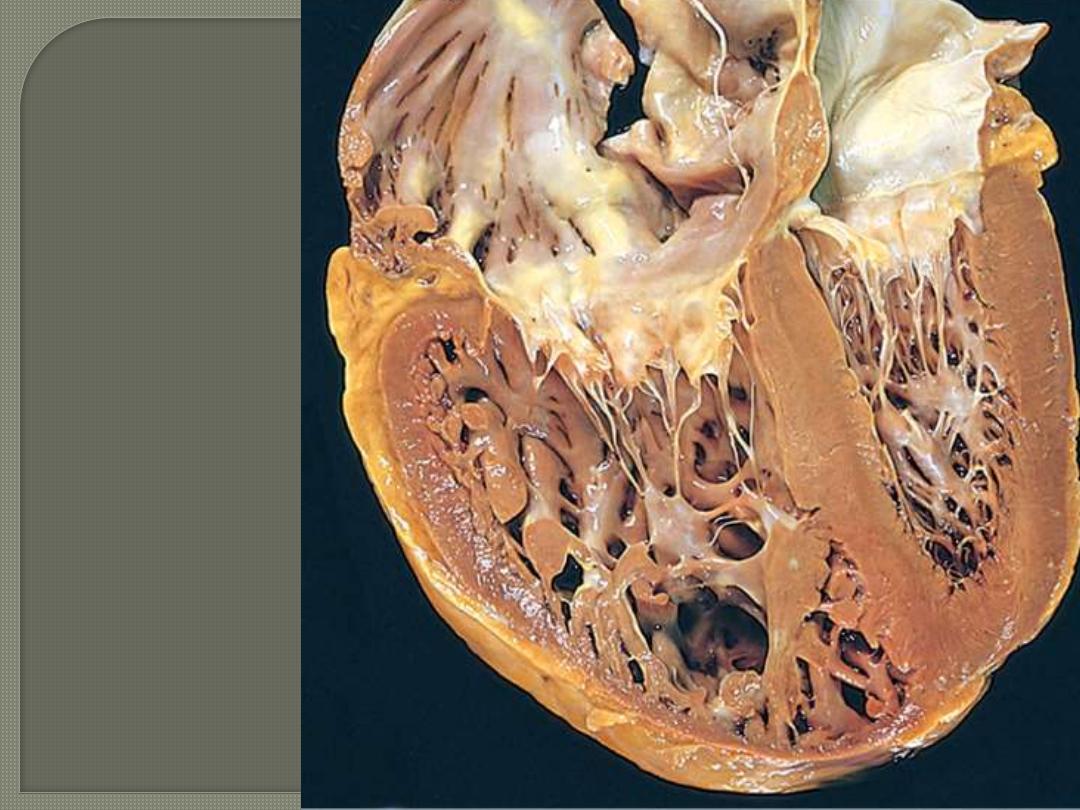
RVH and Right
Atrial dilation in
right sided HHD

RHEUMATIC FEVER AND RHEUMATIC
HEART DISEASE
Rheumatic fever is an immunologically mediated acute
inflammatory systemic disease occurring a few weeks
after an episode of group A streptococcal pharyngitis.
Acute RF appears most often in children between ages
5-15 yrs.
The incidence of RF has declined remarkably in many
parts of the world over the past 40 yrs.

In acute RF, focal inflammatory lesions are found in
various tissues of the body but most distinctively within
the heart.
Diffuse inflammation and Aschoff bodies may be found in
any of the three layers of the heart, pericardium,
myocardium, or endocardium- hence the designation
rheumatic pancarditis.

Aschoff bodies consist of foci of swollen collagen
surrounded by lymphocytes, some plasma cells, and
distinctive (pathognomonic) plump macrophages
which have abundant cytoplasm and central round-
ovoid nuclei in which the chromatin is disposed in a
central, slender, wavy ribbon (hence called
"caterpillar cells"). Some of these macrophages
become multinucleated to form Aschoff giant cells.
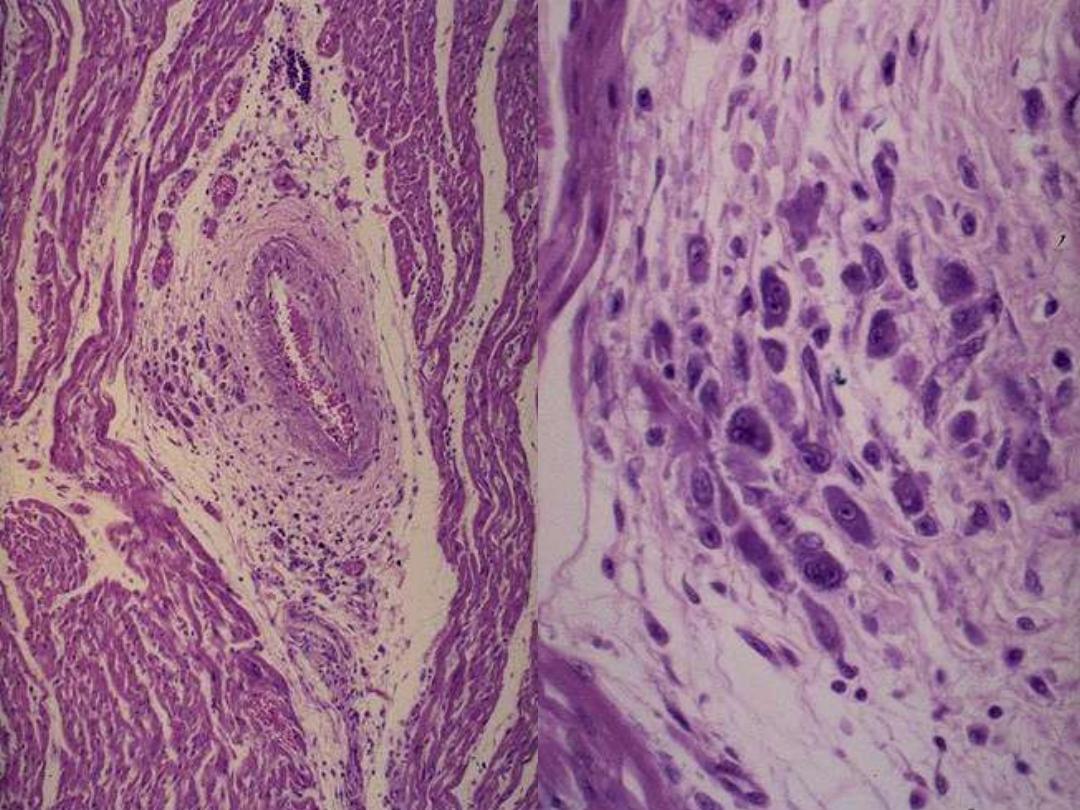
ACUTE RHEMATIC MYOCARDITIS- ASCHOFF BODIES
In the pericardium, the inflammation is accompanied by a
fibrinous or serofibrinous pericardial exudate, described as a
"bread-and-butter" pericarditis
.
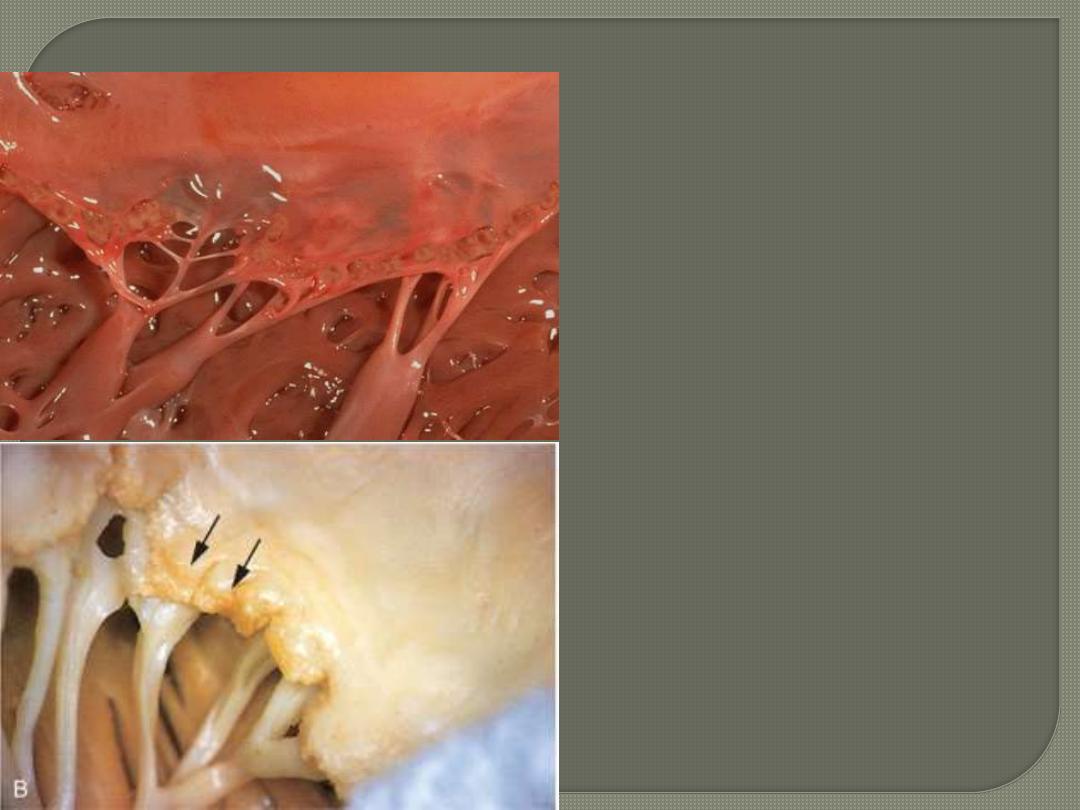
fibrinoid necrosis of
collagen within the cusps (and
along the tendinous cords) on which
sit small vegetations
(verrucae) along the lines of
closure
.
ACUTE RHEUMATIC VALVULITIS


Chronic Rheumatic heart disease
is the result of organization of the acute inflammation
and subsequent fibrosis.
In particular, the valvular leaflets become thickened
and retracted, causing permanent deformity.
the mitral valve is alone (70% of the cases) or
together with the aortic valve (25%).
RHD is the most frequent cause of mitral stenosis
(99% of cases).

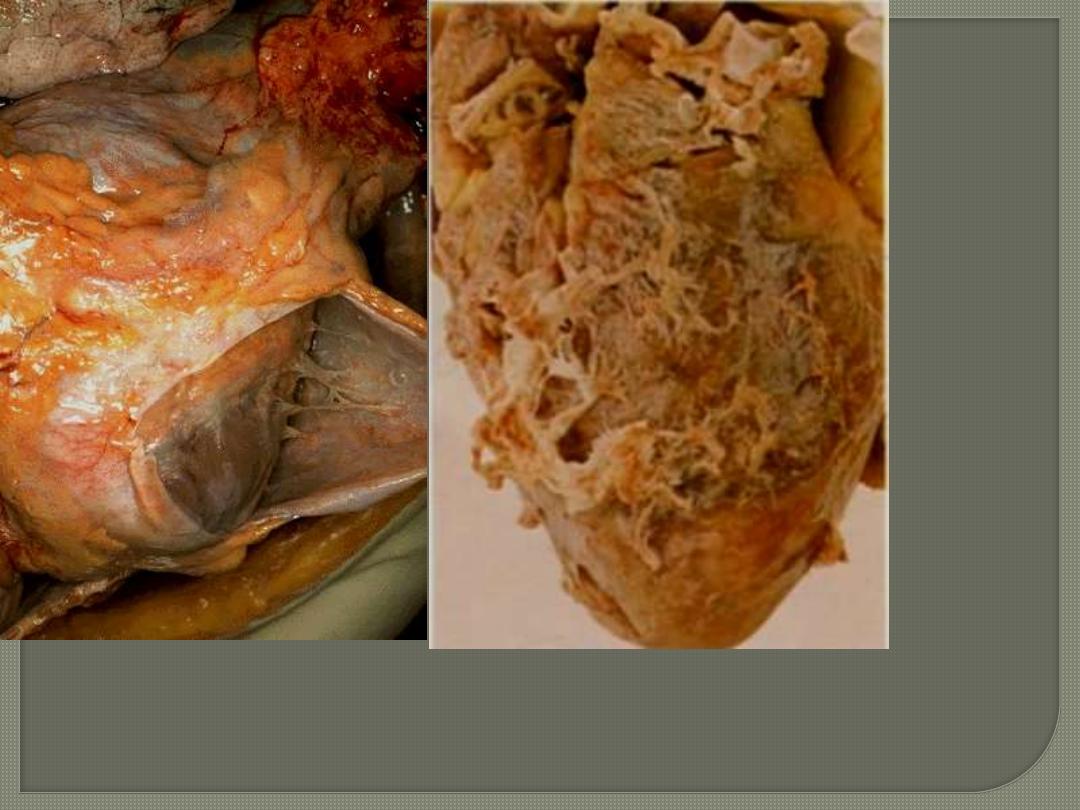
The cardinal anatomic changes of the mitral valve are
1. Leaflet thickening
2. Commissural fusion
3. Shortening, thickening and fusion of the tendinous cords.
Fibrous fusion at the valvular commissures and
calcification create "fish mouth" or "buttonhole" stenoses.
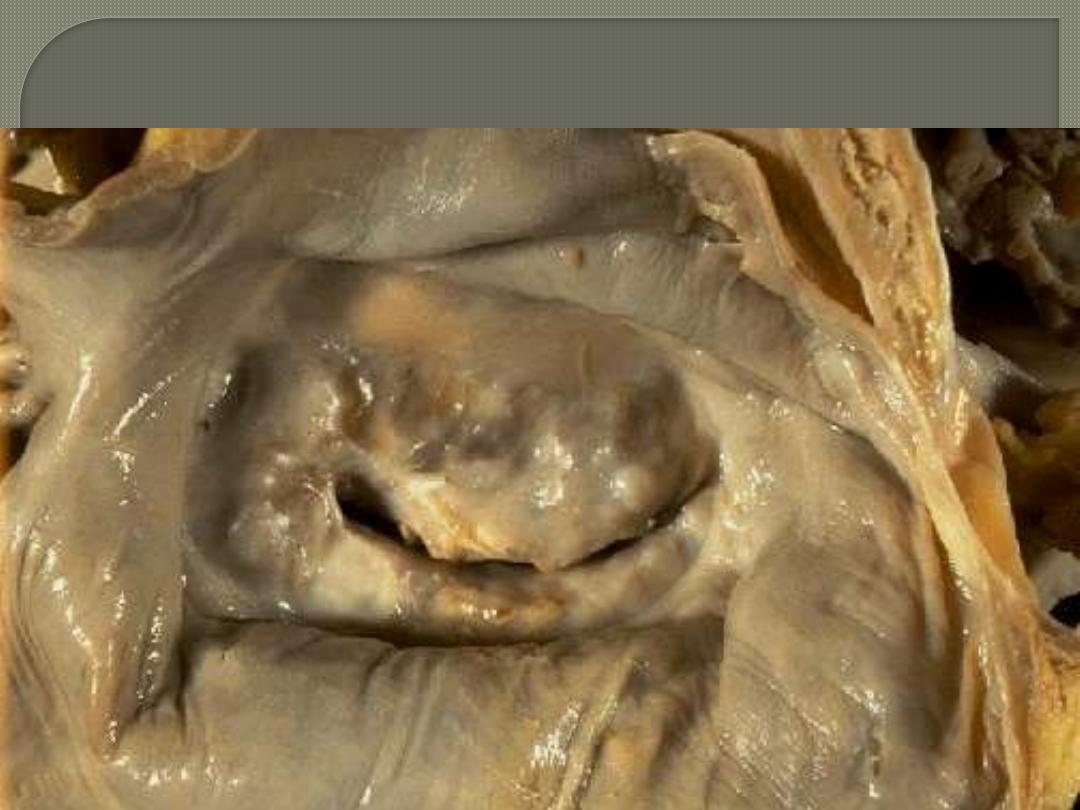
MITRAL VALVE- CHRONIC RHEUMATIC VALVULITIS
Fish mouth (Button hole) appearance

Microscopically diffuse fibrosis with vascularization is
noted in valvular leaflets.
Thrombotic vegetations on the surface and
calcifications may be seen.
Aschoff bodies are rarely seen being replaced by
fibrosis.

Pathogenesis of RF & RHD
RF is due to a hypersensitivity reaction induced by
group A streptococci. It is thought that antibodies
that are originally developed and directed against the
M protein of the offending streptococci also cross-
react with glycoprotein antigens in the heart, joints,
and other tissues.
A genetic predisposition to the disease appears
operating as well, because only a minority of infected
patients (3%) develop RF.
Although pharyngeal cultures for streptococci are
negative by the time the illness begins, antibodies to
one or more streptococcal enzymes, such as
streptolysin O and DNAse B, are present and can be
detected in the sera of most patients.

COMPLICATIONS OF CRHD
1. With recurrent attacks of RF, the chronic valvulitis
is likely to worsen and damage is cumulative.
2. Arrhythmia
3. Embolization primarily from atrial thrombi
4. Infective endocarditis superimposed on deformed
valves.
The manifestations of chronic rheumatic carditis
usually occur years or even decades after the initial
episode of acute RF.

INFECTIVE ENDOCARDITIS
This serious condition signifies
"colonization of the heart valves or the
endocardium by microbes with eventual
formation of bulky, friable vegetations
that often results in destruction of the
underlying cardiac structures".
Bacteria are the most common
offenders (bacterial endocarditis) but
other microorganisms are occasionally
the causative agents e.g. fungi,
rickettsiae of Q fever, and chlamydiae.

• Clinically classified into:
Acute infective endocarditis which signifies an infection that is
– Destructive
– Involving frequently a normal heart valve
– Caused by virulent organism
– Have a rapid clinical course leading to death within days-weeks of
more than 50% of patients despite antibiotics and surgery;
– It is difficult to cure by antibiotics and usually require surgery.


Etiology and Pathogenesis of infective
endocarditis
Two sets of factors predispose to IE
1.
Structural abnormalities of the heart
valves;
IE may develop on previously normal
valves, but a variety of cardiac and vascular
abnormalities predispose to this form of
infection.
a. Rheumatic heart disease
b. myxomatous mitral valves
c. Degenerative calcific aortic stenosis
d. Bicuspid aortic valve (calcified or not)
e. Artificial (prosthetic) valves
2. Host factors particularly those that interfere
with defenses; such as
a. neutropenia
b. immunodeficiency e.g. associated with
malignancy , therapeutic immunosuppression.
c. diabetes mellitus
d. alcoholism
e. intravenous drug abuse.

Gross features of infective endoc
arditis
In both the subacute and acute forms of the disease
there are friable, bulky, and potentially destructive
vegetations.
The mitral and aortic valves are the most common
sites of infection,
Vegetations sometimes erode into the underlying
myocardium to produce an abscess cavity.
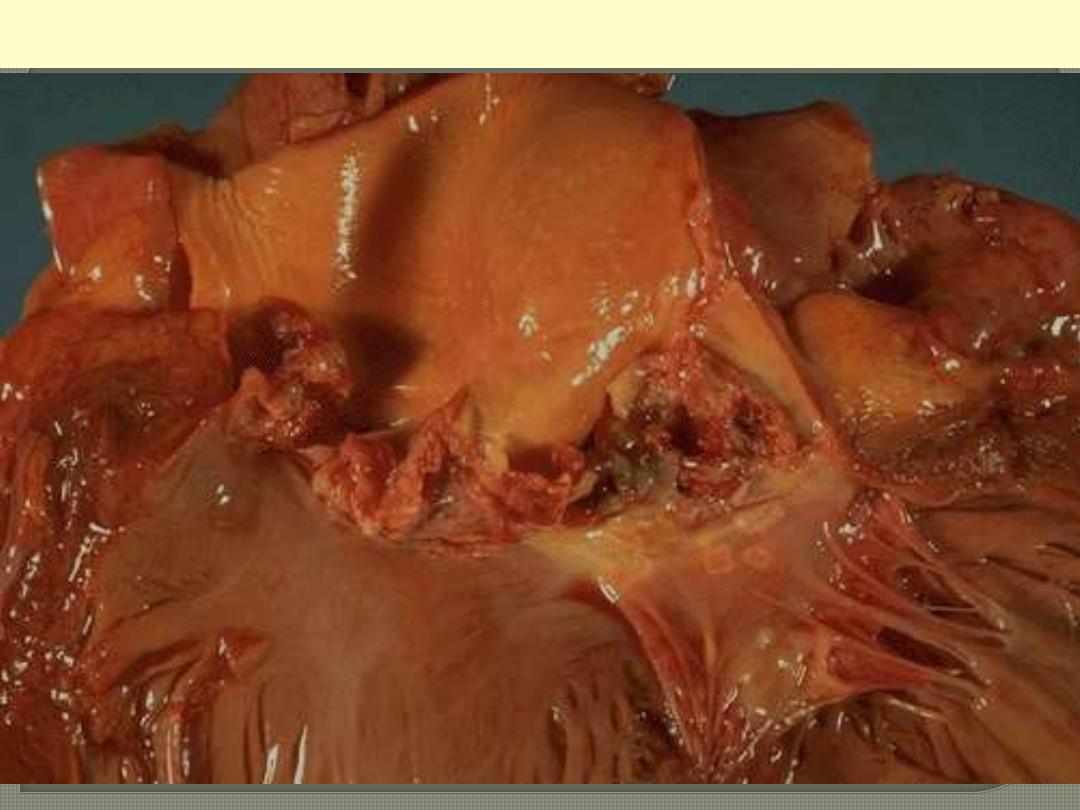
The more virulent bacteria cause the acute form that can lead to serious destruction, as shown here in
the aortic valve. Irregular reddish tan vegetations overlie valve cusps that are being destroyed.
Acute bacterial endocarditis
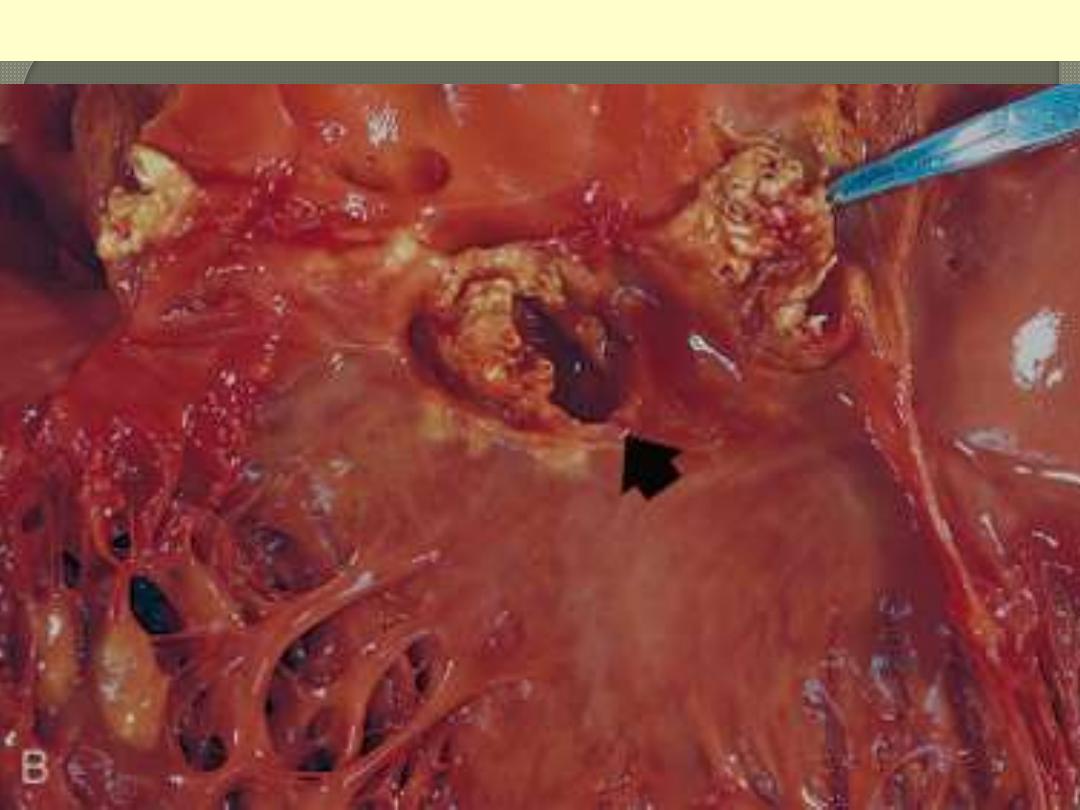
The disease is caused by Staphylococcus aureus with extensive cuspal destruction and ring abscess
(arrow).
Acute endocarditis of congenitally bicuspid aortic valve

Microscopic features of infective endocarditis
The vegetations in general contain fibrin,
inflammatory cells, and bacteria (or other
organisms)
granulation tissue at their bases (suggesting
chronicity).
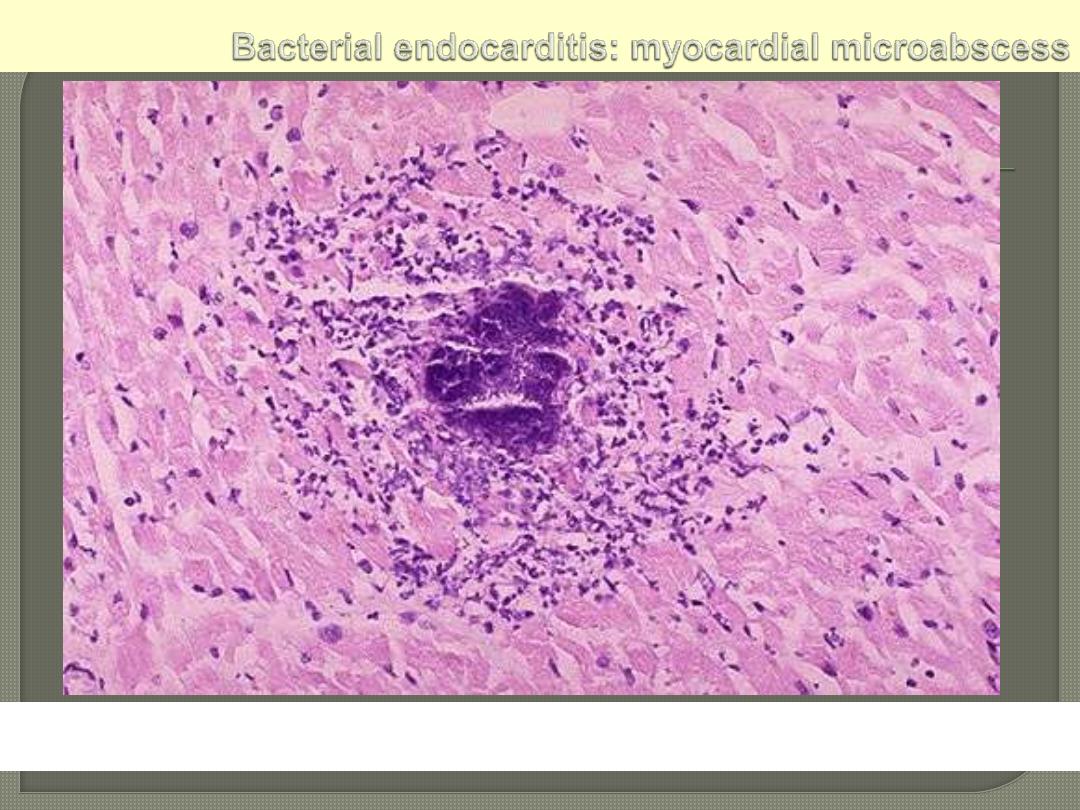
The center consists of blue bacterial colonies and is
surrounded by acute inflammatory cells.

Complications of Infective endocarditis
(whether
acute or subacute)
A. Cardiac
1. Valvular dysfunction (insufficiency or stenosis) that
eventuates in heart failure
2. Myocardial abscesses
3. Suppurative pericarditis
B. Embolic with infarctions
C. Metastatic infections (including septic infarcts) esp.
in acute IE e.g. brain and renal abscesses,
meningitis.
D. Renal complications
1-Embolic infarction that may be multiple and septic
2-Immunologically mediated Glomerulonephritis.
