
TISSUE
REPAIR

Learning objectives
At the end of the session the student should be able to :
1-Outline the meaning of regenerative medicine and
therapeutic cloning.
2-Know the Components of the Extracellular Matrix
3-Know the Repair by connective tissue
4-Describe Angiogenesis (neovascularization) and its
clinical significance.
5-Identify Healing by 1
st
and 2
nd
intention.
6-Know the Pathological aspect of repair.
Learning objectives ;

REGENERATION &
HEALING BY FIBROSIS
Critical to survival is the ability
to repair the damage caused
by injurious agents &
inflammation.

Repair
:
refers to the restoration of
tissue architecture and function after an
injury. This occurs by regeneration &/or
healing.
Regeneration:
complete
reinstitution of the damaged components
of the affected tissue i.e. the tissue
essentially returns to a normal state.

Healing
is a reparative process
characterized by laying down of connective
(fibrous) tissue that results in scar
formation.
This mode occurs when
The injured tissues are incapable of
complete regeneration.
The supporting structures of the tissue are
severely damaged.
Although the resulting fibrous scar is not
normal, it provides enough structural
stability that allows the injured tissue to
function.
Repair involves:
The proliferation of various cells.
Close interactions between cells and the
extracellular matrix (ECM).
Therefore, an understanding of the process of
repair requires some knowledge of the control of
cell proliferation and the functions of the ECM.
GROWTH FACTORS
Cell proliferation can be triggered by
1. Growth factors
2. Hormones
3. Cytokines
4. Signals from the ECM
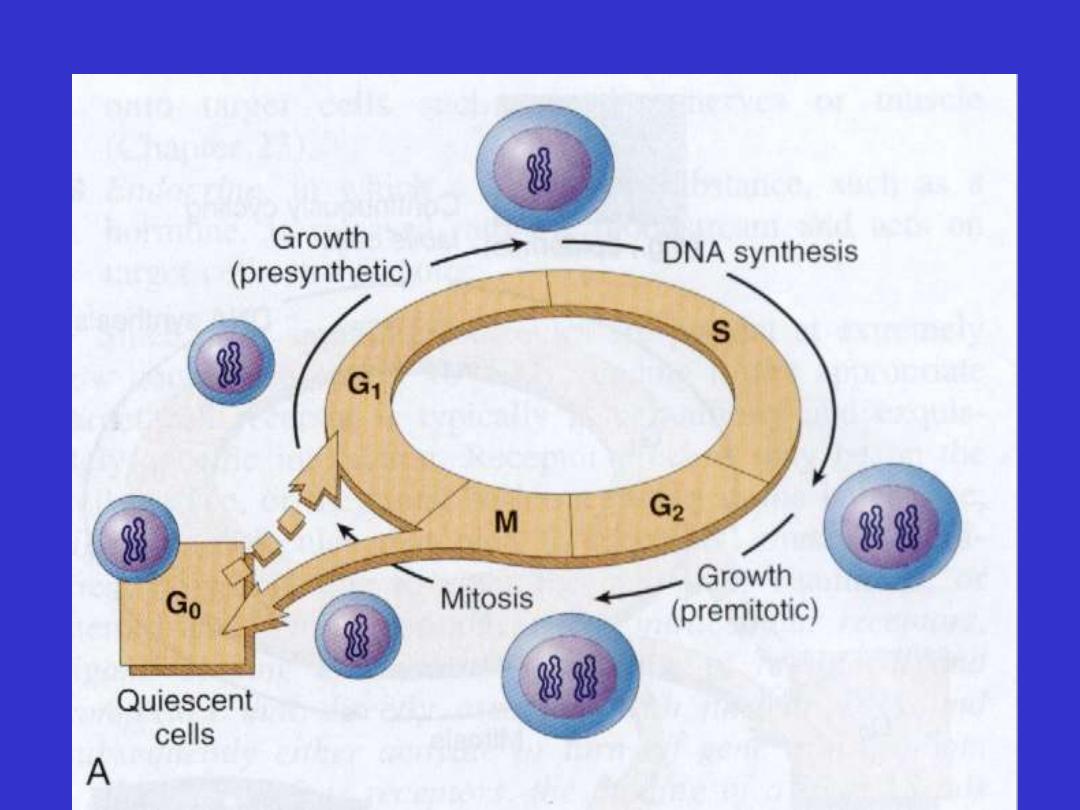
Stages of cell cycle
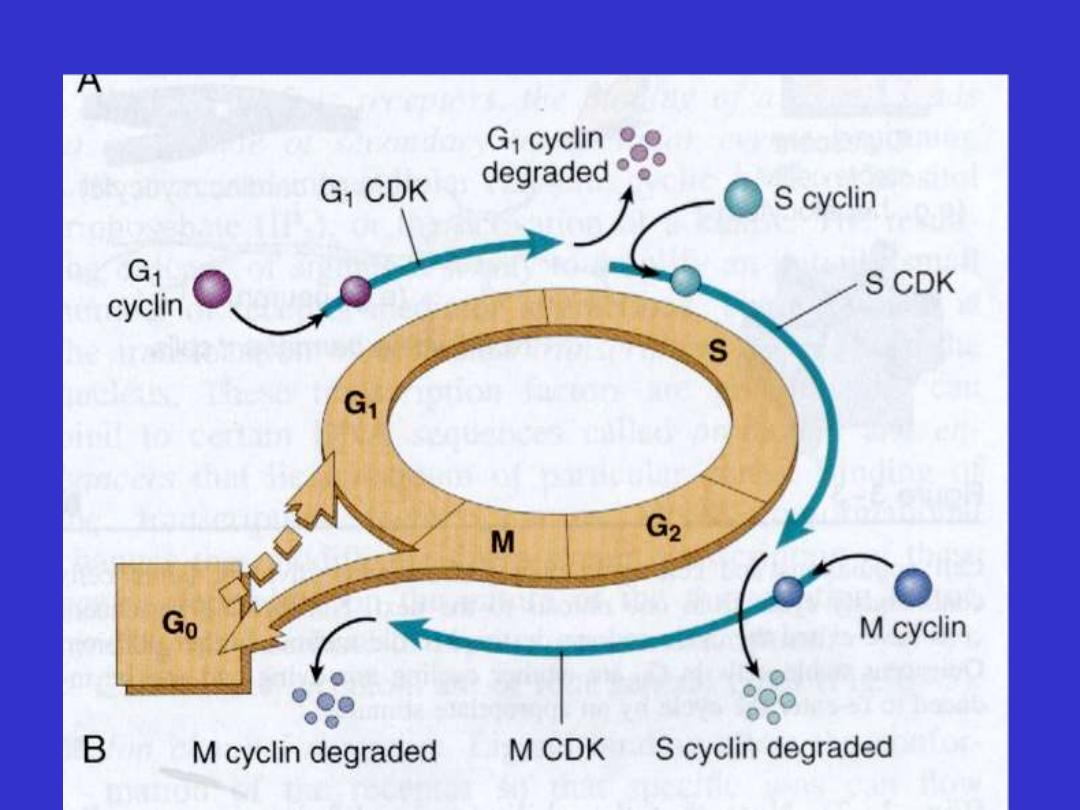
Control of cell cycle progression

Labile cells
• Under normal conditions are continuously dividing &
dying cells
• They include
1. Haemopoietic cells
2. Surface epithelial cells
a. stratified squamous surface of the skin, oral cavity,
vagina, & cervix;
b. cuboidal epithelium of the ducts draining exocrine
organs
c. columnar epithelium of G.I.T, genital tract
d. transitional epithelium of the urinary tract.
• Above tissues have
stem cells
programmed to divide
continuously

• Stem cell gives two daughter cells
1. retains the ability to divide (self-
renewal)
2. differentiates into nonmitotic cell carry
normal function of the tissue.
• Stem cell
- unipotent (skin)
- pleuripotent (bone marrow)
Embrionic ES type and adult type

Regenerative medicine
has a main objective of
regeneration and repopulation of damaged organs
using ES or adult stem cells. One of the most exciting
prospects in this field is the type of stem cell therapy
known as
therapeutic cloning.
Other potential therapeutic strategies using stem cells
involve:
Transplanting stem cells into areas of injury
Mobilization of stem cells from the bone marrow into
injured tissue
The use of stem cell culture systems to produce large
amounts of differentiated cells for transplantation into
injured tissue.

Stable cells
• Quiescent or low-levels of replicative capacity in normal
state
• Rapid division in response to injury
• Include
A. Parenchymal cells of most solid glandular tissues
- liver
- kidney
- pancreas
B. Endothelial cells
C. Mesenchymal cells
- fibroblasts
- smooth muscle cells

Permanent cells
• Specialized, terminally differentiated cells
• No proliferative activities in postnatal life
- cannot be replaced by identical cells.
• Include
- Neurons
- Cardiac muscle cells
- Cells of the lens.
• If injured, they will be replaced by scar tissue.
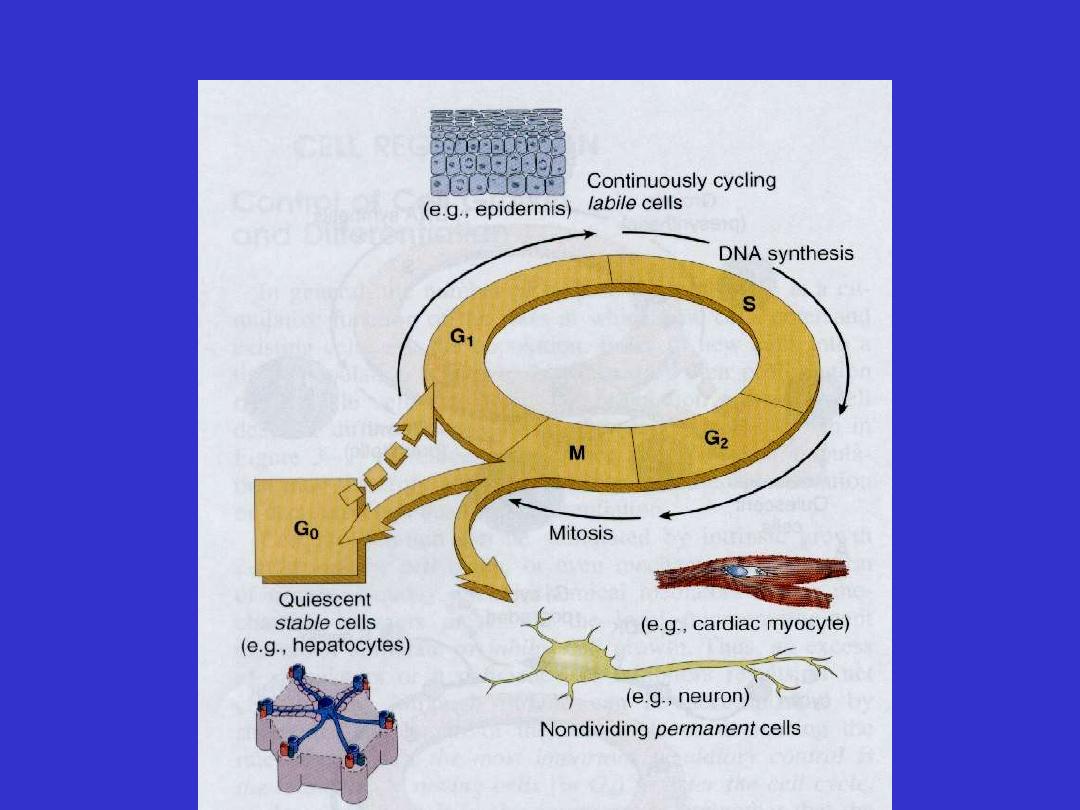
Cell types and cell cycle phases

ECM and
Tissue
Remodeling

Extracellular matrix (ECM)
It is dynamic, constantly remodeling macro-molecular
complexes and synthesized locally
ECM has the following functions
1. Provides mechanical support for cells
2. Determines cell orientation (polarity)
3. Controls and Regulates cell growth & differentiation
4. Provides scaffolding for tissue renewal.
5. Important for storage & presentation of regulatory
molecules

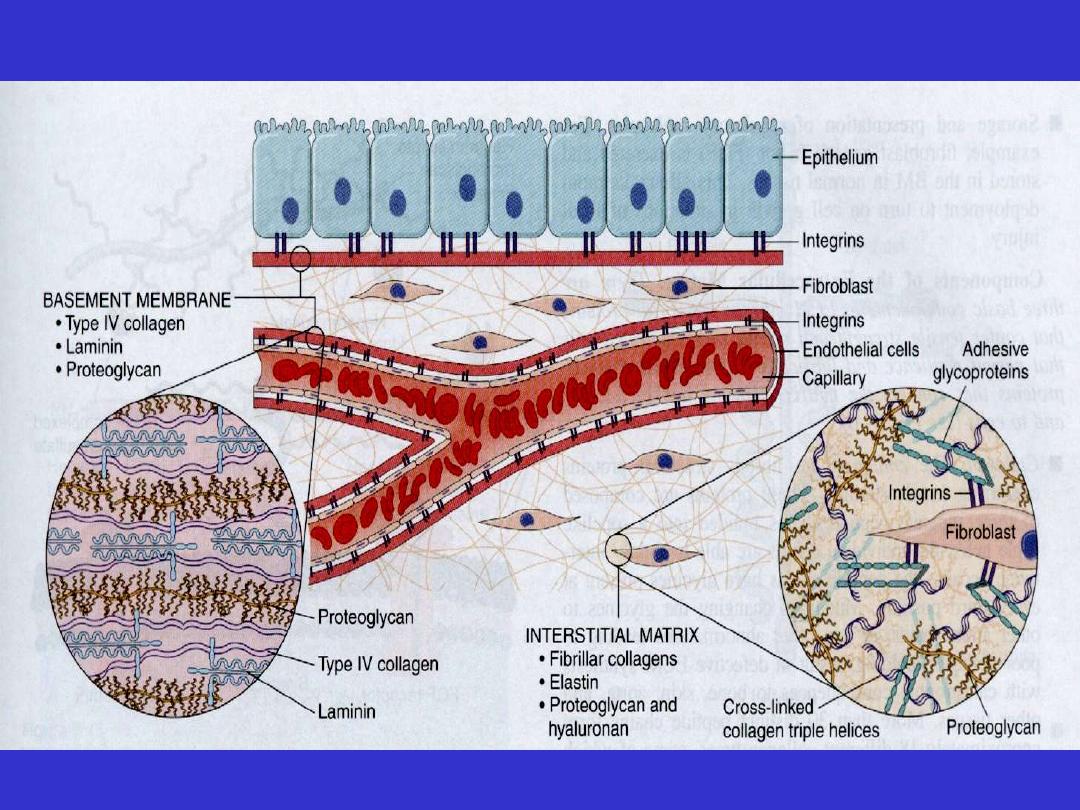
ECM occurs in two basic forms:
1. The interstitial matrix
2. Basement membrane (BM).

Interstitial matrix
• Present in spaces between
a. Cells in connective tissues
b. Epithelium and supportive vascular & smooth
muscle structures

Basement membrane
• Sits beneath the epithelium
• A boundary between the epithelium &
underlying connective tissues
• Synthesized by epithelium & underlying
mesenchymal cells
• Consists of amorphous type IV collagen +
adhesive glycoproteins.
Components of ECM

Components of ECM
Three basic components
1. Fibrous structural proteins
2. Water-hydrated gels
3. Adhesive glycoproteins

Collagen nice to know
• the most abundant of the matrix proteins
• confers tensile strength
• composed of triple helical structure formed from
three peptide chains
• Fibroblasts are principle cells in synthesis
• Synthesis
- starts by secretion of procollagen molecules
- modified by the removal of peptides
- removal by specific peptidases.

- resultant collagen molecules align to form collagen fibrils
- fibrils have banded appearance on EM
- these contribute to the strength of the fibrils
- reinforced by cross-linking of collagen molecules by
covalent bonding.
• Collagen fibers formed by aggregation of collagen fibrils.
• 18 types of collagen
• Tensile strength of fibrillar collagens
- derives from cross-linking
- dependent on Vit.C
- children with ascorbate deficiency
Have skeletal deformities
Bleed easily because of weak vascular wall BM
Show poor healing of injuries

Elastin nice to know
• protein forming core of elastic fibers.
• Molecules are extensively cross-linked producing random
coils
property of recoil after stretching.
• Elastic fibers most abundant in tissues need for recoil
1. Large arteries such as the aorta
2. Dermis of skin
3. Ligaments
4. Uterus
• Defects in elastic fibers lead to skeletal abnormalities &
weakened aortic wall (Marfan’s syndrome)
dissection

Glycosaminoglycans
• water-hydrated gel; negatively charged polysaccharide
chains.
• The most abundant forms are
1. Hyaluronic acid
2. Chondroitin sulphate
3. Dermatan sulphate
4. Heparan sulphate
5. Keratan sulphate.
• Relative amounts vary from tissue to tissue.
• Their molecules are hydrophilic; provide tissue turgor
• Have capacity to bind to collagens and fibronectin
• May participate in structural organization of ECM.

• Cell and tissues regeneration
CELL AND TISSUE REGENERATION
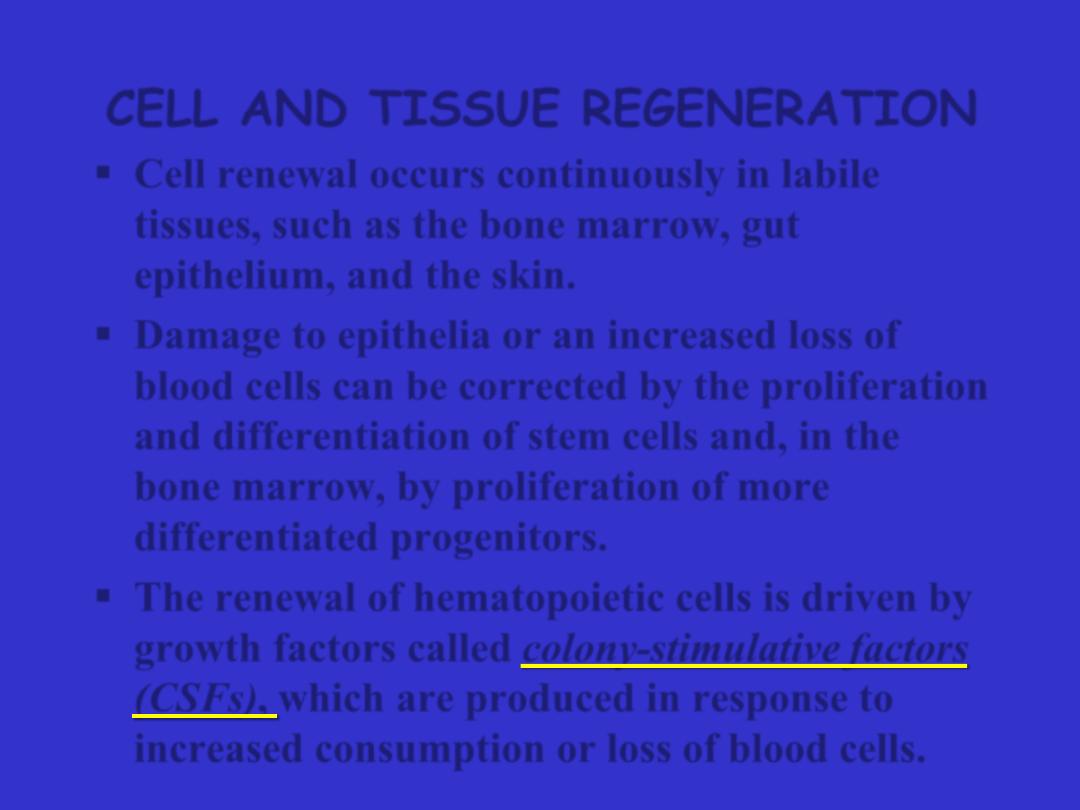
Cell renewal occurs continuously in
labile
tissues
, such as the bone marrow, gut
epithelium, and the skin.
Damage to epithelia or an increased loss of
blood cells can be corrected by the proliferation
and differentiation of stem cells and, in the
bone marrow, by proliferation of more
differentiated progenitors.
The renewal of hematopoietic cells is driven by
growth factors called
colony-stimulative factors
(CSFs),
which are produced in response to
increased consumption or loss of blood cells.

Tissue regeneration can occur in
parenchymal organs with stable cell
populations, but with the exception of the
liver,
this is usually a limited process.
The surgical removal of a kidney elicits in
the
contralateral kidney a compensatory
response that consists of both hypertrophy
and hyperplasia of proximal duct cells.

The regenerative response of the
liver
that occurs after
surgical removal of hepatic tissue is striking.
Up to 60% of the liver may be removed in a procedure
called living-donor transplantation, in which a portion
of the liver is resected from a normal individual and is
transplanted into a recipient with end-stage liver
disease ,
or after partial hepatectomies performed for
tumor removal.
In such cases, the tissue resection triggers proliferation
of the remaining hepatocytes (normally quiescent).
Experimentally, hepatocyte replication after partial
hepatectomy is initiated by
cytokines (e.g., tumor
necrosis factor [TNF] and interleukin 6 [IL-6]).
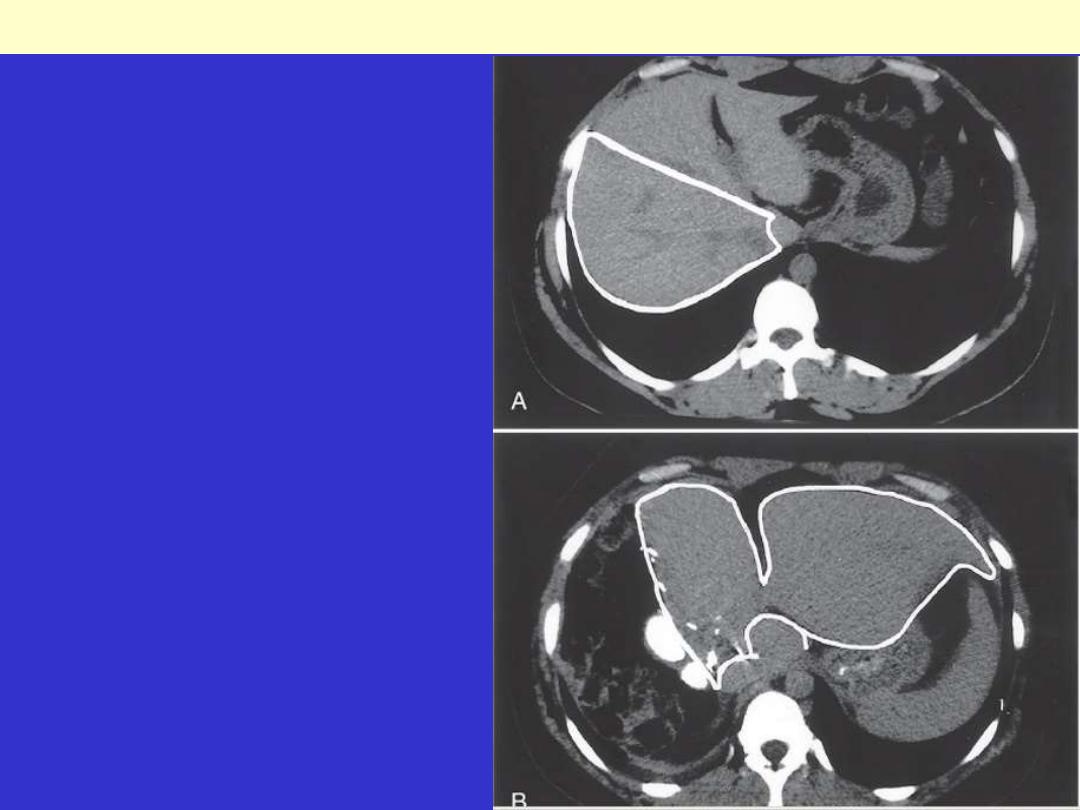
Computed tomography scans of the donor
liver in living-donor liver transplantation. A,
The liver of the donor before the operation.
Note the right lobe (outline), which will be
resected and used as a transplant. B, Scan of
the same liver 1 week after resection of the
right lobe; note the enlargement of the left
lobe (outline) without regrowth of the right
lobe.
Regeneration of human liver.

EGF (epidermal growth factor receptor, or EGFR)
with intrinsic tyrosine kinase activity, is
mitogenic
for
hepatocytes and most epithelial cells, including
keratinocytes.
In cutaneous wound healing EGF is produced by
keratinocytes, macrophages, and other inflammatory
cells.
The main EGFR (referred to as EGFR1) is frequently
overexpressed in lung and some brain tumors
and is an
important therapeutic target for the treatment of these
conditions.
ERB B2 (also known as HER-2/NEU)
has received
great attention because of its overexpression in
breast
cancers
, in which it is a target for effective cancer
control.

REPAIR BY
CONNECTIVE
TISSUE

Healing or repair by connective tissue is
encountered if
1. A severe or persistent (chronic) tissue
injury that result in damage to
parenchymal cells as well as the stromal
framework
2. Injury affects nondividing cells
Under these conditions, repair occurs by
replacement of the nonregenerated cells with
connective tissue, or by a combination of
regeneration of some cells and scar formation.

Repair begins
within 24 hours
of injury by
the emigration of
fibroblasts and the induction of fibroblast and endothelial cell
proliferation.
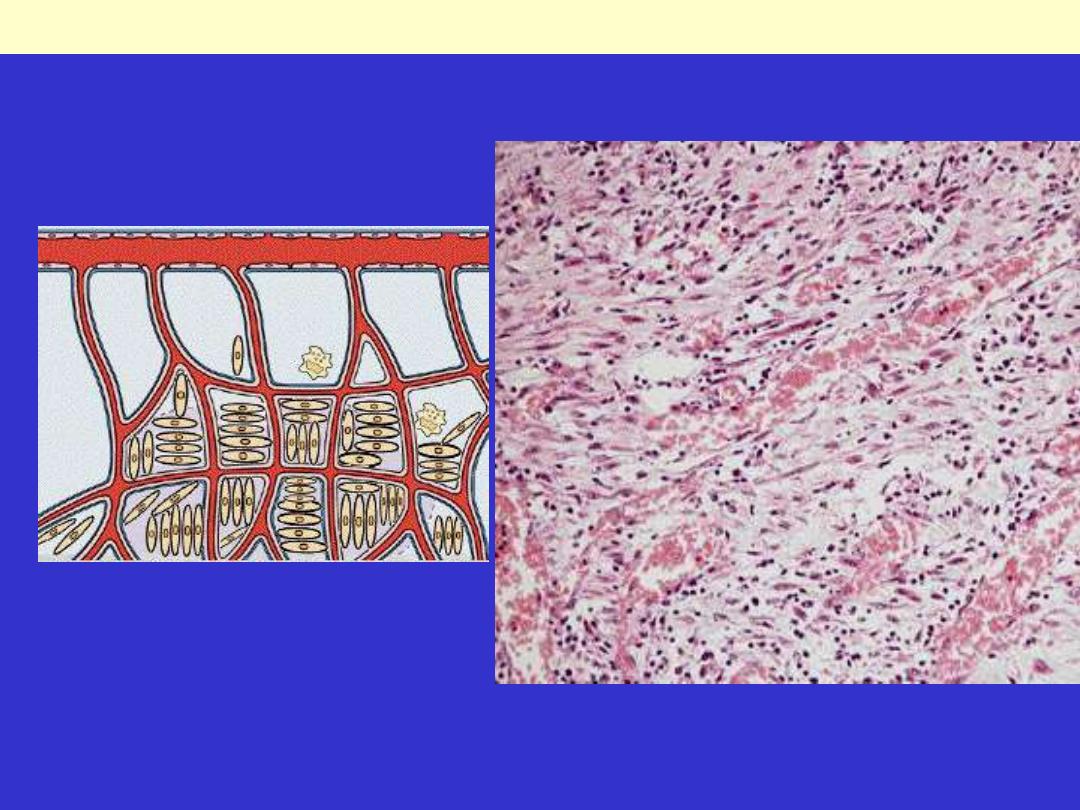
By 3 to 5 days, a specialized type of tissue that is characteristic of
healing, called
granulation tissue
is apparent.
The term granulation tissue derives from
the pink, soft, granular
gross appearance, such as that seen beneath the scab of a skin
wound.
Its microscopic appearance is characterized by
proliferation of
fibroblasts
and
new
thin-walled,
delicate
capillaries
(angiogenesis), in a loose ECM.
Granulation tissue then progressively accumulates connective
tissue matrix, eventually resulting in the formation of a scar
which may remodel over time.
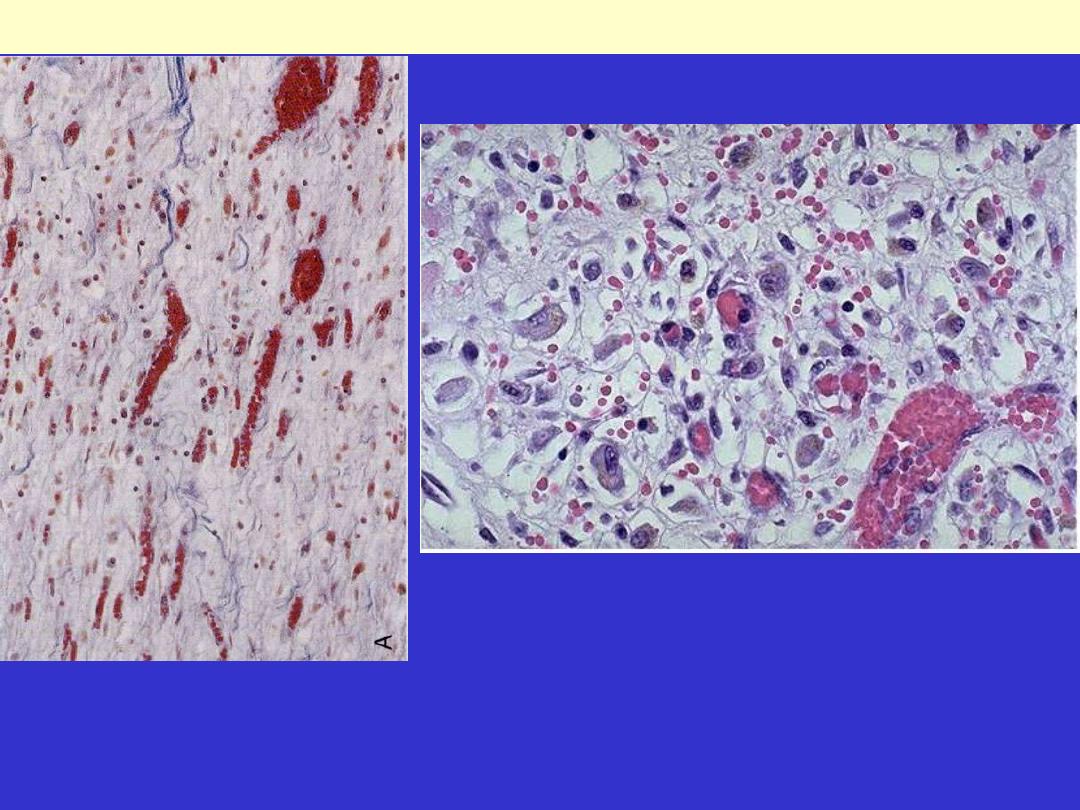
Rt. There are numerous blood vessels, edema, and a loose extracellular matrix containing occasional
inflammatory cells.
Lt. at high magnification, granulation tissue has capillaries, fibroblasts, and a variable amount of
inflammatory cells.
Granulation tissue formation in wound healing

Repair by connective
tissue deposition
consists of four
sequential processes:

Formation of new blood vessels
(angiogenesis)
Migration and proliferation of
fibroblasts
Deposition of ECM (scar formation)
Maturation and reorganization of the
fibrous tissue (remodeling)

Angiogenesis
(neovascularization)

The preexisting vessels send out capillary sprouts to
produce new vessels.
Angiogenesis is a critical process:
in healing at sites of injury
in the development of collateral circulations at sites of
ischemia
and in allowing tumors to increase in size beyond the
limits of their original blood supply.
It has recently been found that
endothelial precursor
cells
may migrate from
the bone marrow
to areas of
injury and participate in angiogenesis at these sites.
Much work has been done to understand the
mechanisms underlying angiogenesis, and therapies
to
either enhance the process (e.g., to improve blood flow
to a heart ruined by coronary atherosclerosis) or
inhibit it (to interfere with tumor growth) are being
developed.

New vessels formed during angiogenesis are
leaky
. This
leakiness explains why granulation tissue is often edematous,
and accounts in part for the edema that may persist in healing
wounds long after the acute inflammatory response has
resolved.
Several factors induce angiogenesis, but the most important
are
VEGF and basic fibroblast growth factor (FGF-2).
VEGF
stimulates both proliferation and motility of
endothelial cells, thus initiating the process of capillary
sprouting.
In angiogenesis involving endothelial cell precursors from the
bone marrow,
VEGF acts through VEGFR-2
to mobilize these
cells from the bone marrow and to induce proliferation and
motility of these cells at the sites of angiogenesis.

Migration of
Fibroblasts and ECM
Deposition (Scar
Formation)

Scar formation builds on the granulation tissue
framework of new vessels and loose ECM that
develop early at the repair site. It occurs in two
steps:
1. Migration and proliferation of
fibroblasts into the site of injury and
2. Deposition of ECM by these cells.

The recruitment and stimulation of fibroblasts is driven by
many growth factors, including
PDGF.
One source of this factor is
the activated endothelium
, but
more importantly, growth factors are also elaborated by
inflammatory cells
.
Macrophages, in particular, are important cellular
constituents of granulation tissue, and besides clearing
extracellular debris and fibrin at the site of injury, they
elaborate a host of mediators that induce fibroblast proliferation
and ECM production.
Mast cells and lymphocytes can contribute directly or
indirectly to
fibroblast proliferation and activation.

As healing progresses, the number of proliferating
fibroblasts and new vessels decrease
the fibroblasts progressively become more synthetic,
and hence there is increased deposition of ECM.
Collagen synthesis, in particular, is critical to the
development of strength in a healing wound site.
Collagen synthesis by fibroblasts begins
early in wound healing (days 3 to 5) and
continues for several weeks, depending
on the size of the wound.
The same growth factors that regulate fibroblast
proliferation also participate in stimulating ECM
synthesis.

Net collagen accumulation, however, depends not only
on increased synthesis but also on diminished collagen
degradation.
Ultimately, the granulation tissue scaffolding evolves
into a
scar
composed of
largely inactive, spindle-shaped
fibroblasts, dense collagen, fragments of elastic tissue,
and other ECM components.
As the scar matures, there is progressive vascular
regression, which eventually transforms the highly
vascularized granulation tissue into a pale, largely
avascular scar.
Many growth factors are involved in the above
processes, including
TGF-β, PDGF, and FGF as well as
cytokines (IL-1 & TNF).

The transition from granulation tissue to scar involves
shifts in the composition of the ECM; even after its
synthesis and deposition, scar ECM continues to be
modified and remodeled.
The outcome of the repair process is, in part, a balance
between ECM synthesis and degradation.
The degradation of collagens and other ECM
components is accomplished by a family of
matrix
metalloproteinases (MMPs),
which
are
dependent on
zinc ions
for their activity.
MMPs include interstitial enzymes that
degrade
collagen,
fibronectin,
proteoglycans, & laminin.
MMPs are produced by a variety of cell
types
(fibroblasts,
macrophages,
neutrophils, synovial cells),
and their
synthesis and secretion are regulated by
growth factors, cytokines, and other
agents.
Their synthesis may be suppressed
pharmacologically with
steroids.

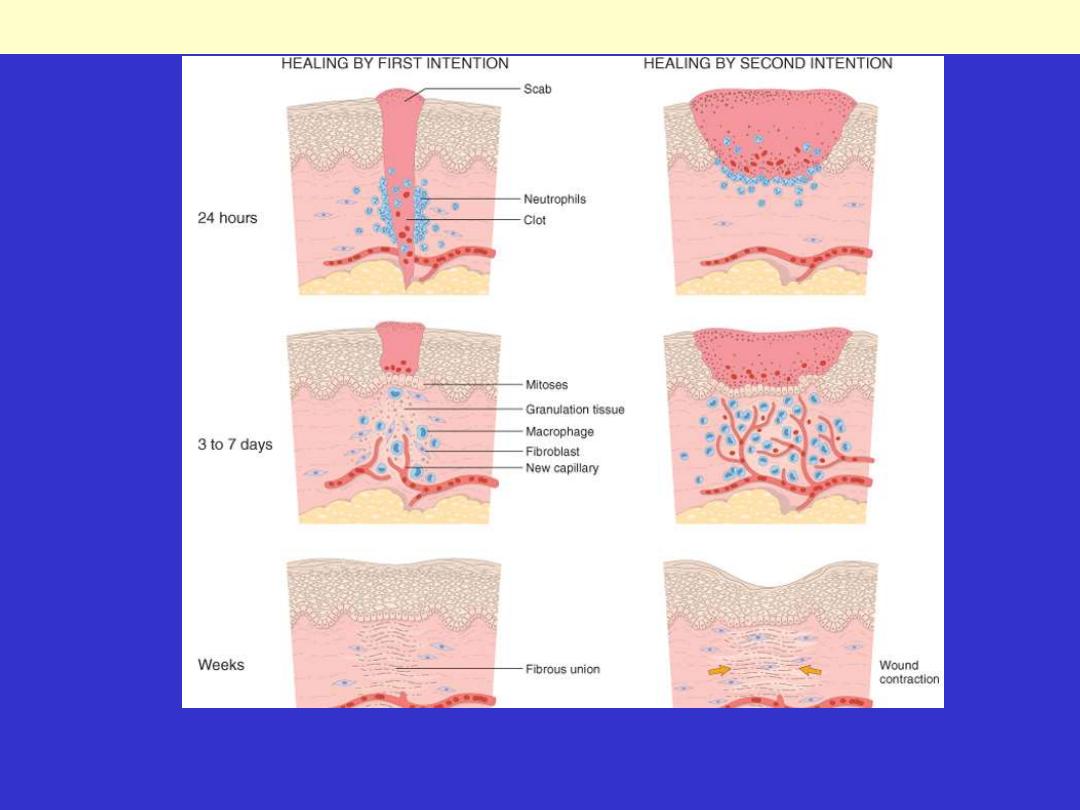
Healing by First Intention
• One of the simplest examples of wound repair is
the
healing of a clean, uninfected surgical incision
approximated by surgical sutures.
• This is referred to as
primary union or healing by first
intention.
• The incision causes only
focal disruption of epithelial
basement membrane continuity and death of a relatively
few epithelial and connective tissue cells.
• As a result, epithelial regeneration predominates over
fibrosis.
• A small scar is formed, but there is minimal wound
contraction.

The narrow incisional space first fills with fibrin-
clotted blood.
Within 24 hours
, neutrophils are seen at the incision
margin, migrating toward the
fibrin clot.
Within 24 to 48 hours
, epithelial cells from both edges
have begun to migrate and proliferate along the
dermis. The cells meet in the midline beneath the
surface scab, yielding a thin but continuous epithelial
layer.
By day 3,
neutrophils have been largely replaced by
macrophages, and granulation tissue progressively
invades the incision space. Epithelial cell proliferation
continues, yielding a thickened epidermal covering
layer.
• By day 5,
neovascularization reaches its
peak as granulation tissue fills the
incisional space. The epidermis recovers
its normal thickness as differentiation of
surface cells yields a mature epidermal
architecture with surface keratinization.
• During the second week,
there is
continued collagen accumulation and
fibroblast proliferation that bridge the
incision. The leukocyte infiltrate, edema,
and increased vascularity are diminished.

By the end of the first month
, the scar
comprises a cellular connective tissue
largely devoid of inflammatory cells and
covered by an essentially normal
epidermis.
The tensile strength of the wound increases
with time. However, the dermal
appendages destroyed in the line of the
incision are permanently lost.
Steps in wound healing by first intention (left) and second intention (right). In the latter, note the large
amount of granulation tissue and wound contraction.
Wound healing
Fibro-vascular granulation tissue
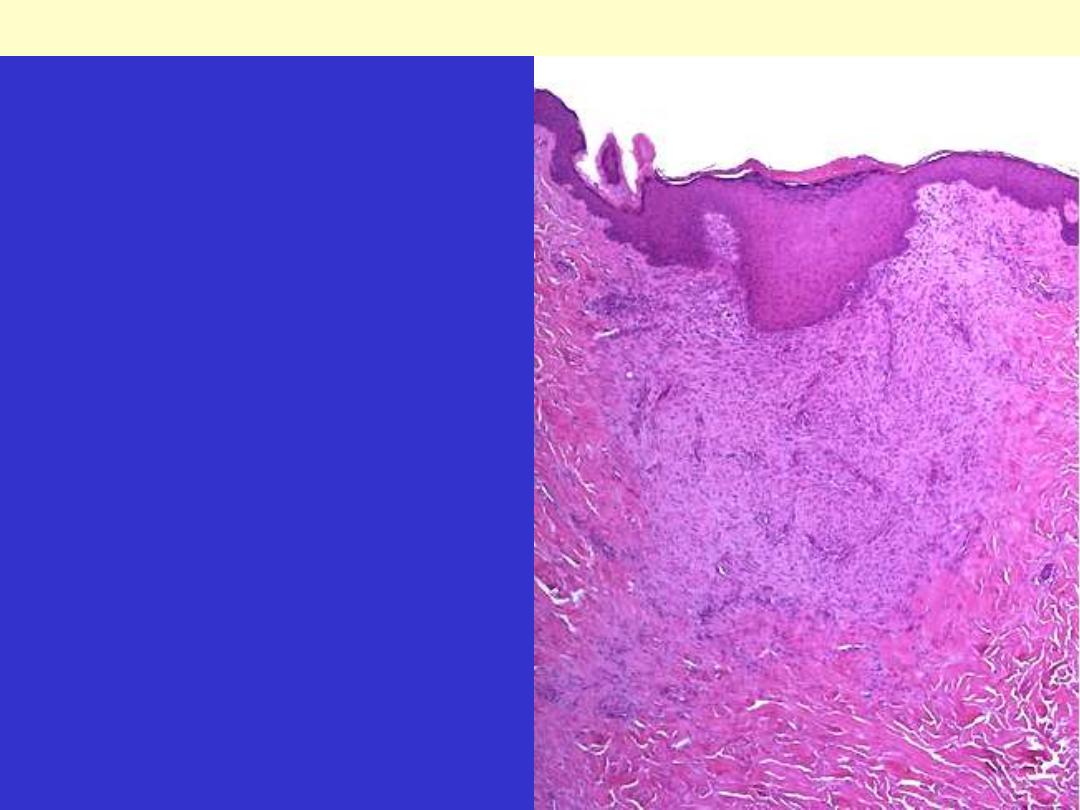
Healing scar, skin
This is a healing biopsy site on the skin seen a
week following the excision, The skin surface has
re-epithelialized, and below this is granulation
tissue with small capillaries and fibroblasts
forming collagen. After a month, just a small
collagenous scar will remain.

Healing by Second Intention
(healing by secondary union)
When cell or tissue loss is more extensive, the repair process is
more complex, the inflammatory reaction is more intense, there is
abundant development of granulation tissue, and the wound
contracts by the action of
myofibroblasts
. This is followed by
accumulation of ECM and formation of a large scar. This mode of
healing occurs in
Large wounds
Abscesses
Ulcerations
After infarction in parenchymal organs.
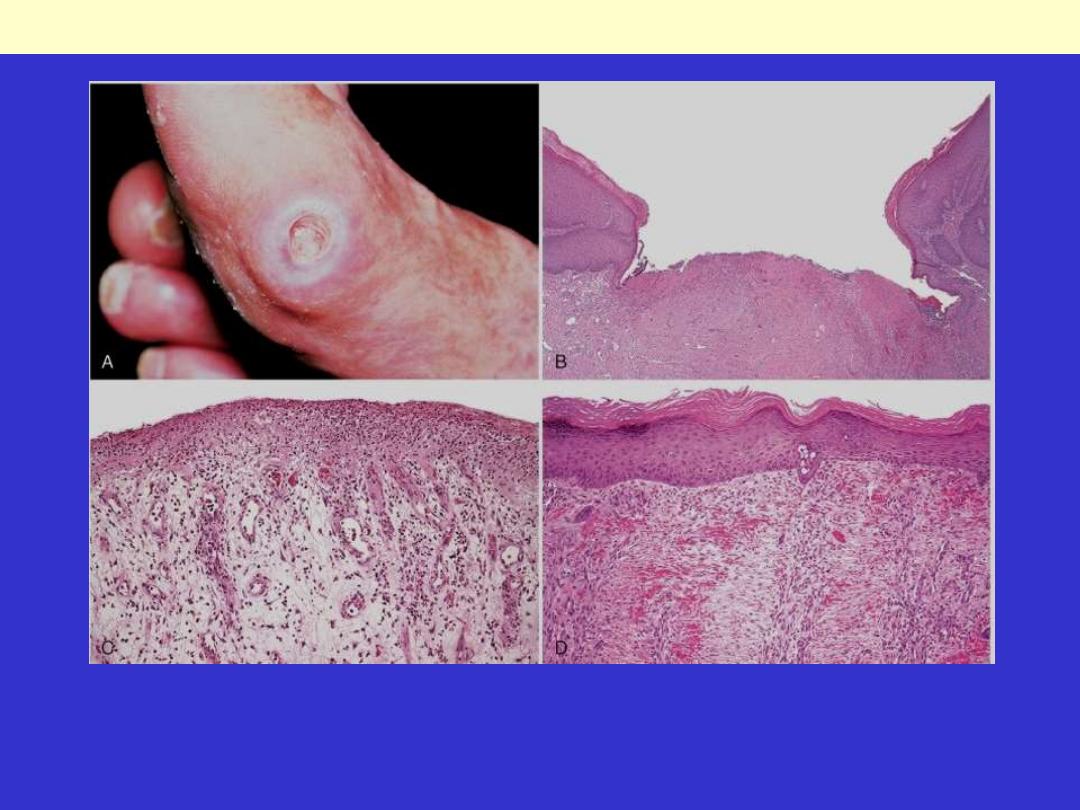
A, Pressure ulcer of the skin, commonly found in diabetic patients. B, A skin ulcer with a large gap
between the edges of the lesion. C, A thin layer of epidermal re-epithelialization, and extensive
granulation tissue formation in the dermis. D, Continuing re-epithelialization of the epidermis and
wound contraction
Healing of skin ulcers

Secondary healing differs from
primary healing in several respects:
A larger clot or scab
rich in fibrin and fibronectin forms at the
surface of the wound.
Inflammation is more intense
because large tissue defects have a
greater volume of necrotic debris, exudate, and fibrin that must be
removed.
Much larger amounts of granulation tissue are formed.
A greater
volume of granulation tissue generally results in a greater mass of
scar tissue.
Secondary healing involves wound contraction.
Within 6 weeks, for
example, large skin defects may be reduced to 5% to 10% of their
original size, largely by contraction. This process has been
ascribed to the presence of
myofibroblasts
, which are modified
fibroblasts exhibiting many of the ultrastructural and functional
features of contractile smooth muscle cells.

Wound Strength
Carefully sutured wounds have approximately
70%
of the
strength of unwounded skin, largely because of the placement of
the sutures.
When sutures are removed, usually
at 1 week
, wound strength is
approximately
10%
of that of unwounded skin, but this
increases rapidly over the next 4 weeks.
The recovery of tensile strength results from collagen synthesis
exceeding degradation during the first 2 months, and from
structural modifications of collagen (e.g., cross-linking and
increased fiber size) when synthesis declines at later times.
Wound strength reaches approximately 70% to 80% of normal by 3
months but usually does not substantially improve beyond that
point
.

Wound healing may be affected by several
external or internal influences that
reduce the quality or adequacy of the
reparative process.
1. Infection
is the single most important
cause of delay in healing; it prolongs the
inflammation phase of the process .
2. Nutrition
has profound effects on wound
healing; protein deficiency & vitamin C
deficiency, inhibits collagen synthesis and
retards healing.

3. Glucocorticoids (steroids)
have anti-
inflammatory effects, and their administration
may result in poor wound strength due to
diminished fibrosis. In some instances,
however, the anti-inflammatory effects of
glucocorticoids are desirable,
When ?
For example, in corneal infections,
glucocorticoids are sometimes prescribed
(along with antibiotics) to reduce the likelihood
of opacity that may result from collagen
deposition.

4. Mechanical variables
such as increased local pressure or
torsion may cause wounds to pull apart, or dehisce i.e. open out or
gape.
5. Poor perfusion
,
due either to arteriosclerosis and diabetes or
to obstructed venous drainage (e.g. in varicose veins), also impairs
healing
6. Foreign bodies
such as fragments of steel, glass, or even bone
impede healing.
7. The type (and volume) of tissue injured
is critical.
Complete restoration can occur only in tissues composed of stable
and labile cells; even then, extensive injury will probably result in
incomplete tissue regeneration and at least partial loss of function.
Injury to tissues composed of permanent cells must inevitably result
in scarring with, at most, attempts at functional compensation by
the remaining viable elements. Such is the case with healing of a
myocardial infarct.

8. The location of the injury and the character of
the tissue in which the injury occurs
are also
important.
For example, inflammation arising in tissue spaces (e.g.,
pleural, peritoneal, synovial cavities) develops extensive
exudates.
Subsequent repair may occur by digestion of the exudate,
initiated by the proteolytic enzymes of leukocytes and
resorption of the liquefied exudate.
This is called
resolution
, and in the absence of cellular
necrosis, normal tissue architecture is generally
restored.
However, in the setting of larger accumulations, the
exudate undergoes
organization:
granulation tissue
grows into the exudate, and a fibrous scar ultimately
forms.

Aberrations of cell
growth and ECM
production
This may occur even in what begins as normal
wound healing.
1. Keloid
refers to the accumulation of
exuberant amounts of collagen that give rise to
prominent, raised scars. There appears to be a
heritable predisposition to keloid formation,
and the condition is more common in blacks.
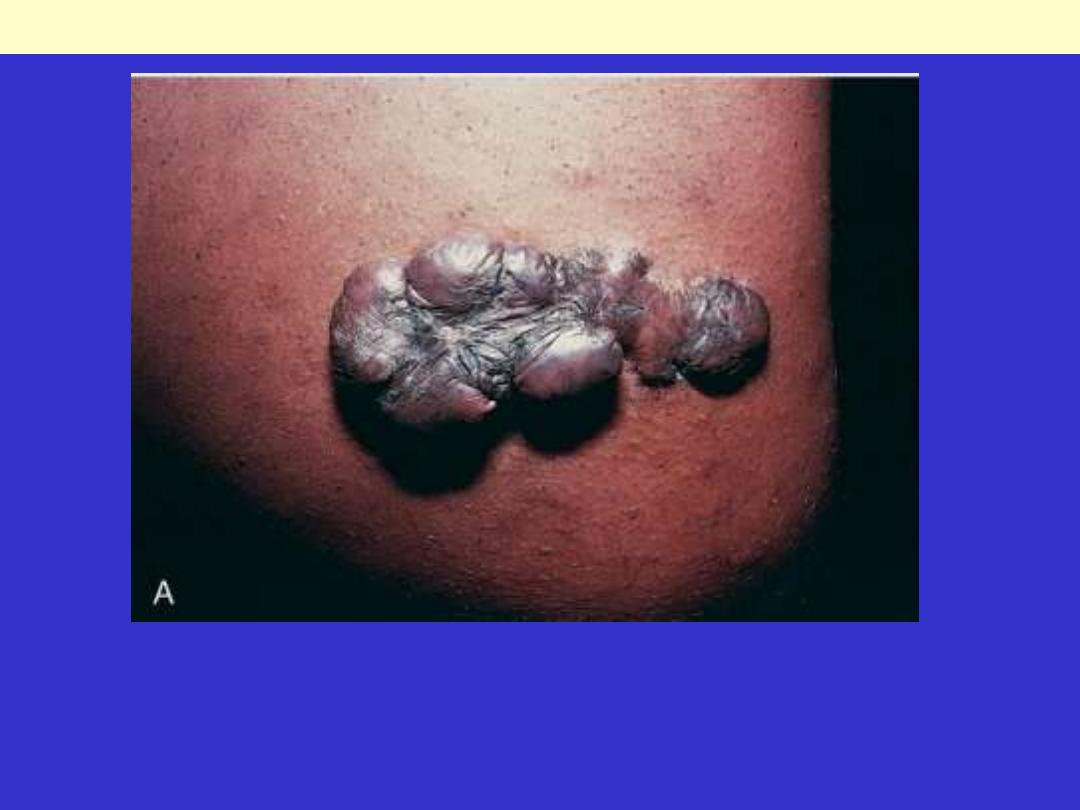
A, Excess collagen deposition in the skin forming a raised scar known as a keloid.
Keloid
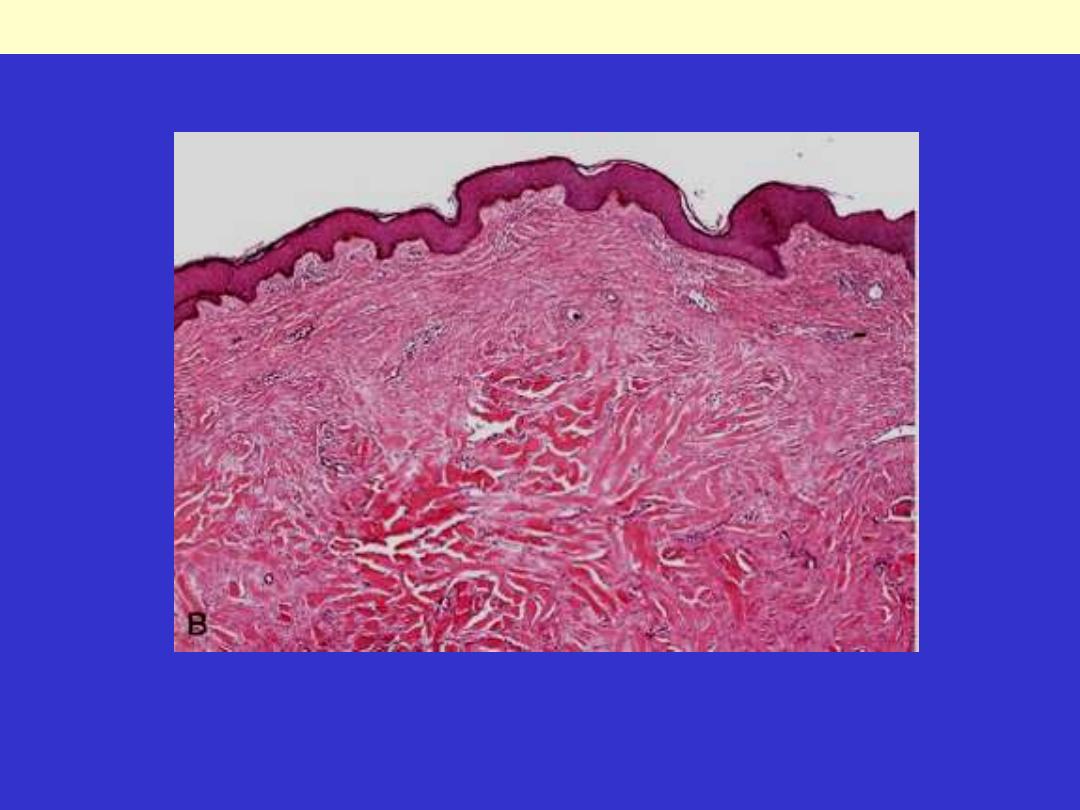
B, Thick connective tissue deposition in the dermis.
Keloid
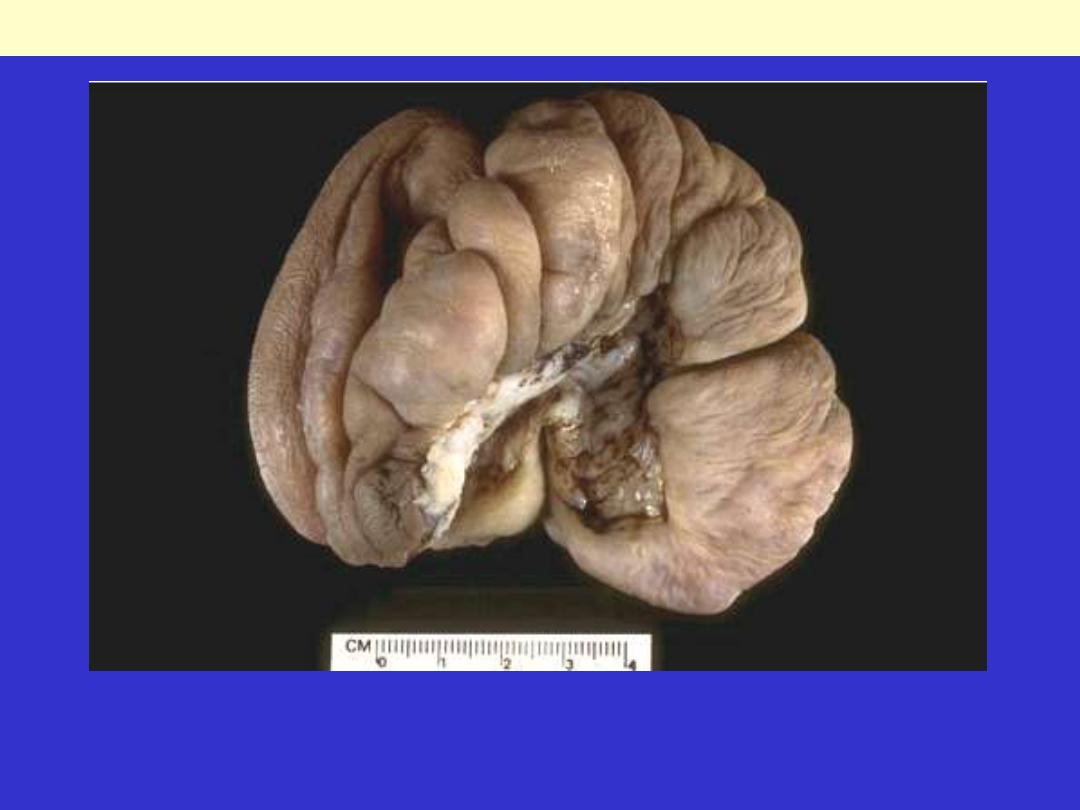
Keloid
This large nodular mass is a keloid excised from the ear in a young male who had previously incurred
trauma with laceration. Ear piercing in women may promote keloid formation. A keloid is an
overgrowth of dermal scar tissue that forms over months following the injury.
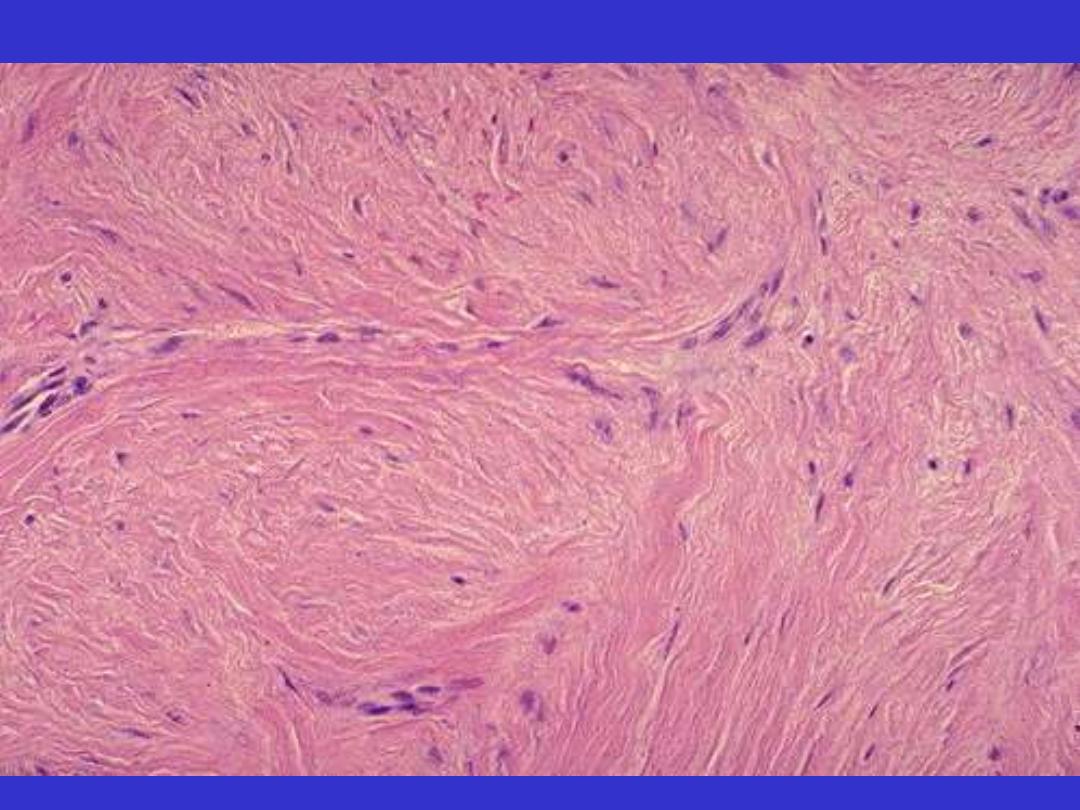
Keloid mic

2. Exuberant granulation:
healing wounds may also generate
excessive granulation tissue that
protrudes above the level of the
surrounding skin and hinders re-
epithelialization.
The restoration of epithelial continuity
requires cautery or surgical resection of
the granulation tissue.

3. Disabling fibrosis:
Associated with chronic inflammatory diseases such as
rheumatoid arthritis, pulmonary fibrosis, and cirrhosis
have many similarities to those involved in normal
wound healing.
In these diseases, however, persistent stimulation of
fibrogenesis results from chronic immune reactions
that sustain the synthesis and secretion of growth
factors, fibrogenic cytokines, and proteases. Collagen
degradation by collagenases, normally important in
wound remodeling, is responsible for much of the joint
destruction seen in rheumatoid arthritis.
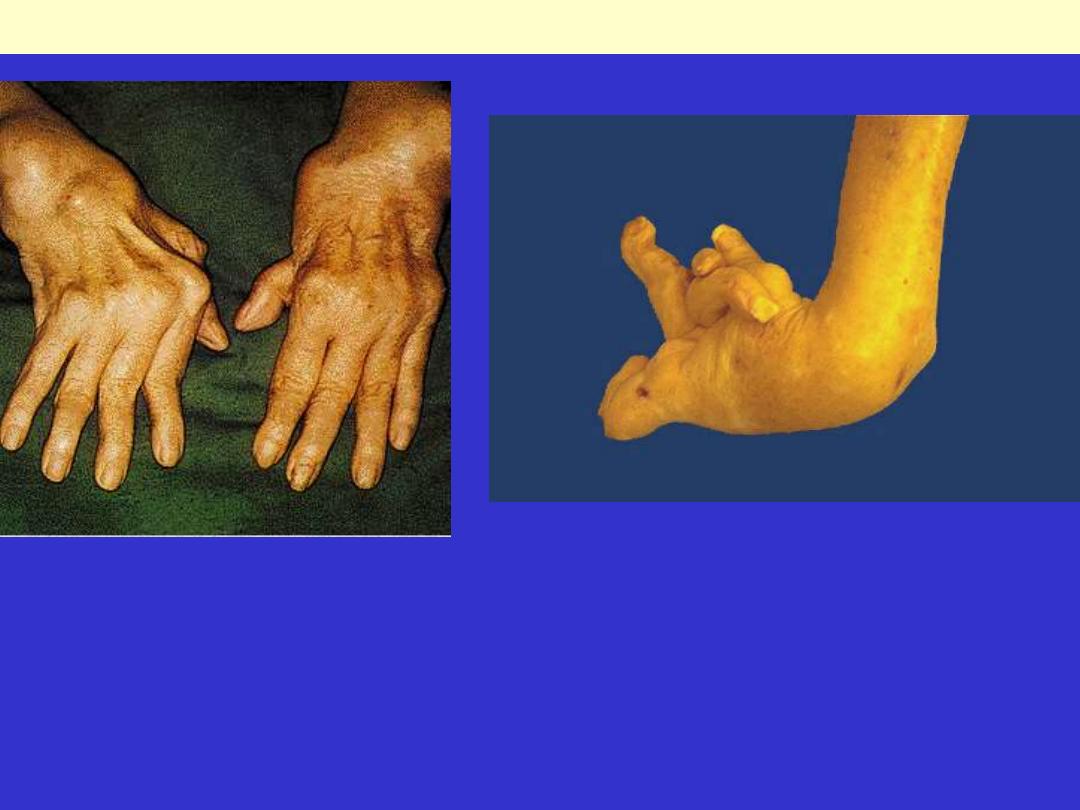
This deformity of the hand is due to rheumatoid arthritis (RA). This autoimmune disease leads to
synovial proliferation and joint destruction, typically in a symmetrical pattern involving small joints of
hands and feet, followed by wrists, ankles, elbows, and knees. Rheumatoid factor can be identified
serologically in most, but not all, RA patients.
Rheumatoid arthritis

End
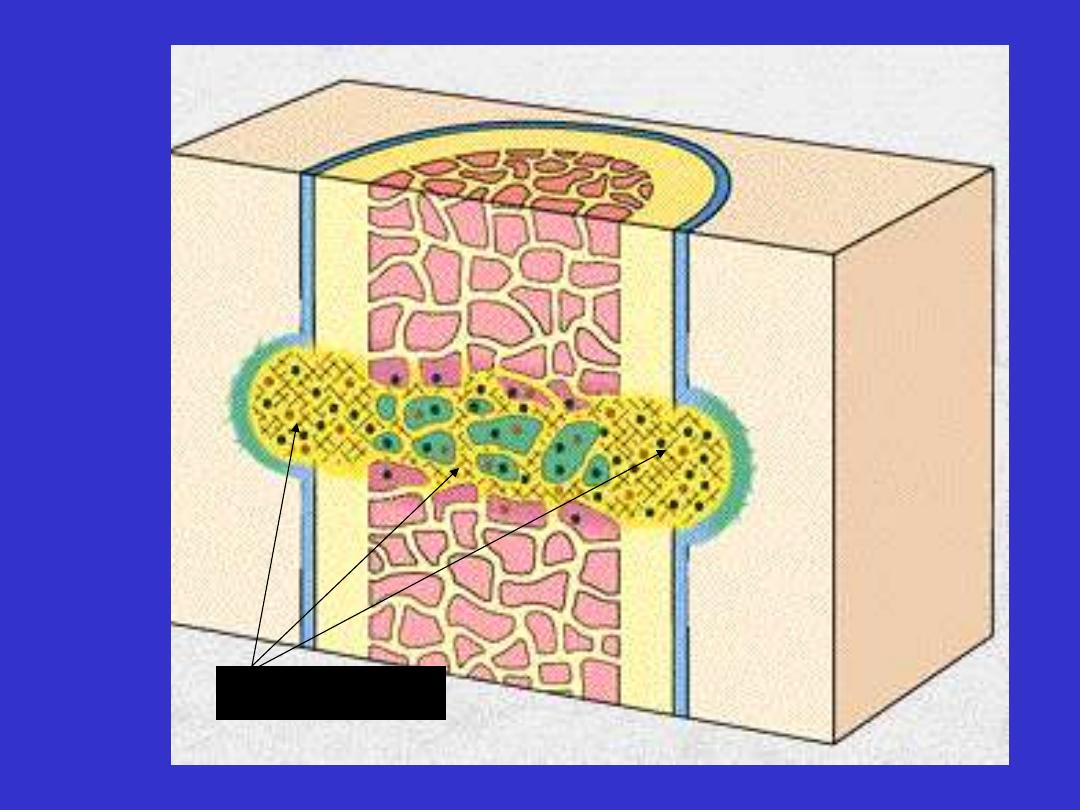
Fracture healing
Tearing of blood vessels
Hematoma
periosteum stripped off
Ischemic necrosis of haemopoietic
marrow with bone trabeculae
• Bone death recognized by empty lacunae
• Organization of the hematoma
- local inflammatory response (neutrophils & macrophages)
- phagocytosis of hematoma & necrotic debris
- in-growth of capillaries & fibroblasts
fibro-vascular granulation tissue
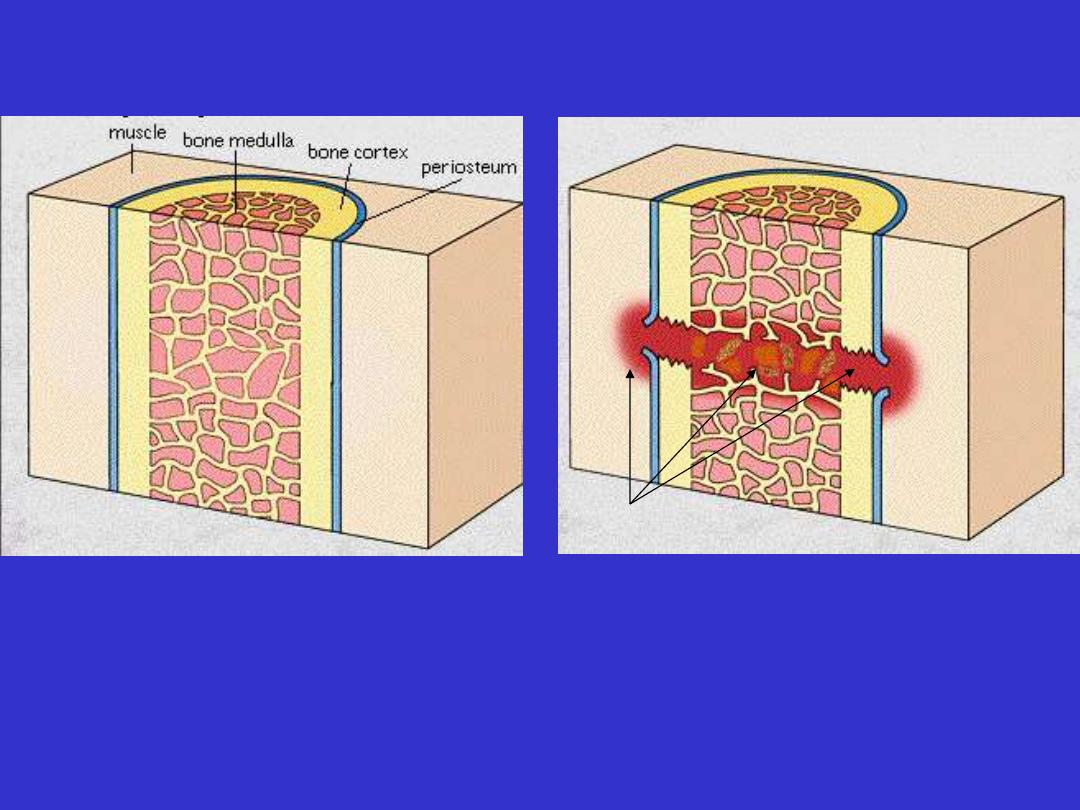
Bone fracture-hematoma formation
Normal bone
Early fracture
Hematoma
End of the 1st week
• Osteoblasts
-derived from inner layer of periosteum
- migrate into granulation tissue
- deposit large quantities of osteoid (woven bone)
External callus
Immobilize bone fracture
• Enlarging cuffs of callus advance towards each other to
bridge fracture gap externally
• Significant gap between bone ends may induce cartilage
formation.
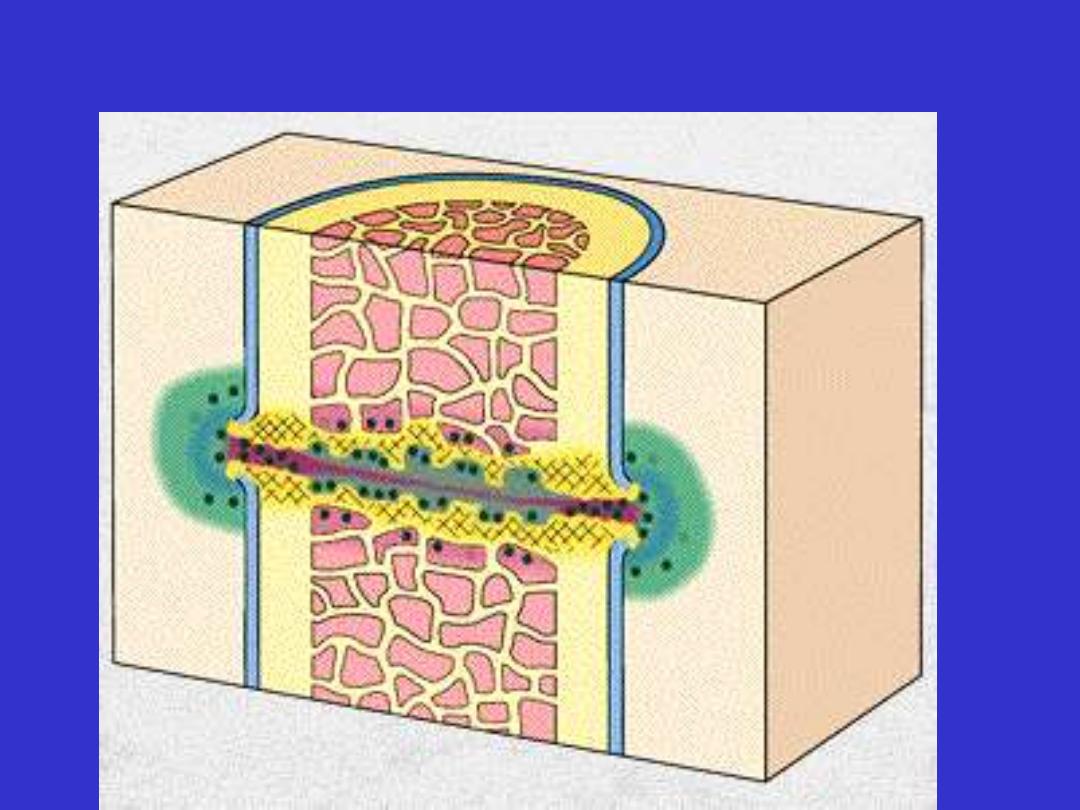
Bone fracture-osteoid (woven bone) formation

callus (internal and external)
• bridges fracture from within the medullary
cavity
• formed by the 3rd week
• union is to begin with by woven bone
(mechanically weak)
• amount of external callus depends on
- site of fracture
- degree of immobilization
- abundant in poorly immobilized fracture
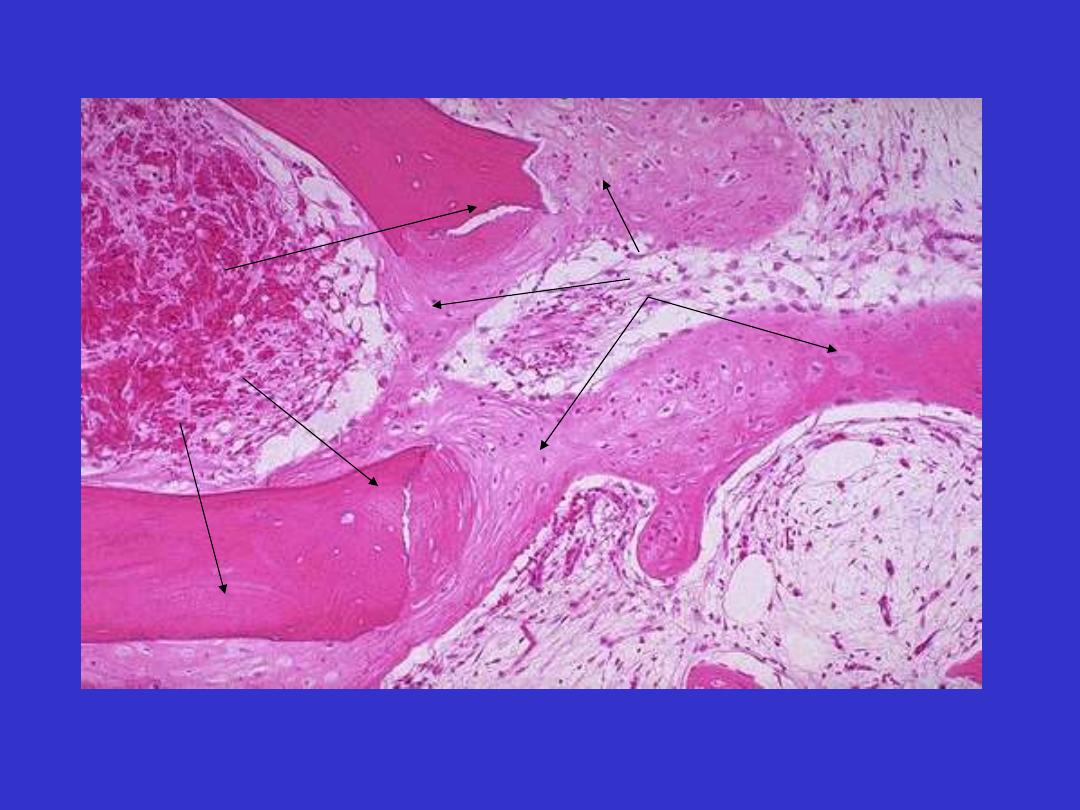
Fracture callus mic
Fractured native
bone trabeculae:
lamellar
Callus: woven bone
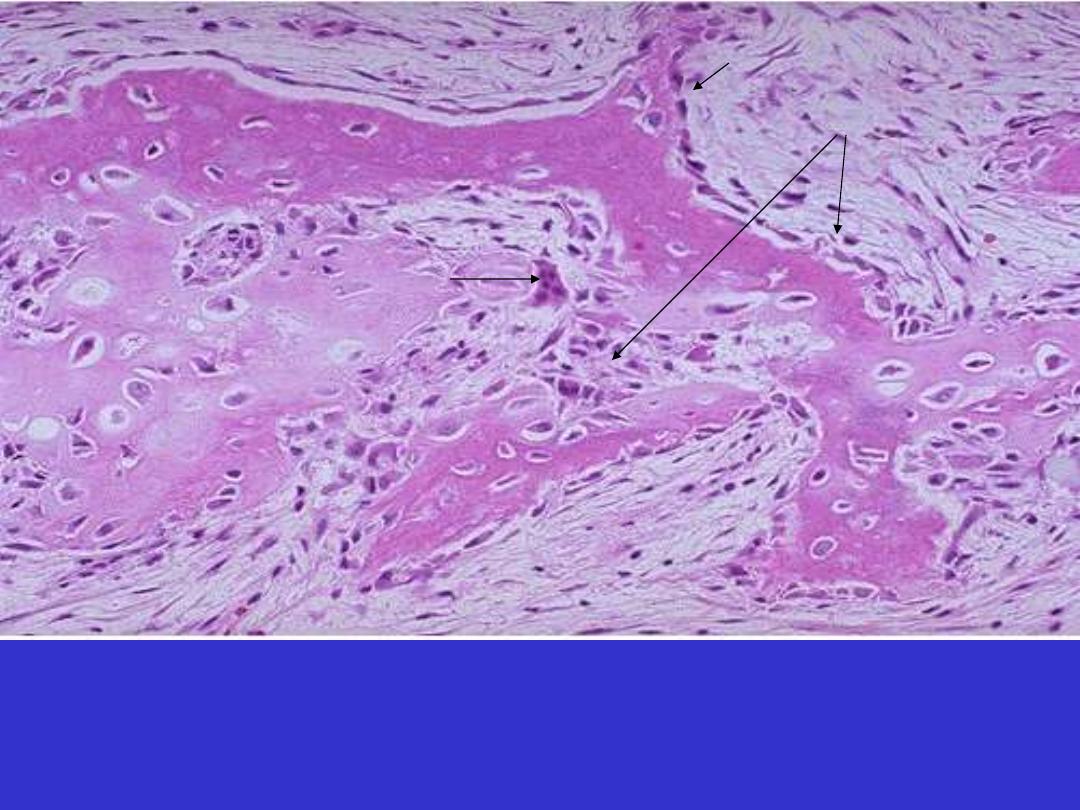
Osteoblasts rimming
trabeculae of woven
bone
osteoclast

Remodeling
• “reconstruction through transformation of woven bone
to lamellar bone and restoration of marrow cavity”
• Aim is to get full mechanical strength.
• Cortical woven bone is resorbed and gradually replaced
by lamellar (compact) bone (compact)
• Medullary callus is removed with restoration of the
marrow cavity
• Remodeling is done by osteoblasts & osteoclastes
• The whole reparative process may take about a year
(more rapid in children)
Callus in third week: replacement by compact mature
lamellar bone
Lamellar bone

Pathological Fracture
• Trivial trauma may cause fracture when the underlying bone
is abnormal
- Osteoporosis that occurs in the elderly (femur & vertebral
column).
- Osteomalacia
- Paget's disease of bone
- Primary or metastatic tumors
carcinoma
- breast
- bronchus
- thyroid
- kidney

Nervous System
• Mature neurons are permanent cells
• damage to brain or spinal cord followed by capillary
ingrowth and Gliosis
• Gliosis
- equivalent to scar formation
- remains permanently.
• In spinal cord injuries, axonal regeneration can be seen
up to 2 weeks
• Neurons in peripheral nervous system can regenerate
their axon
-section of a peripheral nerve may result in complete
functional recovery
- if the cut ends are not in perfect alignment the result is
traumatic neuroma

End
