
Cellular
Adaptations
and
Injury

Objectives
• Understand the concepts of cellular growth ,adaptations---
Hyperplasia, Hypertrophy ,Atrophy, Metaplasia
• List the factors of cell injury and death---O2, Physical, Chemical,
Infection ,Immunologic, Genetic, Nutritional
• Describe the pathologic mechanisms at the SUB-cellular level---
ATP ,Mitochondria, Ca++, Free Radicals ,Membranes
• Compare and differentiate the concepts of APOPTOSIS and
NECROSIS
• Identify common INTRA-cellular accumulations---Fat, Hyaline ,
CA++, Proteins, Glycogen ,Pigments
• Understand aging and differentiate the concepts of
preprogrammed death versus wear and tear.
Cell specificity and homeostasis
•
Each cell has a specific function
Genetic setup
Machinery and metabolic pathways
Cell’s specific function
•
The concept of homeostasis
- equilibrium with external environment
- maintenance of dynamically stable internal
machinery
- input
orchestrated
with output
Concept of adaptation
External disturbances
- Physiologic
- pathologic
changes in cell
machinery
new steady state
counteract external changes
escape from cell injury
preserve viability

What are the stimuli that induce
cellular adaptive reactions?
What is the aim of cellular
adaptation?
Enumerate the types of cellular
adaptive responses?

Cellular adaptations
• Reactions induced by:
- physiological stimuli
- pathological stimuli.
• Aim: to escape cell injury
•
The adaptive responses include:
1.Atrophy
2.Hypertrophy
3.Hyperplasia
4.Metaplasia
5.Dysplasia
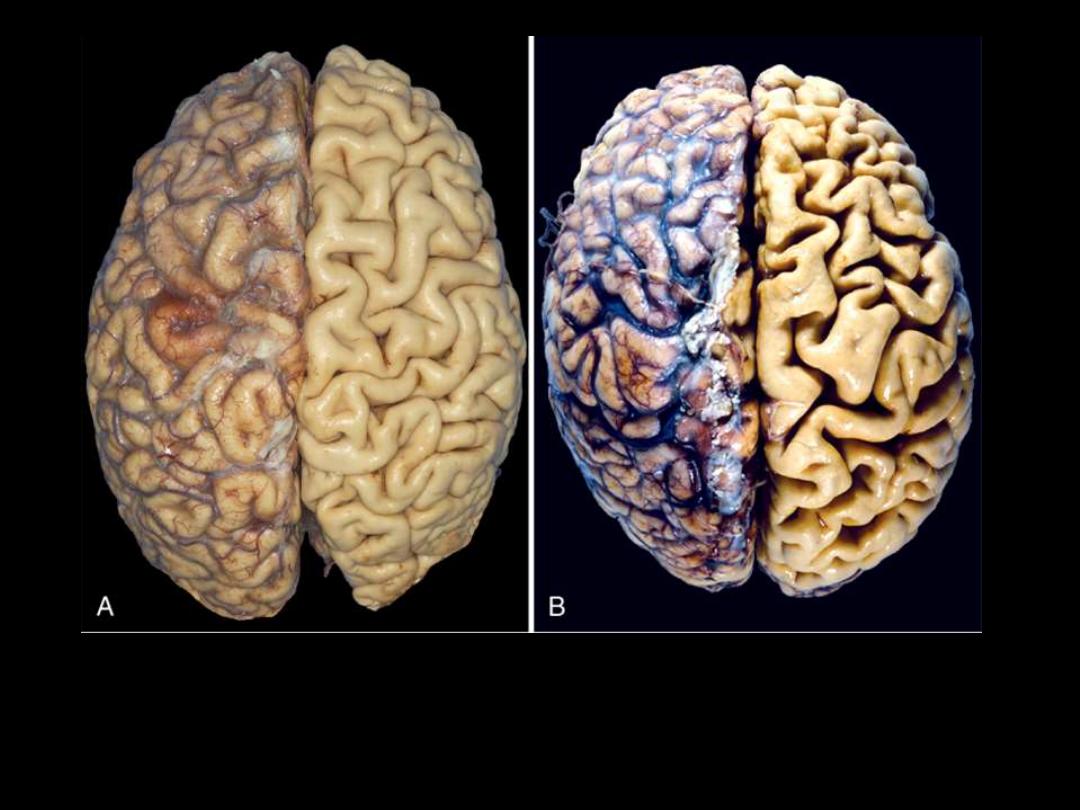
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B
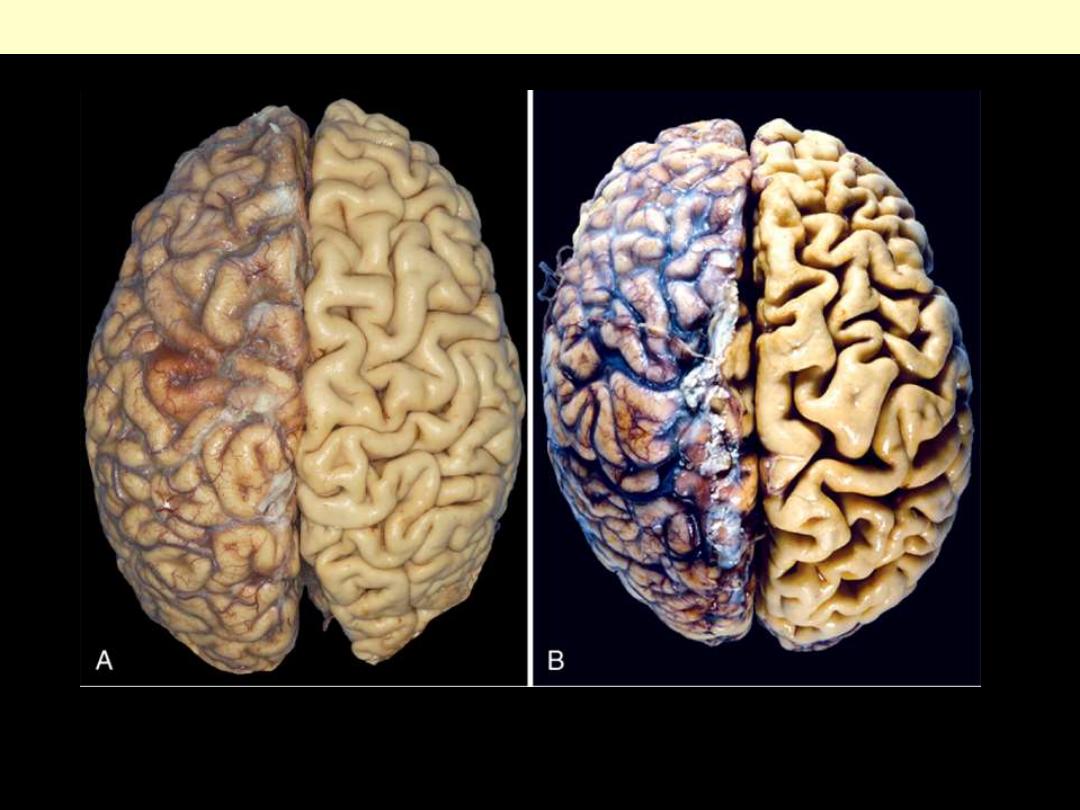
A.
Normal brain of a young adult.
B.
Atrophy of the brain in an 82-year-old male with
atherosclerotic disease. Atrophy of the brain is due to aging and reduced blood supply. Note
that loss of brain substance narrows the gyri and widens the sulci. The meninges have been
stripped from the right half of each specimen to reveal the surface of the brain.
Brain atrophy
A
B
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B
A
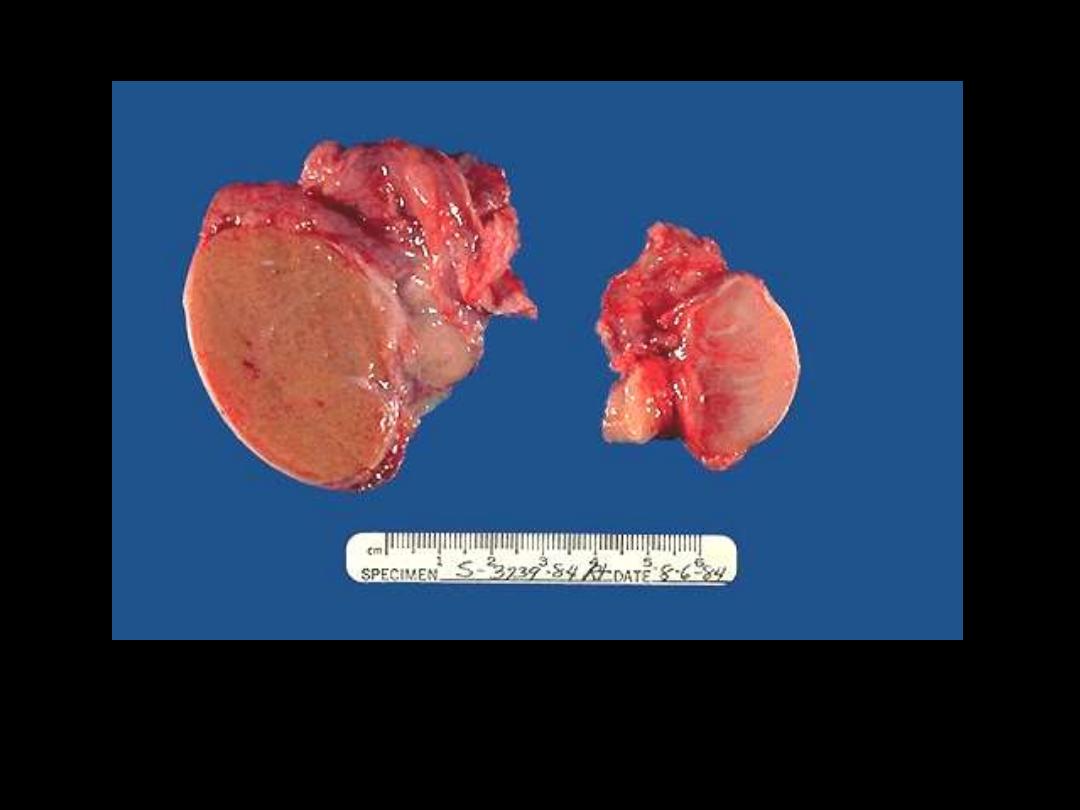
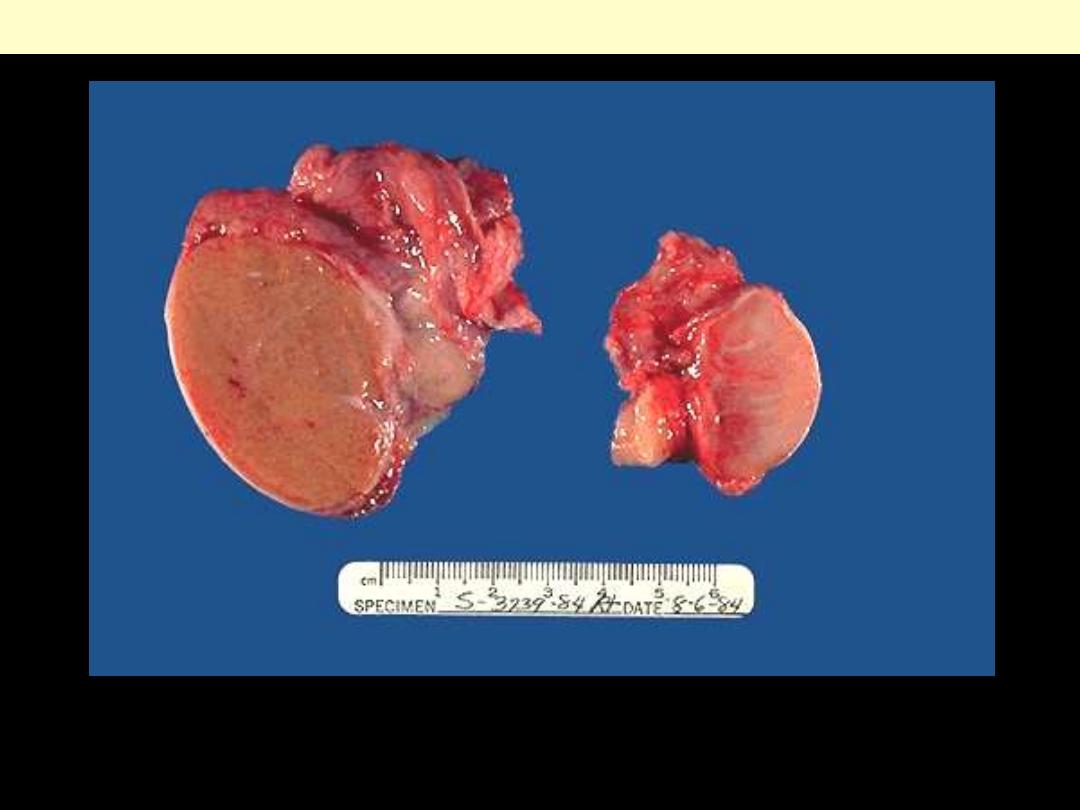
. Is a normal testis.
B
. is a testis that has undergone atrophy. Bilateral atrophy may occur
with a variety of conditions including chronic alcoholism, hypopituitarism, atherosclerosis,
chemotherapy or radiation, and severe prolonged illness. A cryptorchid testis will also be
atrophic.
Testicular atrophy
A
B
Atrophy
• Adaptive response
• Decrease in size and function of cells
↓ size of the organ or tissue

Define atrophy?
Enumerate the causes of atrophy.
Atrophy
• decrease in the size of the cell
↓organ
size (atrophic)
Causes of atrophy
1. Decrease workload
2. Denervation
3. Ischemia
4. Under nutrition
5. Loss of endocrine stimulation
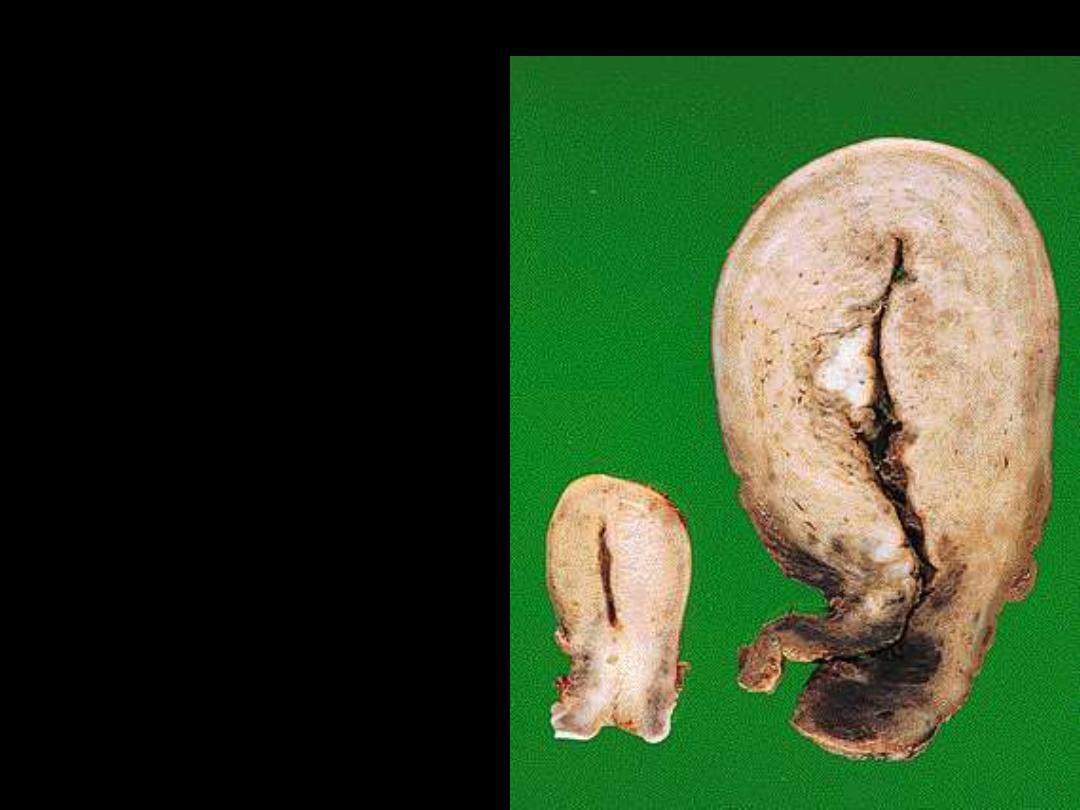
A
B
1. Identify the organ.
2. Describe the gross
abnormality
(pathological changes) in
B
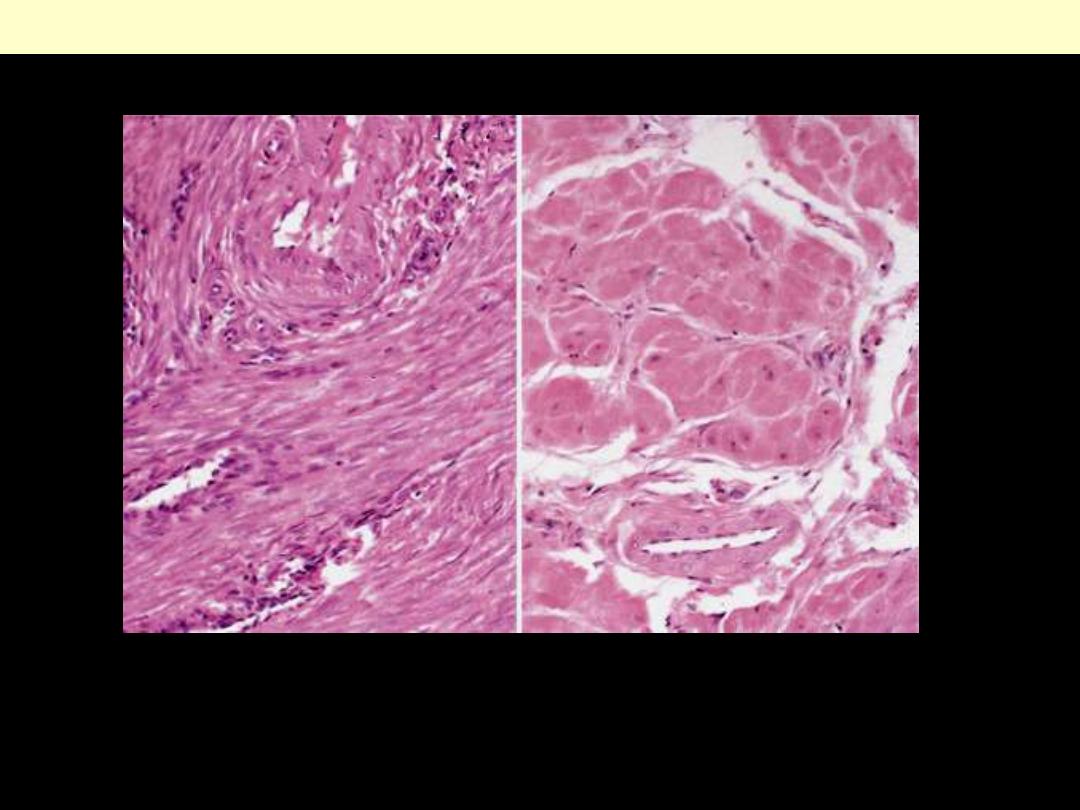
A
. Small spindle-shaped uterine smooth muscle cells from a normal uterus.
Compare this with (
B
) large, plump hypertrophied smooth muscle cells from a gravid
uterus (same magnification).
Normal Vs hypertrophied uterine smooth muscle cells
A
B

A 51-year-old male has a blood pressure of
150/95
mm Hg. If this condition remains untreated for years,
which of the following cellular alterations will be seen
in the heart?
(A) Atrophy
(B) Hyperplasia
(C) Melaplasia
(D) Hemosiderosis
(E) Hypertrophy
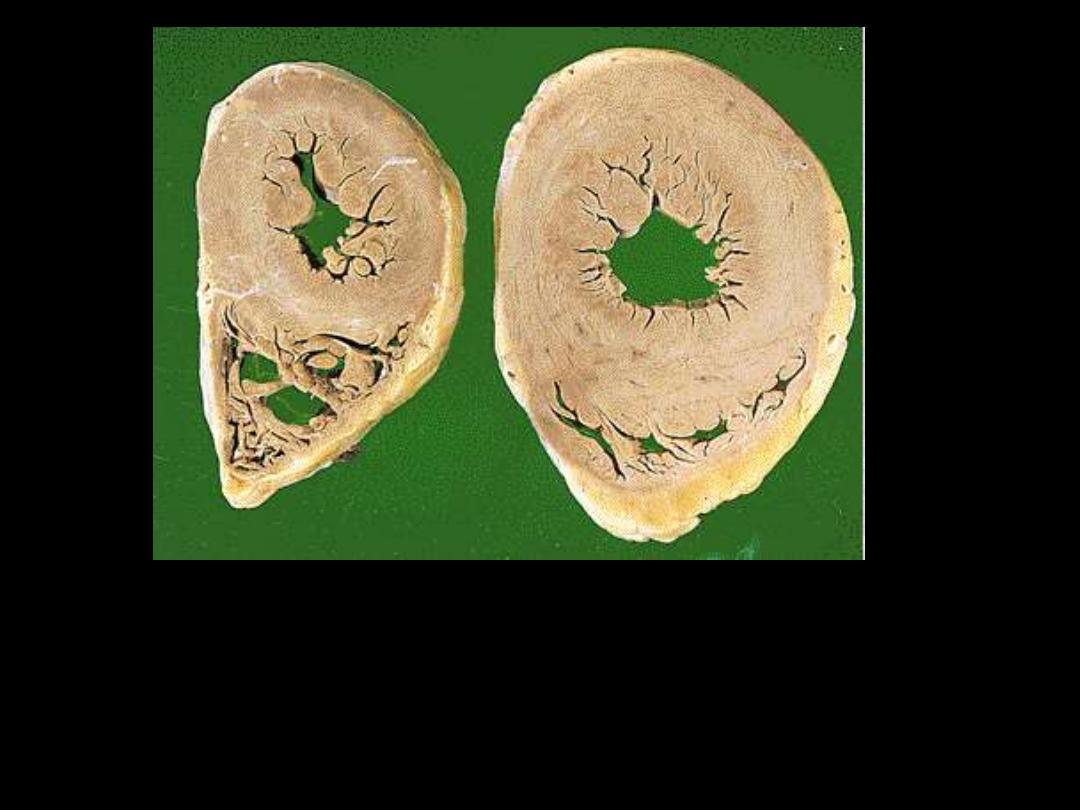
A
B
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B

Athletes as an example of muscular
hypertrophy

What are the types of hypertrophy?
Give an example for each type.

Hypertrophy
Muscular hypertrophy
protective adaptation
Types of hypertrophy:
Physiological e.g.
- athletes
- mechanical workers
- uterus in pregnancy
Pathological e.g.
- LVH in systemic hypertension
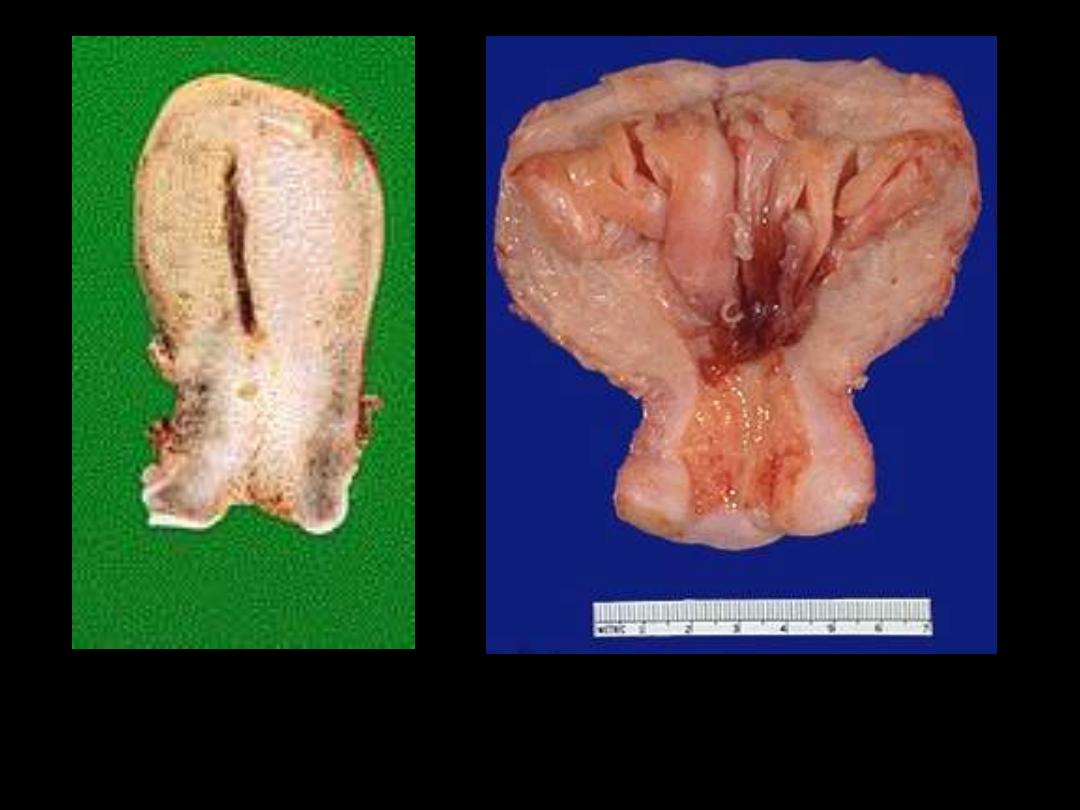
A
B
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B
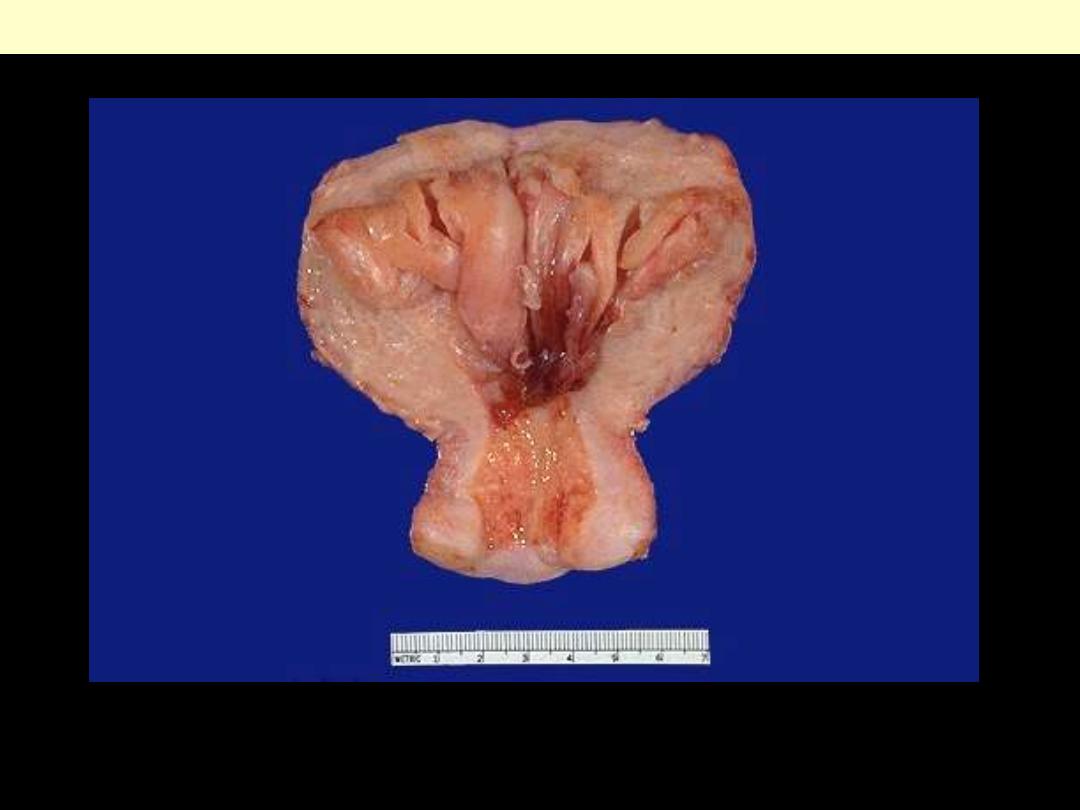
Endometrial hyperplasia
The prominent folds of endometrium in this uterus (opened to reveal the endometrial cavity) are an
example of hyperplasia. The hyperplasia involves both endometrial glands and stroma.

Define hyperplasia?
What are the types of hyperplasia?
Give an example for each type.

Hyperplasia
•
Increase in the number of cells in an organ or
tissue leading to an increase in its size.
•
Hyperplasia and hypertrophy closely related;
often occur together
•
Depending on their potential for hyperplasia
1. Labile cells
2. Stable cells
3. Permanent cells

Physiological and pathological HP
Physiological hyperplasia :
1. Hormonal
2. Compensatory
Pathological hyperplasia:
1. Excessive hormonal stimulation
2. The effect of growth factors on target cells
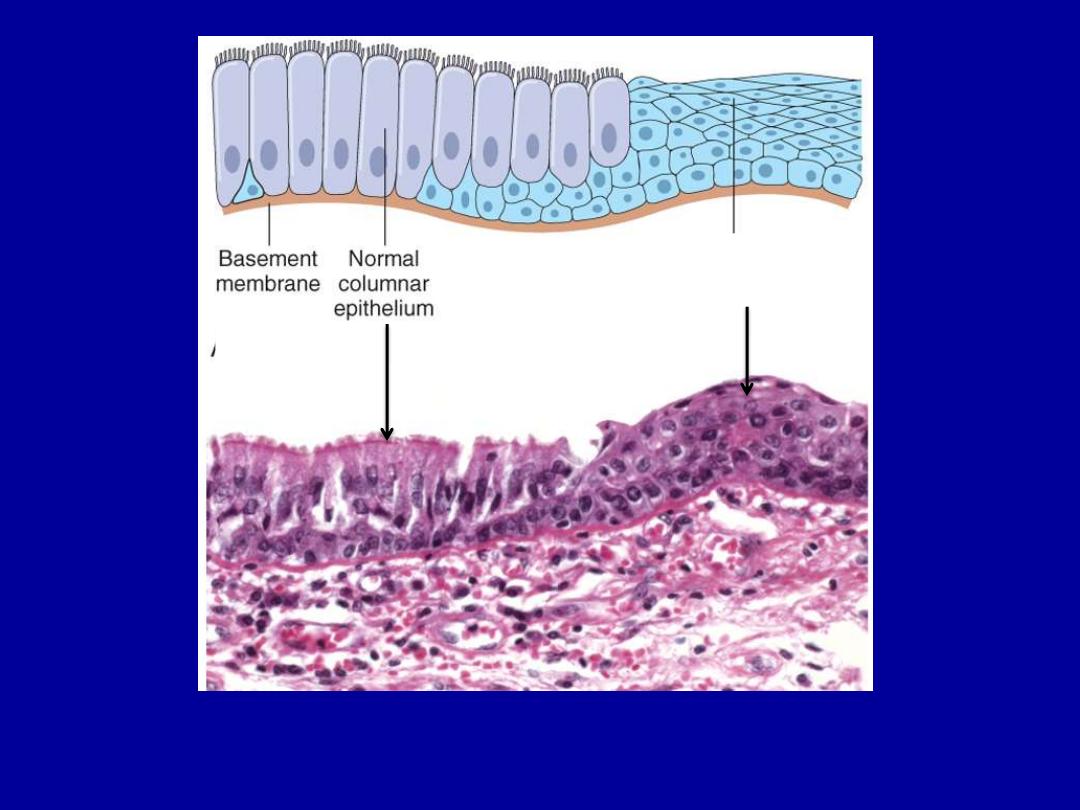
Squamous
epithelium
Describe the histopathological changes?
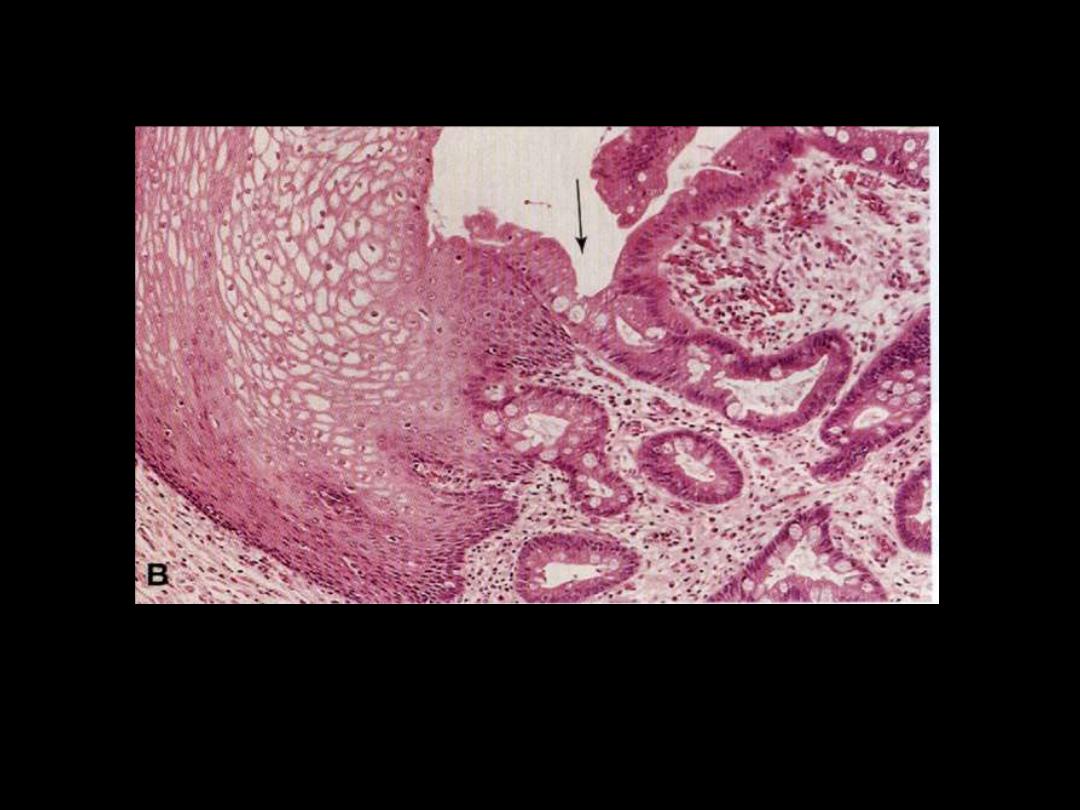
Describe the histopathological changes?
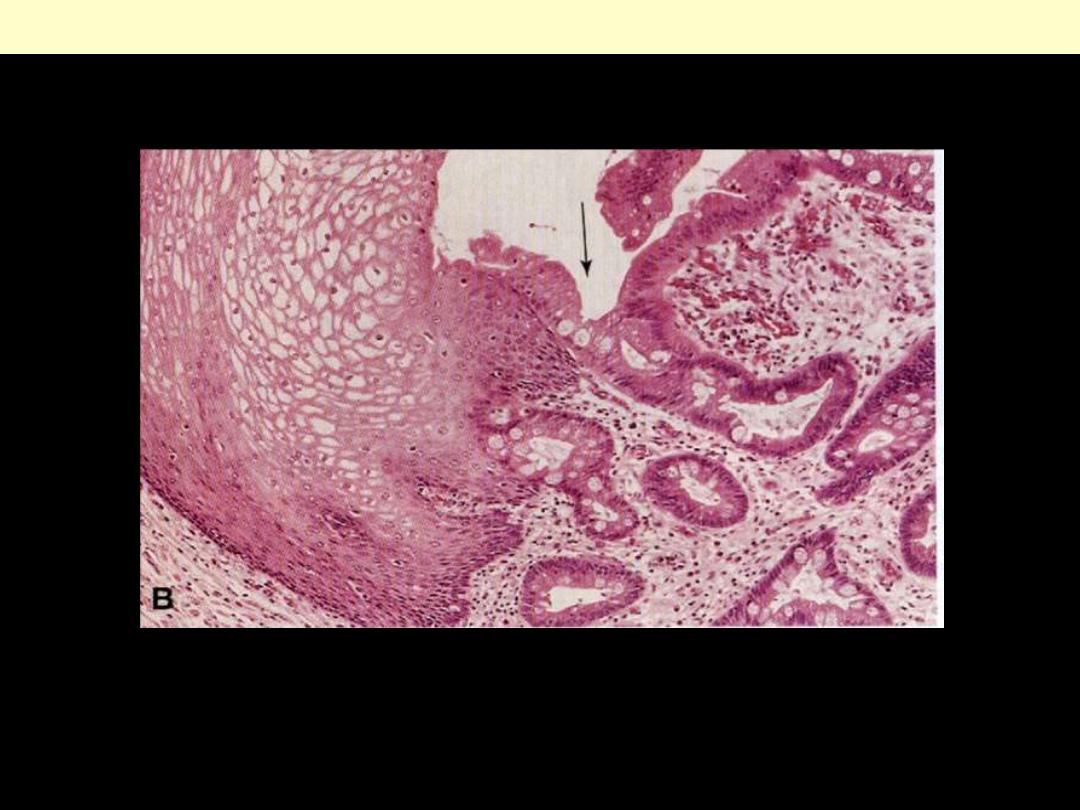
Metaplastic transformation (arrow) of the normal esophageal stratified squamous epithelium (Lt) to
mature columnar epithelium (Barrett esophagus)
Columnar (intestinal) metaplasia esophagus

Define metaplasia?
Give examples

Metaplasia
•
Replacements of one mature cell type by another mature
cell type
•
May represent an adaptation
•
Examples
1. Squamous metaplasia of the
- laryngeal and bronchial respiratory epithelium.
- urothelium in the urinary bladder
2. Columnar metaplasia of esophageal squamous epithelium
3. Mesenchymal metaplasia e.g.
- bone formation cells
Cell injury
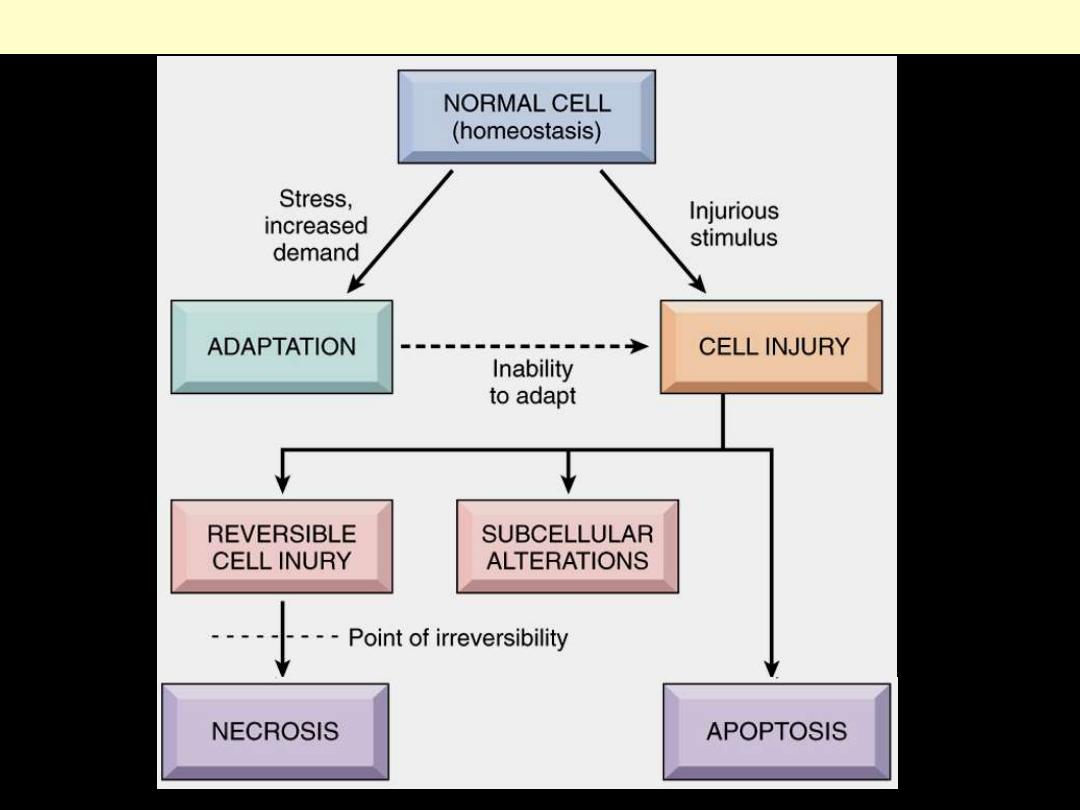
Occurs
1. Limits of adaptive capability exceeded
2. No adaptive response is possible
Cell injury divided
1. Reversible
2. Irreversible
cell death
Cell injury
Reversible
Irreversible
Stages in the cellular response to stress and injurious stimuli

Enumerate the causes of cell injury?

Categorization of injurious agents
Hypoxia
Physical agents
Chemical agents
Infectious agents
Immunological reactions
Genetic derangement
Nutritional imbalances
Aging

What are the factors that influence
the severity of injury?

Factors influencing severity of injury
1. Type and severity of injurious agent
2. Duration of exposure
3. Type of affected cells
- neurons (highly susceptibility)
- myocardial cells-hepatocytes
(intermediate susceptibility)
- Skeletal muscles-epidermis-fibroblasts
(low susceptibility)
Causes of Cell Injury
Most injurious stimuli can be grouped into the following
categories
1. Oxygen Deprivation (Hypoxia):
insufficient supply of
oxygen interferes with aerobic oxidative respiration and is
a common cause of cell injury and death.
Causes of hypoxia include
a. Ischemia
i.e. loss of blood supply in a tissue due to
interference with arterial flow or reduced venous
drainage. This is the most common cause of hypoxia
b. Inadequate oxygenation of the blood
, as in pneumonia
c. Reduction in the oxygen-carrying capacity of the blood
, as
in anemia or carbon monoxide (CO) poisoning.

Causes of Cell Injury
2. Chemical Agents
include concentrated glucose or salt &
Oxygen at high partial pressures. Various poisons cause damage
by affecting either membrane permeability, or the integrity of
enzymes. Environmental toxins as pollutants, insecticides, CO,
and alcohol as well as drugs can cause cell or tissue injury.
3
. Infectious Agents
including viruses, bacteria, rickettsiae,
fungi and parasites.
4. Immunologic Reactions
are primarily defensive in nature but
they can also result in cell and tissue injury. Examples include
autoimmune diseases & allergic reactions in genetically
susceptible individuals.
Causes of Cell Injury
5. Genetic Defects:
including
gross congenital malformations
such as those associated
with Down syndrome or as subtle as
point mutations
e.g. in sickle cell anemia.
Genetic defects may cause cell injury because of
deficiency of functional proteins
, such as enzymes in
inborn errors of metabolism, or
accumulation of damaged DNA
or
misfolded proteins
, both of which trigger cell death when
they are beyond repair.

Causes of Cell Injury
6. Nutritional Imbalances:
Protein-calorie insufficiency
among is the most obvious
example;
vitamin deficiencies
are not uncommon.
Excesses of nutrition
are also important causes of morbidity
and mortality; for example, obesity markedly increases the risk
for type 2 diabetes mellitus. Moreover, diets rich in animal fat
are strongly implicated in the development of atherosclerosis as
well as in increased vulnerability to cancer e.g. that of the colon.

Causes of Cell Injury
7.Physical Agents:
trauma, extremes of temperatures,
radiation, electric shock, and sudden changes in atmospheric
pressure all are associated with cell injury.
8. Aging:
cellular senescence leads to alterations in replicative
and repair abilities of individual cells and tissues. All of these
changes result in a diminished ability to respond to damage
and, eventually, the death of cells and of the organism.

SUBCELLULAR RESPONSES TO INJURY
Certain
agents
and
stresses
induce
distinctive
alterations involving only subcellular organelles.
Autophagy:
refers to lysosomal digestion of the cell's
own components.
It is a survival mechanism whenever there is nutrient
deprivation; the starved cell lives by eating its own
contents.
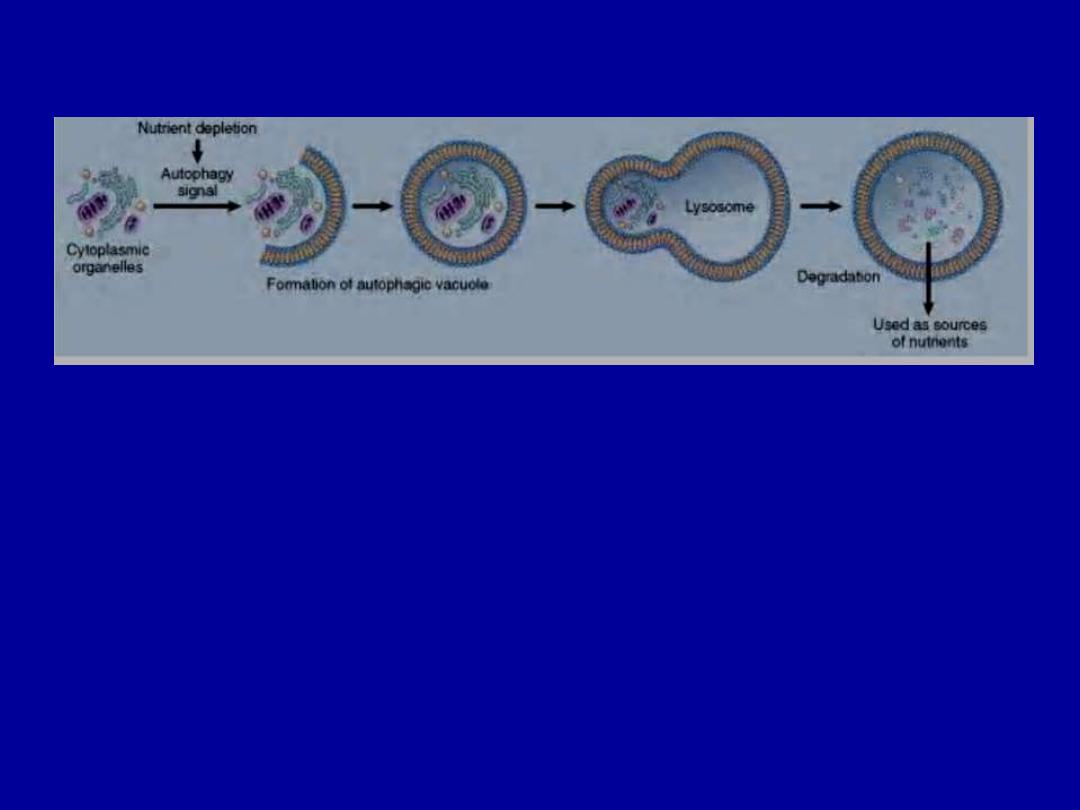
Autophagy. Cellular stresses, such as nutrient deprivation, activate autophagy genes
that create vacuoles in which cellular organelles are sequestered and then degraded
following fusion of the vesicles with lysosomes. The digested materials are recycled to
provide nutrients for the cell.

Induction (Hypertrophy) of Smooth ER:
the smooth ER
(SER) is involved in the metabolism of various
chemicals, and cells exposed to these chemicals show
hypertrophy of the ER as an adaptive response that may
have important functional consequences.
Mitochondrial Alterations:
mitochondrial dysfunction
plays an important role in acute cell injury and death.
In some non-lethal pathologic conditions, there may be
an increase in the number of mitochondria e.g. in
cellular
hypertrophy.
Conversely,
mitochondria
decrease in number during cellular atrophy (probably
via autophagy).

Cytoskeletal
Abnormalities:
Abnormalities
of
the
cytoskeleton may be manifested as an abnormal appearance
and function of cells (e.g.
Mallory bodies
, which represent
intracellular accumulations of fibrillar material in alcoholic
liver disease).
In
Kartagener (immotile cilia) syndrome
there are both
sterility in males and chronic infections of the lung.

MECHANISMS OF CELL INJURY
The outcomes of the interaction between the injurious agent
& the cell depend on
1.The type of injury, its duration, and its severity.
2.The type, adaptability, and genetic makeup of the injured
cell.
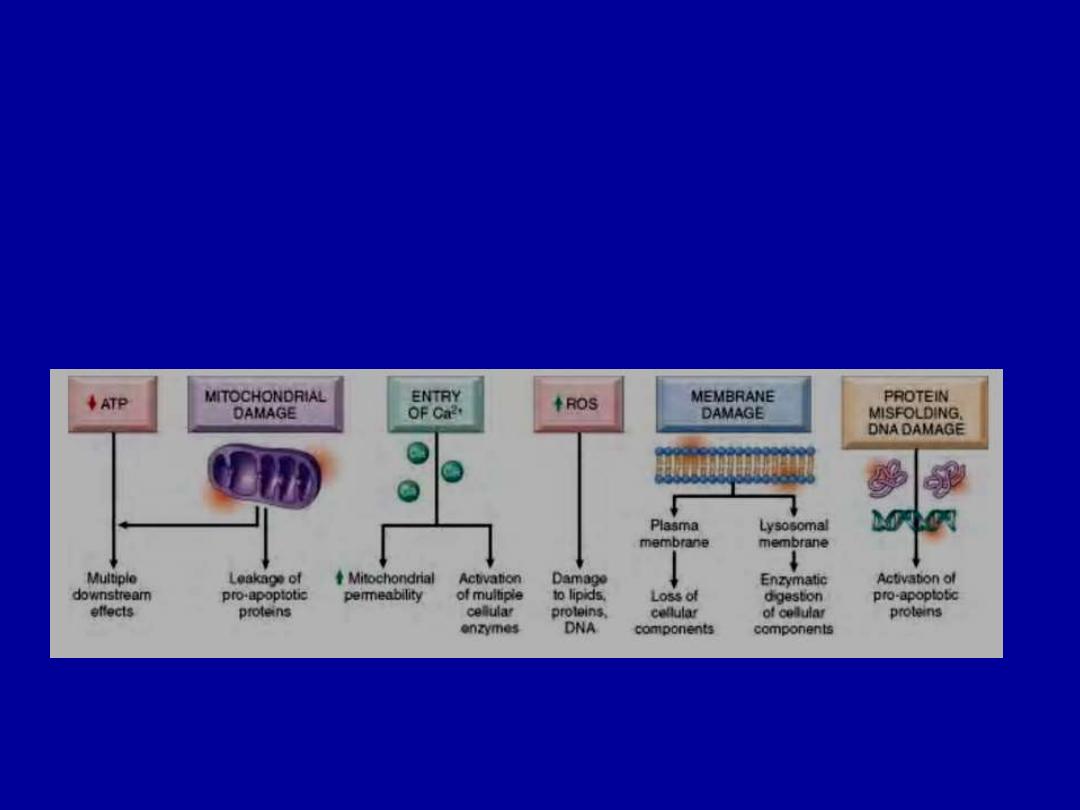
The most important targets of injurious stimuli
are
1.Mitochondria
(the sites of ATP generation)
2.Cell membranes
, which influence the ionic and osmotic
homeostasis of the cell
3.Protein synthesis
(ribosomes)
4.The cytoskeleton
(microtubules, and various filaments)
5.The genetic apparatus of the cell
(nuclear DNA)

ATP Depletion
The major causes of ATP depletion are
1.Reduced supply of oxygen and nutrients
2.Mitochondrial damage
3.The actions of some toxins (e.g., cyanide)
Depletion of ATP to less than 5% to 10% of normal levels
has widespread effects on many critical cellular systems.
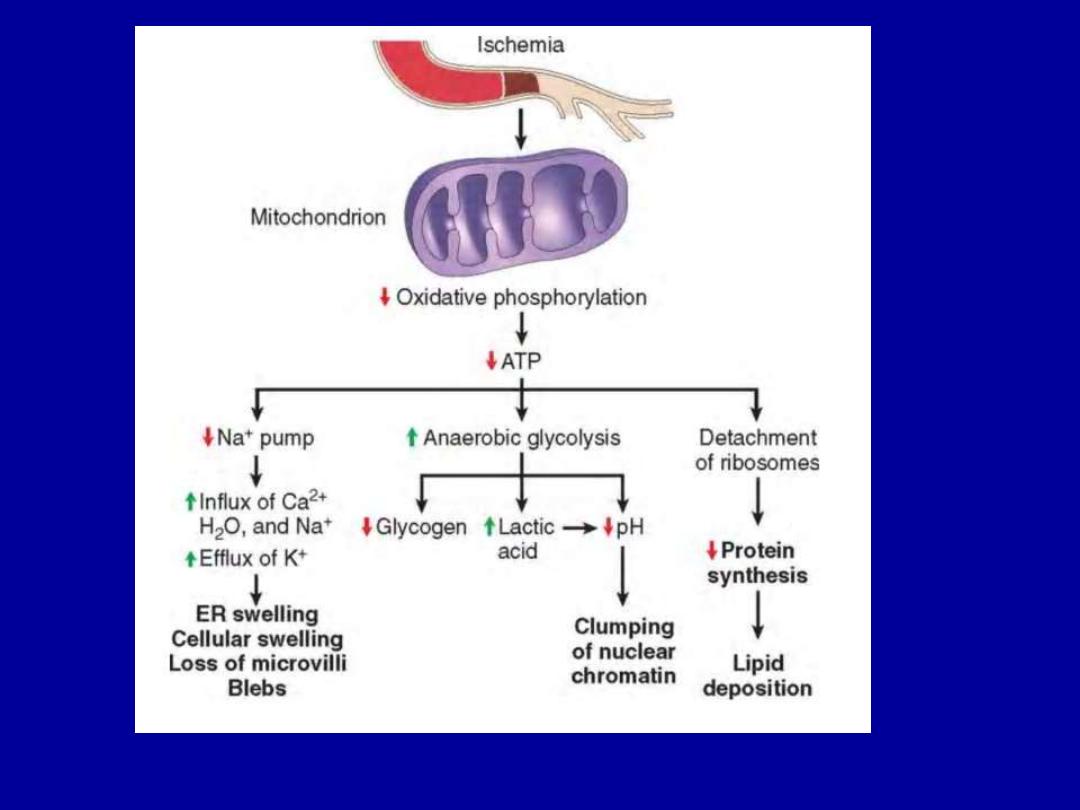

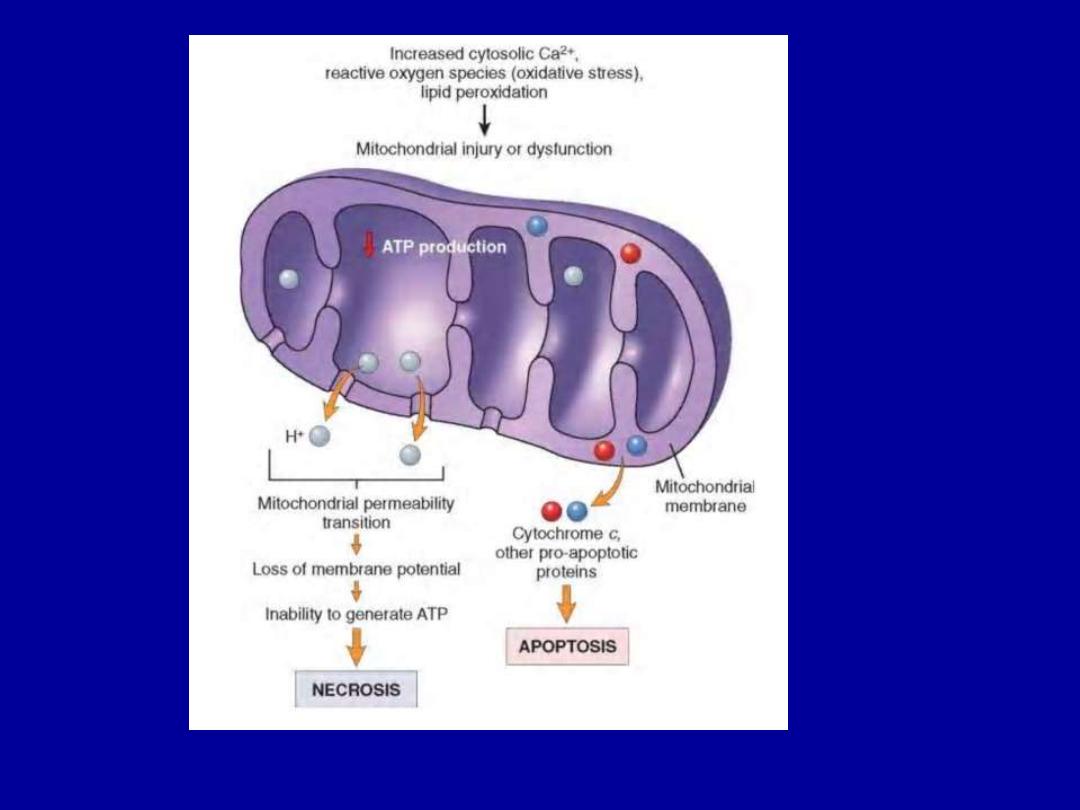
Mitochondrial Damage
There are two major consequences of mitochondrial damage:
1.The formation of a channel in the mitochondrial
membrane, called
the permeability transition pore.
The
opening of this channel leads to the loss of mitochondrial
membrane potential and pH changes, resulting in failure of
oxidative phosphorylation and progressive depletion of ATP,
culminating in necrosis of the cell.
2.Increased permeability of the mitochondrial membrane
may result in
leakage of cytochrome c
(the major protein
involved in electron transport) that are capable of activating
apoptotic pathways.

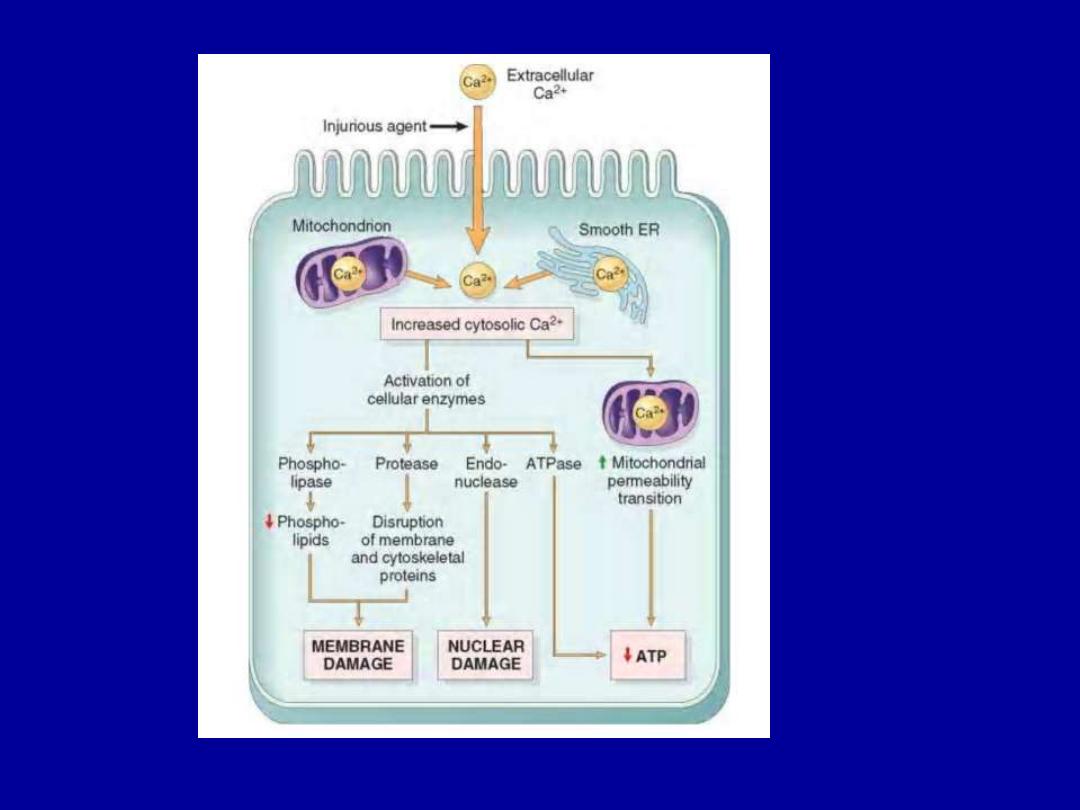
Influx of Calcium
Increased cytosolic Ca
2+
•Activates a number of enzymes
including phospholipases
(which cause membrane damage), proteases (which break
down
both
membrane
and
cytoskeletal
proteins),
endonucleases (which are responsible for DNA and
chromatin fragmentation), and ATPases (worsen ATP
depletion).
•Induction of apoptosis
, by direct activation of caspases.

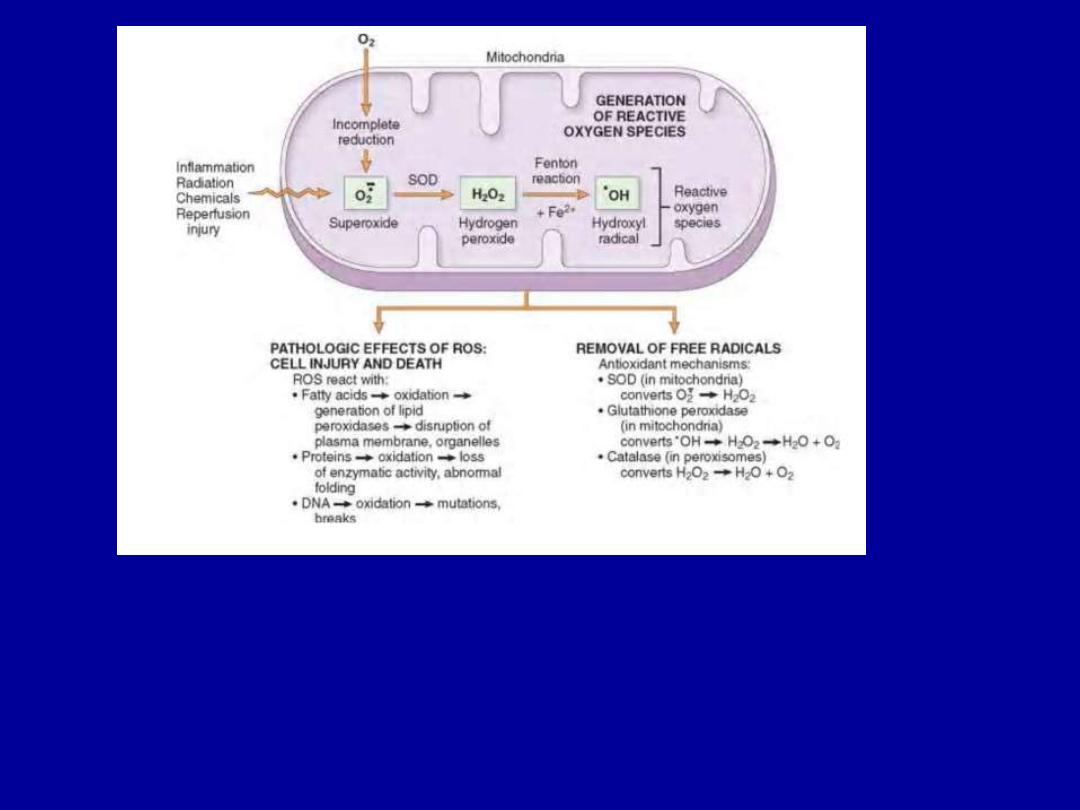
Accumulation of Oxygen-Derived Free Radicals (Oxidative
Stress)
These are designated as reactive oxygen species (ROS) &
are units with a single unpaired electron in their outer
orbit.
When generated in cells they avidly attack nucleic acids,
cellular proteins and lipids.
The molecules that react with free radicals are in turn
converted into free radicals, thus propagating the chain of
damage.

Cell injury in many circumstances involves damage by free
radicals; these include
1.Reperfusion injury
2.Chemical and radiation injury
3.Toxicity from oxygen and other gases
4.Cellular aging
5.Inflammatory cells mediated tissue injury

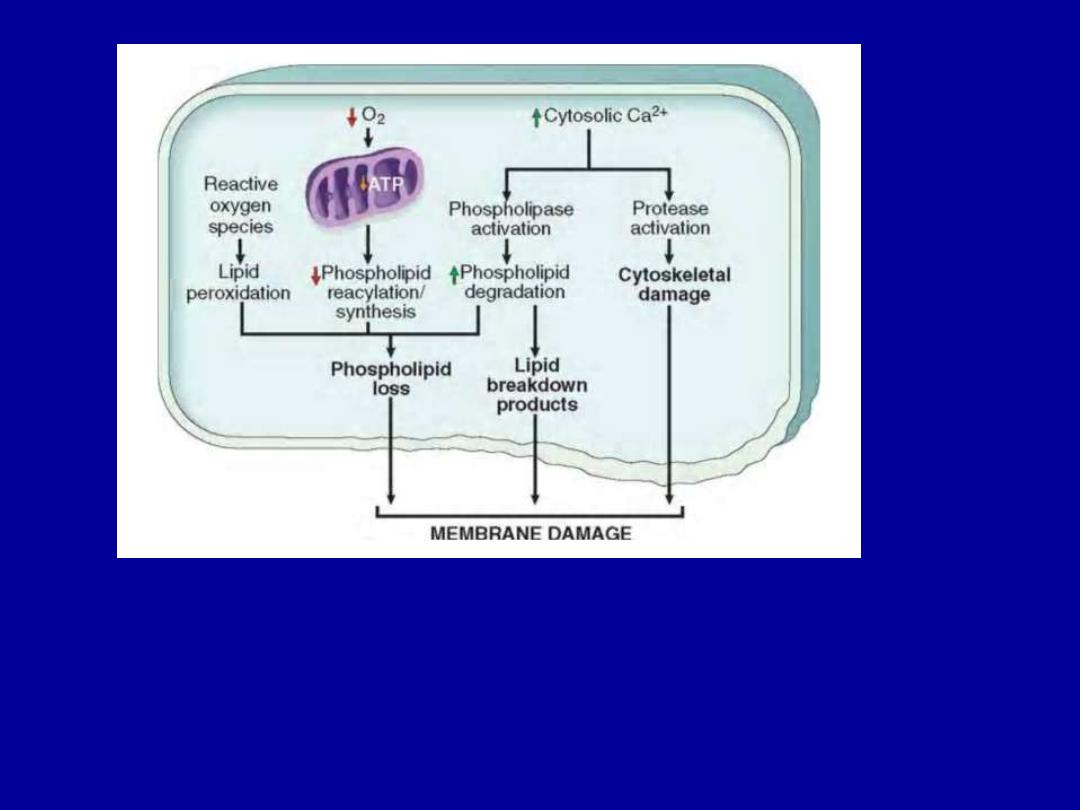
Defects in Membrane Permeability
Early loss of selective membrane permeability leading ultimately
to overt membrane damage is a consistent feature of most forms
of cell injury (except apoptosis). The plasma membrane can be
damaged
by
ischemia,
microbial
toxins,
complement
components-mediated lysis, etc.
The most important sites of membrane damage during cell injury
are
a. the mitochondrial membrane,
b. the plasma membrane, and
c. membranes of lysosomes.

Damage to DNA & Proteins
Cells have mechanisms that repair damage to DNA
, but if this
damage is too severe to be corrected (e.g., after radiation
injury or oxidative stress), the cell initiates its suicide
program and dies by apoptosis.
A similar reaction is triggered by improperly folded
(configured) proteins which may be the result of inherited
mutations or through free radicals.
These mechanisms of cell injury typically cause apoptosis.

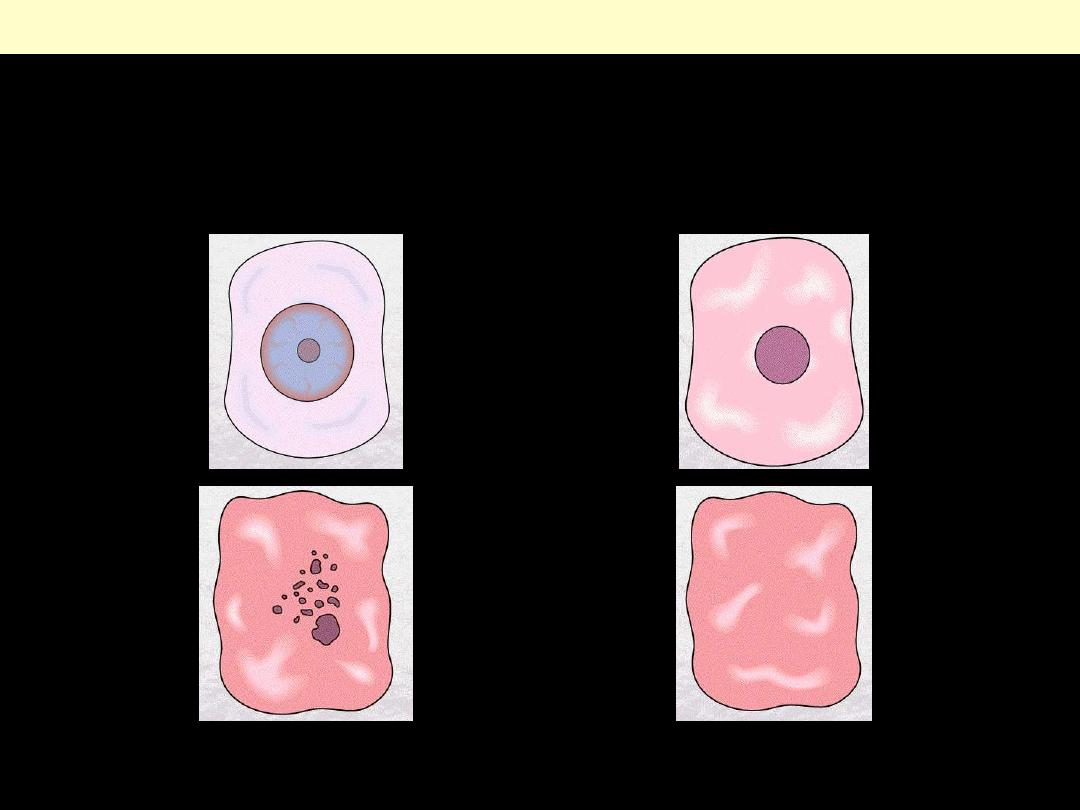
Reversible Cell Injury

Cloudy swelling
(hydropic Swelling)
Normal
A
B
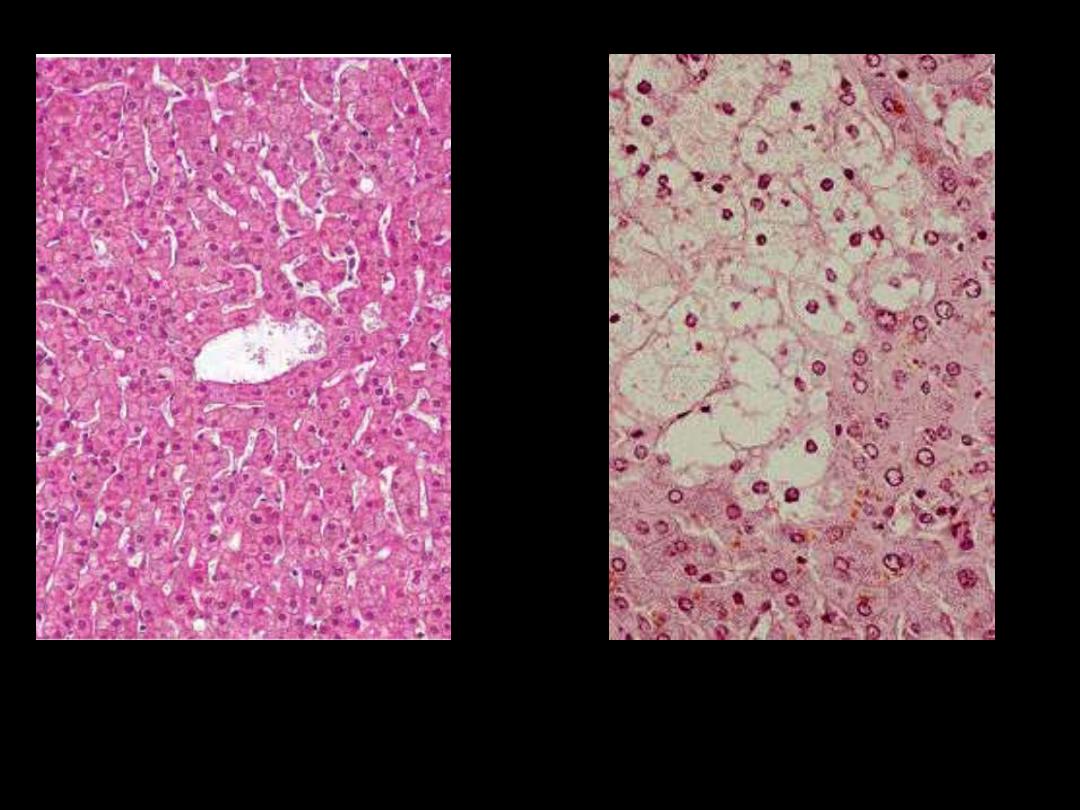
Identify the organ?
Describe the histopathological changes in B?
The affected hepatocytes are distended by accumulated water that imparts
cytoplasmic pallor.
Cellular swelling (hydropic change)

Fatty changes
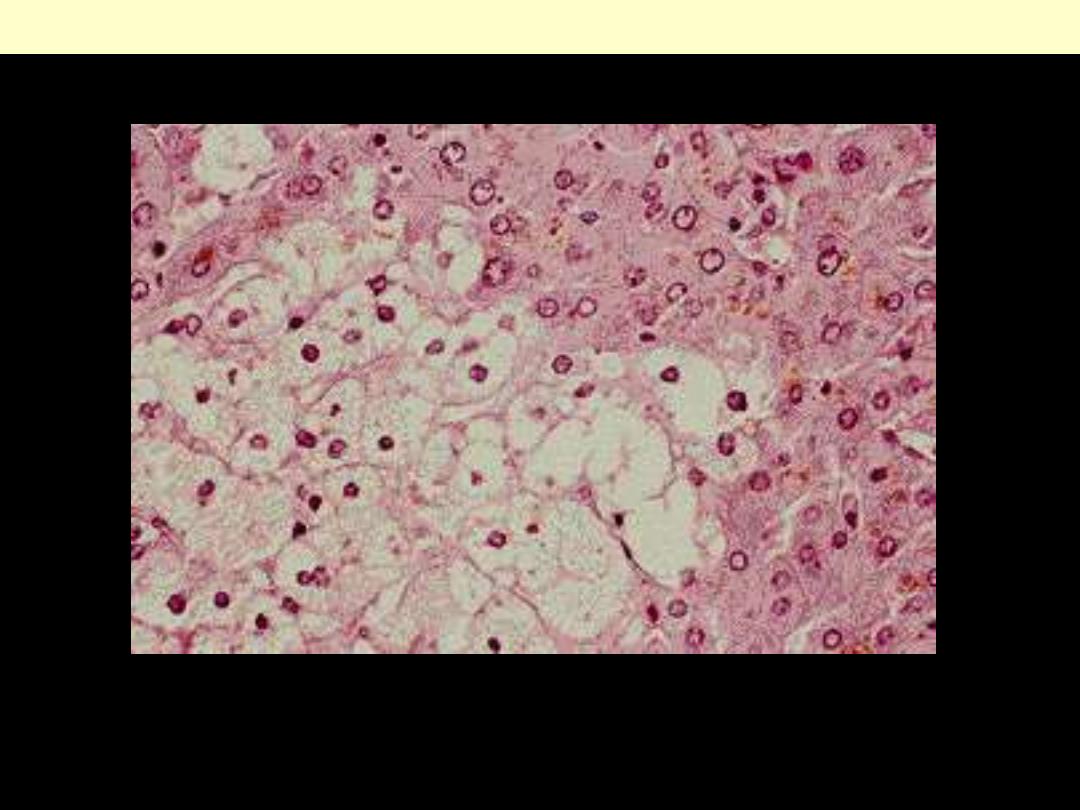
Identify the organ?
Describe the histopathological changes
This is the histologic appearance of hepatic fatty change. The lipid
accumulates in the hepatocytes as vacuoles. These vacuoles have a clear
appearance with H&E staining.

Cell necrosis
Define cell necrosis?
Explain the mechanism?

Cell necrosis
• Morphological changes that follow cell
death
in a living tissue or organ.
• Resulting from
degrading action of enzymes
on irreversibly damaged cells with
denaturation of cellular proteins.
• Morphological changes
- cytoplasmic
- nuclear

Cytoplasmic changes
•
More eosinophilia
- Loss of cytoplasmic RNA
- Increased binding of eosin to the denatured
proteins.
•
More homogeneous appearance
- loss of glycogen particles
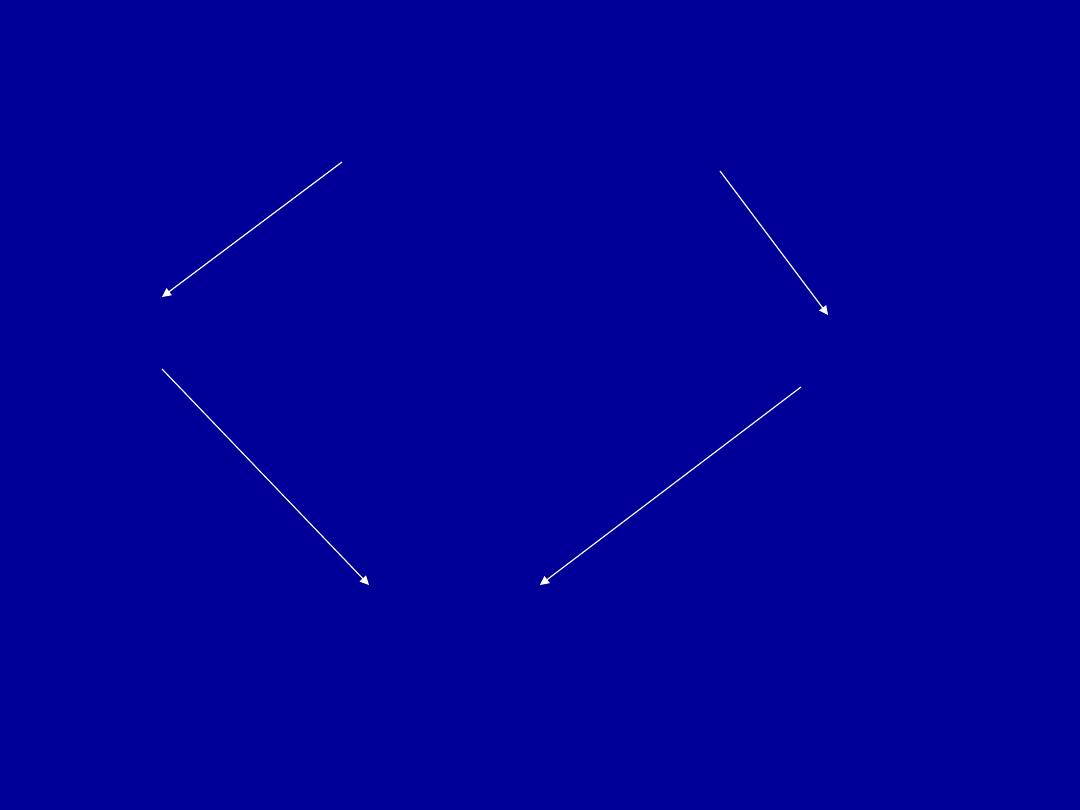
Nuclear changes
chromatin clumping
Pyknosis
karyorrhexis
karyolysis
normal
pyknosis
karyorrhexis
karyolysis
Cell necrosis: Nuclear changes
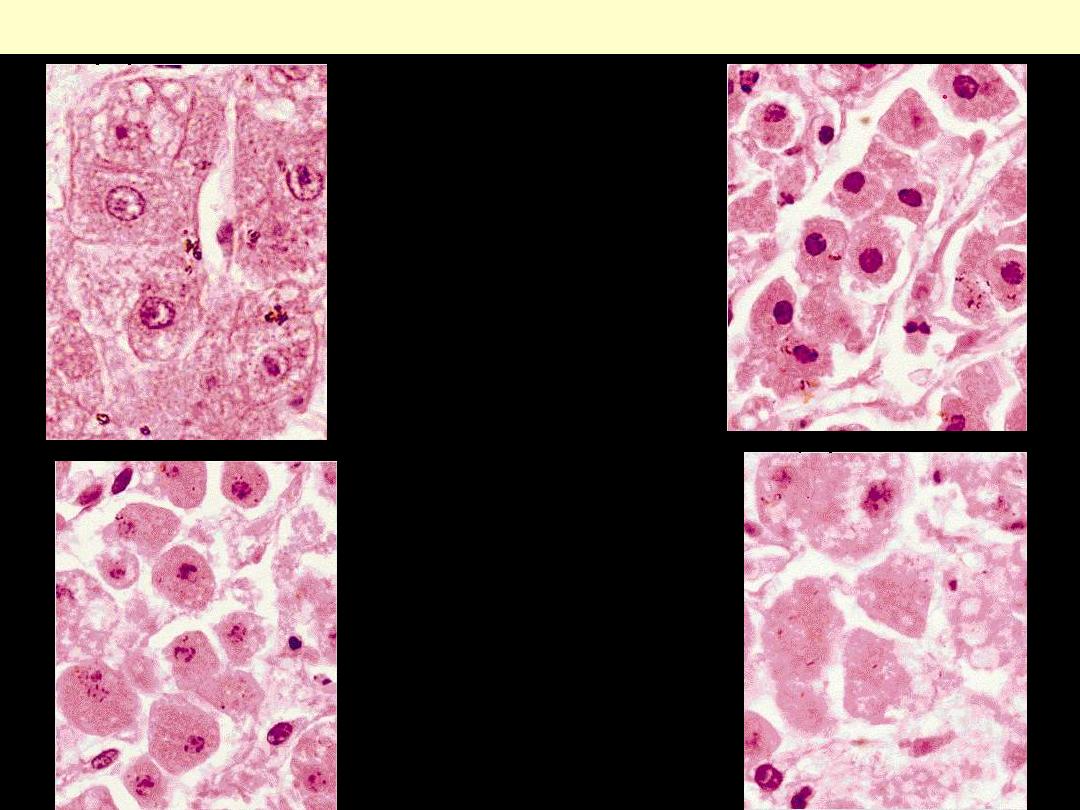
Liver cell necrosis: Nuclear changes
normal
pyknosis
karyorrhexis
karyolysis

What are the morphological types of
necrosis?

Types of cell necrosis
1. Coagulation (coagulative) necrosis.
2. Liquefaction (liquefactive) necrosis.
3. Fat necrosis
4. Caseation (caseous) necrosis
5. Gangrenous necrosis.
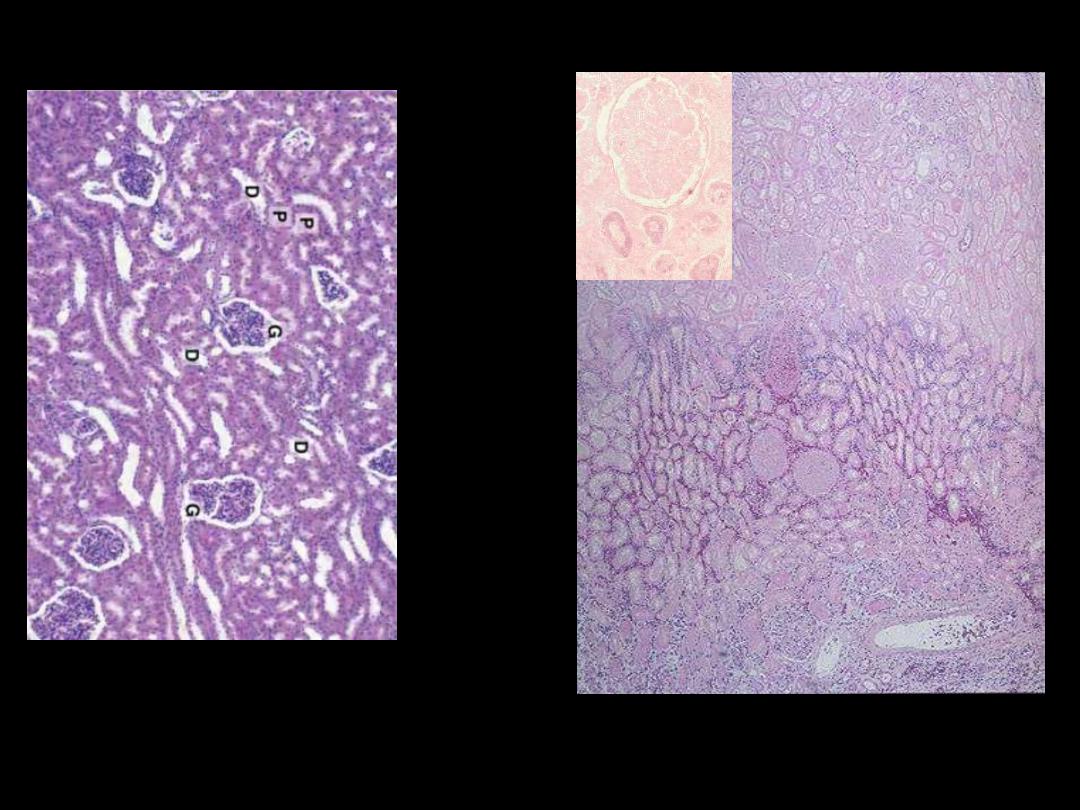
A
B
Identify the organ?
Describe the histopathological changes?
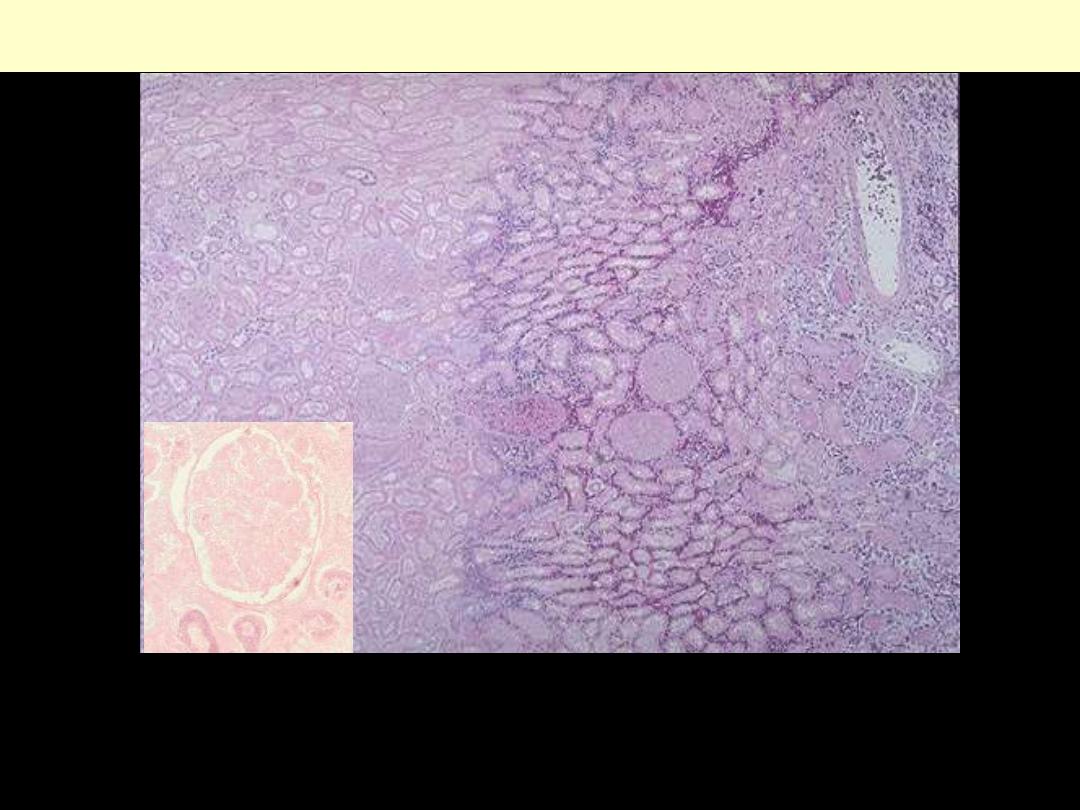
Coagulative necrosis-kidney
Microscopically, the renal cortex has undergone anoxic injury at the left so that the cells appear pale
and ghost-like. There is a hemorrhagic zone in the middle where the cells are dying or have not quite
died, and then normal renal parenchyma at the far right. This is an example of coagulative necrosis
Within the area of necrosis (Lt) the outlines of tubules and glomeruli are still preserved but fine
structural details are lost (inset)
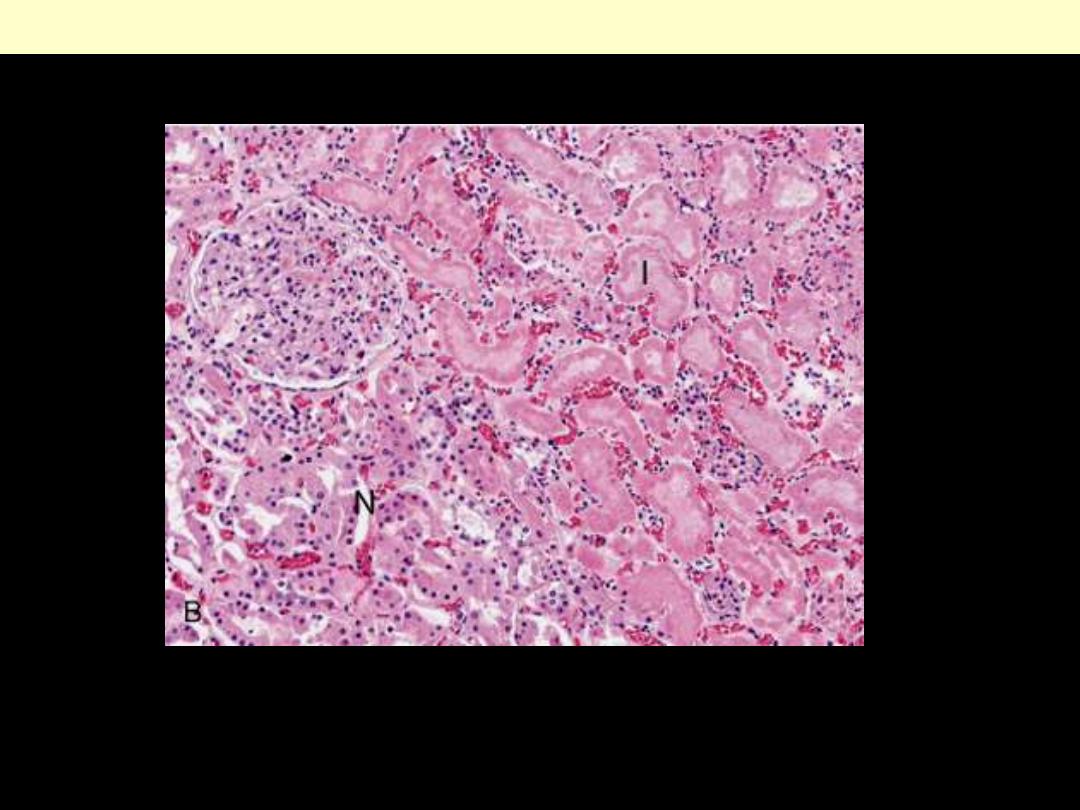
Microscopic view of the edge of the infarct, with normal kidney (N) and necrotic cells in the infarct (I).
The necrotic cells show preserved outlines with loss of nuclei, and an inflammatory infiltrate is present
(difficult to discern at this magnification).
Coagulative necrosis Kidney
A
Normal
B
Identify the organ?
Describe the histopathological
changes in B?
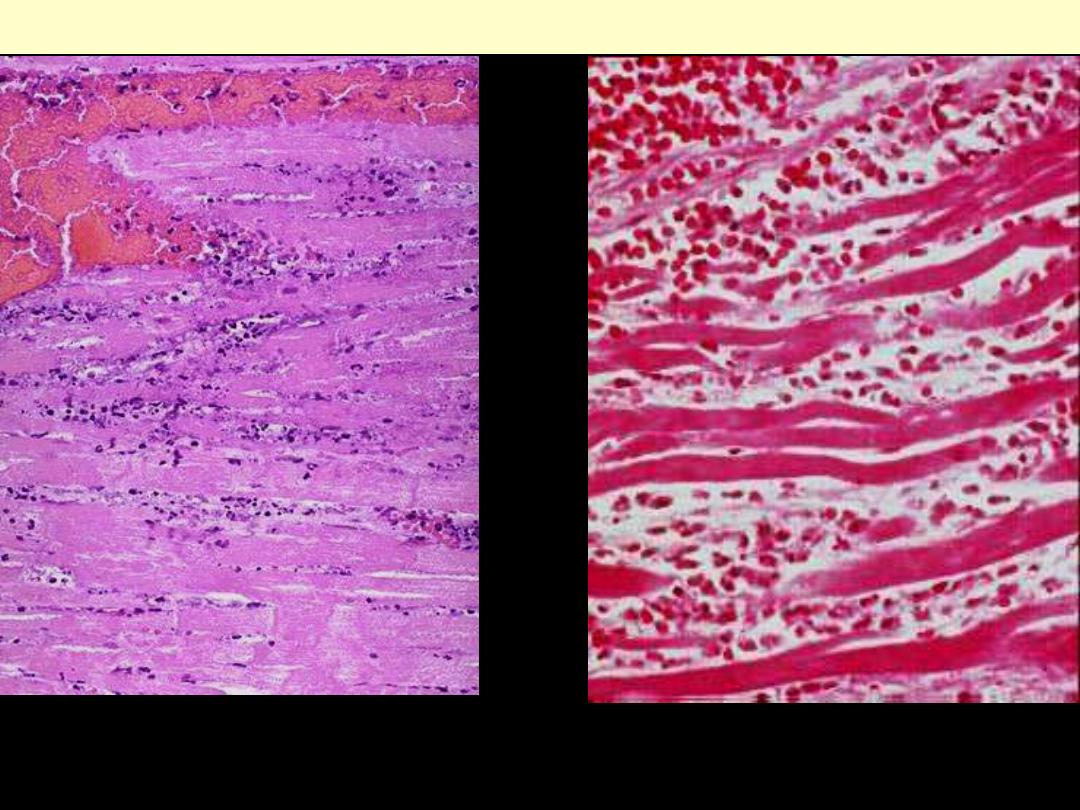
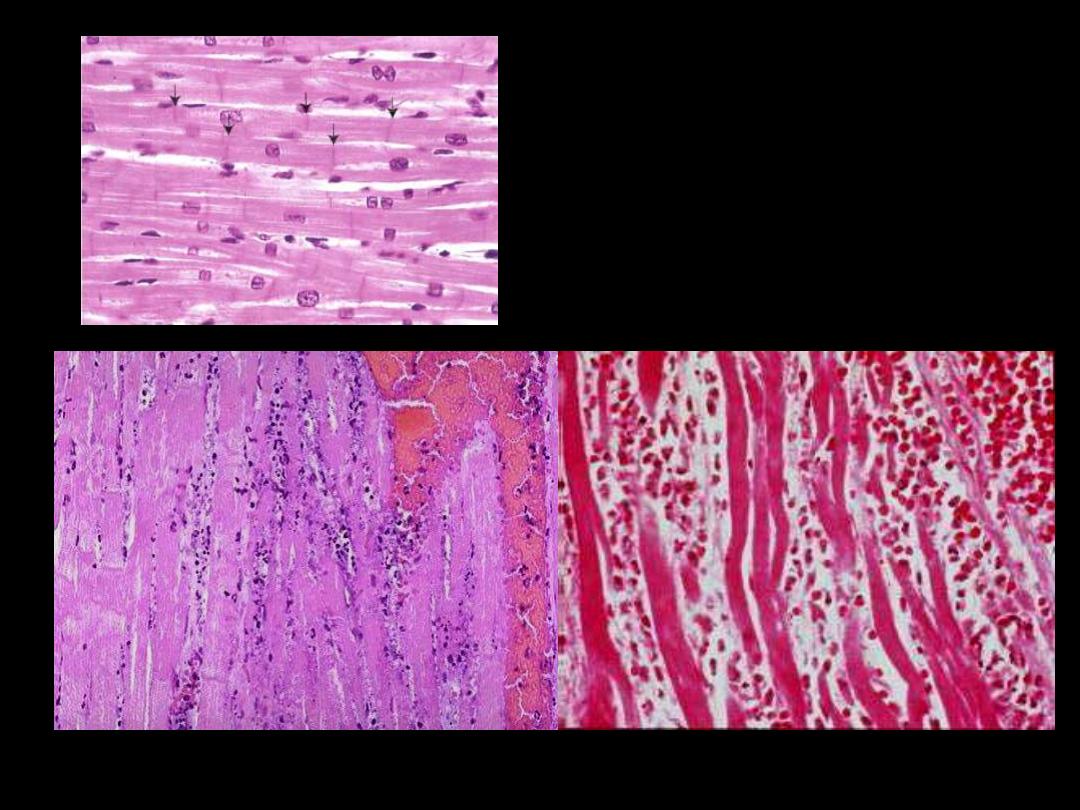
The necrotic myocytes are intensely eosinophilic with loss of both cross striations & nuclei.
The outlines of individual fibres are still maintained. There are inflammatory cells infiltration
& RBCs in-between the necrotic fibers.
Coagulative necrosis myocardium

Coagulative necrosis
• Sudden severe ischemia in organs
• Nucleus: lost
• Cytoplasm: homogeneous deeply eosinophilic
• Outlines of cells are still discernible
• Fine structural details lost
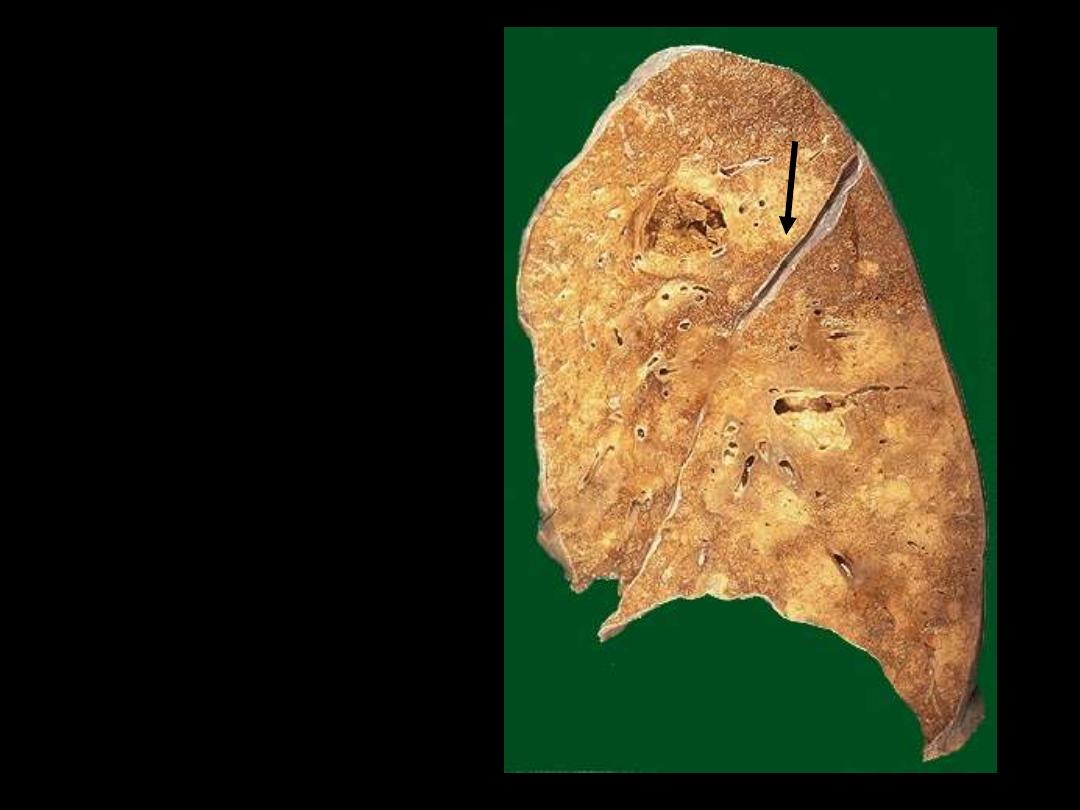
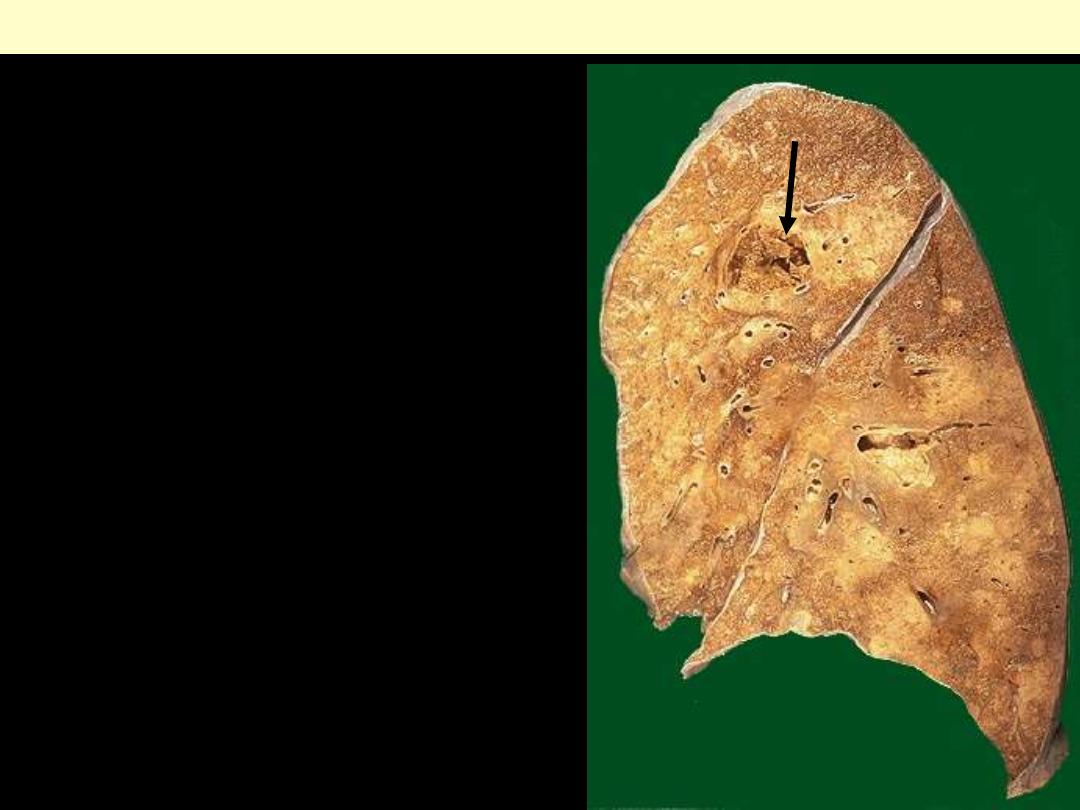
Identify the organ?
Describe the gross
pathological changes?
This is an example of liquefactive
necrosis.
There
is
confluent
bronchopneumonia (scattered pale
areas) complicated by abscess
formation, which is seen here as a
cystic
cavity
(arrow).
The
contained pus poured off during
the sectioning of the lung tissue.
Lung abscess

A 68-year-old female suddenly lost consciousness and,
on awakening an hour later, could not speak or move her
right arm and leg. Two months later, a head computed
tomography (CT) scan showed a large cystic area in her
left parictal lobe. Which of the following pathologic
processes most likely occurred in the brain?
(A) Fat necrosis
(B) Coagulative necrosis
(C) Apoptosis
(1)) Liquefactive necrosis
(E) Karyolysis
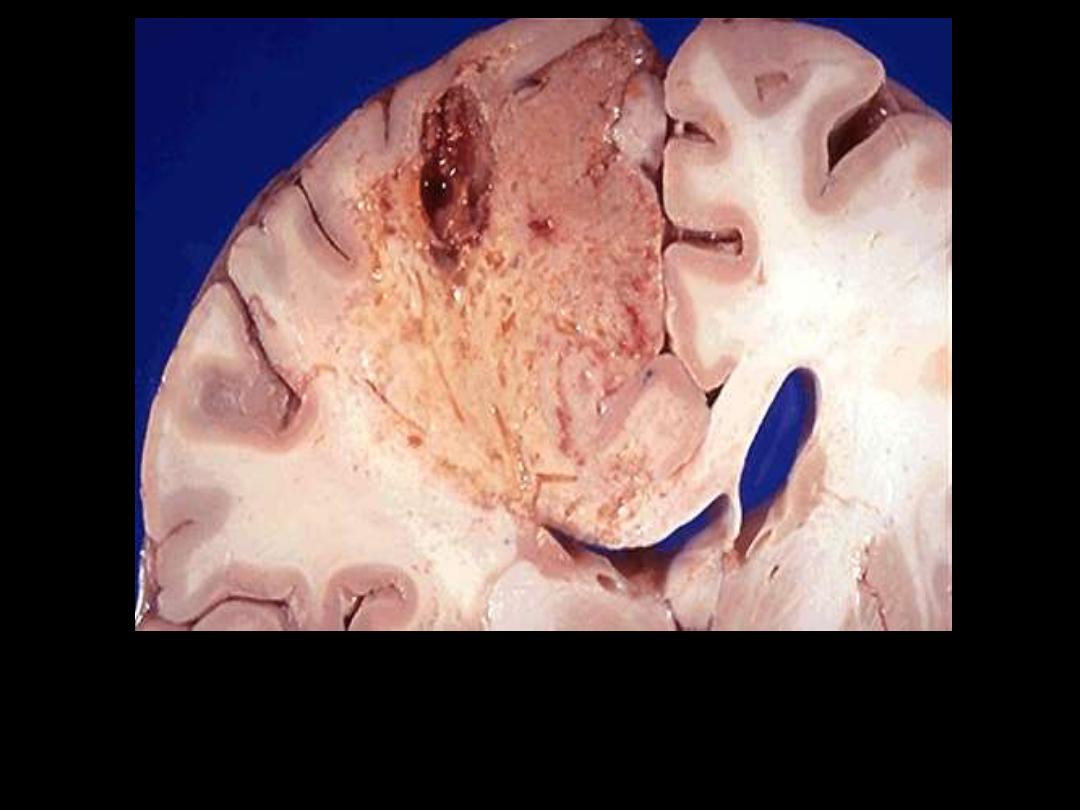
Identify the organ?
Describe the gross pathological changes?
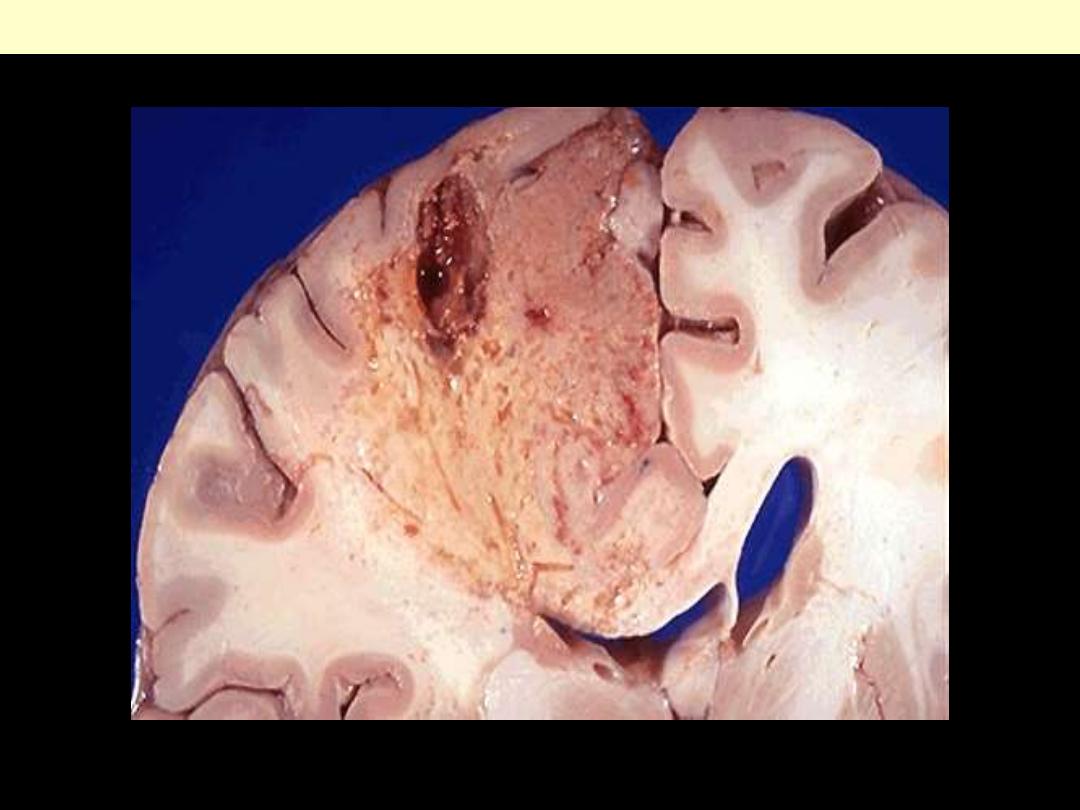
This is an example of liquefactive necrosis; the affected area is wedge-shaped, pale, soft & cystic.
Brain infarction

Mention two situations in which
liquefactive necrosis is seen?
Liquefaction
liquefactive necrosis
•
Seen in two situations
– Ischemic destruction of brain tissue
– Bacterial infections e.g. abscesses
•
complete digestion of dead cells by enzymes
cyst filled with debris + fluid
Identify the organ?
Describe the gross pathological
changes?
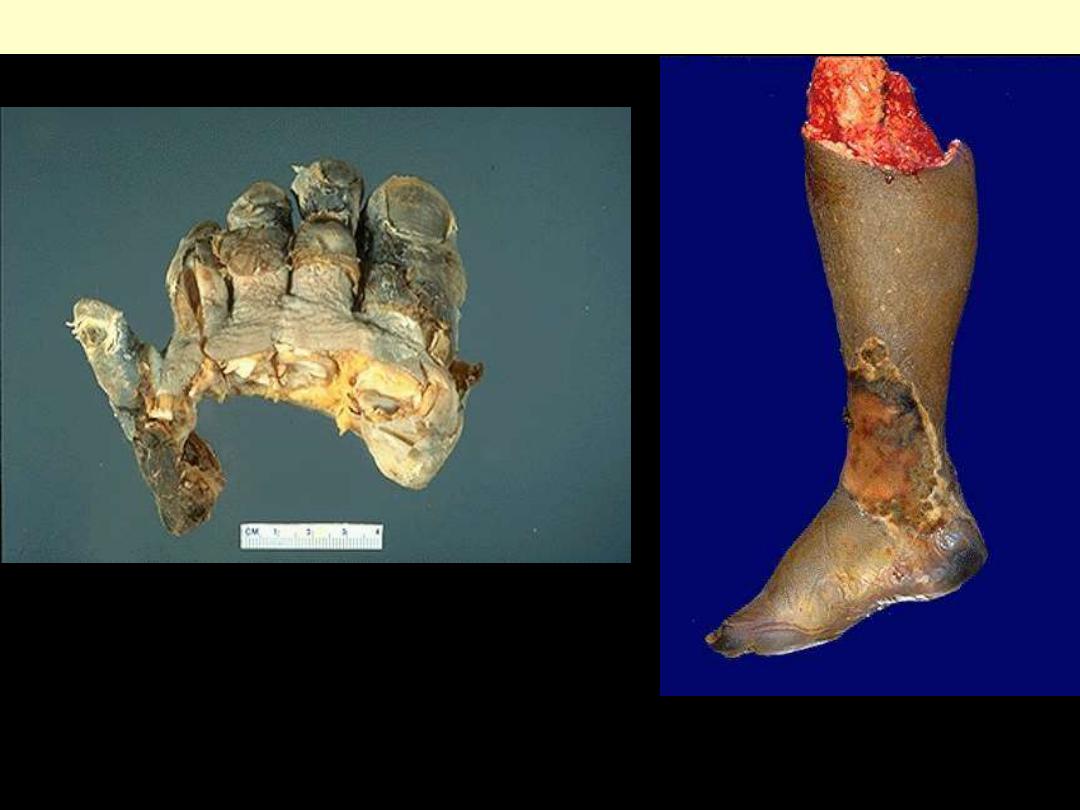
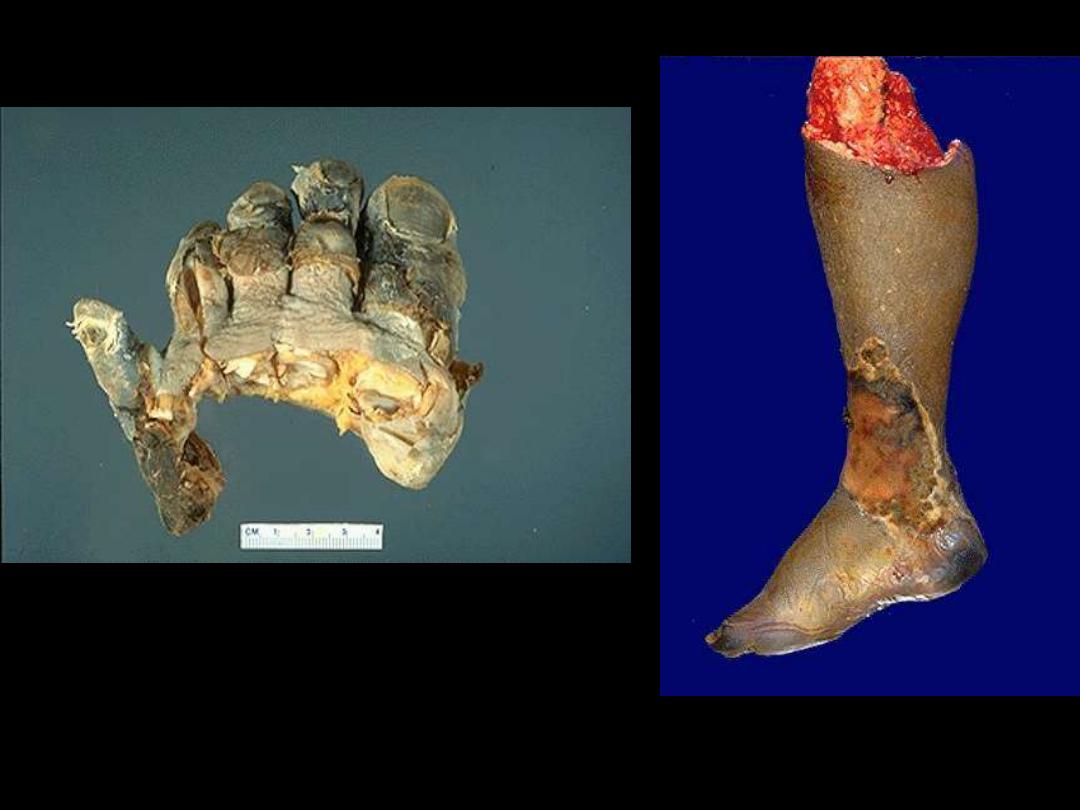
Dry gangrene
Ganagrene of lower limb
Wet gangrene

Mention the subtypes of gangrenous
necrosis
?

Gangrene
Gangrenous necrosis
• Used in surgical practice: lower limb,
intestine
• Gangrene = coagulative necrosis (ischemia)
+ liquefactive necrosis (bacterial infection)
• Two subtypes
1. Dry gangrene
2. Wet gangrene
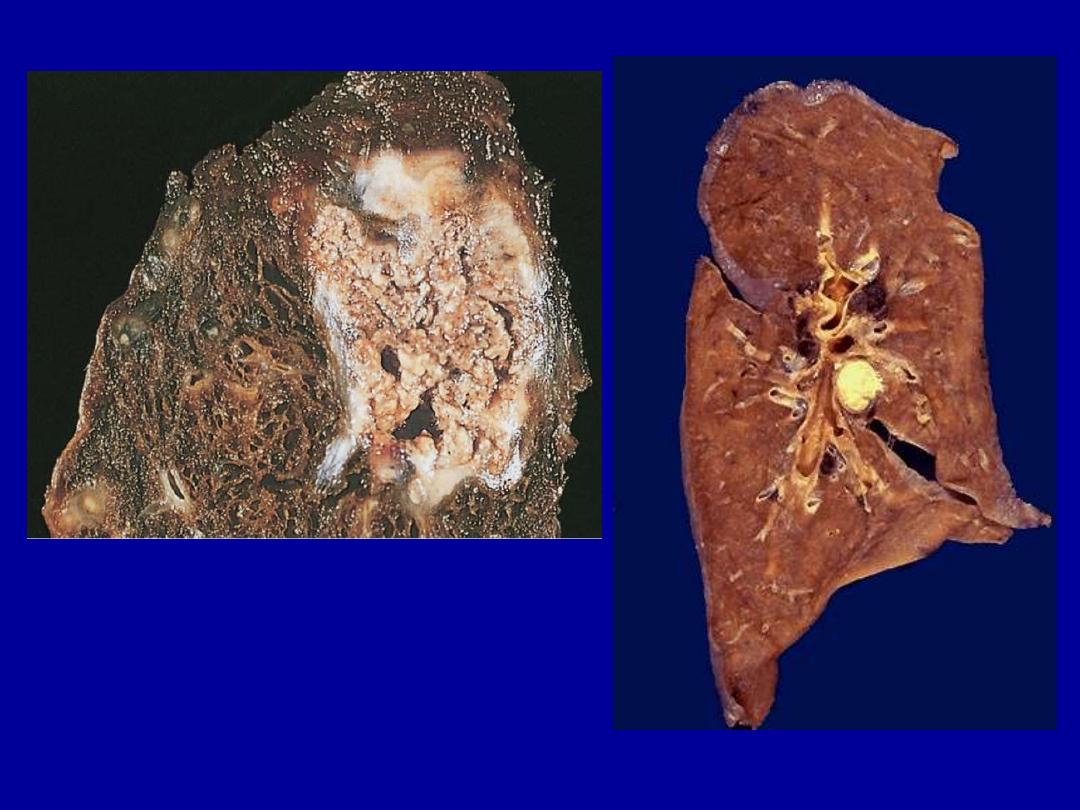
Identify the organ?
Describe the gross pathological
changes in A & B?
A
B
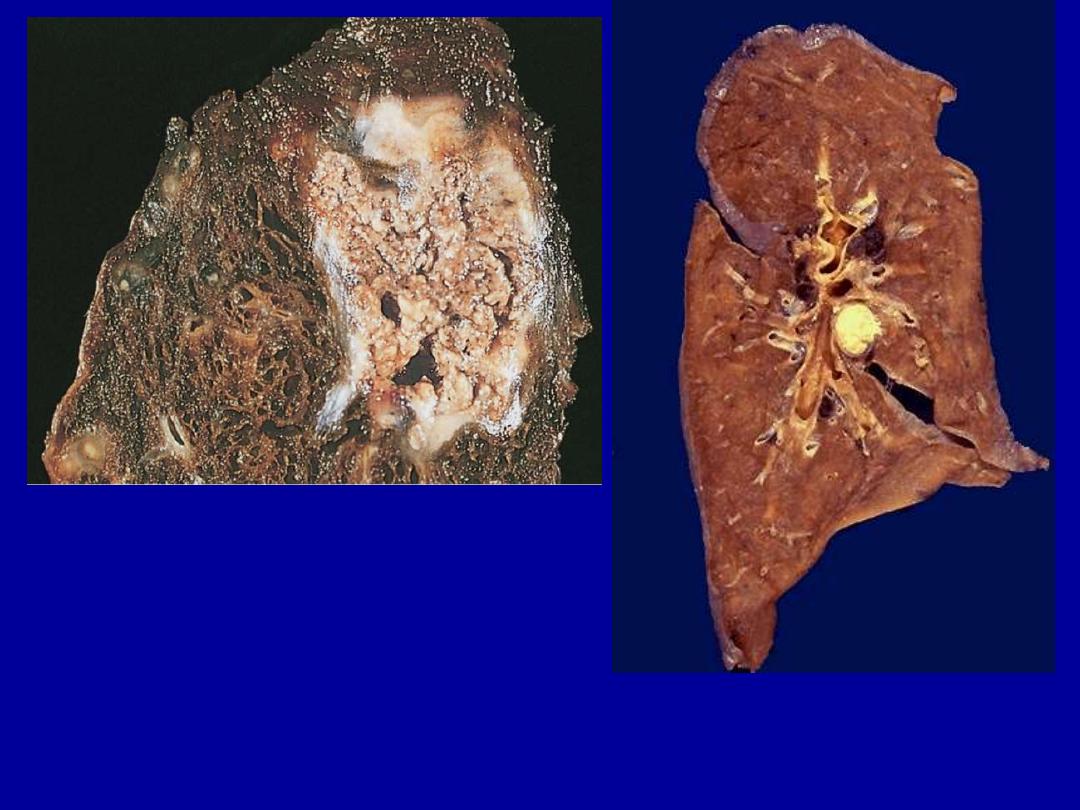
A
B
A tuberculous lung with a large area
of caseous necrosis containing yellow-
white and cheesy debris.
This is the gross appearance of caseous necrosis in a hilar lymph node
infected with tuberculosis. The node has a cheesy yellow to white appearance.
Caseous necrosis is really just a combination of coagulative and liquefactive
necroses
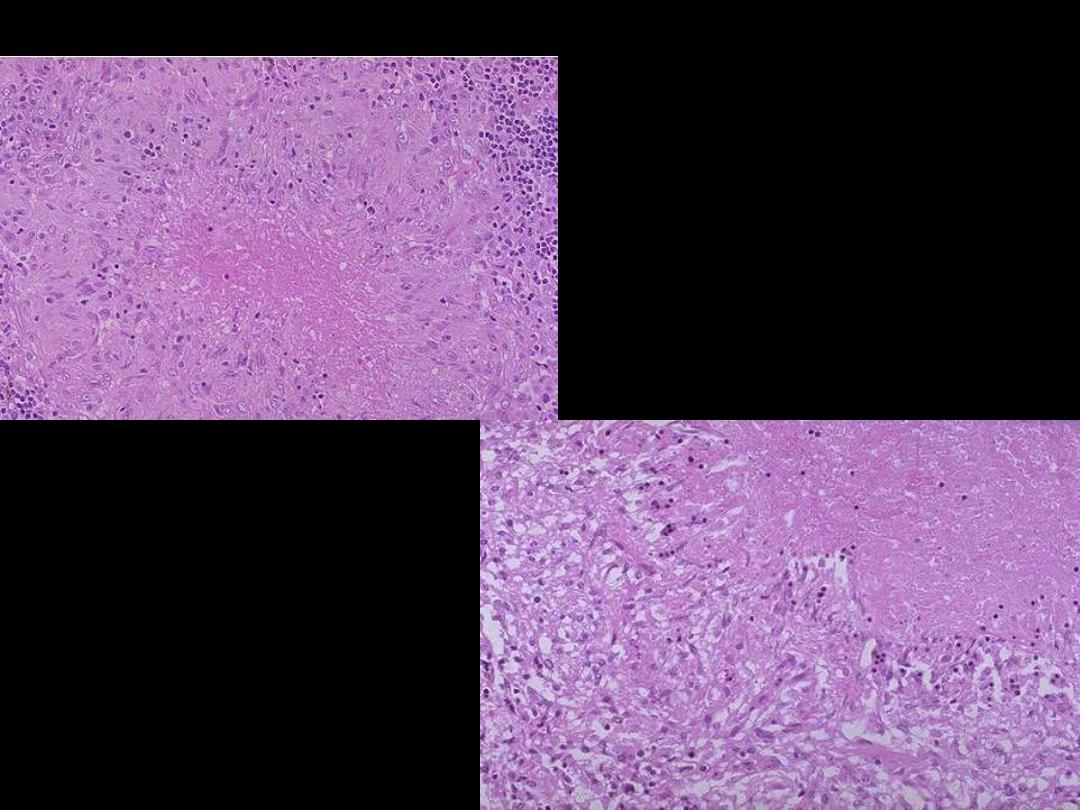
Describe the
histopathological
changes?
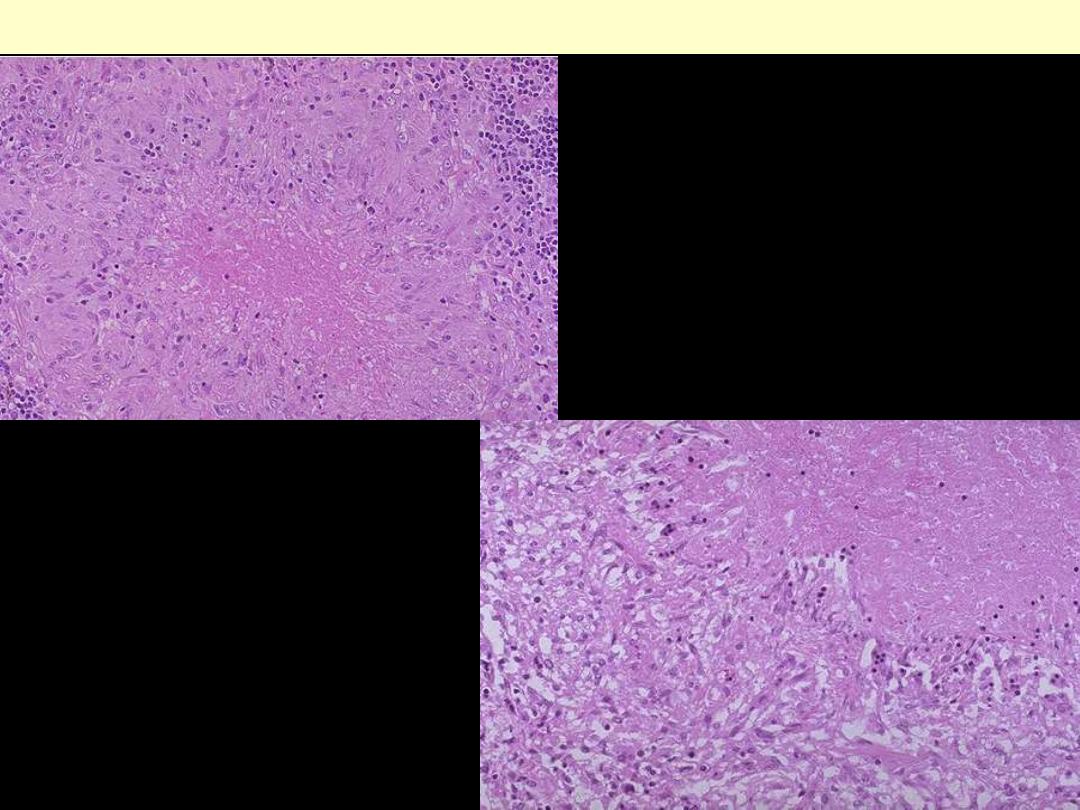
Microscopically, caseous necrosis is
characterized
by
amorphous
(acellular), granular pink areas of
necrosis, as seen here at the upper
Lt, surrounded by a granulomatous
inflammatory process. The lower
Rt. Photo is a close up view
Caseating TB granuloma
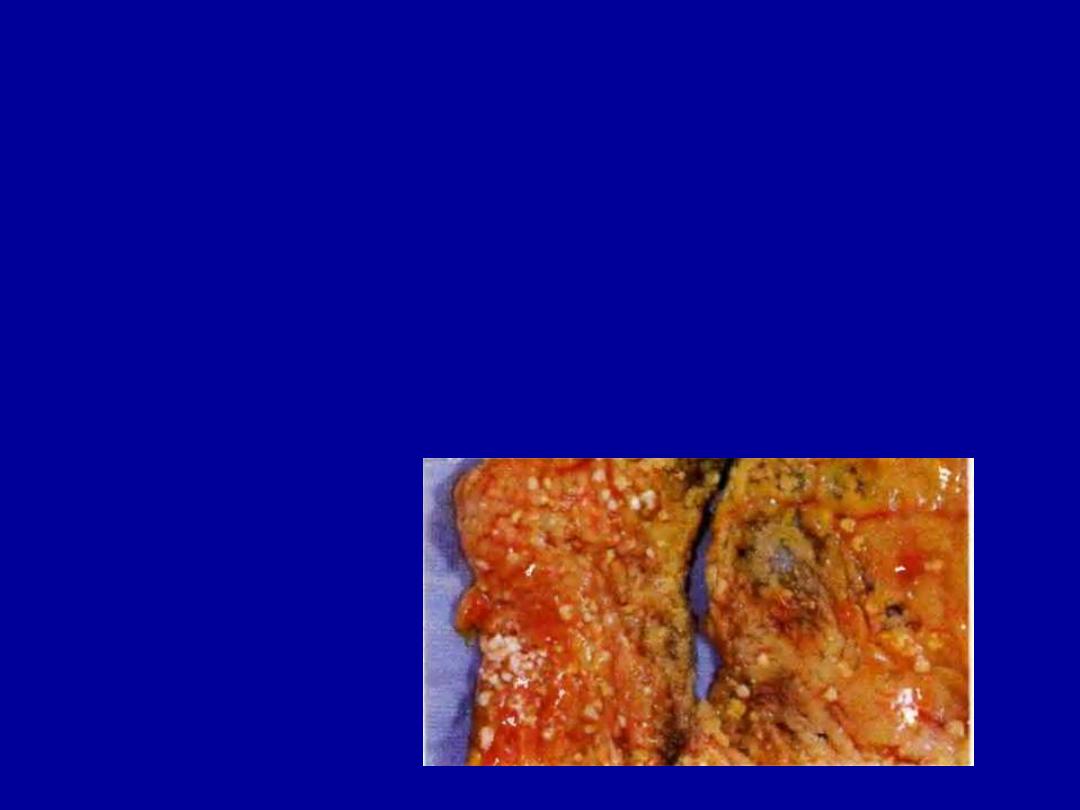
A 38-year-old woman experienced severe abdominal pain
with hypotension and shock that led to her death within 36
hours. From the gross appearance of the mesentery, seen in
the figure, which of the following events has most likely
occurred?
(A) Hepatitis B virus infection
(B) Small intestinal infarction
(C) Tuberculous lymphadenitis
(D) Gangrenous cholecystitis
(E) Acute pancreatitis
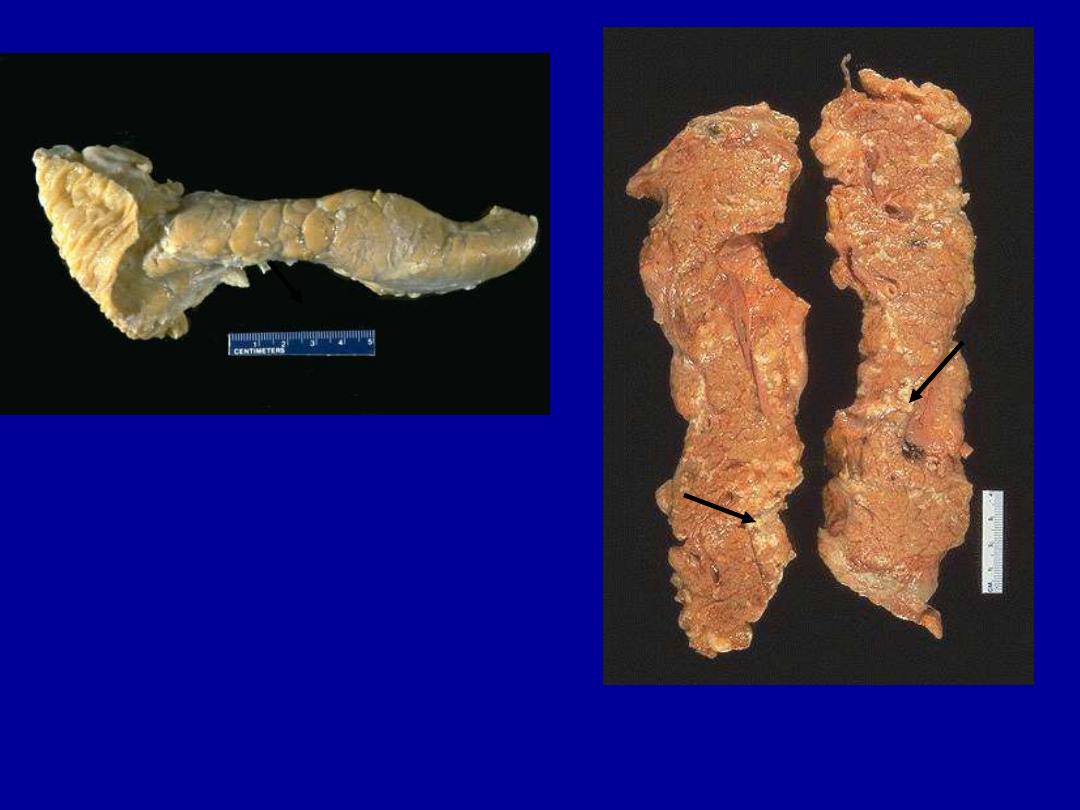
Identify the organ?
Describe the gross pathological
changes in B?
A
B
Normal
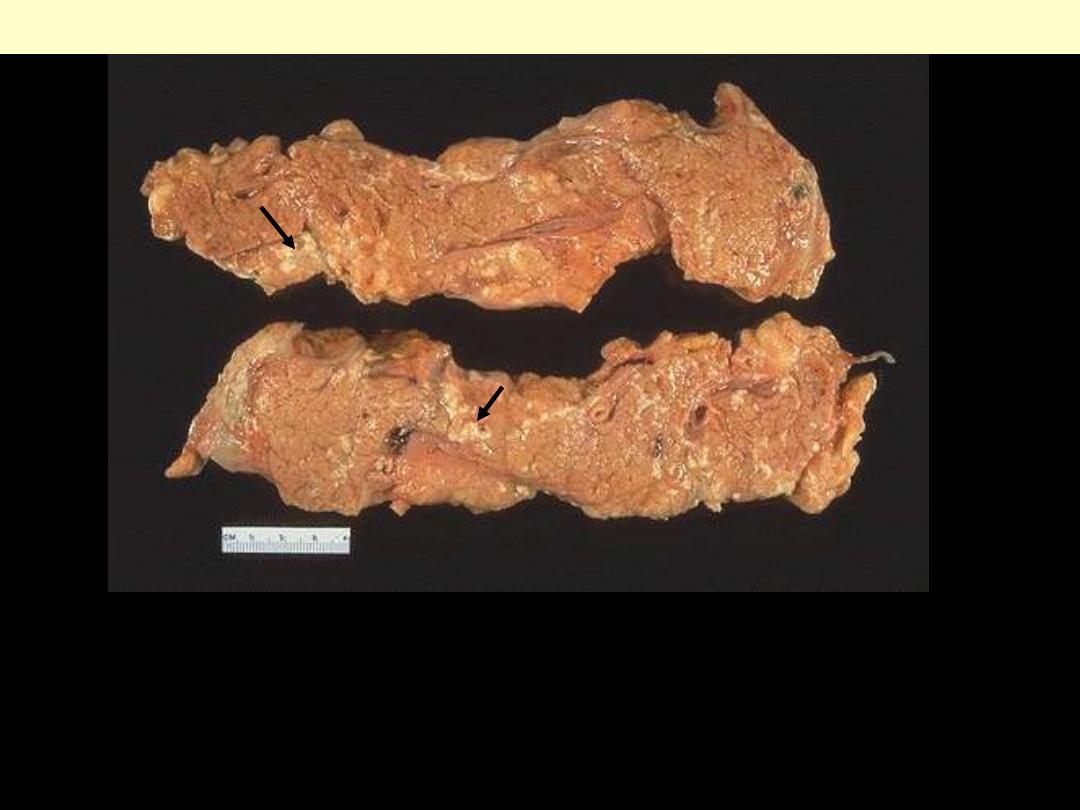
Injury to the pancreatic acini leads to release of powerful enzymes which
damage fat through lipases; these liberate fatty acids which complex with
calcium leading to the production of soaps, and these appear grossly as the
soft, chalky white areas seen here on the cut surfaces.
Fat necrosis of acute pancreatitis
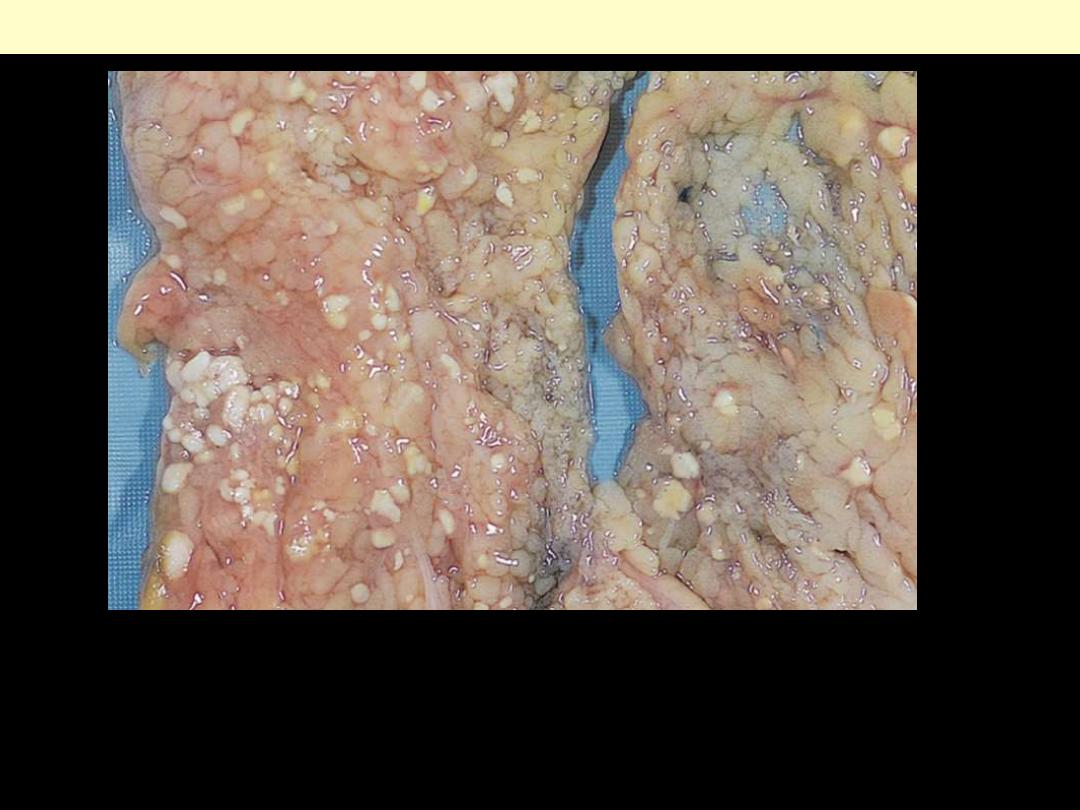
The areas of white chalky deposits represent foci of fat necrosis with
calcium soap formation (saponification) at sites of lipid breakdown in the
mesentery.
Fat necrosis in acute pancreatitis.

Mention the conditions in which fat
necrosis is seen?

Fat necrosis
•
Involves adipose tissue
•
Mediated through lipases
•
Seen in
1.
acute pancreatitis
2.
breast trauma (traumatic fat necrosis)
Mic:
- shadowy outlines of necrotic cells
- surrounding inflammatory cells
- calcium soaps: bluish deposits
Grossly
chalky white
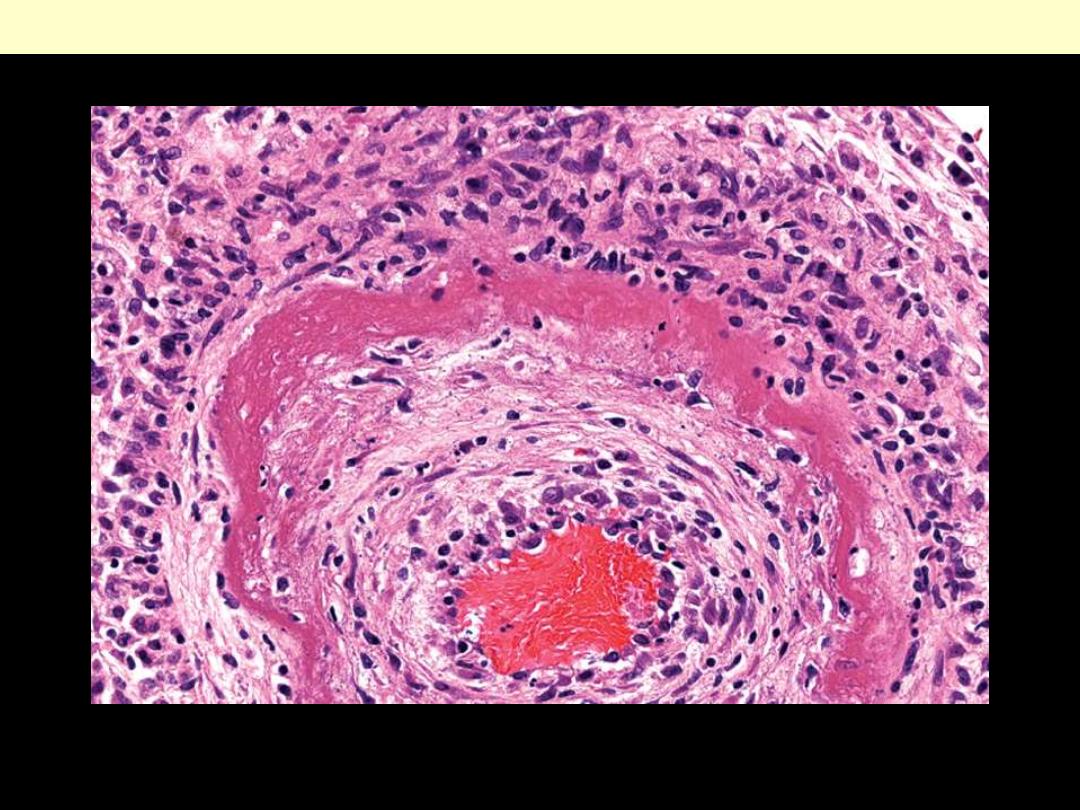
The wall of the artery shows a circumferential bright pink area of necrosis with protein deposition and
inflammation (dark nuclei of neutrophils).
Fibrinoid necrosis of an artery in polyarteritis nodosa.
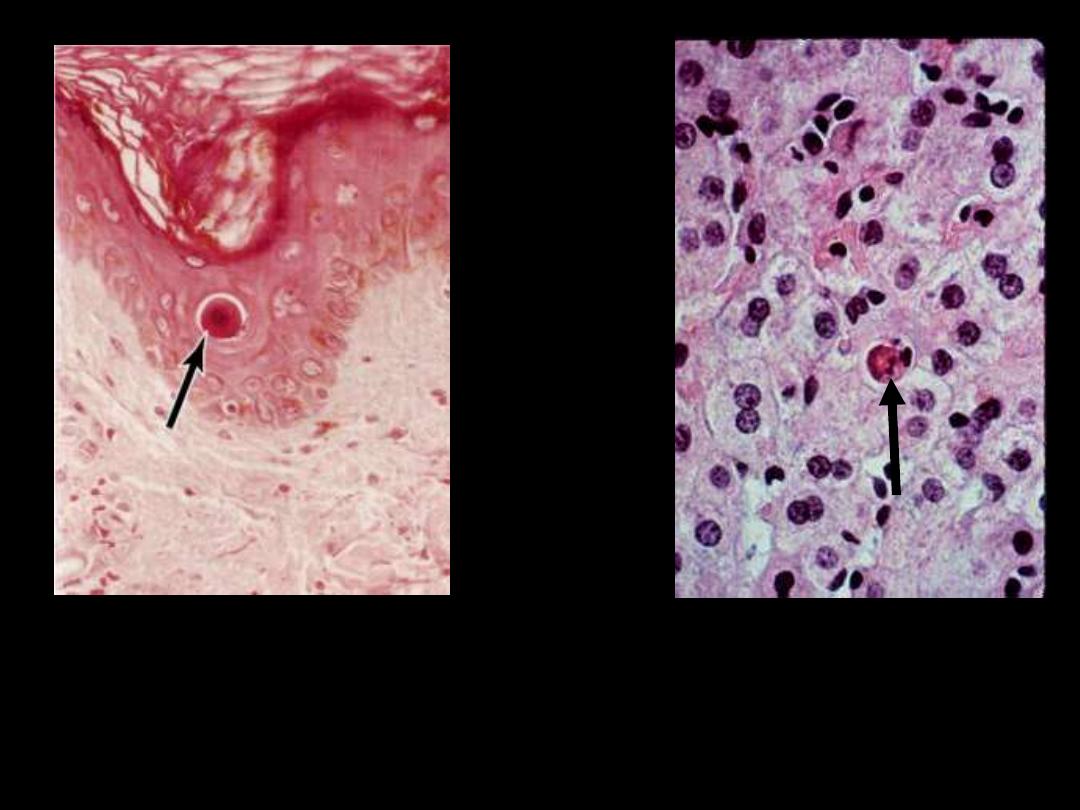
Describe the pathological changes in the arrowed cells
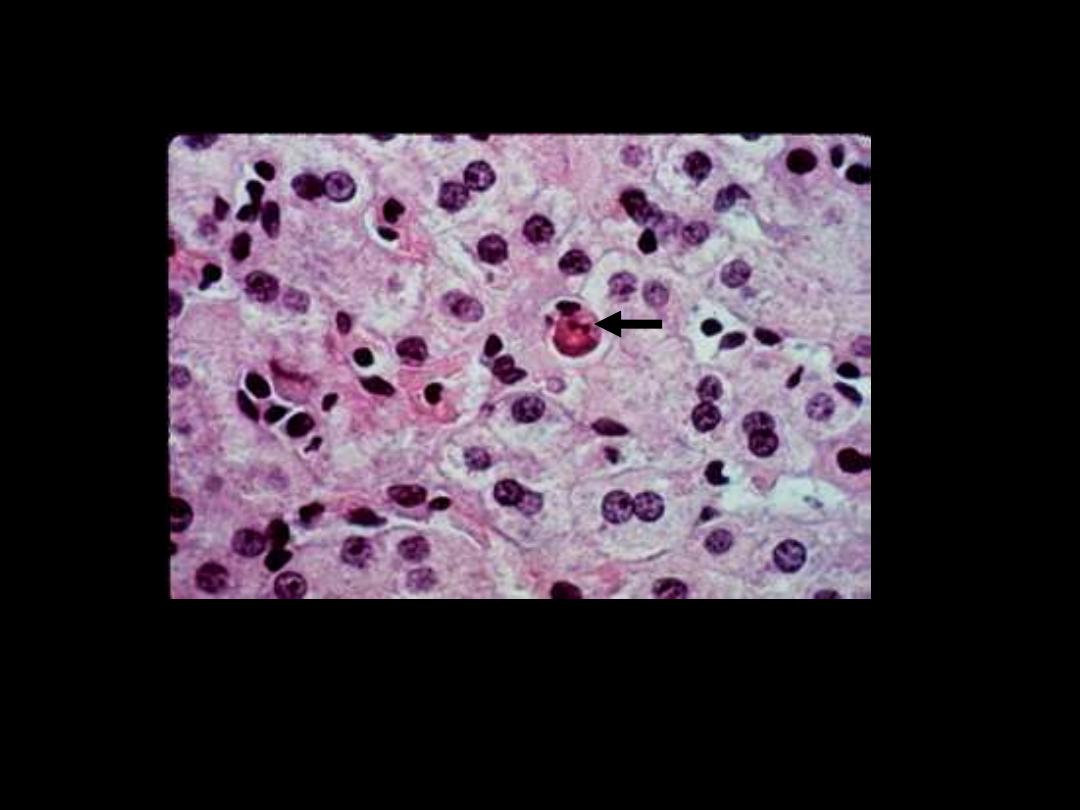
The cell is reduced in size and contains brightly eosinophilic
cytoplasm and a condensed nucleus
.
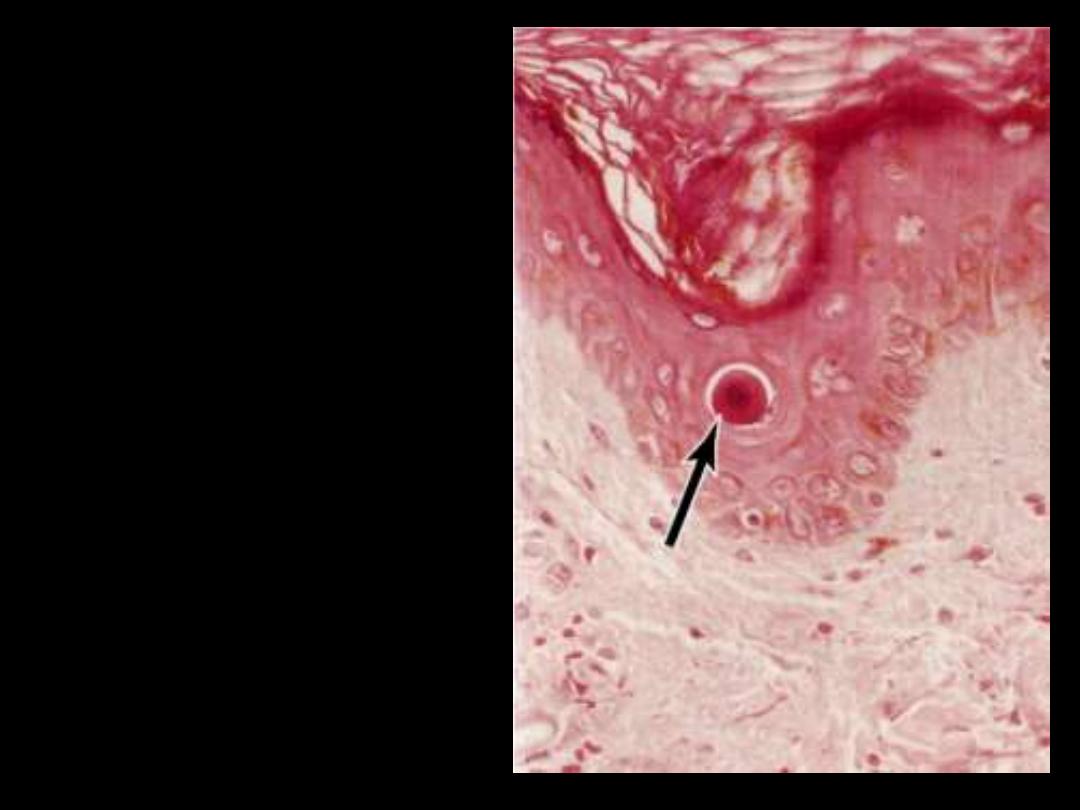
The cytoplasm is intensely
esoniphilic (pinkish) and the
nucleus condensed
(pyknotic)

On day 28 of the menstrual cycle in a 23-year-old female,
there is menstrual bleeding that lasts for a few days She has
had these regular cycles for many years.
Which of the
following processes is most likely happening in the
endometrium just before the onset of bleeding?
(A) Apoptosis
(B) Caseous necrosis
(C) Heterophagocytosis
(D) Atrophy
(E) Liquefactive necrosis

Apoptosis
•
Distinctive pattern of cell death
•
Scattered individual cells or cluster of cells
•
Responsible for programmed destruction of
cells during embryogenensis
•
Seen in
1. Hormone dependent involution
2. Certain pathological conditions

Feature
Necrosis
Apoptosis
Cell size
Enlarged (swelling)
Reduced (shrinkage)
Nucleus
Pyknosis ➙ karyorrhexis ➙
karyolysis
Fragmentation into
nucleosome-size fragments
Plasma
membrane
Disrupted
Intact; altered structure,
especially orientation of
lipids
Cellular contents
Enzymatic digestion; may
leak out of cell
Intact; may be released in
apoptotic bodies
Adjacent
inflammation
Frequent
No
Physiologic or
pathologic role
Invariably pathologic
(culmination of irreversible
cell injury)
Often physiologic, means
of eliminating unwanted
cells; may be pathologic
after some forms of cell
injury, especially DNA
damage
Features of Necrosis and Apoptosis

Intracellular Accumulation

What are the mechanisms responsible for
intracellular accumulations?
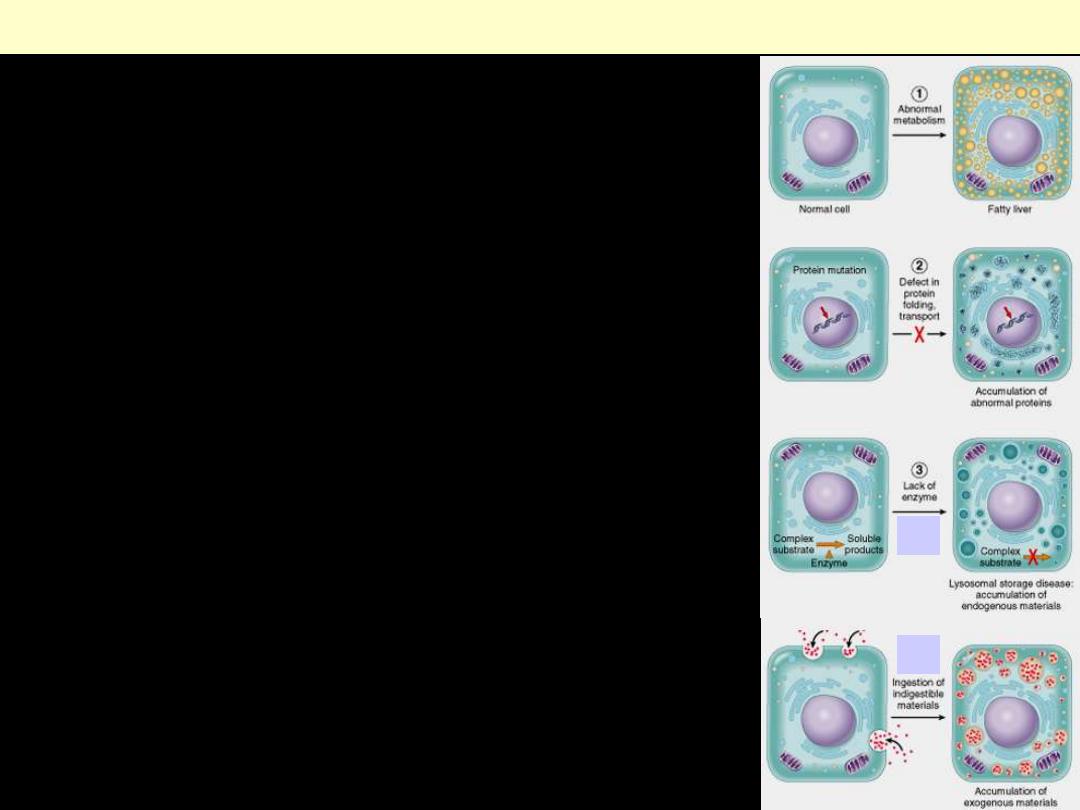
(1) Abnormal metabolism, as in fatty
change in the liver.
(2) Mutations causing alterations in protein
folding and transport, so that defective
molecules accumulate intracellularly.
(3) Failure to degrade a metabolite
a. A deficiency of critical enzymes
responsible for breaking down certain
compounds, causing substrates to
accumulate in lysosomes, as in
lysosomal storage diseases.
b. An inability to degrade phagocytosed
particles, as in carbon pigment
accumulation.
Mechanisms of intracellular accumulation.
b
a

What are the effects of intracellular accumulations?
What are the sites of accumulation?

•
Effects
1. No effect (harmless)
2. Severe toxicity
•
Sites of accumulation
1. Nuclear
2. Cytoplasmic (lysosomal)

Lipid accumulations (fatty change)
•
Abnormal accumulation of fat within
parenchymal cells.
•
Reversible cell injury
•
Often seen in the liver
Gross features
Mild
fatty change: no gross changes
Progressive
accumulation
-
Organ enlargement
-
Increasingly yellow discoloration,
-
Soft and greasy
Normal
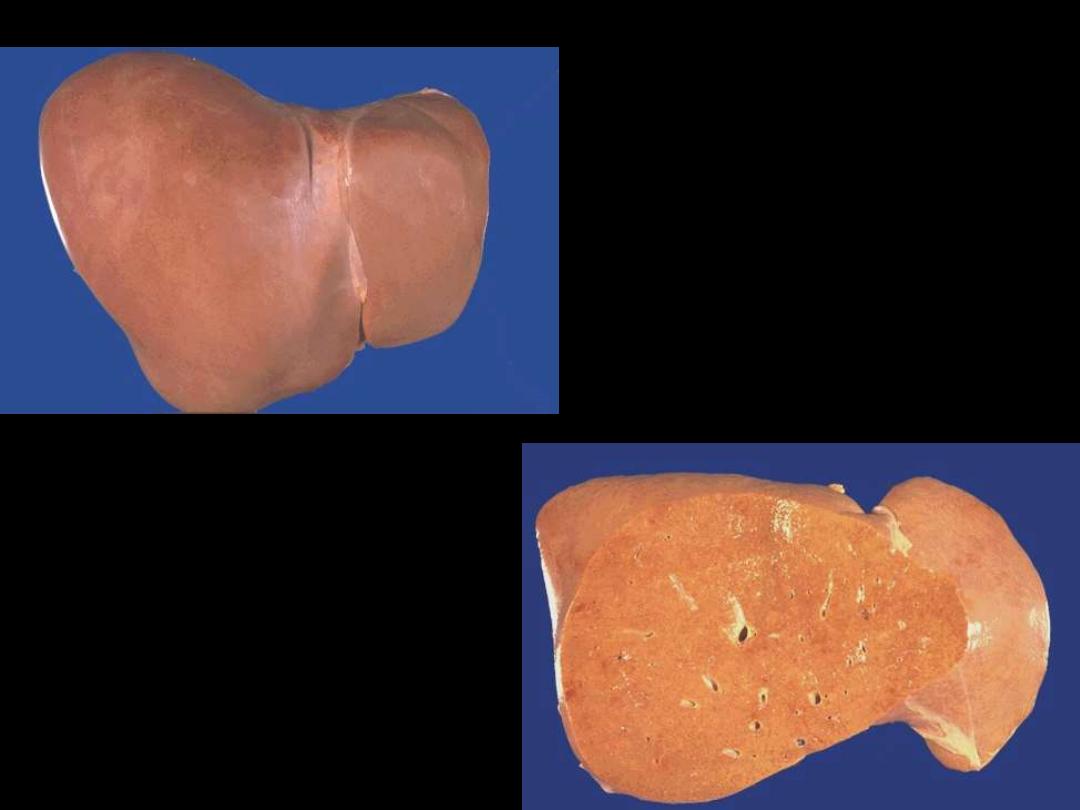
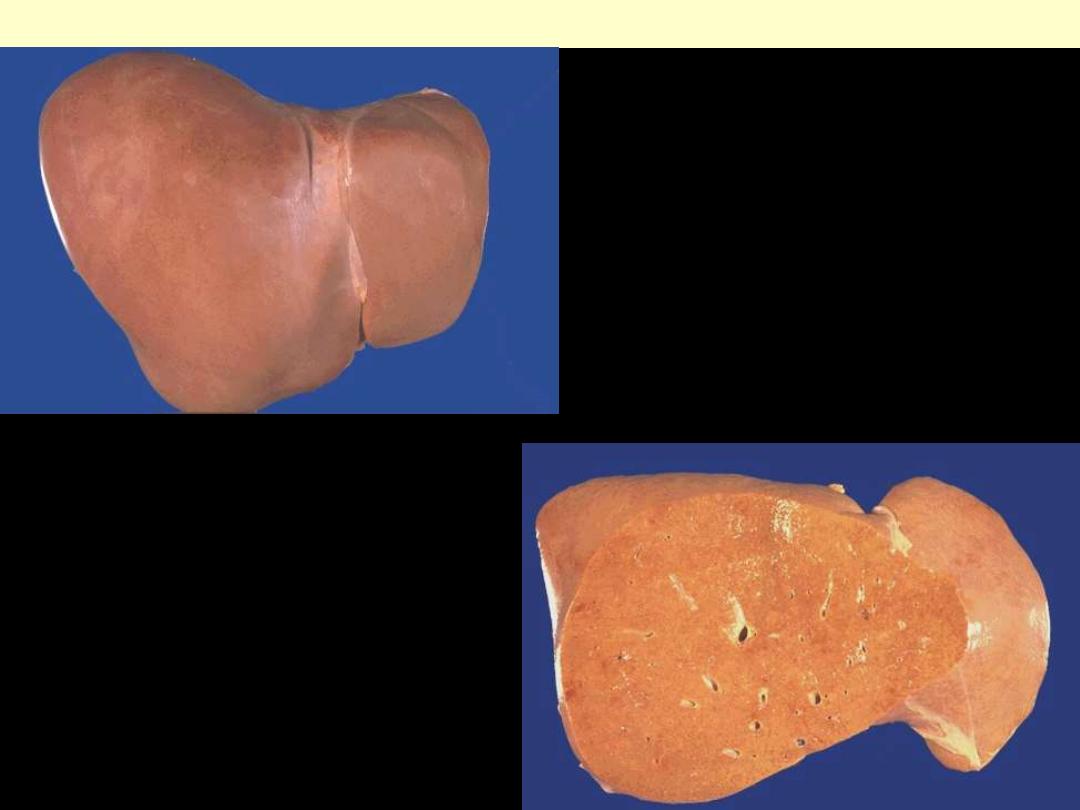
1. Identify the organ.
2. Describe the gross
abnormality
(pathological changes) in
B
A
B
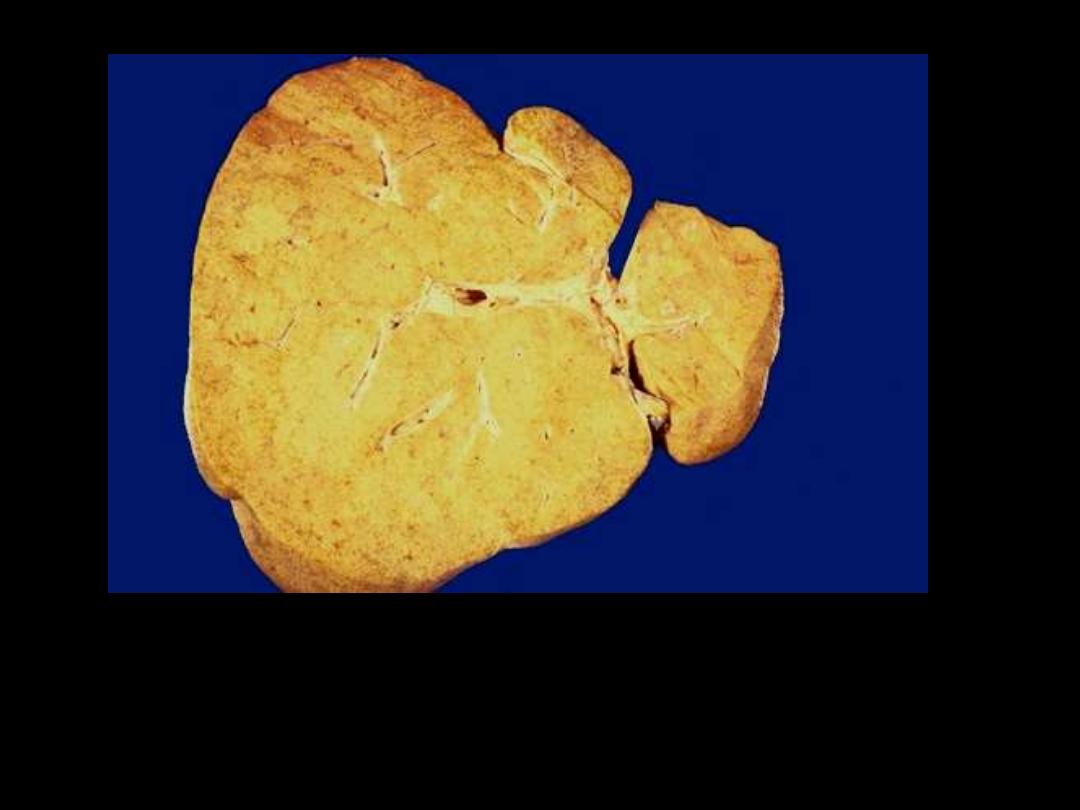
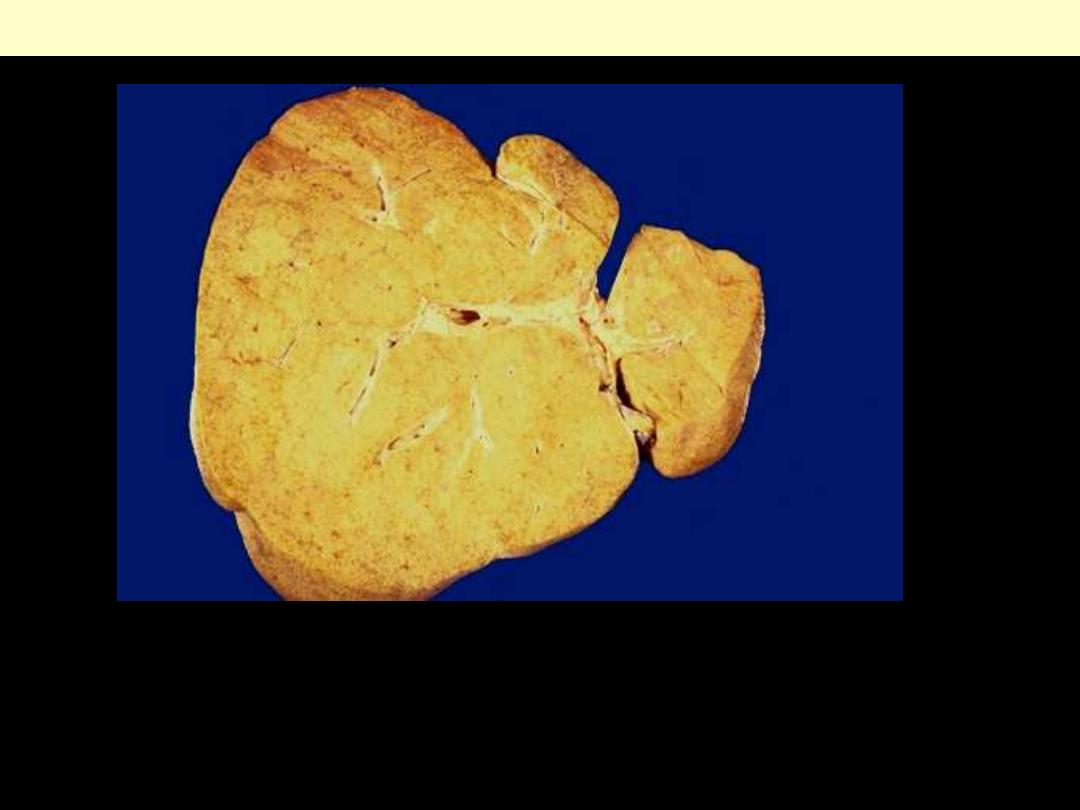
1. Identify the organ.
2. Describe the gross abnormality (pathological changes)
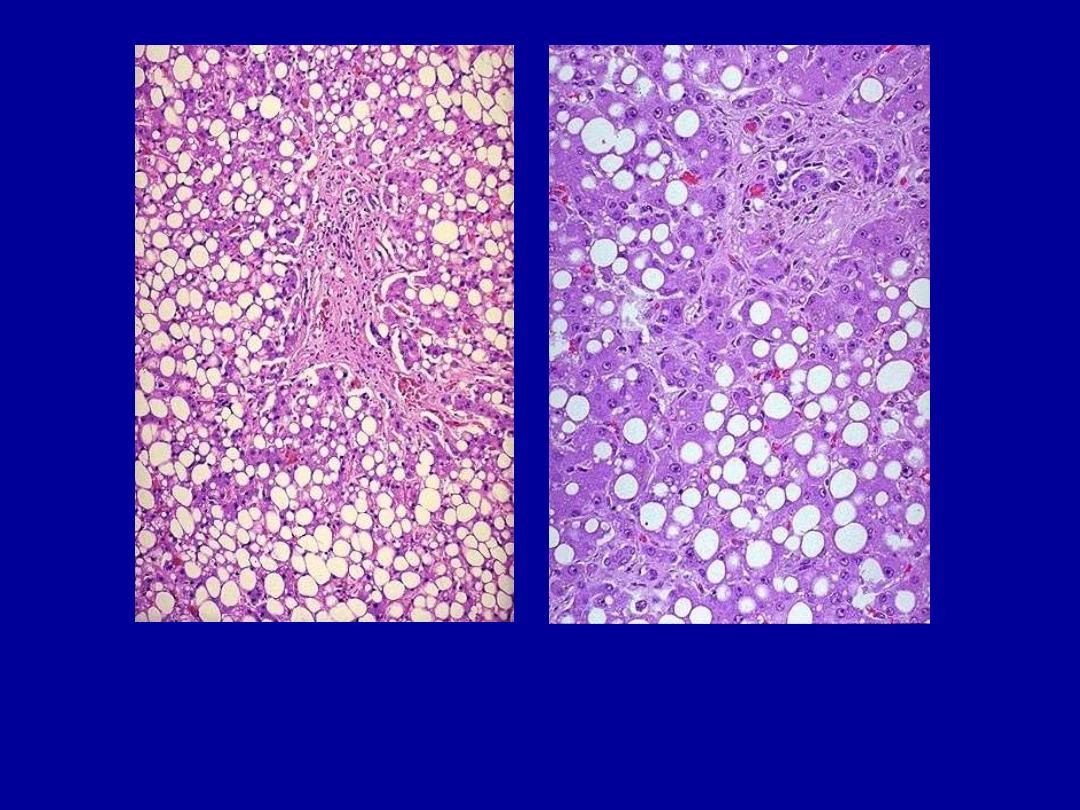
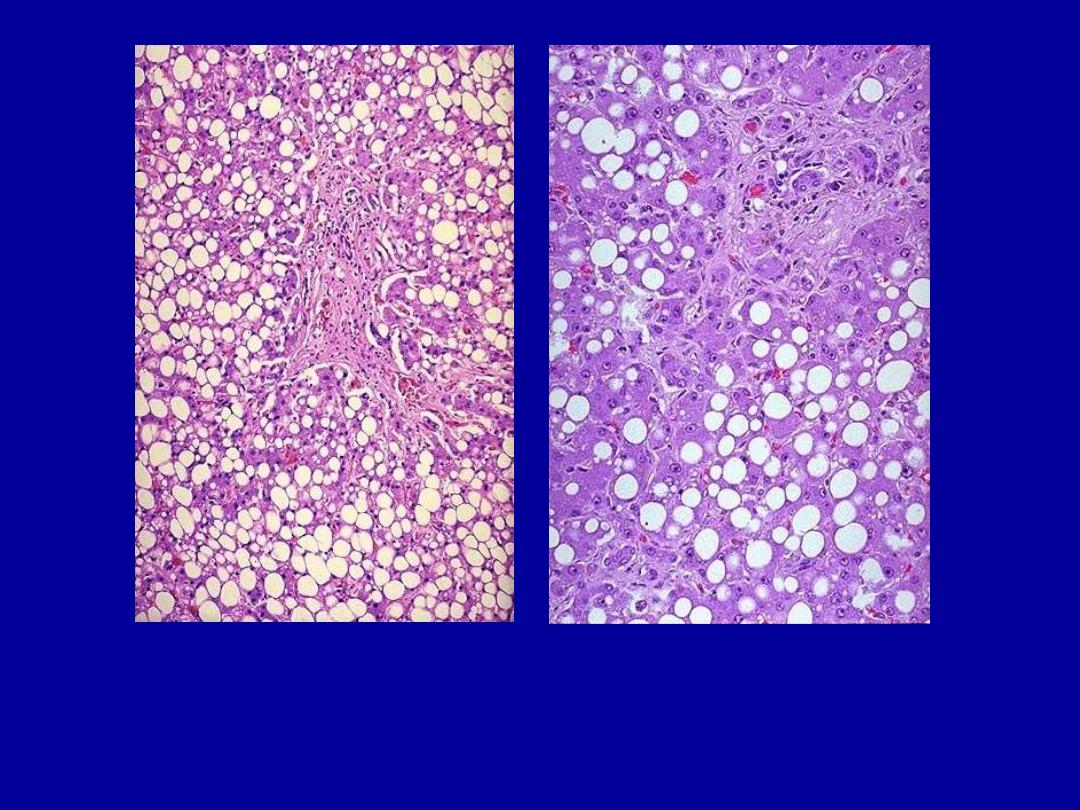
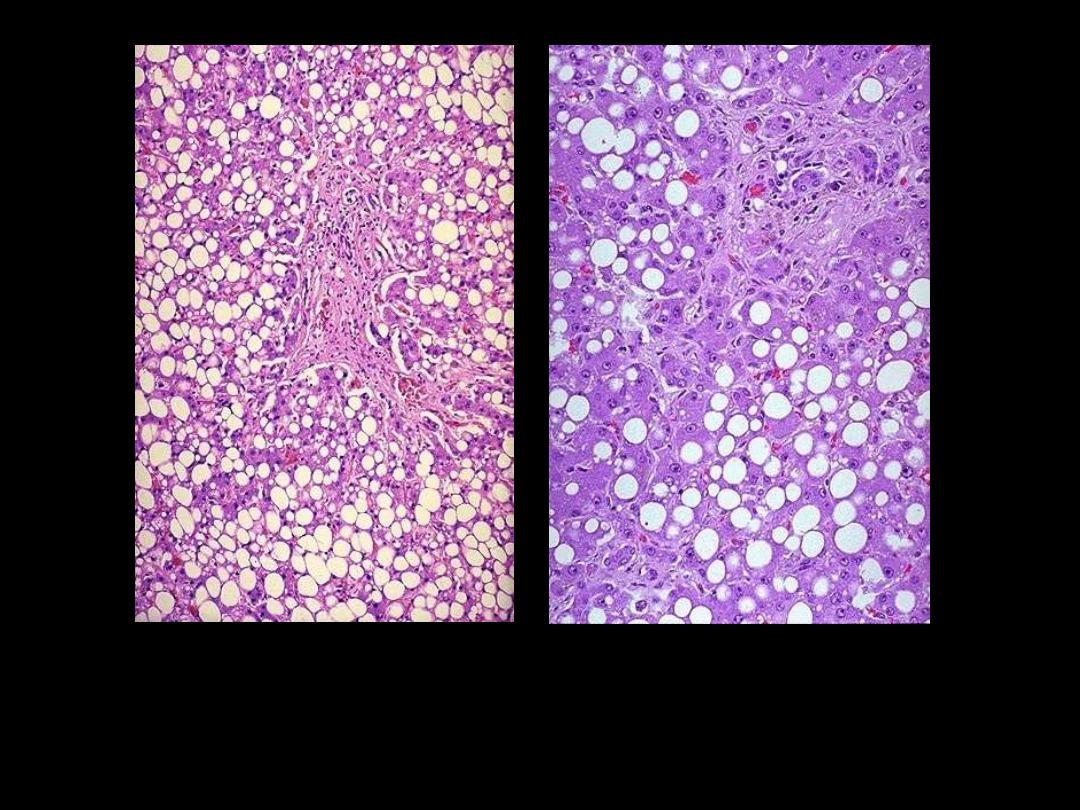
Fatty change liver
Normal
Fatty change
Severe fatty change liver
In the liver mild fatty change shows no gross changes, but
with progressive accumulation, the organ enlarges and
become increasingly yellow, soft and greasy to touch.
Identify the organ?
Describe the histopathological changes
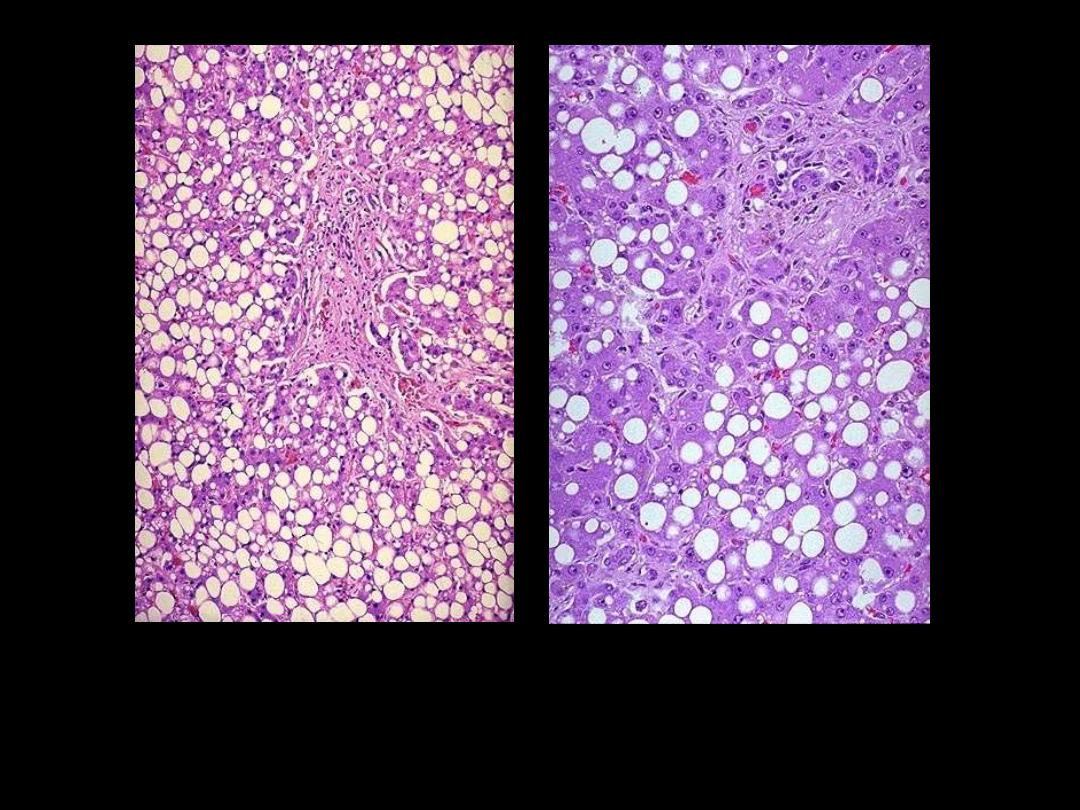
This is the histologic appearance of hepatic fatty change. The lipid
accumulates in the hepatocytes as vacuoles. These vacuoles have a clear
appearance with H&E staining.

A right carotid endarterectomy is performed on a 69- year-old
female who had an audible bruit on auscultation of the neck.
Examination of the curetted atheromatous plaque reveals a
grossly yellow-tan, firm appearance.
Microscopically, which of
the following materials can be found in abundance in the form
of crystals producing long, cleftlike spaces?
(A) Glycogen
(B) Lipofuscin
(C) Hemosiderin
(D) Immunoglobulin
(E) Cholesterol
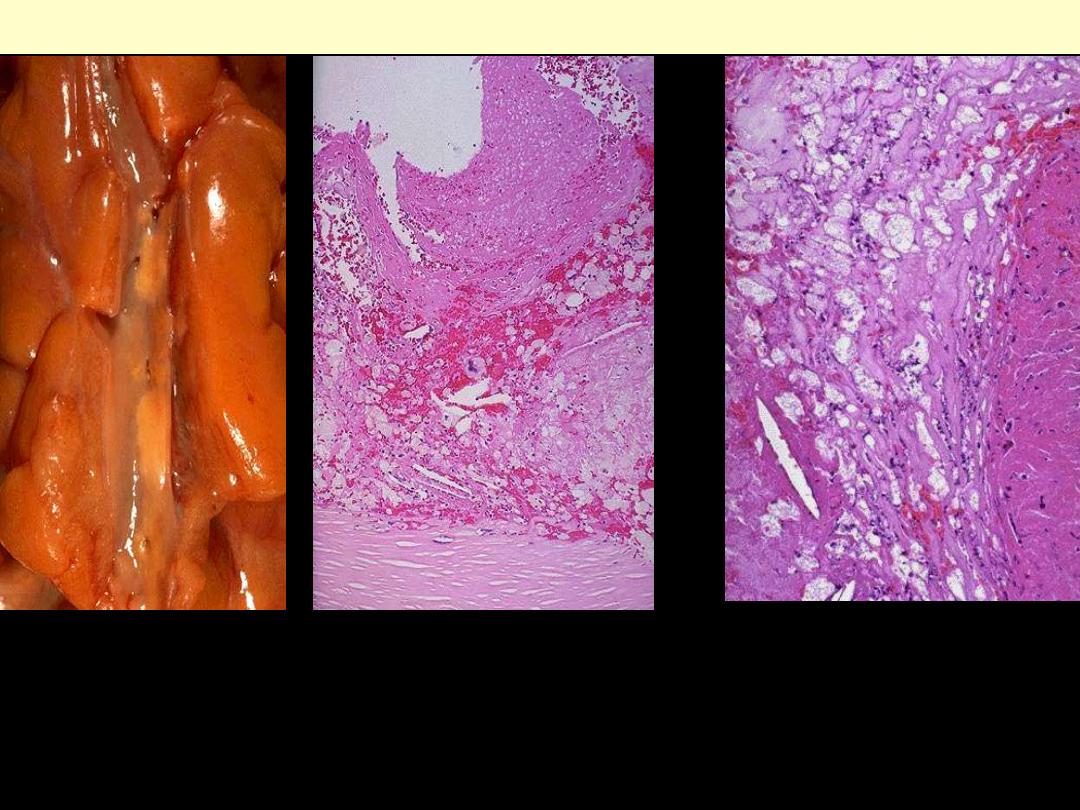
A: A coronary artery has been opened longitudinally; it is surrounded by epicardial fat. This coronary shows
occasional yellow-tan lipid plaque and no narrowing.
B: the lumen of the artery is at the top, and the band of smooth muscle at the bottom is the atrophic media. The intima
is enormously thickened, by the presence of amorphous material containing large numbers of cholesterol crystals (the
unstained clefts). There are many foamy (lipid-filled) macrophages.
C: This high magnification of the atheroma shows numerous foam cells and an occasional cholesterol cleft.
Coronary atherosclerosis
A
B
C
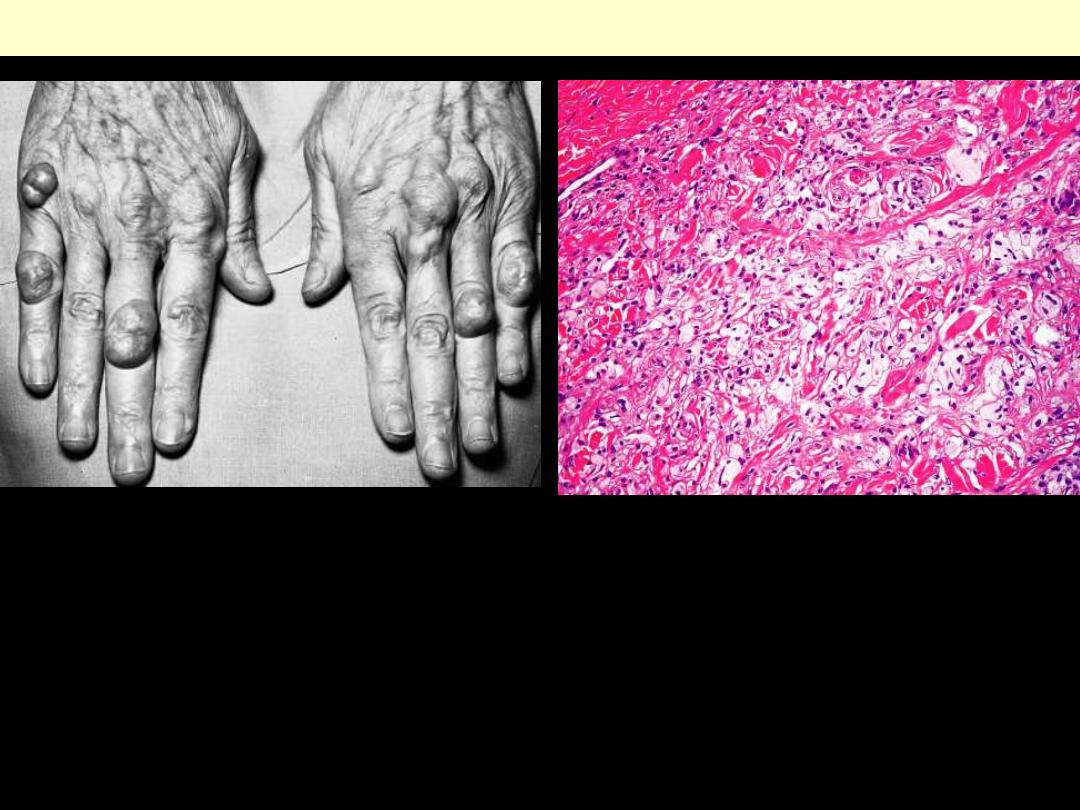
Xanthoma
Cutaneous xanthoma showing ill-defined
collection of foamy macrophages in the dermis.
Xanthoma tuberosum multiplex in patient with
hypercholesterolemia.
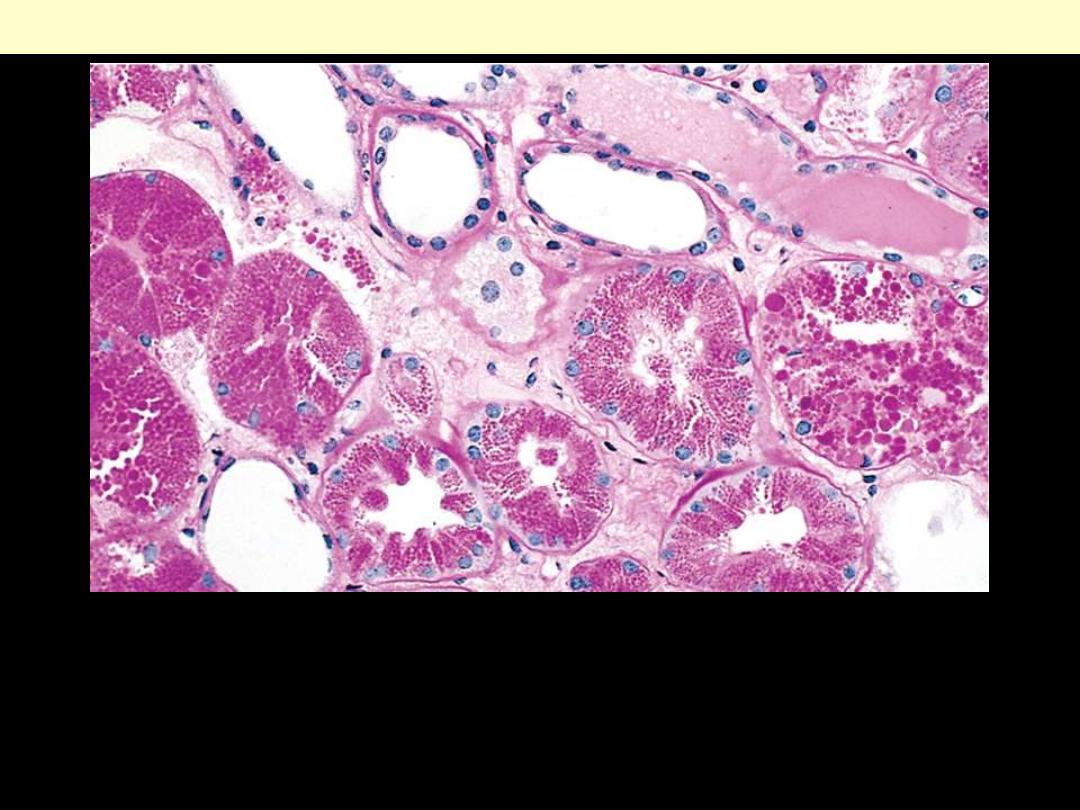
Describe the histopathological changes
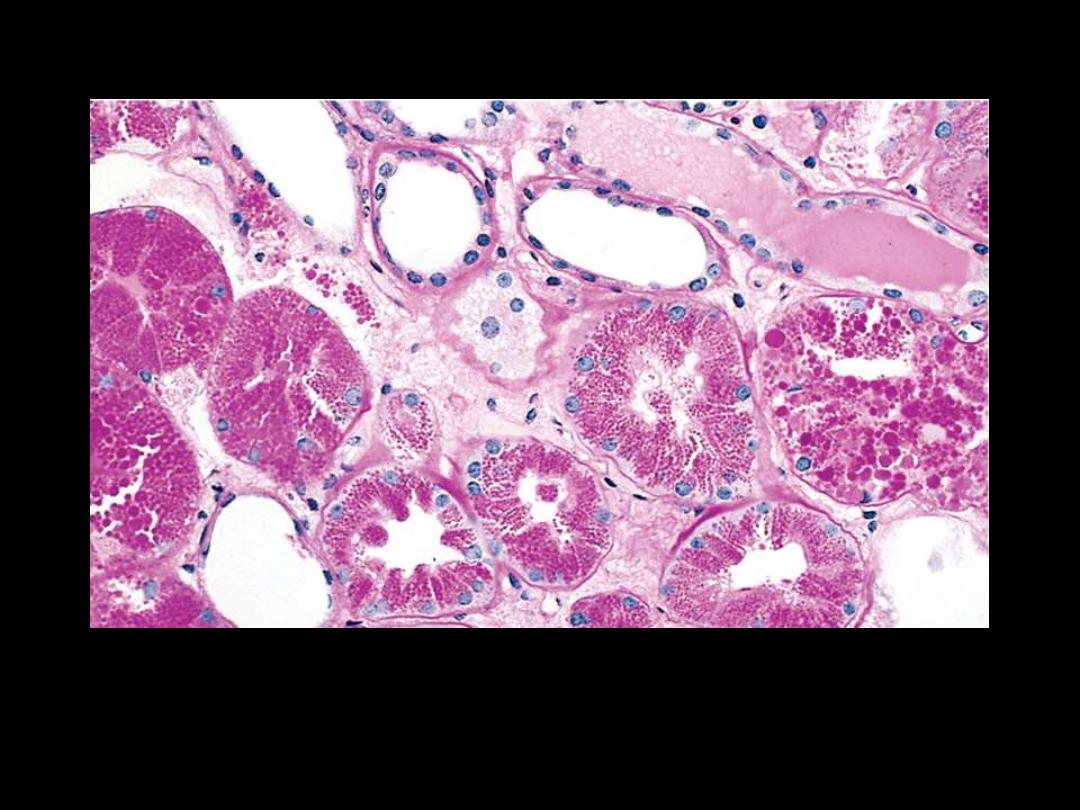
In nephrotic syndrome, there is a abnormally large reabsorption of
the protein. Pinocytic vesicles containing this protein fuse with
lysosomes, resulting in the histologic appearance of pink, hyaline
cytoplasmic droplets
Protein reabsorption droplets in the renal tubular epithelium
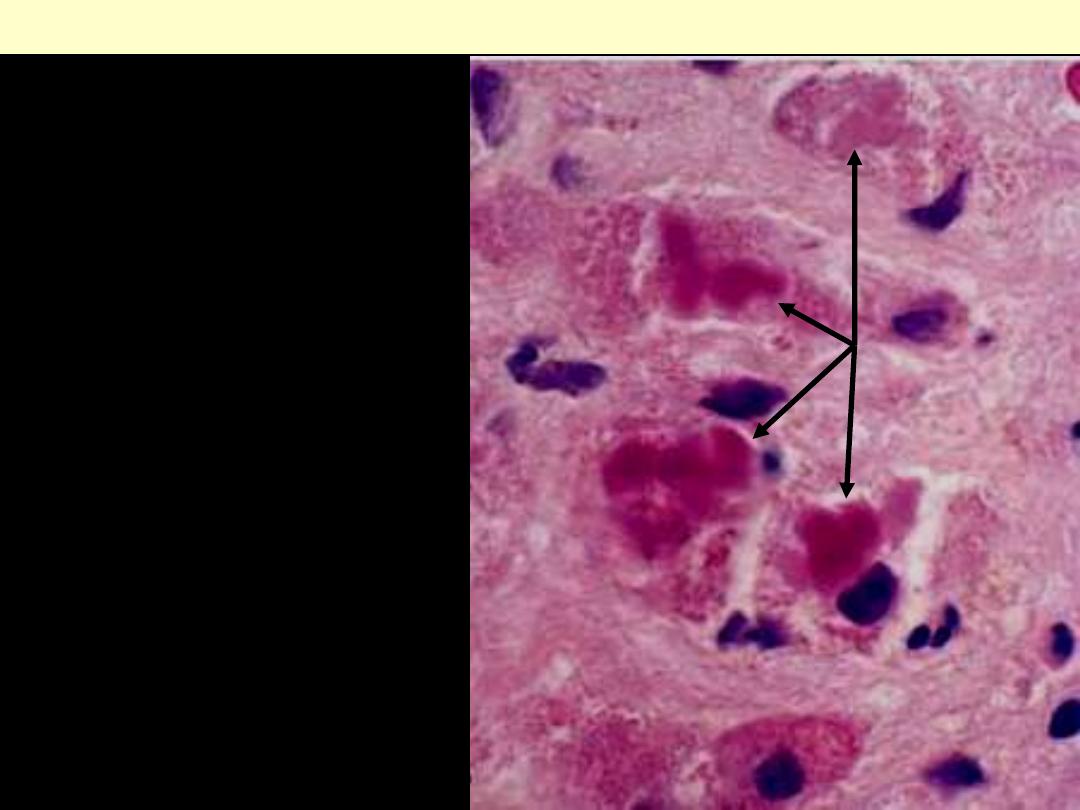
Alcoholic hepatitis
Eosinophilic Mallory bodies
are seen in hepatocytes
(arrows).

Protein accumulations
Occurs principally in
• Epithelial cells
of proximal convoluted renal
tubules (e.g. in proteinuria)
• Plasma cells
Glycogen accumulations
•
Seen as clear cytoplasmic vacuoles.
•
PAS stain routinely used for demonstration
•
Diabetes mellitus
glycogen
accumulation
1. distal renal tubules
2. hepatocytes
3. myocardial cells
4. islet cells of Langerhans
•
Glycogen storage diseases
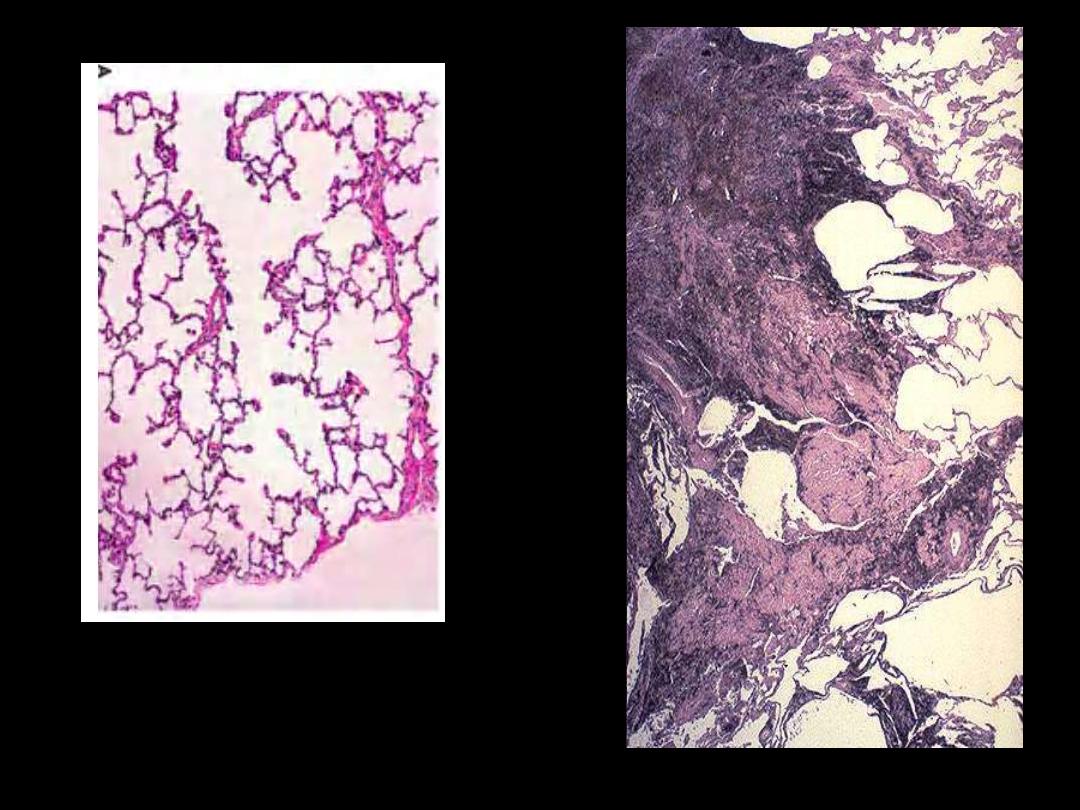
Identify the organ?
Describe the histopathological
changes in B
A
B
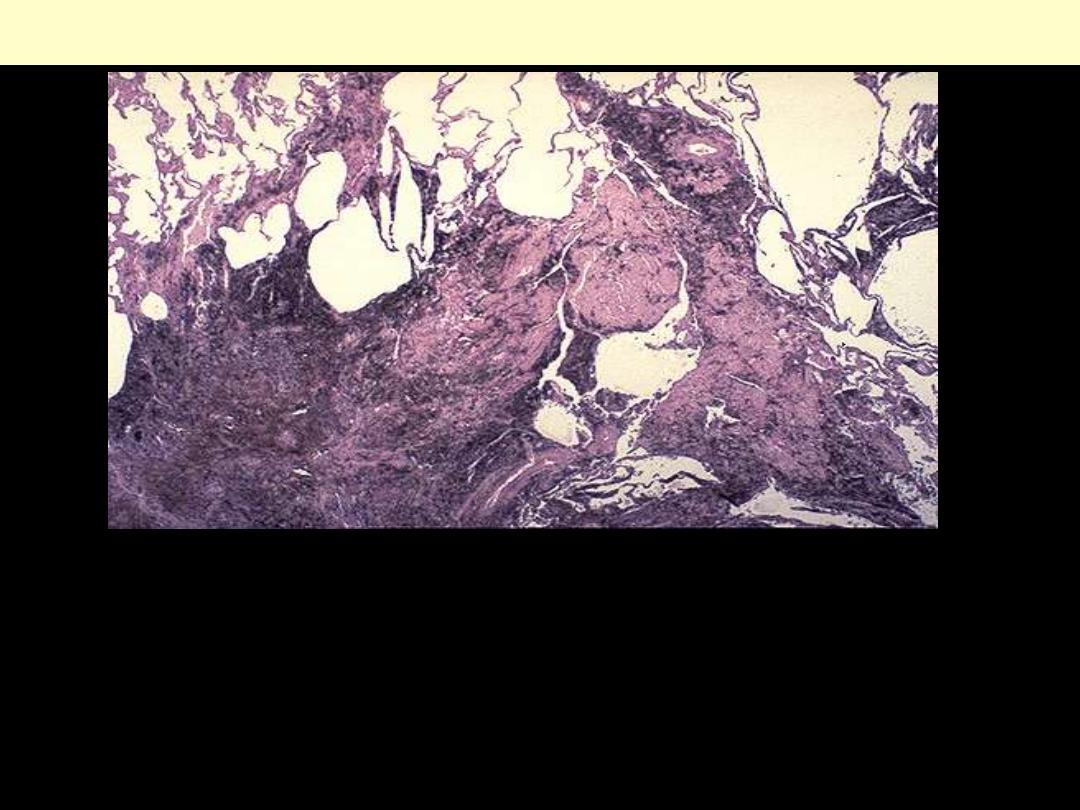
Lung: coal worker’s pneumoconiosis
Anthracotic pigment ordinarily is not fibrogenic, but in massive
amounts (as in "black lung disease" in coal miners) a fibrogenic
response can be elicited to produce excessive collagenous fibrosis
impregnated with the black pigment.
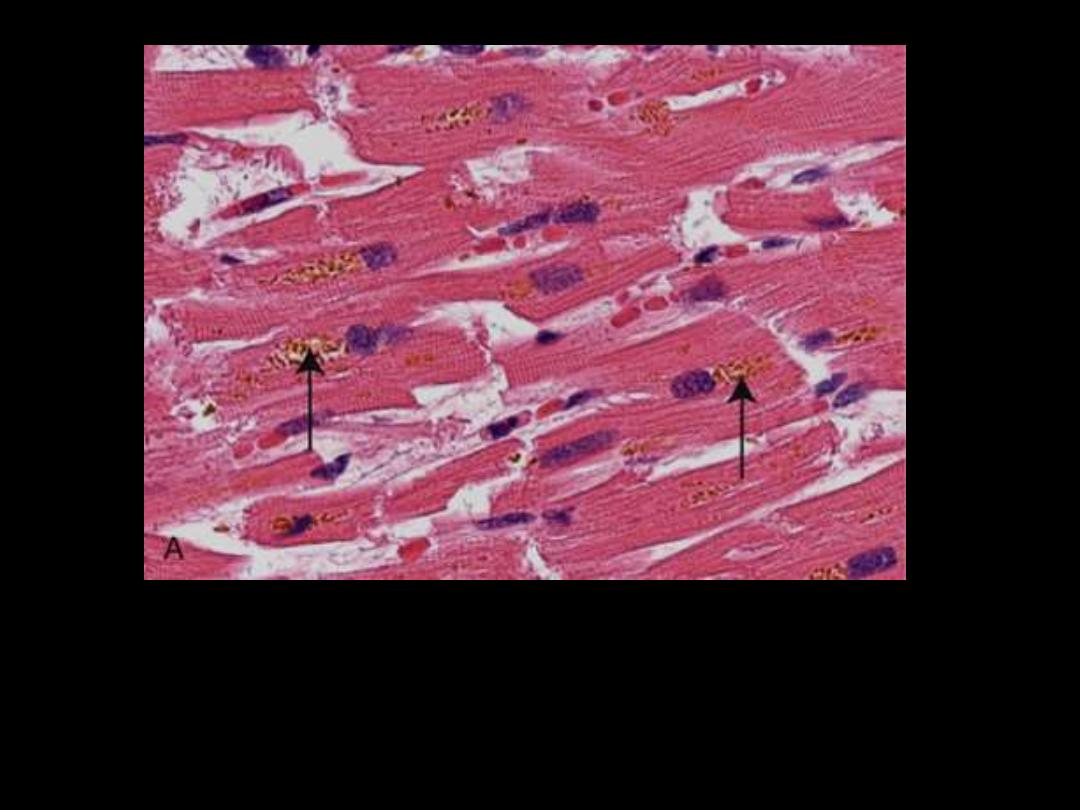
Identify the organ?
Describe the histopathological changes
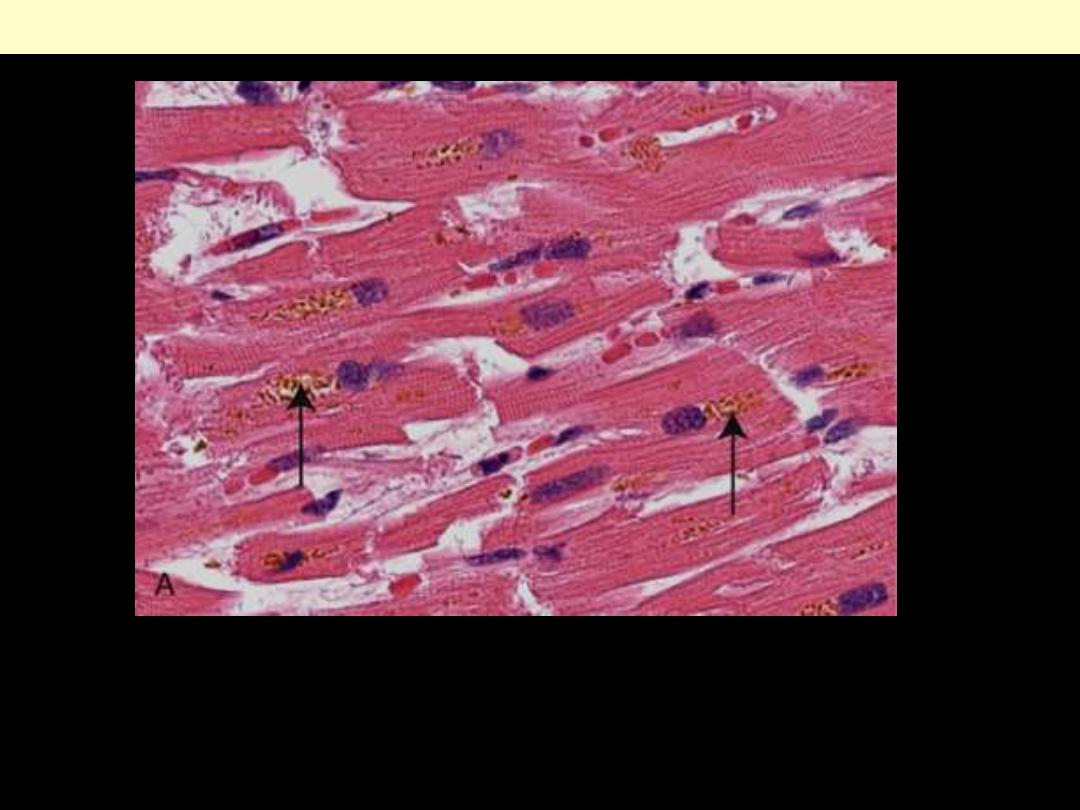
Brownish-yellow granular intracellular material (deposits
indicated by arrows).
Lipofuscin granules in a cardiac myocytes
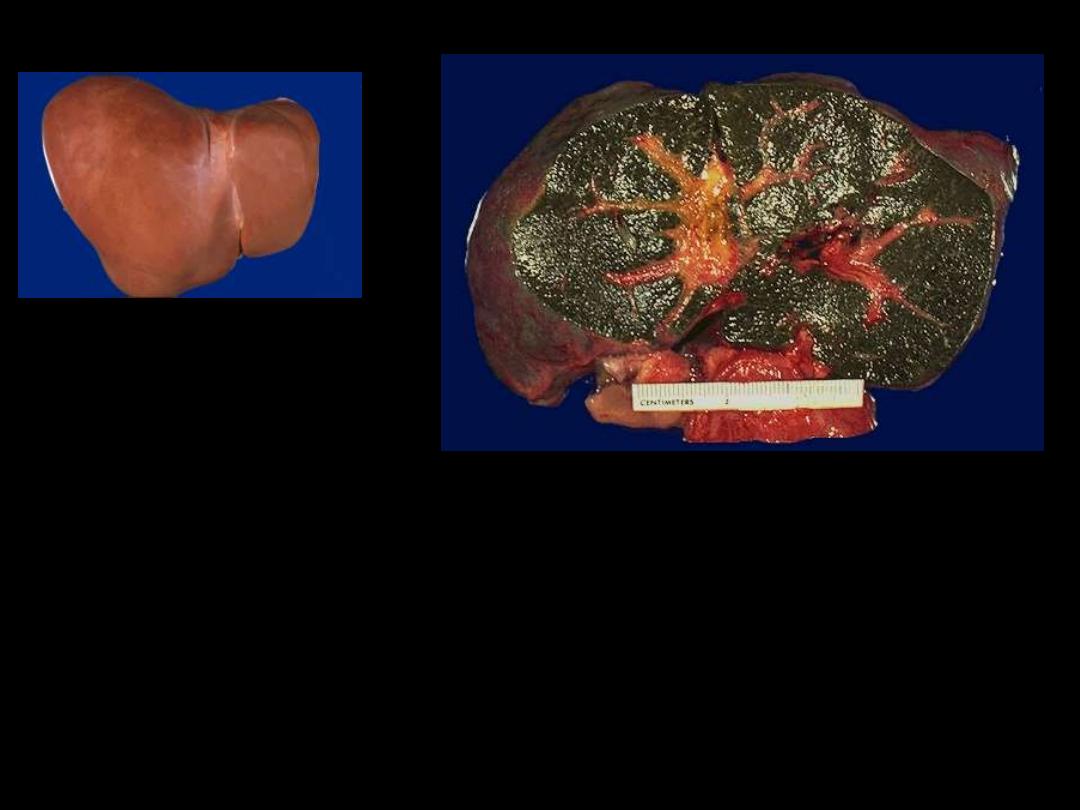
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B
A
B
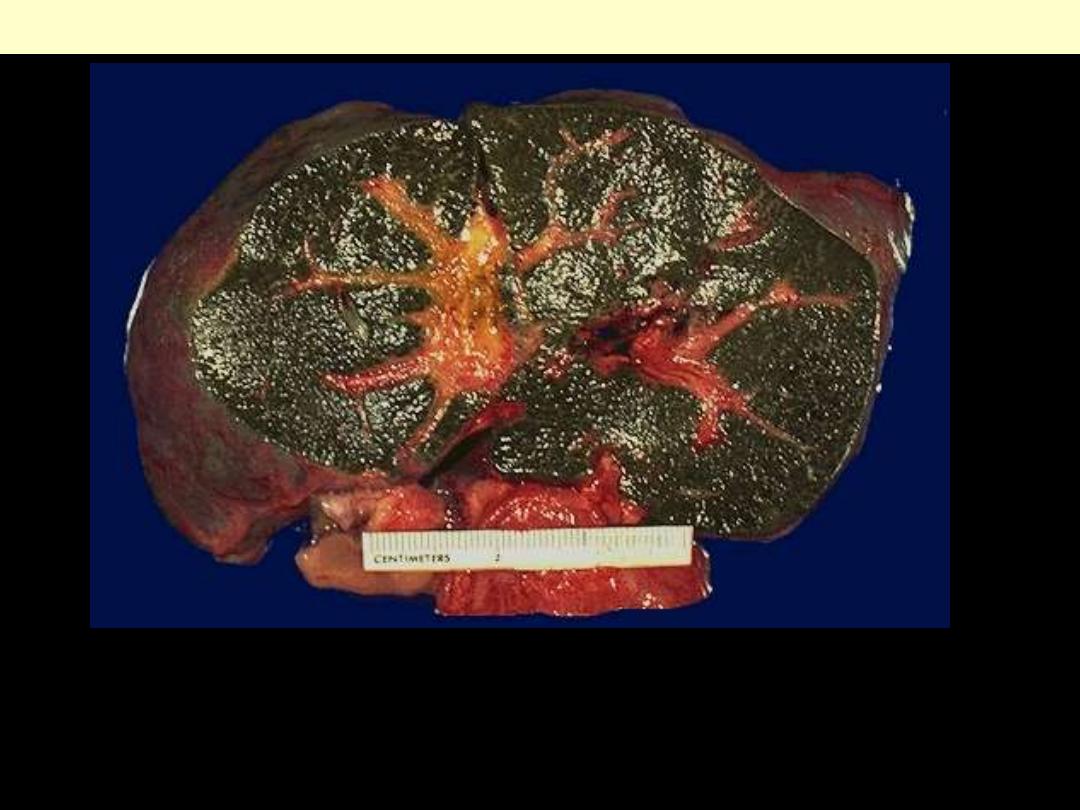
The dark green color comes from formalin acting on bile
pigments in the liver from marked cholestasis, turning bilrubin to
biliverdin.
Bile-stained liver
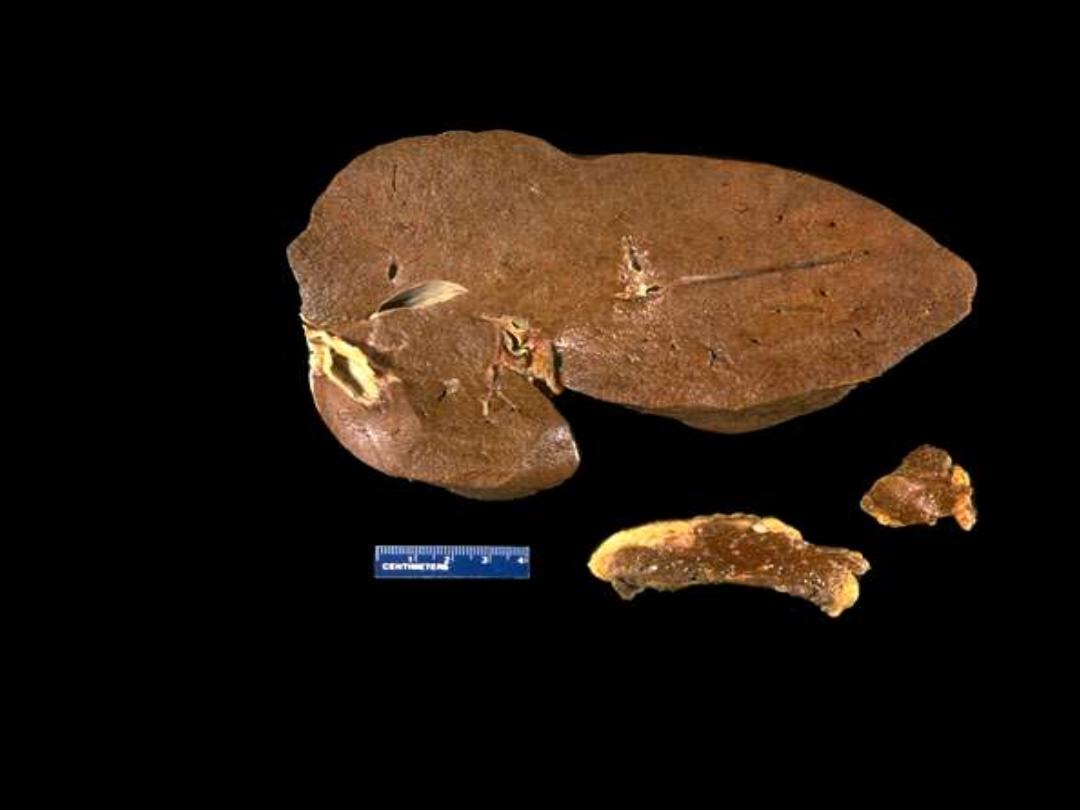
A
B
C
1. Identify the organs: A, B & C.
2. Describe the gross abnormality (pathological changes).

Hemochromatosis: liver, pancreas, and lymph node
The dark brown color of the liver, as well as the pancreas (bottom center) and lymph nodes (bottom
right) on sectioning is due to extensive iron deposition in a middle-aged man with hereditary
hemochromatosis.

A 22-year-old female has a congenital anemia that required
multiple transfusions of red blood cells for many years. She
now has no significant findings on physical examination.
However, her liver function test results are abnormal.
Which
of the following findings would most likely appear in a liver
biopsy?
(A) Steatosis in hepatocytes
(B) Bilirubin in canaliculi
(C) Glycogen in hepatocytes
(D) Amyloid in portal triads
(E) Hemosiderin in hepatocytes
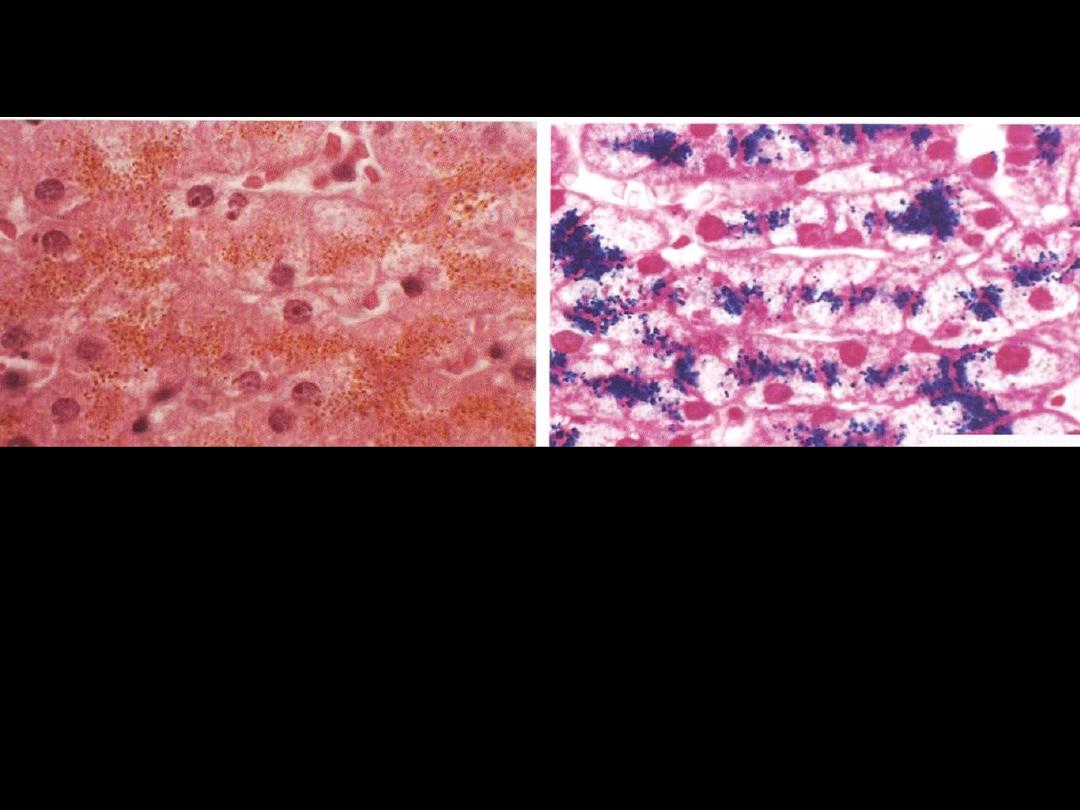
Identify the organ?
Describe the histopathological changes
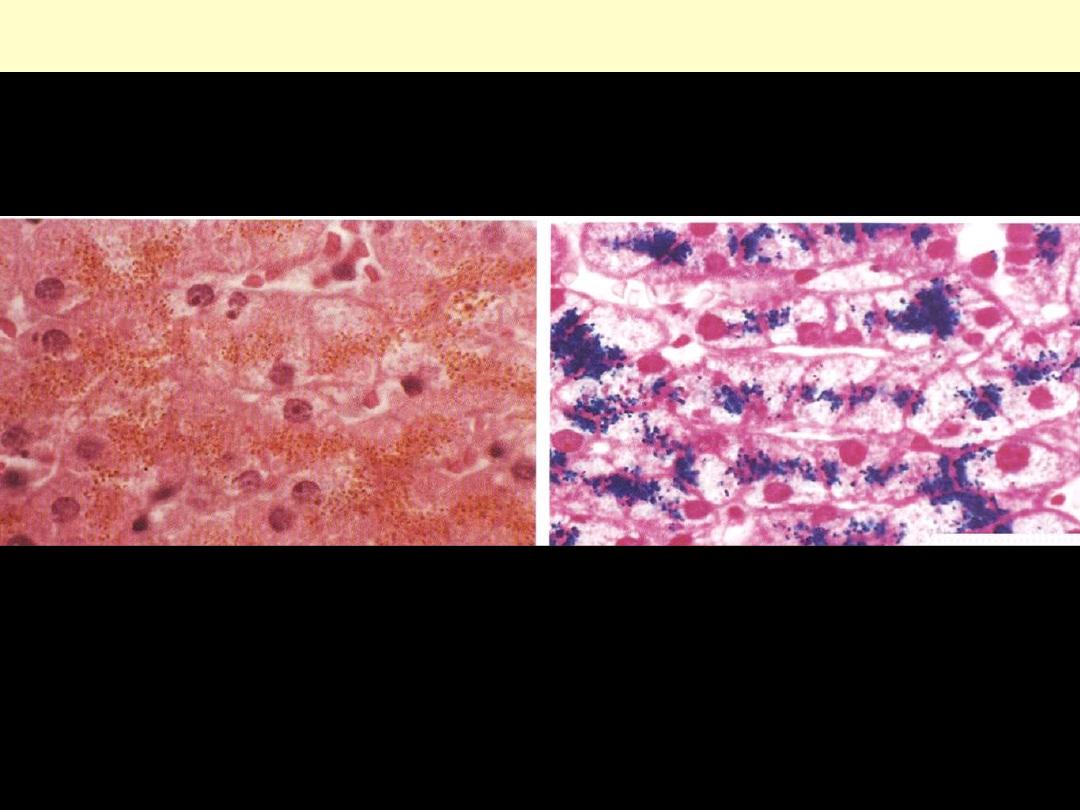
Hemosiderin granules liver cells
A
:
H&E stained section showing hemosiderin as yellow-brown finely granular
pigment within hepatocytes.
B
:
same section stained with an iron stain (Prussian blue); the hemosiderin
granules are deep blue.
A
B
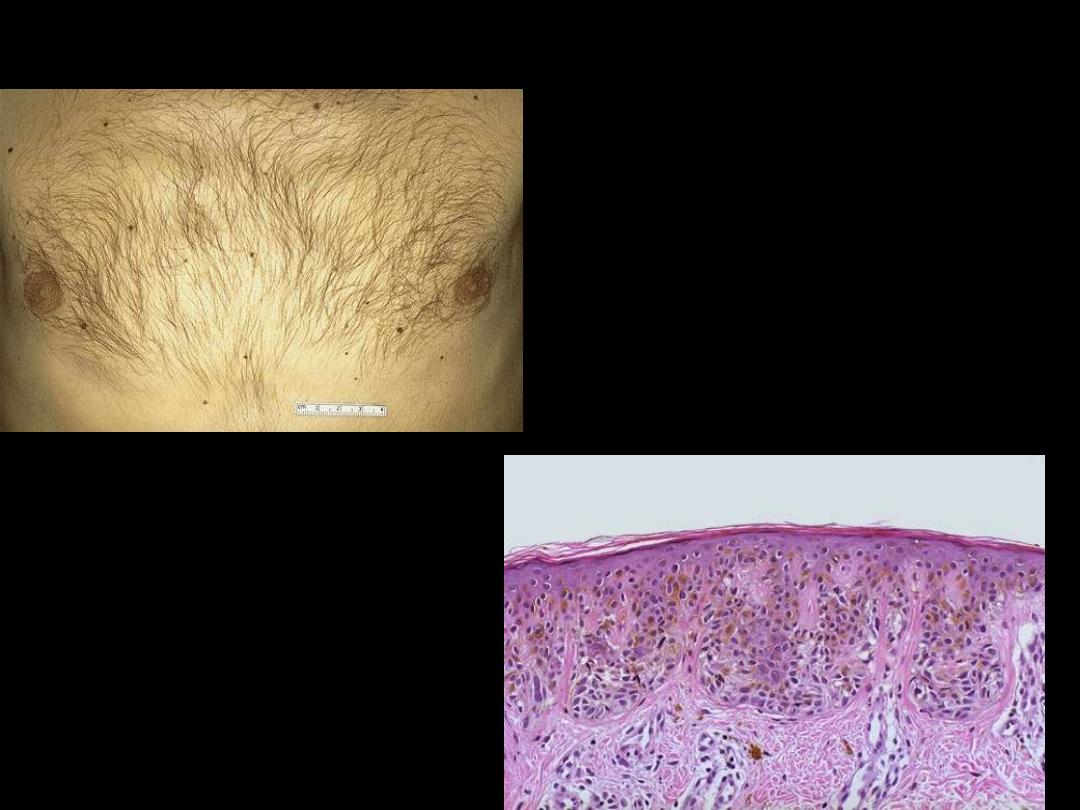
Pigmented nevi

Pigments
• colored substances
• Divided into
1. normal constituents e.g. melanin
2. Abnormal
a. endogenous
b. exogenous.
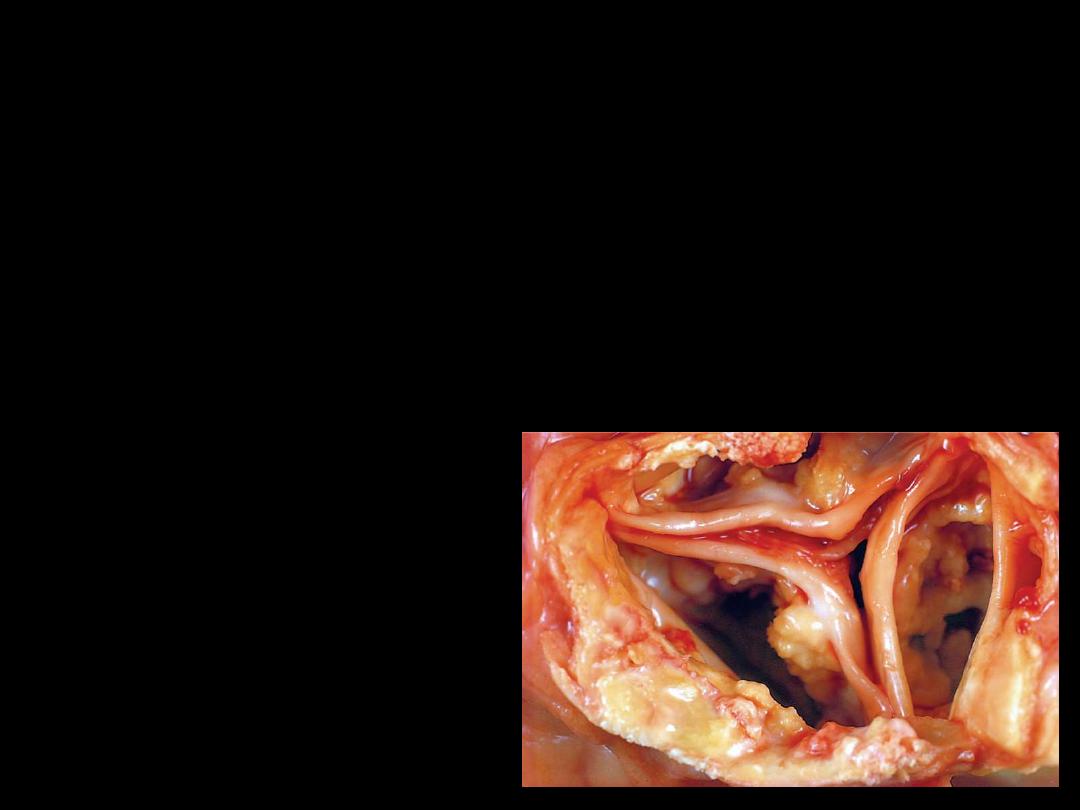
The aortic valve seen in the figure was discovered at the autopsy
of a 72-year-old male. The heart weighed 580 g. with marked
left ventricular hypertrophy and minimal coronary arterial
atherosclerosis. A serum chemistry panel revealed no
abnormalities prior to death from congestive heart failure.
Which of the following pathologic processes counts for the
appearance of the valve?
0(A) Amyloidosis
0(B) Dystrophic calcification
0(C) Lipofuscin deposition
0(D) Hemosiderosis
0(E) Fatty change
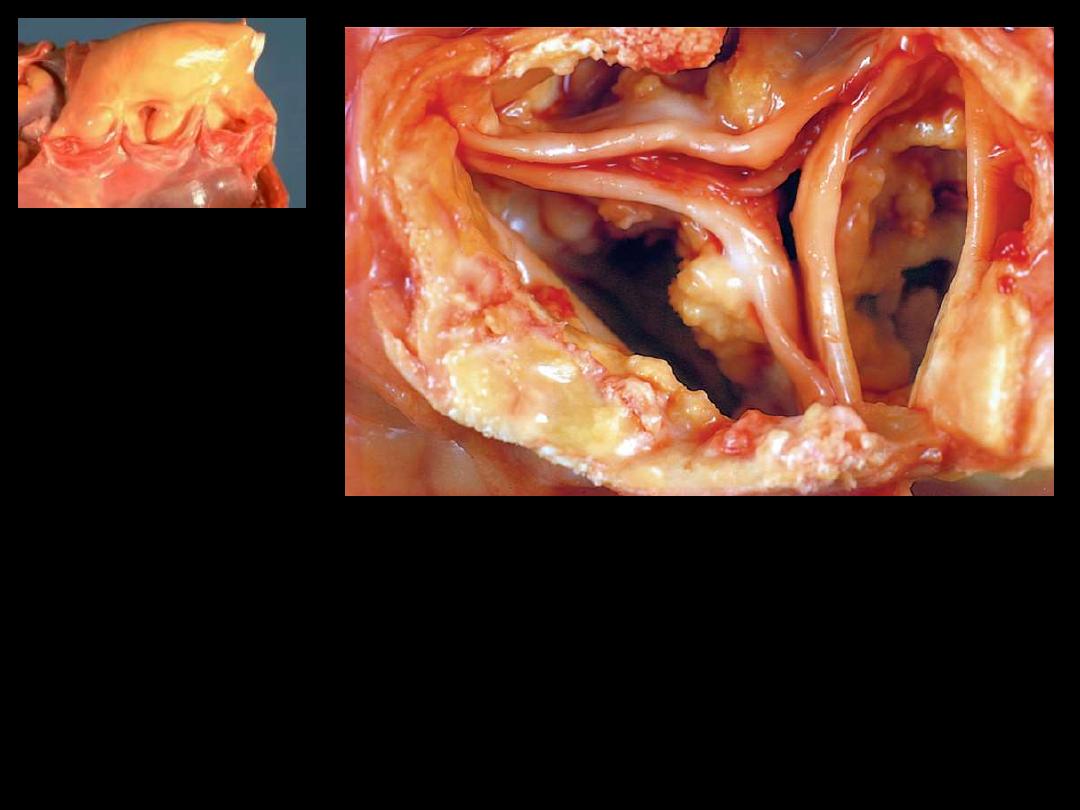
1. Identify the organ.
2. Describe the gross abnormality (pathological changes) in B
A
B
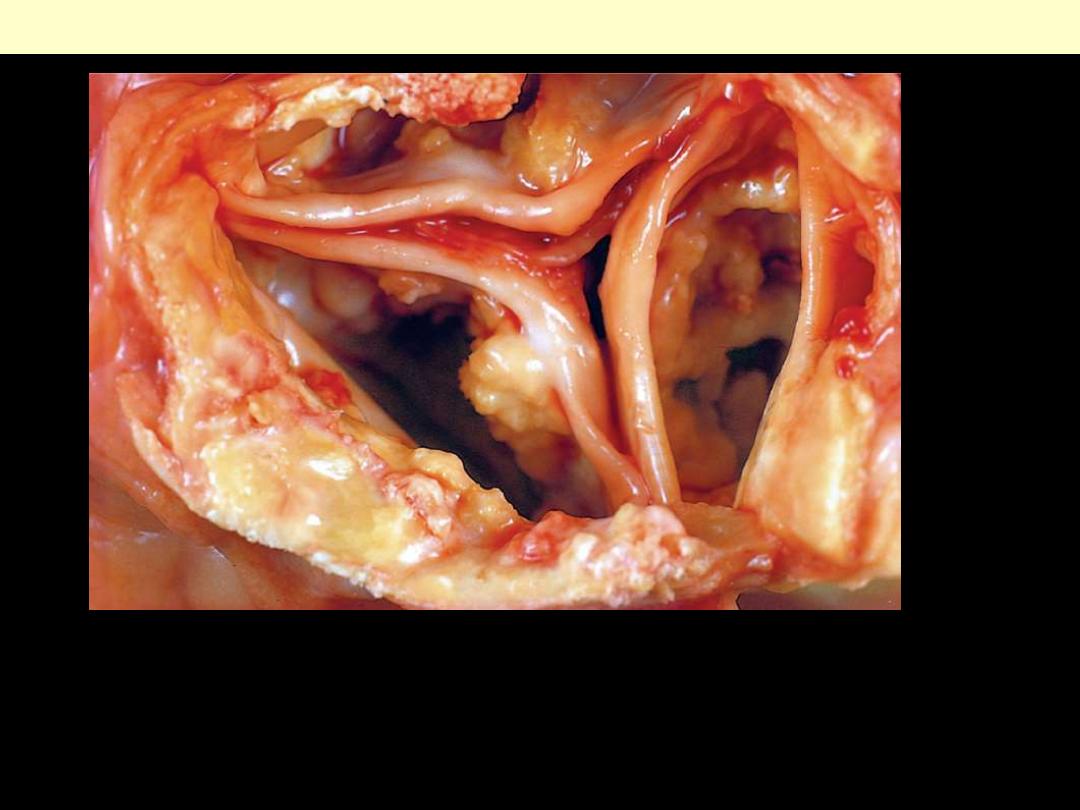
A view looking down onto the unopened aortic valve in a heart with calcific aortic
stenosis. The semilunar cusps are thickened and fibrotic. Behind each cusp are
large, irregular masses of dystrophic calcification that will prevent normal opening
of the cusps.
Calcification of the aortic valve.

Define pathologic calcification.
What are the types of calcification?

Calcification
Pathologic calcification implies the abnormal deposition of
calcium salts.
Dystrophic calcification is noted in
- Areas of necrosis
- Advanced atherosclerosis
- Aging or damaged heart valves
Gross features
- fine, white granules or clumps giving gritty feeling.
Microscopic features
-
basophilic (bluish), amorphous granules that may coalesce to form larger
clumps.
-
Sometimes as rounded lamellar fashion at a nidus of necrotic cells (
psammoma bodies)
- papillary carcinoma
- meningioma

Calcification
Metastatic calcification
• seen in cases of hypercalcemia of any cause
• principally affects
- blood vessels
- kidneys
- lungs
- gastric mucosa.

END
