1
THIRD YEAR
Professor Nada Al Alwan
Diagnostic Cytopathology
Definition: Diagnostic or Clinical C ytology is the study of the normal and disease-
altered cells obtained from various sites of the body (i.e., through the detection of
abnormal morphologic characteristics of the examined dissociated human cells).
Cytopathology in Relation to Histopathology
Advantages of Cytopathology (c
ould be summarized in the following Table):
Limitations of Cytopathology:
As a rule, histopathology remains the gold standard technique in diagnostic pathology.
The whole presented tissue definitely provides a more accurate precise diagnosis of
Histopathology
Cytopathology
Deals with the form and the
structure of the tissue.
Evaluation usually begins with a
tissue biopsy.
More invasive traumatic
procedure is needed; utilizing
surgical instrumentation such as
foreceps, scissors, etc..)
Needles if used should have a
large gauge (i.e., Tru-cut needles
measuring 14, 16 ) .
Diagnosis obtained after days.
Basic stain is H&E
Paraffin blocks are needed
Difficult to identify specific
causative inflammatory pathogen.
Deals with the structural changes
within the nucleus and cytoplasm
of individual cells
Evaluation requires cells only.
Inexpensive simple means of
diagnosis which allows frequent
repetition of cellular sampling
(since it causes no tissue injury).
Fine needles with 22, 23 or 24
gauge are usually preferred.
Rapid diagnosis that could be
obtained within minutes.
Basic stain is Pap stain (however
H&E could be used as well)
Mainly slides are needed
Smears permit better evaluation of
the nature of the inflammatory
process. Fungi and parasites are
usually easier to be diagnosed.

2
the disease in question. The interpretation of the morphological cellular changes is
based mainly on individual observations and often cannot be forced into rigid criteria.
Thus, in many cases the cytological diagnosis could not be final and needs
confirmation by histopathology for the following reasons:
Often the nature of the lesion is not so obvious as in a histological section.
The location of the lesion is d ifficult to be pinpointed by cytology: for example a
squamous cancer cells found in a cytology sputum sample may have originated
from the buccal mucosa, pharynx, larynx or bronchi.
The size of the lesion cannot be approximated by cytology.
The type of the lesion –e.g., in situ carcinoma as compared with early invasion is
more difficult to assess since the relationship of the cells to the surrounding
stroma cannot be determined by cytology.
Sampling Techniques
In general, diagnostic cytology is based upon three basic sampling techniques :
(1) the collection of exfoliated cells,
(2) the collection of cells removed by brushing or similar abrasive techniques,
(3) the aspiration biopsy or removal of cells from non-surface –bearing tissue (or
deep organs) by means of a needle, with or without a syringe.
1- Exfoliative Cytology
Exfoliative cytology is based on spontaneous shedding of cells derived from the lining
of an organ into a cavity, where they can be removed by nonabrasive means.
Typical examples are:
The vaginal smear which is prepared from cells removed from the posterior
fornix ofthe vagina. The cells that accumulate in the vaginal fornix are derived
from several sources: from the squamous epithelium that lines the vagina and
the vaginal portio of the uterine cervix, from the epithelial lining of the
endocervical canal, and from other more distant sources such as the
endometrium. These cells accumulate in the mucoid material and other
secretion from the uterus and the vagina. In addition to the cells derived from
the epithelial linings, the vaginal fornix often contains leukocytes and
macrophages that may accumulate there in response to an inflammatory

3
process, and a variety of microorganisms such as viruses, bacteria, fungi, and
parasites that may inhibit the lower genital tract.
Another example for the use of exfoliative cytology is the s putum
examination. The sputum is a collection of mucoid material that contains cells
derived from the buccal cavity, the pharynx, larynx, trachea, the bronchial tree
and the pulmonary alveoli; as well as inflammatory cells, microorganisms,
foreign material , etc…
The same principle applies to:
Voided urine
Body fluids
Nipple discharge
Exfoliative cytology is the simplest of the three sampling techniques. To prevent the
deterioration of cells by air-drying or by enzymatic or bacterial actions, the material
should be processed immediately without delay. Alternatively, the use of fixatives,
such as alcohol or other cell preservatives is indicated.
2- Abrasive Cytology
The purpose of this procedure is to enrich the sample with cells obtained directly from
the surface of the target of interest. Hence, cell specimens are usually obtained
through superficial scraping of the lesion (artificial mechanical desquamation) .
Examples include:
Cervical scraping or the so called Pap s mear: the cervical scraper introduced
by Ayre in 1947 (i.e., Ayre’s spatula) allows a direct sampling of cells from
the squamous epithelium of the cervix and the adjacent endocervical canal.
Buccal mucosal smear
Skin scraping of various lesions
Brushing techniques through using rigid endoscopic & fibroptic instruments
to collect cell samples from the gastrointestinal tract, bronchial tree, etc…
The Pap Smear Test
Is used mainly to detect precancerous and cancerous lesions of the uterine cervix.It is
based on the fact that cancer of the cervix is one of the most preventable cancers,
because most of the cellular changes which may lead to ca rcinoma can be detected
4
and accordingly treated at an early stage before progression. In most cases, cervical
carcinoma develops slowly ( over a period of up to 10 yea rs ); whereby it passes into
different pre-neoplastic conditions before it reaches the cancer stage; termed
dysplasia
or
Ce rvical
Intraepithelial
Neoplasia
(CIN):
Mild Dysplasia --► Moderate Dysplasia --► Severe Dysplasia--►
CIN I CIN II CIN III
Carcinoma in Situ --► Carcinoma
Nomenclature of Dysplastic Lesions according to The Bethesda System
Squamous & Glandular abnormalities
The term squamous intraepithelial lesions (SIL) encompasses the spectrum of the
precursors to invasive squamous cell carcinoma previously called dysplasia,
carcinoma in situ, border line lesions and CIN .
Grading squamous intraepithelial lesions
The Bethesda System recommends a low-grade /high grade approach in classifying
SIL. Most LSILs are secondary to transient infections that carry little risk of transition
into carcinoma, whereas most HSILs are associated with viral persistence and a
significant potential for progression to invasive cancer.
Low- grade squamous intraepithelial lesions
LSILs encompasses lesions that were previously described separately as
"koilocytosis" (signifying Human Papilloma Virus infection) and mild dysplasia
(CIN 1).
High- grade squamous intraepithelial lesions
HSILs encompasses lesions that were previously described as moderate dysplasia
(CIN 2) and severe dysplasia (CIN 3)
Microscopical examination of the exfoliated cells from the uterine cervix through Pap
Smear test should therefore lead to the detection of these pre-neoplastic lesions in
their earliest stages (i.e., stage of mild dysplasia ). Accordingly, giving the
appropriate
treatment
would
eventually
prevent
cervical
cancer.
Pap Smear test is a simple procedure in which cells are collected from the cervix and
examined cytologically to exclude abnormal changes. No anaesthesia is required, and
the diagnosis could be obtained within few minutes. The procedure could be easily
carried out in any clinic or specialized lab. It is recommended that all women

5
between the ages of 18 and70 years, who have ever had sexual activity, to have the
test every 2 years.
Pap smear could also allow the diagnosis of other microbiological agents such as
Monilia, Trichomonas Vaginalis, Herpes Simplex or Human Papillomavirus “HPV”.
The latter might cause progression of dysplasias to carcinoma when untreated.
3- Fine Needle Aspiration Cytology (FNAC)
In general, the definitive diagnosis of any mass can be established by:
- Open biopsy
- Tissue core needle (Tru-cut) biopsy
- Fine needle aspiration biopsy.
Compared to FNA, Tru-cut biopsy is a more traumatic procedure which should be
performed under local anaesthesia. It requires more time and special equipments
that are more expensive. Pain, discomfort and bleeding are common complications.
FNAC, on the other hand, provides many advantages to the surgeons being an easy,
reliable, cost effective diagnostic technique which could give rapid results. The
procedure could be performed in an office setting without anaesthesia. It is usually
not more painful than a venipuncture and can be repeated immediately if the acquired
material is inadequate.
The National Health Service Breast Screening Program (NHSBSP) displayed that
many centers wished to include FNA as an additional test to provide preoperative
diagnosis of breast cancer and to reduce the number of operations for benign breast
diseases.
When reduced to its simplest terms, FNAC consists of:
Using a needle and syringe to remove material from a mass.
Smearing it on a glass slide.
Applying a routine stain.
Examining it under the microscope.
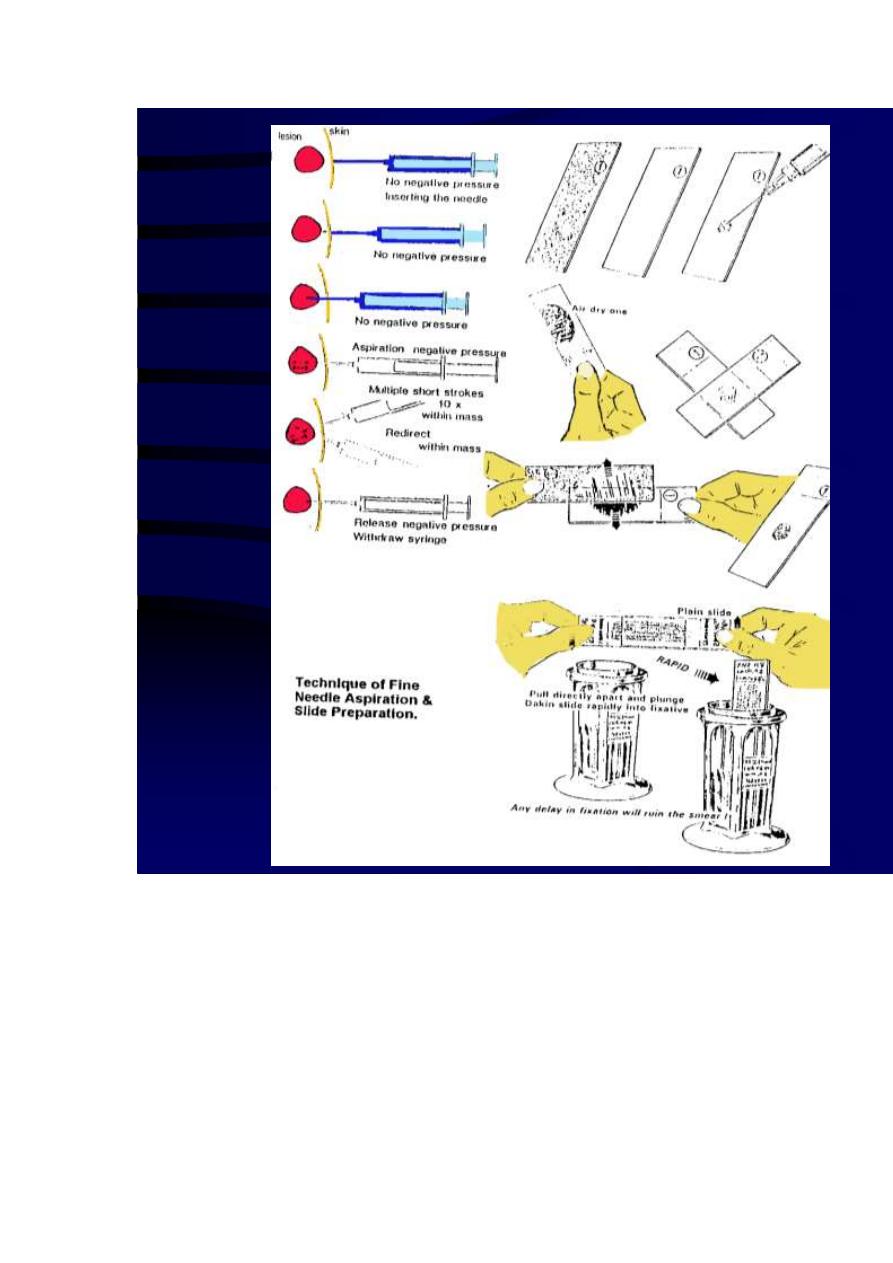
Technique of FNAC:
The skin over the palpable mass (within the breast, lymph node, or any other organ) is
sterilized with an antiseptic. Utilizing the index and the middle finger or the thumb and the

6
index finger, the mass is localized with the non-dominant hand. With firm downward pressure
on the skin over the mass, it should be compressed against a rib and stabilized.
Aspirates should be obtained using preferably a 23 gauge, 1 ½ inch disposable needle
mounted on a 10 ml plastic syringe, held by the dominant hand. Larger needles (22 gauges)
are used in aspirating material from hard fibrous masses and in cases of suspected cyst or
abscess.
Without using anesthesia, the needle should be gently introduced through the skin passing to
the level of the dominant mass. Having confirmed the position of the needle within the mass,
negative pressure should be created within the syringe by pulling back the plunger. The
needle should move back and forth through the mass, in different rotational directions using
sewing-like-motion. Suction should be maintained throughout the process by outward
pressure of the right thumb on the underside of the syringe. By this method the needle can cut
loose many small pieces of tissue that are then readily aspirated due to the suction applied by
syringe. These to and fro strokes should be repeated at least 6-8 times. In general most of the
aspirations are terminated when material begins to appear in the needle hub.
The following step should be designed to protect the material that has been obtained. All
suction should be released before removing the needle from the mass. This is accomplished
by allowing the syringe plunger to return gently to its resting position. Then the needle should
be withdrawn gently from the mass. To limit haematoma formation from the site of the
puncture, firm pressure should be applied with a piece of cotton for two minutes.
7
Equipments needed for FNAC procedure:
Syringes
The key feature in a syringe is lightness in weight, adequate in volume, and one hand control. Modern
sterile disposable plastic syringes are perfect for use in FNAC. Most practitioners use the 10 cc size,
while some prefer the 20 cc size (specifically when a cyst is suspected).
Needles
Several variables should be considered in choosing the proper length and gauge. In general the length
should be at least 1.5 inch (3-8 c m). Th is is desirable in the breast because many lesions are found
deeper than expected. It has also been demonstrated that small gauges (22-23) are prefe rred since they
are rigid enough and at the same time cause less sensation and haematoma . Needles with a p lastic hub
rather than a metal hub are pre ferred as we ll. Th is permits the aspirator to mon itor the recovery of
tissue, blood or other fluids as they appear in the hub.

8
Glass Slides
Co mmon routine slides measure 75 mm X 25 mm X 1mm. An important procedure is slide labe ling at
the time o f sa mpling. That is why slides wh ich have one frosted end are preferred. Various substances
are used to increase the adherence of specimens to the slide surface, the most common ly used being
albumin.
Fixatives
These are applied to the smears as a spray or by imme rsion of the slide into a liquid. The most
commonly used is 95 % Ethanol. This ine xpensive readily availab le liquid provides exce llent
cytological details. Its only disadvantage is the need to store and transport slides in a container of liquid
and to have them immersed in for at least 20 minutes.
Routine Stains
For cytological d iagnosis, fixed material is usually stained by Papanicolaou Stain (wh ich is prefe rred)
or by He mato xylin and Eosin (H&E) method. Both e mploy one of the He mato xy lins for staining of the
nuclei. This dye colors the nucle ic ac ids as a dark purple blue co lor. The counter staining with Eosin is
designed to show cytoplasmic characteristics. With the Pap stain one hallmark is the use of Orange-G,
which stains cytoplasmic keratin with a bright orange color.
Air-dried smea rs on the other hand are usually stained by any Ro manowsky stains, which are various
combinations of methylene blue and its breakdown products (Azure A, B, &C) with Eos in. Most rely
on Methanol fixation.
Papanicolaou Stain
All slides should be wet fixed within 95% or absolute Ethanol before staining:
95% ethanol 10 dips
80% ethanol 10 dips
70% ethanol 10 dips
50% ethanol 10 dips
Distilled water 10 dips
Hematoxylin Stain 1-2 min.
Running Tap water 10 dips
50% ethanol 10 dips
70% ethanol 10 dips
80% ethanol 10 dips
95% ethanol 10 dips
Orange-G Stain 2 min.
95% ethanol 10 dips
Eosin Stain 2 min.
95% ethanol 10 dips
95% ethanol 10 dips
Absolute ethanol 10 dips
Absolute ethanol: Xylol (50:50 mixture) 5 min.
Xylol 20 min. Mount with Canada Balsam
Pitfalls to Reliable Results from FNAC
No mass
Vague mass
Mass being too small ( less than 1 cm )
Mass being too deep
Gross blood into the syringe (i.e., RBCs can mask the picture)
Scanty cellularity in the aspirated mass
Dense fibrosis
Gross fluid

9
Indications for Cytopathology
1- Differentiation between benign and malignant lesions.
2- Diagnosis of malignancy and its type, as well as the identification of the
neoplastic cells in primary, metastatic (secondary) or recurrent tumors.
3- Diagnosis of premalignant diseases: i.e., detection of precancerous or
dysplastic cellular changes (figure 5). As explained earlier, dysplasia may be
graded as mild, moderate and severe. In the uterine cervix for example, those
lesions could be eliminated by treatment of the associated inflammation and
causative pathogen or by excision of the lesion.
4- Detection of inflammation and certain types of pathogenic agents : i.e., the
cytologist could prove that the cause of vaginal secretion is vaginitis due to
Trichomonus vaginalis, Moniliasis or the cytology could show changes
suggestive of Human Papilloma Virus infection (HPV – Figure 6). Similarly
the cause of cystitis may be related to bilharziasis ( through the detection of
Bilharzial ova in smears of urine samples).
5- Study of the hormonal patterns and evaluation of the gonadal hormonal
activity: through the examination of the squamous cells in vaginal smears;
which are under the influence of ovarian hormones (figure 7).
6- Follow- up and monitoring of response to chemotherapy and irradiation; the
latter producing certain cellular features which could be diagnostic on
cytologic examination.
7- The identification of sex chromosome : if a newborn presents with ambiguous
genitalia, one can not tell whether the sex is male or female. The presence of a
dark dot attached to the nuclear membrane from inside (Barr body +ve)
indicates that a sex chromosome is present, i.e., the genotype of the baby is
XX (♀). Conversely the absence of this Barr body indicates that there is no X
chromosome and accordingly the newborn is genotypically a male ((XY ♂).
8- Tumour markers study on cytological specimens.

11
Malignancy
Criteria of
Cytological
A) Nuclear Changes:
Nuclear hypertrophy: nuclear enlargement that leads to increased N/C ratio.
Nuclear size variation
Nuclear shape variation
Hyperchromatism and chromatin irregularity: refers to increased chromatin
materials. In malignant cells the chromatin is not evenly distributed within the
nucleus; it is also distributed as coarse, clumps. This in contradistinction to
normal cells, which have evenly distributed chromatin.
Multinucleation: malignant cells may contain more than one nucleus. However,
some normal cells such as hepatocytes and histiocytes may contain more than
one nucleus. Multinucleated malignant cells differ from nonmalignant
multinucleated cells by the fact that the nuclei of malignant cells are unequal
in size (in contrast to that of normal cells).
Irregularity of the nuclear membrane.
Irregular and prominent nucleoli: giant nucleoli or multiple nucleoli may be
present that differ in their sizes and shapes. It should be remembered, however,
that normal columnar and goblet cells may contain 2 nucleoli.
B) Cytoplasmic Changes:
Scantiness of cytoplasm: in consequence to the high N/C ratio.
Cytoplasmic boundries: sharp and distinct in Squamous cell carcinomas and
indistinct in undifferentiated carcinomas.
Variation in size .
Variation in Shape.
Cytoplasmic staining: deep orange in keratinizing squamous ca rcinomas
or basophoilic in immature poorly differentiated carcinomas.
Cytoplasmic inclusions: e.g. melanin pigments in melanoma.
Cytoplasmic and nuclear membrane relationship: cytoplasmic borders of
malignant cells could be tightly molded against the nucleus, touching it in
more than one place.

11
C) Changes in Malignant Cells as a Group:
Cellular phagocytosis or Cannibalism: i.e., one cell appearing to be contained
in a vacuole of the cytoplasm of another epithelial cell; indicating rapid
growth of cells within a narrow cavity.
Variation in the size and shape of the cell clusters or sheets.
Lack of cellular adhesion: due to abnormalities in the desmosomes.
Abnormal mitosis: malignant nuclei may show multiple poles e.g. three poles
(tripolar mitosis; triploidy) or four poles (tetrapolar mitosis; tetraploidy). This
is abnormal since normal mitosis has two poles (bipolar). Thus nor mal cells
show euploid or diploid pattern on flow or image cytometry whereas
malignant cells may show abnormal aneuploid patterns.
Bloody background: fresh blood is meaningless, but malignancy is suspected
when the blood is old, partially ingested by histeocytes or present within a
specimen which is obtained without trauma.
Foreign cellular structures: e.g. psammoma bodies in a routine vaginal smear.
Degeneration and inflammation as a result of erosion or necrosis within the
malignant tumour which could indicate invasion (i.e., tumour diathesis).
Perhaps the best demonstrable tool to observe the various types of malignant cells
which could be encountered cytologically is through studying the different variants of
Bronchogenic Carcinoma.
A routine recommendation for these patients is the examination of three successive
early morning sputum samples.
Histologically, there are four major different types of bronchial cell carcinoma could
be recognized on cytological examination:
1. Squamous Cell Carcinoma
Is the most common and easiest type to be diagnosed by cytologic examination of
sputum because of its central position within the bronchial tree. Cytologically, most
malignant cells exfoliate singly but some may be observed in clusters or sheets.
Cells often assume variable sizes and shapes; could be amoeboid in shape, assuming
pseudopod- like projections. Spindle or tadpole cells could be also detected. The

12
background is usually obscured by necrotic debries, mucous and RBCs (tumour
diathesis).
The cytoplasm is abundant, dense and granular with a glassy orange appearance in
well-differentiated types due to the presence of keratin (stained by Orane G).
Anucleated deep orange squames may be evident in long standing cases. The nucleus
may show nuclear size and shape variation with irregularity of the nuclear membrane.
Marked hyperchromasia is often present; the nuclei appearing very dark or pyknotic.
Cannibalism is common. Red prominent nucleoli could be present.
2. AdenoCarcinoma
Usually periphral in position. Cells exfoliate in clusters, sheets or acini; rarely
singly. They are often round, oval and irregular in shape. Cytoplasmic vacuoles are
observed in well-differentiated types due to the presence of mucous droplets. Large
vacuoles may push or compress the nucleus to one side giving an irregular moon-
shaped appearance: signet-ring pattern. Neutrophils maybe seen within these
vacuoles. Nuclei are often round or oval with moderate hyperchromatism and
irregular nuclear membrane. Single or multiple diagnostic nucleoli are usually
found. Multinucleatin may occur. Cells stain positive with PAS stain (due to the
presence of mucin).
3. Undifferentiated Small Cell Carcinoma
The WHO classification recognizes three histological subtypes:
-
Oat cell carcinoma.
-
Small cell carcinoma – intermediate type.
-
Combined Oat cell carcinoma (seen with foci of squamous cell
and/or adenocarcinoma).
The cellular morphology of small cell carcinoma varies with the method of specimen
collection. In sputum, the exfoliated cells are often small in association with long
strands of mucous. Dark lines of elongated compact masses of numerous small
hyperchromatic neoplastic cells are seen with satellite single cells scattered around.
The nucleus could be degenerated and pyknotic. In brushings and FNA samples, cells
are usually better preserved, appearing larger and displaying more variations in
nuclear chromasia and chromatin patterns.

13
4. Undifferentiated Large Cell Carcinoma
These are epithelial tumours comprised of malignant cells with large nuclei and
prominent nucleoli that show no evidence of squamous, glandular or small cell
differentiation. In the WHO classification, two variants are recognized :
-
Giant cell carcinoma which contains a prominant component of highly
pleomorphic multinucleated cells which maybe actively phagocytic. The
hyperchromatic nuclei shows irregular chromatin clumping and large
prominent macronucleoli.
-
Clear cell carcinoma which is a rare variant composed of large cells with
clear or foamy cytoplasm without mucin.
In general, during the evaluation of a smear, the cytopathologist should look for the
following changes
Modification of the normal nuclear and cytoplasmic cells.
Maturation status.
The quantity of the cells (hypo- or hyper-cellular)
The mode of desquamation (clusters, sheets or scattered individually).
The smear background (i.e., the presence of leukocytes, histiocytes, RBCs,
necrotic cells and/or protein deposits).
