
Pneumocystis carinii
Pneumocystis carinii is an important cause of
pneumonia in immunocompromised individuals. The
classification and life cycle of Pneumocystis are unclear.
Many aspects of its biochemistry indicate that it is yeast,
but it also has several attributes of a protozoan. An
analysis of rRNA sequences and mitochondrial DNA and
of various enzymes supports the idea that it is a fungus.
However, the findings that it does not grow on fungal
media and that antifungal drugs are ineffective have
delayed acceptance of its classification as a fungus. Each
mammalian species is thought to have its own species of
Pneumocystis.

Pathogenesis & Epidemiology
Transmission occurs by inhalation, and infection is
predominantly in the lungs. The presence of cysts in the
alveoli induces an inflammatory response consisting
primarily of plasma cells, histiocytes, lymph resulting
in a frothy exudate that blocks oxygen exchange. (The
presence of plasma cells has led to the name "plasma
cell pneumonia.") The organism does not invade the
lung tissue. P. carinii is distributed worldwide. It is
estimated that 70% of people have been infected
.
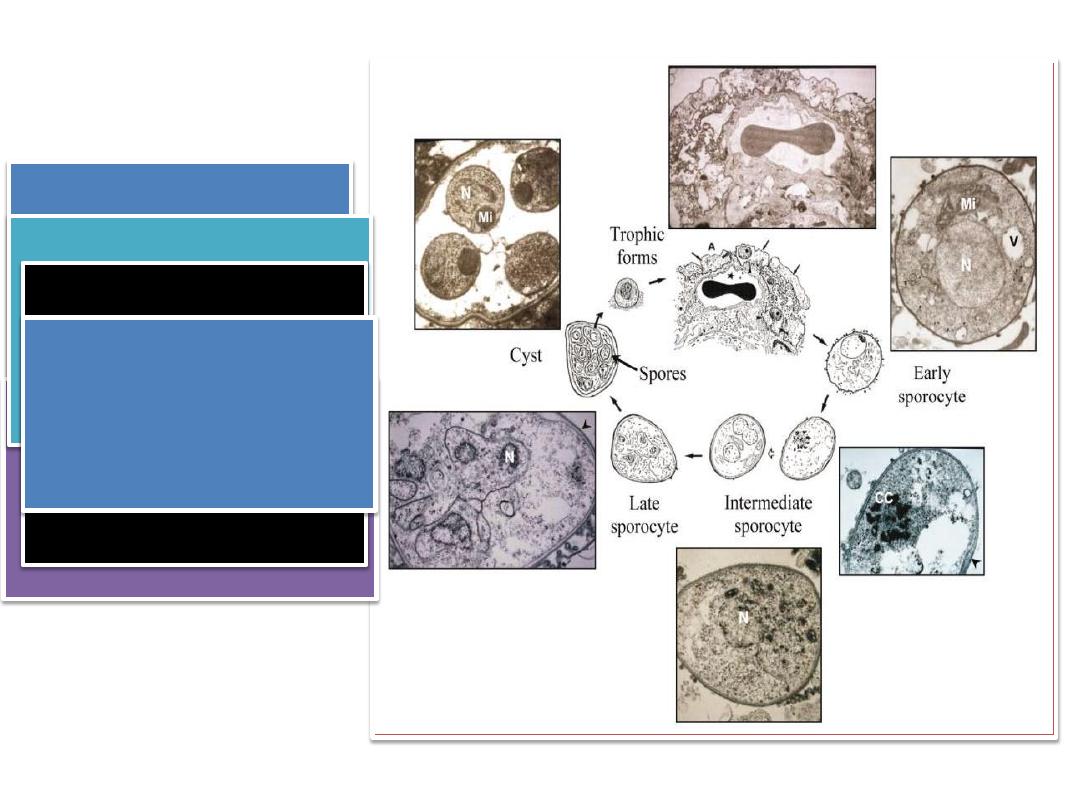
• Ultrastructural studies
suggest the existence of
mating types and the
probable conjugation of
Pneumocystis trophic
forms
• Following the fusion of
mating types, a round,
thin-walled, mononuclear
and probably diploid
sporocyte is produced
• Trophic forms,
sporocytes and mature
cysts are the three main
morphological forms in
the Pneumocystis life
cycle.
• Trophic forms : These
vegetative forms appear
as mononuclear, 2-8
µm in diameter with
thin cell wall ameboid
in shape cytoplasmic
projections known as
filopodia.
mature cyst, eight
individual spores are
clearly delineated
Meiosis is followed by an
additional mitotic
replication resulting in
eight nuclei in the late
sporocyte stage

Clinical Findings
The sudden onset of fever, nonproductive
cough, dyspnea, and tachypnea is typical of
Pneumocystis pneumonia. In infants, the
disease usually has a more gradual onset. The
mortality rate of untreated Pneumocystis
pneumonia approaches 100%.

Laboratory Diagnosis
Diagnosis is made by finding the typical cysts by
microscopic examination of lung tissue or fluids
obtained by bronchoscopy, bronchial lavage, or open
lung biopsy. Sputum is usually less suitable. The cysts
can be visualized with methenamine-silver, Giemsa the
cyste appear as a round masses of unstained cytoplasm
containing 2-8 purple stained nuclei.

Fluorescent-antibody staining is also commonly
used for diagnosis. The organism stains poorly
with Gram stain. There is no serologic test, and in
culture uninucleated forms present in the
inoculum so its impractical. PCR-based tests are
being developed.
Treatment
The treatment of choice is a combination of
trimethoprim and sulfamethoxazole (Bactrim, Septra).
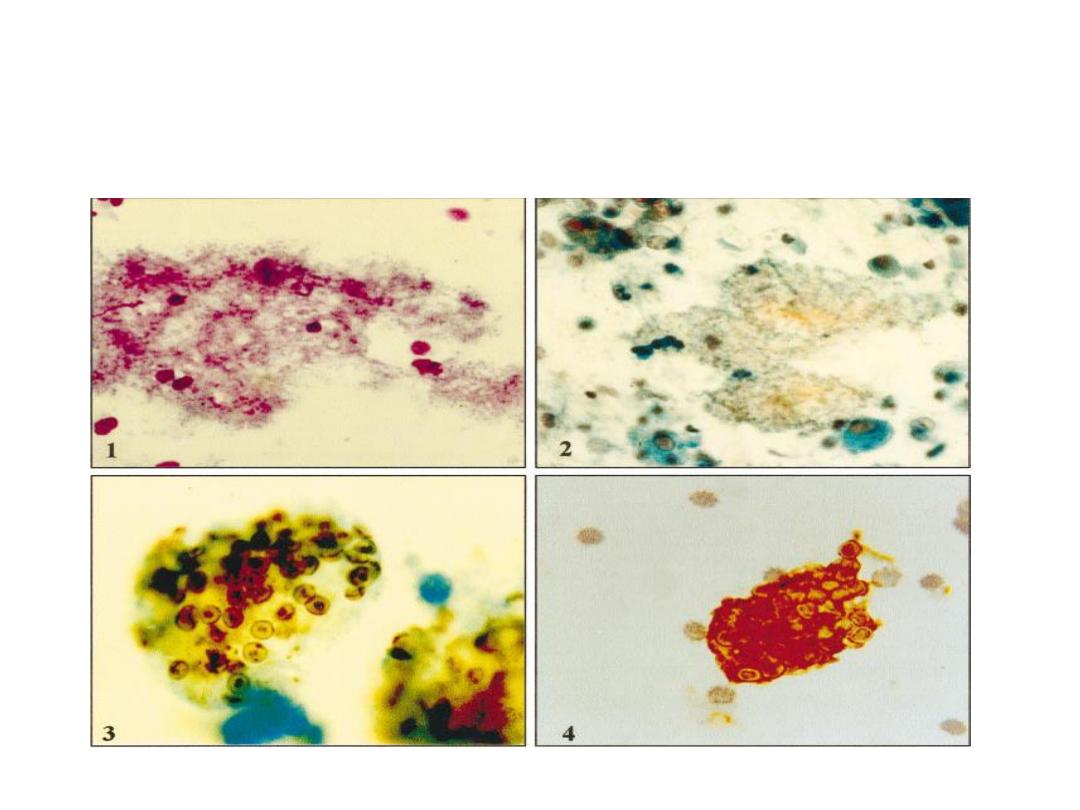
Giemsa staining of tissue or smear reveals the uninucleated
trophozoites as well as the organism within the cysts
Sarcocystis
Causative agent of sarcocystosis
Sarcocystis hominis
Sarcocystis suihominis
Sarcocystis lindemani

Sarcocystis hominis and S. suihominis are known
as human intestinal parasites. Infection results from
ingestion of raw or insufficiently heated meat from
cattle (Sarcocystis hominis) or pigs (Sarcocystis
suihominis), which frequently contains muscle cysts of
these species. The prevalence of intestinal sarcocystosis
in humans is low and is only rarely associated with
illness, except in volunteers who ingest large numbers
of sarcocysts. Cases of infection of humans as
intermediate hosts, with intramuscular cysts, number
less than 100 and are of unknown origin

Life cycle:
Man gets infection by eating raw or
undercooked meat that contained sarcocyst, in the
small intestine of definitive host (man) the
brodyzoites are released and they migrate to sub
epithelial lining and develop to male and female
gametocyte. Fertilization occur to form thin-walled
oocysts that sporulate in the intestinal wall. Once the
frail oocyst wall has burst, free sporocysts containing
four sporozoites each are excreted with stool in most
cases. The sporozoites are infectious for intermediate
hosts.

When the intermediate host get these sporocysts
it infect the endothelial cells of blood vessels and
schizogony cycle occurs. Those schizont rupture
releases merozoites in the blood vessels and go to
striated muscles and develop into tissue cyst (
sarcocyst). In case of sarcocytis lindemani similar man
can act as intermediate host and asexual cycle may
occur in the striated muscle.
Clinical manifestations:
Both species can cause short-lived (6
to 48 hours) symptoms within 24 hours of
eating meat containing cysts, for example
nausea, vomiting, and diarrhea as well as a
mild fever.
S.suihominis is more pathogenic than S.
hominis.

Diagnosis:
An intestinal infection with Sarcocystis can be
diagnosed by detection of sporocysts (14 X 9 μm), or
more rarely oocysts (approx. 20 X 13 μm) in stool
using the SAFC or the flotation method. The two
Sarcocystis species cannot be differentiated.
Treatment:
An effective therapy has not yet been
developed.
Prevention:
consists in boiling or deep-freezing (–20 C
for three days) of pork and beef.

THANK YOU
