Isosporabelli
Isospora
belli
is
an
intestinal
protozoan that causes Isosporiasis (
is a human intestinal disease caused
by this parasite.
cause
diarrhea,especiallyin
innunocomprpmised
patients,
eg, those with AIDS

• It is world wide, especially in tropical and
• subtropical area. Its life cycle parallels that of
other members of the coccidia. The organism
is acquired by fecal – oral transmission of
oocysts from either human or animal sources.
CausalAgent
The
parasite Isospora
belli infects the
the small intestine, and is the least
common of the three intestinal
coccidia
that
infect
humans
(
,
and Isospora
Lifecycle
At time of excretion, the immature
(more
rarely
two).
In
further
maturation
after
excretion,
the
sporoblast divides in two, so the oocyst
now contains two sporoblasts. The
sporoblasts secrete a cyst wall, thus
becoming sporocysts

and the sporocysts divide twice to
produce four sporozoites each.
Infection occurs by ingestion of
sporocyst-containing oocysts: the
sporocysts excyst in the small
intestine and release their
sporozoites, which invade the
epithelial cells and initiate
.
Upon rupture of the
, the
, and continue
the cycle of asexual multiplication.
develop into
merozoites.

After a minimum of one week, the
sexual stage begins with the
development of male and female
gametocytes.
the development of oocysts that are
excreted in the stool. Isospora belli
infects both humans and animals
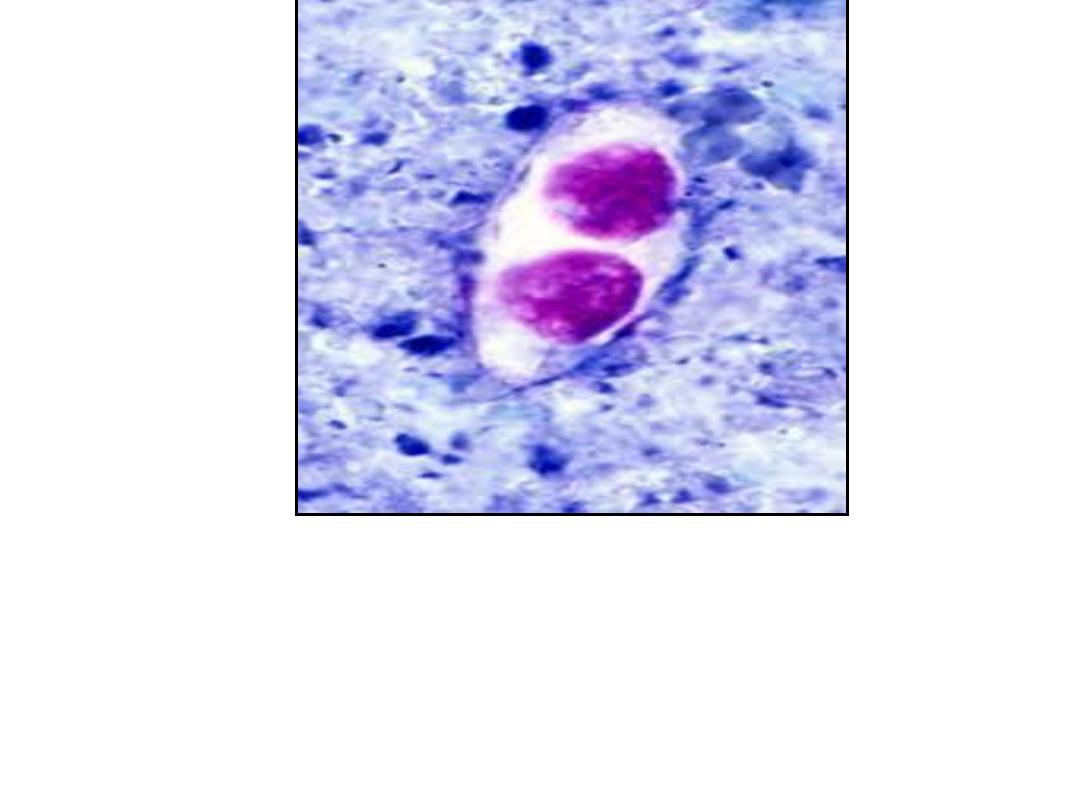
Unsporulated oocysts of Isospora
belli (human feces; acid fast
stain)
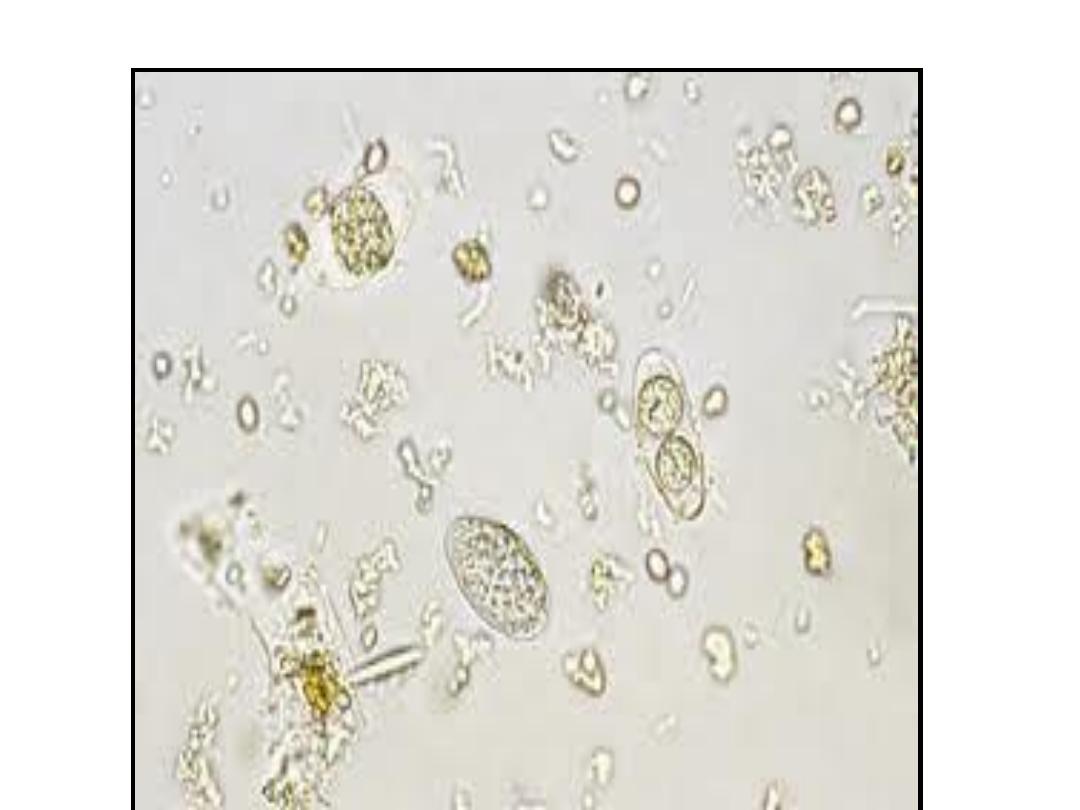
Isospora belli. Wet mount
preparation. When passed in feces
ClinicalFeatures
Infection causes acute, non-bloody
with crampy abdominal
pain, which can last for weeks and
result in
and weight
loss.

In immunodepressed patients, and
in infants and children, the diarrhea
can be severe.
present (differently from other
protozoan infections).

LaboratoryDiagnosis
Microscopic demonstration of the
large typically shaped oocysts is the
basis for diagnosis.

Because the oocysts may be passed in
small amounts and intermittently,
repeated stool examinations and
concentration procedures are
recommended.
If stool examinations are negative,
examination of
biopsy or string test (Enterotest) may
be needed. The oocysts can be
visualized on wet mounts by
microscopy with bright-field,
differential interference contrast (DIC),
and
. They can also be
stained by modified

Typicallaboratoryanalyses include:
Microscopy
Morphologic
comparison
with
otherintestinalparasites
Bench aids for Isospora

Treatment
is
the usual treatment choice. See
recommendations in The Medical
Letter (Drugs for Parasitic Infections)
for complete information.

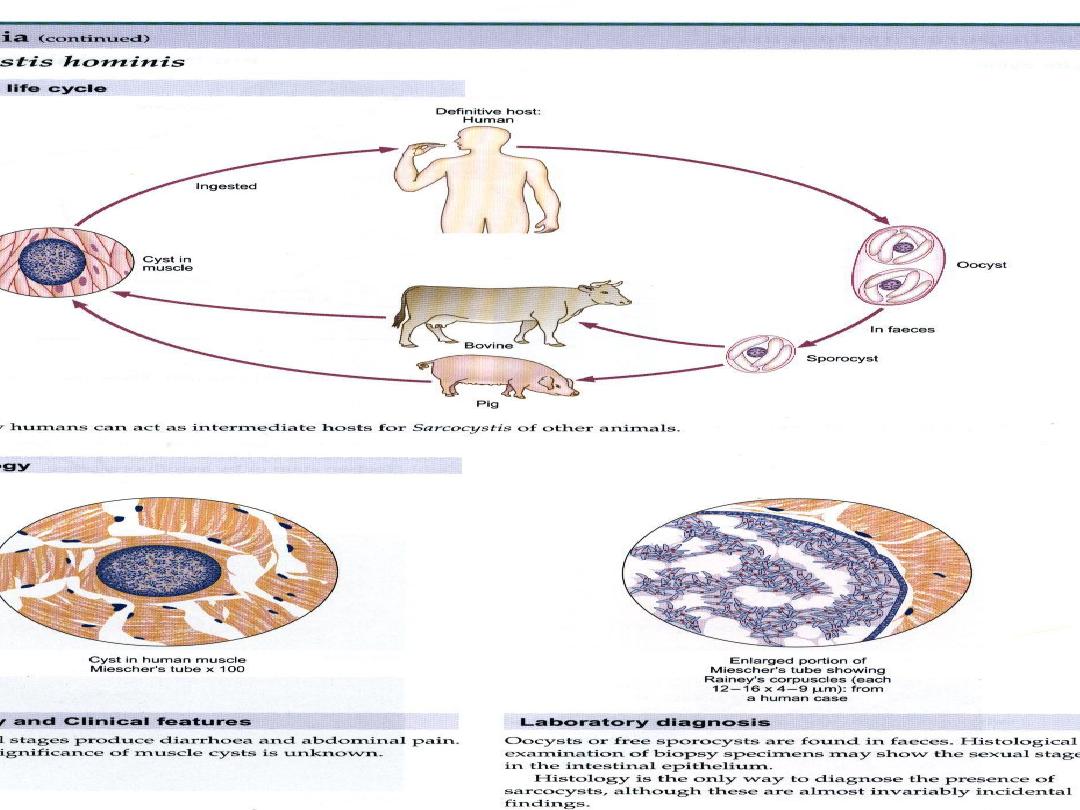
Sarcocystis:
(Sarcocysts were first isolated by
the
Swiss
scientist
Friedrich
Miescher in 1843. When examining
a
house
mouse,
Miescher
discovered white, “threadlike”
structures (sarcocysts) in its muscle
tissue.

These cysts came to be called
“Miescher’s tubules” for many
years after their discovery. Over the
next few decades, similar cysts
were found in other animals such as
pigs, but it was not until 1889 when
they were finally given a name –
Sarcocystis miescheriana .

As much was still unknown about the
organism, scientists were unsure
whether to classify the species as
protozoa or as fungi, since only the
sarcocyst stage had been identified at
that time. However, in 1967 through
the use of electron microscopes,

bradyzoites
(crescent-shaped
structures found in several protozoa)
were found in sarcocyst cultures, and
the
debate
was
resolved

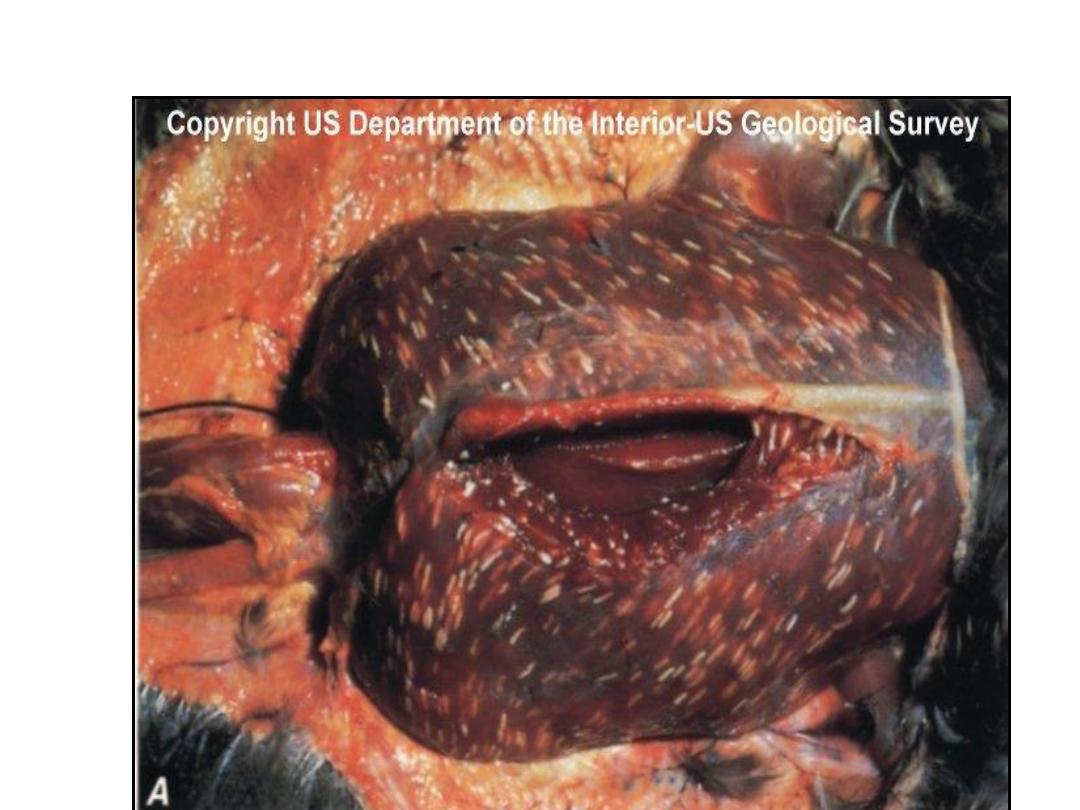
Sarcocystis species are intracellular
protozoan parasites with an
intermediate-definitive host life
cycle based on a prey-predator
relationship.

Asexual stages develop in
intermediate hosts after they ingest
the oocyst stage from definitive-
host feces and terminate with the
formation of intramuscular cysts
(sarcocysts).

Sarcocysts in meat eaten by a
definitive host initiate sexual stages
in the intestine that terminate in
oocysts excreted in the feces. Most
Sarcocystis species infect specific
hosts or closely related host
species.

For example, humans and some
primates are definitive hosts for
Sarcocystis hominis and S. suihominis
after eating raw meat from cattle and
pigs, respectively. The prevalence of
intestinal sarcocystosis in humans is
low and is only rarely associated with
illness, except in volunteers who ingest
large numbers of sarcocysts

We have 3 species that infect
man:
S
hominis
causes
coccidiosis
hominis.
S suihominis causes coccidiosis
suihominis.
S lindemanni causes sarcocytosis

Specificity for Definitive Hosts
Similar specificity relationships
have been found for definitive
hosts of some species. Dogs and
coyotes serve as definitive hosts
for S. cruzi, but humans and cats
do not .

Humans, baboons, and rhesus
monkeys can serve as definitive
hosts for S. hominis, and humans,
chimpanzees, and rhesus and
cynomolgus monkeys can serve as
definitive hosts for S. suihominis .
No other definitive hosts have
been identified for S. hominis or S.
suihominis.

The infective stage of these parasites to
man
is:
1-
bradyzoites or cystizoites within cyst or
true cyst ( sarcocyst), we have
larger number of these bradyzoites which
are fusiform, elongated& cylindrical with
a cystic wall, either smooth or striated &
divided into many compartments ( the
infective stage reach the man by eating
undercooked meats).

The infective stage of man is called
mieschers tube which are elongated
, fusiform or cylindrical bodies
forming the encapsulated cystic
intramascular of the protozoan
Sarcocystis
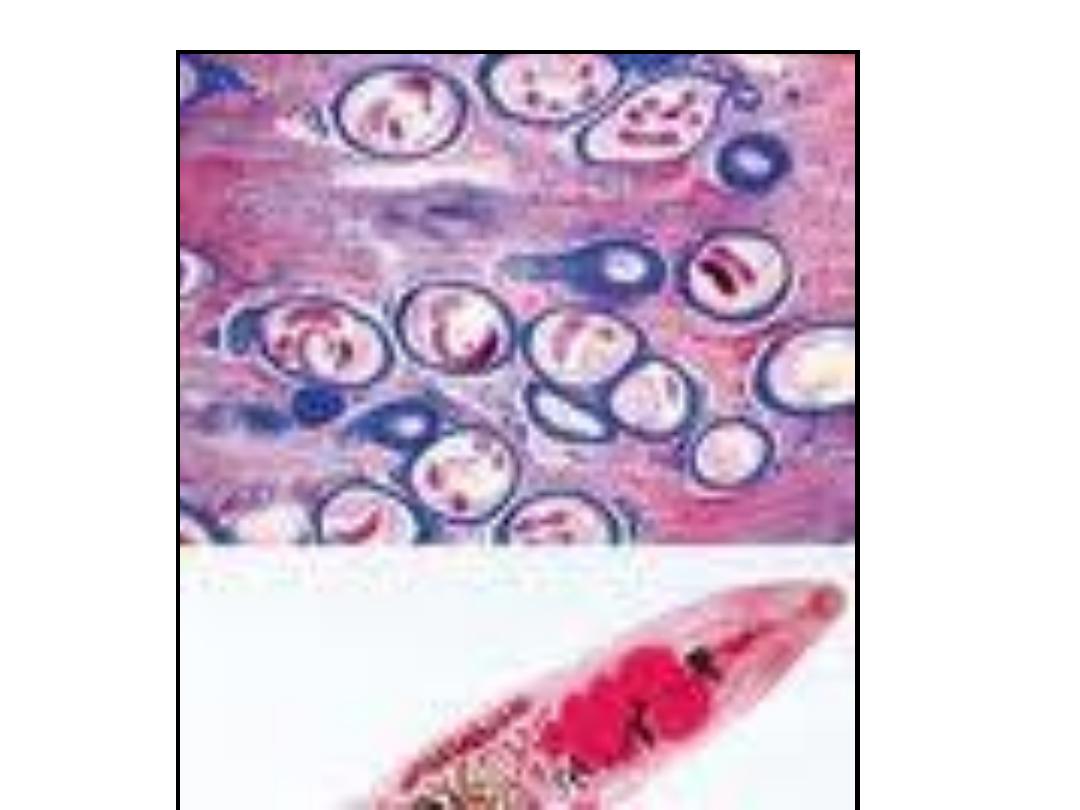

(the white threadlike sarcocysts
were once called Miescher's
tubules)

B-mature oocyst: containing the
sporocyst with
4
sporozoites.
Sometimes upon rupture we may
find feces with free sporocyst each
containing 4 sporoziotes, these are
found in stool of infected man ( as
final host).

TRANSMISSION FROM ANIMALS
TO HUMANS
Eating raw or undercooked beef and
pork containing mature oocysts of S.
hominis and S. suihominis. respectively,
has resulted in humans acquiring
intestinal sarcocystosis

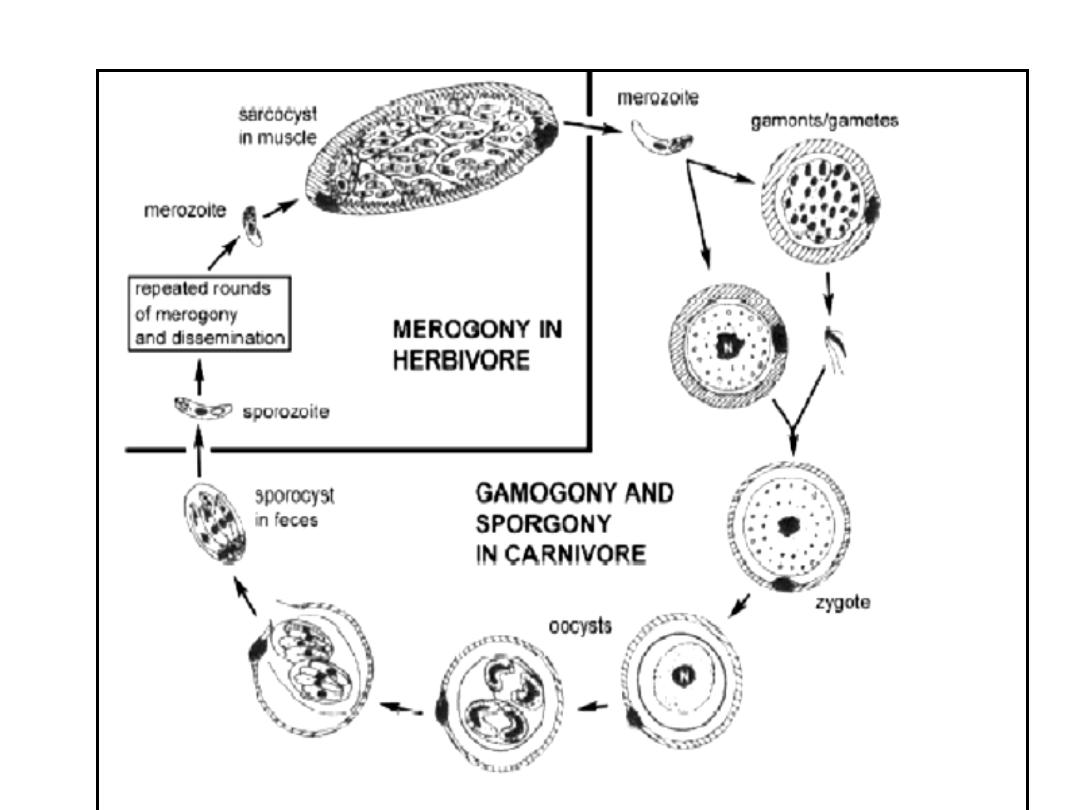
LIFECYCLES
Sarcocystis species are intracellular
protozoan parasites with a requisite
two-host life cycle based on a prey-
predator (intermediate-definitive) host
relationship.The life cycle involve 2
obligatory hosts, bearing the sexual &
asexual cycles is completed in man or
predator ( carnivorous) while the
asexual stage take place in prey (
herbivorous

or intermediate host In the small
intestine of man ( final host) the
bradyzoites are released & they
migrate to sub – epithelial lining of
small intestine & develop to male
& female gametocytes.

One male gametocytes fertilize the
female gametocyte (ovum) to form
immature
oocyst.
But
this
maturation occurs within the tissue
of intestine & pass with feces &
some of them are ruptured, so we
may see within the stool free
sporocyst or mature oocyst.

When intermediate host get these
sporocyst it infect endothelial cells
of blood vessels and schizogony (
asexual reproduction by multiple
fission, found in some protozoa
especially parasitic sporozoan),

these
schizonts
rupture
releases
merozoites infect blood vessels & go
to striated muscle & develop into
tissue cyst ( sarcocyst). But in case of
Sarcocystis lindmani is similar to this
life cycle but differ in that asexual
cycle in the muscle of man while the
sexual cycle is the unknown final host,
so the sexual cycle is a blind end.
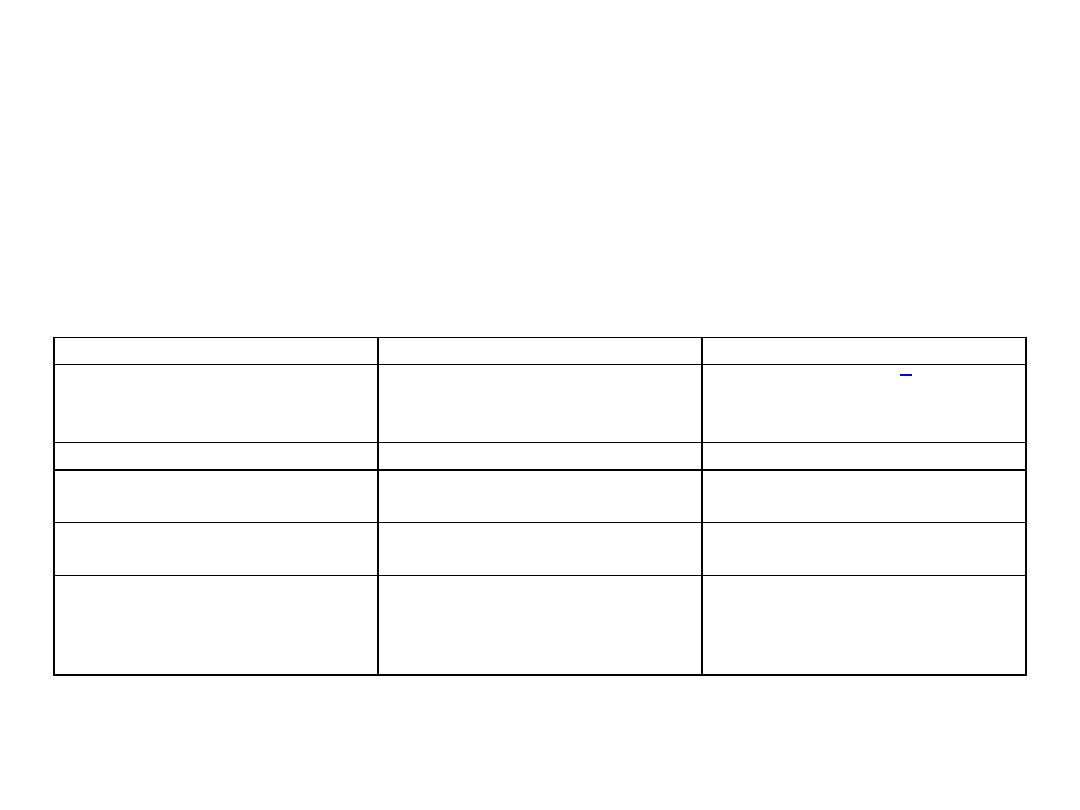
Characteristic
Muscular infection
Intestinal infection
Source of infection
Water or food contaminated with
feces from unknown carnivore or
omnivore
Raw or undercooked meat
Infective stage
Oocyst or free sporocysts
Sarcocyst containing bradyzoites
Developmental stages
Intravascular schizonts (not seen);
intramuscular sarcocysts
Sexual stages in lamina propria;
oocysts excreted in feces
Time from ingestion of infective
stage to symptoms
Weeks to months, lasting months to
years
3-6 h, lasting 36 h
TABLE 1. Symptoms of sarcocystosis are summarized in Table
Characteristic
Muscular infection
Intestinal infection
Source of infection
Water or food contaminated with
feces from unknown carnivore or
omnivore
Raw or undercooked meat
Infective stage
Oocyst or free sporocysts
Sarcocyst containing bradyzoites
Developmental stages
Intravascular schizonts (not seen);
intramuscular sarcocysts
Sexual stages in lamina propria;
oocysts excreted in feces
Time from ingestion of infective
stage to symptoms
Weeks to months, lasting months to
years
3-6 h, lasting 36 h
TABLE 1. Symptoms of sarcocystosis are summarized in Table
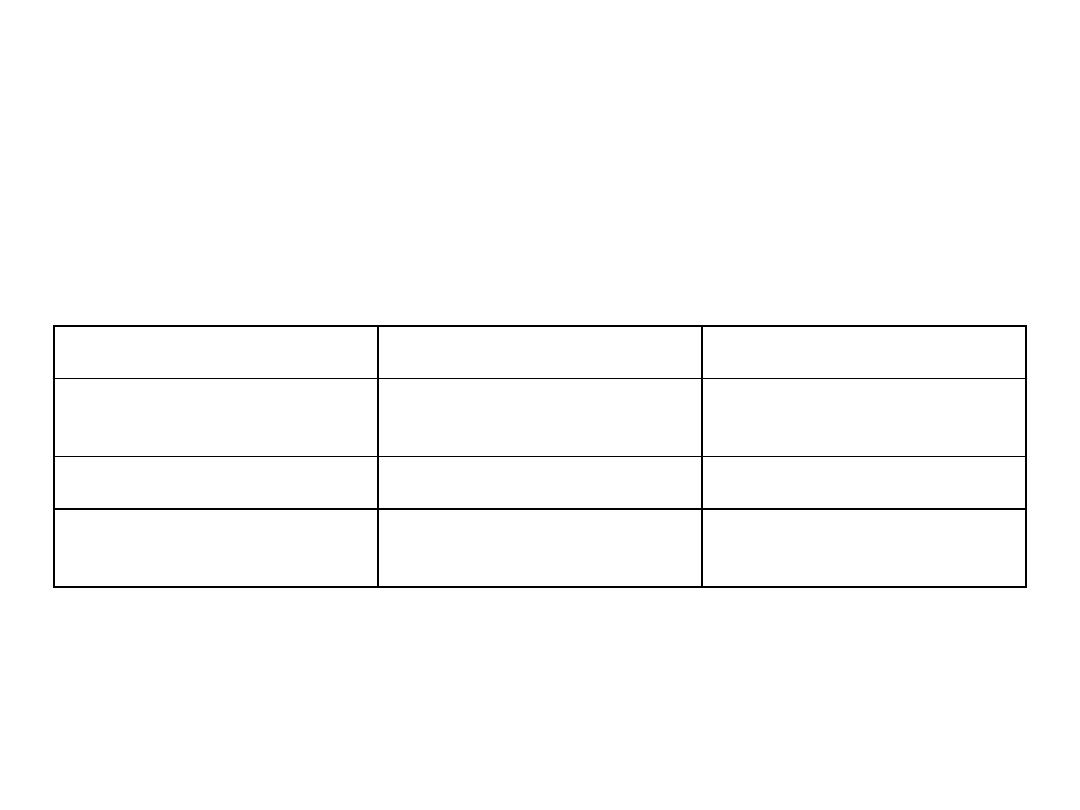
Time from ingestion of infective
stage to symptoms
Weeks to months, lasting months to
years
3-6 h, lasting 36 h
Symptoms
Musculoskeletal pain, fever, rash,
cardiomyopathy, bronchospasm,
subcutaneous swelling
Nausea, loss of appetite, vomiting,
stomach ache, bloat, diarrhea,
dyspnea, and tachycardia
Diagnosis
Biopsy specimen containing
sarcocyst; antibodies to bradyzoites
Oocysts or sporocysts in feces,
beginning 5-12 days after ingestion
Therapy (none approved)
Co-trimoxazole, furazolidone,
albendazole, anticoccidials,
pyrimethamine, anti-inflammatories
None

DIAGNOSIS
Presumptive diagnosis of human
intestinal sarcocystosis is based on
symptoms and a history of recently
having eaten raw or undercooked
meat. Definitive diagnosis,
requiring identification of
sporocysts in feces,

might require several stool
examinations beginning several
days after having eaten the meat.
Sporocysts of S. hominis are first
excreted 14 to 18 days after
ingesting beef, and those of S.
suihominis are excreted 11 to 13
days after ingesting pork

Sporocysts can be seen by bright-field
microscopy in a fecal flotation wet
mount just beneath the coverslip.
Flotation based on high-density
solutions incorporating sodium
chloride, cesium chloride, zinc sulfate,
sucrose, Percoll, Ficoll-Hypaque, and
other such density gradient media is
preferred to formalin-ethyl acetate and
other sedimentation methods.

Because sporocysts of different
species overlap in size and shape,
species cannot be distinguished
from one
another solely by
microscopy.

Treatment
There is no known prophylaxis or
therapeutic treatment for intestinal
sarcocystosis. Infections are self-
limiting, of short duration, and
often asymptomatic.

The efficacy of co-trimoxazole or
furazolidone
remains
to
be
demonstrated. For six persons in
Thailand with segmental necrotizing
enteritis associated with sexual stages
of
Sarcocystis
and
gram-positive
bacilli, surgical resection of the small
intestine was followed by antibiotic
treatment. This extremely aggressive
course of treatment has not been
applied
in
other
cases.