Sunday 10/ 5 / 2015
©Ali Kareem 2014-2015
Name
:
______________________________
Class
:
_______________________________
مكتب اشور لالستنساخ
IMMUNOSUPPRESSANTS
Lecture 11 & 12
Total lectures NO. 60 & 61
Dr. Mohammed Rashad

1
Immunosuppressants
Dr.Mohammed Rashad
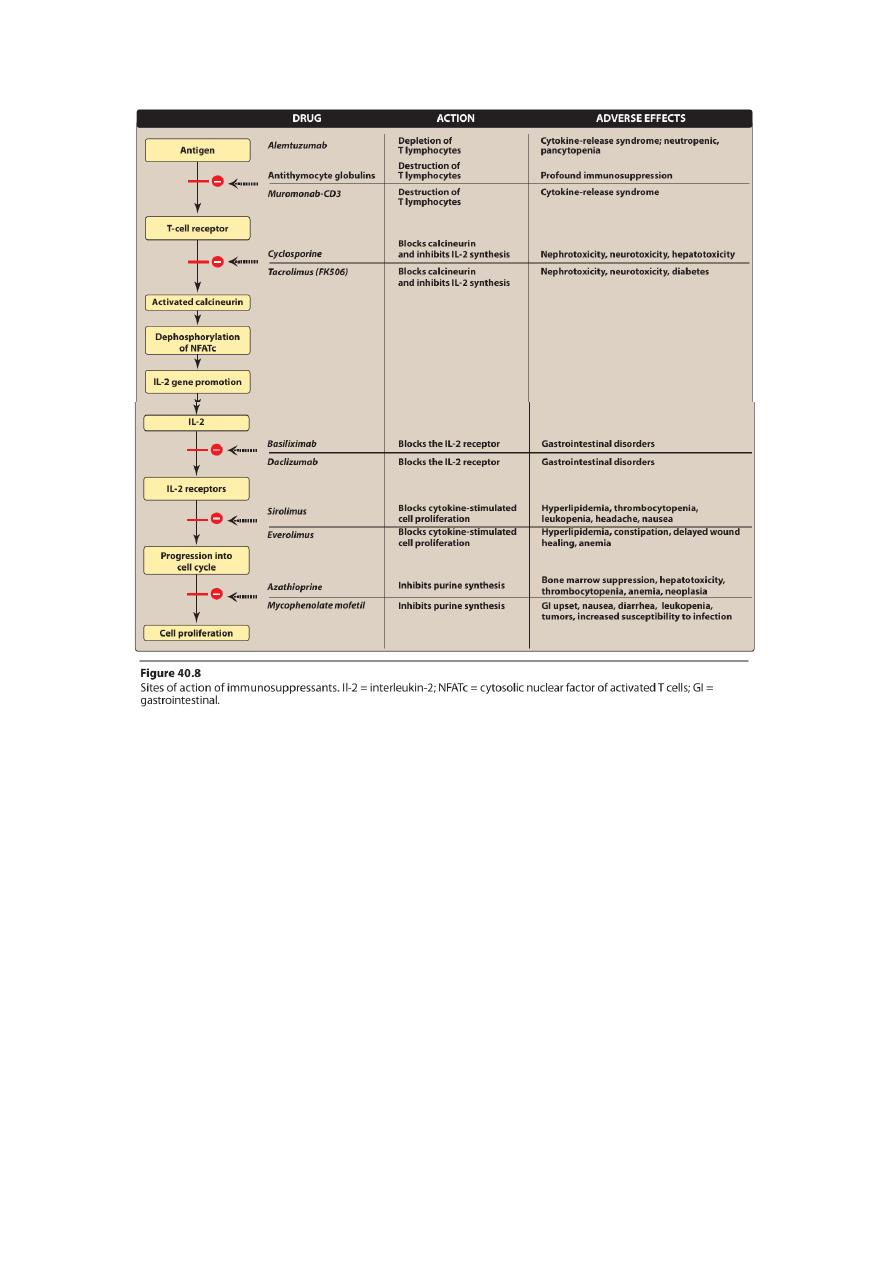
• Today, the principal approach to immunosuppressive therapy is to
alter lymphocyte function using drugs or antibodies against
immune proteins.
Immunosuppressive drugs can be categorized according to their
mechanisms of action:
• Some agents interfere with cytokine production or action
• others disrupt cell metabolism, preventing lymphocyte
proliferation
• mono- and polyclonal antibodies block T-cell surface molecules.
SELECTIVE INHIBITORS OF CYTOKINE PRODUCTION AND FUNCTION
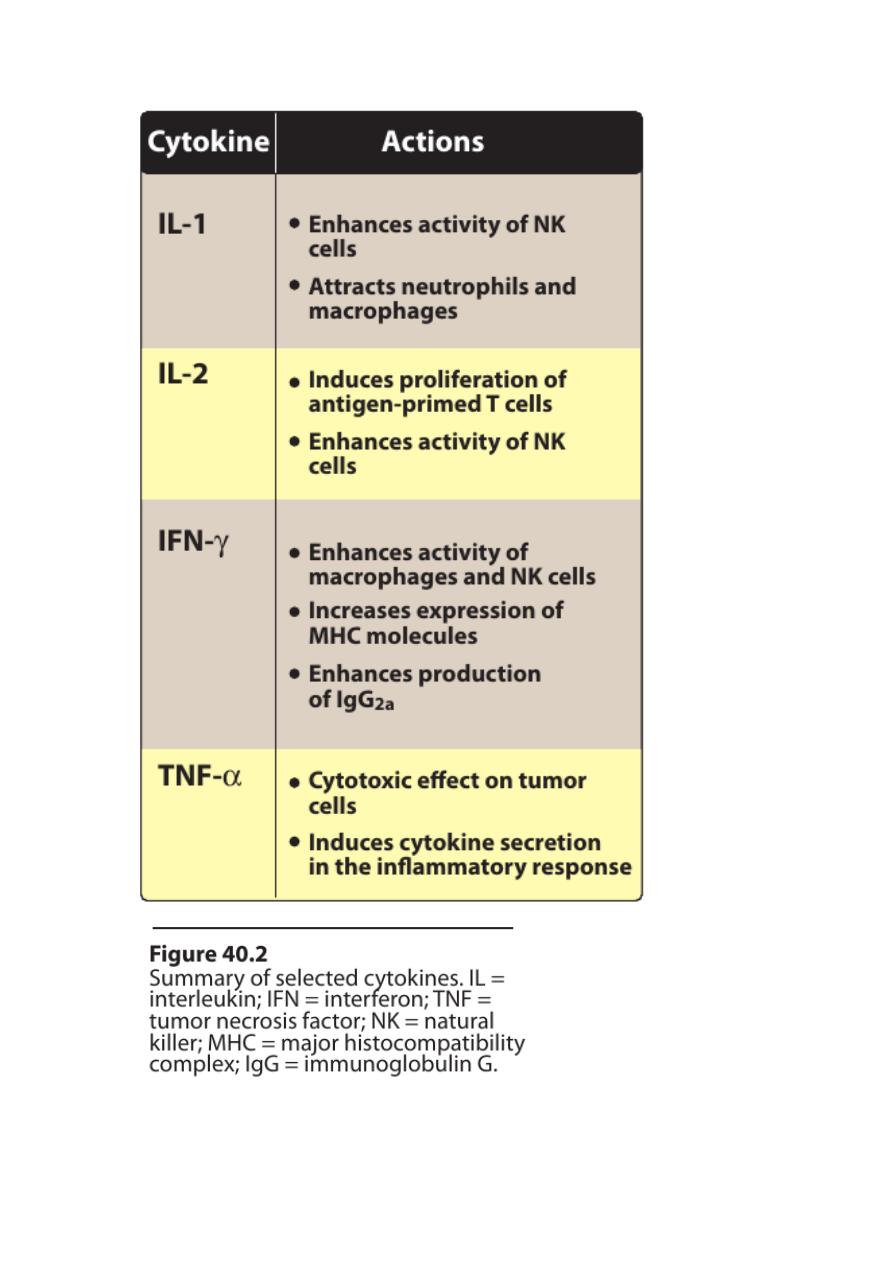
• Cytokines are soluble, antigen-nonspecific, signaling proteins that
bind to cell surface receptors on a variety of cells.
• The term cytokine includes the molecules known as interleukins
(ILs), interferons (IFNs), tumor necrosis factors (TNFs),
transforming growth factors, and colony-stimulating factors.
• These cytokines collectively activate natural killer cells,
macrophages, and cytotoxic T lymphocytes.
• Clearly, drugs that interfere with the production or activity of IL-2,
such as cyclosporine, will significantly dampen the immune
response and, thereby, decrease graft rejection.
A.Cyclosporine
• Cyclosporine is a lipophilic cyclic polypeptide.
• Cyclosporine is used to prevent rejection of kidney,l iver

2
• Cyclosporine is most effective in preventing acute rejection of
transplanted organs when combined in a double-drug or triple-
drug regimen
• Cyclosporine is an alternative to methotrexate for the treatment
of severe, active rheumatoid arthritis.It can also be used for
patients with recalcitrant psoriasis. it is also used for
xerophthalmia.
1.Mechanism of action:
• Cyclosporine binds to a cyclophilin to form a complex that binds
to calcineurin.
• The latter is responsible for dephosphorylating NFATc (cytosolic
Nuclear Factor of Activated T cells).
• NFATc cannot enter the nucleus to promote the reactions that are
required for the synthesis of a number of cytokines, including IL-2.
• The end result is a decrease in IL-2,which is the primary chemical
stimulus for increasing the number ofT lymphocytes.
2.Pharmacokinetics:
• Cyclosporine may be given either orally or by intravenous (IV)
infusion.
• About 50 percent of the drug is associated with the blood fraction.
Half of this is in the erythrocytes,and one tenth is bound to the
lymphocytes.
• Excretion of the metabolites is through the biliary route.
3.Adverse effects:
• dose dependent.
• Nephrotoxicity is the most common. Nephrotoxicity may be
irreversible in 15 percent of patients.
• Hepatotoxicity (liver function should be periodically assessed.)

3
• Infections are common (herpes group and cytomegalovirus (CMV)
are prevalent)
• Lymphoma may occur in all transplanted
• Anaphylactic reactions
• hypertension, hyperlipidemia, hyperkalemia
• tremor, hirsutism,
glucose intolerance, and gum hyperplasia.
B. Tacrolimus
• Tacrolimus is approved for the prevention of rejection of liver and
kidney transplants
• An ointment preparation has been approved for moderate to
severe atopic dermatitis
1.Mechanism of action:
• Tacrolimus exerts its immunosuppressive effect in the same
manner as cyclosporine
2.Pharmacokinetics:
• Tacrolimus may be administered orally or IV
• Absorption is decreased with high-fat or high-carbohydrate meals.
• Tacrolimusis from 10- to 100-fold more potent than cyclosporine
• Renal excretion is very low, and most of the drug is found in the
feces.
3.Adverse effects:
• Nephrotoxicity and neurotoxicity (tremor, seizures,and
hallucinations) tend to be more severe

4
• Developmentof post transplant, insulin-dependent diabetes
mellitus is a problem.
• tacrolimus does not cause hirsutism or gingival hyperplasia
• tacrolimus has a lower incidence of hypertension and
hyperlipidemia.
• Anaphylactoid reactions
C. Sirolimus
• Sirolimus is approved for use in renal transplantation, together
with cyclosporine and a corticosteroids
• Sirolimus-coated stents inserted into the cardiac vasculature
inhibit restenosis of the blood vessels.
1.Mechanism of action:
• Sirolimus binds to mTOR.
• Binding of sirolimus to mTOR blocks the progression of activated T
cells from the G1 to the S phase of the cell cycle
• sirolimus does not lower IL-2 protduction but rather inhibits the
cellular responses to IL-2.
2.Pharmacokinetics:
• The drug is available only as oral preparations
• Sirolimus has a long half-life (57 to 62 hours) and a loading dose is
recommended
• Sirolimus also increases the drug concentrations of cyclosporine
• The parent drug and its metabolites are predominantly eliminated
in feces.

5
3.Adverse effects:
• A common side effect of sirolimus is hyperlipidemia.
• The combination of cyclosporine and sirolimus is more
nephrotoxic. Although the administration of sirolimus and
tacrolimus appears to be less nephrotoxic
• Other untoward problems are headache,nausea and diarrhea,
leukopenia, and thrombocytopenia.
• Impaired wound healing
D. Everolimus
• Everolimus (another mTOR inhibitor) was recently approved for
use in:
• renal
• second-line treatmentin patients with advanced renal cell
carcinoma.
1. Mechanism of action:
• Everolimus has the same mechanism of actionas as sirolimus.
2. Pharmacokinetics:
• Everolimus is rapidly absorbed, attaining maximal concentrations
in 1 to 2 hours post dose
• It has a much shorter half-life than does sirolimus at30 ± 11 hours
and requires twice-daily dosing.
• Everolimus increases drug concentrations of cyclosporine, thereby
enhancing the nephrotoxicity.
3. Adverse effects:
• Everolimus has similar side effects to sirolimus
6
• An additional adverse effect is angioedema, which may increase
with concomitant use of angiotensin-converting enzyme
inhibitors.
• There is also an increased risk of kidney arterial and venous
thrombosis, resultingin graft loss.
IMMUNOSUPPRESSIVE ANTIMETABOLITES
Immunosuppressive antimetabolite agents are generally used in
combination with corticosteroids and the calcineurin inhibitors,
cyclosporine and tacrolimus.
A. Azathioprine
• Azathioprine was the first for use in organ transplantation.
• The immunosuppressive effects of azathioprine are due to
nucleotide analog.
• The drug has little effect on suppressing a chronic immune
response.
• Its major toxicity is bone marrow suppression.
• Concomitant use with angiotensin-converting enzyme inhibitors
or cotrimoxazole can lead to an exaggerated leukopenic response.
• Allopurinol significantly inhibits the metabolism of azathioprine.
• Nausea and vomiting are also encountered.
B. Mycophenolatemofetil
• Mycophenolatemofetil has replaced azathioprine because of its
safety and efficacy in prolonging graft survival.

7
• It has been successfully used in heart, kidney, and liver
transplants.
• Like 6-MP, it deprives the rapidly proliferating T and B cells of a
key component of nucleic acids
• The most common adverse effects include diarrhea, nausea,
vomiting,abdominal pain
• leukopenia, and anemia.
• Higher doses of (3 g/day) were associated with a higher risk of
CMV infection.
• [Note: mycophenolic acid is less mutagenic or carcinogenic than
azathioprine.]
• Concomitant administration with antacids containing magnesium
or aluminum
C. Enteric-coated mycophenolate sodium
• In an effort to minimize the GI effects associated with
mycophenolatemofetil, enteric-coated mycophenolate sodium
was developed.
• The active drug (mycophenolic acid) is contained within a delayed-
release formulation
• The new formulation was found to be equivalent to
mycophenolatemofetil in the prevention of acute rejection
episodes in kidney transplant recipients.
• However, the rate of GI adverse events was similar to that with
mycophenolatemofetil.

8
ANTIBODIES
• The use of antibodies plays a central role in prolonging allograft
survival.
• The names of monoclonal antibodies conventionally contain
“muro” if they are from a murine (mouse) source and “xi” or “zu”
if they are chimerized or humanized, respectively.
• The suffix “mab” (monoclonal antibody) identifies the category of
drug.
• The polyclonal antibodies, although relatively inexpensive to
produce, are variable and less specific, which is incontrast to
monoclonal antibodies, which are homogeneous and specific.
A. Antithymocyte globulins
• They are primarily used, together with other immunosuppressive
agents, at the time of transplantation to prevent early allograft
rejection
• they may be used to treat severe rejection episodes or
corticosteroid-resistant acute rejection.
• The antibody-bound cells are phagocytosed in the liver and
spleen, resulting in lymphopenia and impaired T-cell responses.
• The antibodies are slowly infused intravenously, and their half-life
extends from 3 to 9 days.
• Because the humoral antibody mechanism remains active,
antibodies can be formed against these foreign proteins. [Note:
This is less of a problem with the humanized antibodies.]
• Other adverse effects include chills and fever, leukopenia and
thrombocytopenia, infections due to CMV or other viruses,and
skin rashes.

9
B. Muromonab-CD3 (OKT3)
• Muromonab-CD3 is a murine monoclonal antibody that is
synthesized by hybridoma technology and directed against the
glycoprotein CD3 antigen of human T cells.
Muromonab-CD3 is used for:
• treatment of acute rejection of renal allografts
• corticosteroid-resistant acute allograft rejection in cardiac and
hepatic transplant patients.
• It is also used to deplete T cells from donor bone marrow prior to
transplantation.
Adverse effects:
• Anaphylactoid reactions may occur.
• Cytokine release syndrome may follow the first dose. (The
symptoms can range from a mild, flu-like illness to a life-
threatening, shock-like reaction.)
• High fever is common.
• Central nervous system effects, such as seizures, encephalopathy,
cerebral edema, aseptic meningitis, and headache.
• Infections can increase (some due to CMV).
• Muromonab-CD3 is contraindicated
• in patients with a history of seizures
• in those with heart failure
• in pregnant women, and in those who are breast-feeding.

11
C. IL-2-receptor antagonists
• Basiliximabis said to be “chimerized” because it consists of 25
percent murine and 75 percent human protein.
• Daclizumab is 90 percent human protein, and is designated
“humanized.”
• Both agents have been approved for prophylaxis of acute
rejection in renal transplantation
• They are not used for the treatment of ongoing rejection
1.Mechanism of action:
• Both compounds are anti-CD25 antibodies and bind to the α chain
of the IL-2 receptor on activated T cells.They thus interfere with
the proliferation of these cells.
• Basiliximabis about 10-fold more potent than daclizumab as a
blocker of IL-2 stimulated T-cell replication.
• Blockade of this receptor foils the ability of any antigenic stimulus
to activate the T-cell response system.
2.Pharmacokinetics:
• Both antibodies are given IV.
• The serum half-life of daclizumab is about 20 days, and the
blockade of the receptoris 120 days.
• Five doses of daclizumab are usually administered, the first at 24
hours before transplantation, and the next four doses at14-day
intervals.
• The serum half-life of basiliximab is about 7 days.Usually, two
doses of this drug are administered, the first at 2 hours prior to
transplantation, and the second at 4 days after the surgery.
3.Adverse effects:
• Both daclizumab and basiliximab are well tolerated.
11
• Their major toxicity is GI.
• No clinically relevant antibodies to the drugs have been detected,
and malignancy does not appear to be a problem.
D. Alemtuzumab
• Alemtuzumab, a humanized monoclonal antibody, exerts its
effects by causing profound depletion of T cells from the
peripheral circulation.
• This effect may last for up to 1 year.
• Alemtuzumab is currently approved for the treatment of
refractory B-cell chronic lymphocytic leukemia.
• Although it is not currently approved for use in organ
transplantation, it is being used incombination with sirolimus and
low-dose calcineurin inhibitors in corticosteroid-avoidance
• protocols at many transplant centers.
Side effects include:
• first-dose cytokine-release syndrome,requiring premedication
with acetaminophen, diphenhydramine, and corticosteroids
• neutropenia, anemia, and, rarely, pancytopenia.
• B-cell mediated rejection and development of autoimmune
disorders in a small number of patients and, thus, this agent
should be used with caution.
CORTICOSTEROIDS
• The corticosteroids were the first pharmacologic agents to be
used as immunosuppressives both in transplantation and in
various autoimmune disorders.

12
• They are still one of the mainstays for attenuating rejection
episodes.
• For transplantation, the most common agents are prednisone or
methylprednisolone
• prednisone or prednisolone are used for autoimmune conditions.
• The steroids are used to suppress acute rejection of solid organ
allografts and in chronic graft-versus-host disease.
• they are effective against autoimmune conditions, including
refractory rheumatoid arthritis, systemic lupus erythematosus,
temporal arthritis, and asthma.
• The exact mechanism responsible for the immunosuppressive
action of the corticosteroidsis unclear.
• The steroids are able to rapidly reduce lymphocyte populations.
• They bind to the glucocorticoid receptor. The complex passes into
the nucleus and regulates the translation of DNA.
• adverse effects, for example, are diabetogenic and can cause
hypercholesterolemia, cataracts, osteoporosis, and hypertension
with prolonged use.
13
14
