@Ali Kareem 2014-2015
Name
:
______________________________
Class
:
_______________________________
مكتب اشور لالستنساخ
General Pharmacology
Lectures 1 – 2 – 3
Total lectures NO. 1
Dr. Ahmad Al-Zohyri
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
References:
1. Basic & clinical pharmacology by Katzung
2. Lippincott’s illustrated reviews by Finkel, Cubeddu & Clark
3. Clinical pharmacology by Laurence
Pharmacology:
Is the science that studies the manner in which the function of the living system is
affected by a chemical agent (drug).
It's divided into two major categories:
1. MEDICAL PHARMACOLOGY:
Deals with materials used to "prevent, diagnose and treat" diseases.
2. TOXICOLOGY:
Deals with undesirable effects of chemicals in biological systems.
NATURE OF DRUGS:
A drug is any small molecule that when introduced into the body ALTERS the
body's functions by interaction at the molecular level (sub-cellular level).
Note: ALTERS means: causes a change in a certain parameter.
CHARACTERISTICS OF DRUGS:
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
1. MOLECULAR SIZE:
(Either small as carbon monoxide or large as enzymes).
The majority of drugs have molecular weight range of (100-1000) which enables
them for convenient administration and efficient absorption and distribution.
2. MOLECULAR SHAPE:
Drugs vary in shape and that's of extreme importance because the majority of drugs
interact with specific sites within the target tissue called "RECEPTOTS".
The shape of the receptor site determines what kind of drug molecule may interact
with it.
Also the shape of the drug molecule must be "Complementary" to the shape of the
receptor site to produce an optimal fit.
As the optimal fit increased, the response to the drug increased too.
3. CHEMICAL NATURE:
Drugs also differ in their chemical nature; they are either highly active or inert
substances. Many drugs are either weak acids or weak bases.
DRUG-BODY INTERACTION
1. PHARMACO-DYNAMIC INTERACTION:
Means the effect of drug(s) on the body.
2. PHARMACO-KINETIC INTERACTION:

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
Means the way the body handle the drug(s) i.e. Absorption Distribution
Metabolism Elimination (Excretion) ... (ADME)
PHARMACO-DYNAMIC INTRACTIONS:
Mechanisms of drug action:
1. ON THE CELL MEMBRANE:
Mechanisms:
A- by acting on specific receptors:
For example: agonist and antagonist on adrenoceptors.
Histamine Receptors.
ACH Receptors.
B- by interference with selective passage of ions across membrane:
For example: Ca
+2
entry (channel blockers).
C- by inhibition of membrane bound enzymes or pumps:
For example: Membrane bound ATPase is inhibited by "Cardiac Glycoside".
Amine Pump (in nerve cells) is inhibited by "Tricyclic Anti-depressant".
For example: membrane bound ATPase is inhibited by “Cardiac Glycoside”.
Amine Pump (in nerve cells) is inhibited by “tricyclic Anti-depressant”.
D- physiochemical interaction:

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
for example :General and Local Anesthetics and Alcohol, they appear to act on
lipid constituents of the membrane of the nerve cells.
2. ON METABOIC PROGRESS (WITHIN THE CELL CYTOPLASM):
A- Enzyme Inhibition:
for example: (MAO "Mono Amino Oxidase") is inhibited by "Phenelizine"
(Cholinesterase) is inhibited by "Pyridostiqmine"
(Xanthine Oxidase) is inhibited by "Allopurinol'"
B- inhibition of transport process that carry substances across cells:
For example: Anion Transport is inhibited in the renal tubules by "Probenecid "
and this may cause:
*A delay in "Penicillin "excretion
*enhancing the elimination of Urates by inhibiting reabsorption.
C- Incorporation into larger molecules:
For example: 5-flurouracil (which is an anti-cancer) into mRNA in place of amino
acid Uracil.
I.e. the drug gets into the structure of the large molecules by taking the place of
another group e.g. amino acid.
D- in case of antimicrobial agents, they act by altering the metabolic processes
unique to the microorganism such as "Penicillin" which interferes with the
bacterial cell wall formation (inhibits the wall formation).
** Affects the bacteria only not the host

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
3. OUTSIDE THE CELL:
A- Direct Chemical Interaction as in case of "Antacids" or "Acid Neutralizers"
Happens in the stomach cavity itself not in the cells...
B- Osmosis as in case of "Purgatives" such as MgS04 and "Diuretics" such
"Manitol".
RECEPTOR:
Is the component of α cell or α microorganism that interacts with α drug and
initiates the chain of biochemical events leading to the drug's observed effect.
Most of the receptors are proteins and when an agonist binds to them these
proteins undergo alternation in conformation which induces changes in systems
within the cell leading to α response.
DOWN-REGULATION:
When the tissues are continuously exposed to an agonist, the number of receptors
decreases, this phenomena is called "Down-Regulation" a causes of
"Tachyphylaxis".
Tachyphylaxis: loss of efficacy with frequently repeated doses of a certain drug
(only agonist).
UP-REGULATION:
Prolonged contact of receptors with antagonists leads to the formation of new
receptors.
For example: {up-regulation} following abrupt withdrawal of β-adrenoceptors
blockers causes Angina Pectoris worsening.

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
RELATION BETWEEN RECEPTORS AND CLINICAL USAE OF
DRUGS:
1)) Receptors determine the "Qualitative" relation between dose or (concentration)
of a drug and its pharmacological effect.
I.e. the receptors' affinity for binding determines the concentration of the drug
required while the total number of receptors limits the maximum effect a drug
may produce.
2)) Receptors are responsible for "Selectivity" of a drug action (Good Drugs are
the selective ones i.e. less side effects).
The Molecular SIZE, SHAPE and ELECTRICAL CHARGE of the drug will bind
to a particular receptor among the huge number of chemically different binding
sites available in a cell, an animal or a patient.
(i.e. .the molecular SIZEJ the SHAPE and the CHARGE determines the
"Selectivity"}.
3)) Receptors mediate the action of pharmacological antagonism; it means that it
prevents the action of an agonist.
The effect of a pure antagonist on a cell or in a patient depends entirely upon
preventing the action of agonist molecule from binding to the receptor.
MAJOR RECEPTOR FAMIUES:
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
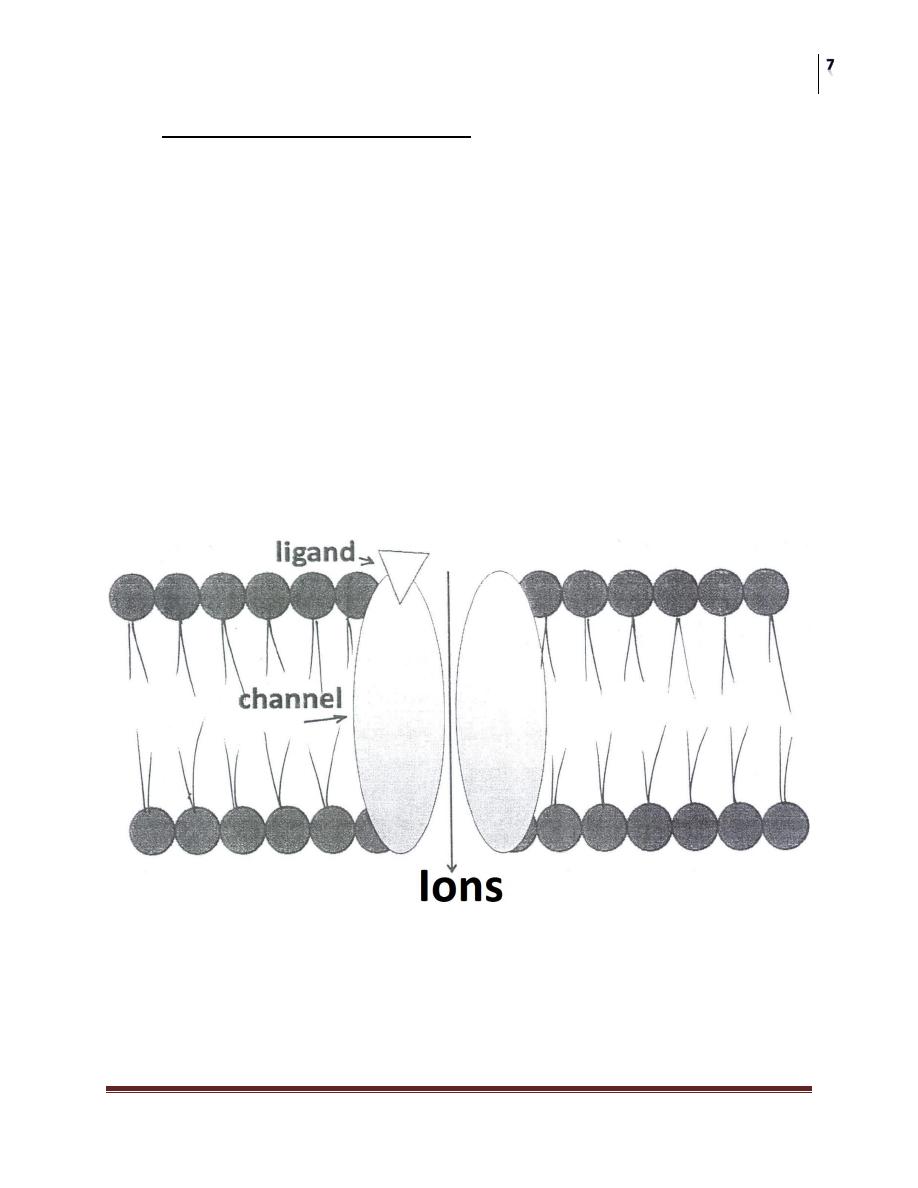
A- LIGAND-GATED ION CHANNEL:
Are responsible for regulation of the flow of ions across cell membrane.
The activity of these channels is regulated by the binding of a ligand to the
channel.
The Response is very rapid (few milliseconds).
For example: Nicotinic receptors and aminobuteric acid (GABA) receptors.
Stimulation of the nicotinic receptors by Acetylcholine results in Na
+
influx
generation an action potential & activation & contraction of skeletal muscle cells.
Benzodiazepines enhances the stimulation of GABA receptors, this will result in
increased CI
-
influx & hyper-polarization of the receptive cell.
Change in the membrane potential leading to an intra-cellular effect
Continuation of:

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
Major Receptor families:
The Figure Below shows the four major families:
A) Ligand-gated ion channel
B) G protein-coupled receptor
C) Enzyme-linked receptors
D) Intracellular receptors

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
B- G PROTElN-COUPLED RECEPTORS:
These are comprised of a single peptide that has seven membrane-spanning
regions.
• These receptors are linked to a G Protein (Gs and others) having three subunits,
alpha (α) subunit (binds guanosine triphosphate GTP) and a beta-gamma (βY)
subunit.
• Binding of appropriate ligand to extracellular region of the receptor activates the
G Protein, so that GTP replaces guanosine diphosphate GDP on the alpha subunit.
• Dissociation of G Protein occurs, and both the alpha-GTP subunit and the βY
subunit interact with other cellular effectors (an enzyme or an ion channel).
• Effectors then change the concentration of the 2
nd
messenger that are responsible
for further actions within the cell.
• Stimulation of these receptors results in responses last several "seconds to
minutes".
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
SECOND MESSENGERS:
Are essential in conducting and amplifying signals coming from G Protein-
Coupled receptors.
A common pathway turned on by Gs and other types of G Protein, is the
activation of adenylyl Cyclase by α-GTP subunit, which results in the
production of cAMP a second messenger that regulates protein
phosphorylation.
G Protein also activates phospholipase C (responsible for the generation of
two other 2
nd
messengers, "inositol-1,4,5 triphosphate" and
"diacylglycerol").
These effectors are responsible for the regulation of intracellular free
Calcium concentration and other proteins as well.
This family of receptors transduces signals derived from: Odor, Light,
Noradrenaline, dopamine, 5-HT and Acetylcholine
C- ENZYME LINKED RECEPTORS:
These receptors have cytosolic enzyme activity as an integral component of their
structure or function.
Binding of a ligand to an extracellular domain activates or inhibits this
cytosolic enzyme activity.
Duration of responses to stimulation of these receptors is in order of
"minutes to hours".
Examples of these receptors are the Insulin receptors and others having a
Tyrosine Kinase activity as part of their structure.
When the ligand binds to the receptor subunit, the receptor undergoes
conformational changes (converting from its inactive form into an active
Kinase form).
The activated receptor autophosphorylates " = phosphorylates itself" and
phosphorylates Tyrosine residues on specific proteins.

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
The addition of a phosphate group can modify the 3D structure of the target
protein, by acting as molecular switch.
Example: when insulin binds to two of its receptor subunits, their intrinsic
tyrosine kinase activity causes autophosphorylation of the receptor itself
in turn the phosphorylated receptor phosphorylates target molecules (insulin
receptor substrate peptides) that activates other important cellular signals {lP
3
}
and the Mitogen-activated protein kinase system leading to multiplication of the
initial signal.
SPARE RECEPTORS:
Some receptor types characterized by their ability to amplify signal duration and
intensity (G Protein Linked Receptors).
Two phenomena account for the amplification of the ligand-receptor signal:
1- A single ligand-receptor complex can interact with many G Protein, thereby
multiplying the original signal many folds.
2- The activated G Proteins persist for longer duration than the original ligand-
receptor complex.
Because of this amplification, only a fraction of the total receptors for a specific
ligand may need to be occupied to elicit a maximum response. (99% of insulin
receptors are spare receptors).

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
DESENSITIZATION OF RECEPTORS:
(de-sensitize-ation means make
something less sensitive)
Repeated or continuous administration of an agonist (or an antagonist) may
lead to changes in the responsiveness of the receptor.
Repeated administration causes "tachyphylaxis", receptors are still present
but unresponsive to the ligand.
Also occur when receptors are "down-regulated" (due to molecular changes
in the receptors, endocytosis then sequestered from further agonist
interaction).
Receptors may be recycled to surface, restoring sensitivity or may be further
processed and degraded, decreasing the total number of receptors available.
Some receptors require rest period after stimulation before they can be
activated again. During this recovery phase they are said to be "Refractory"
or" Unresponsive".
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
DRUG RECEPTORS BINDING FORCES:
They're either:
1- Weak forces like a) H
+
bonds, b) Van Der Wall and, c) electrostatic bonds.
These bindings are REVERSIBLE.
2- Strong forces: or Covalent bonds. These bonds are IRREVERSIBLE.
THEORIES OF DRUG-RECEPTOR INTERACTIONS:
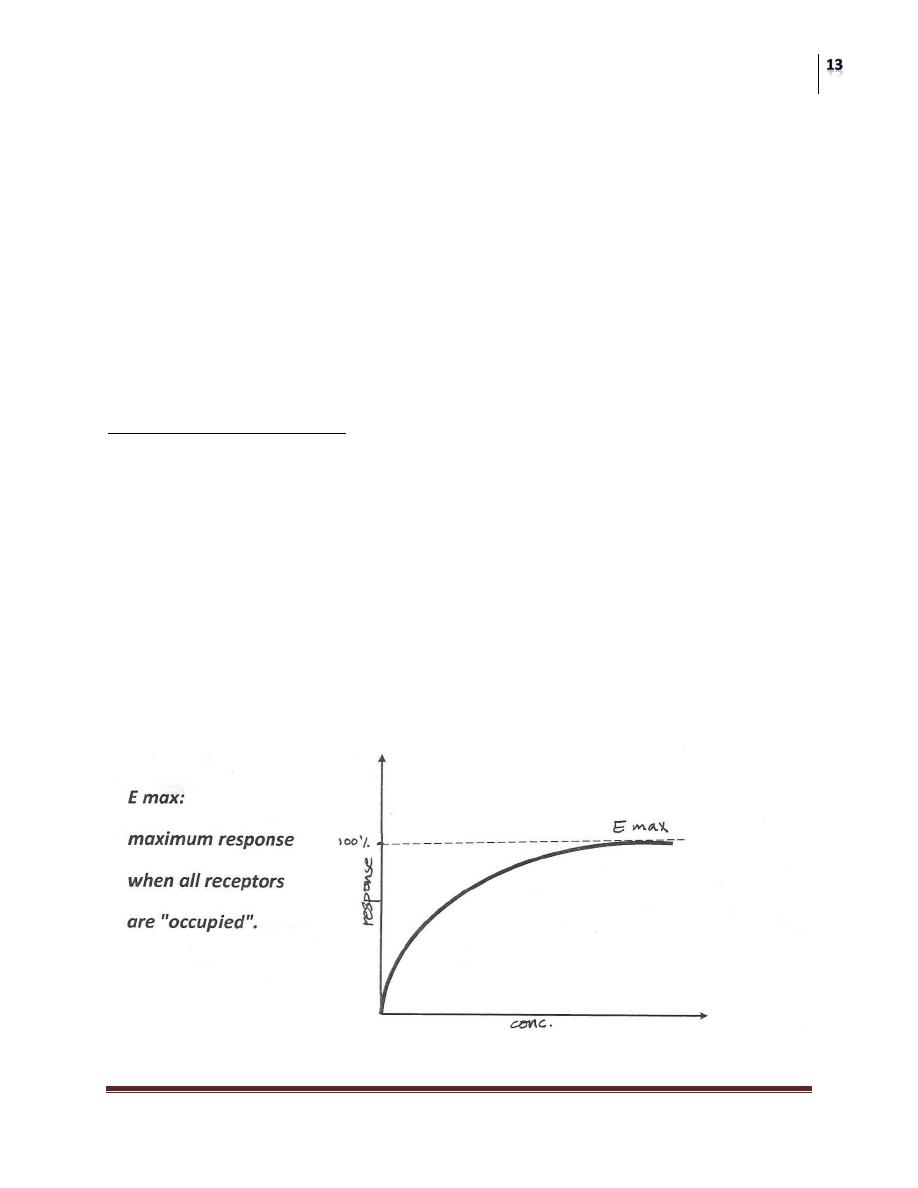
1) OCCUPATION THEORY:
"RESPONSE TO AN AGONIST IS A FUNCTION OF THE NUMBER OF
RECEPTORS OCCUPIED BY THAT AGONIST"
This means:
This response will increase as the concentration increases. This is true until all
receptors are occupied
So further increase in the dose (concentration) of the drug causes an increase in
response till reaching maximum response after that there is no further increase in
response because no more receptors left available for binding with the drug.
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
2) RATE THEORY:
"RESPONSE TO AN AGONIST IS PROPORTIONAL TO THE RATE OF
ASSOCATION OF AGONIST WITH RECEPTORS"
This means that the more the dose the more the rate of association and the more the
response until it reaches the maximum response
Note:
Agonist drugs have "fast" rates of association and dissociation with receptors,
while antagonists have "low" rate of both and accordingly antagonists have small
or no effect.

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
Dose-response relationships
AGONIST DRUG:
"An agent that can bind to a receptor producing a biological response".
The magnitude of the drug effect depends on the drug's concentration at the
receptor site (determined by the dose of drug administrated and by factors
characterized of the drug kinetic profile. i.e. the rate of absorption,
distribution and metabolism.
Characteristics:
1) A drug that interacts with a receptor.
2) Causes a response.
3) It has affinity for the receptor.
4) It has efficacy (intrinsic activity).
5) It has "fast" rate of association and dissociation from the receptor.
Agonist drugs are known to activate receptors because they resemble the
natural transmitters or hormones.
Their importance depends on their capacity to resist degradation inside the
body and to act for longer than the natural substances they mimic. For
example: Bronchodilation produced by "salbutamol" lasts longer than that
induced by "adrenaline".
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
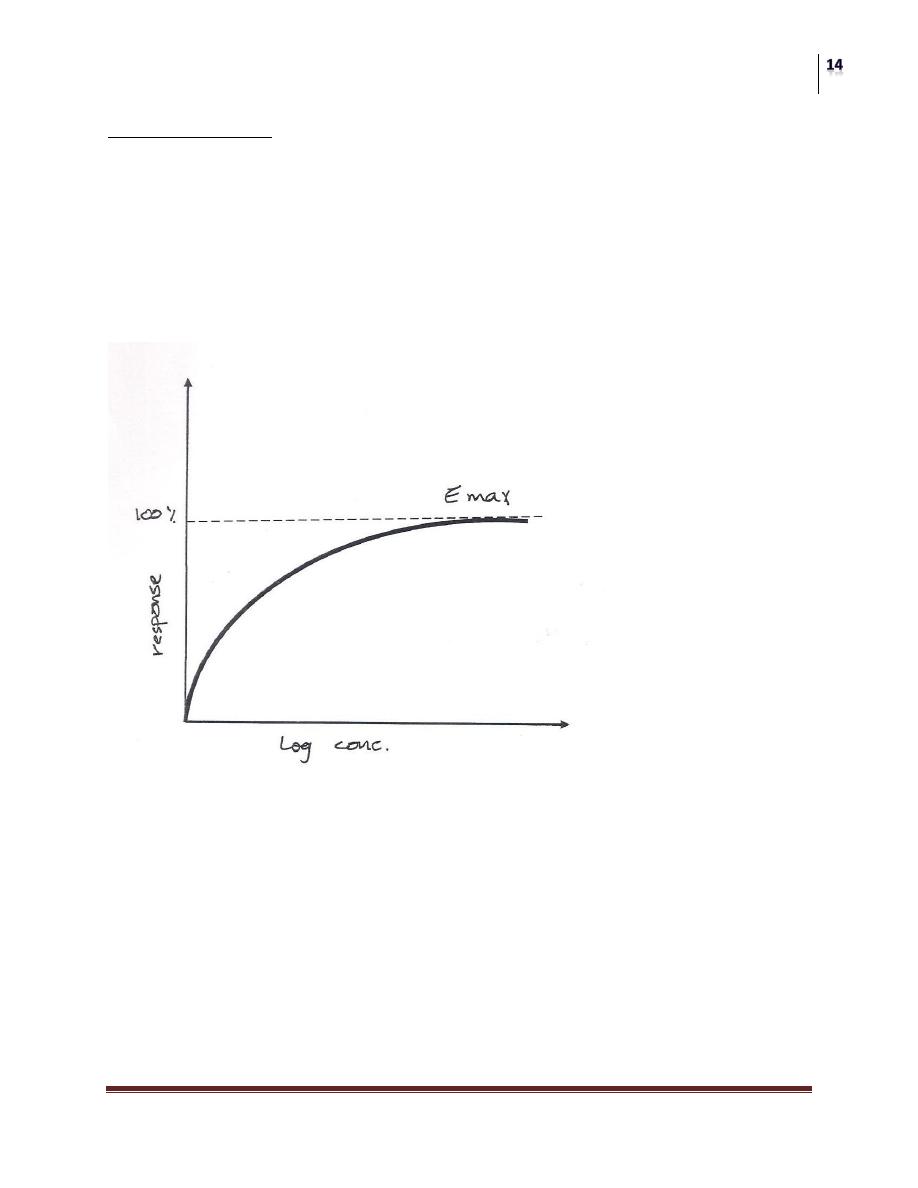
GRADED DOSE RESPONSE RELATION:
As the concentration of a drug increased, the magnitude of its pharmalogical effect
also increases. The relationship between dose and response is a continuous one:
[drug] + [receptor] [drug-receptor complex]
The response is a graded effect (continuous and gradual).
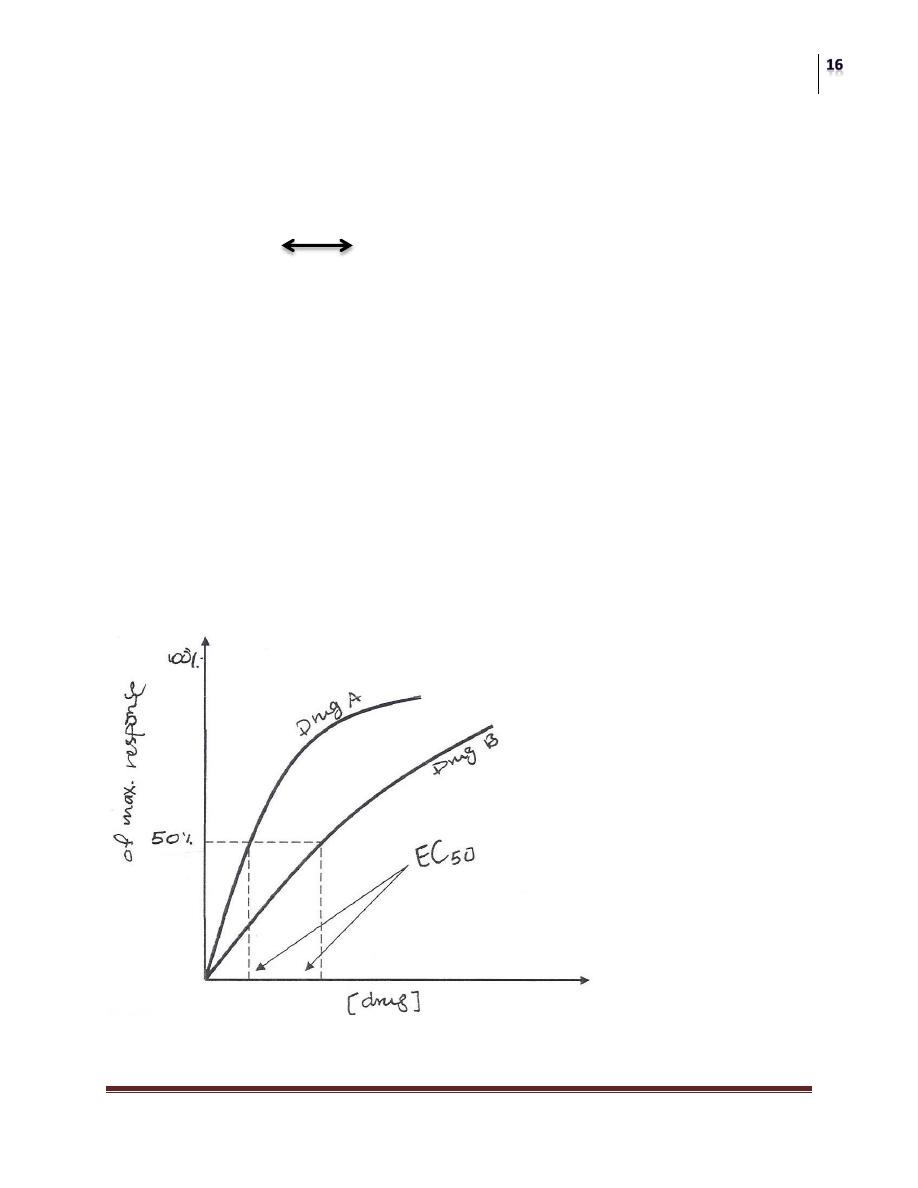
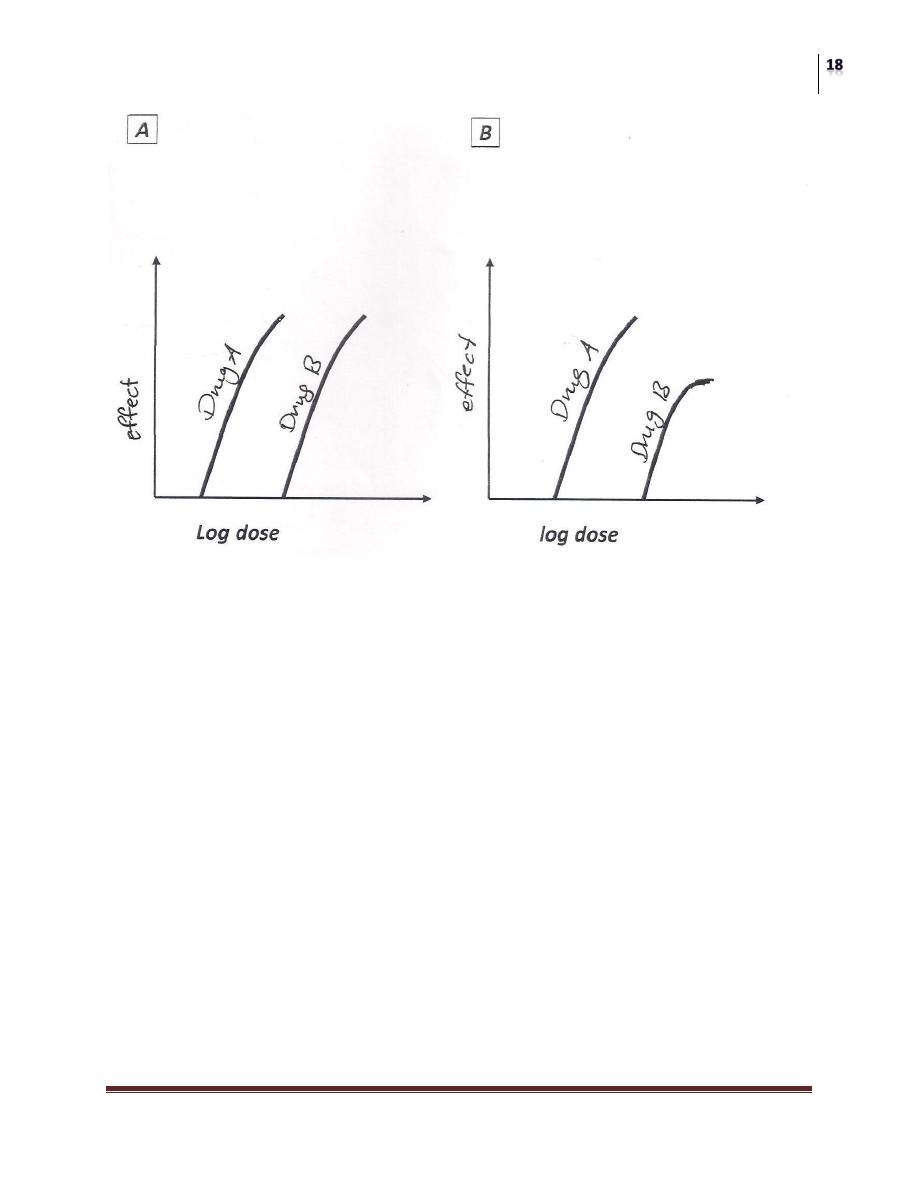
POTENCY:
A measure of the amount of drug necessary to produce an effect of a given
magnitude.
The concentration producing an effect that is 50% of the maximum is used
to determine potency; it commonly designated as the "EC
50
" as the diagram
below shows;

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
The EC
50
is the concentration of the drug that produces a response equal to
50% of the maximum response.
The EC
50
for drugs A and B are indicated (Drug A is more potent than Drug
B) because less dose of drug A is needed to obtain 50% effect.
AFFINITY AND INTRINSIC ACTIVITY (EFFICACY):
The magnitude of the response is the function of number of receptors occupied.
AFFINITY
"Is the tendency of a drug to form a combination with the receptor".
The response is not only the function of concentration of drug-receptor complex
but it also depends on the intrinsic activity or "efficacy".
EFFICACY
"Is the capacity to stimulate for a given receptor occupancy”
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
Log dose-response curves showing the difference between potency and efficacy.
Panel A: drug A is much more potent than drug B but both have the same
maximum response.
Panel B: drug A is not only more potent but also has a greater efficacy (higher
Peak Effect) than drug B.

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
ANTAGONISTS:
"Are drugs that decrease the activity of another drug or endogenous ligand".
Characteristics:
1) A compound or a drug that interacts with receptors preventing the action of
agonist.
2) Produces very small or no response.
3) It has affinity for receptors.
4) It has NO efficacy.
5) Slow rate of association and dissociation
Antagonists are similar to some extent to the natural agonists to be
recognized by receptors and to occupy these receptors without activating
them, thereby preventing (blocking) the natural agonist from exerting its
effect.
PARTIAL AGONIST:
1) Agents (drugs) act on the same receptors as powerful agonists.
2) Do NOT produce the same maximum effect regardless of their concentration.
3) Have HIGH affinity.
4) Have LOW efficacy.
Some drugs in addition to blocking access of the natural agonist to receptors
are capable of low degree of activation. i.e. (have the action of both agonist
and antagonist).
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
TYPES OF DRUG ANTAGONISM
:
1) PHARMACOLOGICAL ANTAGONISM:
Antagonist competes with Agonist for the same receptor site so reducing or
preventing the action of the Agonist.
Pharmacological Antagonism is further subdivided into:
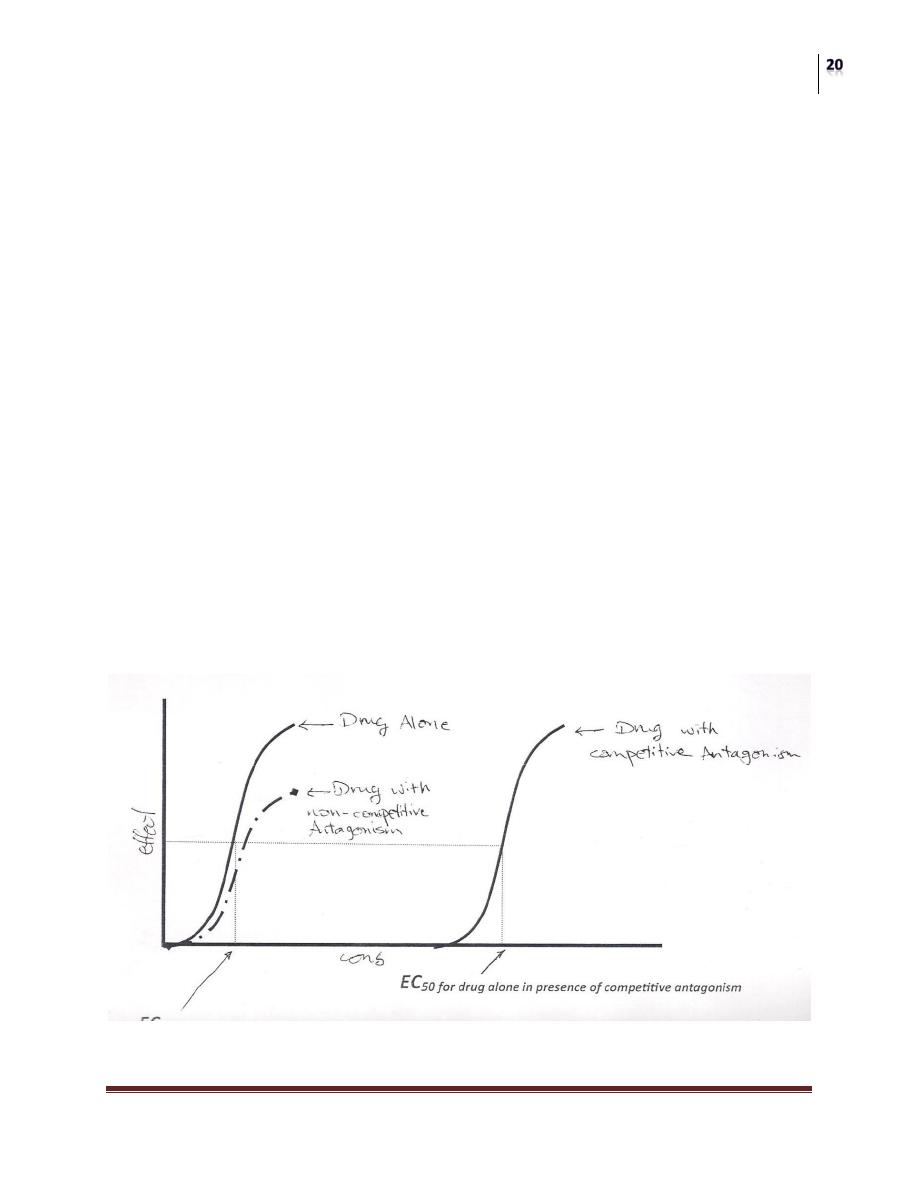
A) COMPETITIVE REYERSIBLE ANTAGONISM:
In this case the antagonist combines "reversibly" with the receptor so this
combination can be overcomed by increasing the concentration of the agonist.
B) NON-COMPETITIVE IRRIVERSIBLE ANTAGONISM:
Antagonist combines "irreversibly" with the receptor by a covalent bond so
increasing the dose of agonist will (never) overcome the inhibition

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
2) CHEMICAL ANTAGONISM:
A mechanism at which one drug antagonize the actions of a 2nd drug by
binding to it and causes its inactivation.
For example: "Protamine" a protein positively charged at physiological pH, can be
used to counter the effects of "Heparin ", an anticoagulant negatively charged.
3) PHYSIOLOGICAL (FUNCTIONAL) ANTAGONISM:
A mechanism at which one compound or drug opposes the physiological action of
another drug.
For example: "Glucocorticoid Hormones" increase blood sugar while "insulin"
decrease it.
MEDIAN TOXIC DOSE (TD 50):
"Is the dose required to produce a particular toxic effect in 50% of experimented
animals".
MEDIAN LETHAL DOSE (LD 50):
"Is the dose required to cause death to 50% of experimented animals",
THERAPEUTIC EFFICACY:
"Is the capacity of a drug to produce an effect and refers to the maximum of such
effect".
This means if drug A can produce a therapeutic effect that can’t be obtained by
drug B however much of drug B is given then drug A has the higher therapeutic
efficacy.
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
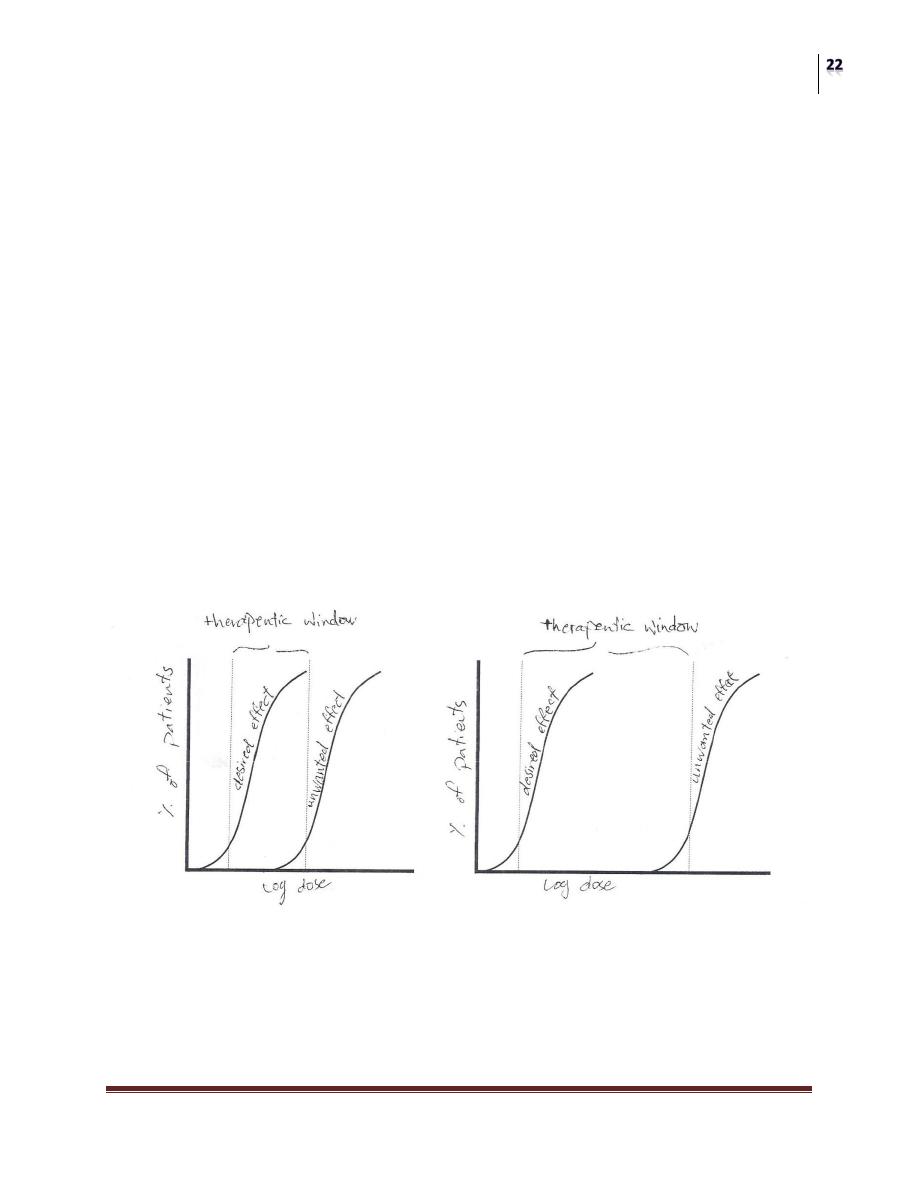
THERAPEUTIC INDEX (TI):
"Is the ratio of the dose that causes toxicity to the dose that causes a clinically
desired effect (response) in a population of individuals".
Therapeutic Index = TD
50
/ ED
50
Where, TD is the Toxic Dose
ED is the Effective Dose
“THE GREATER THE INDEX, THE MORE SAFE IS THE DRUG”
(TI) is a measure of the drugs' safety, because Larger Value of (TI) indicates a
wide margin between doses that are effective and doses that are toxic.
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
TOLERANCE:
A condition when developed, it becomes necessary to increase the dose of a drug
to obtain an effect which is previously obtained by a smaller dose.
Tolerance is either:
1) NATURAL:
It’s not induced by the drug but it happens due to "inherited factors"
(Pharmacogenetic).
2) ACQUIRED:
Which is developed after a prolonged time of using a drug, due to:
a) Reduced efficacy at receptor sites as with "opioids".
b) Enzyme induction (increased metabolism) as with "alcohol".
I.e. An increase in enzymes causes an increase in drug metabolism so the drug
concentration is decreased, rapidly
c) Cross- Tolerance between drugs of similar structure or metabolized by the same
enzyme, and sometimes between dissimilar drugs. For example: "Antibiotics".
PHARMACOKINETIC INTERACTIONS:
Means the way in which the body handles the drug(s) by means of
(Absorption Distribution Metabolism Elimination) (Abb.
ADME)

Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
ORDERS OF REACTIONS CAN BE DIVIDED INTO:
1) 1ST ORDER REACTIONS (PROCESSES OR KINETICS):
Drugs taken into the body are subjected to absorption, distribution, metabolism and
elimination.
In most instances, the rate at which the above processes (ADME) occur are
PROPORTIONAL TO THE CONCENTRATION OF THE DRUG (high at high
concentration of drug and falls in proportion at low concentration).
SO, PROCESSES FOR WHICH RATE IS PROPORTIONAL TO
CONCENTRATION ARE CALL£D "FIRST ORDER".
2) ZERO ORDER KINETICS:
As the amount of drug in the body rises, any metabolic processes that have limited
capacity become "saturated", (I.e. the rate of the process reaches a maximum
amount at which these processes remain constant).
And this may happen due to the "limiting amount of enzymes" (i.e. no more
enzymes are available to react).
Further increase in the rate is impossible after saturation despite the increase in
dose of the drug.
So, THE RATE OF REACTION IS NOT PROPORTIONAL TO DOSE OR
CONCENTRATION ( A RATE-LIMITED OR DOSE-DEPENDENT OR
ZERO-ORDER PROCESS) DUE TO "SATURATION".
It takes place with certain drugs especially these used at high doses for example:
"Aspirin" and "Ethanol".
Enzyme-mediated metabolic reactions are the most likely to show rate limitation
(enzyme saturation).
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
Example on 1st and 2nd order: Alcohol (Ethanol):
Rate of Metabolism = 10 ml/hr. or 8 gm. /hr. for a 70 kg person.
Half-life = 1 hr. at plasma concentration below 10 mg/dl = (2/3 glass of wine)
If a 70 kg man drinks 1/2 bottle of Scotch whiskey = 375 ml (40% alcohol) = 150
ml pure alcohol
Then, alcohol concentration in blood = 250 mg/dl
If metabolism is 1
st
order (t
1/2
= 1 hr.)
Next morning (8:00 am) he would've less than 1 mg/dl
But alcohol is subjected to zero-order kinetics due to enzyme saturation, so
metabolizing 10 ml/hr.
After 8 hours leaving 70 ml alcohol in blood = 120 mg/dl
Legal limit = 80 mg/dl
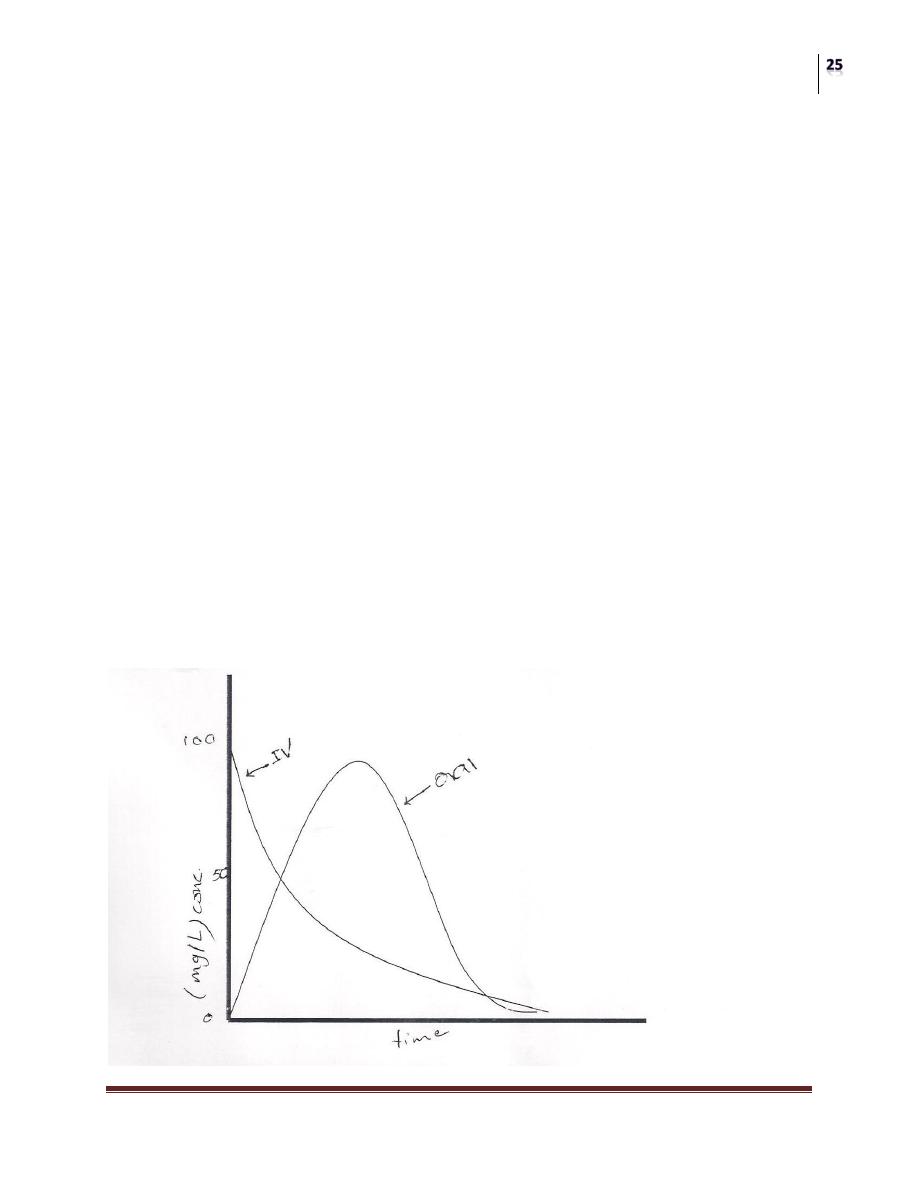
The figure below shows plasma concentration-time curve for a drug given IV and
orally.
Pharmacology
General Pharmacology
Dr. Ahmad Al-Zohyri
Lec.1-2-3
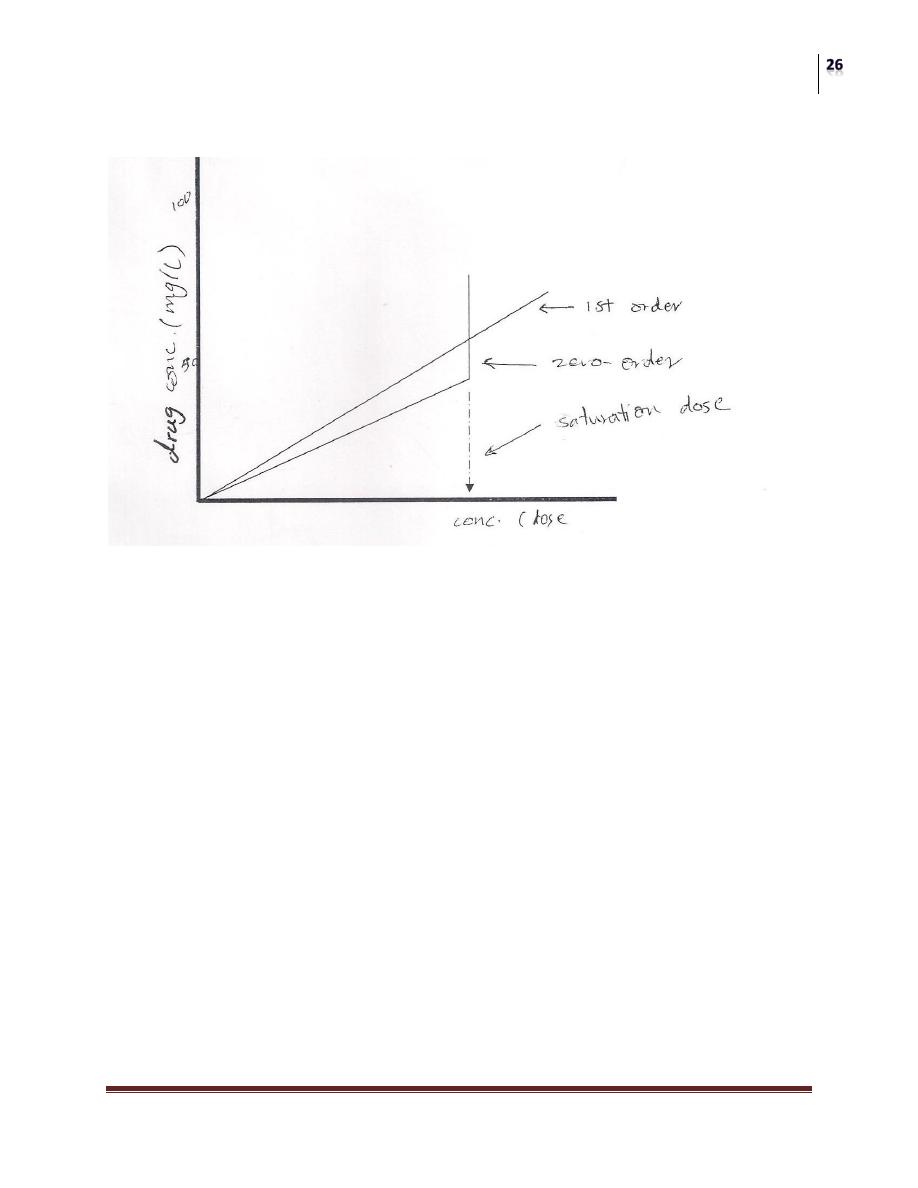
Comparison of effect of increasing dose in plasma concentration of drugs subjected
to 1st and zero order (saturation) kinetics.
The end
Ali Mazin
Ibrahim Ghazi
