
Dr. Ayad Abbas
Lec. 3
ELECTROLYTE BALANCE
Tues. 7 / 10 / 2014
Published by : Ali Kareem
مكتب اشور لالستنساخ
5102
-
5102

Surgery
ELECTROLYTE BALANCE
د. اياد عباس
Lec 3:
7/10/2014
Sodium balance
total body sodium Sodium is the principal cation content of the
extracellular fluid. The amounts to approximately 5000 mmol, of which
44 per cent is in the extracellular fluid,
9 per cent in the intracellular fluid
47 per cent in bone.
The sodium housed in bone merits special notice: a little more than half of it is
osmotically inactive and requires acid for its solution; the remainder is water
soluble and exchangeable. Thus, there is a large storehouse of sodium ready to
compensate abnormal loss from the body.
The daily intake of sodium is inconstant. On average it is 1 mmol/kg sodium
chloride or 500 ml of isotonic 0.9 per cent saline solution. An equivalent amount
is excreted daily, mainly in the urine and some in the faeces. The loss in
perspiration normally is negligible; however, in people not acclimatized to
tropical heat, prolonged profuse sweating results in a considerable loss of sodium
— as much as 85 mmol/hour. If water alone is given to counter-balance the fluid
loss, serious sodium depletion can occur from excessive sweating.
Sodium, with its equivalent anions, accounts for about 90 per cent of
the osmotic pressure of the plasma. Changes in the sodium content coincide
with changes in the osmolality of all the body fluids. The serum sodium value
is normally between 137 and 147 mmol /litre.
The sodium excretion shut down of trauma

Following trauma/surgery there is a variable period of reduced excretion
of sodium. For this reason it may be inadvisable to administer large quantities of
isotonic (0.9 per cent) saline solution after an operation. The period of sodium
excretion shut down can last for up to 48 hours and is due to increased
adrenocortical activity.
Sodium excess (syn. hypernatraemia)
This is likely to arise if a patient is given an excessive amount of 0.9 per cent
saline solution intravenously during the early postoperative period when, as has
been described, some degree of sodium retention is to be expected. The result is
an overloading of the circulation with salt and its accompanying water.
Clinical features:
Slight puffiness of the face is the only early sign.
Pitting oedema should be sought, especially in the sacral region, but for
pitting oedema to be present at least 4.5litres of excess fluid must have
accumulated in the tissue spaces.
The patient’s weight increases with the water-logging.
There is cellular dehydration.
Restlessness, lethargy, hyperreflexia and progress to seizure, comma and
alternately death.
Signs of overhydration in infancy (infants are very susceptible) are:
Increased tension in the anterior fontanelle.
Increased weight.
An increase in the number of urinations.
Oedema.
Hypernatraemia
:
it is of 3 types:
A● Hyper Na
+
with low total body Na
+
:
in which there is loss of H
2
O + Na
+
but (H
2
O > Na
+
).
Causes:
Renal: e.g.osmotic diuresis (hyperglycaemia, mannitol).
Osmotic diarrhoea
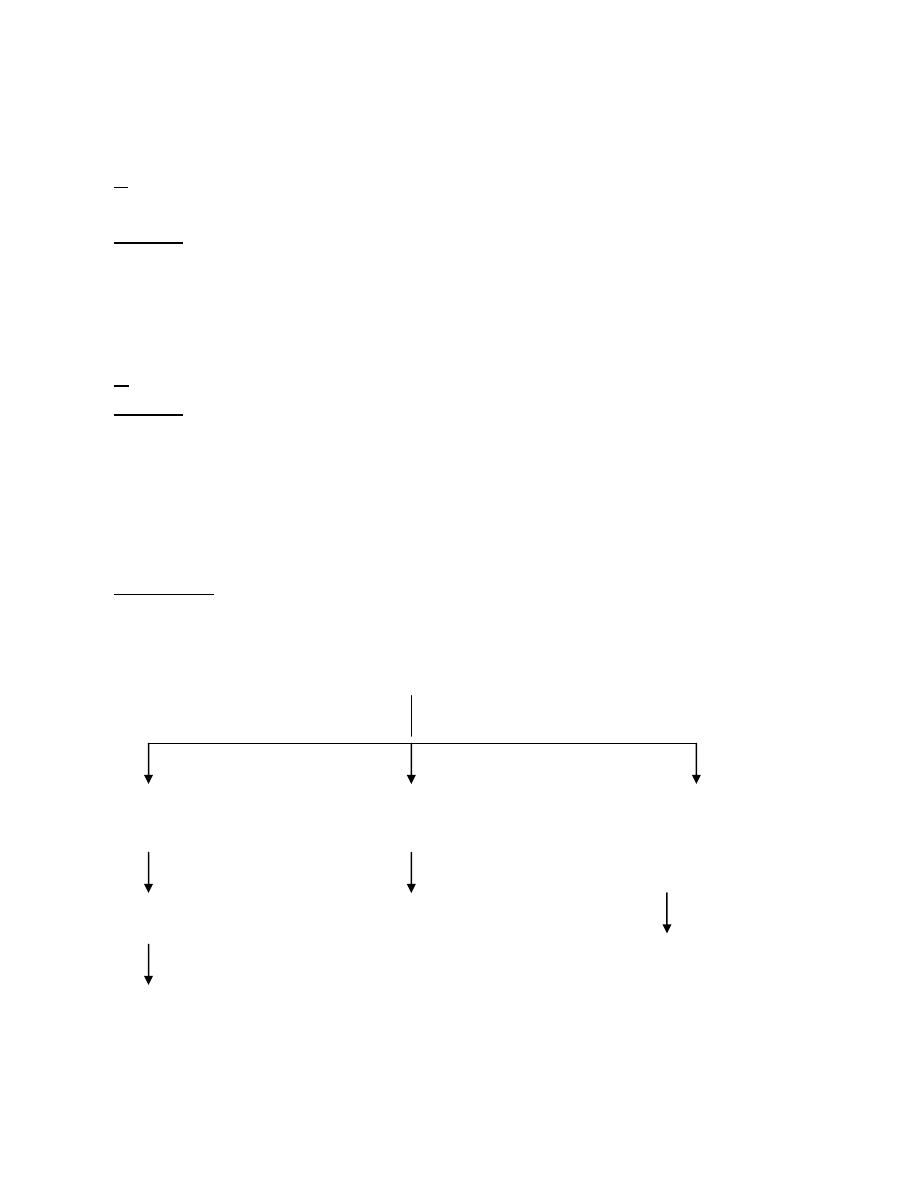
Sweating
B● Hyper Na
+
with normal total body Na
+
:
in which there is loss of H
2
O.
Causes:
Renal: Diabetes Inspidus
Burn
↑ respiratory loss
C● Hyper Na
+
with ↑ total body Na
+
Causes:
Massive salt ingestion
IV hypertonic saline
NaHCO
3
therapy
1
○
hyperaldosteronism
Cushing syndrome
Treatment:
H
2
O deficit should be corrected over 48 hrs and rapid correction may → seizure,
brain oedema and death, so plasma concentration of Na
+
should not ↓ > 0.5
mmol/L/hr.
Type 1 Type 2 Type 3
H
2
O +Na
+
loss H
2
O loss Loope diuretic
Lazix (frusemide)
Replace isotonic loss Replace H
2
O with
Glucose water Replace any H
2
O
Deficit by
Replace H
2
O loss by Glucose water
Glucose water
Sodium depletion (syn. hyponatraemia)
The most frequent cause of sodium depletion seen in surgical practice is
obstruction of the small intestine, with its rapid loss of gastric, biliary,
pancreatic and intestinal secretions by antiperistalsis and ejection, whether by
vomiting or aspiration. Duodenal, total biliary, pancreatic and high intestinal
external fistulae also are all notorious for bringing about early and profound
hyponatraemia. Severe diarrhoea due to dysentery, cholera, ulcerative colitis or
pseudomembranous colitis will cause hyponatraemia with acidosis. The finding of
hyponatraemia with elevated potassium would suggest adrenocortical
insufficiency. Hyponatraemia is also seen in SIADH.
There is one other less obvious, and surreptitious, means whereby the
patient is robbed of sodium and that is by gastric aspiration combined with
allowing the patient to drink as he or she pleases and promptly aspirating the fluid
swallowed. The act of drinking excites the flow of gastric juice, and this is also
aspirated. During this form of therapy, should the patient be receiving intravenous
dextrose solution to maintain fluid balance, he or she will soon become a victim of
hyponatraemia.
Clinical features:
Clinical features of hyponatraemia with salt and water depletion are due to
extracellular dehydration.
Neurological(↑ IC water)
Mild-moderate (Na
+
> 125) → asymptomatic
Early symptom: nausea, anorexia and weakness
Progress (Na
+
< 120) → cerebral oedema, lethargy, confusion, seizure,
comma and death.
The eyes are sunken and the face is drawn.
In infants the anterior fontanelle is depressed.
The tongue is coated and dry; in advanced cases it is brown in colour.
The skin is dry and often wrinkled, making the patient look older than his or
her years.
The subcutaneous tissue feels lax.
Peripheral veins are contracted and contain dark blood.
The arterial blood pressure is likely to be below normal.
The urine is scanty, dark in colour, of a high specific gravity and, except in
cases of salt-losing nephritis, contains little or no chloride.
Presuming that the haemoglobin level before the dehydra-tion
commenced was normal, the haematocrit reading (PCV) provides an index of the
degree of haemoconcentration. However, haemoconcentrations can be masked by
pre-existing anaemia.
Laboratory investigations would show normal or slightly reduced serum sodium
with low urinary output and low urinary sodium.
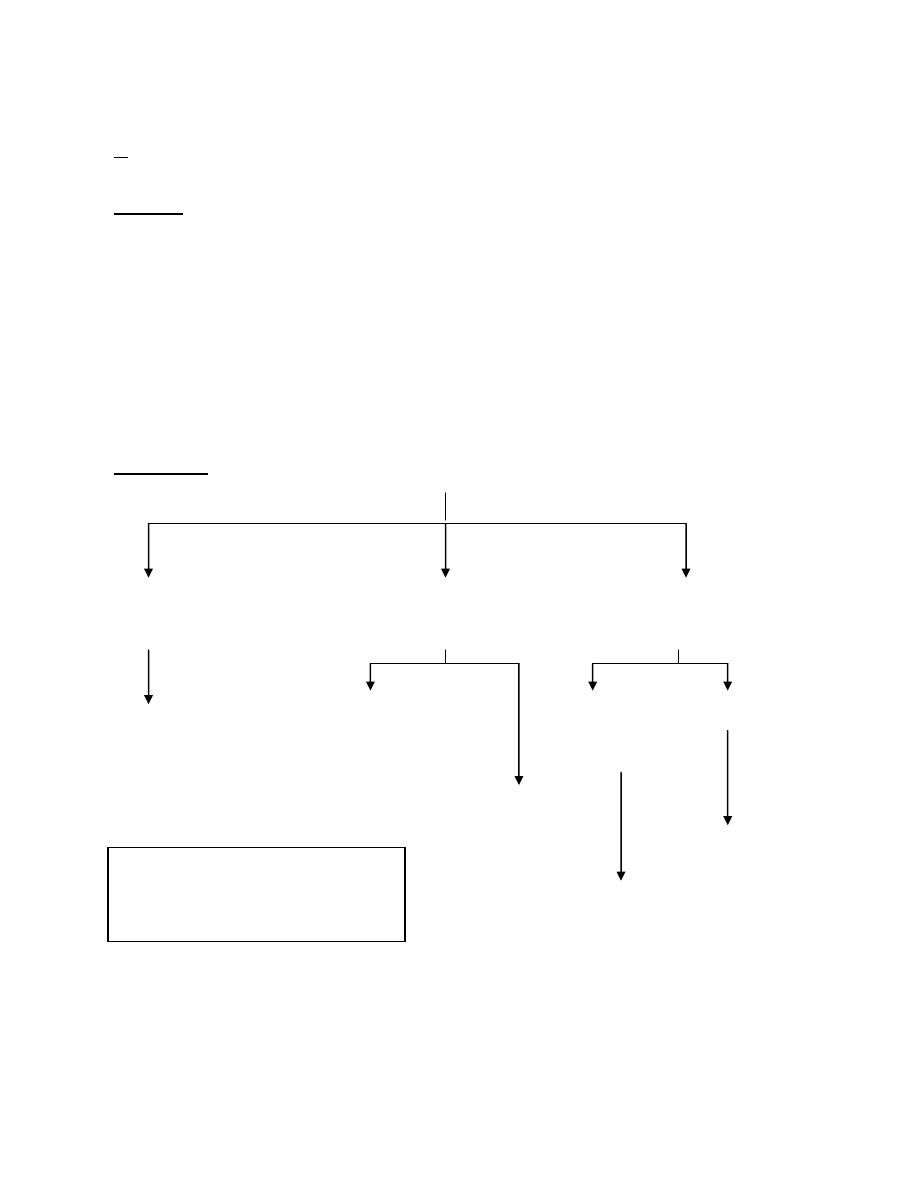
Hyponatraemia
and it is of 3 types:
A● With low total body Na
+
(↓ extracellualr volume).
Causes:
Renal diuretic
↓ aldosterone
Renal tubular acidosis
Vomiting
Diarrhoea
External Fistulae.
Intestinal Obstruction.
Gastric Aspiration.
B● With normal total body Na
+
(normal EC volume)
Causes:
Inappropriate ADH syndrome
Glucocorticoid deficiency
Hypothyroidism
Drug induced : chlorpramide, cyclophosphamide, vincristin,
carbamazepine
Na
+
deficit = BW * Na
+
deficit
=BW * (Na
+
desired-Na
+
present)
C● With ↑ total body Na
+
(↑ extracellular volume)
Causes:
CHF
Renal failure
Cirrhosis
Nephrotic syndrome
Treatment:
↓ EC vol. Normal EC vol. ↑EC vol.
cortisol or thyroid hor. HF& cirrhosis RF
Replace isotonic deficit + nephrotic synd.
Replace Na
+
deficit
Restrict H
2
O
Restrict fluid
*Restrict fluid
*Loop diuretic

Acute symptomatic Hyponatraemia
Requires prompt treatment
● Correct plasma Na
+
to 130 mmol/L
● Very rapid correct associated with demyelinating lesion in pons → serious
neurological sequelae, so according to severity
mild symptoms 0.5 mmol/L/hr or less.
moderate 1 mmol/L/hr or less.
severe 1.5 mmol/L/hr or less.
Postoperative hyponatraemia
Hyponatraemia with a normal or increased extracellular fluid volume
arises as a result of too prolonged administration of a sodium-free solution (cf.
Water intoxication, above).
Potassium balance
Potassium is almost entirely intracellular. No less than 98 per cent is
intracellular, and only 2 per cent is present in the extracellular fluid. Three
quarters of the total body potassium (approximately 3500 mmol) is found in
skeletal mus-cles. When the body needs endogenous protein as a source of energy,
potassium, as well as nitrogen, is mobilised. The mobilised potassium passes to
the extracellular fluid, but the surplus over and above the normal content is so
rapidly excreted by healthy kidneys that the concentration of potassium in the
serum remains unaltered. Each day a normal adult ingests approximately 1.0
mmol/kg of potassium in food; fruit, milk and honey are rich in this cation. Except
for a very small quantity in formed faeces, and a still smaller quantity in sweat, an
amount corresponding to the intake is excreted in the urine.

Hypokalaemia:
Clinical features
Most patients are asymptomatic. K
+
< 3.5 mmol/L
Symptoms usually occur < 2.5 mmol/L
Symptoms of hypokalaemia may include:
1.CVS:
ECG changes:
ST depression
PR & QT prolongation
T inversion
U wave
Disarrhythmia
Myocardial dysfunction
Cardiac arrest may occur
Orthostatic hypotension
2.Neuromuscular:
Skeletal muscle weakness.
Tetany.
Rhabdomyositis.
Listlessness and slurred speech.
Muscular hypotonia
Depressed reflexes.
Abdominal distension as a result of a paralytic ileus.
3.Renal:
Polyurea, nephrogenic DI
↑ absorption of HCO
3
-
→ metabolic alkalosis
↑ Na
+
retension
4.Postoperative pulmonary complications:
Weakness of the respiratory muscles may result in rapid, shallow, gasping
respirations.
Causes:

↓ intake
Excessive loss
Renal:
1. solute diuresis e.g. saline, Glc, mannitol
2. Diuretic treatment
3. Hypoaldosteronism and disorder of rennin-angiotensin
4. Cushing syndrome
5. Diuretic phase of acute renal failure
GIT: diarrhoea, vomiting, fistula, prolonged gastroduodenal aspiration
with fluid replacement by intravenous isotonic saline solution,
postoperative period following extensive resections for carcinoma of the
alimentary tract, because often the operation has to be undertaken after
months of weight loss and potassium depletion.
Movement of K
+
into cells
Alkalosis
Drug: insulin, cathecolamine
Diabetic coma treated by insulin and prolonged infusion of saline solution.
Treatment
Oral supplements: 60-80 mmol/day up to 200 mmol/day
I.V. KCl :
not > 40 mmol/L fluid
not > 20 mmol/hr rapid adminstration may → V.F.
should be under ECG monitoring
not given in unurea
Oral potassium. Potassium can be given in the form of milk, meat
extracts, fruit juices and honey. However, in hospital practice, effervescent tablets
of potassium chloride 2 g can be given by mouth 6-hourly.
Intravenous potassium. Rapid intravenous supplementation (especially
when renal function is impaired) carries the risk of dysrhythmias and cardiac
arrest if the serum concen-tration rises to a dangerous level. Administration
should be properly controlled; the level of potassium should be checked daily; the
urine output must be adequate. When there is no associated alkalosis, the
potassium deficit can be restored by adding 40 mmol potassium chloride to each
litre of 5per cent glucose, glucose—saline or 0.9 per cent saline solution, which is
given 6—8-hourly. Severe hypokalaemia should be treated in a high dependency
or intensive care environment.
Hyperkalaemia:
K
+
> 5 mmol/L
Causes:
↑ K
+
intake: I.V adminstration, rapid blood transfusion.
↓ renal output
1. Renal failure (acute, chronic an GFR < 15 ml/min
2. Adrenocortical insufficiency
3. Drug: ACE inhibitor, K
+
sparing diuretic
Movement of K
+
out of cell
1. Acidosis
2. Trauma
3. Rhabdomyositis
Effect:
● Nausea, vomiting, diarrhoea, muscle weakness
● ECG changes:
1.peaked T wave
2.absent P wave
3.widened QRS complex
4.sturring ST into T wave
● VF com. > 7 mmol/L
● Cardiac arrest may occur in diastole
Treatment:
● Polystyren sulphate resins: 15 gm 6-8 hourly orally or 30 g rectally
● Insulin 5-10 u in 100 ml of 10-20 dextrose i.v. over 30-60 min (shift K
+
to
cell).

● Bicarbonate: 50 mmol i.v (exchange K
+
for H
+
across cell membrane)
● Calcium gluconate: 5-10 ml i.v if severe ( act as physiological antagonist of K
+
● Salbutamol: 5 mg nebulized or i.v (50 Mc bolus and to 5-10 Mc /min infusion
(↑ cellular uptake)
●Dialysis
Calcium
Calcium is an extracellular cation with a plasma concentration of 2.2—
2.5 mmol/litre. It exists in three forms: bound to protein, free nonionised and free
ionised — the last form being the component necessary for blood coagulation and
affecting neuromuscular excitability. The ionised proportion falls with increasing
PH; thus in respiratory alkalosis due to hyperventilation there may be tetany —
with an apparently normal total serum calcium level. In the urine, the ionisation
and the solubility of calcium are similarly depressed if the pH is elevated, thus
promoting stone formation. The serum level of calcium is likely to be modified by
any factor promoting or inhibiting its absorption from the bowel, its storage in
bone or its elimination by the kidneys: such factors include vitamin D and phytic
acid, parathormone and calcitonin, and the state of renal and small-bowel
function.
Hypocalcaemia
occurs when total Ca
+2
< 2 mmo/L and/or due to ↓ plasma ionized Ca
+2
.
Although total Ca
+2
↓ in hypoproteinaemia → the ionized portion is normal and
no clinical features.
Causes:
↓ parathyroid hormone activity:
1.hypoparathyroidism 2.hypomagnesaemia
↓ vit. D activity:1.Inadequate 2.malabsorption 3.or altered vit. D
metabolism: renal failure, hepatic insufficiency
Hyperphosphataemia
Precipitation of Ca
+2
: rhabdomyosistis, pancreatitis

Chelation of Ca
+2
: multiple transfusion, rapid infusion of albumin
Clinical Features:
● Paraesthesia
● Muscle cramps, spasms, tetany
● Stridor
● Chvostek's sign (facial spasm following trapping on facial nerve)
● Trousseau's sign (carpopedal spasm following flat of tourniquet around arm)
● Mental excitability → convulsion
● ECG: prolong QT interval
Treatment:
● Predisposing cause
● i.v calcium if severe 5-10 ml of Ca
+2
chloride or < 10-20 ml of gluconate slowly
● Magnesium if deficient
Hypercalcaemia:
symptom presents when Ca
+2
> 3.5 mmol/L
Causes:
Hyperparathyroidism
Malignancy both 1
○
and bony metastasis
less common:
↑ intake of vit. D or vit A
Other:
Paget disease of bone
hyperthyroidism
adrenocorticoid insufficiency
Sarcoidosis
Thiazide diuretics
Effect:
● Psychatric disturbance
● Dehydration : polyurea, polydipsia
● Nausea, vomiting & constipation

● Muscle weakness
● Drowsiness & comma
● ECG shorten QT interval, prolong interval
● may → renal calculi
Treatment:
● Symptomatic hyper Ca
+2
needs rapid treatment by brisk diuresis (200-300 ml/h)
with i.v normal serum infusion and loop diuretic.
● If severe:
1.Calcitonin 5-10 u/kg/d (i.m subcutaneous) in 1-2 divided doses.
2.Biphosphonate e.g. Na
+
pamidornate 15-60 mg
● Dialysis may be necessary.
Magnesium
Magnesium is an intracellular cation which shares some of the properties
of potassium and some of calcium. The normal magnesium concentration is 0.7—
0.9 mmol /litre. The average daily intake is approximately 10 mmol. Magnesium
deficien-cy may occur when there is prolonged loss of gastrointestinal secretions
due to fistulae or ulcerative colitis, very prolonged administration of intravenous
fluids without magnesium supplements, following massive small bowel
resections, and in some cases of cirrhosis of the liver or disease of the
para-thyroids. The clinical picture of magnesium deficiency is marked by central
nervous system irritability, ECG changes, lowered blood pressure and lowered
protein synthesis. Postoperative cardiac arrythmias (e.g. de novo atrial fibrillation)
are commonly associated with both hypokalaemia and hypomagnesaemia.
Treatment. For the treatment of mild hypomagnesaemia 20 mmol as
magnesium sulphate can be added to 5percent-dextrose or normal saline over a
24-hour period. Magnesium supplements are essential in hyperalimentation.
Hypomagnesaemia:
Mg
+2
< 0.75 mmol/L
Causes:
↓ Mg
+2
intake
Malabsorption
↑ loss GIT (diarrhoea, vomiting), renal (diuretic, DM)

Effect:
● Arrhythmia
● Neurological: confusion, irritability, tremor, convulsion
Treatment:
● 1
○
cause
● Acutely: 10-20 mmol/L MgSO
4
i.v over 1-2 h and repeated as required up to 50
mmol/ day
Hypermagnesaemia:
Mg
+2
> 1.05 mmol/L
Causes:
Renal failure
Mg
+2
adminstration
Laxatavie, antiacid
Adernocorticoid insufficiency, hypothyroidism
Effect:
● Vasodilatation, hypotension, cardiac conductive defect
● Sedation, comma, weakness & respiratory depression
Treatment:
● Diuresis
● i.v Ca
+2
Printed by :
Ali Malik
Special greetings to: Marwan Zuhair, Mohamed Esam, Sabah Nasir,
Yasir Kasim and to Zaid Fareed
