Dr. Ghassan Endocrine Physiology
1
Pancreas
:
After studying these lectures, you should be able to . . .
1. Kew the endocrine function of pancreas.
2. Understand the physiological effects of insulin on different body
parts.
3. Use your physiological knowledge to predict the cause of signs and
symptoms of diabetes mellitus.
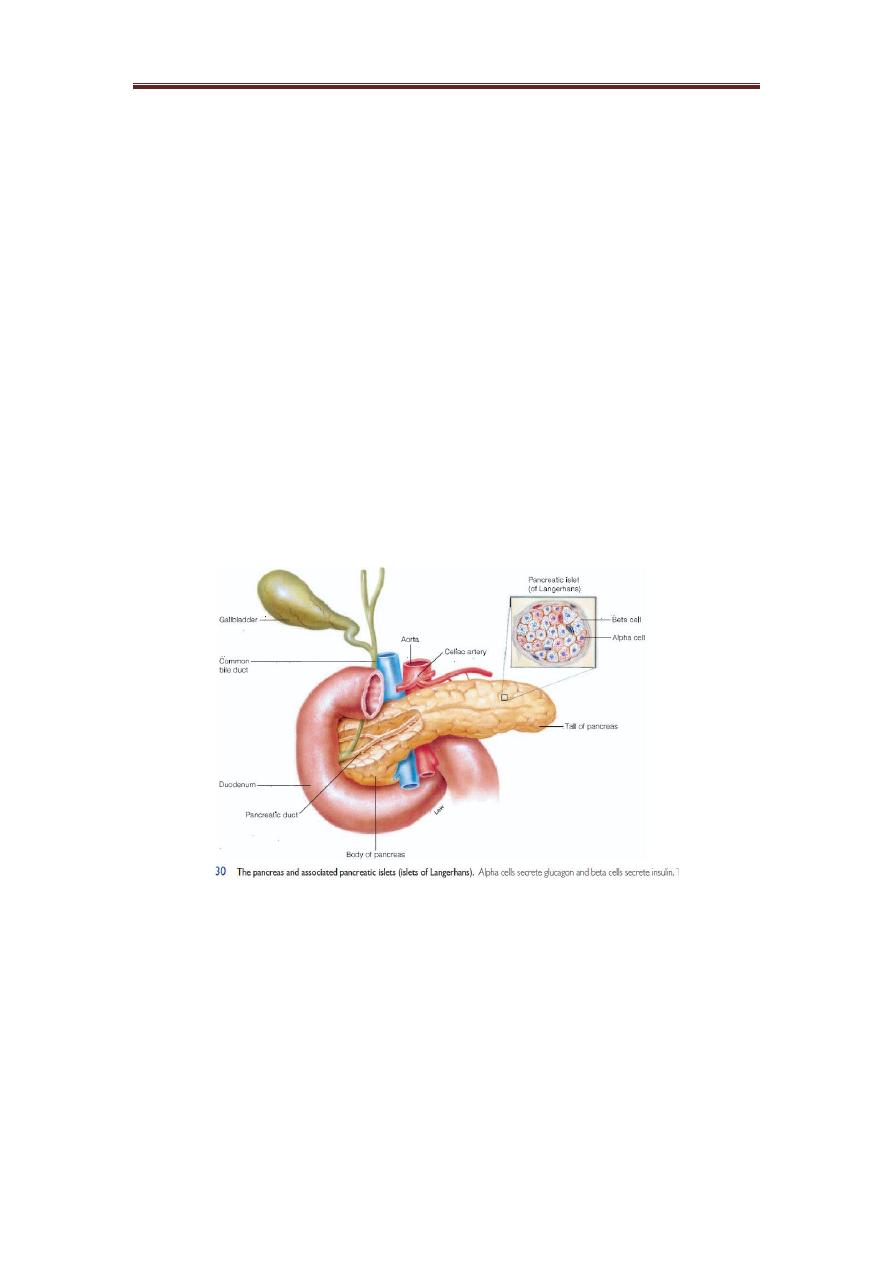
An endocrine and an exocrine gland:
The endocrine portion of the pancreas consists of scattered
clusters of cells called the pancreatic islets or islets of
Langerhans. These endocrine structures are most common in
the body and tail of the pancreas Pancreatic Islets (Islets of
Langerhans). On a microscopic level, the most conspicuous cells
in the islets are the alpha and beta cells.
Alpha cells secrete glucagon in response to a fall in blood
glucose concentrations. Glucagon stimulates the liver to
hydrolyze glycogen to glucose (glycogenolysis), which causes
the blood glucose level to rise. This effect represents the
completion of a negative feedback loop. Glucagon also
stimulates the hydrolysis of stored fat (lipolysis) and the
consequent release of free fatty acids into the blood. This
effect helps to provide energy substrates for the body during
Dr. Ghassan Endocrine Physiology
2
fasting. Glucagon also Increases Gluconeogenesis: Even after all
the glycogen in the liver has been exhausted under the
influence of glucagon, continued infusion of this hormone still
causes continued hyperglycemia. This results from the effect of
glucagon to increase the rate of amino acid uptake by the liver
cells and then the conversion of many of the amino acids to
glucose by gluconeogenesis.
Beta cells secrete insulin in response to a rise in blood glucose
concentrations. One of the most important of all the effects of
insulin is to cause most of the glucose absorbed after a meal to
be stored almost immediately in the liver in the form of
glycogen. Then, between meals, when food is not available and
the blood glucose concentration begins to fall, insulin secretion
decreases rapidly and the liver glycogen is split back into
glucose, which is released back into the blood to keep the
glucose concentration from falling too low.
When the quantity of glucose entering the liver cells is more
than can be stored as glycogen or can be used for local
hepatocyte metabolism, insulin promotes the conversion of all
this excess glucose into fatty acids. These fatty acids are
subsequently packaged as triglycerides in very-low-density
lipoproteins and transported in this form by way of the blood
to the adipose tissue and deposited as fat.
Insulin increases glucose transport into and glucose usage by
most other cells of the body (with the exception of the brain
cells). The brain is quite different from most other tissues of the
body in that insulin has little effect on uptake or use of glucose.
Instead, the brain cells are permeable to glucose and can use
glucose without the intermediation of insulin.
The brain cells are also quite different from most other cells of
the body in that they normally use only glucose for energy.
Therefore, it is essential that the blood glucose level always be
maintained above a critical level, which is one of the most
important functions of the blood glucose control system.

Dr. Ghassan Endocrine Physiology
3
The normal blood glucose when the person is fasting is range
from 80 t0 120 mg/100ml while post-meal or called random
blood glucose is between 130 to 170 mg/100ml.
When the blood glucose falls too low, into the range of 20 to
50 mg/100ml, symptoms of hypoglycemic shock develop,
characterized by progressive nervous irritability that leads to
fainting, seizures, and even coma.
Insulin deficiency causes lipolysis of storage fat and release of
free fatty acids. The absence of insulin causes hydrolysis of the
stored triglycerides, releasing large quantities of fatty acids and
glycerol into the circulating blood. Consequently, the plasma
concentration of free fatty acids begins to rise within minutes.
This free fatty acid then becomes the main energy substrate
used by essentially all tissues of the body.
the excess fatty acids in the liver cells causes excessive
amounts of acetoacetic acid to be formed so in the
mitochondria, oxidation of the fatty acids then proceeds very
rapidly, releasing extreme amounts of acetyl-CoA. A large part
of this excess acetyl-CoA is then condensed to form acetoacetic
acid, which in turn is released into the circulating blood. The
absence of insulin also depresses the utilization of acetoacetic
acid in the peripheral tissues. Thus, so much acetoacetic acid is
released from the liver that it cannot all be metabolized by the
tissues.
Some of the acetoacetic acid is also converted into b-
hydroxybutyric acid and acetone. These two substances, along
with the acetoacetic acid, are called ketone bodies, and their
presence in large quantities in the body fluids is called ketosis.
Insulin Promotes Protein Synthesis and Storage. During the
few hours after a meal when excess quantities of nutrients are
available in the circulating blood, not only carbohydrates and
fats but proteins as well are stored in the tissues; insulin is
required for this to occur though:

Dr. Ghassan Endocrine Physiology
4
1. Insulin stimulates transport of many of the amino acids
into the cells.
2. Insulin inhibits the catabolism of proteins, thus decreasing
the rate of amino acid release from the cells, especially
from the muscle cells.
3. In the liver, insulin depresses the rate of gluconeogenesis.
Insulin Lack Causes Protein Depletion and Increased Plasma
Amino Acids: Virtually all protein storage comes to a halt when
insulin is not available. The catabolism of proteins increases,
protein synthesis stops, and large quantities of amino acids are
dumped into the plasma.
The plasma amino acid concentration rises considerably, and
most of the excess amino acids are used either directly for
energy or as substrates for gluconeogenesis.
This degradation of the amino acids also leads to enhanced
urea excretion in the urine. The resulting protein wasting is one
of the most serious of all the effects of severe diabetes
mellitus. It can lead to extreme weakness as well as many
deranged functions of the organs.
Insulin and Growth Hormone Interact Synergistically to
Promote Growth: Because insulin is required for the synthesis
of proteins, it is as essential for growth of an animal as growth
hormone is. It appears that the two hormones function
synergistically to promote growth, each performing a specific
function that is separate from that of the other.
Diabetes Mellitus:
Diabetes mellitus is a syndrome of impaired carbohydrate, fat,
and protein metabolism. There are two general types of
diabetes mellitus:
1. Type I diabetes, also called insulin-dependent diabetes
mellitus (IDDM), is caused by lack of insulin secretion.

Dr. Ghassan Endocrine Physiology
5
2. Type II diabetes, also called non–insulin-dependent diabetes
mellitus (NIDDM), and is caused by decreased sensitivity of
target tissues to the metabolic effect of insulin. This reduced
sensitivity to insulin is often called insulin resistance.
In both types of diabetes mellitus, metabolism of all the main
foodstuffs is altered. The basic effect of insulin lack or insulin
resistance on glucose metabolism is to prevent the efficient
uptake and utilization of glucose by most cells of the body,
except those of the brain. As a result, blood glucose
concentration increases, cell utilization of glucose falls
increasingly lower, and utilization of fats and proteins
increases.
Type I Diabetes—Lack of Insulin Production by Beta Cells of
the Pancreas
Injury to the beta cells of the pancreas or diseases that impair
insulin production can lead to type I diabetes. Viral infections or
autoimmune disorders may be involved in the destruction of
beta cells in many patients with type I diabetes. In some
instances, there may be a hereditary tendency for beta cell
degeneration even without viral infections or autoimmune
disorders.
The usual onset of type I diabetes occurs at about 14 years of
age, and for this reason it is often called juvenile diabetes
mellitus. Type I diabetes may develop very abruptly, over a
period of a few days or weeks, with three principal sequelae:
(1) increased blood glucose, (2) increased utilization of fats for
energy and for formation of cholesterol by the liver, and (3)
depletion of the body’s proteins.
1- Blood Glucose Concentration Rises to Very High Levels in
Diabetes Mellitus. The lack of insulin decreases the efficiency
of peripheral glucose utilization and augments glucose
production, raising plasma glucose to 300 to 1200 mg/100 ml.

Dr. Ghassan Endocrine Physiology
6
The increased plasma glucose then has multiple effects
throughout the body.
2- Increased Blood Glucose Causes Loss of Glucose in the
Urine: The high blood glucose causes more glucose to filter into
the renal tubules than can be reabsorbed, and the excess
glucose spills into the urine. This normally occurs when the
blood glucose concentration rises above 180 mg/100 ml, a level
that is called the blood “threshold” for the appearance of
glucose in the urine.
3- Increased Blood Glucose Causes Dehydration The very
high levels of blood glucose (sometimes as high as 8 to 10 times
normal in severe untreated diabetes) can cause severe cell
dehydration throughout the body. This occurs partly because
glucose does not diffuse easily through the pores of the cell
membrane without insulin, and the increased osmotic pressure
in the extracellular fluids causes osmotic transfer of water out
of the cells. In addition to the direct cellular dehydrating effect
of excessive glucose, the loss of glucose in the urine causes
osmotic diuresis. causing massive loss of fluid in the urine,
causing dehydration of the extracellular fluid, which in turn
causes compensatory dehydration of the intracellular fluid.
Thus, polyuria (excessive urine excretion), intracellular and
extracellular dehydration, and increased thirst are classic
symptoms of diabetes.
4- Chronic High Glucose Concentration Causes Tissue
Injury: When blood glucose is poorly controlled over long
periods in diabetes mellitus, blood vessels in multiple tissues
throughout the body begin to function abnormally and undergo
structural changes that result in inadequate blood supply to the
tissues. This in turn leads to increased risk for heart attack,
stroke, end-stage kidney disease, retinopathy and blindness,
and ischemia and gangrene of the limbs.
Chronic high glucose concentration also causes damage to
many other tissues. For example, peripheral neuropathy, which
is abnormal function of peripheral nerves, and autonomic

Dr. Ghassan Endocrine Physiology
7
nervous system dysfunction are frequent complications of
chronic, uncontrolled diabetes mellitus. These abnormalities
can result in impaired cardiovascular reflexes, impaired bladder
control, decreased sensation in the extremities, and other
symptoms of peripheral nerve damage.
5- Diabetes Mellitus Causes Increased Utilization of Fats
and Metabolic Acidosis: The shift from carbohydrate to fat
metabolism in diabetes increases the release of keto acids,
such as acetoacetic acid and b-hydroxybutyric acid, into the
plasma more rapidly than they can be taken up and oxidized by
the tissue cells. As a result, the patient develops severe
metabolic acidosis from the excess keto acids, which, in
association with dehydration due to the excessive urine
formation, can cause severe acidosis. This leads rapidly to
diabetic coma and death unless the condition is treated
immediately with large amounts of insulin and fluids.
All the usual physiologic compensations that occur in metabolic
acidosis take place in diabetic acidosis. They include rapid and
deep breathing, which causes increased expiration of carbon
dioxide; this buffers the acidosis. The kidneys compensate by
decreasing bicarbonate excretion and generating new
bicarbonate that is added back to the extracellular fluid.
6- Diabetes Causes Depletion of the Body’s Proteins: Failure
to use glucose for energy leads to increased utilization and
decreased storage of proteins as well as fat. Therefore, a
person with severe untreated diabetes mellitus suffers rapid
weight loss and asthenia (lack of energy) despite eating large
amounts of food (polyphagia).
Type II Diabetes—Resistance to the Metabolic Effects of
Insulin:
Type II diabetes is far more common than type I, accounting for
about 90% of all cases of diabetes mellitus. In most cases, the
onset of type II diabetes occurs after age 30, often between the
ages of 50 and 60 years, and the disease develops gradually.

Dr. Ghassan Endocrine Physiology
8
Therefore, this syndrome is often referred to as adult-onset
diabetes. In recent years, however, there has been a steady
increase in the number of younger individuals, some less than
20 years old, with type II diabetes. This trend appears to be
related mainly to the increasing prevalence of obesity, the most
important risk factor for type II diabetes in children as well as in
adults.
Insulin resistance is part of a cascade of disorders that is often
called the “metabolic syndrome.” Some of the features of the
metabolic
syndrome
include:
(1)
obesity,
especially
accumulation of abdominal fat; (2) insulin resistance; (3) fasting
hyperglycemia; (4) lipid abnormalities such as increased blood
triglycerides and decreased blood high-density lipoprotein-
cholesterol; and (5) hypertension. All of the features of the
metabolic syndrome are closely related to excess weight gain,
especially when it is associated with accumulation of adipose
tissue in the abdominal cavity around the visceral organs.
In the type II diabetes, the pancreatic beta cells become
“exhausted” and are unable to produce enough insulin to
prevent more severe hyperglycemia, especially after the person
ingests a carbohydrate-rich meal.
In many instances, type II diabetes can be effectively treated, at
least in the early stages, with exercise, caloric restriction, and
weight reduction, and no exogenous insulin administration is
required. Drugs that increase insulin sensitivity or drugs that
cause additional release of insulin by the pancreas may also be
used. However, in the later stages of type II diabetes, insulin
administration is usually required to control plasma glucose.
Physiology of Diagnosis of Diabetes Mellitus
1- Urinary Glucose: Simple tests used to determine the
quantity of glucose lost in the urine. In general, a normal
person loses undetectable amounts of glucose, whereas a
person with diabetes loses glucose in small to large amounts, in

Dr. Ghassan Endocrine Physiology
9
proportion to the severity of disease and the intake of
carbohydrates.
2- Fasting Blood Glucose: The fasting blood glucose level in
the early morning is normally 80 to 90 mg/100 ml, and 110
mg/100 ml is considered to be the upper limit of normal. A
fasting blood glucose level above this value often indicates
diabetes mellitus or at least marked insulin resistance.
3- Glucose Tolerance Test: when a normal, fasting person
ingests 1 gram of glucose per kilogram of body weight, the
blood glucose level rises from about 90 mg/100 ml to 120 to
140 mg/100 ml and falls back to below normal in about 2
hours.
In a person with diabetes, the fasting blood glucose
concentration is almost always above 110 mg/100 ml and often
above 140 mg/100 ml. Also, the glucose tolerance test is almost
always abnormal above 200 mg/100 ml.
