Prof.Dr.H.D.El-Yassin 2014
1
Lectuctre6
Biochemistry and Disorders of Hormones of the Pancreas
Objectives
1. List the hormones synthesized and secreted from the pancreas and state
their functions and clinical significance
2. Understand the mechanism of synthesis and release of insulin and
glucagon
3. Understand the mechanism of interaction of insulin with its receptor which
is the platform for developing medications for type 1 and type 2 DM
4. Understand the mechanism of interaction of glucagon with its receptor
5. define insulinoma and the laboratory results obtained in the assessment of
the disease
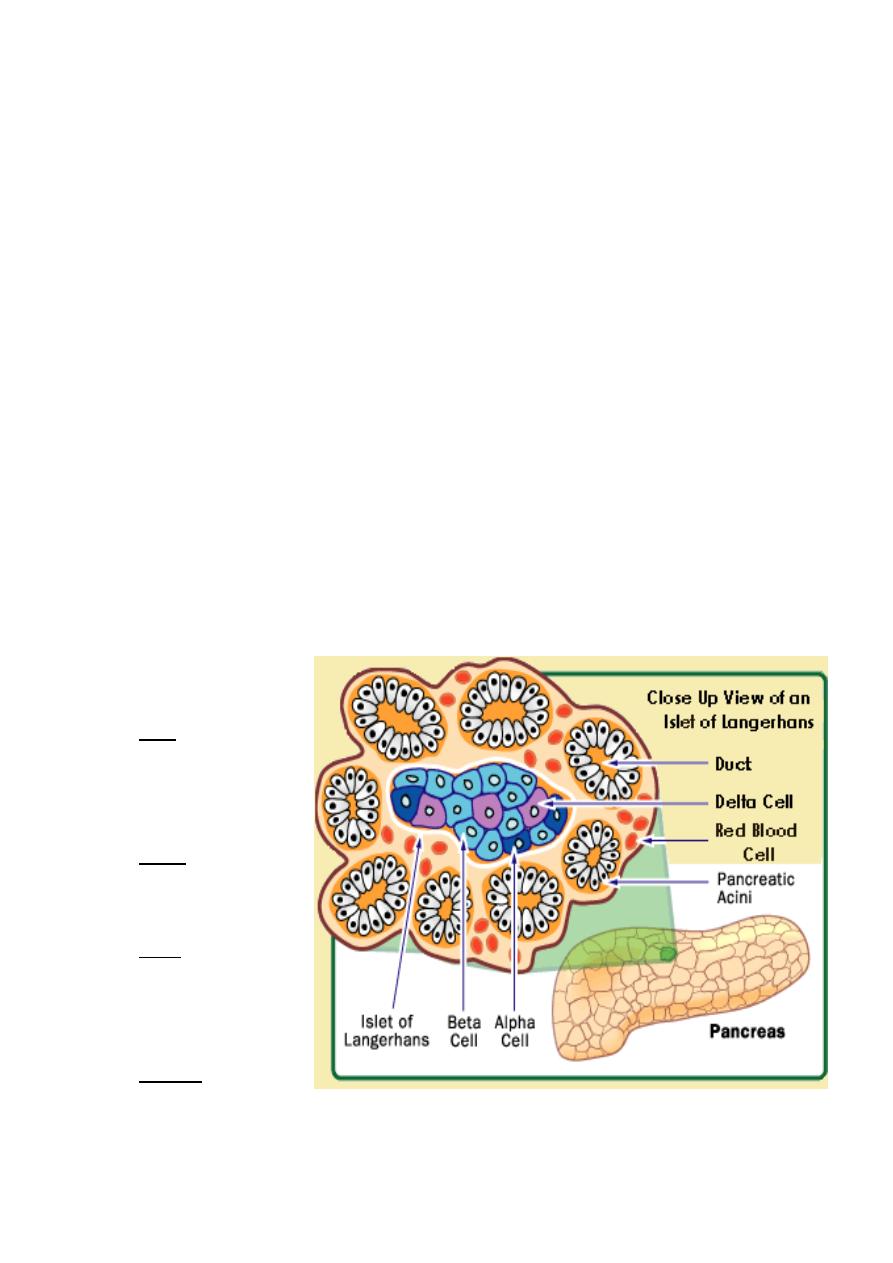
The bulk of the pancreas is an exocrine gland secreting pancreatic fluid into the duodenum
after a meal.
However, scattered through the pancreas are several hundred thousand clusters of cells
called islets of Langerhans. The islets are endocrine tissue containing four types of cells.
In order of abundance,
they are the:
1.
beta cells, which
secrete insulin,
amylin and preptin.
2.
alpha cells, which
secrete glucagon;
3.
delta cells, which
secrete
somatostatin, and
4.
gamma cells, which
secrete pancreatic polypeptide (PP).
Prof.Dr.H.D.El-Yassin 2014
2
Gluconeogenesis
Glucogenolysis
Lipolysis
Ketogenesis
Proteolysis
Uptake of ions (especially
K
+
and PO
4
-3
Protein synthesis
Glycogen synthesis
Glycolysis
Glucose uptake in muscle
and adipose tissue
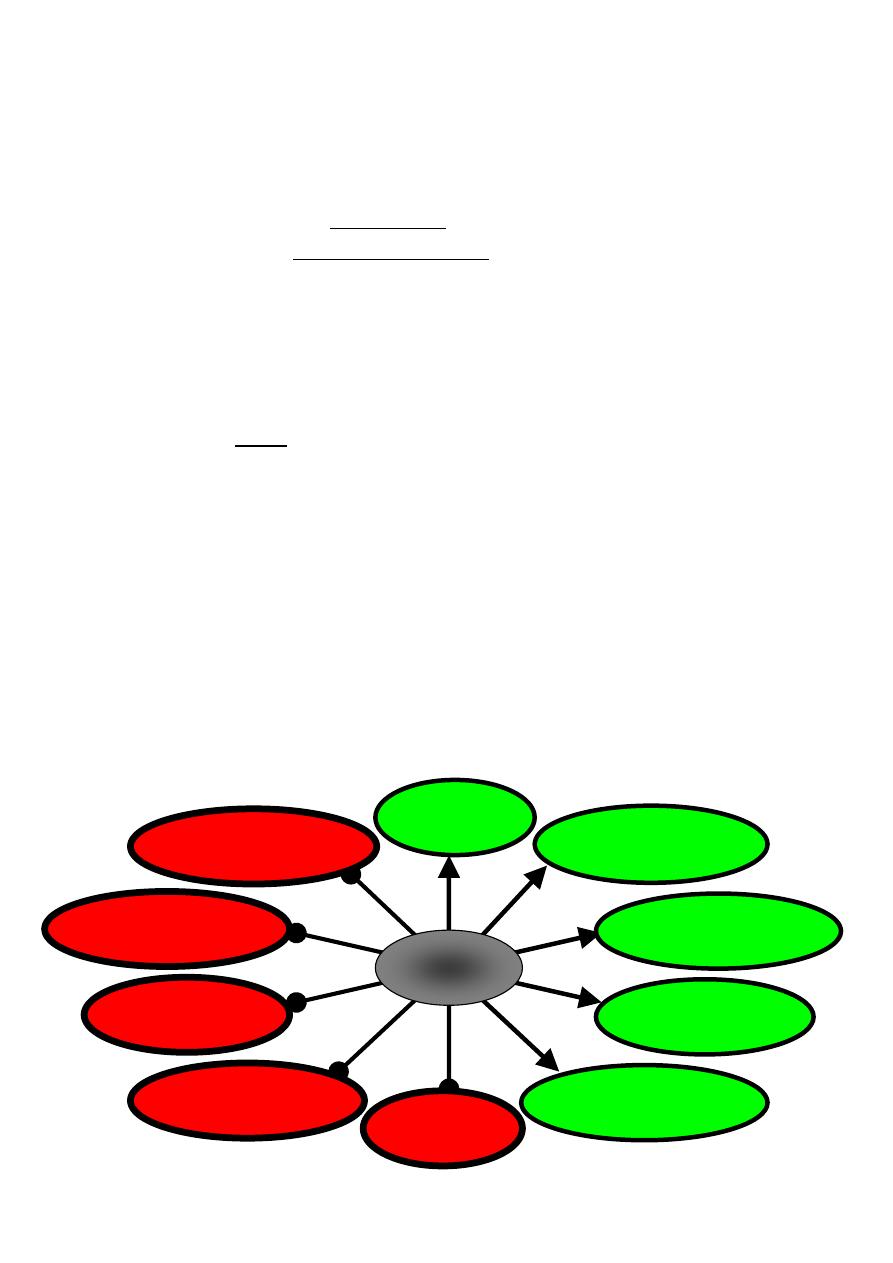
Insulin
Alpha Cells
The alpha cells of the islets secrete glucagon, a polypeptide of 29 amino acids.
Glucagon acts principally on the liver where it stimulates the conversion of
glycogen into glucose ("glycogenolysis") and
fat and protein into intermediate metabolites that are ultimately converted into
glucose ("gluconeogenesis")
In both cases, the glucose is deposited in the blood.
Glucagon secretion is
stimulated by low levels of glucose in the blood;
inhibited by high levels of glucose in the blood, and
inhibited by amylin.
The physiological significance of this is that glucagon functions to maintain a steady level
of blood sugar level between meals.
Beta Cells
Insulin is a small protein consisting of
an alpha chain of 21 amino acids linked by two disulfide (S
—S) bridges to a
beta chain of 30 amino acids.
Beta cells have channels in their plasma membrane that serve as glucose detectors. Beta
cells secrete insulin in response to a rising level of circulating glucose ("blood sugar").

Prof.Dr.H.D.El-Yassin 2014
3
Insulin affects many organs.
1.
It stimulates skeletal muscle fibers to
take up glucose and convert it into glycogen;
Take up amino acids from the blood and convert them into protein.
2.
acts on liver cells
stimulating them to take up glucose from the blood and convert it into glycogen
inhibiting production of the enzymes involved in breaking glycogen back down
("glycogenolysis") and
inhibiting "gluconeogenesis"; that is, the conversion of fats and proteins into glucose.
3.
acts on fat (adipose) cells to stimulate the uptake of glucose and the synthesis of fat.
4.
acts on cells in the hypothalamus to reduce appetite.
Actions of Insulin
Metabolic process
Reaction
consequence
glycogenesis
Glucose to Glycogen
(-) Blood glucose
glycogenolysis
Glycogen to Glucose
(+) Blood glucose
gluconeogenesis
Amino acids to Glucose
(+) Blood glucose
Protein synthesis
Amino acids to protein
(-) Blood amino acids
Protein degradation
Protein to Amino acids
(+) Blood amino acids
Fat synthesis (lipogenesis or
triglyceride synthesis
Fatty acids and glycerol to
triglycerides
(-) Blood fatty acids
Fat breakdown (lipolysis or
triglycerides degradation
Triglycerides to Fatty acids and
glycerol
(+) Blood fatty acids
(-) decrease in
(+) increase in
Amylin
Amylin is a peptide of 37 amino acids, which is also secreted by the beta cells of the
pancreas.
Some of its actions:
inhibits the secretion of glucagon;
slows the emptying of the stomach;
sends a satiety signal to the brain.
Amylin (IAPP) was identified independently by two groups as the major component of
diabetes-associated islet amyloid deposits in 1987
Prof.Dr.H.D.El-Yassin 2014
4
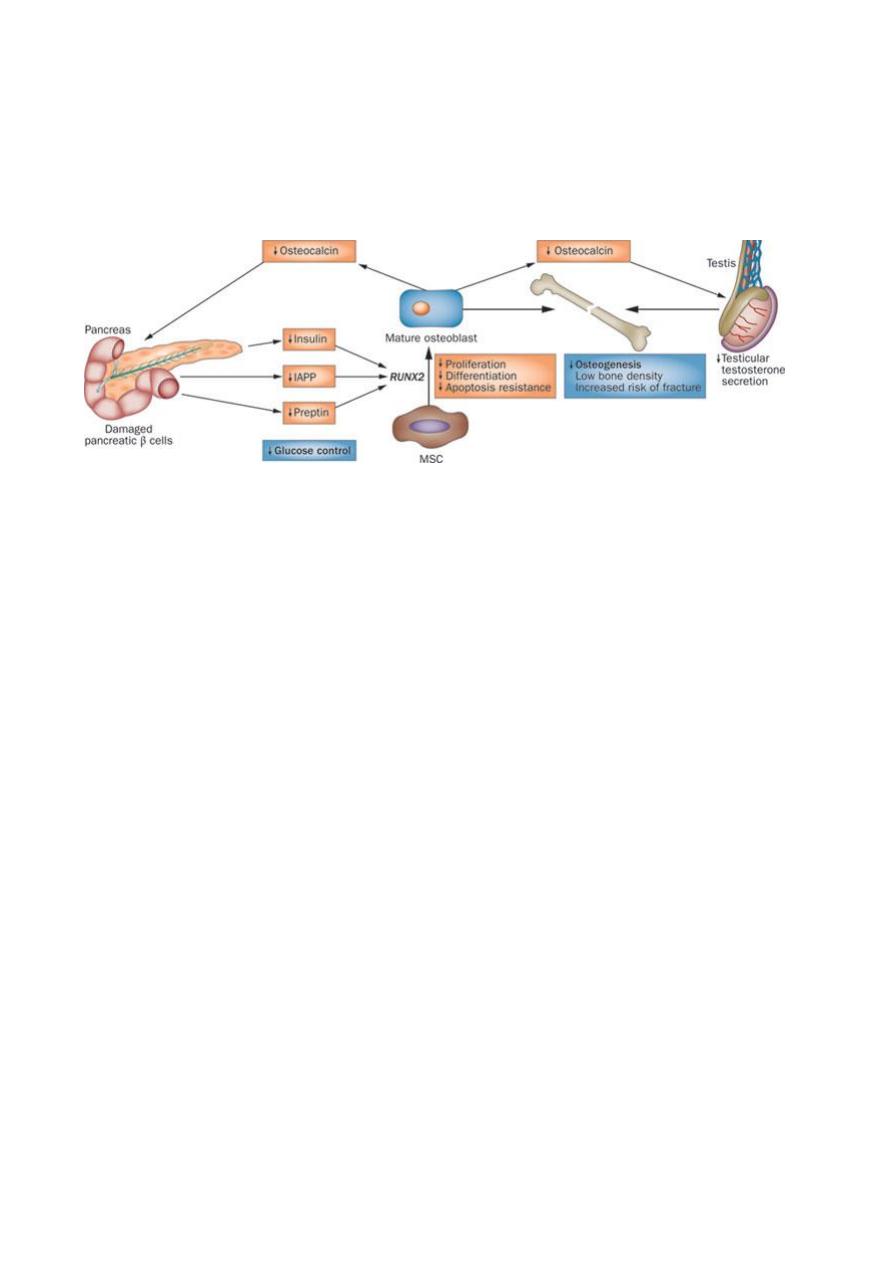
Preptin is a peptide of 34 amino acids co-secreted with insulin and amylin.
Some of its actions:
Stimulates proliferation of primary fetal osteoblast
Reduces osteoblsat apoptosis
Nice to know:
Pancreatic β-cell destruction in patients with T1DM prevents secretion of insulin, IAPP and
preptin, thereby reducing their effects on the RUNX2 (transcriptional factor associated with
osteoblast differentiation) gene. This reduction decreases proliferation and differentiation
of MSCs (marrow stroma cells) into osteoblasts and their resistance to apoptosis
—
preventing osteogenesis and bone mass accrual. Moreover, reduced insulin secretion in
patients with T1DM prevents stimulation of osteoblasts to produce osteocalcin, which
stimulates β-cell proliferation and acts on the testes to produce testosterone, a hormone
that increases osteogenesis.
Delta Cells
The delta cells secrete somatostatin. This consists of two polypeptides, one of 14 amino
acids and one of 28.
Somatostatin has a variety of functions. Taken together, they work to reduce the rate at
which food is absorbed from the contents of the intestine.
Somatostatin is also secreted by the hypothalamus and by the intestine.
Gamma Cells
The gamma cells of the islets secrete a 36-amino-acid pancreatic polypeptide. Its function
is to self regulate the pancreas secretion activities . it also has effects on hepatic glycogen
levels and gastrointestinal secretions.
Its secretion human is increased after a protein meal, fasting, exercise and acute
hypoglycemia and is decreased by somatostatin.,.
Prof.Dr.H.D.El-Yassin 2014
5
Synthesis and release of insulin and glucagon
Insulin and glucagon are synthesized in different cell types of the endocrine pancreas, which
consists of microscopic clusters of small glands (the islets of Langerhans)
. The α cells secrete
glucagon, and the
β cells secrete insulin into the hepatic portal vein via the pancreatic veins.
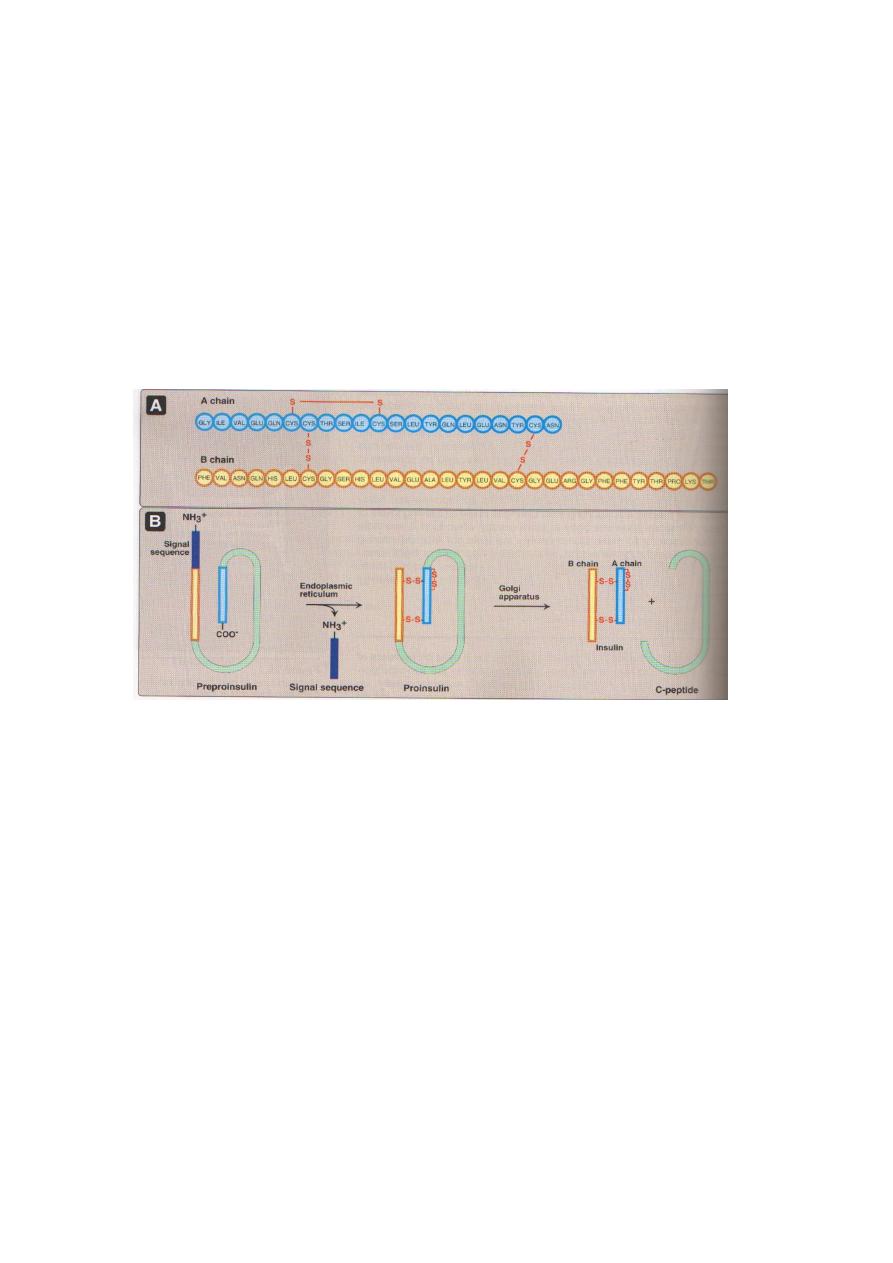
Synthesis and secretion of Insulin
Insulin is a polypeptide hormone. The active form of insulin is composed of two polypeptide
chains (the A-chain and the B-chain) linked by two interchain disulfide bonds. The A-chain has
an additional intrachain disulfide bond.
Insulin, like many other polypeptide hormones, is synthesized as a preprohormone that is
converted in the rough endoplasmic reticulum (RER) to proinsulin. The "pre" sequence, a short
hydrophobic signal sequence at the N-terminal end, is cleaved as it enters the lumen of the
RER. Proinsulin folds into the proper conformation and disulfide bonds are formed between
the cysteine residues. It is then transported in microvesicles to the Golgi complex. It leaves
the Golgi complex in storage vesicles, where a protease removes the C-peptide (a fragment
with no hormonal activity) and a few small remnants, resulting in the formation of biologically
active insulin. Zinc ions are also transported in these storage vesicles. Cleavage of the C-
peptide decreases the solubility of the resulting insulin, which then coprecipitates with zinc.
Exocytosis of the insulin storage vesicles from th
e cytosol of the β cell into the blood is
stimulated by rising levels of glucose in the blood bathing the β cells.
Prof.Dr.H.D.El-Yassin 2014
6
Quick quiz: The metal ion required during crystalizatio of inulin is:
(a) Zinc
(b) Calcium
(c) Copper
(d) Chromium
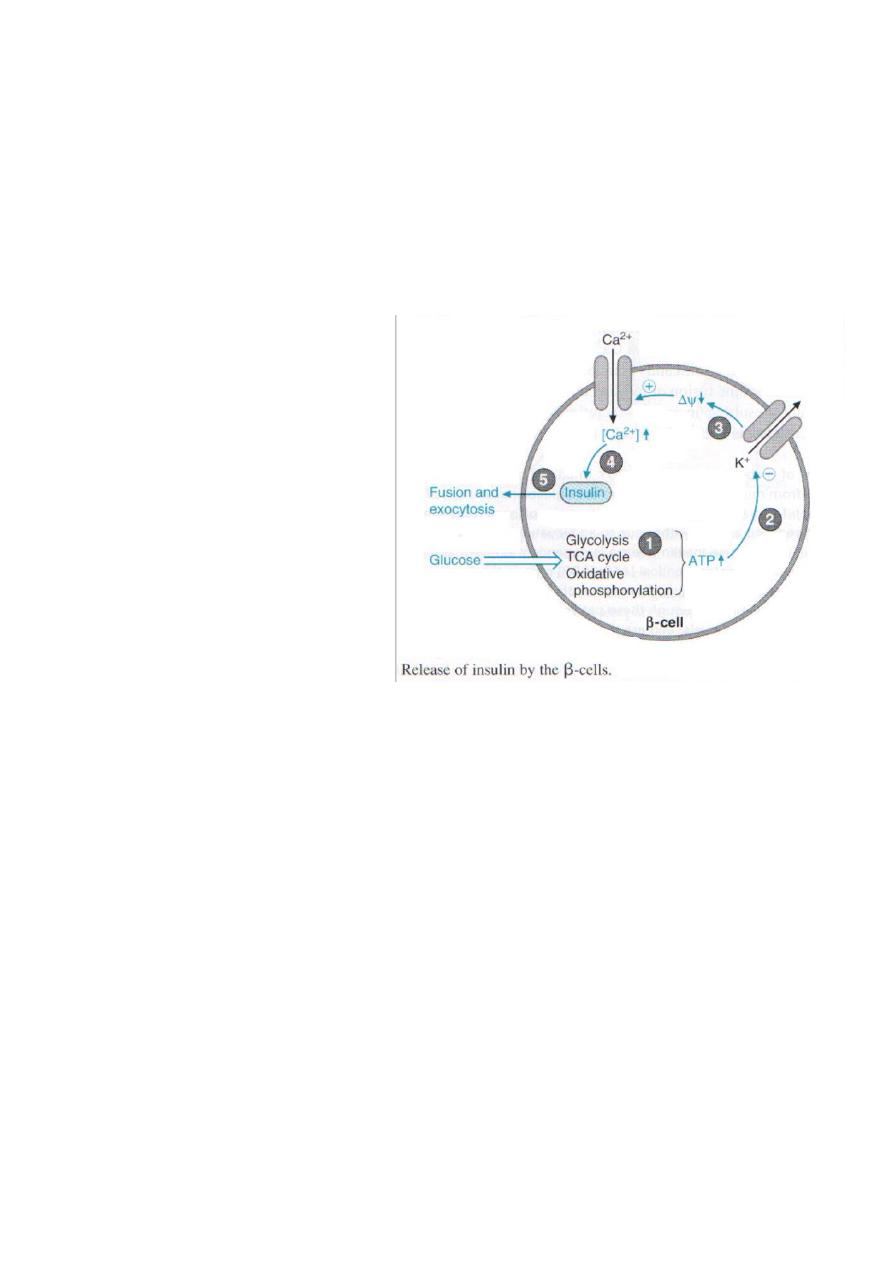
Glucose enters the β cell via specific
glucose transporter proteins known
as
GLUT2.
Glucose
is
phosphorylated through the action of
glucokinase to form glucose 6-
phosphate, which is metabolized
through glycolysis, the TCA cycle,
and oxidative phosphorylation. These
reactions result in an increase in ATP
levels within the β cell. As the β cell
[ATP|/ [ADP] ratio increases, the
activity of a membrane-bound, ATP-
dependent K
+
channel is inhibited (i.e.,
the channel is closed). The closing of this channel leads to a membrane depolarization,
which activates a voltage-gated Ca
2+
channel that allows Ca
+
to enter the β cell such that
intracellular Ca
2+
levels increase significantly. The increase in intra-cellular Ca
2+
stimulates
the fusion of insulin containing exocytotic vesicles with the plasma membrane, resulting in
insulin secretion. Thus, an
increase in glucose levels within the β cells initiates insulin
release.
Stimulation and inhibition of insulin release
The release of insulin occurs within minutes after the pancreas is exposed to a high glucose
concentration. The threshold for insulin release is approximately 80 mg/glucose /dL. Above
80 mg/dL, the rate of insulin release is not an all-or-nothing -response but is proportional to
the glucose concentration up to approximately 300 mg/dL glucose. As insulin is secreted,
the synthesis of new insulin molecules is stimulated, so that secretion is maintained until
blood glucose levele fall. Insulin is rapidly removed from the circulation and degraded by the
liver and to a lesser extent by kidney and skeletal muscle) so that blood insulin levels decrease
rapidly
Prof.Dr.H.D.El-Yassin 2014
7
A number of factors other than the blood glucose concentration can modulate insulin such
as:
1. neural signals
2. certain amino acids
3. gastric inhibitory polypeptide (GIP, a gut hormone released after the ingestion of
food)
4. epinephrine secreted in response to fasting, stress, trauma and vigorous exercise
decrease the release of insulin
Synthesis and secretion of Glucagon
Glucagon a polypeptide hormone, is synthesized in
the α cells of the pancreas by cleavage
of the much larger preproglucagon, a 160-amino acid peptide. Like insulin
preproglucagon is produced on the rough endoplasmic reticulum and is converted to
proglucagon as it enters the ER lumen. Proteolytic cleavage at various sites produce the
mature 29-amino acid glucagon and larger glucagon-containing fragments (named
glucagon-like peptides I and 2). Glucagon is rapidly metabolized, primarily in the liver and
kidneys. Its plasma half-life is only about 3 to 5 minutes.
Insulin
Glucagon Epinephrine
Glucagon secretion is regulated principally by circulating levels of glucose and insulin.
Increasing levels of each inhibit glucagon release. Glucose probably has both a direct
suppressive effect on secretion of glucagon from the α cell as well as an indirect effect, the latter
being mediated by its ability to stimulate the release of insulin.
Certain hormones stimulate glucagon secretion. :
1) catecholamines (epinephrine)
2) cortisol
3) gut hormones
4) Many amino acids also stimulate glucagon release.
Glycogenolysis
Gluconeogenesis
Ketogenesis
Glycogenolysis
Gluconeogenesis
Ketogenesis

Prof.Dr.H.D.El-Yassin 2014
8
Quick quiz:
Most effective stimulation factor to the secretion of glucagons is:
(a) High carbohydrate diet
(b) Hyperglycemia
(c) Hypoglycemia
(d) High fat diet
Metabolic effects of glucagon
1. Effects on carbohydrate metabolism: The intravenous administration of glucagon leads to an immediate
rise in blood glucose. This results from an increase in the breakdown of liver (not muscle) glycogen and
an increase in gluconeogenesis.
2. Effects on lipid metabolism: Glucagon favors hepatic oxidation
OF
fatty acids and the
subsequent formation of ketone bodies acetyl CoA. The lipolytic effect of glucagon in adipose
tissue is minimal in humans.
3. Effects on protein metabolism: Glucagon increases uptake of amino acids by the liver,
resulting in increased availability of carbon skeletons for gluconeogenesis. As a
consequence plasma levels of amino acids are decreased.
Quick quiz:
All the statements are true for glucagons EXCEPT
(a) Its secretion is inhibited by hyperglycemia
(b) It will stimulate only glycogenolysis in muscle
(c) It ill bind to membrane receptors in liver and adipose tissue
(d) It will stimulate lipolysis with the help of hormone sensitive triacylglycerol (TAG) lipase
Prof.Dr.H.D.El-Yassin 2014
9
Insulin and Glucagon receptors
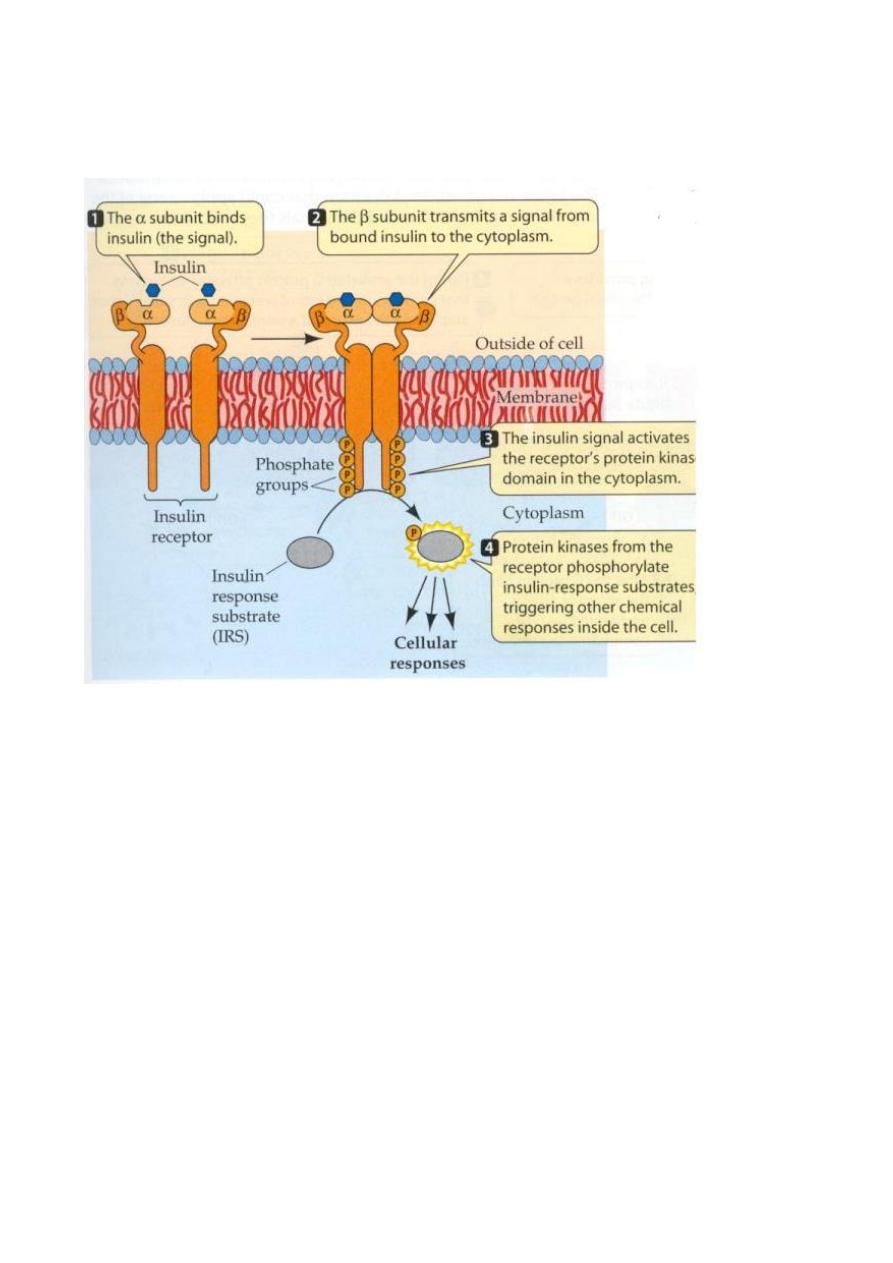
1. Insulin Receptor
Insulin Binding to it's Receptor Followed by Activation of the Receptor:
1.
Insulin binds switching ON the receptor. Once the receptor is ON (catalytically active)
insulin may disaccociate and be degraded.
2.
The Tyr Kinase domain is phosphorylated.
3.
A "cascade" of events takes place (see below).
4.
Biochemical / Physiological Responses:
How insulin binds to its receptor, was not yet known until recently (Jan 013).
They described it as resembling the "handshake"
For more than 20 years, scientists have been trying to solve the mystery of how insulin
binds to the insulin receptor.
The generation of new types of insulin have been limited by our inability to see how insulin
interacts with its receptor in the body.
Prof.Dr.H.D.El-Yassin 2014
10
Quick quiz: the interaction of insulin with its receptor
1. cause a conformational change in the receptor only
2. cause a conformational change in the hormone only
3. cause a conformational change in both hormone and receptor.
4. cause no conformational change
Understanding how insulin interacts with the insulin receptor is fundamental to the
development of novel insulins for the treatment of diabetes
The importance of this finding is that:
insulin is a key therapy for type 1 and type 2dm diabetes mellitus and pharmaceutical
industries are interested in making insulin that have varying properties so that:
people might not have to inject insulin quite often or
might ingest insulin in different ways or
might be interested in making insulin that can be stored in normal temps.
It shows which part of insulin you could alter , which parts you have to leave the same and
suggests ways in which you could treat the insulin molecule to generate new properties
that could make beneficial therapeutic for patients of type1 and type 2 DM
Quick quiz: How can this finding benefit diabetics
(a) by providing more injected insulin
(b) by curing their damaged B cells
(c) generating new properties to the insulin molecule that shall be used theraputicaly
(d) will have no benefit at all
Prof.Dr.H.D.El-Yassin 2014
11
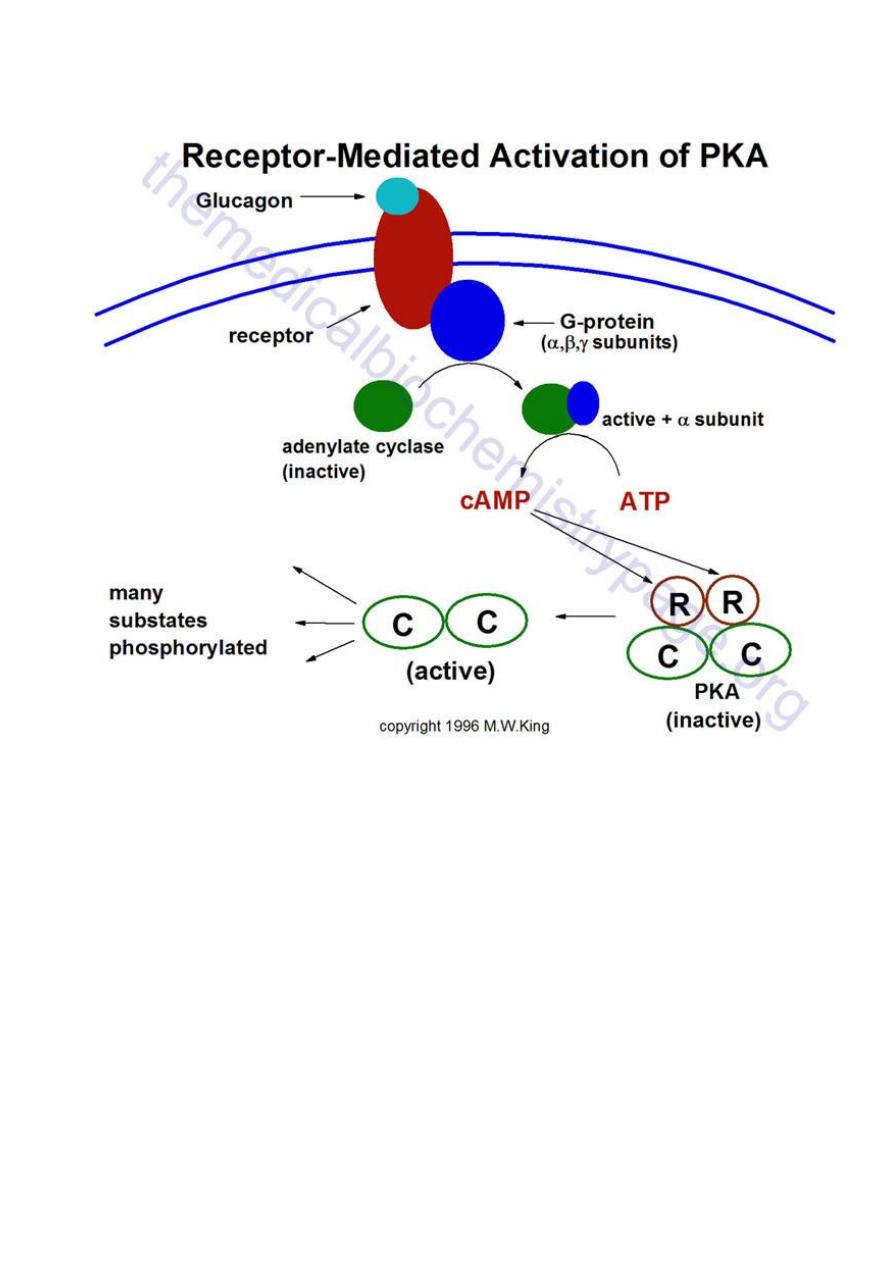
2. Glucagon Receptor
Representative pathway for the activation of cAMP-dependent protein kinase, PKA. In this
example glucagon binds to its' cell-surface receptor, thereby activating the receptor.
Activation of the receptor is coupled to the activation of a receptor-coupled G-protein
(GTP-
binding and hydrolyzing protein) composed of 3 subunits. Upon activation the α-
subunit dissociates and binds to and activates adenylate cyclase. Adenylate cylcase then
converts ATP to cyclic-AMP (cAMP). The cAMP thus produced then binds to the
regulatory subunits of PKA leading to dissociation of the associated catalytic subunits. The
catalytic subunits are inactive until dissociated from the regulatory subunits. Once released
the catalytic subunits of PKA phosphorylate numerous substrate using ATP as the
phosphate donor

Prof.Dr.H.D.El-Yassin 2014
12
Clinical cases and correlations
Insulinoma
A 36-year-old woman was referred to a university hospital for evaluation of spells of
dizziness and weakness. These spells typically lasted for 10 min and were occurring with
increasing frequency. The spells usually came on after a large meal and could be
terminated by her eating candy or drinking fruit juice. After each episode the patient was
hungry and tired, and her memory was blurred. The patient's physical examination was
within normal limits except for mild obesity. She claimed to have gained 20 kg during the
preceding 2 yr. After a 13-hr fast her blood glucose concentration was 2.1 mmollL. After a
5-hr glucose tolerance test, her blood glucose was 2.6 mmol/L.
Celiac angiography revealed an abnormality in the body and tail of the pancreas. The
patient developed one of her spells while a medical student was in her room, and he was
able to obtain a blood sample during the episode. This sample contained 1.1mmol/L of
glucose. The patient was transferred to the surgical service, and an insulin-secreting
pancreatic adenoma (tumor) was removed, requiring resection of 90% of the pancreas.
Biochemical questions
1. An insulinoma is an insulin-secreting tumor. How did the presence of such a tumor
explain the patient's symptoms?
2. Proinsulin was found in large quantities in this patient's plasma. What is the
relationship of proinsulin to insulin? What is preproinsulin?
3. What effects of increased insulin secretion might have predisposed this woman to
obesity?
4. What digestive problems might result from excision of 90% of the pancreas?
The insulinoma was producing insulin. Because of the excessive amount of insulin-
secreting tissue, too much insulin was released after dietary carbohydrate intake. This
caused hypoglycemia during the 5-hr glucose tolerance test and after meals, producing
the spells of weakness and dizziness. In addition, to this normal insulin release when
carbohydrate was ingested, the tumor also was secreting some insulin continuously.
This inappropriate insulin release caused the low blood glucose concentration during
prolonged fasting.
Proinsulin is the prohormone form of insulin that is made in the
-cells of the pancreatic
islets. It has no insulin-like action. After synthesis on the ribosomes, the initial precursor,
preproinsulin, penetrates through the endoplasmic reticulum into the lumen of this
organelle. The leader sequence is removed in this process, forming proinsulin, which is

Prof.Dr.H.D.El-Yassin 2014
13
transported to the Golgi apparatus and stored in granules.
Proinsulin is converted to insulin in these granules by proteolytic cleavage, but the
conversion is incomplete. When insulin is discharged from the
-cell, some proinsulin that
remains in the granule also is released. Likewise, C-peptide that is split out in the
conversion of proinsulin to insulin is released during insulin secretion, but it too, has no
insulin-like activity.
Insulin acts on adipocytes, enhancing fatty acid storage as triglyceride. It binds to specific
receptors on the cell surface and facilitates glucose entry into the adipocyte, increasing the
availability of the triose backbone, glycerol 3-phosphate, needed for triglyceride synthesis.
This also provide glucose carbon atoms for fatty acid syntesis.
In addition, it increases the content of lipoprotein lipase in the adipose tissue. This enzyme
catalyzes the hydrolysis of chyromicron and VLDL triglycerides, a step that is required to
transfer their fatty acids into the adipocytes for resynthesis into triglyceride. Much of the
fatty acid stored in the adipose tissue is delivered to the adipocytes in the form of
lipoprotein triglycerides, either VLDL from the liver or chylomicrons from the intestine.
Therefore the elevated lipoprotein lipase activity also favors triglyceride formation in the
adipose tissue. Those adipose tissue effects that were mediated by the excessive insulin
production could have contributed to he recent weight gain noted by this patient.
In addition to polypeptide hormones, the pancreas making many digestive enzymes.
These include amylase for dietary starches, lipase for triglycerides, chymotrypsin and
trypsin for proteins, as well as several others. Since 90%, of the pancrease was excised,
the remaining 10% may not produce sufficient amounts of these enzymes to adequately
digest large meals. This might lead to malnutrition and weight toss in spite of an adequate
diet. Because of this possibility, six or more smaller meals rather than three regular meals
each day might be recommended.
Question: Binding of insulin to its receptor:
a) Occurs on the ß-subunit.
b) Induces autophosphorylation.
c) Reduces binding of cytosolic substrate proteins.
d) Leads only to phosphorylation of proteins.
e) Does not lead to release of a second messenger.
Answer:
B This occurs on tyrosine residues of the ß-
subunit. A: Binding is to the α-
subunit. C: Autophosphorylation facilitates binding. D: Some proteins are
dephosphorylated. E: A second messenger may account for short-term metabolic effects.
