FATTY
ACIDS
Objective:
Describe the pathways of fatty
acids synthesis, oxidations and energy production.
Identify lipogensis (TG synthesis) & lipolysis (TG
degradation) in the liver, adipose tissues and
muscles
with
hormonal
regulation.
Illustrate
ketone
bodies
production
and
utilization in physiological & pathological
conditions like in D M.

Fatty
acids
synthesis
Fatty Acids (FAs) are synthesized endogenously by:
1. De novo synthesis from Acetyl-CoA, mainly the synthesis
of
Palmitic
acid
C16
2. Elongation system; synthesis of vey long FAs needed in
the CNS from the previously synthesized FA in de Novo
pathway.
De
novo
synthesis
of
FAs:
It occurred in the cytoplasm, starting from Acetyl-CoA and
need
for
ATP
and
NADPH,
and
involved:

1. First step is activated by Acetyl-CoA carboxylase
(ACC) enzyme which is the regulatory enzyme of FAs
synthesis
pathway
Acetyl-CoA(C2) + CO2 (as HCO3
-
)+ATP = Malonyl-
CoA(C3)
2. Malonyl-CoA (FROM STEP 1)+ another molecule of
C2(acetyl-CoA) with removal of CO2, results in gaining
of two carbon fragment and formation of C4 molecule
(acetoacetyl-CoA). This step is stimulated by
Multienzyme complex, the Fatty acid synthase and
involved four steps and needed for NADPH, AND
repeated several times with

addition of C2 (from malonyl-CoA) with each turn till
formation the required FA, the C10, C12, C14,C16, C14,
C18.
Acetyl-CoA(C2)+CO2=malonyl-CoA(C3)
Malonyl-CoA+ C2=C4+CO2 (four steps)
C4+Malonyl-CoA(C3)=C6+CO2 (four steps)
C6+C3=C8+CO2 (four steps)
C8+C3=C10+CO2……….. C16. (four steps)

NADPH molecules are derived mainly from PPP
(PENTOSE PHOSPHATE PATHWAY) of CHO metabolism
(which occurred in the cytoplasm) and also from Malic
acid
pathway
.
Regulation
of
FA
synthesis:
It regulated via the Acetyl-CoA carboxylase enzyme,
this enzyme is stimulated by Citrate and inhibited by
long chain FA (or long chain acyl-CoA), this regulation of
enzyme activity is achieved by phosphoralation and
dephosphoralation via cAMP. Moreover, the same
enzyme is stimulated by Insulin and inhibited by
Glucagon by their effects on enzyme gene expression
(hormonal
regulation).

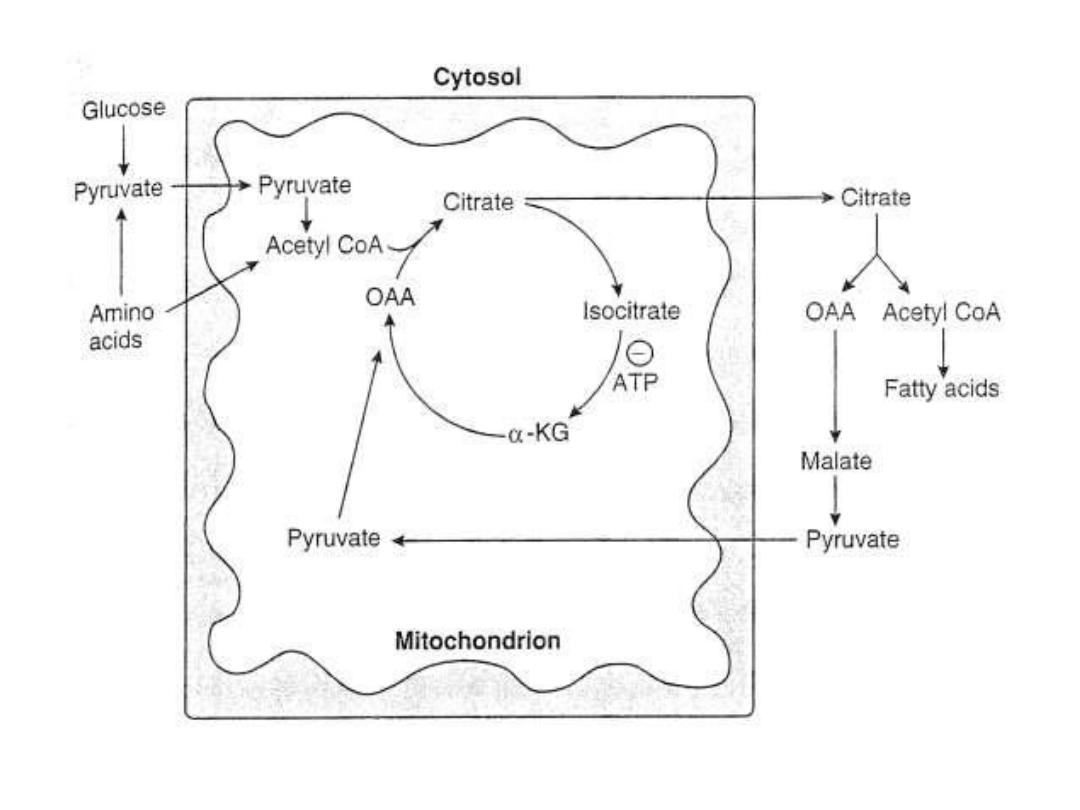
FA synthesis, Excess CHO, and Obesity:
You must now
that, acetyl-CoA substrate of FA synthesis is derived
from
CHO:
Excess amount of ingested CHO= Increased blood
glucose=Glycolysis
(cytoplasm)=Pyruvate(cytoplasm)+in presence of O2=
Pyruvate (mitochondria) BY PDH enzyme= Acetyl-CoA
(mitoch.)+
oxaloacetate(OxA)=citrate
Citrate…..CAC…..=ATPs, when the requirement of ATPs
is achieved by CAC, the ATPs inhibit the ICD enzyme of
this
cycle..=accumulation
of
citrate
(mitoch.)
…=..citrate (cytoplasm), here by ATP-citrate Lyase
enzyme=
acetyl-CoA+
OxA.

By these steps excess CHO will be converted into acetyl-CoA
in the cytoplasm which will be entered into the FA pathway
synthesis.
So, excess CHO will leads to increased in FA synthesis ????.
Synthesized FAs in the cytoplasm (as 3Acyl-CoA)+
glycerol (as glycerol-3-phosphate=TG=VLDL….blood
transported mainly to sk-M, CM, and adipose tissues
for
uptake
of
FFAs.
In the adipose tissues, the taken FFA will be reacted in
the form of acyl-CoA WITH glycerol-3-P= TG that stored
there with resultant increased the size of
adipocytes=obesity.

FA
Oxidations
These are the pathways that deal with degradation of
FAs in order to produce the ATPs, in other words, they
are the ways for utilization of body from the
endogenous and exogenous lipid. They are of several
types; alpha, beta, and gamma, of which the Beta is
the
most
important
one
in
human.
In case of dietary lipids, liver, muscles use the lipid in
alternative with CHO for energy production. The dietary
energetic lipid is TG which is firstly hydrolyzed into:
TG =glycerol+FFA, which occurred in the cytoplasm.
The produced FFAs are the lipid substrates of ATPs
production

Beta-oxidation
pathway:
This pathway occurred in the mitochondrial matrix
and
starting
from
acyl-CoA
(for
example;
CH3(CH2)14COCoA)
and
not
the
FFA(CH3(CH2)14COOH). So, the entered FFA in the
cytoplasm of Muscle for example is (steps of FA Beta-
oxidation):
1. activation of FFA (inactive substance)=acyl-CoA
(active substance) CH3(CH2)14COCoA, by thiokinase
enzyme
and
2ATP

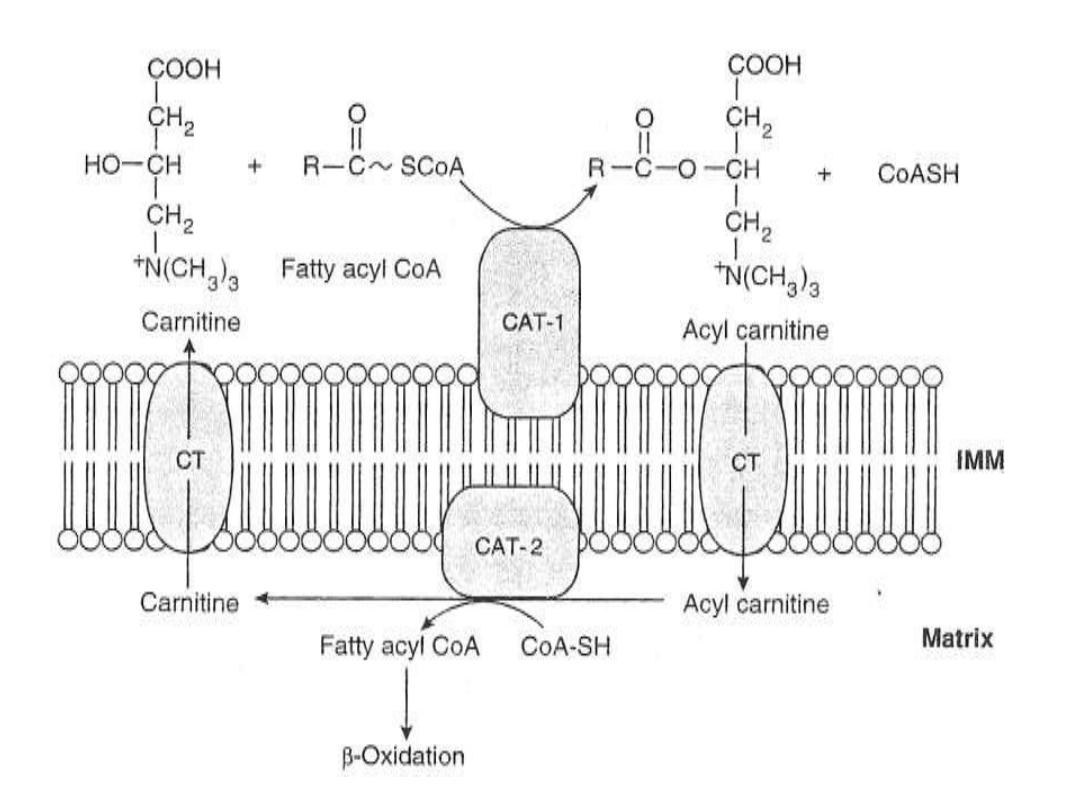
2. the activated acyl-CoA must be transported into the
mitoch. across the mitoch.membrane which only
occurred in the presence of CARNITINE SHUTTLE
SYSTEM with exception for short chain FFA(C2-C4) for
which the mitochondrial membrane is permeable. This
SYSTEM
is
composed
of
:
a.
Acyl-Carnitine Transferase I (ACTI, APTI) located in
the
outer
mitochondrial
memebrane
b
.
Translocase which spanning the inner mitochondrial
membrane
c
.
Acyl-Carnitine Transferase (ACT II, APT II)
and
d
.
Carnitine substance.
CARNITINE SUBSTANCE is
C4 synthesized endogenously

from lysine and metheonine amino acids in the liver
and derived exogenously from meat product, its
deficiency will lead to impairment of beta-oxidation
such as in malnutrition.
3. In the matrix of
mitochondria, the beta-oxidation take place by
cleavage of two-carbon fragment from the entered
acyl-CoA with each turn (each turn involved 4 steps
and
produced
reducing
equivalents
1NADH+1FADH2=5ATPs).
So, in case of C16=C14+ acetyl-CoA , AND, then the
formed C14 will undergoes the same four steps and
produced=C12+ acetyl-CoA+1NADH+1FADH2 , and so
continued till formation of C4=2acetyl-CoA.

The second carbon also named alpha, the C3 is beta, C4
gamma.
3 2 1 C16:
CH3…….
CH2CH2COCoA
Cleavage
beta alpha
between C2(alpha and C3(beta), so the name beta-
oxidation
c14: CH3..CH2CH2CoA (C12)+Acetyl-CoA
C12: CH3…CH2CoA(C10)+Acetyl-CoA
With production of 1NADH+1FADH2 with each
cleavage.

So the number of turns=(Number of Carbon/2)-1, for
C16=(16/2)-1=7x5ATPs=35 ATPs.
Moreover, the cleaved
two-carbon is produced as acetyl-CoA. The number of
acetyl-CoA =Number of carbon chain/2=C16/2=8
acetyl-CoA
which entered in the CAC pathway and
produced 12 ATPs(3NADH+1FADH2+GTP), So, 8 Acetyl-
CoAx12 ATPs=96 ATPs.
Thus total produced ATPs from
beta-oxidation of C16=35+96=131 ATPs-2(for activation
of FFA , RCOOH=RCOCoA,step 1)=129 ATPs.

So, in general, the number of ATPs produced from
Beta-oxidation only are=No. of turnx5, while from
complete oxidation of FFA (Beta-oxidation+CAC for
produced acetyl-CoA)=No. of turnsx5ATPs+No. of
acetyl-CoAx12 ATPs. The entered FFAs in the cytoplasm
of muscle (Sk-M & CM) are for production of local
energy. In the liver, the control
between the re-
esterification of the entered FFA in the cytoplasm with
formation of TG and the mitochondrial beta-oxidation
of FFA is very important and regulable and the two
pathways cannot be proceeded simultaneously.

The control is dependent on Nutritional state: in normal
feeding, Insulin secretion increased ?=stimulation of
acetyl-CoA carboxylase enzyme?, the??=increased of FA
and TG synthesis, while in fasting=insulin decreased and
antagonist hormones like glucagon increased ?=decreased
the activity of enzyme??=inhibition of FA pathway
synthesis and stimulation of Beta-oxidation pathway.
The
produced FFA from synthesis pathway either 1. In the liver
and in the feeding of normal meal, FA derived from
remnant chylomicron will be reesterified with glycerol to
form TG=VLDL= and transported to the Muscles and
adipose
tissues.

2. In the fasting state & prolonged exercise, uncontrolled D M,
and lipid rich meal, the body will depend mainly on the stored
adipose
tissue
TG.
In
the
latter state, the stored TG , and under the effect of glucagon, GH,
ACTH, TSH…etc, and the absence of insulin, will be mobilized
from
adipose
tissue:
TG=FFAs+glycerol, by the enzyme Hormone-sensitive(HS-LPL, the
adipocytes intracellular enzyme which is active only in the
presence of glucagon, adrenaline…..). The released or mobilized
FFAs transported in the blood as FA-albumin. The FAs will be
taken by Muscles and other tissues with exception of RBCs and
Brain ?? which proceeded them in beta-oxidation for energy
production.
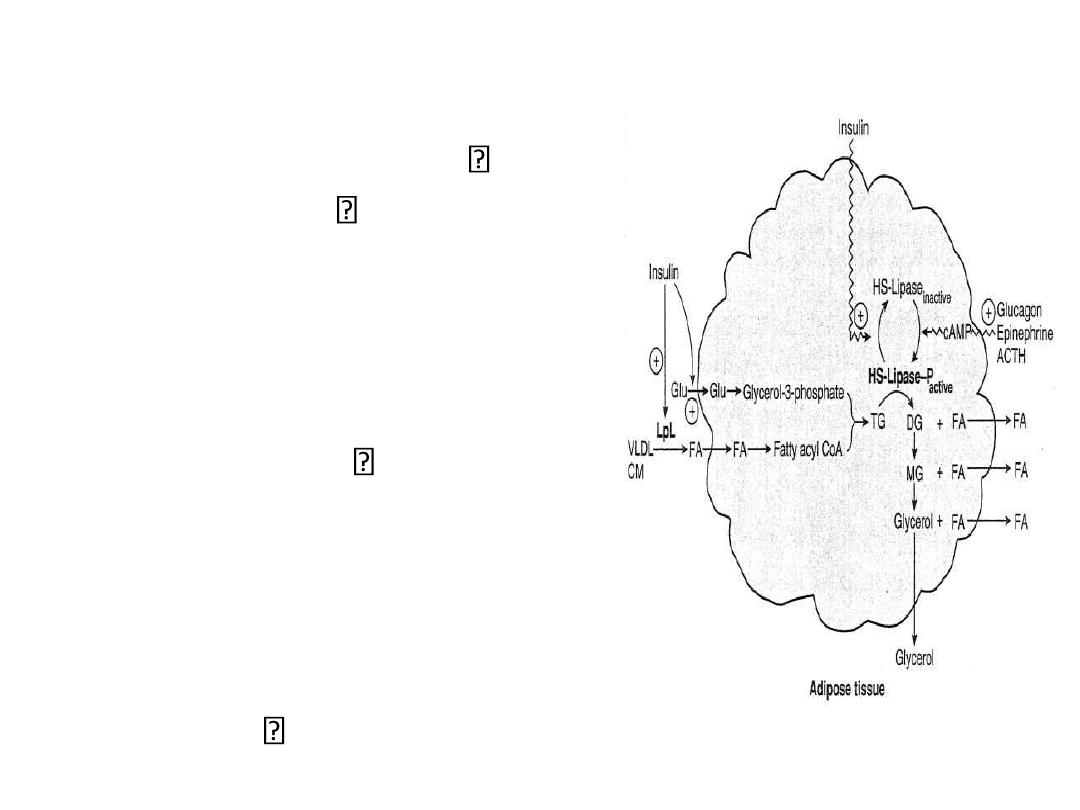
Lipid Metabolism in Fat Cells:
Fed State
Insulin
stimulates LPL coated
on the vascular endothelial
– increased uptake of FA
from chylomicrons and
VLDL
stimulates glycolysis
– increased glycerol
phosphate synthesis
– increases esterification
– inactivates HSL
net effect: TG storage
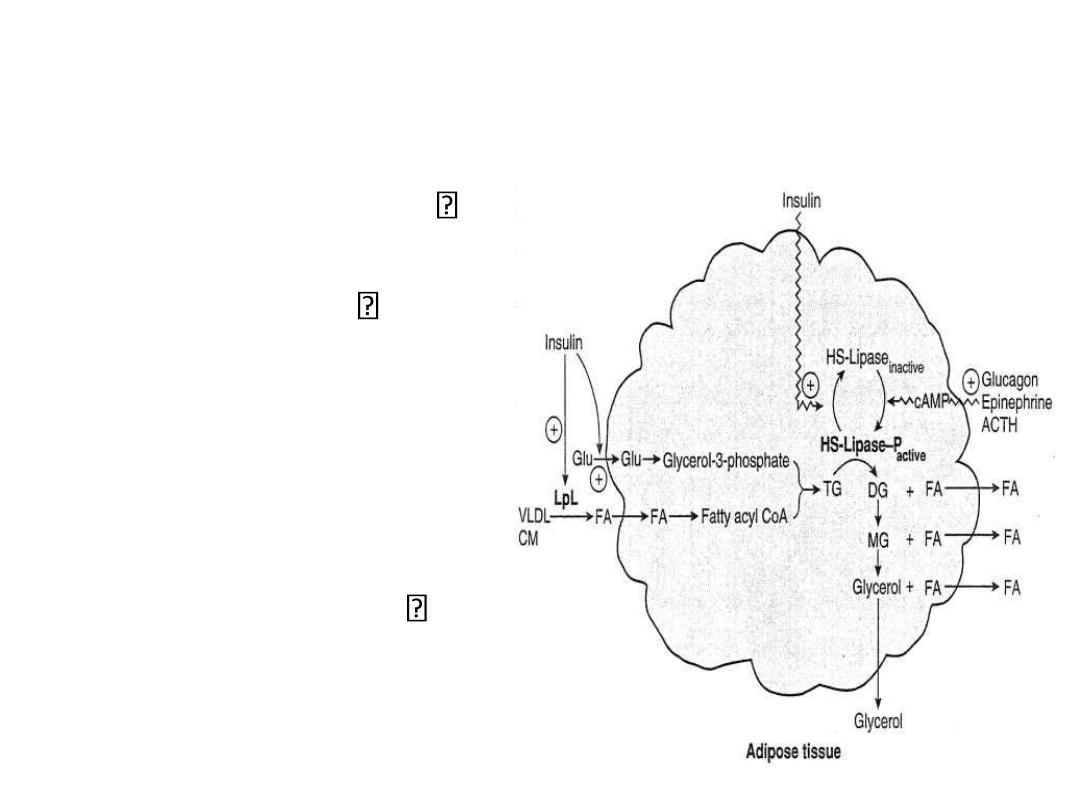
Lipid Metabolism in Fat Cells:
Starved or Exercising State
Glucagon,
epinephrine
activates adenylate
cyclase
– increases cAMP
– activates protein
kinase A
– activates HSL
net effect: TG
mobilization and
increased FFA

The regulation of Beta –oxidation is at CAT-I(CPT-I) of
Carnitine shuttle system. This enzyme (ACT-I) is stimulated by
long chain FAs (or long chain acyl-CoA) and inhibited by
malonyl
–CoA
of
FA
synthesis
and
Insulin.
So, in feeding state , the citrate concentration increased which
stimulates acyl-CoA carboxylase (enhanced FA synthesis
pathway), also, the malonyl-CoA the product of acyl-CoA
carboxylase is increased which inhibits CAT-I and so inhibits
the B-oxidation pathway, the net: enhanced synthesis of FAs
and inhibition of oxidation pathways. The vice versa in
prolonged Fasting state , starvation& prolonged exercise
(physiological conditions)and uncontrolled D M (pathological
condition).

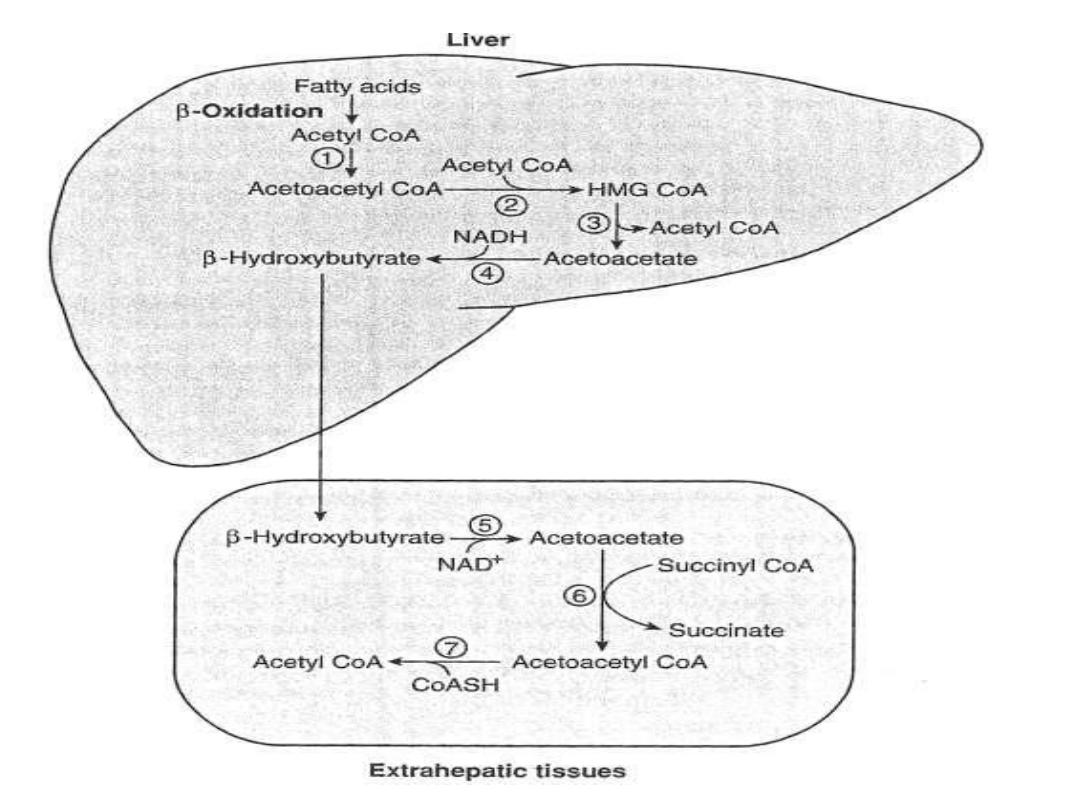
Ketone bodies:
These are three substances,
acetoactate, beta-hydroxybutyrate and acetone. They
are produced from acetyl-CoA that are derived from
beta-oxidation of FA, so their blood and tissues levels
are increased in parallel to the blood concentrations of
FAs. These substances by themselves are not harmful
but the produced of H+ with them are the harmful and
so prolonged oxidation of FFAs results in increased of
ketone bodies formation and H+ concentration
(decreased the PH) and the disturbance of body buffer
system
which
if
not
treated
is
fatal.

Ketone bodies are synthesized exclusively in the liver
(the two required enzymes HMG-CoA synthase and
lyase are found only in the liver), but are utilized in the
extrahepatic tissues , the Muscle and brain (which have
transferase enzyme for activation of acetoacetate).
In
feeding state
blood concentration of ketone bodies are
increased or decreased?, while in fasting are increased
or
decreased?.
Although from their associated ion H+ harmful, ketone
bodies are important as alternative energy fuel for
glucose in the brain in case of starvation and D M when
the blood levels of glucose are low or unutilized,
respectively.

In the latter cases, the brain will be depend on
ketone bodies for production of ATPs and spares
the limited available glucose for RBCs. Brain
cannot
use
FFAs
beta-oxidation?.
In starvation: ketone bodies blood levels
increased up to 2.5 times after 3 weeks compared
with
that
after
3
days
of
starvation.
Ketonemia: means the increased blood levels of
ketone bodies. Ketonuria, means the increased of
urinary excretion of ketone bodies. Ketosis
included both ketonemia and ketonuria
.