
Antineoplastic Agents
Lecture 2
1
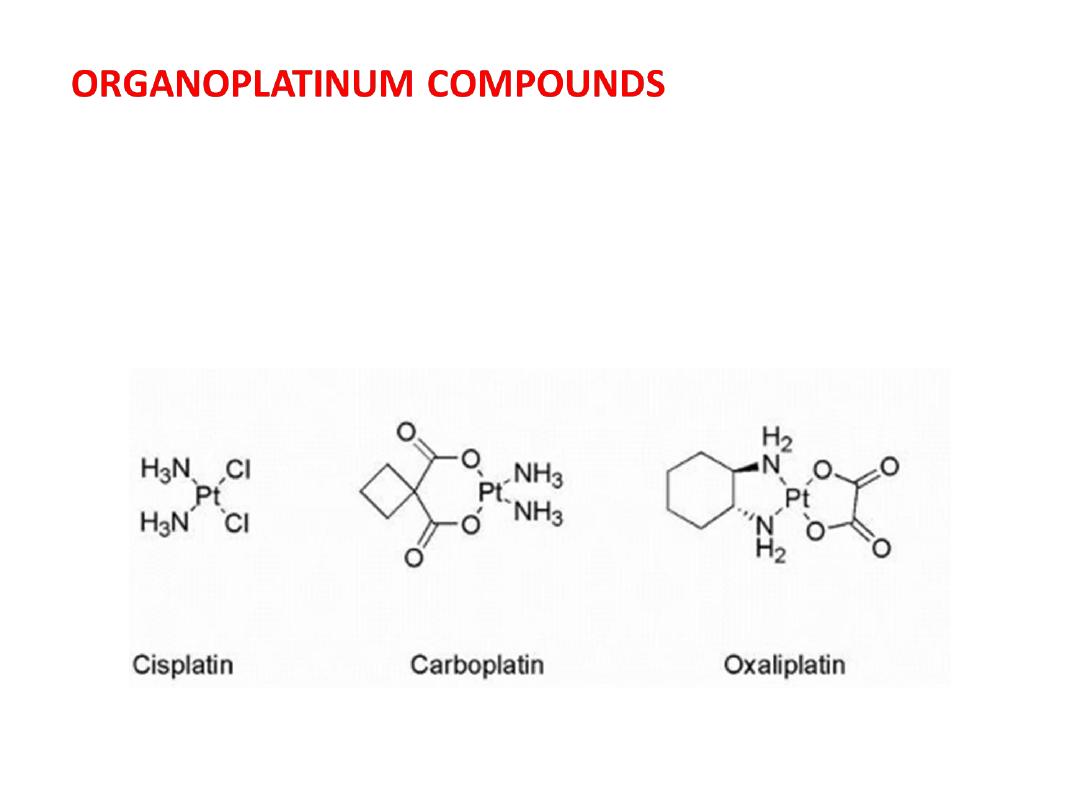
Is compounds based on platinum that play a central role
in many cancer treatment protocols
2

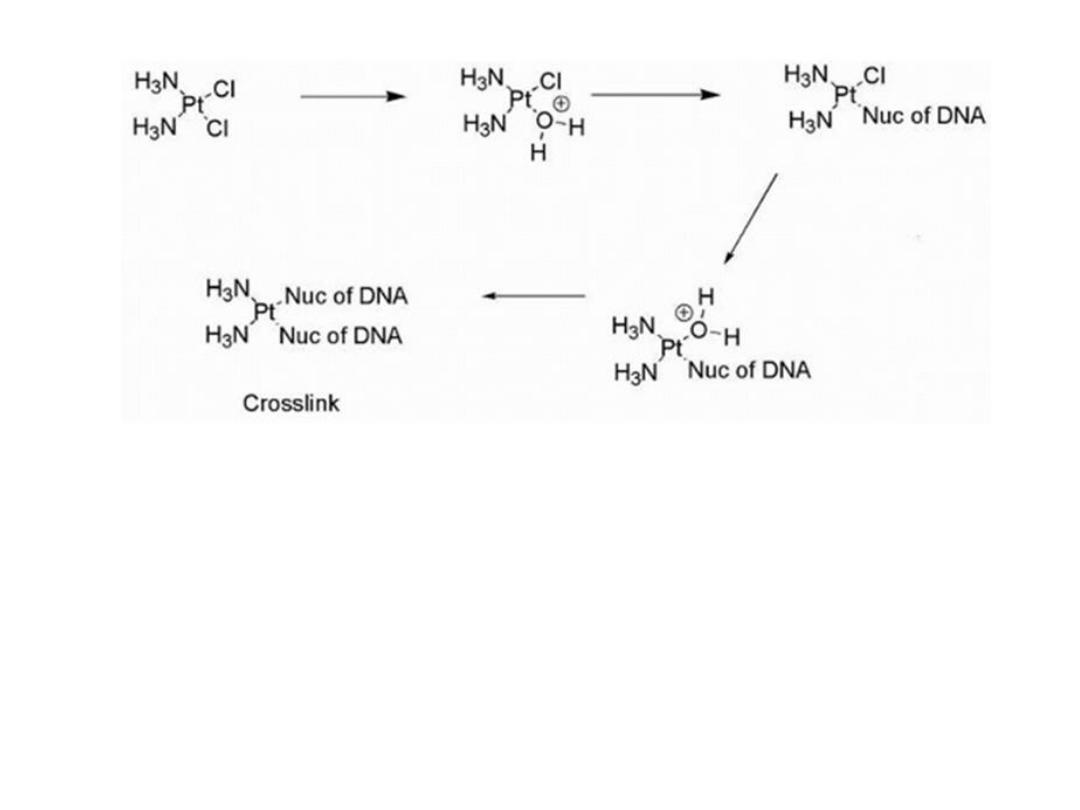
The dichloro species is maintained in the blood stream
as a result of the relatively high chloride concentration .
Movement into the tumor cells is accomplished by
passive diffusion or carrier-mediated transport. Once
inside the tumor cell, the drug encounters a lower
chloride concentration and one chloro group is
substituted by a water molecule in a process known as
aquation. This serves to “trap” the molecule in the cell
as a result of ionization. Reaction with DNA occurs
preferentially at the N-7 of guanine of two adjacent
guanine residues resulting in primarily (95%) intrastrand
cross-links
3
Platinum (II) is considered to be a “soft” electrophile and
as a result, its complexes are subject to attack by “soft”
nucleophiles such as thiol groups found on proteins. This
can result in significant protein binding (88%-95%) and
inactivation caused by the presence of thiols in albumin,
glutathione, and other proteins.
4
Cisplatin administration is also associated with significant
nephrotoxicity and neurotoxicity that is dose limiting. These
factors lead to the development of less reactive platinum
compounds such as carboplatin and oxaliplatin in which the
leaving group was incorporated into achelate.
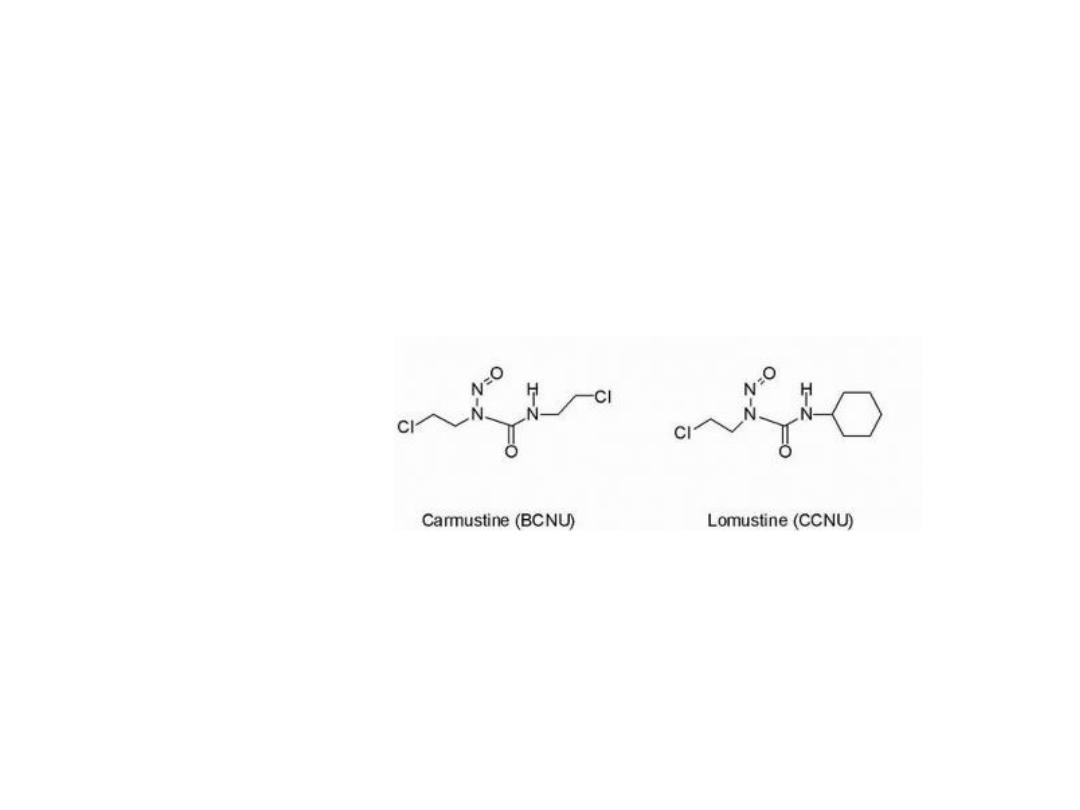
NITROSOUREAS
General structural requirement
1. Urea moiety.
2. Nitroso group linked to the urea.
3. Electronegative atom with good leaving properties.
5

These compounds are reasonably stable at pH = 4.5 but
undergo both acid and base catalyzed decomposition at
lower and higher pH, respectively
alkylation of DNA involves abstraction of the NH proton,
which is relatively acidic (pKa = 8-9), followed by
rearrangement
to
give
an
isocyanate
and
a
diazohydroxide.
The diazohydroxide, upon protonation followed by loss of
water, yields a diazo species that decomposes to a reactive
carbocation . The isocyanate functions to carbamylate
proteins and RNA, whereas the carbocation is believed to
be the agent responsible for DNA alkylation.
6
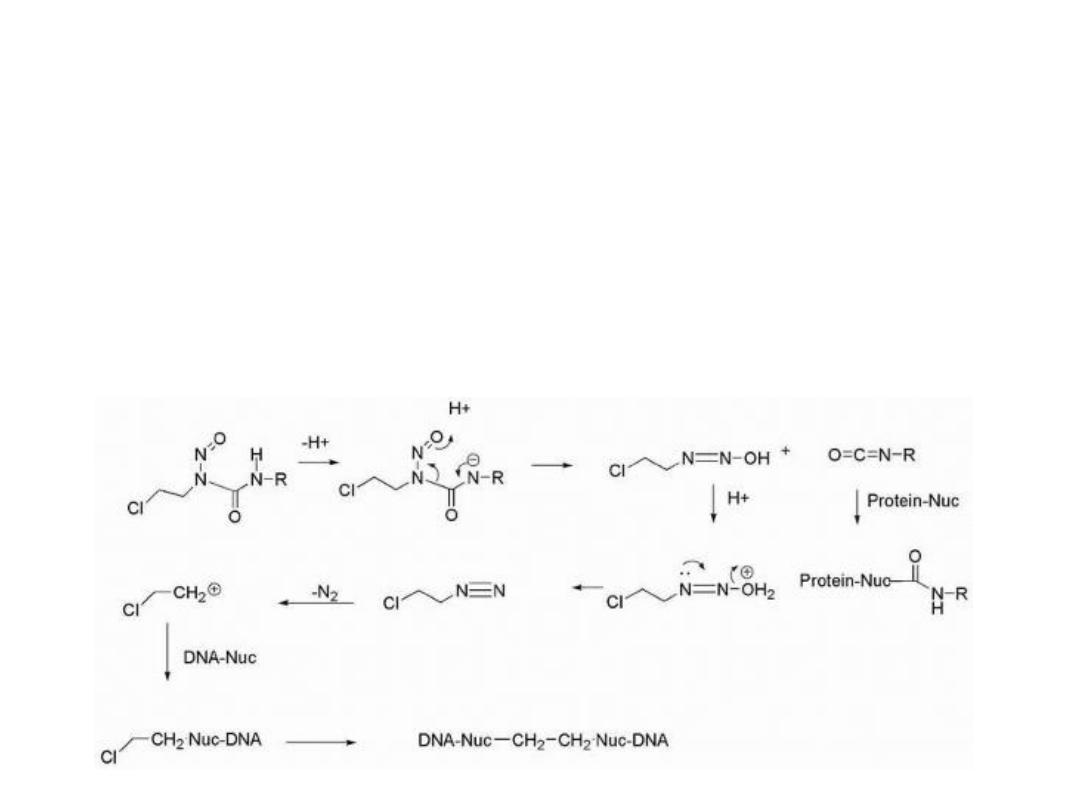
Alternative mechanisms of decomposition have also
been proposed involving formation of chlorovinyl . In
those cases where there is a chloroethyl moiety attached
to the N-nitroso urea functionality, crosslinking of DNA
occurs. Alkylation occurs preferentially at the N-7
position of guanine with minor amounts of alkylation at
O guanine .
7
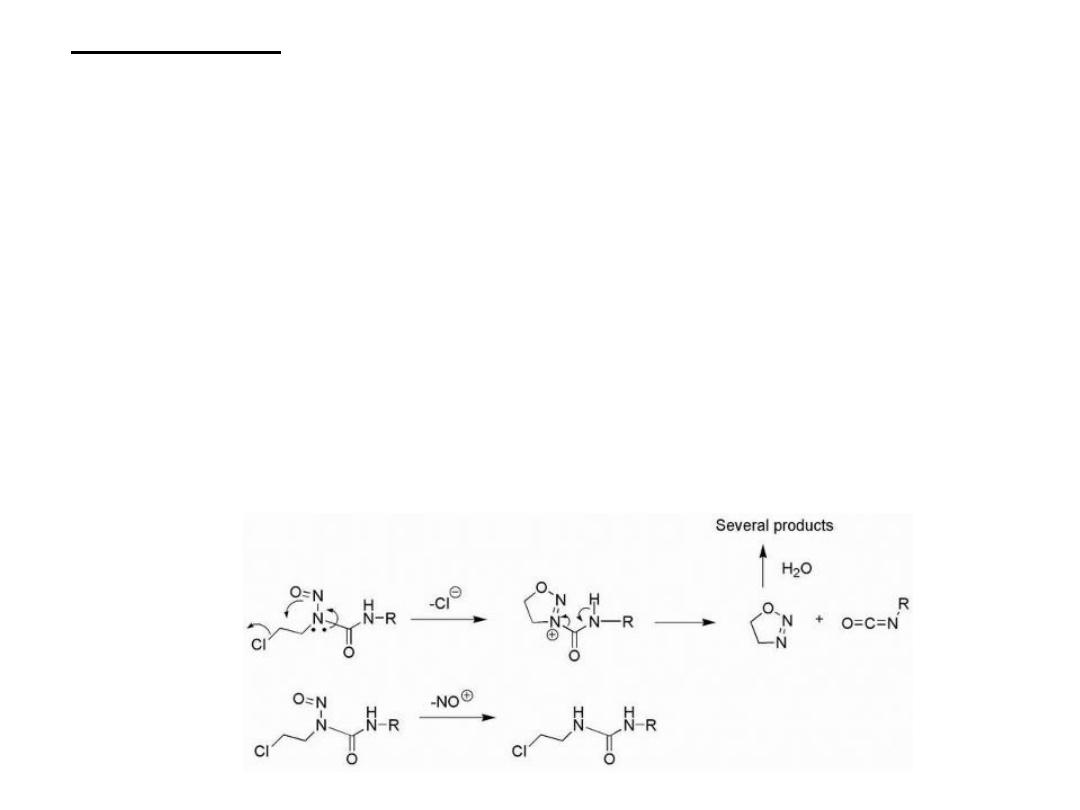
Detoxification pathways of the nitrosoureas are also possible
and can play a role in resistance to this group of agents. Two
major routes of inactivation have been identified and are
indicated in The first of these involves dechlorination, which
is facilitated by CYP participation and involves cyclization to
give 4,5-dihydro-[1,2,3] oxadiazole and the isocyanate, which
is still capable of carbamylating proteins.The oxadiazole can
be further degraded by hydrolysis to give several inactive
products. The second route involves denitrosation, which in
the case of BCNU (carmustine) has been shown to be
catalyzed by CYP monooxygenases and glutathione-S-
reductase.
8
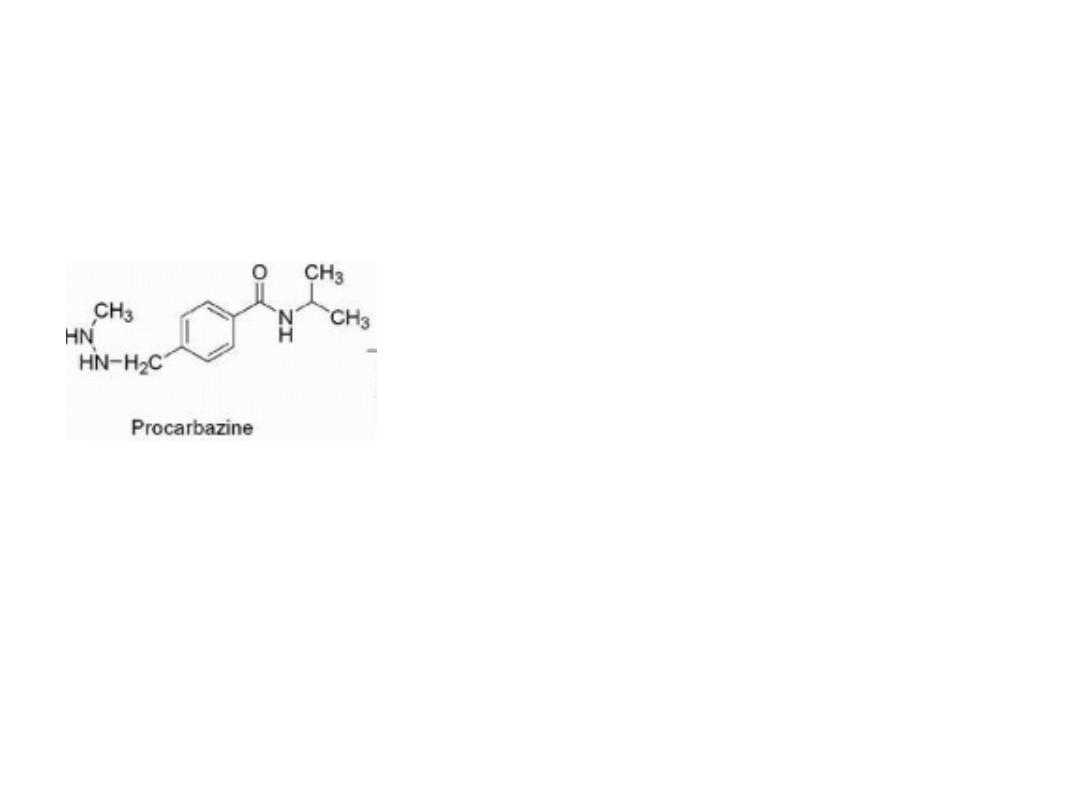
PROCARBAZINE, DACARBAZINE, AND TEMOZOLOMIDE
Procarbazine
oxidation of procarbazine does occur in the liver and is
mediated by CYP and monoamine oxidase to give azo-
procarbazine. This compound may also be generated
nonenzymatically in an aerobic environment
9

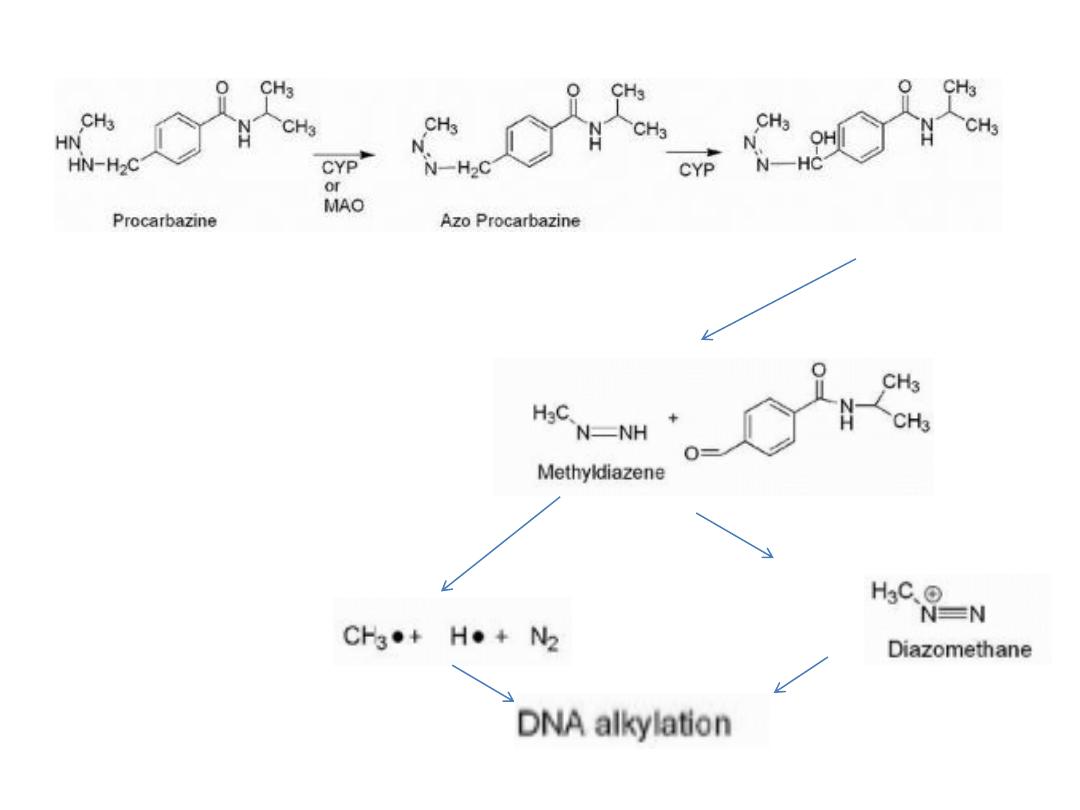
There are several chemical and metabolic pathways that azo-
procarbazine may undergo, one such route involves CYP-
mediated oxidation of the benzylic methylene carbon with
subsequent decomposition to give methyldiazine and the
aldehyde. The methyldiazine may then decompose by
hemolytic bond cleavage to give methyl and hydrogen
radicals along with nitrogen gas or be further oxidized to give
the diazo compound, which can decompose to give the
methyl carbocation
10
11
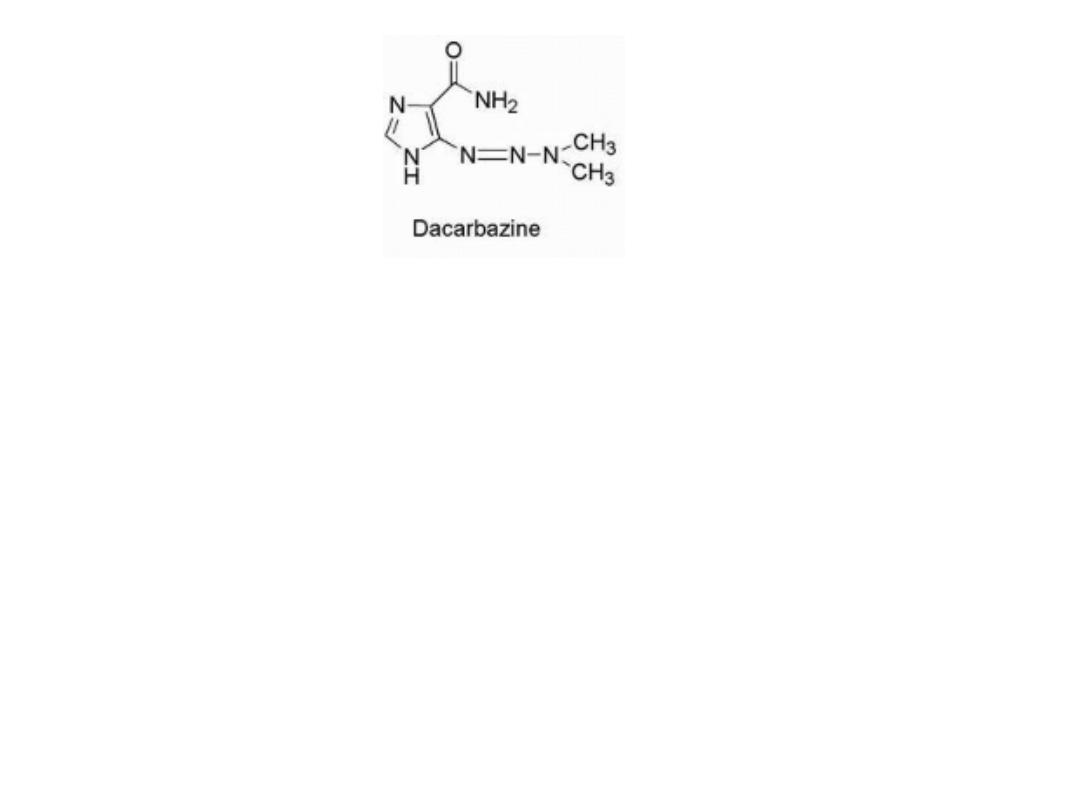
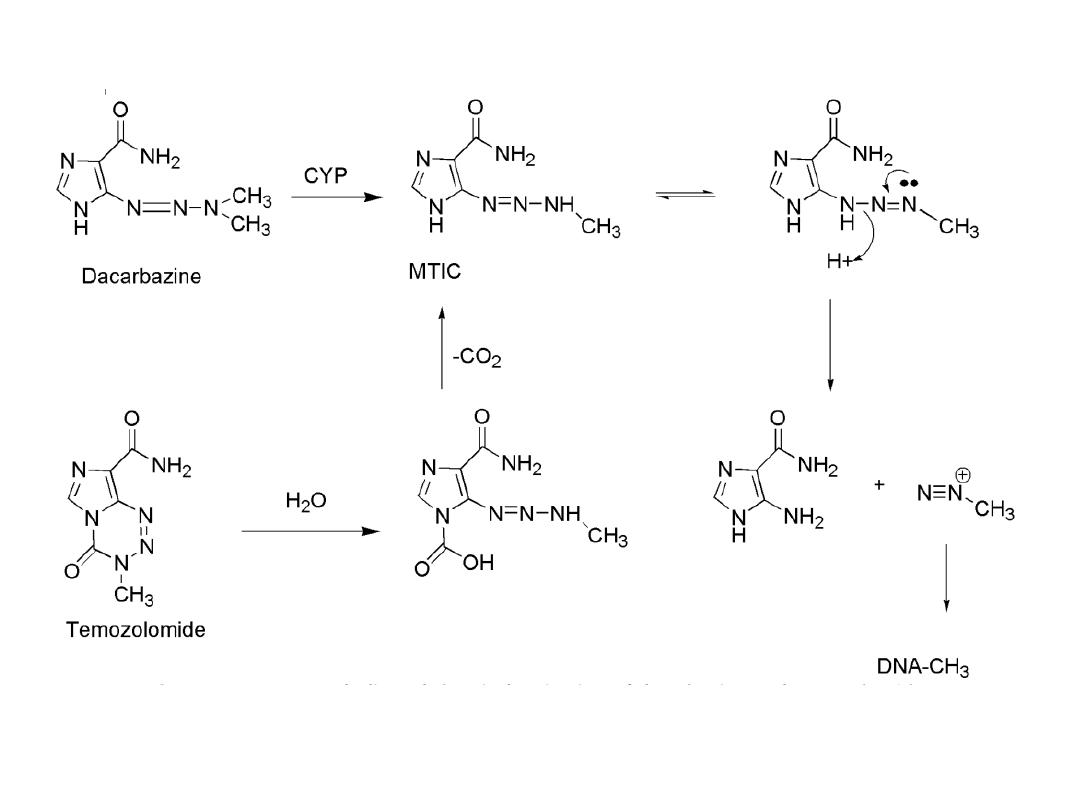
dacarbazine
Activation of the agent occurs through the action of CYP to
give the demethylated product monomethyl triazeno
imidazole carboxamide (MTIC) Tautomerization allows for
decomposition to give the aminocarboxamido-imidazole
and diazomethane, which is capable of alkylating DNA .
12
13
Temozolomide
Hydrolysis of temozolomide gives the carboxy-triazene,
which spontaneously loses CO
2
to give MTIC. Temozolomide
may be administered orally.
14
