
Antineoplastic Agents
Lecture 1
1

Introduction
Cancer occurs after normal cells have been transformed
into neoplastic cells through alteration of their genetic
material and the abnormal expression of certain genes.
Neoplastic
cells
usually
exhibit
chromosomal
abnormalities and the loss of their differentiated
properties. These changes lead to uncontrolled cell
division and many result in the invasion of previously
unaffected organs, a process called metastasis.
2

Malignant – grow more rapidly; often called
“cancer”
- Not cohesive; seldom have capsule
- Irregular shape; disrupted architecture
- Invade surrounding cellsm“Metastasis”
Benign – “noncancerous”
-Local; cells cohesive, well-defined borders
-Push adjacent tissue away
-Doesn’t spread beyond original site
-Often has capsule of fibrous connective tissue
Two major types: benign, malignant

• Poorly differentiated = disorganized
May be:
• Well-differentiated = retain normal cell
function
Mimic normal tissue
Cancer (Neoplastic) Cells
5

Anticancer Drugs
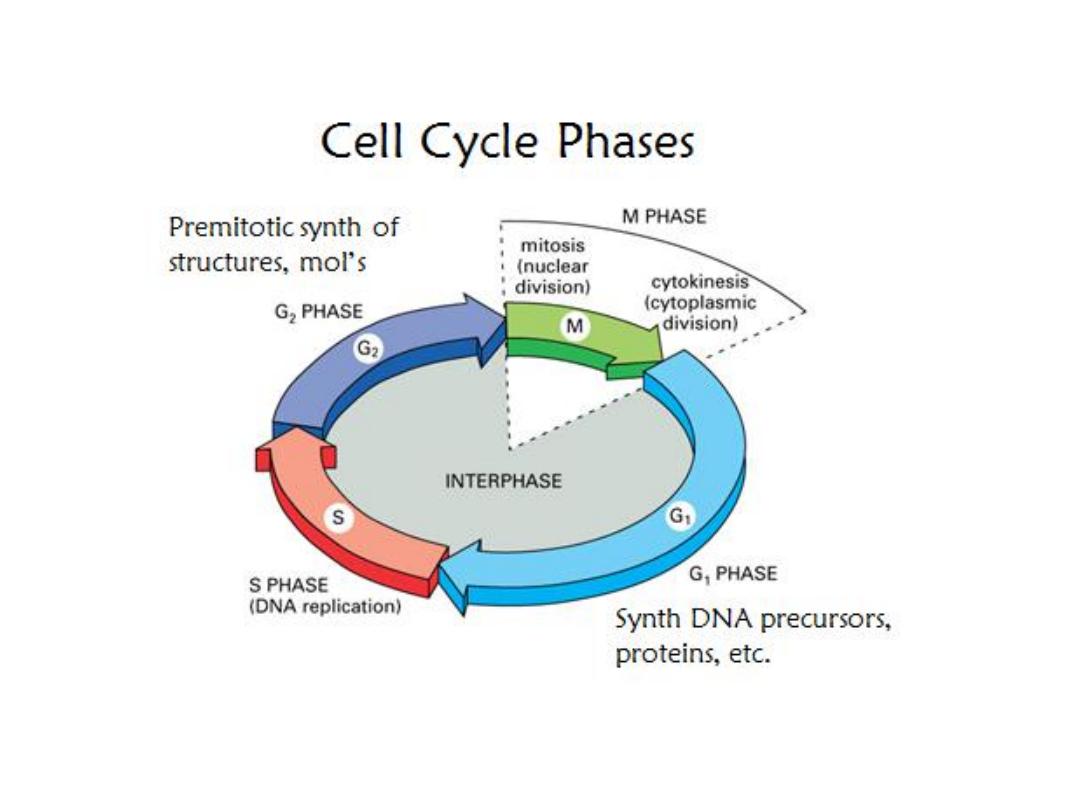
Affect cell division
Active on rapidly dividing cells
Most effective during S phase of cell cycle
Many cause DNA damage
Damage DNA initiate apoptosis
Side effects greatest in other rapidly-dividing cells
Bone marrow toxicity
Impaired wound healing
Hair follicle damage
GIT epithelial damage
May themselves be carcinogenic
6

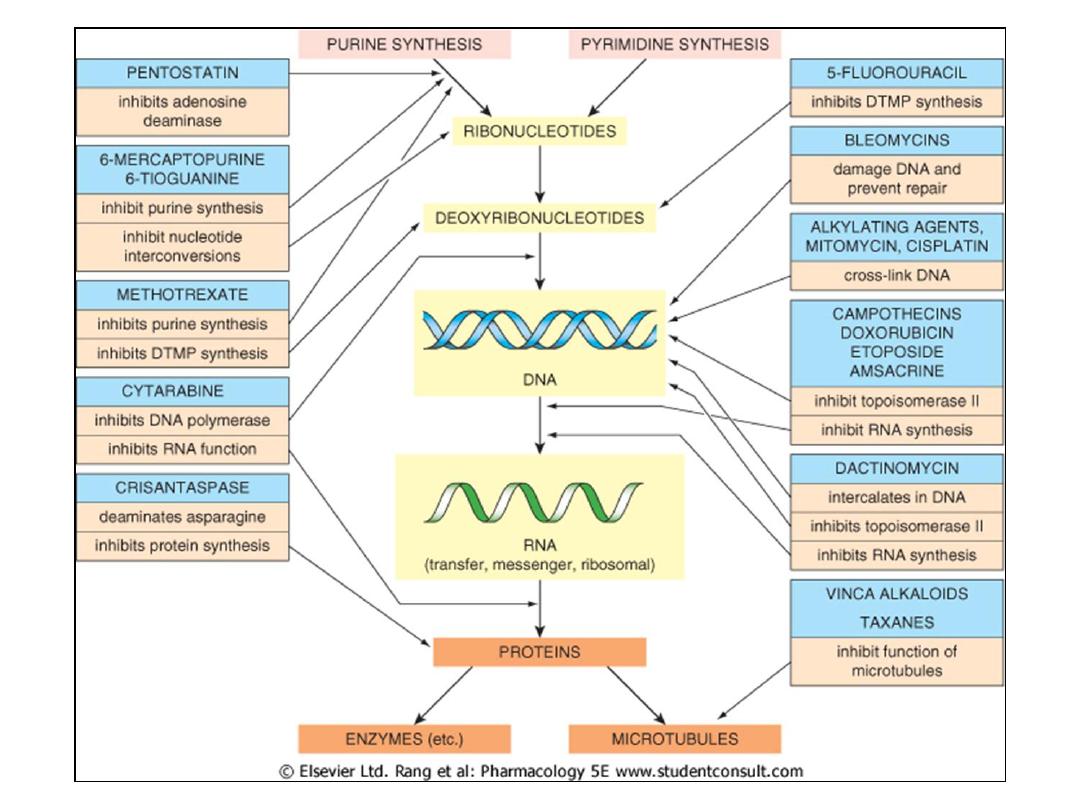
Drugs Used in Cancer Chemotherapy
Cytotoxic Agents:
Alkylating Agents
Antimetabolites
Cytotoxic antibiotics
Plant derivatives
Hormones
Suppress hormone secretion or antagonize
hormone action
Miscellaneous (mostly target oncogene products)
7
8

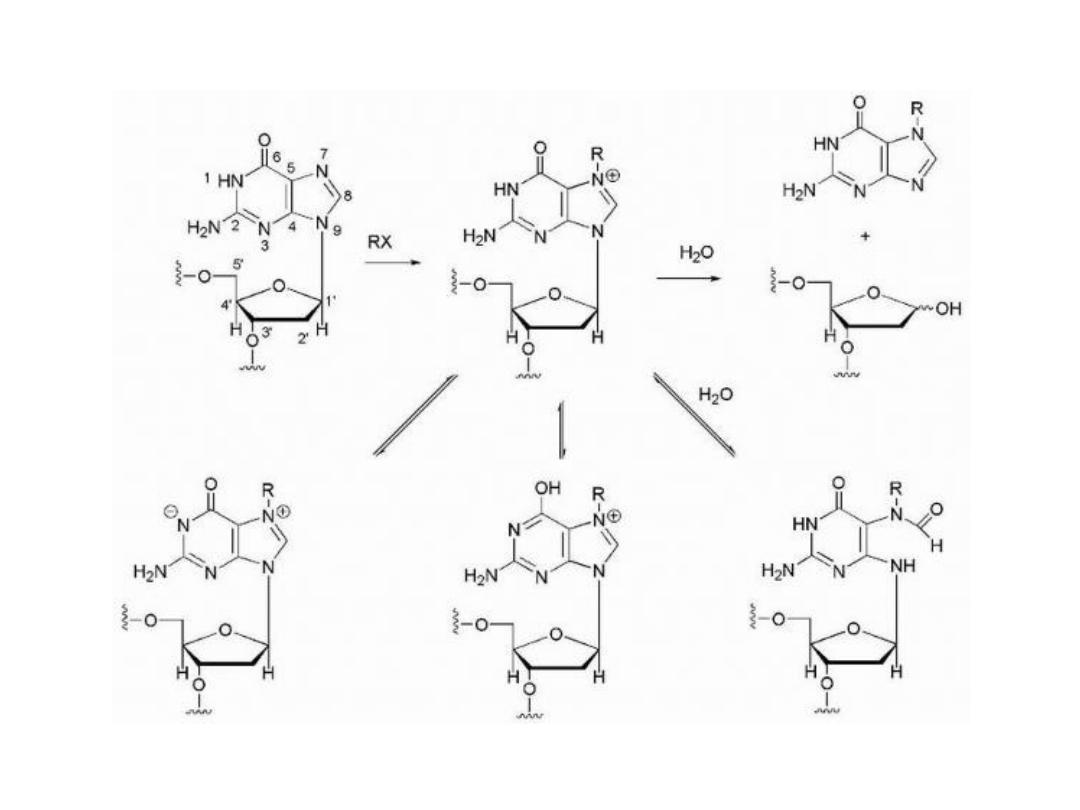
Alkylating Agents
The alkylating agents are a class of drugs that are capable of
forming covalent bonds with important biomolecules. The
major targets of drug action are nucleophilic groups present
on DNA (especially the 7-position of guanine); however,
proteins and RNA among others may also be alkylated.
Alkylation of DNA is thought to lead to cell death.
Potential mechanisms of cell death include activation of
apoptosis caused by p53 activation and disruption of the
template function of DNA.
• Alkylation7-position of guanine converts the base to an
effective leaving group so that attack by water leads to
depurination and the loss of genetic information if the
resulting depurination is not repaired by the cell.
9

• Additionally, alkylation has been proposed to result in
altered base pairing away from the normal G-C: A-T
hydrogen
bonds
because
of
alterations
in
tautomerization.
• The alkylation also leads to increased acidity of the N-1
nitrogen reducing the pKa giving rise to a zwitterionic
form that may also mispair.
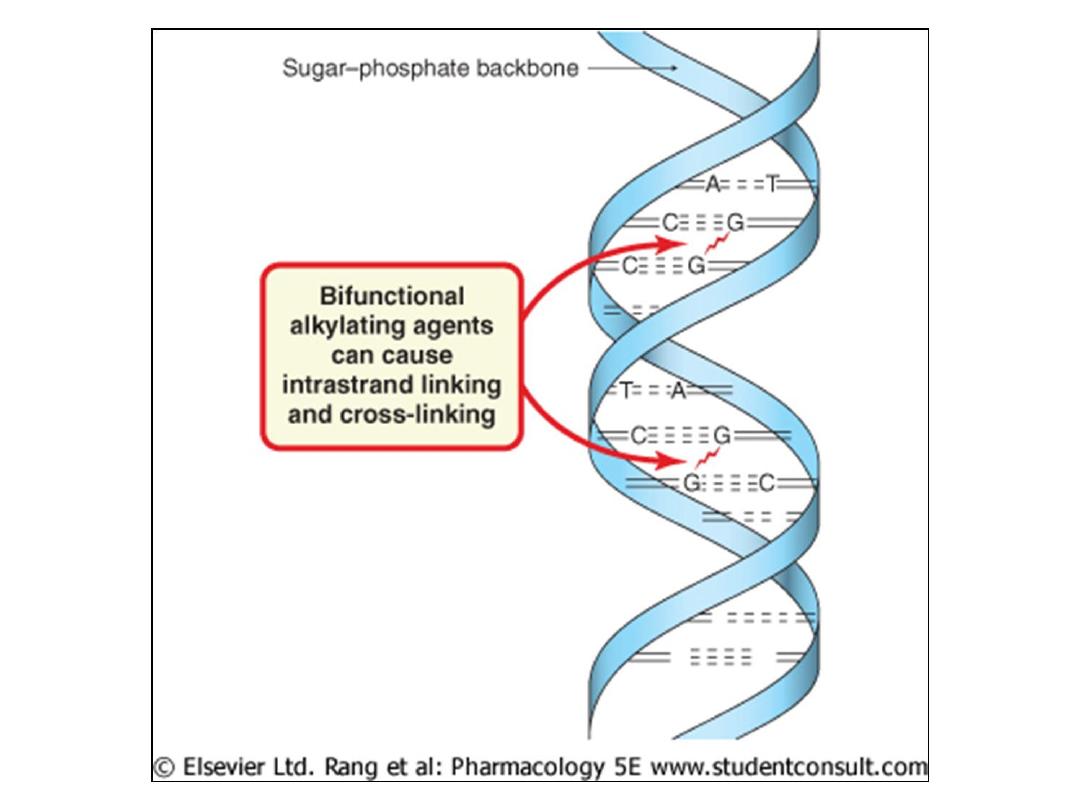
• For those agents that possess two reactive functionalities,
both interstrand and intrastrand cross-linking becomes
possible. When interstrand links occur, separation of the
two strands during replication is prevented and therefore
replication is blocked.
10
11
12
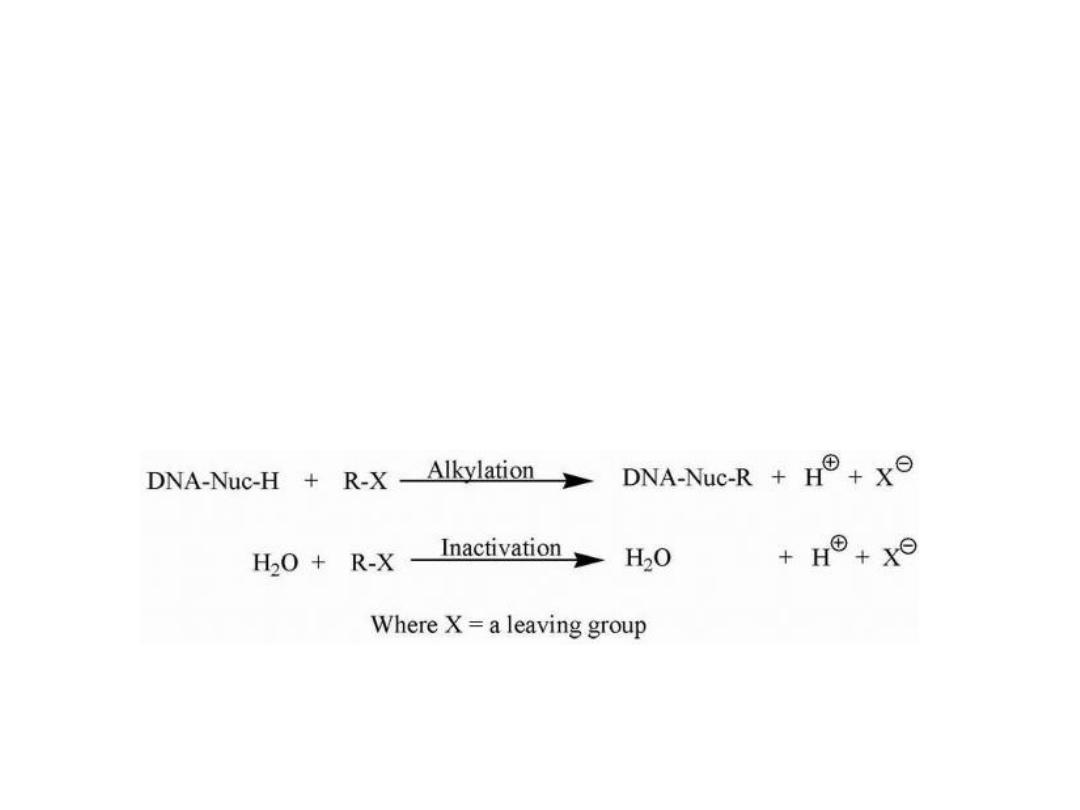
Most of the currently used alkylating agents are nonselective
regarding the sequence of DNA with which they react.
The general mechanism for alkylation involves nucleophilic
attack by —N=, —NH2, —OH, —O—PO
3
H of DNA and RNA,
while additional nucleophiles (—SH, COOH, etc.) present on
proteins may also react
13

Resistant to Alkylating Agents
1.Decreased cellular uptake,
2.Increased inactivation by detoxifying nucleophilic
thiols such as glutathione
3. Increased DNA repair processes
4. Decreased drug activation when this is necessary
for generation of an alkylating species
14

NITROGEN MUSTARDS
Mustards such as mechlorethamine are classified as
dialkylating agents in that one mustard molecule can
alkylate two nucleophiles.
The initial acid-base reaction is necessary to release the
lone pair of electrons on nitrogen, which subsequently
displaces chloride to give the highly reactive aziridinium
cation.
Nucleophilic attack can then occur at the aziridinium
carbon to relieve the small ring strain and neutralize the
charge on nitrogen.
15
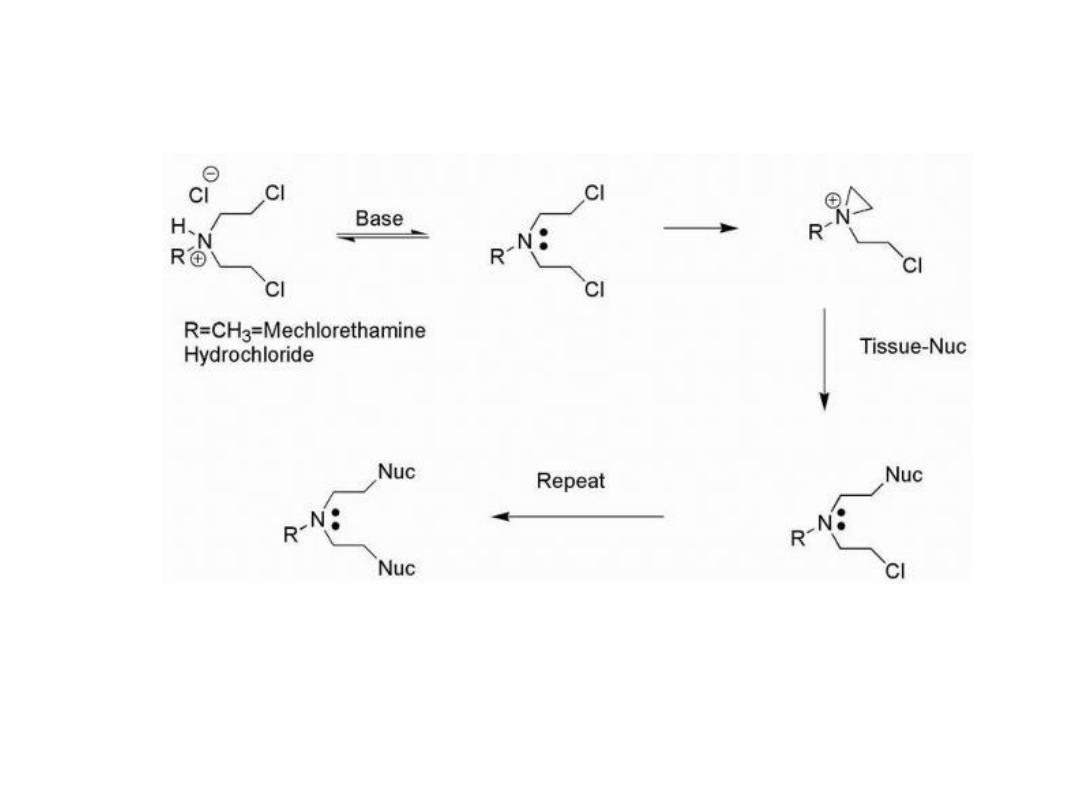
16
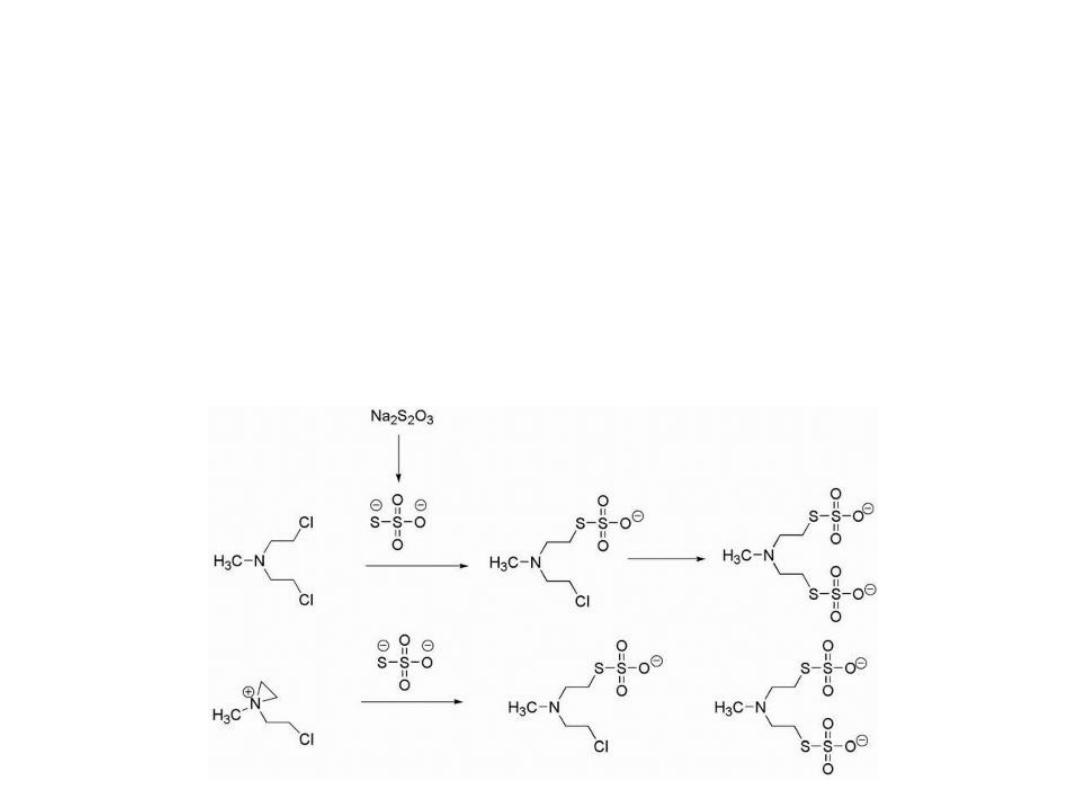
Mechlorethamine is highly reactive, in fact, too reactive
and therefore nonselective, making it unsuitable for oral
administration and necessitating direct injection into the
tumor.
In cases of extravasation (drug escapes from the tumor
into the underlying tissue), the antidote sodium
thiosulfate (Na
2
S
2
O
3
), a strong nucleophile, may be
administered.
17

The lack of selectivity of mechlorethamine
One rationale was to reduce the reactivity by reducing the
nucleophilicity of nitrogen, thereby slowing aziridinium
cation formation.
This could be accomplished by replacement of the weakly
electron-donating methyl group with groups that were
electron withdrawing.
This is seen in the case of chlorambucil and melphalan by
attachment of nitrogen to a phenyl ring. Reactivity was
reduced such that these compounds could be administered
orally.
In the case of melphalan, attachment of the mustard
functionality to a phenylalanine moiety was not only an
attempt to reduce reactivity but also an attempt to increase
entry into cancer cells by utilization of carrier-mediated
uptake. Melphalan was found to utilize active transport to
gain entry into cells.
18
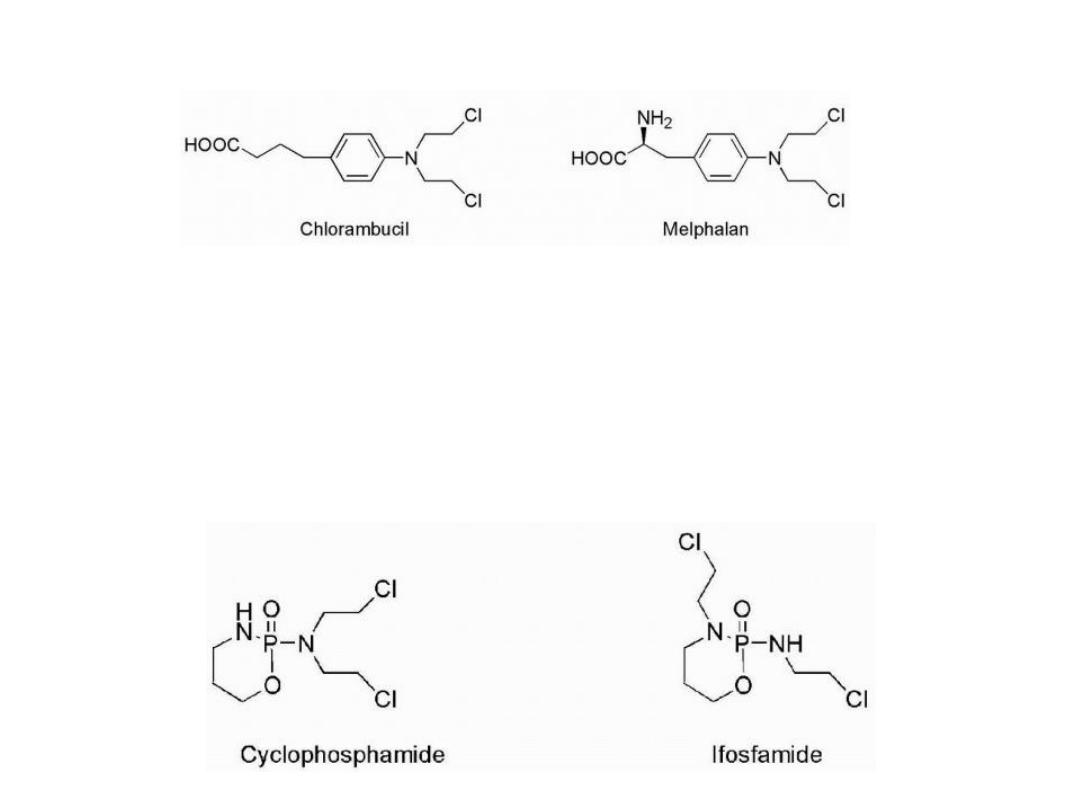
Attachment
of
more
highly
electron-withdrawing
functionalities
was
utilized
in
the
case
of
cyclophosphamide and ifosfamide.
In these cases, aziridinium cation formation is not possible
until the electron-withdrawing function has been altered.
19
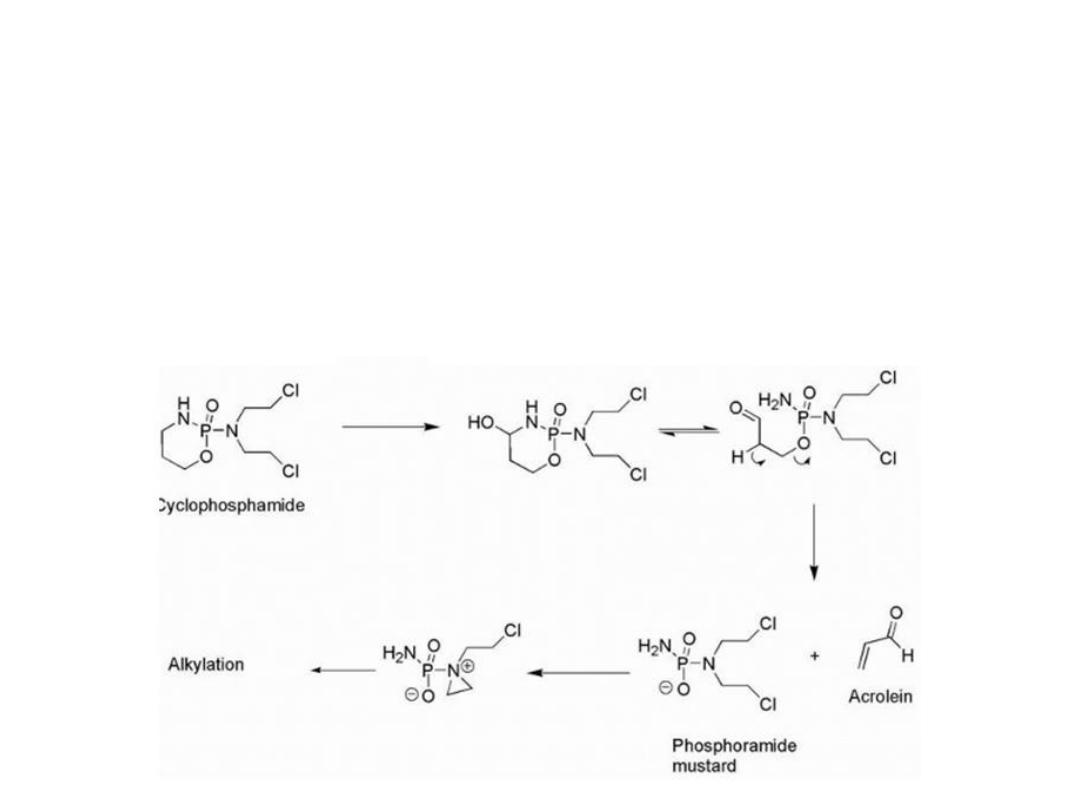
The drug was activated by cytochrome P450 (CYP)
isozymes to give a carbinolamine that could undergo ring
opening to give the aldehyde. The increased acidity of the
aldehyde α- hydrogen facilitates a retro-Michael
decomposition .The ionized phosphoramide is now
electronreleasing via induction and allows aziridinium
cation formation to proceed.
20
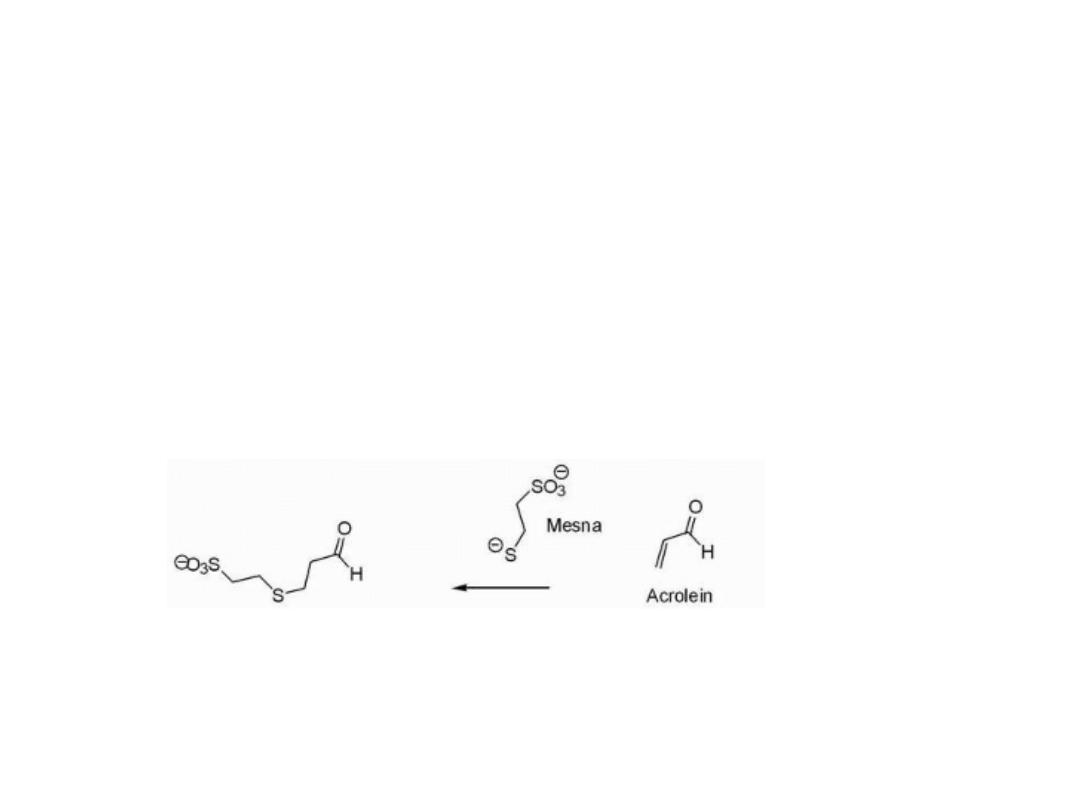
Acrolein is also formed as a result of this process, which
may itself act as an electrophile that has been associated
with bladder toxicity.
To decrease the incidence of kidney and bladder toxicity,
the sulfhydryl (—SH) containing agent mesna may be
administered and functions to react with the electrophilic
species that may be present in the kidney.
21
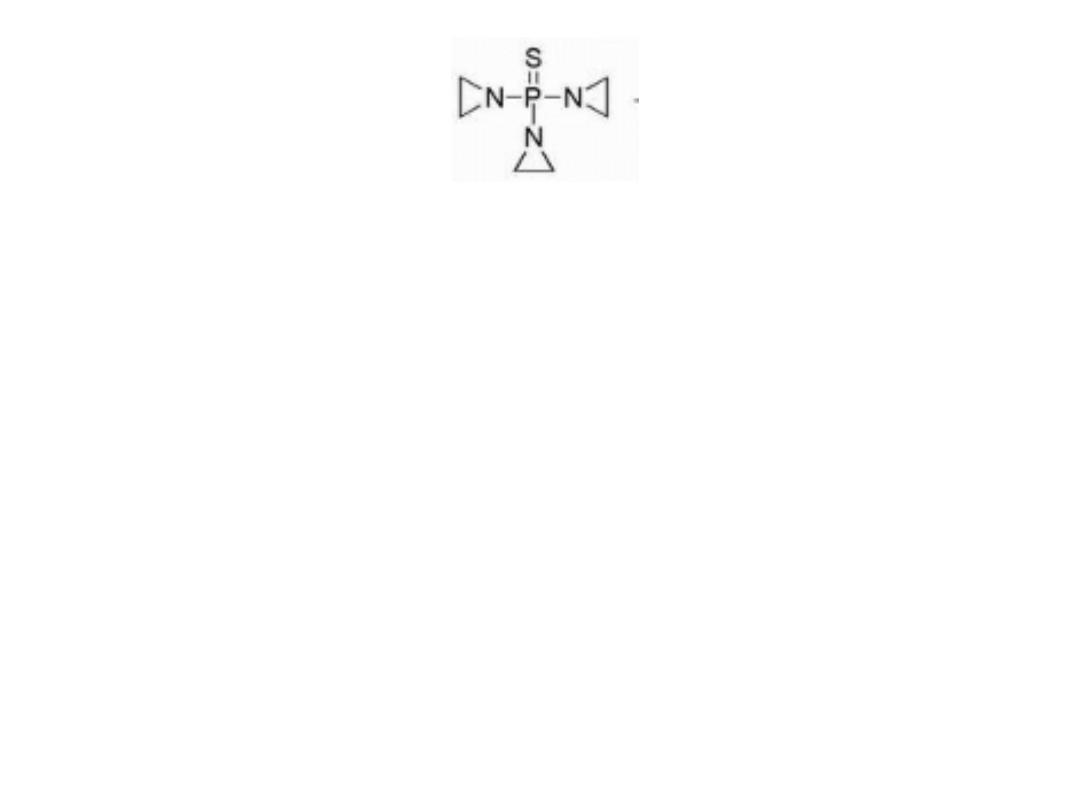
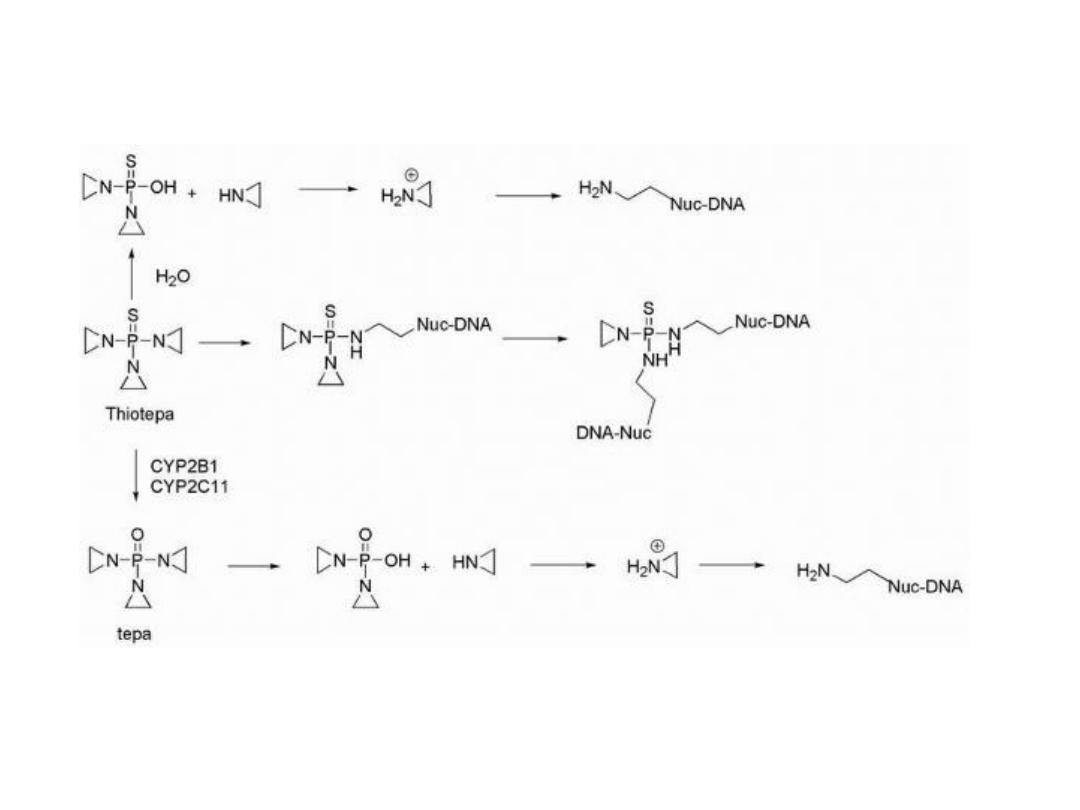
THIOTEPA
Thiotepa containing the thiophosphoramide functionality
was found to be more stable than the oxa-analog.
Thiotepa incorporates a less reactive aziridine ring
compared with that formed in mechlorethamine. The
adjacent thiophosphoryl is electron withdrawing and,
therefore, reduces the reactivity of the aziridine ring
system.
Monoalkylation is also possible as a result of aziridine
formation via hydrolysis of thiotepa.
22
23

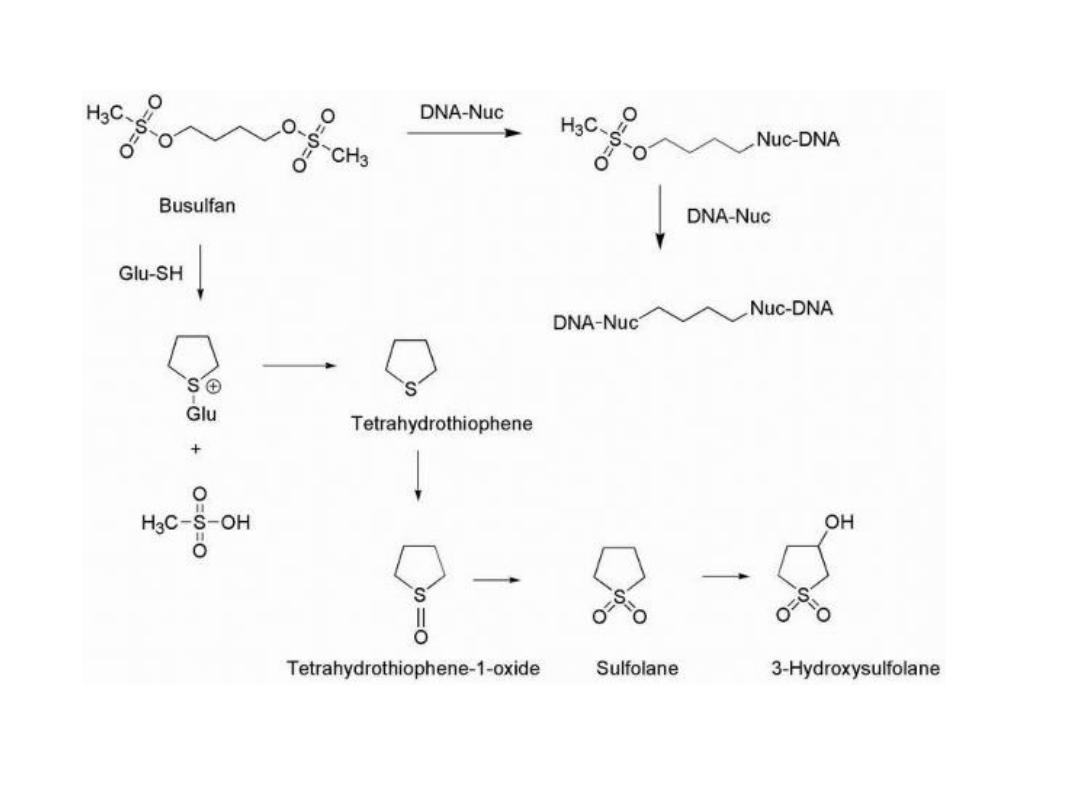
BUSULFAN
Busulfan utilizes two sulfonate functionalities as
leaving groups separated by a four-carbon chain that
reacts with DNA to primarily form intrastrand cross-
link at 5′-GA-3′ sequences.
The sulfonates are also subject to displacement by the
sulfhydryl functions found in cysteine and glutathione,
and metabolic products are formed as a result of
nucleophilic attack by these groups to generate
sulfonium species along with methane sulfonic acid.
This is followed by conversion to tetrahydrothiophene,
and further oxidation products are subsequently
produced to give the sulfoxide and sulfone. The cyclic
sulfone known as sulfolane may be further oxidized to
give 3-hydroxysulfolane.
24
25
