
للإل
طالع
-
اختياري
Page 1 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
Proteins
Protein Structure
Proteins
are the major components of living
organisms and perform a wide range of essential
functions in cells.
•
While DNA is the information molecule, it is
proteins that do the work of all cells
-
microbial, plant ,
animal.
•
Proteins
regulate
metabolic activity
,
catalyze
biochemical reactions
and
maintain structural
integrity of cells and organisms.
Proteins
can be formed using
02
different
building blocks called
amino acids.
Each of these amino acid building blocks has a
different chemical structure and different
properties.
Each protein has a unique amino acid sequence
that is genetically determined by the order of
nucleotide bases in the DNA ,the genetic code.
Since each protein has different numbers and
kinds of the twenty available amino acids ,
each protein has a unique chemical
composition and structure
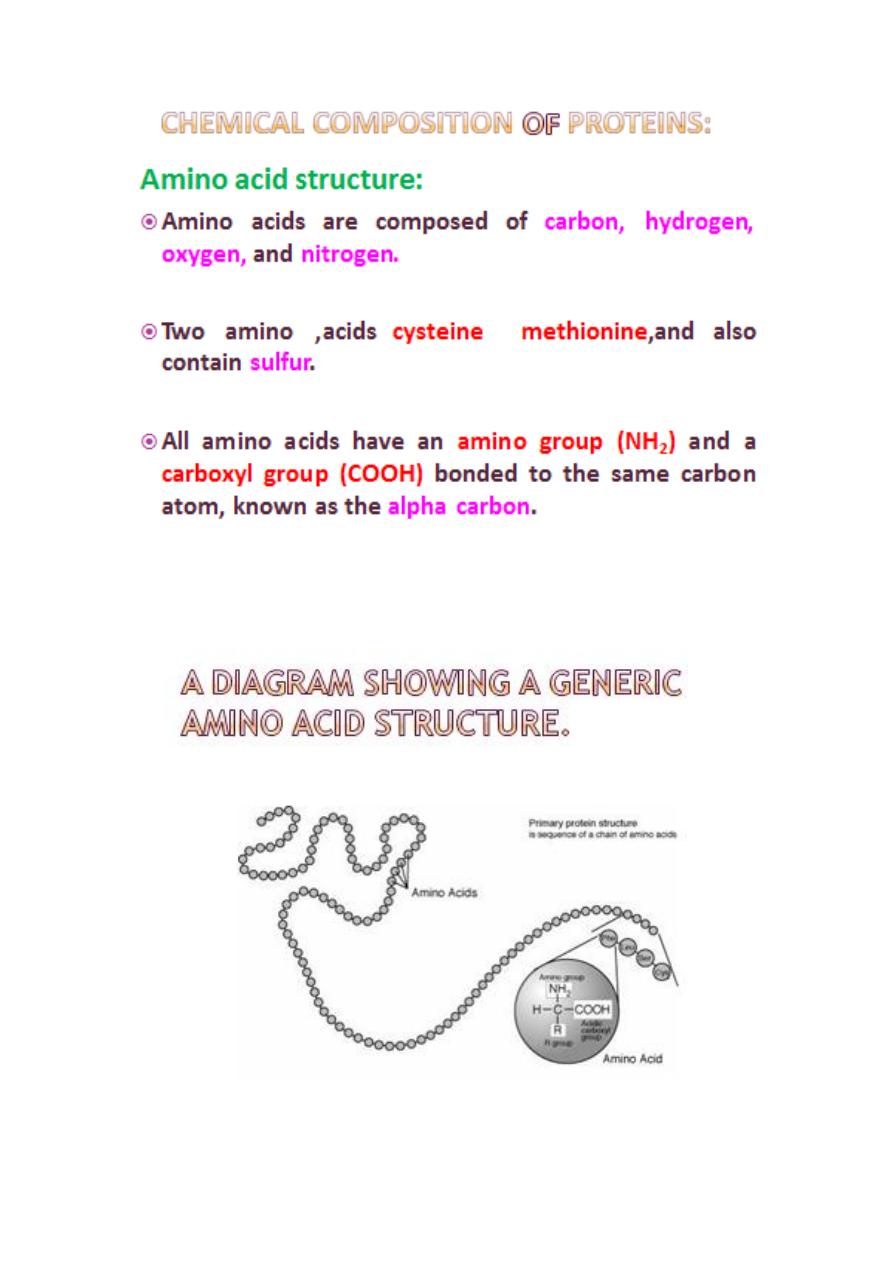
للإل
طالع
-
اختياري
Page 2 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
للإل
طالع
-
اختياري
Page 3 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
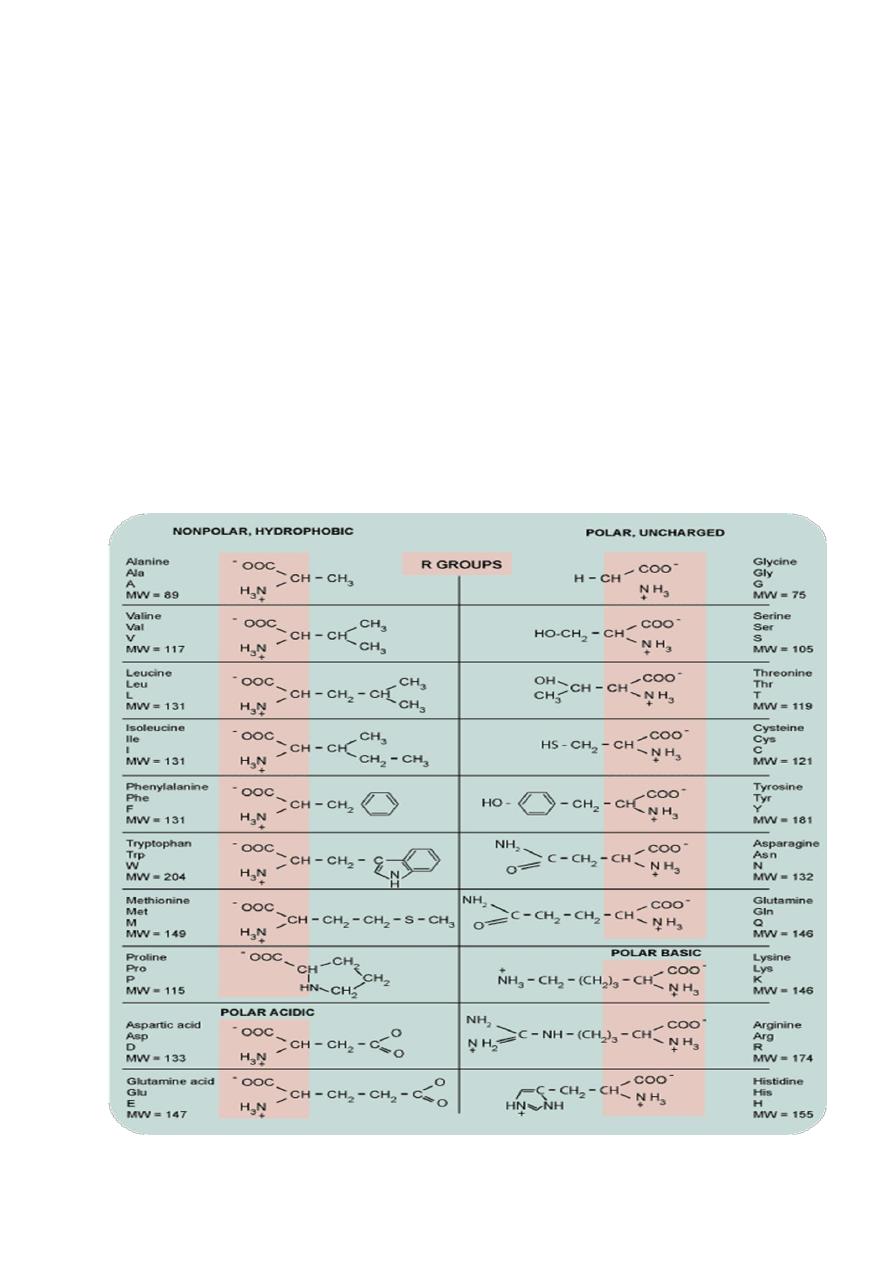
Amino acids
differ in the side chain or
R group
that
is bonded to the alpha carbon .
Glycine
,the simplest
amino acid has a single
hydrogen
atom as its R
group
.
-
Alanine
has a
)
methyl
– CH
(
3
group.
ž
The chemical composition of the unique R
groups is responsible for the important
characteristics of amino acids such as
chemical
reactivity
,
ionic
charge
and
relative
hydrophobicity.
للإل
طالع
-
اختياري
Page 4 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
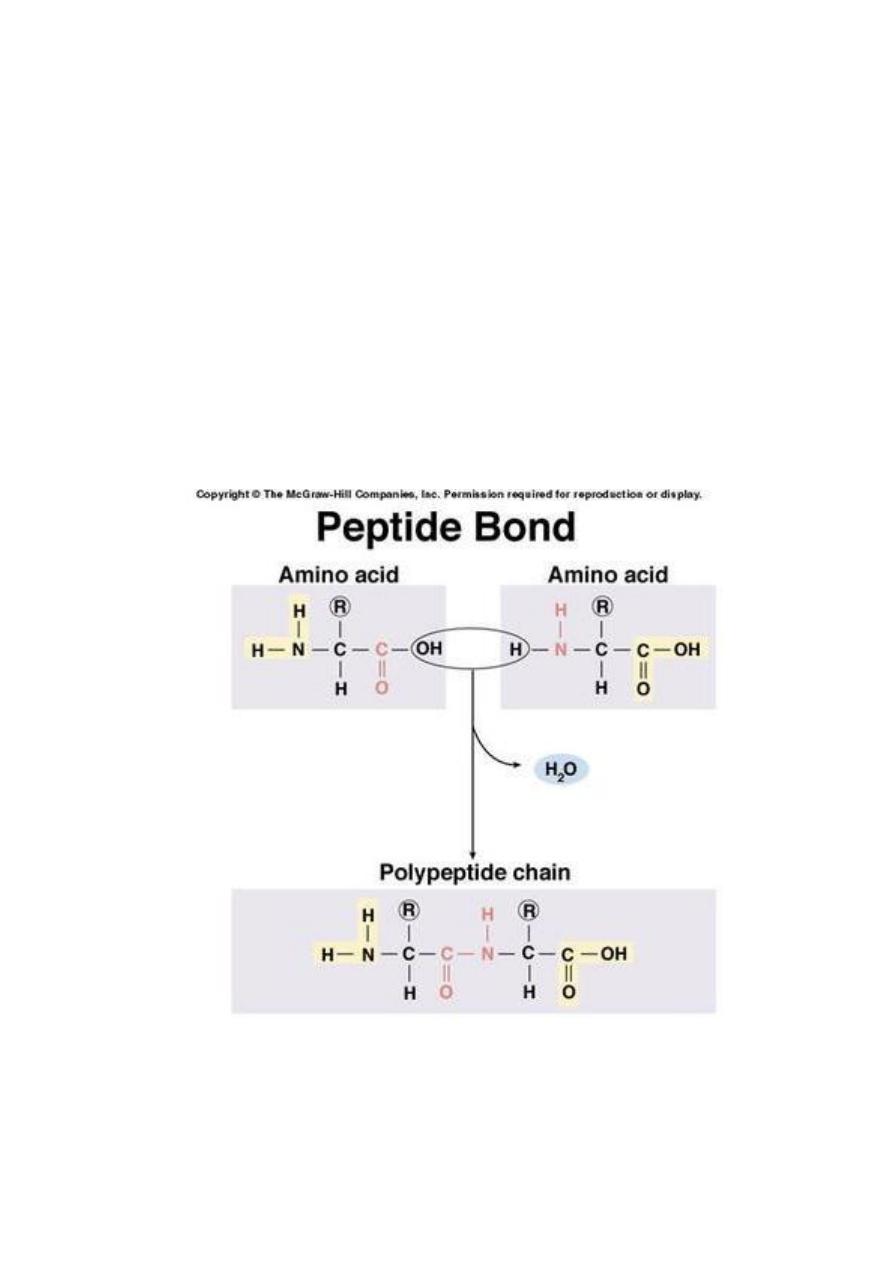
A protein is formed by amino acid subunits
linked together in a chain.
The bond between two amino acids is formed
by the removal of a H
2
O molecule from two
different amino acids ,forming a
dipeptide
.
The bond between two amino acids is called a
peptide bond
and the chain of amino acids is
called a peptide (20 amino acids or smaller) or
a polypeptide.
للإل
طالع
-
اختياري
Page 5 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
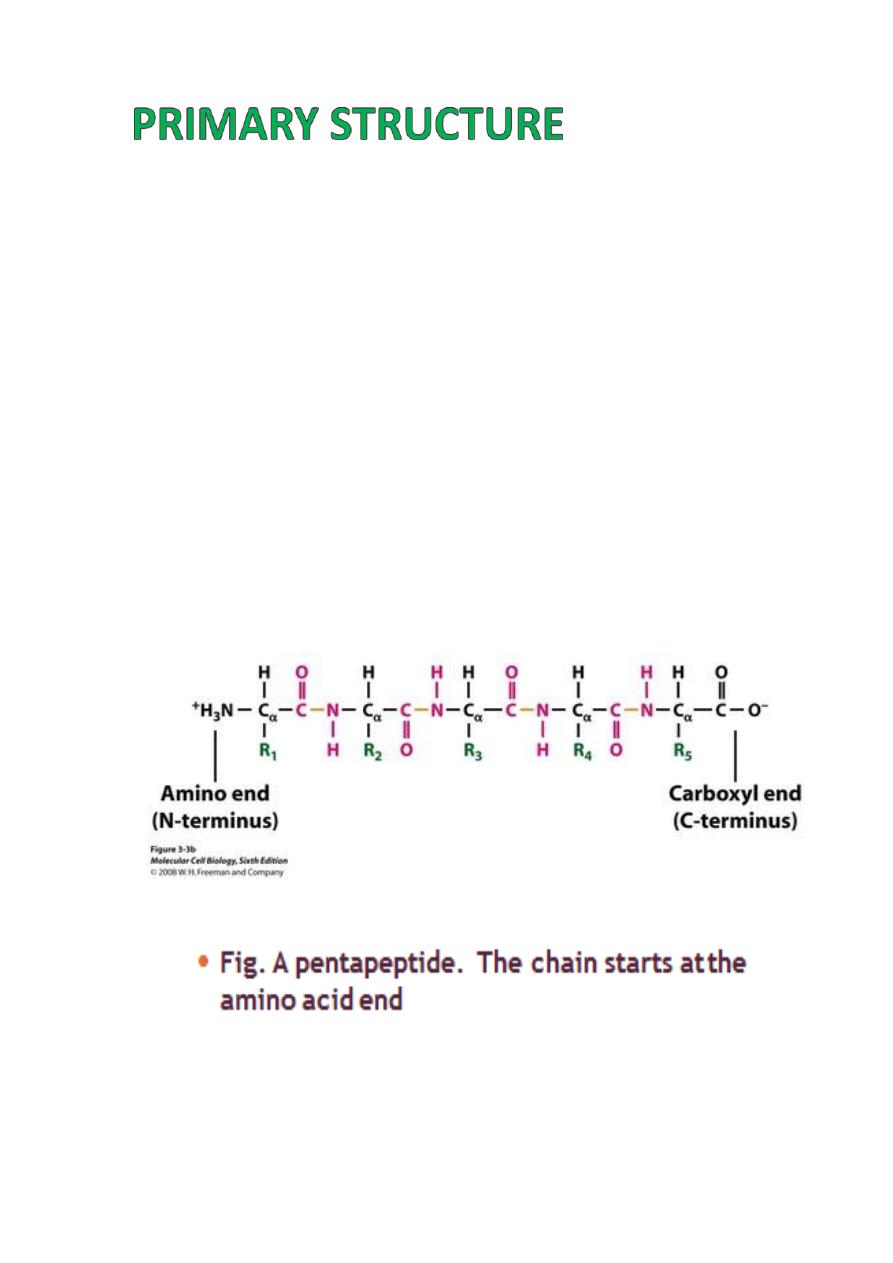
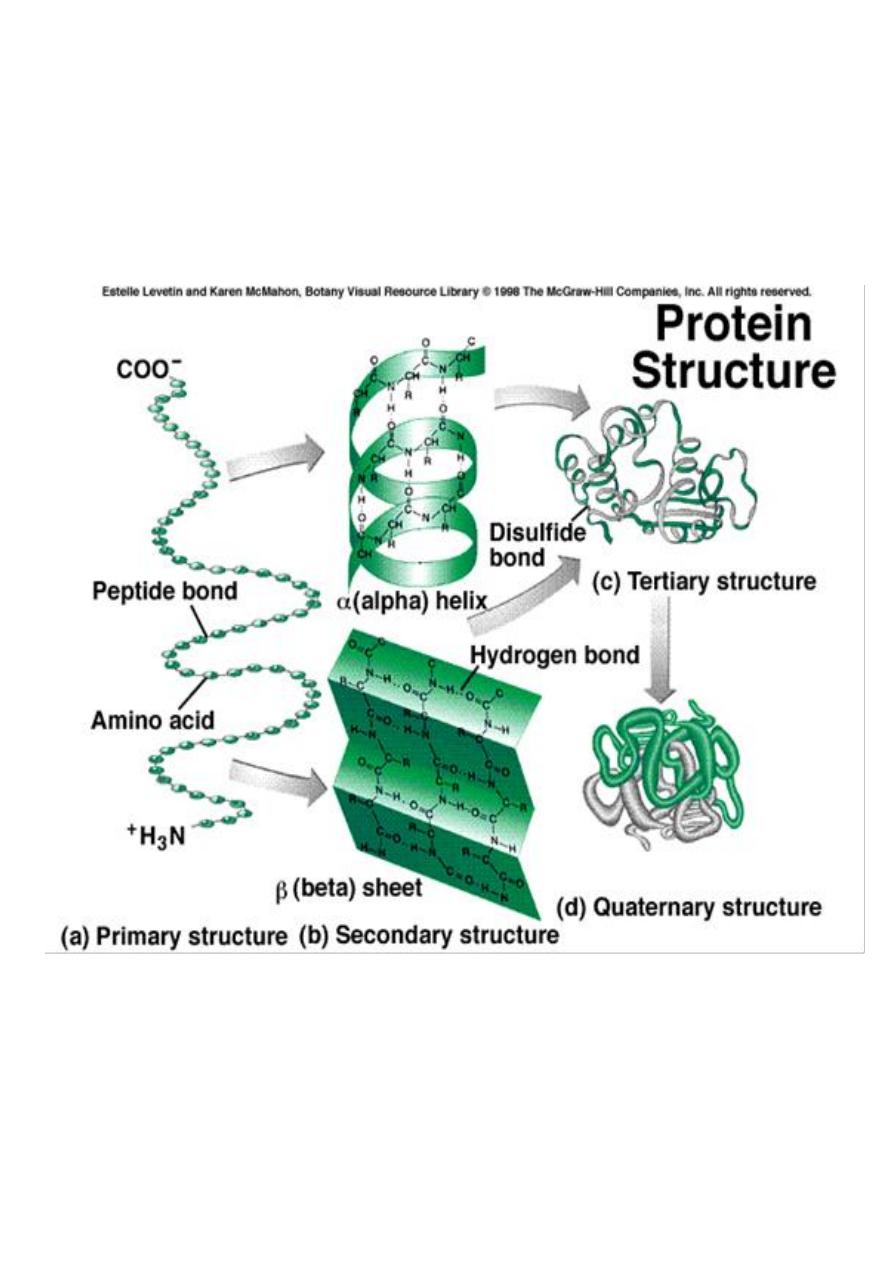
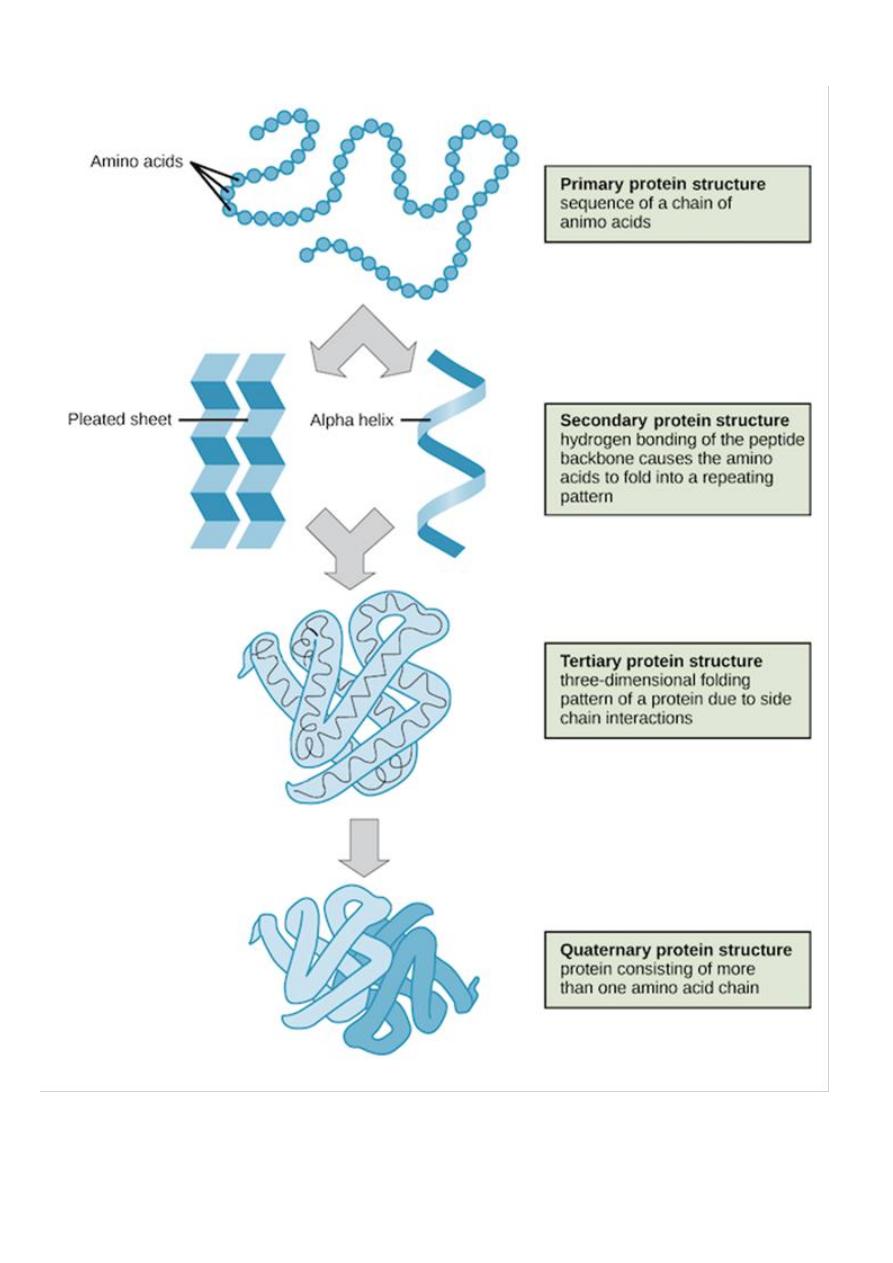
Primary Structure
refers to the
linear
sequence
of amino acids that make up the polypeptide chain.
This sequence is determined by the genetic
code, the sequence of nucleotide bases in the
DNA.
The sequence of amino acids determines the
positioning of the different R groups relative to
each other.
This positioning therefore determines the way
that
the protein folds and the final structure of
the molecule.
للإل
طالع
-
اختياري
Page 6 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
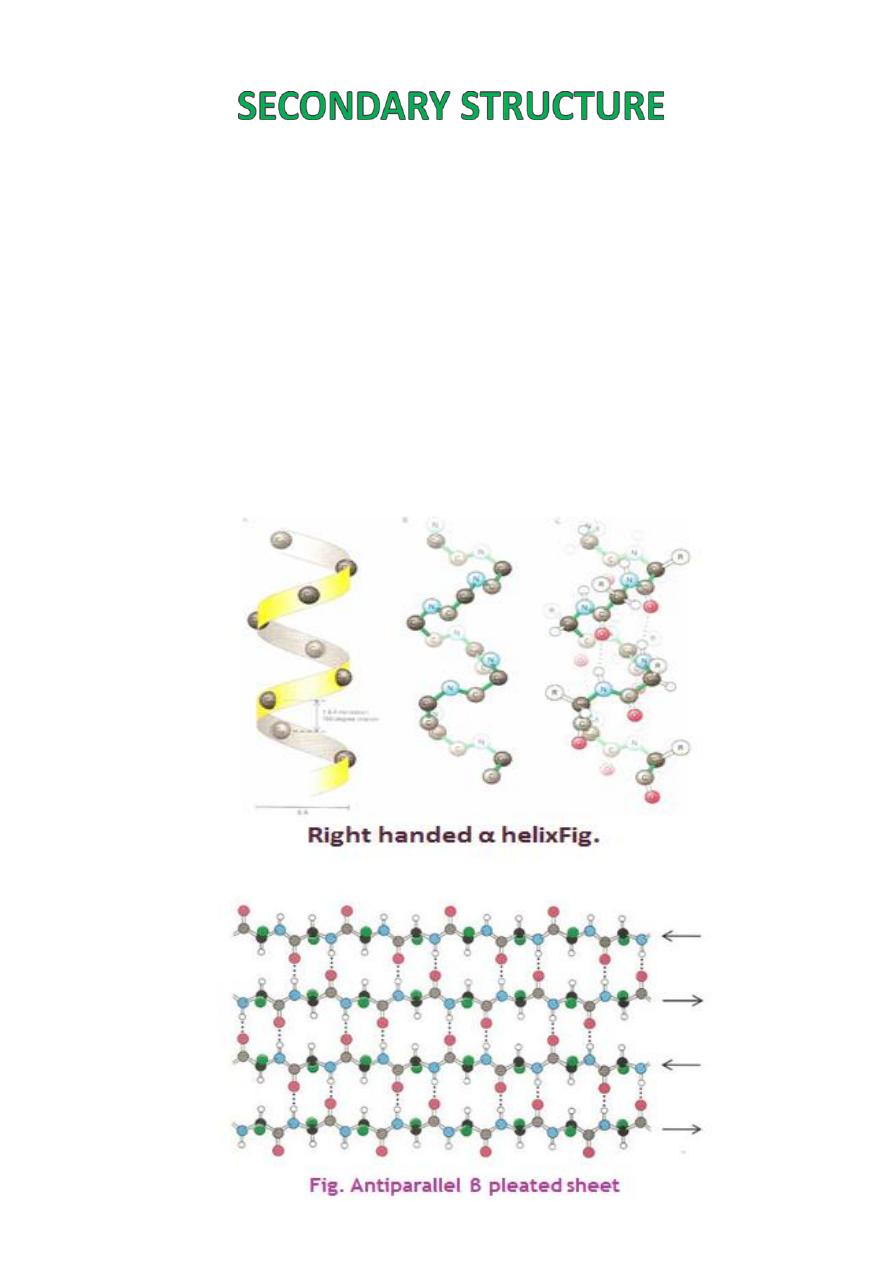
The
secondary structure
of protein molecules
refers to the formation of a regular pattern of
twists or kinks
of the polypeptide chain.
The regularity is due to hydrogen bonds
forming between the atoms of the amino acid
backbone of the polypeptide chain.
The two most common types of secondary
structure are called the
alpha helix
and
ß
pleated sheet
.

للإل
طالع
-
اختياري
Page 7 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
Tertiary structure
refers to the
three dimensional
globular structure
formed by
bending and twisting
of the polypeptide chain.
This process often means that the linear
sequence of amino acids is folded into a
compact globular structure.
The folding of the polypeptide chain is
stabilized by multiple weak ,non covalent
interactions .These interactions include:
Hydrogen bonds
that form when a Hydrogen
atom is shared by two other atoms.
Electrostatic interactions
that occur between
charged amino acid side chains .Electrostatic
interactions are attractions between positive
and negative sites on macromolecules.
Hydrophobic interactions
:During folding of
the polypeptide chain ,amino acids with a
polar
( water soluble)
side chain are often
found on the surface of the molecule while
amino acids with non-polar
(water insoluble)
side chain are buried in the interior .This
means that the folded protein is soluble in
water or aqueous solutions.
Covalent bonds
may also contribute
to
tertiary
structure.
The amino acid ,
cysteine
,has an SH group as
part of its R group and therefore ,the
disulfide

للإل
طالع
-
اختياري
Page 8 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
bond (S-S)
can form with an adjacent cysteine .
For example ,insulin has two polypeptide
chains that are joined by two disulfide bonds.
Quaternary structure
refers to the fact that some
proteins contain more than one polypeptide
chain ,adding an additional level of structural
organization :the association of the polypeptide
chains.
Each polypeptide chain in the protein is called
a
subunit
.
The subunits can be the same polypeptide
chain or different ones .For example ,the
enzyme
ß-galactosidase
is a tetramer ,meaning
that it is composed of
four subunits
,and, in this
case ,the subunits are identical
-
each
polypeptide chain has the same sequence of
amino acids.
Hemoglobin
,the oxygen carrying protein in the
blood ,is also a
tetramer
but it is composed of
two polypeptide chains of one type (141 amino
acids) and two of a different type
641
)
amino
acid).
In chemical shorthand ,
hemoglobin
is referred
to as
a
0
ß
0
.
للإل
طالع
-
اختياري
Page 9 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
For some proteins, quaternary structure is
required for full activity (function) of the
protein.
للإل
طالع
-
اختياري
Page 10 of 10
محاضرة من النت/ اشكال البروتين/صف اول طبية
