
First Class/Practical Medical Chemistry
Page 1 of 10
By : Dr. Tamathir Abass
Carbohydrates
By : Dr.Tamathir Abbas
POLYSACCHARIDES
Consists of repeat units of monosaccharaides (hundruds and
thousands) or their derivatives held together by glycosidic bonds.
Linear and branched polymers.
Homopolysaccharides and hetropolysaccharides
HOMOPOLYSACCHARIDES
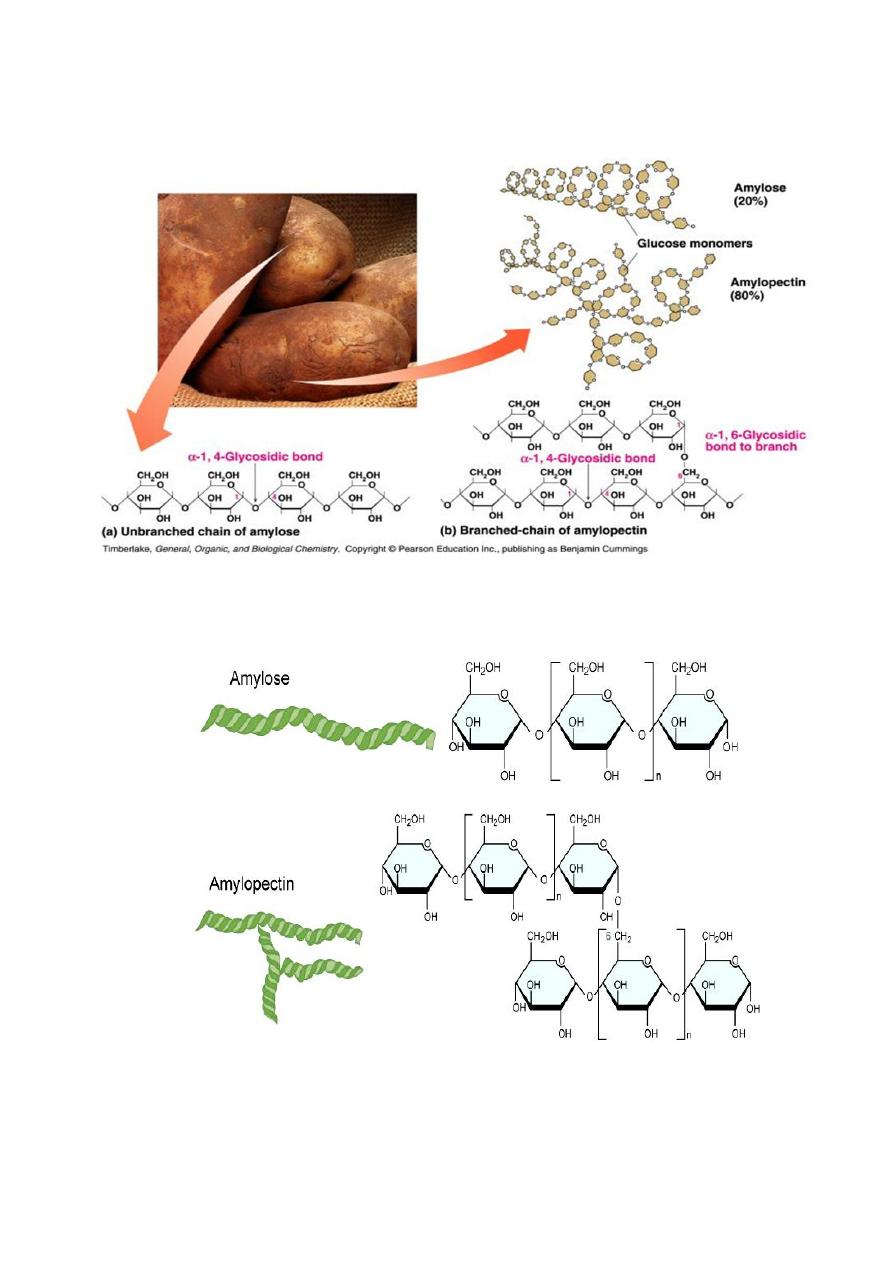
STARCH.
Starch is a polymer consisting of D-glucose units.
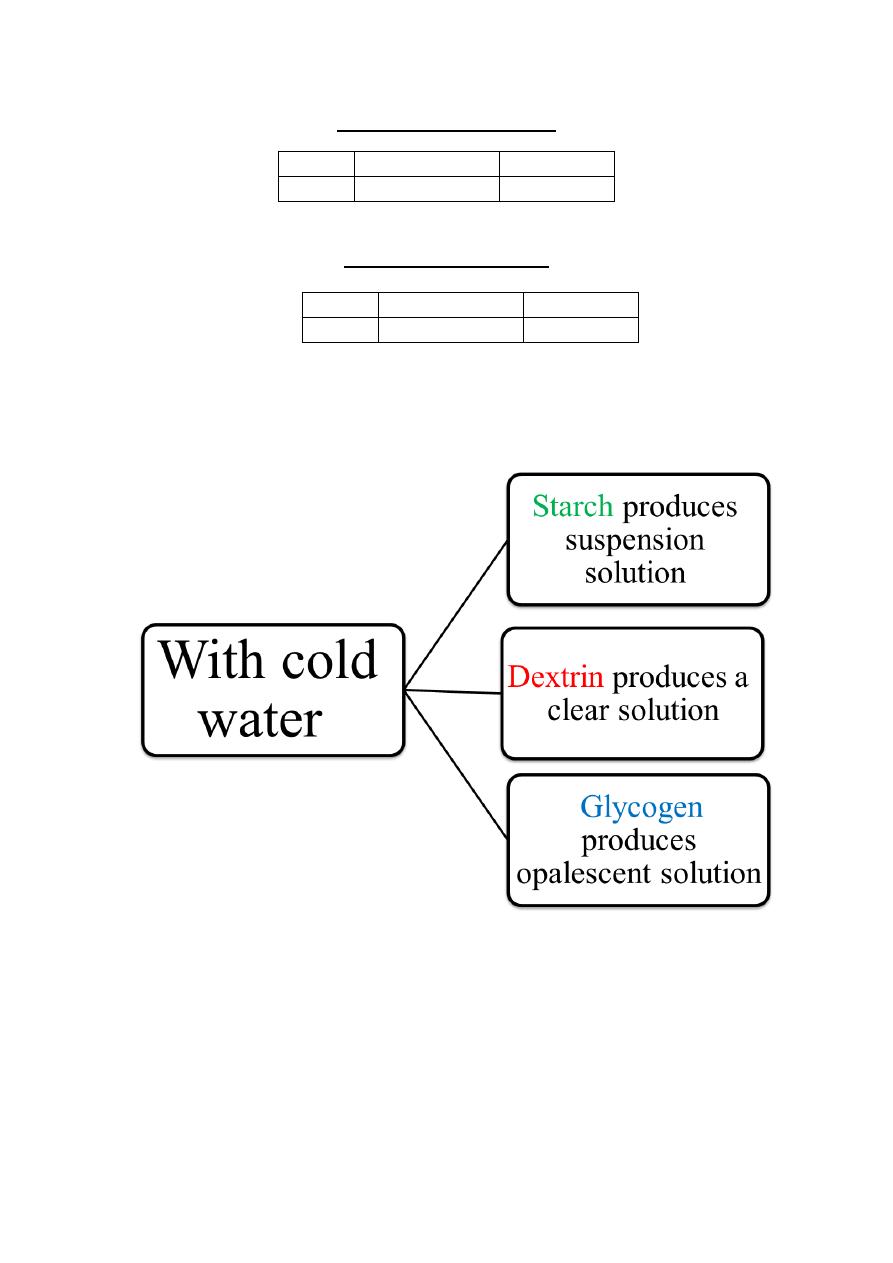
Starches (and other glucose polymers) are usually insoluble in
water because of the high molecular weight, but they can form
thick colloidal suspensions with water.
2 main constituents: amylose (15-20%) and amylopectin (80-85%)
Amylose: straight chain (unbranched) glucose polymers joined by
α-1→4 linkage
Amylopectin: Branched polymer of glucose composed of 24-30
glucose residues. Branch is joined to the chain by α-1→6 linkage.
First Class/Practical Medical Chemistry
Page 2 of 10
By : Dr. Tamathir Abass
Structure of starch

First Class/Practical Medical Chemistry
Page 3 of 10
By : Dr. Tamathir Abass
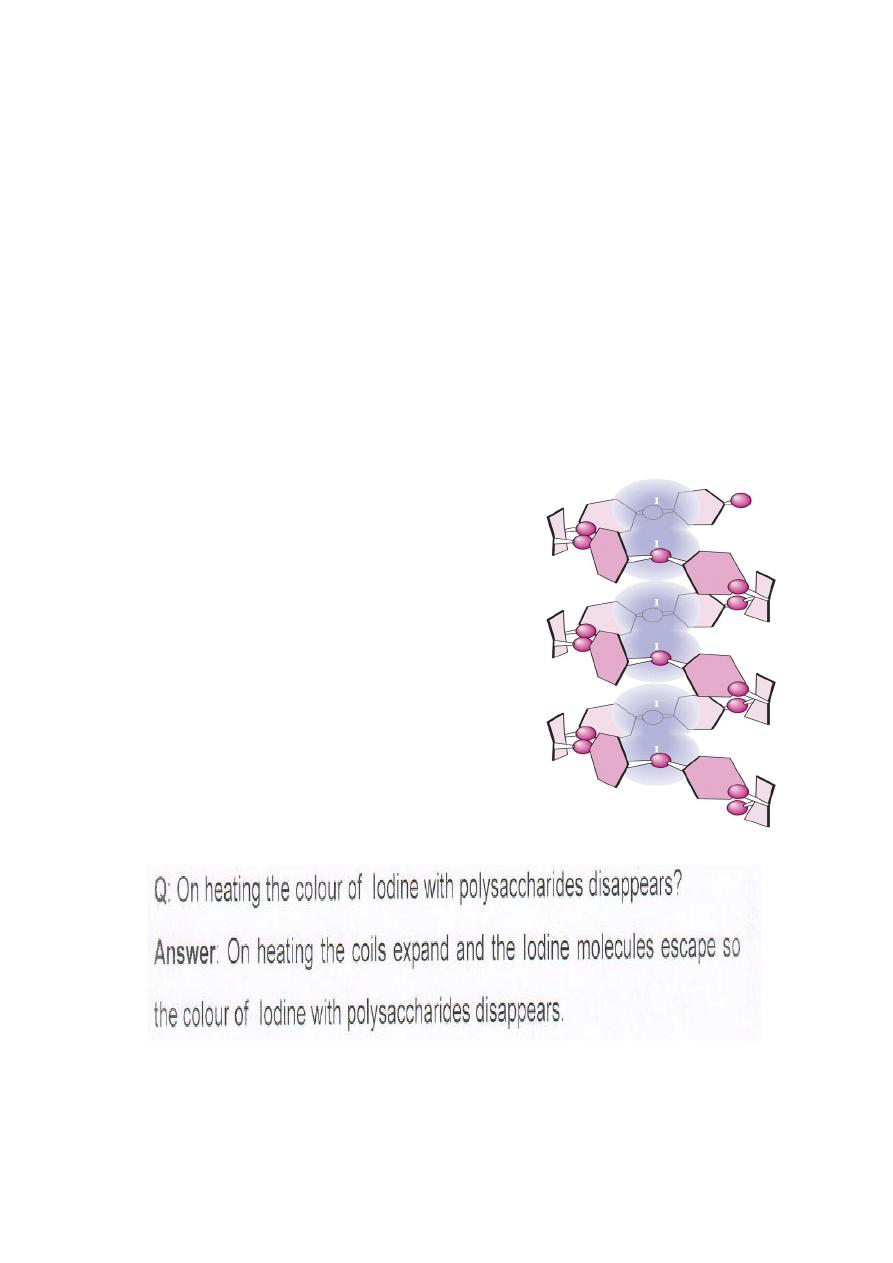
IODINE REACTION
• This is a test for polysaccharides
Principle :
Iodine forms a coordinate complex between the helically
coiled polysaccharide chain and iodine centrally located within
the helix due to adsorption. The color obtained depends upon
the length of the unbranched or linear chain available for
complex formation.
Left to right: Lugol's iodine, starch solution, starch solution
with iodine.
Yellow-orange - negative. Purple-black -positive.
Interpretation
Amylose- A linear chain component of starch, gives a deep blue
color
Amylopectin- A branched chain component of starch, gives a
purple color
Glycogen- Gives a reddish brown color
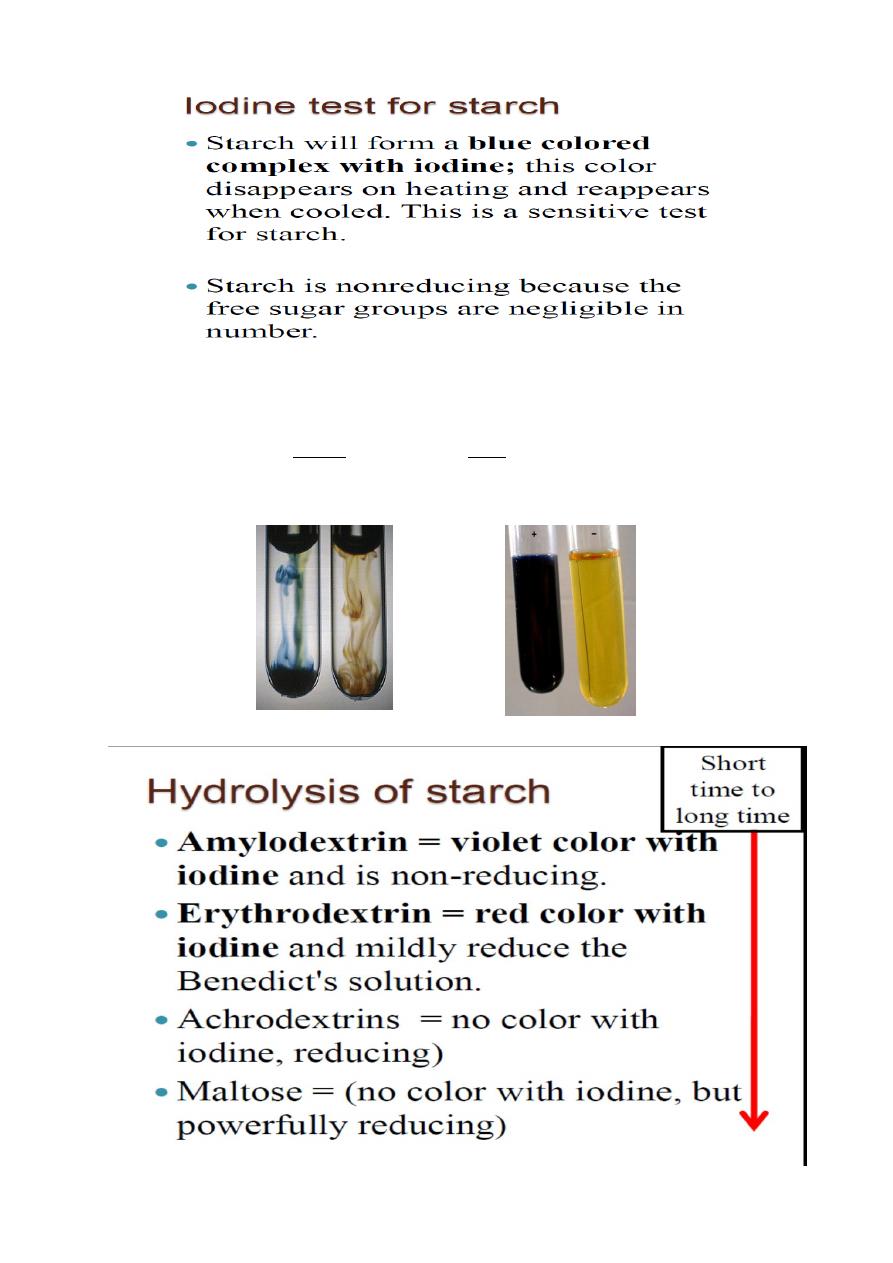
Dextrins- Amylo, Eryhthro and Achrodextrins, formed as
intermediates during hydrolysis of starch give violet, red and no
color with iodine respectively.
First Class/Practical Medical Chemistry
Page 4 of 10
By : Dr. Tamathir Abass
• The presence of starch can easily be identified using iodine
(I
2
)
• Rows of iodine atoms form in the core of the
-helix of
amylose, forming a dark blue complex
• Because amylopectin, glycogen and cellulose do not form
-helices, they do not complex well with iodine, so do not
show the blue color (they show a purple or brown color)
• Monosaccharides do not interact with the iodine, so no
color is produced
Starch
Suspensions of Amylose
in water adopt a helical
conformation
Iodine (I
2
) can insert in
the middle of the Amylose
helix to give a blue color
that is characteristic and
diagnostic for starch
First Class/Practical Medical Chemistry
Page 5 of 10
By : Dr. Tamathir Abass
Iodine Test
• Test for Cellulose, Starch, Dextrin
Procedure: Add a drop of dil. HCl to 1 ml test solution to acidify the
solution. Add few drops of iodine solution. Record the colours observed.
Gently warm the solution and then cool it. Note he change.
First Class/Practical Medical Chemistry
Page 6 of 10
By : Dr. Tamathir Abass
Sucarase
boiling 1 min
boiling 1 min
boiling 30 second
dil. CH
3
COOH
dil. CH
3
COOH
Lactas
e
Maltase
dil. HCl
Hydrolysis of C.H (Di and polysaccharides)
There are two types of hydrolysis:
1- Hydrolysis of C.H by acid and a period of boiling.
2- Hydrolysis of C.H by digestive enzymes.
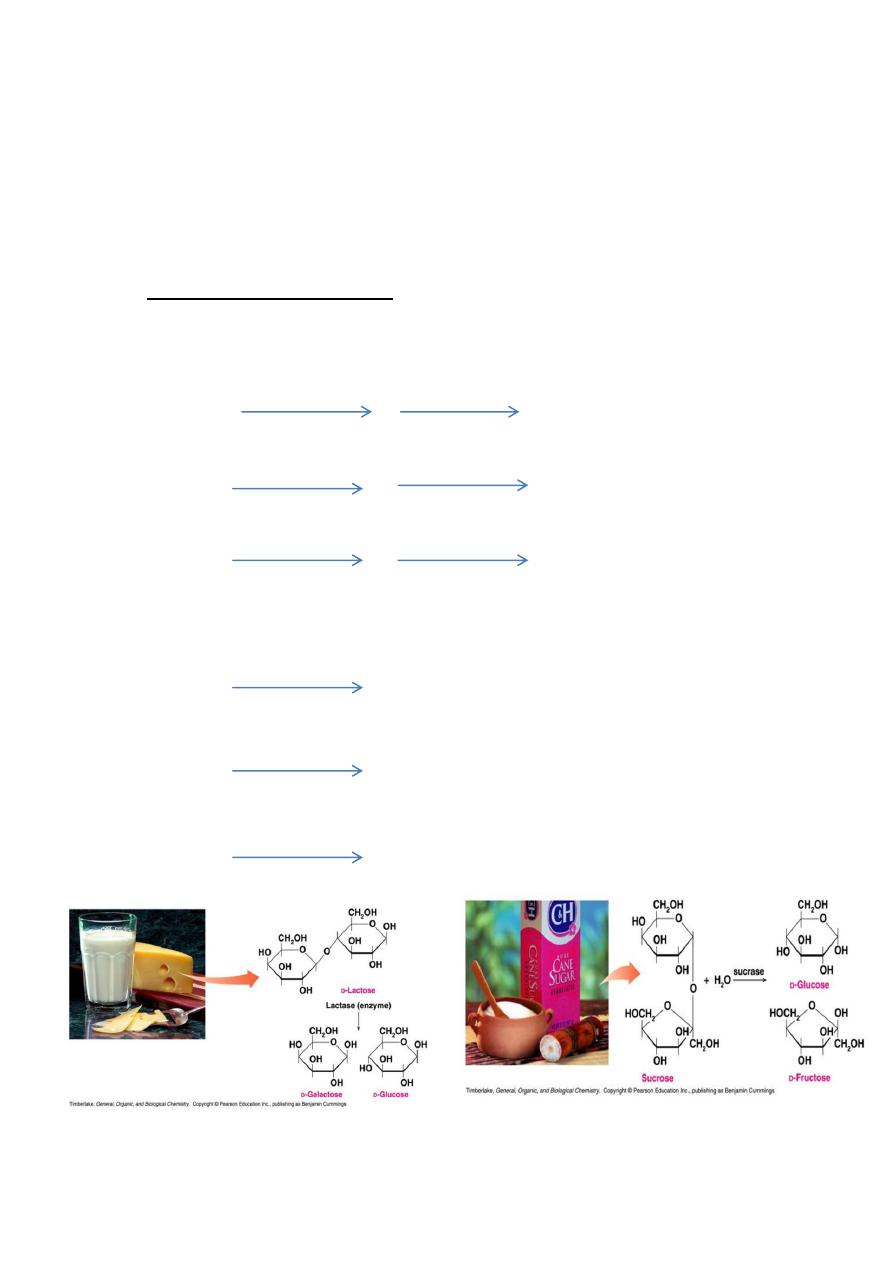
A-Hydrolysis of disaccharides (Lactose, Maltose and Sucrose).
1- By acid and a period of boiling.
Lactose
glucose + galactose
Maltose
2 glucose
Sucrose
glucose + fructose
2- By digestive enzymes.
Lactose glucose + galactose
Maltose 2 glucose
Sucrose
glucose + fructose

First Class/Practical Medical Chemistry
Page 7 of 10
By : Dr. Tamathir Abass
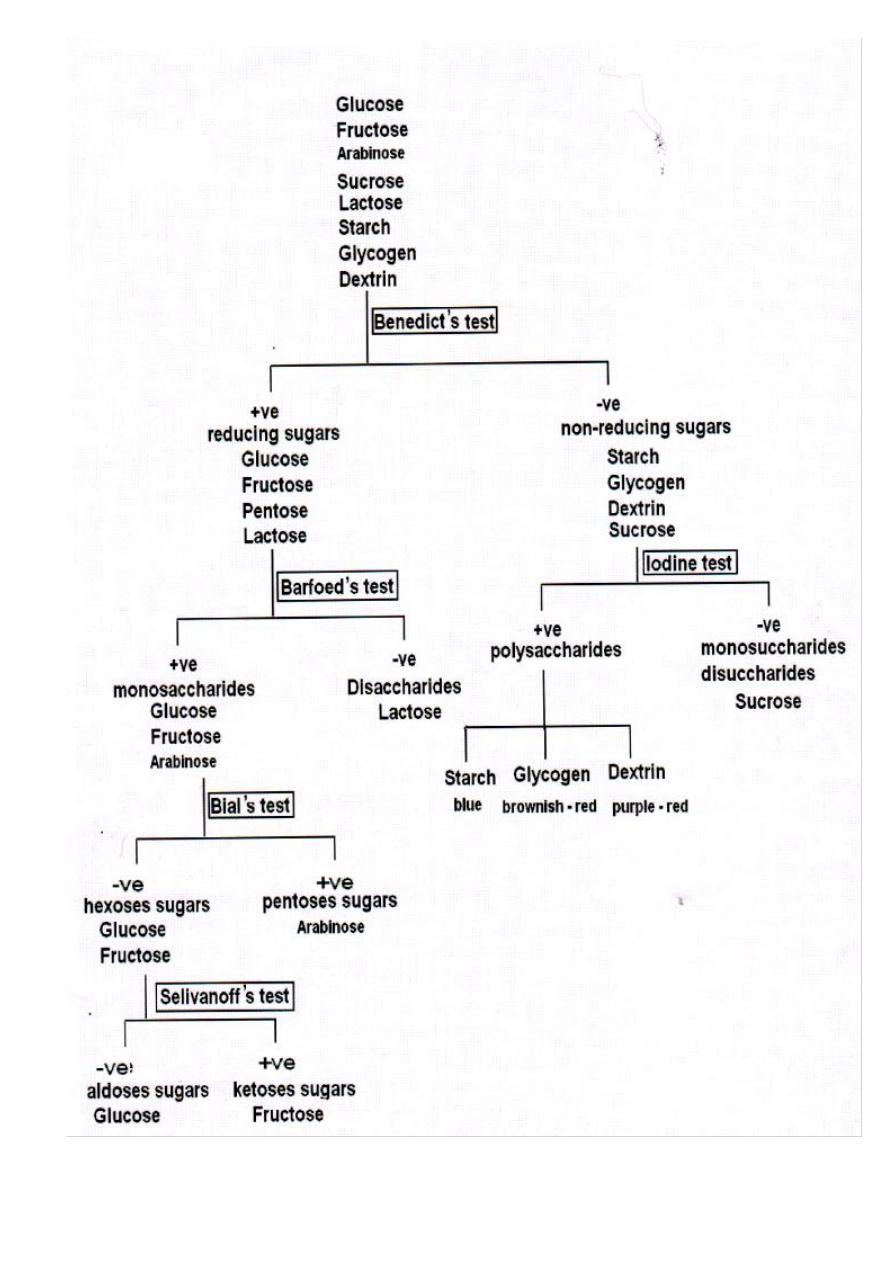
Disaccharides before hydrolysis
Sugar
Benedict's test Barfoed's test Seliwanoff's test
Lactose
+ve
-ve
-ve
Maltose
+ve
-ve
-ve
Sucrose
-ve
-ve
-ve
Disaccharides after hydrolysis
B- Hydrolysis of polysaccharides (starch)
1- Hydrolysis of starch by HCl.
2- Hydrolysis of starch by enzyme α-amlyase present in saliva and
pancreatic juice.
Starch amylose dextrin maltose glucose
While enzymatic hydrolysis occurs at a much lower temperature (37
º
C) .
Furthermore, the end product of enzymatic hydrolysis is maltose rather
than glucose.
Sugar
Benedict's test Barfoed's test Seliwanoff's test
Lactose
+ve
+ve
-ve
Maltose
+ve
+ve
-ve
Sucrose
+ve
+ve
+ve
First Class/Practical Medical Chemistry
Page 8 of 10
By : Dr. Tamathir Abass
Starch before hydrolysis
Sugar Benedict's test Iodine test
Starch
-ve
+ve
Starch after hydrolysis
After hydrolysis the ability of reducing power increases while the ability
of starch to react with Iodine decreases.
Sugar Benedict's test Iodine test
Starch
+ve
-ve
First Class/Practical Medical Chemistry
Page 9 of 10
By : Dr. Tamathir Abass

First Class/Practical Medical Chemistry
Page 10 of 10
By : Dr. Tamathir Abass
