Hyperlipidemia as a risk factor and its treatment
ByDr. salim al-rubaae
Associate professor & consultant internist-endocrinologist
C.A.B.M D.M M.B.C.H.B
College of medicine-university of Baghdad
Medical department
Chief of endocrine-sector
Senior-lecturer
Lipoproteins, Lipid Metabolism and Inflammatory Markers
Structure of Lipoproteins
Free cholesterolPhospholipid
Triglyceride
Cholesteryl ester
Apolipoprotein
Types of Lipoprotein Particles
Triglyceride-rich lipoproteins
Chylomicrons
Very low-density lipoprotein (VLDL)
Cholesterol-rich lipoproteins
Low-density lipoprotein (LDL)High-density lipoprotein (HDL)
Chylomicrons and Very Low-density Lipoproteins
Chylomicrons are much larger than VLDLChlylomicrons contain more triglyceride per particle than VLDL
They contain apolipoproteins on their surface such as:
B apolipoproteins (B100 – VLDL; B48 – chylomicrons)
C apolipoproteins (CII and CIII)
A apolipoproteins (A-I and A-II)
ApoE
LDL cholesterol
Strongly associated with atherosclerosis and CVD events10% increase results in an approximate 20% increase in CHD risk
Most of the cholesterol in plasma is found in LDL particles
Smaller denser LDL are more atherogenic than larger, less dense particles
Risk associated with LDL-C is increased by other risk factors:
low HDL-C
smoking
hypertension
diabetes and the metabolic syndrome
HDL cholesterol
HDL-C has a protective effect for risk of atherosclerosis and CHD
Epidemiological studies show the lower the HDL-C level, the higher the risk for atherosclerosis and CHD
low level (<40 mg/dL, 1 mmol/L) increases risk
HDL-C tends to be low when triglycerides are high
HDL-C is lowered by smoking, obesity and physical inactivity
ApoA-I is the major apolipoprotein in HDL and an elevated ApoA-I is linked to reduced CVD risk
Triglycerides
May be associated with increased risk of CHD eventsLink with increased CHD risk is complex
may be direct effect of smaller TG-rich lipoproteins and/or
may be related to:
low HDL levels
highly atherogenic forms of LDL-C
hyperinsulinaemia/insulin resistance
procoagulation state
hypertension
abdominal obesity
Apolipoproteins
Protein content of lipoproteinsApoB levels used to estimate LDL particle number and increased CVD risk
ApoA-I – major apolipoprotein in HDL and is linked to reduced CVD risk
Functions of apolipoproteins include:
facilitation of lipid transport
activation of three enzymes in lipid metabolism
lecithin cholesterol acyltransferase (LCAT)
lipoprotein lipase (LPL)
hepatic triglyceride lipase (HTGL)
binding to cell surface receptors
ApoB, ApoA-I and ApoB/ApoA-I as Predictors of Risk: AMORIS
Objective: to determine the relationship between levels of ApoB, ApoA-I and other lipids and death from MI
175,553 individuals screened
Follow-up approximately 65 months
Conclusions:
ApoB, ApoA-I and the ratio ApoB/ApoA-I have stronger predictive value than TG, TC and LDL-C
ApoB and ApoA-I better than LDL-C at all levels of LDL-C, but especially valuable in patients with average/low LDL-C
ApoB and ApoA-I help to identify subjects with the metabolic syndrome
Walldius G et al. Lancet 2001;358:2026-2033.
Intestine
Skeletal muscleAdipose
tissueChylomicron
Chylomicronremnant
Remnant
receptor
Liver
Dietarytriglycerides
and cholesterol
LP lipase
to atheromaFFA
Exogenous Pathway of Lipid Metabolism
IDL
Large
VLDLSmall
VLDL
LDL
receptor
Liver
LPL Lipoprotein lipase
HL Hepatic lipase
LDL
LPL
LPL
LPL
HL
HL
HL
Endogenous Pathway of Lipid Metabolism
Reverse Cholesterol Transport
Peripheraltissues
Cell
membraneVLDL, IDL, LDL
LDL
receptor
LCAT
CETPFC
CE
CE
TG
HDL
HDL3
TGCE
Free cholesterol
Cholesteryl estersCETP
Cholesteryl ester transfer proteinLCAT
Lecithin cholesterol acyltransferaseSRB1
FCABCA1
LiverScavenger receptor class B, member 1
Triglycerides
SRB1
ATP-binding cassette, sub-family A, member 1ABCA1
Classification of Dyslipidaemiasand Pathogenesis of Atherosclerosis
Classification of Dyslipidaemias:Fredrickson (WHO) ClassificationLDL – low-density lipoprotein; IDL – intermediate-density lipoprotein; VLDL – very low-density lipoprotein. (High-density lipoprotein (HDL) cholesterol levels are not consideredin the Fredrickson classification.)
Phenotype
I
IIa
IIb
III
IV
V
Lipoprotein
elevated
Chylomicrons
LDLLDL and VLDL
IDLVLDL
VLDL andchylomicrons
Atherogenicity
None seen
++++++
+++
+
+
Prevalence
Rare
CommonCommon
IntermediateCommon
RareSerum
cholesterol
mean to
mean to
mean to
Serum
TGmean
• Yeshurun D, Gotto AM. Southern Med J 1995;88(4):379–391Abetalipoprotinemia
ARC&TG are extremely low
chylomicron, VLDL, LDL, APO-B are negative
Parents (heterozygote) have normal plasma lipid and APO-B
C/F : onset in early childhood
diarrhea / failure to thrive
fat mal absorption
spinocrebellar degeration ( ataxia, dismateria,
spastic-gait ( 3rd , 4th decades ), loss of
tendon-reflaxes, vibration & position senses
pigmented retinopathy- loss night & color vision
( blindness )
acanthocytosis
cardiomyophathy ( rare ) : HF & arrhythmias
DD: Friedreichs ataxia
C/F related to decrease fat soluble vit.
RX : low fat , high caloric high vit. Diet + vit. E
Familial chylomicronaemia
Atosomal – recessive 1/1000000defect is either lipoprotein lipase or APO-C2
chylomicron is very high in the cir. hypertriglyceridaemia( chylomicron&VLDL)
C/F : 1. lipema- retnalis ( fundoscopy )
2. Recurrent abd. Pain (pancreatitis )
3. Eruptive-xanthomas buttouskes
4. Hepatospleeniomigaly ( reuptake of chylomicron by RE cells of live and spleen
5. no premature IHD
RX: a. in LPL def. 1. low lipid diet 15 gm /d
2. fat-soluble vit. ( A,D,K & E supply )
3. fish oil
4. plasmapharesis
b. in APO-C2 plasma infusion ( FFP)
Familial dysbetalipoprotenemia FDL ( type 3 , F.broad beta disease)
APO-E defect & deficiency ( E2/E2 genotype ) is the most one & constitute 1% of general populationmixed hyper lipoprotenemia ( IDL , C , TD )
PPT. factors ( high caloric and fat diet , DM, obesity, hypothyroidism, renal disease, alcohol , estrogen def. and presence of FHC
C/F : seldom occur before menopause in adulthood
1. zanthomas ( tuberoerubtive and palmerxanthoma )
2. premature IHD peripheral vascular disease
3. C&TG are high lipoprotein electrophoresis ( broadbeta disease )
VLDL-C /TG >= 0.3
RX should be aggressive due to IHD
1. treatment of the precipitating factor if any
2. dietary fat restriction
3. Statins, fibrates , niacin
4. Combination of drug therapy
Familial Hypercholesterolaemia (FH)
Most common genetic disorder in Europe and the
US
Higher incidence in Afrikaners , French Canadian & Christian Lebanese
Caused by a mutation of the LDL receptor
Increases risk of CVD
coroneal- arcus
eyelid xanthelasmas
Aortic-stenosis
Tendon xanthoma
Two types of FH:
Heterozygous FH
one LDL-receptor gene affected
affects about 1 in 500 people
TC 9.0-14.0 mmol/L (360-560 mg/dL) in adulthood
Homozygous FH
both LDL-receptor genes affected
rare – affects about 1 in 1,000,000 people
TC 15.0-30.0 mmol/L (600-1200 mg/dL) in adulthood
RX dietary fat restrictions
lipid lowrind drugs ( statin , resins , nicotinic acid, combination therapy , ezetimbe(inhibit intestinal cholestrol –c absorption 10mg per day , LDL- aphaeresis )Relationship Between Cholesterol and CHD Risk: Framingham Study
Castelli WP. Am J Med. 1984;76:4-12.
0
25
50
75
100
125
150
<204
(<5.3)
205-234
(5.3-6.1)
235-264
(6.1-6.8)
265-294
(6.8-7.6)
>295
(>7.6)
CHD incidence per 1000
Serum total cholesterol, mg/dL (mmol/L)
Relationship of Serum Cholesterol to Mortality: Seven Countries Study
Verschuren WM et al. JAMA 1995;274(2):131–13635
Serum total cholesterol, mmol/L (mg/dL)
30
25
20
15
10
5
0
Death rate from CHD/1000 men
2.60
(100)
3.25
(125)
3.90
(150)
4.50
(175)
5.15
(200)
5.80
(225)
6.45
(250)
7.10
(275)
7.75
(300)
8.40
(325)
9.05
(350)
Northern Europe
United States
Southern Europe, inland
Southern Europe, Mediterranean
Japan
Serbia
Cholesterol: A Modifiable Risk Factor
Plasma cholesterol at levels >200 mg/dL cause 4.4 million deaths a year1
Incidence of plasma cholesterol >200 mg/dL in:
51% (107 million) adults in the USA2
58% of patients with established CHD in EUROASPIRE II3
10% reduction in plasma cholesterol results in:
15% reduction in CHD mortality (p<0.001)
11% reduction in total mortality (p<0.001)4
LDL-C is a major target to prevent CHD
• 1. International CVD Statistics 2005 AHA;
• 2. Heart and Stroke Statistical Update 2004 AHA;
• 3. EUROASPIRE II Study Group. Eur Heart J 2001;22:554-572;
• 4. Gould AL et al. Circulation 1998;97:946–952.
Risk Factors for Cardiovascular Disease
ModifiableSmoking
Dyslipidaemia
Raised LDL-C
Low HDL-C
Raised triglycerides
Raised blood pressure
Diabetes mellitus
Obesity
Dietary factors
Thrombogenic factors
Lack of exercise
Excess alcohol consumption
Non-modifiable
Personal history of CVD
Family history of CVD
Age
Gender
Pyörälä K et al. Eur Heart J 1994;15:1300–1331.
Levels of Risk Associated with Smoking, Hypertension and Hypercholesterolaemia
x1.6
x4x3
x6
x16
x4.5
x9
Hypertension
(SBP 195 mmHg)
Serum cholesterol level
(8.5 mmol/L, 330 mg/dL)
Smoking
Poulter N et al., 1993
The Metabolic Syndrome andAssociated CVD Risk Factors
Insulin Resistancehigh TGs
small dense LDL
low HDL-C
Atherosclerosis
Endothelial Dysfunction
Hypertension
Abdominal obesity
HyperinsulinaemiaDyslipidaemia
DiabetesHypercoagulability
Deedwania PC. Am J Med 1998;105(1A);1S-3S.
Overweight and Obesity as a Risk Factor• Associated with significant mortality and morbidity
• Now reached epidemic proportions in Western society and causes:
• 220,000 deaths per year in US and Canada
• 320,000 deaths per year in Western Europe
• An independent risk factor for CVD
• Abdominal obesity associated with the metabolic syndrome which also includes:
• dyslipidaemia, hypertension and insulin resistance
The World Health Report 2002 and International Cardiovascular Disease Statistics 2003; AHA.
The Progression from CV Risk Factors to Endothelial Injury and Clinical Events
Risk factors
Oxidative stressEndothelial dysfunction
NO
Local mediators
Tissue ACE-Ang IIPAI-1
VCAM
ICAM cytokines
Endothelium
Growth factors matrix
Proteolysis
LDL-C
BP
Heart failure
Smoking
Diabetes
Vasoconstriction
Vascular lesion and remodelling
Plaque ruptureInflammation
ThrombosisClinical endpoints
NO Nitric oxideGibbons GH, Dzau VJ. N Engl J Med 1994;330;1431-1438.
Normal Arterial Wall
LumenMedia:
Smooth muscle cell
Matrix proteins
Internal elastic membrane
Endothelium
Intima:
External elastic membrane
Historical Model of Atherogenesis
healthy
subclinicalsymptomatic
Threshold
DecadesYears-Months
Months-Days
Plaque
Intima
MediaLumen
Stable anginaStable plaques with narrowing
Simple diagnostic (ECG, angiography)
Rare MI
Easy to treat
Antischkow N. Beitr Path Anat Allg Path 1913;56:379-404.
Pathogenesis of Atherosclerotic Plaques
Protective response results in production ofcellular adhesion molecules
Monocytes and T lymphocytes attach to
‘sticky’ surface of endothelial cells
Migrate through arterial wall to subendothelial space
Lipid-rich foam cells
Endothelial damage
Macrophages take up oxidised LDL-C
Fatty streak and plaque
‘activated’ endothelium
CELLULAR ADHESION MOLECULESinduces cell proliferation and a prothrombic state
attracts monocytes and T lymphocytes
which adhere to endothelial cells
cytokines (eg. IL-1, TNF-)
chemokines (eg.MCP-1, IL-8)growth factors (eg. PDGF, FGF)
Koenig W. Eur Heart J Suppl 1999;1(Suppl T);T19-26.
The ‘Activated’ Endothelium
Upregulation of endothelial
adhesion moleculesIncreased endothelial permeability
Migration of leucocytes into the artery wall
Leucocyte adhesion
Lipoprotein infiltration
Endothelial Dysfunction in Atherosclerosis
Formation of foam cellsAdherence and entry of leucocytes
Activation of T cells
Migration of smooth muscle cells
Adherence and aggregation of platelets
Fatty Streak Formation in Atherosclerosis
Formation ofthe fibrous cap
Accumulation of
macrophages
Formation of
necrotic core
Formation of the Complicated Atherosclerotic Plaque
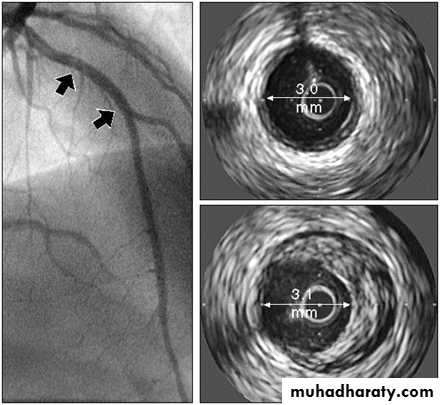
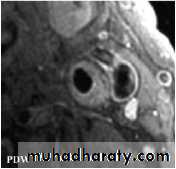
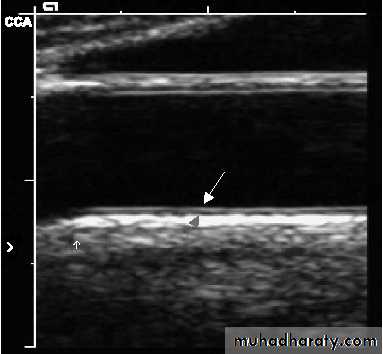
Imaging Techniques Used to Assess Atherosclerosis
• Invasive techniques• Intravascular ultrasound (IVUS)
• Coronary angiography
• Non-invasive techniques
• Magnetic resonance imaging (MRI)
• Computed tomography (CT)
• Ultrasound (B-mode)
Intravascular Ultrasound (IVUS) Showing Atheromatous Plaque
Reproduced from Circulation 2001;103:604–616, with permission from Lippincott Williams & Wilkins.Angiogram
IVUSatheroma
normal vesselCoronary Angiographyof Stenotic Coronary Artery
6
Arrow indicates atherosclerosis (stenosis) of the coronary artery
Magnetic Resonance Image (MRI) of a Stenotic Carotid Artery Bifurcation
left carotid artery bifurcation with an atherosclerotic plaque with a necrotic corerelatively normal artery
Chu B et al. Stroke 2004;8:2444–2448.
Computed Tomography (CT)Showing Atherosclerotic Artery
B-mode UltrasoundReproduced with permission from Kastelein, JJP et al. Am Heart J 2005;149:234–239.
Clinical Manifestations of AtherosclerosisCoronary heart disease
Angina pectoris, myocardial infarction, sudden cardiac death, congestive heart failure (CHF), and arrhythmias
Cerebrovascular disease
Transient ischaemic attack, stroke
Peripheral vascular disease
Intermittent claudication, gangrene, cold feet, painful feet, impotenceUnmet Need and Guidelines
Benefits of Cholesterol LoweringMeta-analysis of 38 primary and secondary intervention trials
% in cholesterol reductionTotal mortality (p=0.004)
CHD mortality (p=0.012)
Mortality log odds ratio
0
4
8
12
16
20
24
28
32
36
-1.0
-0.8-0.6
-0.4
-0.2
-0.040
Gould AL et al. Circulation. 1998;97:946-952.
Relationship Between Changes in LDL-C and HDL-C Levels and CHD RiskThird Report of the NCEP Expert Panel. NIH Publication No. 01-3670 2001. http://hin.nhlbi.nih.gov/ncep_slds/menu.htm
1% decreasein LDL-C reduces CHD risk by1%
1% increasein HDL-C reduces CHD risk by3%Many patients fail to achieve NCEP ATP II LDL-C Goal on Lipid-modifying Therapy: L-TAP
020
40
60
80
100
Percentage of patients
85%
of patientsreceived lipid-modifying therapy
39%
of patients receiving lipid-modifying therapy reached their NCEP ATP II LDL-C goal
(n=4888)
(n=4137)
<20%
of CHD patients who receiving lipid-modifying therapy reached NCEP ATP II LDL-C goal (100 mg/dL; 2.6 mmol/L)
(n=1352)
Pearson TA et al. Arch Intern Med 2000;160:459-467.
Many patients fail to reach European TC goal: EUROASPIRE II
020
40
60
80
100
Percentage of patients
61%
of high-risk patients* received lipid-modifying therapy
51%
of patients reached Joint European TC goal**
*CABG, PTCA, MI or ischaemia, ** TC<5 mmol/L (190 mg/dL)
EUROASPIRE II. Euro Heart J 2001;22:554-572.
Many patients who are treated are still not getting to goal
LDL-C=low-density lipoprotein cholesterol; CHD=coronary heart disease; HDL-C=high-density lipoprotein cholesterol†Patients with LDL-C >100 mg/dL, HDL-C <45 mg/dL, CHD and/or diabetes mellitus
*LDL-C goal of <100 mg/dL
Foley KA et al. Am J Cardiol 2003; 92: 79–81
2829 patients†
1464
not at goal on
initial dose
1365
at goal on initial dose
813 not titrated
651titrated
448 not at goal
203at goal52%
48%
55%
45%
31%
69%
Overall:
1568 (55.4%) patients achieve goal
1261 (44.6%) patients do not achieve goal
Even With Dose Titration, Many Patients Fail to Achieve LDL-C Goals: ACCESS
Ballantyne CM et al. Am J Cardiol 2001;88:265–269.
At week 54, n=2543 CHD patients
Patients at LDL-C goal (%)0
20
40
60
80
atorvastatin 10–80 mg
simvastatin 10–40 mglovastatin 20–80 mg
fluvastatin 20–80 mgpravastatin 10–40 mg
72%
52%
44%
30%
25%
n=1286
n322
n=303
n=332
n=300
Estimate total CVD risk of fatal
CVD event in 10 years using SCORE chartTotal CVD risk <5%
TC 5 mmol/L (190 mg/dL)Total CVD risk 5%
TC 5 mmol/L (190 mg/dL)
Measure fasting lipids, give lifestyle
advice, with repeat lipids after
3 months
Lifestyle advice
Aim: TC<5 mmol/L (190 mg/dL)
LDL-C <3.0 mmol/L (115 mg/dL)
Follow-up at 5-year intervals
TC <5 mmol/L (190 mg/dL)
and LDL-C <3.0 mmol/L (115 mg/dL)
Maintain lifestyle advice with annual
follow-up. If total risk remains 5%,
consider drugs to lower TC to <4.5 mmol/L
(175 mg/dL) and LDL-C to
<2.5 mmol/L (100 mg/dL)
TC 5 mmol/L (190 mg/dL) or
LDL-C 3 mmol/L (115 mg/dL)Maintain lifestyle
advice and start drug
therapy
De Backer G et al. Eur Heart J 2003;24:1601–1610.
2003 European Guidelines:Guide to lipid management in asymptomatic subjectsNCEP ATP III Guidelines
* TLC: therapeutic lifestyle changesAdapted from NCEP, Adult Treatment Panel III. JAMA 2001;285:2486-2497.
Patients with
Drug therapy
considered if LDL
-
C
Initiate TLC*
if LDL
-
C
LDL
-
C
treatment
goal
0
-
1 risk factors
³
160 mg/dL†
³
190 mg/dL
(160
-
189
mg/dL: drug
optional)
<160 mg/dL†
³
2 risk factors
(10
-
year risk
£
20%)
³
130 mg/dL†
10-yr risk 10-20%:
³
130 mg/dL
10-yr risk <10%:
³
160 mg/dL
<130 mg/dL†
CHD and CHD risk
equivalents
(10
-
year risk >20%)
³
100 mg/dL†
³
130 mg/dL
(100
-
129
mg/dL: drug
optional)
<100 mg/dL†
† 100 mg/dL = 2.6 mmol/L; 130 mg/dL = 3.4 mmol/L; 160 mg/dL = 4.1 mmol/L; 190 mg/dL = 5 mmol/L
NCEP ATP III: LDL-C Goals
Adapted from NCEP, Adult Treatment Panel III, 2001. JAMA 2001:285;2486-2497.CHD or CHD risk equivalents
< 2 risk factors≥ 2 risk factors
LDL-C level
100 -160 -
130 -
190 -
goal
100mg/dL
goal
130
mg/dL
goal
160
mg/dL
100 mg/dL = 2.6 mmol/L; 130 mg/dL = 3.4 mmol/L; 160 mg/dL = 4.1 mmol/L
NCEP ATP III: LDL-C Goals (2004 proposed modifications)*Therapeutic option
70 mg/dL =1.8 mmol/L; 100 mg/dL = 2.6 mmol/L; 130 mg/dL = 3.4 mmol/L; 160 mg/dL = 4.1 mmol/LHigh Risk
CHD or CHD risk equivalents
(10-yr risk >20%)
LDL-C level
100 -160 -
130 -
190 -
Lower Risk
< 2 risk factors
Moderately High Risk
≥ 2 risk factors
(10-yr risk 10-20%)
goal
160mg/dL
goal
130mg/dL
70 -
goal100
mg/dL
or optional
70 mg/dL*Moderate Risk
≥ 2 risk factors
(10-yr risk <10%)
goal
130
mg/dL
or optional
100 mg/dL*Grundy SM et al. Circulation 2004;110:227-239.
Existing LDL-C goals
Proposed LDL-C goalsPrimary target
LDL-C <100 mg/dL (2.6 mmol/L)<70 mg/dL (1.8 mmol/L)#
…and if TG 200 mg/dL (2.2 mmol/L), a secondary target is:
Non-HDL-C <130 mg/dL (3.4 mmol/L)#An optional goal for high-risk patients with diabetes and overt CVD
NCEP ATP III Recommendations for Lipid Goals in Patients With Diabetes
NCEP ATP III. JAMA 2001;285:2486–2497.
LDL-C <100 mg/dL (2.6 mmol/L) or a reduction of 30-40%
<70 mg/dL (1.8 mmol/L)#
HDL-C* >40 mg/dL (1.15 mmol/L)
TG <150 mg/dL (1.7 mmol/L)
*For women, HDL value should be increased by 10 mg/dL
#An optional goal for high-risk patients with diabetes and overt CVDADA. Diabetes Care 2005;28(suppl 1):S4–S36.
ADA Recommendations for Lipid Goals in Patients With Diabetes
Lipid-modifying Therapies and Statins
Effect of Lipid-modifying Therapies on LipidsTherapy
Bile acid
sequestrantsNicotinic acid
Fibrates
Probucol
Statins*Ezetimibe
TC–total cholesterol, LDL–low density lipoprotein, HDL–high density lipoprotein, TG–triglyceride. *Daily dose of 40 mg of atorvastatin, simvastatin, pravastatin and fluvastatin.TC
Down
20%Down
25%Down
15%Down
25%Down
19–37%
-
LDL
Down
15–30%Down
25%Down
5–15%Down
10–15%Down
25–50%Down
18%
HDL
Up
3–5%
Up
15–30%
Up
20%
Down
20–30%Up
4–12%
Up
1%
TG
Neutral or up
Down
20–50%Down
20–50%Neutral
Down14-29%
Down
8%Patient
tolerability
Poor
Poor toreasonable
Good
Reasonable
GoodGood
Yeshurun D, Gotto AM. Southern Med J 1995;88(4):379–391. Knopp RH. N Engl J Med 1999;341:498–511. Product Prescribing Information. Gupta EK, Ito MK. Heart Dis 2002;4:399-409.,
Main Effects of Statins
Effects on lipids:Reduce LDL-C, TC and TG
Increase HDL-C
Pleiotropic effects:
Improve or restore endothelial function
Enhance the stability of atherosclerotic plaques
Decrease oxidative stress
Decrease vascular inflammation
Anti-thrombotic effects
Takemoto M, Liao JK. Arterioscler Thromb Vasc Biol 2001;21:1712-1719.
Mechanism of Action of Statins: Cholesterol Synthesis Pathway
acetyl CoA
HMG-CoAmevalonic acid
mevalonate pyrophosphate
isopentenyl pyrophosphate
geranyl pyrophosphate
farnesyl pyrophosphate
squalene
cholesterol
dolichols
ubiquinones
HMG-CoA synthase
HMG-CoA reductaseSqualene synthase
X
Statins
Pharmacokinetics of Statins
Horsmans Y. Eur Heart J Supplements 1999;1(Suppl T):T7–12, Vaughan CJ et al. J Am Coll Cardiol 2000;35:1–10. Rosuvastatin data from Core Data Sheet.Statin
Protein
binding (%)
Metabolised
by CYP450
Lipophilic
Half-
life (h)
rosuvastatin
atorvastatin
simvastatin
pravastatin
fluvastatin
~90%
>98%
95–8%
~50%
>98%
Yes
Yes
No
Yes
No
Yes
Yes
No
~19
~15
~3
~2
~3
* intermediate between hydrophilic and lipophilic
intermediate*minimal
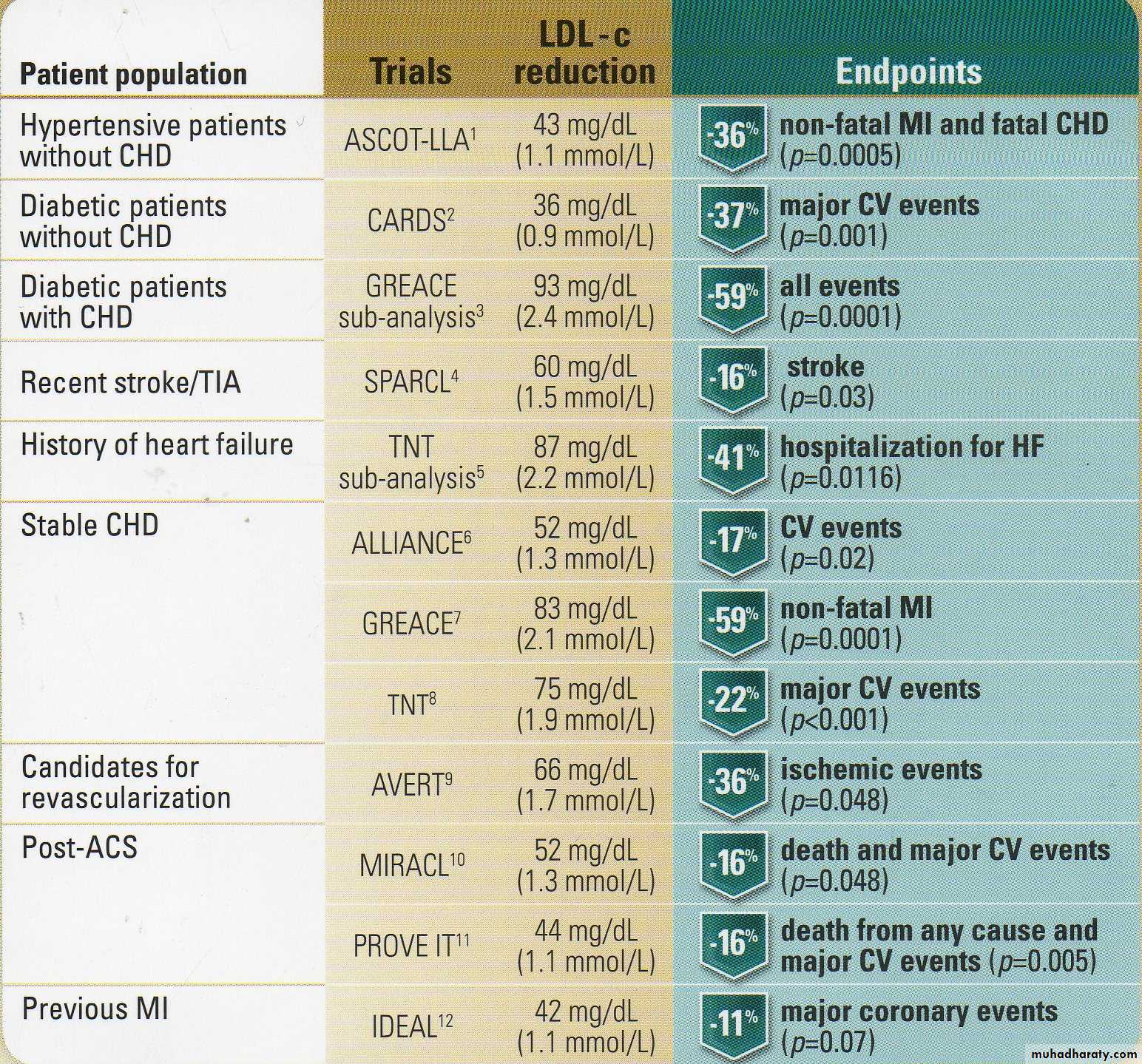
Key Statin Trials
Making an impact in abroad range of patiantsAtorvastatin LDL-c reduction & clinical results in landmark trials
Summary
• Atherosclerosis is associated with CVD, which is a major cause of death in developed countries• Dyslipidaemia, in particular elevated LDL-C and low HDL-C, is associated with increased risk for CVD
• Large statin trials have shown that the lower the level of LDL-C achieved the greater the reduction in CV events
• Diabetes is a risk factor for CVD, which is the leading cause of death amongst people with diabetes
• Dyslipidaemia is associated with diabetes and the metabolic syndrome
• Guidelines recommend lipid levels to reduce the morbidity and mortality caused by dyslipidaemia, and proposed recommendations suggest even more stringent levels for the future
63
ASCOT STUDY(Anglo-Scandinavian Cardiac Outcomes Trial)
Aim:
Investigating effects of atorvastatin on nonfatal MI and fatal CHD in patients with total cholesterol ≤ 250 mg/dl.
Comprehensively investigating effects of atorvastatin in primary prophylaxis of coronary heart disease
Patient Profile:
Disease: Having at least 3 of below written risk factors in addition to hypertension
Left ventricular hypertrophy
ECG abnormalities
Diabetes
Previous stroke or transient ischemic attack
Peripheral vascular disease
Age above 55 years
Male
Proteinuria or microalbuminuria
Smoking
64
ASCOT STUDY(Anglo-Scandinavian Cardiac Outcomes Trial)
Number of patients:
10.305 patients
Duration:
3.3 years
Groups:
Patients received atorvastatin 10 mg/day or placebo (in addition, all groups received anti-hypertensive treatment).
65
Conclusions:
Study is prematurely ended in September of 2002 by ethics committee due to positive results about atorvastatin.
No serious side effect is observed.
Decrease observed in cardiovascular events occurred earlier in comparison with many other studies.
ASCOT STUDY (Anglo-Scandinavian Cardiac Outcomes Trial)
CV Event Reduction with Statin Therapy: ASCOT-LLA
Sever PS et al. Lancet 2003;361:1149-1158.RRR relative risk reduction
0
1
2
3
4
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
Years
Cumulative incidence of nonfatal
MI plus fatal CHD)(%)
36%
RRR
p=0.0005
atorvastatin
placebo67
Comments:
In hypertensive patients with risk to develop moderate cardiovascular event, a serious reduction in risk is obtained with Atorvastatin 10 mg.
Although baseline dose of atorvastatin (10mg) was not titrated, said results could be obtained; if dose was increased, benefit would be much higher.
If the study could be sustained for 5 years, the resultant risk reduction would reach to 50 percent.
Lack of adequate efficiency in diabetes sub-group can be explained regarding low event number in this group.
ASCOT STUDY (Anglo-Scandinavian Cardiac Outcomes Trial)
Lancet. 2003;361:1149-1158.
68GREACE Study (GREek Atorvastatin and Coronary Heart disease Evaluation study)
1600 patients with coronary heart disease are randomized to receive atorvastatin (10-80 mg/day) or general care.
Duration of study:3 years
69
%
TC
LDL-C
HDL-C
CM
Mortality
MI
UA
Subjects: 1600
78% male, 58 years old
22% female, 59 years old
Treatment: atorvastatin 10-80 mg/day
Duration: 3 yearsCM=coronary mortality
MI=nonfatal myocardial infarction;
UA=unstable angina;
Stroke
GREACE Study (GREek Atorvastatin and Coronary Heart disease Evaluation study)
70
AVERT Study (Atorvastatin versus revascularization treatment)
It has significant position in improving percutaneous coronary revascularization symptoms and exercise performance in patients with ischemic heart disease and angina pectoris.
In this study, it is compared with lipid lowering treatment in reducing ischemic event incidence of percutaneous coronary revascularization.
71
N Engl J Med. 1999;341:70-76.
0
5
10
15
20
25
Atorvastatin
Angioplasty/GB
Patients
Experiencing ischemic event (%)
13%
21%
-36% difference*
(P=0.048)
n=22 (164)
n=37 ( 177)
AVERT Study (Atorvastatin versus revascularization treatment)
72
Aggressive lipid lowering therapy with atorvastatin in patients with stable CAD:
Reduces ischemic events at rate of 36 %
Delays time to occurrence of first event
It is safe
It delays or prevents need to percutaneous revascularization.
N Engl J Med. 1999;341:70-76
Comments
AVERT Study (Atorvastatin versus revascularization treatment)
73
Aim of the study is to determine whether atorvastatin 80 mg/day administered within 24-96 hours following acute coronary syndrome would reduce fatal and non-fatal ischemic events.
Study enrolled 3086 patients with unstable angina or with acute myocardial infarction not associated with Q wave.
MIRACL Study (Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering)
74
0
5
10
15
0
4
8
12
16
week
Cummulative
incidence(%)
RR = 0.84P = 0.048
Atorvastatin
Placebo
17.4
14.8
• JAMA. 2001;285:1711-1718.
Time to First Ischemic EventMIRACL Study (Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering)
75
Comments
Aggressive lipid lowering treatment and atorvastatin, started at acute phase of unstable angina and non-Q MI, reduces early recurrent ischemic events.
It is observed that benefit is gained in patients with low – normal baseline LDL-C levels.
Conclusions provides support to the idea that treatment should be considered at hospital irrespective of baseline LDL-C levels.
Treatment is safe and well tolerated.
JAMA. 2001;285:1711-1718.
MIRACL Study (Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering)
76
ASAP Study
325 patients with familial hypercholesterolemia were monitored for 2 years under treatment with atorvastatin or simvastatin.
Baseline values:
TC: 386 mg/dL (atorvastatin), 397 mg/dL (simvastatin)
LDL-C: 309 mg/dL (atorvastatin), 322 mg/dL (simvastatin)
Patients received atorvastatin at dose of 40 mg/day or simvastatin at dose of 20 mg/day; doses were doubled 4 weeks later.
Parimary evaluation criterion was change in carotid and femoral intimal media thickness.
77
REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering)
Aim:
It examined influence of aggressive treatment on progression or regression of coronary atherosclerosis.
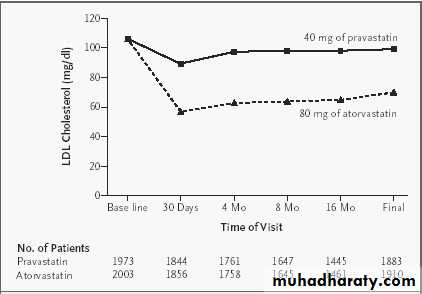
The main aim was to measure, using intravascular ultrasonography (IVUS), the effect of treatment with Atorvastatin 80 mg/day (aggressive treatment) or pravastatin 40 mg/day (moderate treatment) on coronary atherosclerotic lesions in coronary arteries of patients CHD.
Effects of LDL and CRP levels on progression or regression of atherosclerosis were also evaluated.
78
REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering)
Patient Profile:
654 patients with history of CHD and diagnosed using coronary angiography or percutaneous coronary intervention
Duration:
18 months
Groups:
Atorvastatin 80mg vs Pravastatin 40mg (Highest doses at market when the study was started)
79
LDL-C Baseline
150.2 mg/dL
AHA 2003, Orlando, FL
REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering)
LDL-C Completion
79 mg/dLLDL-C Completion
110 mg/dL
Atorvastatin
Pravastatin
46.3%
25.2%
80
REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering)
Conclusions:
LDL-C reduced at rate of 25 % in Pravastatin group, while corresponding figure was 46 % in the group receiving atorvastatin.
The volume of atheroma, the primary evaluation criterion determined with IVUS, had 0.4 % regression with atorvastatin, while 2.7 % increase was observed in pravastatin group; the difference between two groups was significant (p=0.02)
CRP level reduced at rate of 5.2 % in pravastatin group, while it regressed at rate of 36.4 % in atorvastatin group (p<0.0001).
81
REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering)
Comments:
This is the first study comparing highest doses of two popular statins in market.
Progression of coronary heart disease stopped in atorvastatin group, while it persisted in pravastatin group.
Mean LDL level reached in pravastatin group was 110, higher than the guideline target which was 100.
CRP level, important indicator of arterial inflammation, might have contributed to this result.
Selection of statin is important. Highest dose of an efficient statin should be used.
82
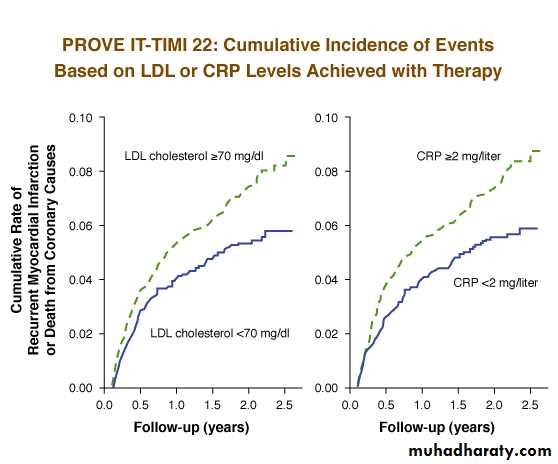
PROVE IT Study (PRavastatin Or atorVastatin Evaluation and Infection Therapy)
Reducing lipid levels using statins decreases cardiovascular events, but the target reduction level in CRP is not clear.
Aim of this study is to examine whether there is any difference between pravastatin at standard dose and atorvastatin at high dose in terms of following incidences considered as primary evaluation criteria: all-cause death, myocardial infarction, hospitalization due to unstable angina, revascularization and stroke.
83
Atorvastatin 62 mg /dL
Pravastatin 95 mg/dLMean LDL-C levels following the study
PROVE IT Study (PRavastatin Or atorVastatin Evaluation and Infection Therapy)
84
PROVE IT Study (PRavastatin Or atorVastatin Evaluation and Infection Therapy)
Recurrent event rates were found at lowest level (1.9 %) in patients with CRP level below 1 mg/dl and LDL cholesterol level below 70 mg/dl following treatment with statin.85
In Atorvastatin Group;
16% : Death, MI, Stroke, hospitalization
due to UE or revascularization14% : CHD-related death, MI or revascularization
25% : Death, myocardial infarction or emergency revascularization
PROVE IT Study (PRavastatin Or atorVastatin Evaluation and Infection Therapy)
86ALLIANCE (Aggressive Lipid Lowering Initiation Abates New Cardiac Events)
Aim:
It is to compare patients receiving aggressive treatment with atorvastatin at dose of 10-80 mg/day with patients receiving conventional care in reducing cardiovascular complications.
Patient Profile:
2242 CHD patients with at least one of following conditions were enrolled:
Acute myocardial infarction
Percutaneous transluminal coronary angioplasty
Coronary artery bypass graft
Unstable angina
J Am Coll Cardiol. 2004;44:1772-1779
87ALLIANCE (Aggressive Lipid Lowering Initiation Abates New Cardiac Events)
Duration:
52 months
Groups:
Atorvastatin 10-80mg (Atorvastatin dose was titrated to 80 mg/day or to ensure LDL-K <80 mg/dL) vs standard treatment (Initially, 2/3 of patients were receiving lipid lowering therapy)
Conclusions:
Mean atorvastatin dose was 40.5 mg in the study.
In the atorvastatin group, 45 % of patients received dose of 80 mg.
J Am Coll Cardiol. 2004;44:1772-1779
88
LDL-C Baseline
147 mg/dL
LDL-C Completion
95 mg/dLLDL-C Completion
111 mg/dL
Atorvastatin
Standard Treatment
34.3 %
23.3 %
ALLIANCE (Aggressive Lipid Lowering Initiation Abates New Cardiac Events)
LDL-C Baseline
146 mg/dL
J Am Coll Cardiol. 2004;44:1772-1779.
89
Patients reaching the target of 100mg/dL
Atorvastatin
Standard Treatment72 %
40 %
ALLIANCE (Aggressive Lipid Lowering Initiation Abates New Cardiac Events)
J Am Coll Cardiol. 2004;44:1772-1779
90
Atorvastatin 80 mg
n=4,995
Primary evaluation criteria: Major cardiovascular event defined as CHD, nonfatal MI, resuscitated cardiac arrest and fatal and nonfatal stroke.
TNT Study
Presented at ACC 2005
Atorvastatin 10 mg
n=5,006
10.003 patient with stable coronary heart disease
35-75 years old, LDL : 130 -250 mg/dL, triglyceride ≤ 600 mg/dL
In the 8-week open period at the beginning of the study, patients received atorvastatin 10 mg.
6 yıl
91TNT Study
LDL-C values
Presented at ACC 2005
92
TNT Study
Major cardiovascular events
Hazard Ratio [HR]=0.78
p<0.001
Presented at ACC 2005
93
TNT Study - Summary
It is demonstrated that in patients with stable coronary heart disease, reducing LDL-C values below 100 mg/dL with high dose atorvastatin further reduces major cardiovascular events at the Year 6 in comparison with keeping LDL-C levels around 100 mg/dL with atorvastatin 10 mg.
Presented at ACC 2005
94
High dose atorvastatin
80 mg/day
If LDL-C<40 mg/dL at Week 24, dose is reduced to 40 mg/day.(13%)
n=4,439
Primary Eva. Criteria: Major coronary event defined as coronary death, hospitalization due to non-fatal acute MI or resuscitated cardiac arrest.
IDEAL Study (Incremental Decrease in Clinical Endpoints Through Aggressive Lipid Lowering Trial)
Presented at AHA 2005
Standard-dose simvastatin
20 mg/day
If cholesterol>190 mg/dL at Week 24, dose is increased to 40 mg/day (23%)
n=4,449
8.888 patients aged at or below 80 years; patients with previous myocardial infarction
Patients receiving statin treatment at baseline: baseline LDL 121.5 mg/dL; total cholesterol 196 mg/dL; mean follow-up period 4,8 years
95
IDEAL Study
Major coronary events
p = 0.07
Presented at AHA 2005%
* Major coronary event; defined as coronary death, hospitalization due to non-fatal acute MI or resuscitated cardiac arrest.
%11
96
IDEAL Study
In patients with previous myocardial infarction, a significant, although not significant, reduction is ensured in cardiovascular events with high dose atorvastatin for 5 years in comparison with standard dose simvastatin treatment.
With high dose atorvastatin treatment, significant reduction in recurrent MI, one of secondary evaluation criteria, is observed.
Hepatic enzyme elevation was higher in high dose atorvastatin group.
Presented at AHA 2005
97
ARMYDA-3 Study (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery)
Aim:
Investigating influences of statin treatment on atrial fibrillation occurring after cardiac operations.
It is considered that inflammatory mechanisms may play a role in post-operative atrial fibrillation.
In addition to reducing LDL-C, statins also have other pleitropic effects and modulation of inflammatory response may be one of those effects. Thus, they may possibly reduce incidence of atrial fibrillation.
98
Study Design:
200 patients naive to statins were treated with atorvastatin 40 mg or placebo.
All patients were scheduled for elective CABP operation or heart valve replacement / correction.Patients with history of atrial fibrillation were excluded.
Patients included in the study were preoperatively treated with atorvastatin or placebo for 1 week.
ARMYDA-3 Study (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery)
99
Primary endpoint:
Post-operative atrial fibrillation lasting more than 5 minutes.
Secondary endpoints:
Incidence of major adverse cardiovascular and cerebral event occurring within 30 daysCorrelation between CRP levels and occurrence of atrial fibrillation.
ARMYDA-3 Study (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery)
100
Atrial tachy-arrhythmia (Atrial fibrillation or atrial flutter):
It is a frequent complication observed following cardiac operations.
It is closely related with hemodynamic disorder, stroke, other thromboembolic events , prolonged hospitalization period and increased treatment costs.
Post-operative atrial fibrillation occurs in 40 % of patients undergoing to CABG.
This incidence is more frequent than valve operations and combined CABG + valve surgery.
ARMYDA-3 Study (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery)
101
Results:
In comparison with placebo, atorvastatin treatment significantly reduced incidence of postoperative atrial fibrillation and shortened hospitalization period.
It is found that group treated with atorvastatin showed reduction in atrial fibrillation risk at rate of 601 % in comparison with placebo.
ARMYDA-3 Study is the first randomized study indicating that a specific statin treatment may have positive effects on incidence of atrial fibrillation occurring after cardiac operations.
Moreover, it is also reported that shortened hospitalization period is an important contribution.
ARMYDA-3 Study (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery)
102
CURVES Study
Aim:
Comparing efficiency and safety of atorvastatin, simvastatin, pravastatin, lovastatin and fluvastatin on lipids in patients with baseline LDL-C 160 mg/dL and TG 400 mg/dL.
Patient Profile:
534 patients with baseline LDL-C 160 mg/dL and TG 400 mg/dL
Duration:
8 weeks
Groups:
Atorvastatin 10,20,40,80mg, simvastatin 10,20,40mg, pravastatin 10,20,40mg, lovastatin 20,40,80mg and fluvastatin 20,40mg
103
10 mg
20 mg40 mg
10 mg
20 mg
40 mg
80 mg
10 mg
20 mg40 mg
20 mg
40 mg
20 mg
40 mg
80 mg
6-weeks
diet8-weeksactive treatment
Fluvastatin (24 patients)
Pravastatin (82 patients)
Atorvastatin (198 patients)
Simvastatin (187 patients)
RandomizationLovastatin (43 patients)
Am J Cardiol 1998; 81:582-587
CURVES Study104
Comparison of LDL-C Reductions
Am J Cardiol 1998; 81:582-587
Mean change in LDL-C
-50-40
-30
-20
-10
Dose range (mg)
-60
20
40
80
10
Fluvastatin
PravastatinSimvastatin
*
*
†
†
†
*
*
*
*
*
‡
‡
‡
Lovastatin
(40 mg bid)
Atorvastatin(80 mg qd)
CURVES Study*atorvastatin less than 10 mg (P<0.02)
†.atorvastatin less than 20 mg (P<0.01)
‡ higher than mg equivalent doses of comparison agents (P0.01)
105
Am J Cardiol 1998; 81:582-587
Results: CommentAt general baseline and general initial titration doses, atorvastatin produced higher reduction in LDL-C levels in comparison with other statins.
All statins are well tolerated and serious side effect is observed with none of study drugs.
CURVES Study
106
TARGET/TANGIBLE Study
Aim:
Aim of this study is to determine efficiency and safety level of atorvastatin treatment conducted in comparison with simvastatin in terms of reducing LDL cholesterol levels to < 100 mg/dl in a large patient group with coronary heart disease.
Totally, 2586 patients were enrolled
107
Sixty seven (67) percent of patients in atorvastatin group and 53% of patients in simvastatin group could achieve to reach LDL cholesterol levels at or below 100 mg/dl and there was significant difference between groups.
In patients under secondary prevention due to coronary heart disease, both atorvastatin and simvastatin are safe drugs and adverse event rates are similar with both drugs.
TARGET/TANGIBLE Study
108
It is the largest safety study conducted, to date, in order to compare atorvastatin treatment with another statin in a CHD patient population.
In comparison with simvastatin, atorvastatin ensured ability to reach LDL cholesterol targets under secondary prevention in a larger population and in addition, it offered equivalent safety profile.
TARGET/TANGIBLE Study
109
WATCH Study (Women’s Atorvastatin Trial on Cholesterol)
In previous studies, it was demonstrated that female with high risk of cardiovascular disease gained similar benefits, in comparison with that of male, from cholesterol lowering therapy. In women with defined risk factor, ability to reach LDL-C target was not examined in details.
Aim of the study is to examine ability to reach treatment targets for LDL-C with lipid lowering therapy in 318 women with CHD or CHD risk factor.
Patients received atorvastatin at dose of 10-80 mg in order to reach LDL-C targets.
110
Sixty three (63) % of patients receiving 10 mg atorvastatin and 79 % of patients receiving 20 mg atorvastatin reached the target LDL cholesterol levels.
Of female patients with CHD, 34 % reached target LDL cholesterol levels (<100 mg/dL) with 10 mg atorvastatin, while 60 % of patients reached the target with 20 mg atorvastatin.
Eighty seven (87) of patients without CHD and 80 % of patients with cardiovascular disease reached target LDL value with maximum dose of atorvastatin (80 mg).
WATCH Study (Women’s Atorvastatin Trial on Cholesterol)
111
Comments:
Atorvastatin offers efficient treatment in reaching target LDL-C levels in women with CHD or CHD risk factor.