د. حسين محمد جمعة
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2010
Coughs Overview
SARS
Avian influenza
Vaccines
A cough is an action your body takes to get rid of substances that are irritating to your air passages,. A cough occurs when special cells along the air passages get irritated and trigger a chain of events. The result? Air in your lungs is forced out under high pressure.
You can choose to cough (a voluntary process), or your body may cough on its own (an involuntary process
Coughs Overview
Causes of Coughs
The list of possible causes of cough is long and highly varied. Doctors classify coughs into 2 categories.acute and chronic. Many doctors define an acute cough as one that been present for
less than 3 weeks.
Chronic coughs are those present for
more than 3 weeks
Acute coughs
Infectious causes of acute cough include viral
Upper respiratory infections the common cold sinus infections, pneumonia .SARS, and whooping cough
Noninfectious
causes of cough include flare-ups of the
Following chronic conditions: chronic bronchitis, emphysema, asthma, and environmental allergies.
Is to divide them into their locations with respect to
the lungs. The categories are• environmental irritants,
• conditions within the lungs,
• conditions along the passages
• conditions within the chest cavity but outside of the lungs,
• and digestive causes.
Chronic coughs
environmental substance that irritatesthe air passages or the lungs is capable of producing a chronic cough with continued exposure.
Cigarette smoke is the most common cause of chronic cough.
Other cough-producing irritants include dusts, pollens, pet dander, particulate matter, industrial chemicals and pollution, cigar and pipe smoke, and low environmental humidity.
Within the lungs
Common causes
asthma, emphysema, and chronic bronchitis.
Less common causes
cancer, sarcoidosis, diseases of the lung tissue,
and congestive heart failure
The upper respiratory tract
Chronic sinus infections,chronic postnasal drip,
diseases of the external ear,
infections of the throat, and
use of ACEI.
diseases elsewhere within the chest cavity may also be responsible for chronic cough. Conditions within the chest known to cause chronic cough include cancer,
unusual growth of a lymph node, and an abnormal enlargement of the aorta,
within the chest cavity
An often-overlooked cause of the chronic cough is GERD.
GERD occurs when acid from the stomach travels up the esophagus. This abnormal condition can cause irritation of the esophagus and larynx resulting in the reflex production of a cough.GERD
When to Seek Medical Care
Cough is associated with a fever and sputum production
Cough fails to get better after other symptoms go away
Cough changes in character
Trial therapy shows no signs of reducing the cough
You begin coughing up blood
Cough interferes with the activities of daily living or sleep cycles
Call your doctor immediately if you have shortness of breath or difficulty breathing.
Studies in the United States report a 20% incidence of Bordetella pertussis infection among adults with a persistent cough.
neither infection nor immunisation results in lifelong immunity .
We made a diagnosis of a recent Bordetella pertussis infection if we found a fourfold change in IgG antibody titre to pertussis toxin in paired samples or a single IgG titre to pertussis toxin that was greater than 100 ELISA units/ml.
Previous research in several countries has shown that pertussis is an endemic disease among adolescents and adults.
Our research suggests that in the United Kingdom pertussis is also endemic among younger school age children.
pertussis immunisation policy in the UK was changed from an accelerated primary course (2, 3, and 4 months) of the whole cell vaccine to an accelerated primary course of the acellular vaccine with a fourth preschool booster dose. Some countries administer five doses of pertussis vaccine.
Little evidence indicates that administering erythromycin to children with pertussis two weeks after they have contracted the infection either reduces symptoms or prevents transmission. However, a secure diagnosis of pertussis will allow general practitioners to give parents an indication of the likely length of cough and prevent them prescribing unnecessary drugs for asthma or referring children for further investigations.
What is whooping cough
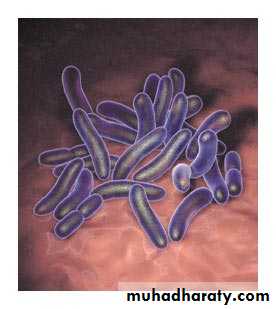
acute, highly contagious infection of the upper respiratory system —specifically, the area where the nasal passages meet the back of the throat (nasopharynx). The incubation period is about 7 to 14 days, The infection causes irritation in breathing passages, resulting in severe coughing spells. caused by Bordetella pertussis is a Gram-negative coccobacillus , is non-motile.humans are its only host it's marked by the symptom that gives the disease its name: a severe, hacking cough followed by a high-pitched intake of breath that sounds like "whoop."
The first outbreaks of pertussis were described in the 16th century. after the introduction of a vaccine in the 1940s, the number of cases gradually declined, reaching a low in the 1980s.
Since then, however, the incidence of whooping cough has been increasing, primarily among children too young to have completed the full course of vaccinations and teenagers whose immunity has faded.
With proper care, most teenagers and adults recover from whooping cough without complications. Whooping cough is more serious in children, especially infants younger than 6 months of age. The bacterium responsible for the infection, Bordetella pertussis, was not isolated until 1906. The incidence of pertussis has been steadily increasing since the 1980's.
Severe coughing attacks that bring up thick phlegm.
Coughing attacks — up to 15 coughs in a row — that end with a high-pitched whoop sound as you gasp for air. These may be so severe that your child vomits or turns red or blue from the effort.Fatigue from coughing so much.
In adults, signs and symptoms of whooping cough may resemble those of bronchitis, a respiratory infection that causes a nagging cough — you may have heard it referred to as the "100-day cough." Babies and infants with whooping cough may not whoop at all, or at least not as loudly as older children do. Some children with whooping cough may experience choking spells and turn blue in the face as they struggle to breathe after a coughing fit.
Severe coughing can result in tiny red spots caused by ruptures in blood vessels at the skin's surface (petechiae) in your upper body, as well as small areas of bleeding in the whites of your eyes. You may even bruise or break a rib if your coughing episodes are severe. Coughing may be worse at night.
Some people may even experience recurring episodes of coughing over the course of a year, especially when they contract a cold or other respiratory infection.
Halperin S. N Engl J Med 2007;356:110-113
Photomicrograph of Bordetella pertussisCan adults get whooping cough
Although whooping cough is considered to be an illness of childhood, adults may also develop the disease.The illness usually is milder in adults than in children, but the duration of the paroxysmal cough is just as long as in children.
The characteristic “whoop” that occurs after paroxysmal bouts of coughing is recognized in only 20-40% of adults with whooping cough.
Adults who do become infected may have retained a partial degree of immunity against the infection, and this can result in a milder illness.
Whooping cough in adults is more common than usually appreciated, accounting for up to 7% of adult illnesses that cause coughing each year.
Infected adults are a reservoir (source) of infection for children.
How is whooping cough diagnosed
clinical history.laboratory tests
Culture of the bacterium Bordetella pertussis from nasal secretions can establish the diagnosis.
PCR test that can identify genetic material from the bacterium in nasal secretions.
How is whooping cough treated
Antibiotics directed against Bordetella pertussis can be effective in reducing the severity of pertussis when administered early in the course of the disease.Antibiotic therapy can also help reduce the risk of transmission of the bacterium to other household members as well as to others who may come into contact with an infected person.
Unfortunately, most people with pertussis are diagnosed later with the condition in the second (paroxysmal) stage of the disease.
Treatment with antibiotics is recommended for anyone who has had the disease for less than 21 days.
It is unclear whether antibiotics have any benefit for persons who have been ill with pertussis for longer periods, although antibiotic therapy is still often considered for this group.
There is no proven effective treatment for the paroxysms of coughing
Severe coughing spells can significantly decrease the blood's oxygen supply.
If blood oxygen levels, measured with a pulse oximeter, are low, oxygen may be given for a short time through a nasal cannula or an oxygen mask.Infants, especially those younger than 4 months of age, are typically hospitalized.
Hospitalization allows health professionals to ensure the baby is getting enough fluids and nutrients.
If needed, a baby also may receive oxygen therapy and have mucus suctioned from the nose and throat.
Because suctioning mucus may trigger coughing spells, it is only done in specific situations.
Home Treatment
If your child has whooping cough, the coughing spells can be frightening. You may be able to help manage the symptoms by:Creating a quiet, calm, restful environment.
Keeping stimulation to a minimum can help reduce the number of coughing spells.
Controlling possible triggers of a coughing episode, such as smoke, dust, sudden noises or lights, or changes in temperature.
Giving your child frequent, small sips of fluids and nutritious foods to provide needed energy that coughing depletes.
Using a cool-mist humidifier in your child's room. But watch closely to see its effect; sometimes humidity makes coughing spells worse, in which case it should be avoided. Dry, hot, or polluted air may make coughing spells worse.
Frequent handwashing is important to help prevent the spread of infection. Keep children away from people who have a severe cough, especially if it is possibly related to whooping cough.
Complications of whooping cough pertussis
Young infants are at highest risk for whooping cough (pertussis) and for pertussis-associated complications. The most common complication and the cause of most pertussis-related deaths, is secondary bacterial pneumoniaoccurred among 5.2% of all reported pertussis cases, and among 11.8% of infants less than six months of age.
Pertussis can cause death in young children; Most of the pertussis-related deaths have occurred in children who have not been vaccinated or who are too young to have received the vaccine.
Seizures, encephalopathy (abnormal function of the brain due to decreased oxygen delivery to the brain,
asthma,
malnutrition.
excessive coughing may cause a bruised or broken rib or a hernia
Ear infections,
Nosebleeds and subconjunctival haemorrhages.
Treatment
Treatment for whooping cough varies, depending on your age and the severity of signs and symptoms.
Older children, teens and adultsbed rest along with an antibiotic such as azithromycin or erythromycin.
Although antibiotics won't cure whooping cough, they can shorten the duration of the illness and they shorten the period of communicability.
Vaccination Schedule for Adults and Adolescents
Tetanus and diphtheria toxoids (Td(Influenza vaccine (flu shot(
Pneumococcal polysaccharide vaccine
Hepatitis A Vaccine
Hepatitis B Vaccine
MMR (Measles-Mumps-Rubella(
Poliovirus
Varicella (chickenpox) vaccine
TETANUS AND DIPHTHERIA TOXOIDS (TD) VACCINE
combination vaccine to prevent tetanus and diphtheria.Adults who had received recommended childhood immunizations should have a Td shot every 10 years
Adults who have never received childhood vaccinations should receive three Td shots (the second shot in 4-8 weeks after the first, and the third shot at 6-12 months after the second shot(
Adults at higher risk of contracting tetanus (adults working with farm manure or home garden manure fertilizers) should have a Td shot every 5 years.
Individuals with a clean, minor wound who has had less than three Td shots or of uncertain vaccination history should receive a Td shot .
Individuals with more serious wounds (wounds from burns, crushing, frostbite) or wounds contaminated with dirt, feces, or saliva should have both Td and tetanus immune globulin if the individual had less than 3 shots or uncertain vaccination history, and Td alone if he/she had 3 or more shots but the last one was more than 5 years ago .The vaccine can be given during pregnancy and lactation except during first trimester of pregnancy.
INFLUENZA VACCINE (also known as "flu shot)Who should receive the influenza vaccine
Adults 50 years or olderAll residents and staff of chronic care facilities, including nursing homes, board and care facilities, and institutions for the mentally and physically disabled .
Adults of any age with chronic diseases such as asthma, chronic bronchitis, emphysema, congestive heart failure, angina, previous heart attack, heart valve conditions, heart inflammation, diabetes mellitus, kidney disorders, sickle cell disease and other hemoglobin abnormalities, cancers, and diseases or treatments that weaken immunity (such as AIDS, cancer chemotherapy, prednisone or other corticosteroid medications(
All care givers, nursing and medical staff and institutional employees and household contacts of those with the chronic diseases noted above
Children and adolescents (ages 6 months to 18 years) on long-term aspirin therapy and therefore at risk for developing Reye's Disease if infected with the influenza virus
The influenza vaccine only protects against three specific strains of influenza virus ,which are chosen each year because they are expected to be the predominant strains in the next flu season. Because one or more of the expected predominant strains changes each year, an influenza immunization is recommended each year.
The influenza vaccine should be given to women who will be at 2nd or 3rd trimesters of pregnancy during the influenza season. not given to women during the 1st trimester .
Individuals with severe allergy to eggs (such as anaphylactic reactions) should not receive influenza vaccine
Individuals currently having an acute illness with fever should postpone their immunization until they have recovered from the acute illness Influenza vaccine only protects against infection by the three particular strains of influenza virus that are selected for the vaccine that year
Vaccinated individuals can still catch other virus infections resulting in cough, fever, and other symptoms that resemble influenza.
Side effects of flu shots include transient soreness at the site of the injection, muscle aches, fever, and feeling unwell. Serious allergic reactions are rare.
VARICELLA (chickenpox) VACCINE
Who should receive the varicella vaccineAll susceptible individuals of any age should be vaccinated. Susceptible individuals are individuals who have not had chickenpox or whose blood tests indicate lack of immunity.
It is especially important for the following individuals to be vaccinated:
Susceptible adolescents and adults living with childrenSusceptible healthcare workers
Susceptible family contacts of patients with weakened immunity such as those with AIDS, those receiving chemotherapy or taking prednisone or other corticosteroid medication
Susceptible international travelers
Susceptible nonpregnant women of childbearing age
Susceptible individuals living or working in environments where transmission is likely (such as teachers of young children, residents or staff of institutional settings, college students(
2doses separated by 4-8 weeks
If more than 8 weeks go by after the 1st dose, the 2nd dose can be given without repeating the 1st dosePregnant women should not receive the varicella vaccine. Pregnancy should be avoided for 1 month after varicella vaccination
The following individuals should not receive the varicella vaccine:
Individuals with anaphylactic allergy to gelatin or neomycinIndividuals with untreated active tuberculosis
Individuals with weakened immunity such as HIV infection, or taking medications such as corticosteroids that weaken immunity, or have diseases that weaken immunity
Pregnant women
Individuals who have received immune globulin or blood product in prior 5 months The varicella vaccine is highly effective in preventing chickenpox.
It is not certain whether the varicella vaccine prevents shingles.
PNEUMOCOCCAL POLYSACCHARIDE VACCINE (also known as lobar pneumonia vaccine (
Adults 65 years and older
Adults with chronic lung and heart problems, and diseases such as diabetes, chronic liver disease, alcoholism, lymphoma, multiple myeloma, kidney diseases, organ transplant recipients, chronic cerebrospinal fluid leak, AIDS and other conditions or treatments which lower body immunityAdults with lack of adequate spleen function such as sickle cell anemia, spleen absent surgically or from an accident
Alaskan Natives and certain American Indian populations who are at higher than normal risk of developing pneumococcal infections and related complications
Pneumococcal vaccine currently in use is a one-time vaccine. However, the following groups should receive revaccination if ≥5 years have passed since the first dose:
Individuals vaccinated prior to age 65
Individuals with lack of spleen function, chronic kidney disease, weakened immunity, or have undergone organ transplantation
The vaccine can be given at the same time as the Influenza vaccine, but has to be given in the opposite arm.
Pneumococcal polysaccharide vaccine is effective against pneumococcal infection, especially against the more serious blood stream infections
Common side effects include pain and redness at the injection site
Fever, muscle aches and severe local reactions are rare
Anaphylaxis is very rare.
Women in first trimester of pregnancy, and individuals who have known allergy to the vaccine or its ingredients should not receive the vaccine
HEPATITIS A VACCINE
Two hepatitis A vaccines are available in the US: Havrix and Vaqta Who should receive the hepatitis A vaccine?Individuals at increased risk of acquiring hepatitis A and individuals with chronic liver disease should be vaccinated.
Individuals at increase risk of acquiring hepatitis A are:
Travelers to countries where hepatitis A is common
Men who have sex with men
Illegal drug users (either injection or non-injection drug use.
Researchers working with hepatitis A virus or primates that are susceptible to hepatitis A infection .
Patients with clotting factor disorders receiving clotting factor concentrates.
Individuals with chronic liver disease such as cirrhosis or hepatitis C are not at increased risk of acquiring hepatitis A, but they can develop serious (sometimes fatal) liver failure if infected with hepatitis A, thus they should be vaccinated.
Some local health authorities or private companies may require hepatitis A vaccination for food handlers.
Hepatitis A vaccines should be given in the muscle
in 2 doses. For adults receiving Vaqta, the seconddose should be given 6 months after the first dose.
For adults receiving Havrix, the second dose should
be given 6-12 months after the first dose.
Safety of hepatitis A vaccine in pregnancy has not
been established, though risk to the fetus is
believed to be low Individuals with past allergic
reaction to vaccine ingredients such as alum or
phenoxyethanol preservative should not receive the
vaccine
Common side effects are soreness at the injection site, headache, and malaise.
After the first dose, protective antibodies develop in 70% of vaccine recipients in 2 weeks and more than 95% of recipients in 4 weeksAfter two doses of the hepatitis A vaccine, immunity against hepatitis A infection is believed to be long lasting.
Because protective antibodies take weeks to develop, travelers to countries where hepatitis A is common should be vaccinated at least 4 weeks before departure. The Centers for Disease Control (CDC) recommends immunoglobulin be given in addition to vaccination if departure is prior to 4 weeks. Immunoglobulin provides quicker protection than the vaccines but the immunoglobulin protection is short-lived.
HEPATITIS B VACCINE
EngerixB and Recombivax HB are two vaccines available in the USWho should receive the hepatitis B vaccine?
All infants
Adolescents under 18 years of age who did not receive hepatitis B
People occupationally exposed to blood or body fluids
Residents and staff of institutions for the developmentally disabled
Patients receiving kidney hemodialysis
Hemophiliacs and other receiving clotting factor concentrates
Household contacts and sex partners of hepatitis B infected
patients .
patients who test positive for hepatitis B surface antigen(
Travelers who will spend more than 6 months in regions with high
Hepatitis infection rates,Injection drug users and their sex partners
Men who have sex with men, men or women with multiple sex
partners,
recent infection with a sexually transmitted infection
Hepatitis B vaccines should be given in three doses, with the second dose 1-2 months after dose, and the third dose 4-6 months after dose.
For best results the vaccinations should be given in the deltoid muscles, not in the buttocks.
If the three-dose schedule is interrupted, it is acceptable to just complete all 3 doses later; it is not necessary to start over.
Engerix B and Recombivax HB can be used interchangeably any point during the vaccination schedule.
All pregnant women should have a blood test for the hepatitis B surface antigen (HBsAg). Women who test positive for HBsAg risk transmitting the virus to their infants during labor.
Therefore infants born to HBsAg-positive mothers should receive hepatitis immune globulin (HBIG) in addition to hepatitis B vaccine at birth.
Even though hepatitis B vaccine can offer long lasting immunity, immunity from the vaccine takes time to develop. While HBIG is an antibody solution that can offer more rapid though short lived immunity.
Hepatitis B vaccinations are safe for pregnant and nursing mothers.
Side effects of Hepatitis B immunization are usually minor: pain at the injection site and low-grade fever.
The hepatitis B vaccines are 95% effective. Five percent of vaccinated individuals will fail to develop the necessary antibodies for immunity after the three doses.
Patients with weakened immunity (such as HIV infection), elderly patients, and patients undergoing kidney hemodialysis are more likely to fail to respond to the vaccinations.
Hepatitis B vaccination takes time to become effective.
Therefore unvaccinated individuals exposed to infected materials (such as a healthcare worker stuck by a contaminated needle) will need hepatitis immune globulin (HBIG) in addition to hepatitis B vaccine.
HBIG is an antibody solution that offers more rapid (though short lived) immunity than the vaccine.
MMR is made from live viruses that have been modified from the viruses that cause disease. Even though there is no evidence of vaccine-induced birth defects, pregnant women should not receive MMR vaccine.
Women should also avoid pregnancy for 28 days after receiving MMR or any rubella-containing vaccine
The following individuals should not receive MMR:
Patients with allergy to eggs or have had anaphylactic reaction to neomycin
Pregnant women
Patients with weakened immunity
Patients with untreated active tuberculosis
Vaccination should be delayed in the following groups:
Adults who have acute illness with feverPatients who have received immune globulin injection in prior 3-11 months
Patients on high doses of corticosteroid, such as prednisone, until at least 3 months after stopping the corticosteroid
What is the effectiveness of the MMR vaccine, and what about side effects
MMR vaccine is greater than 95% effective in conferring long lasting immunity.
Side effects of MMR include joint aches or arthritis that can occur 1-3 weeks after vaccination and lasting for days to weeks.
Other side effects include rash, fever and swollen lymph glands.
POLIOVIRUS VACCINES
There are two poliovirus vaccines; oral livepoliovirus vaccine (OPV) and
inactivated poliovirus vaccine (IPV (
OPV contains live virus that has been modified from the wild virus (the virus that causes polio and paralysis (
IPV contains inactivated (dead) virus and does not cause paralysis or polio. Therefore IPV is currently the recommended vaccine for children and adults.
Unvaccinated adolescents and adults should be given three IPV doses; the second dose should be given 4-8 weeks after the first dose, and the third dose be given 6-12 months after the second dose.
Partially vaccinated adolescents and adults should receive the rest of their vaccine series to total 3 doses.
Adults who have already received childhood immunization but who have had possible exposure to wild poliovirus should be given one dose of IPV.
No adverse effects have been seen in pregnancy, generally avoided in pregnant women unless immediate protection is required.
IPV should not be given to those with prior anaphylactic reaction to a previous dose, or to those with a prior anaphylactic reaction to streptomycin, polymyxin B, or neomycin.
OPV can very rarely cause polio and paralysis. (1 in 6 million doses.)
IPV does not cause paralysis. IPV can cause minor local pain and swelling at injection site.
Severe Acute Respiratory Syndrome ( SARS )
SARS shook the world. By some standards, the first emerging and readily transmissible disease of the
21st century was not a big killer, but it caused more fear and social disruption than any other outbreak of our time.
Before SARS, communicable diseases had been given insufficient
attention, with doctors more interested in high-tech fields such as neurosurgeryand molecular biology. Awareness levels were low and infection-controlprocedures had become slack. In sum, public-health systems were simply notprocedures had become slack. In sum, public-health systems were simply not
What we have learnt from SARS(Severe Acute Respiratory Syndrome) epidemics in China
The mainland of China experienced three outbreaks of SARS between November 2002 and May 2004.The first outbreak resulted in a pandemic and caused huge financial loss and social panic, but rigorous policies and control measures that were established circumvented further pandemics. Such efforts mean that SARS is currently under control.
However, these outbreaks revealed some problems in the health system and in public understanding of emerging infectious diseases.
The lessons we learn while facing up to these events can improve our medical performance in the future for management of new epidemics, such as human avian influenza
Lesson one: honesty is needed
The first case of SARS appeared in Guangdong province, China, in November 2002, but information about it was not broadcast on Central TV, the official Chinese television station, until February 2003, though rumours spread via cell phones and the internet.It was not until three months after the breakout of epidemics that a group of healthcare officials were sent to investigate.
Before this, the absence of open news was an attempt to maintain social stability.
But the silence led to panic buying of vinegar and some Chinese herbal drugs that were believed to help prevent this "mysterious" disease.The public needs to know the truth; concealing what happens may lead to a panic rather than to social stability.
Lesson two: controversy can lead to lost chances
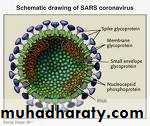
SARS-associated coronavirus (SARS-CoV) is new to humans. Scientists have sequenced the genome of the virus and noted that SARS-CoV is not closely related to any of the previously characterised coronaviruses.
28 February 2003, the health authorities announced that Chlamydia was the cause of "atypical pneumonia": it had been isolated from lung tissue in two of three autopies ;
Clinicians and epidemiologists did not agree. The disease was too highly contagious (with family and hospital clustering) to be of chlamydial origin; it had progressively deteriorating symptoms within a short period of time—and it did not respond to any standard treatment for Chlamydia.
Clinicians claimed that no macrolide-resistant (or quinolone-resistant) strains of Chlamydia had so far been reported. Chlamydia might be one of the pathogens causing death rather than causing the disease.
Arguments continued until April, when US and Hong Kong scientists announced that SARS-CoV was the cause of SARS, when the pathogen was looked at again and found to be SARS-CoV.
Scientists at the Military Academy of Medicine had had pictures of the new coronavirus on 26 February, more than a month before the discovery by US researchers, but they kept silent about their findings because the controversy was raging at that time.5 China lost the chance to announce the primary discovery of SARS-CoV.
Lesson three: conclusions may be premature
The first and the second outbreaks of SARS clearly showed that SARS-CoV in humans was linked to small wild animals, civet cats in particular.6-8Professor Kathryn Holmes, a former president of the American Society for Virology, told the 2005 annual meeting of American Association for the Advance of Science in February: "the SARS epidemic strain has not been seen in nature since June 2003...the human epidemic strain is not being harbored in animals" and thus "SARS no longer exists in wildlife and has essentially disappeared as a threat.”
Several factors lead us to believe that it is too early to conclude that SARS-CoV is eradicated outside laboratories:
The genomic sequences of SARS-CoV from humans and civet cats in the 2003-4 outbreak were nearly identical; there was a cross host evolution of SARS-CoV (spike protein in particular) in civets and humans.
High positive rates of serum SARS-CoV in civets were found at Guangzhou wildlife markets in 2004, but not in other wildlife markets in other cities or provinces.
Civets can be infected experimentally with SARS-CoV
Other SARS-related coronaviruses have been identified in Chinese horseshoe bats. Bats are a natural reservoir of SARS-like coronavirus.
Lesson four: some centres may be flouting regulations
During the second SARS outbreak (March 2004), a young postgraduate who was working at an institute of virology in Beijing developed symptoms of pneumonia on 25 March. When she returned home to Hefei, the capital city of Anhui Province, she was diagnosed as having SARS, and she had transmitted the disease to seven people, including her parents and healthcare workers both in Beijing and Hefei
The institute was shut down immediately .
acquired cases of SARS reflect the ways regulations can be flouted in research institutes: allowing non-professionals to be on SARS research projects; downplaying biosafety regulations; using methods with unconfirmed efficacy to inactivate viruses; improper technical processing in P3 laboratories (those set up according to biosafety level 3 regulations of the World Health Organization);
Strict scientific regulations are urgently needed
Wildlife markets represent a dangerous source of possible new infections that could undermine the prevention of SARS Game foods are believed to "enhance the vitality of the body," and as Cantonese people consume a substantial amount of game as a tonic in cold weather, the wildlife markets in Guangzhou thrive in winter.
Many markets are poorly managed and insanitary, so cross infection, interspecies transmission, amplification, genetic convergence, and mixing of coronavirus may be taking place.
Animal traders standing in close proximity to these infected animals may be affected, as may the food processors who slaughter infected animals in restaurant kitchens, causing SARS-CoV to spread from wildlife to humans—after which it may spread from human to human, principally by drop-let transmission.
It is too early to conclude that the SARS threat is over.
If no action is taken to control wildlife markets, the SARS-CoV organism may develop into an epidemic strain.
During early 2004, when four new cases were identified in Guangdong, the government took strong action on strict control of wildlife markets, including a ban on rearing, sales, transport, slaughter, and food processing of small wild mammals, and implemented "four earlies" (early identification, early reporting, early isolation, and early management) to stop transmission from human to human.
This control strategy seems to have been effective in preventing the second SARS outbreak from evolving into an epidemic.
This policy also holds true in the management of human avian flu, especially in dealing with febrile patients who have a history of contact with live poultry or birds.
Lessons taught by SARS have given us a new outlook on a devastating human health crisis.
these lessons are not confined to China, and they have important implications worldwide. As Franklin P Jones said, experience is the marvellous thing that enables you to recognise a mistake when you make it again.
What has happened with the spread of SARS-CoV must not be allowed to happen again with H5N1
How can we do better next time?
SARS has not been eradicated, and humans remain vulnerable to emerging infectious diseases like bird fluThree aspects may influence future strategies.
Firstly, laboratory workers, epidemiologists, preventive medicine professionals, and clinicians must collaborate closely to contain any emergent infections. Clinical trials using inactivated whole SARS-CoV vaccine are underway, and multidisciplinary research on genotherapy using small interfering RNA (SiRNA) shows promise. Lack of coordinated effort could compromise any advances in science. Also, biosafety should be emphasised when conducting studies related to highly pathogenic micro-organisms.
Secondly, constant consultation with healthcare professionals would provide the evidence that an authority needs for developing appropriate, rather than arbitrary, policies. As early as the 2002-3 SARS outbreak, Guangdong's local government and department of public health summoned leading scientists and respiratory specialists to set up an anti-SARS steering committee. Implementation of measures endorsed by this committee achieved the lowest case fatality rate from SARS in the world (3.8%).
Thirdly, an international monitoring system with a far reaching network is crucial for the early alerting of infectious diseases.
Basic Information About SARS
Severe acute respiratory syndrome (SARS) is a viral respiratory illness caused by a coronavirus, called SARS-associated coronavirus (SARS-CoV). SARS was first reported in Asia in February 2003. Over the next few months, the illness spread to more than two dozen countries in North America, South America, Europe, and Asia before the SARS global outbreak of 2003 was contained.
According to the World Health Organization (WHO), a total of 8,098 people worldwide became sick with SARS during the 2003 outbreak. Of these, 774 died.
Symptoms of SARS
In general, SARS begins with a high fever (temperature greater than 100.4°F [>38.0°C]). Other symptoms may include headache, an overall feeling of discomfort, and body aches. Some people also have mild respiratory symptoms at the outset. About 10 percent to 20 percent of patients have diarrhea. After 2 to 7 days, SARS patients may develop a dry cough. Most patients develop pneumonia.How SARS spreads
The main way that SARS seems to spread is by close person-to-person contact.The virus that causes SARS is thought to be transmitted most readily by respiratory droplets (droplet spread) produced when an infected person coughs or sneezes.
Droplet spread can happen when droplets from the cough or sneeze of an infected person are propelled a short distance (generally up to 3 feet) through the air and deposited on the mucous membranes of the mouth, nose, or eyes of persons who are nearby.
The virus also can spread when a person touches a surface or object contaminated with infectious droplets and then touches his or her mouth, nose, or eye(s).
In addition, it is possible that the SARS virus might spread more broadly through the air (airborne spread) or by other ways that are not now known.
What does “close contact” mean
close contact means having cared for or lived with someone with SARS or having direct contact with respiratory secretions or body fluids of a patient with SARS.Examples of close contact include kissing or hugging, sharing eating or drinking utensils, talking to someone within 3 feet, and touching someone directly.
Close contact does not include activities like walking by a person or briefly sitting across a waiting room or office.
How long is a person with SARS contagious?
Available information suggests that persons with SARS are most likely to be contagious only when they have symptoms, such as fever or cough. Patients are most contagious during the second week of illness.
However, as a precaution against spreading the disease, CDC recommends that persons with SARS limit their interactions outside the home (for example, by not going to work or to school) until 10 days after their fever has gone away and their respiratory (breathing) symptoms have gotten better.
What are the symptoms and signs of SARS?
The illness usually begins with a high fever (measured temperature greater than 100.4°F [>38.0°C]). The fever is sometimes associated with chills or other symptoms, including headache, general feeling of discomfort, and body aches. Some people also experience mild respiratory symptoms at the outset. Diarrhea is seen in approximately 10 percent to 20 percent of patients. After 2 to 7 days, SARS patients may develop a dry, nonproductive cough that might be accompanied by or progress to a condition in which the oxygen levels in the blood are low (hypoxia). In 10 percent to 20 percent of cases, patients require mechanical ventilation. Most patients develop pneumonia.Laboratory confirmation of SARS-CoV infection is based on
Detection of any of the following by a validated test, with confirmation in a reference laboratory: Serum antibodies to SARS-CoV in a single serum specimen, or A four-fold or greater increase in SARS-CoV antibody titer between acute- and convalescent-phase serum specimens tested in parallel, or Negative SARS-CoV antibody test result on acute-phase serum and positive SARS-CoV antibody test result on convalescent-phase serum tested in parallel; or Isolation in cell culture of SARS-CoV from a clinical specimen, with confirmation using a test validated by CDC;Or Detection of SARS-CoV RNA by RT-PCR validated by CDC, with confirmation in a reference laboratory, from:
Two clinical specimens from different sources, or
Two clinical specimens collected from the same source on two different days
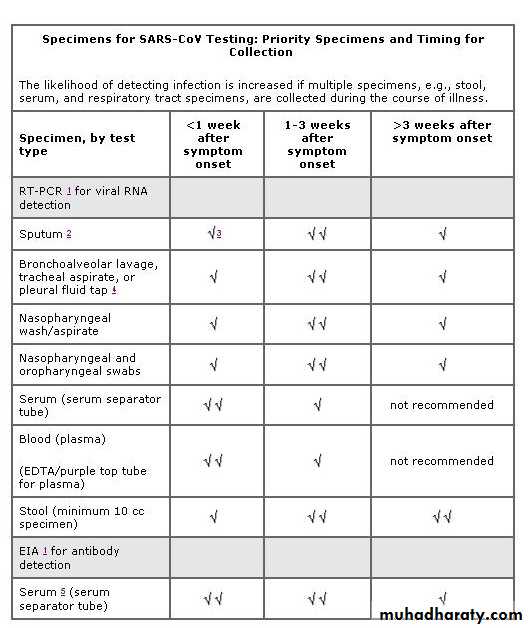
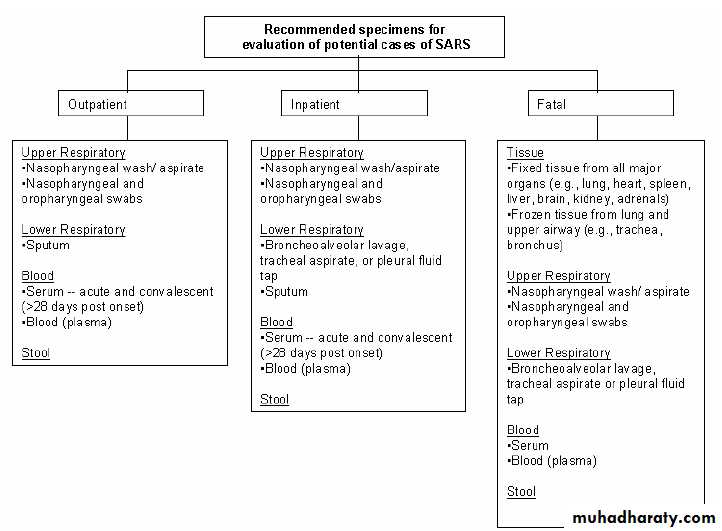
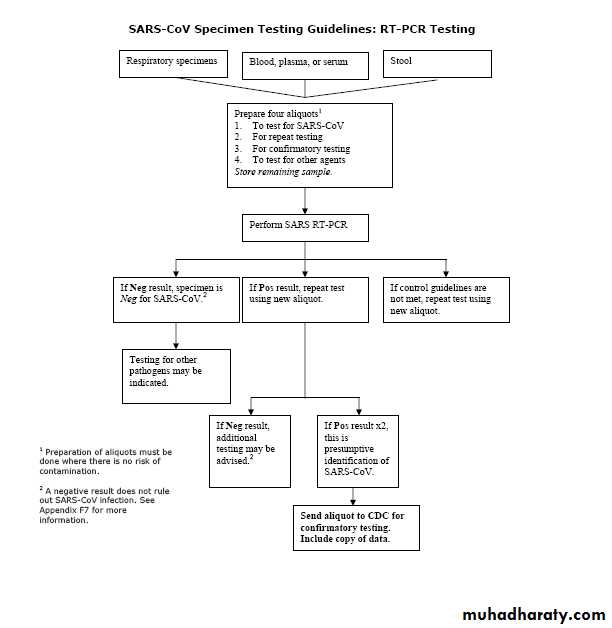
Guidelines for Collecting Specimens from Potential SARS Patients
RT-PCR DiagnosticsAlthough studies to date have not definitively determined the best specimens for SARS RT-PCR(reverse transcription-polymerase chain reaction ) diagnostics, it is reasonable to collect:
During the first week of illness: Nasophyaryngeal (NP) swab plus oropharygeal (OP) swab and a serum or plasma specimen
After the first week of illness: NP swab plus OP swab and a stool specimen
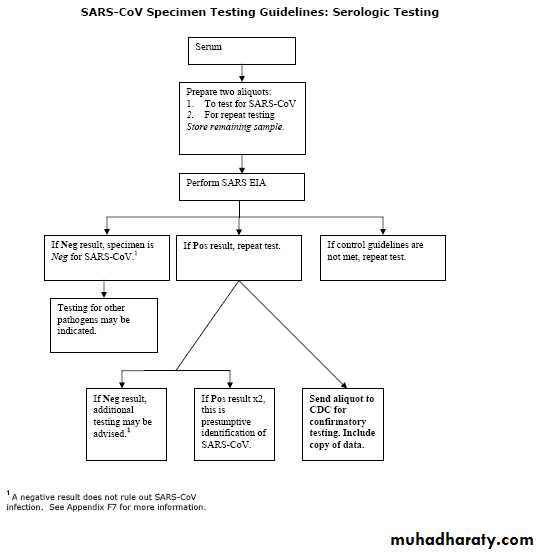
Serologic Diagnostics
Serum specimens for SARS-CoV antibody testing should be collected when the diagnosis is first suspected and at later times if indicated. An antibody response is occasionally detected during the first week of illness, likely to be detected by the end of the second week of illness, and sometimes may not be detected until > 28 days after onset of symptoms.Collecting Respiratory Specimens
Eight types of respiratory specimens may be collected for viral and/or bacterial diagnostics:
1) nasopharyngeal wash/aspirates,
2) nasopharyngeal swabs,
3) oropharyngeal swabs,
4) broncheoalveolar cleavage,
5) tracheal aspirate,
6) pleural fluid tap,
7) sputum; and
8) post-mortem tissue.
Nasopharyngeal wash/aspirates are the specimen of choice for detection of most respiratory viruses and are the preferred specimen type for children under age 2 years.
In contrast to most respiratory pathogens for which respiratory specimens are optimally collected within 72 hours after the onset of symptoms, levels of SARS-CoV may be higher later in the course of the illness
Collecting specimens from the upper respiratory tract
Nasopharyngeal wash/aspirate Have the patient sit with head tilted slightly backward. Instill 1 ml-1.5 ml of nonbacteriostatic saline (pH 7.0) into one nostril. Flush a plastic catheter or tubing with 2 ml-3 ml of saline. Insert the tubing into the nostril parallel to the palate. Aspirate nasopharyngeal secretions. Repeat this procedure for the other nostril. Collect the specimens in sterile vials. Label each specimen container with the patient's ID number and the date collected. If shipping domestically, use cold packs to keep the sample at 4°C. If shipping internationally, pack in dry ice.Nasopharyngeal or oropharyngeal swabs Use only sterile dacron or rayon swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden sticks, as they may contain substances that inactivate some viruses and inhibit PCR testing.
Nasopharyngeal swabs -- Insert a swab into the nostril parallel to the palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nostrils.
Oropharyngeal swabs -- Swab the posterior pharynx and tonsillar areas, avoiding the tongue.
Place the swabs immediately into sterile vials containing 2 ml of viral transport media. Break the applicator sticks off near the tip to permit tightening of the cap. Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
Collecting specimens from the lower respiratory tract
Broncheoalveolar lavage, tracheal aspirate, pleural fluid tapCentrifuge half of the specimen, and fix the cell pellet in formalin. Place the remaining unspun fluid in sterile vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . Label each specimen container with the patient's ID number and the date the sample was collected. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, ship fixed cells at room temperature and unfixed cells frozen.
SputumEducate the patient about the difference between sputum and oral secretions. Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile screw-cap sputum collection cup or sterile dry container. If shipping domestically, use cold packs to keep sample at 4°C. If shipping internationally, pack in dry ice.
Serum and blood (plasma) should be collected early in the illness for RT-PCR testing. The reliability of RT-PCR testing performed on blood specimens decreases as the illness progresses.
Both acute and convalescent serum specimens should be collected for antibody testing. To confirm or rule out SARS-CoV infection, it is important to collect convalescent serum specimens >28 days after the onset of illness.
Collecting Blood Components
A. Collecting serum for antibody or RT-PCR testing
Collect 5 ml-10 ml of whole blood in a serum separator tube. Allow the blood to clot, centrifuge briefly, and collect all resulting sera in vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . The minimum amount of serum preferred for each test is 200 microliters, which can easily be obtained from 5 mL of whole blood.A minimum of 1 cc of whole blood is needed for testing of pediatric patients. If possible, collect 1 cc in an EDTA tube and in a clotting tube. If only 1cc can be obtained, use a clotting tube.
Label each specimen container with the patient's ID number and the date the specimen was collected. If unfrozen and transported domestically, ship with cold packs to keep the sample at 4°C. If frozen or transported internationally, ship on dry ice.
B. Collecting EDTA blood (plasma) for RT-PCR
Collect 5 ml-10 ml of blood in an EDTA (purple-top) tube. Transfer to vials with external caps and internal O-ring seals. If there is no internal O-ring seal, then seal tightly with the available cap and secure with Parafilm ® . Label each specimen container with patient's ID number and date of collection. Store and ship blood specimens with cold packsCollecting Stool Specimens for PCR
Begin collecting stool specimens as soon as possible in the course of the illness. Although collecting earlier specimens is ideal, SARS-CoV has been detected in stool as late as one month after the onset of symptoms.Place each stool specimen - as large a quantity as can be obtained (at least 10 cc) - in a leak-proof, clean, dry container, and refrigerate at 4°C. Patients may drape plastic kitchen wrap across the back half of the toilet, under the toilet seat, to facilitate collection of stool specimens.
IMPORTANT
Refrigerate or freeze tubes after specimens are placed in them. If specimens will be examined within 48 hours after collection, they can be refrigerated. If specimens must be held longer than 48 hours, freeze them as soon as possible after collection. Although storage in an ultra-low freezer (-70°C) is preferable, storage in a home-type freezer (if properly set at -20°C) is acceptable for short periods.
Specimens from possible and known SARS cases must be packaged, shipped, and transported according to the current edition of the "International Air Transport Association (IATA).
Specimens for SARS-CoV Testing: Priority Specimens and Timing for Collection
Guidelines for Laboratory Diagnosis of SARS-CoV Infection
Laboratory confirmation of SARS-CoV infection is based on:• Detection of any of the following by a validated test, with confirmation in a reference laboratory:
o Serum antibodies to SARS-CoV in a single serum specimen, or
o A four-fold or greater increase in SARS-CoV antibody titer between acute- and convalescent-phase serum specimens tested in parallel, or
Negative SARS-CoV antibody test result on acute-phase serum and positive SARS-CoV antibody test result on convalescent-phase serum tested in parallel; or
• Isolation in cell culture of SARS-CoV from a clinical specimen, with confirmation using a test validated by CDC; or
• Detection of SARS-CoV RNA by RT-PCR validated by CDC, with confirmation in a reference laboratory, from:
o Two clinical specimens from different sources, or
o Two clinical specimens collected from the same source on two different days .
Does a negative SARS-CoV test result affect patient management?
the interpretation of negative SARS-CoV test results varies depending on the type of specimen, the timing of specimen collection, and the test that was performed. With the exception of a >28-day negative serologic test result, a negative SARS-CoV test result should not affect patient isolation or management decisions. The clinical features of the illness and the type and risk of exposure are the keys to making decisions on patient management and isolation.
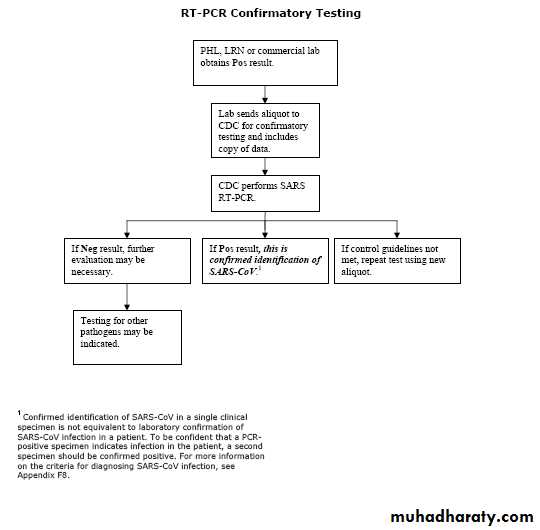
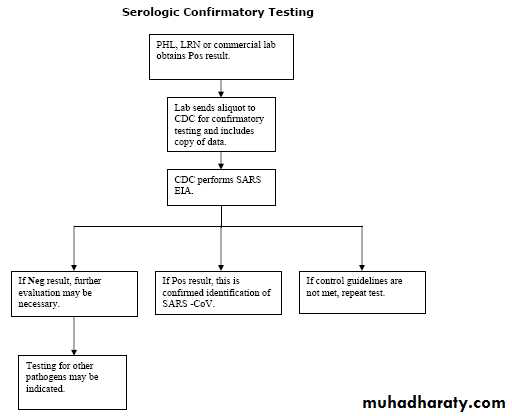
How is a SARS-CoV test confirmed?
Positive antibody and RT-PCR test results should be confirmed by repeat testing of the original specimen AND by testing of the same specimen in an independent laboratory using a validated assay.What tests for SARS-CoV are available?
Both the EIA and the RT-PCR tests are sensitive and highly specific for SARS-CoV. The ability to diagnose SARS-CoV infection in a patient is often limited, however, by either the low concentration of virus in most clinical specimens (RT-PCR assays) or the time it takes a person to mount a measurable antibody response to SARS-CoV (serologic assays). The likelihood of detecting infection is increased if multiple specimens (e.g., stool, serum, respiratory tract specimens) are collected at several times during the course of illness.What tests for SARS-CoV are available?
CDC considers detection of SARS-CoV antibody to be the most reliable indicator of infection. Since previous infection is still rare in most populations, seroconversion is not needed to diagnose infection. Therefore, the presence of SARS-CoV antibody in someone without a previous history of SARS is indicative of recent infection.A negative serologic test can rule out SARS-CoV infection if the serum specimen is collected >28 days after onset of illness. Some persons do not mount an antibody response (test positive) until more than 28 days after onset of illness. Patients with a negative antibody test result whose specimens were obtained 28 days before illness onset or before should have another serum specimen collected >28 days after onset of symptoms.
RT-PCR for SARS-CoV RNA is a very sensitive and specific assay when performed appropriately. This test can detect SARS-CoV RNA in serum, stool, upper and lower respiratory specimens, various tissues, and occasionally urine specimens. Testing of multiple specimen types at several times during the course of illness should increase the likelihood of detecting infection.
Management of asymptomatic healthcare workers with unprotected high-risk exposures
An unprotected high-risk exposure occurs when a healthcare worker is in a room with a SARS patient during an aerosol-generating procedure or event and the recommended infection control precautions are either absent or breached. If a healthcare worker has an unprotected high-risk exposure but has no symptoms of SARS-CoV disease, the worker:Should be excluded from duty (e.g., administrative leave) for 10 days after the date of the last high-risk exposure.
Should be vigilant for the development of fever and/or respiratory symptoms.
Should be actively monitored for the development of fever and/or respiratory symptoms for 10 days after the date of the last high-risk exposure. Decisions regarding activity restrictions, (e.g., quarantine home/work restrictions) outside the facility should be discussed with the health department, Supplement D.
Management of asymptomatic healthcare workers with unprotected exposures that are not high risk
Unprotected exposures that are not high risk occur when a healthcare worker is in a room or patient-care area with a SARS patient (not during a high-risk procedure) and the recommended infection control precautions are either absent or breached.
If a healthcare worker has an unprotected, non-high-risk exposure and has no symptoms of SARS-CoV disease, the healthcare worker:
Need not be excluded from duty.
Should be vigilant for the development of fever and/or respiratory symptoms (i.e., measure and record body temperature twice daily for 10 days following the date of last unprotected exposure, and immediately notify the healthcare facility if symptoms develop.
Should be actively monitored for the development of fever and lower respiratory symptoms before reporting to duty.
Management of symptomatic healthcare workers
Any healthcare worker who has cared for or been exposed to a SARS patient and who develops fever and/or respiratory symptom(s) within 10 days after exposure or patient care should: Immediately contact infection control, occupational health or designee in each facility where s/he works;And Report to the predetermined location for clinical evaluation. (During periods of increased SARS activity in the healthcare facility and/or community, this recommendation extends to all symptomatic personnel working in the facility, regardless of whether they have had contact with a SARS patient.
Any healthcare worker who develops symptoms or fever while at work should immediately put on a surgical mask and notify the appropriate facility contact (e.g., occupational health, infection control, or other designee) and then report to the designated location for clinical evaluation.
Symptomatic healthcare personnel should be managed in accordance with the recommendations in Clinical Guidance on the Identification and Evaluation of Possible SARS-CoV Disease among Persons Presenting with Community-Acquired Illness. Decisions on return to work should be guided by policies or regulation defined by the facility or health department.
General Guidelines for Preventing Transmission
Respiratory Hygiene/Cough Etiquette in Healthcare SettingsShould be incorporated into infection control practices as one component of standard precautions
Hand Hygiene in Health Care SettingsHand hygiene (i.e., hand washing or use of an alcohol-based hand rub) should be performed after contact with a patient.
Guideline for Isolation Precautions in Hospitals
Intended primarily for use in the care of patients in acute-care hospitals, although some recommendations may be applicable for some patients receiving care in subacute-care or extended-care facilities.
Respiratory Hygiene/Cough Etiquette in Healthcare Settings
To prevent the transmission of all respiratory infections in healthcare settings, including influenza, the following infection control measures should be implemented at the first point of contact with a potentially infected person.
Respiratory Hygiene/Cough Etiquette
The following measures to contain respiratory secretions are recommended for all individuals with signs and symptoms of a respiratory infection.Cover the nose/mouth when coughing or sneezing;
Use tissues to contain respiratory secretions and dispose of them in the nearest waste receptacle after use;
Perform hand hygiene (e.g., hand washing with non-antimicrobial soap and water, alcohol-based hand rub, or antiseptic handwash) after having contact with respiratory secretions and contaminated objects/materials.
Healthcare facilities should ensure the availability of materials for adhering to Respiratory Hygiene/Cough Etiquette in waiting areas for patients and visitors.
Provide tissues and no-touch receptacles for used tissue disposal.
Provide conveniently located dispensers of alcohol-based hand rub; where sinks are available, ensure that supplies for hand washing (i.e., soap, disposable towels) are consistently available.
Masking and Separation of Persons with Respiratory Symptoms
During periods of increased respiratory infection activity in the community (e.g., when there is increased absenteeism in schools and work settings and increased medical office visits by persons complaining of respiratory illness), offer masks to persons who are coughing. Either procedure masks (i.e., with ear loops) or surgical masks (i.e., with ties) may be used to contain respiratory secretions (respirators such as N-95 or above are not necessary for this purpose). When space and chair availability permit, encourage coughing persons to sit at least three feet away from others in common waiting areas. Some facilities may find it logistically easier to institute this recommendation year-round.Droplet Precautions
Advise healthcare personnel to observe Droplet Precautions (i.e., wearing a surgical or procedure mask for close contact), in addition to Standard Precautions, when examining a patient with symptoms of a respiratory infection, particularly if fever is present. These precautions should be maintained until it is determined that the cause of symptoms is not an infectious agent that requires Droplet PrecautionsPrevention-cont
Precautions During Specific ProceduresPrehospital Emergency Care and Ground Transport
of Persons with Possible SARS
Early Detection and Isolation of Patients Potentially
at Risk for SARS-CoV Disease (Triage and
Disposition) Guidance on Air Medical Transport for
SARS Patients Performing Aerosol-Generating
Procedures on Patients with SARS
Handling of Human Remains of Patients with SARS.
Respirators
Interim Domestic Guidance on the Use of Respirators to Prevent Transmission of SARS
Understanding Respiratory Protection against SARS
Cleaning andDisinfection
Disinfection of the SARS Patient Environment
Management of Exposures to SARS-CoV in Healthcare Settings.
Laboratory Guidance
The identification of SARS-CoV led to the rapid development of enzyme immunoassays (EIA) and immunofluorescence assays (IFA) for serologic diagnosis and reverse-transcription PCR (RT-PCR) assays for detection of SARS-CoV RNA in clinical samples.These assays can be very sensitive and specific for detecting antibody and RNA, respectively, in the later stages of SARS-CoV infection. However, both are less sensitive for detecting infection early in illness. The identification of SARS-CoV led to the rapid development of enzyme immunoassays (EIA) and immunofluorescence assays (IFA) for serologic diagnosis and reverse-transcription PCR (RT-PCR) assays for detection of SARS-CoV RNA in clinical samples. These assays can be very sensitive and specific for detecting antibody and RNA, respectively, in the later stages of SARS-CoV infection. However, both are less sensitive for detecting infection early in illness.
Real-Time RT-PCR Assays
Many laboratories have developed SARS-CoV real-time RT-PCR assays (which have several advantages over traditional RT-PCR assays. Because real-time RT-PCR assays use internal probes as well as amplification primers, they can be designed to be very specific for SARS-CoV RNA (or cDNA). They can also be very sensitive, with consistent detection limits of between 1 and 10 SARS-CoV RNA copies per reaction. Real-time PCR assays can be performed faster than traditional RT-PCR assays and, because they operate as closed systems, with reduced risk of contamination in the laboratory. Finally, real-time RT-PCR assays can give an accurate estimate of the quantity of virus present in a sample.False-negative results can arise from poor sample collection or degradation of the viral RNA during shipping or storage.
The most common cause of false-positive results is contamination with previously amplified DNA Liberal use of negative control samples in each assay and a well-designed plan for confirmatory testing can help ensure that laboratory contamination is detected and that specimens are not inappropriately labeled as SARS-CoV positive. To decrease the possibility of a false-positive result, testing should be limited to patients with a high index of suspicion for having SARS-CoV disease. In addition, any positive specimen should be retested in a reference laboratory to confirm that the specimen is positive. Finally, all laboratory results should be interpreted in the context of the clinical and epidemiologic information available for the patient.
Antibody Assays
The most commonly used serologic assays are based on cultured SARS-CoV antigen as either inactivated whole virus lysate for EIA or inactivated virus in cells fixed for IFA. These assays have proven to be highly specific, with no cross-reactivity with paired serum specimens from patients infected with the other known human coronaviruses (229E and OC43) or from healthy blood donors and other persons without clinical or epidemiologic evidence of SARS-CoV disease.Antibody assays have been the most reliable indicators of SARS-CoV infection when applied to convalescent-phase serum specimens collected >28 days after onset of illness, antibody becomes detectable within 8 to 10 days, and most have detectable antibody by 2 weeks. However, some persons do not develop detectable antibodies until 28 days after onset of illness .
Other Assays
Among the other methods used to detect SARS-CoV are: isolation in cell culture, electron microscopy for CoV-like particles, and immunohistologic or in situ probe hybridization studies on tissue specimens. These methods are less likely to detect SARS-CoV infection than are RT-PCR or antibody assays. Although isolation of SARS-CoV in cell culture represents a definitive diagnosis, it is not recommended for routine detection as it lacks sensitivity compared to RT-PCR and also requires more restrictive Biosafety Level (BSL) 3 conditions.
Most patients in the early stages of SARS-CoV disease have a low titer of virus in respiratory and other secretions and require time to mount an antibody response.
In one study (in patients treated with high-dose steroids and ribavirin), nasopharyngeal (NP) aspirates were found to be PCR positive in <40% of patients during the first week of illness and in >50% of patients during the second week of illness (Peiris 2003).
During the second week of illness, stool specimens were found to be PCR positive in a higher percentage of patients than were NP aspirates.
Limited data suggest that serum may be the best specimen for SARS-CoV PCR diagnostics during the first few days of illness.
During early 2004, when four new cases were identified in Guangdong, the government took strong action on strict control of wildlife markets, including a ban on rearing, sales, transport, slaughter, and food processing of small wild mammals, and implemented "four earlies" (early identification, early reporting, early isolation, and early management) to stop transmission from human to human.
This control strategy seems to have been effective in preventing the second SARS outbreak from evolving into an epidemic.
This policy also holds true in the management of human avian flu, especially in dealing with febrile patients who have a history of contact with live poultry or birds.
Lessons taught by SARS have given us a new outlook on a devastating human health crisis.
these lessons are not confined to China, and they have important implications worldwide. As Franklin P Jones said, experience is the marvellous thing that enables you to recognise a mistake when you make it again.
What has happened with the spread of SARS-CoV must not be allowed to happen again with H5N1
Animal-to-Human SARS–associated Coronavirus Transmission
Ferrets and domestic cats not only can be infected by SARS-CoV in the laboratory, but also can shed SARS-CoV from the pharynx at 2 days postinfection and continuing through 10 and 14 days postinfection, respectively. No clinical signs were observed in six cats that were injected with SARS-CoV, whereas three of six ferrets that were injected with SARS-CoV became lethargic within 2 to 4 days postinfection, and one of the three ferrets died at day 4 postinfection.This finding indicates that domestic cats may not only be a useful animal model for evaluating candidate vaccines and drugs against SARS but may also be good reservoirs of SARS-CoV.domestic cats can be naturally infected with SARS-CoV from humans infected with SARS, although how this SARS-CoV transmission occurs is unclear. Unfortunately, however, the transmission capability of the SARS-CoV strain transmitting from domestic animal to human, despite the widely accepted hypothesis of the animal origin of SARS-CoV, cannot be ascertained.
Inhibition of SARS CoronavirusInfection In Vitro with ClinicallyApproved Antiviral Drug
The disease can produce severe pneumonia with a reported fatal outcome of 15% to 20%Currently, The urgency of the outbreak has led to the empiric use of broad- spectrum antibiotics and antiviral agents in affected patients in several countries . Developing effective and safe vaccines and chemotherapeutic agents against SARS CoV, however, may take years.Currently, no effective drug exists to treat SARS-CoV infection In this study, we investigated whether a panel of commercially available antiviral
drugs exhibit in vitro anti–SARS-CoV activity .
Tested were 19 clinically approved compounds from several major antiviral pharmacologic classes: nucleoside analogs, interferons, protease inhibitors, reverse transcriptase inhibitors, and neuraminidase inhibitors. Complete inhibition of cytopathic effects of SARS-CoV in culture was observed for interferon subtypes, β -1b, α -n1, α-n3, and human leukocyte interferon α.
. In conclusion, interferon β-1b, α-n1, α-n3, and humanleukocyte interferon α exhibit antiviral activity in an invitro model and are potential drugs for in vivo research and clinical management of SARS-CoV infection.
SARS Vaccine Development
An ideal SARS vaccine should 1) elicit highly potent neutralizing antibody responses against a broad spectrum of viral strains; 2) induce protection against infection and transmission; and 3) be safe by not inducing any infection-enhancing antibodies or harmful immune or inflammatory responses. Currently, an inactivated SARS-CoV vaccine is in clinical trials in China.Safety is the major concern for this type of vaccine The S protein is the major inducer of neutralizing antibodies. Recombinant vector-based vaccines expressing full-length S protein of the late SARS-CoV are under development. These vaccines can induce potent neutralizing and protective responses in immunized animals but may induce antibodies that enhance infection by early human SARS-CoV and animal SARS-CoV–like viruses
The vaccine was obtained from a human strain and is intended for immunizing people 18 through 64 years of age who could be at increased risk of exposure to the H5N1 influenza virus contained in the vaccine. H5N1 influenza vaccine immunization consists of two intramuscular injections, given approximately one month apart. The manufacturer, sanofi pasteur Inc., will not sell the vaccine commercially. Instead, the vaccine has been purchased by the federal government for inclusion within the National Stockpile for distribution by public health officials if needed.
A clinical study was conducted to collect safety information and information on recipient's immune responses and to determine the appropriate vaccine dose. A total of 103 healthy adults received a 90 microgram dose of the vaccine by injection followed by another 90 microgram dose 28 days later. In addition, there were approximately 300 healthy adults who received the vaccine at doses lower than 90 micrograms and a total of 48 who received a placebo injection.
The vaccine was generally well tolerated, with the most common side effects reported as pain at the injection site, headache, general ill feeling and muscle pain. The study showed that 45 percent of individuals who received the 90 microgram, two-dose regimen developed antibodies at a level that is expected to reduce the risk of getting influenza. Although the level of antibodies seen in the remaining individuals did not reach that level, current scientific information on other influenza vaccines suggests that less than optimal antibody levels may still have the potential to help reduce disease severity and influenza-related hospitalizations and deaths.
sanofi pasteur and other manufacturers are working to develop a next generation of influenza vaccines for enhanced immune responses at lower doses, using technologies intended to boost the immune response. Meanwhile, the approval and availability of this vaccine will enhance national readiness and the nation's ability to protect those at increased risk of exposure .
Recent studies have demonstrated that recombinant RBD(receptor-binding domain) consists of multiple conformational neutralizing epitopes that induce highly potent neutralizing antibodies against SARS-CoV. Unlike full-length S protein, RBD does not contain immunodominant sites that induce nonneutralizing antibodies. RBD sequences are relatively conserved. Thus, recombinant RBD or vectors encoding RBD may be used as safe and efficacious vaccines for preventing infection by SARS-CoV with distinct genotypes.
Prevalence of IgG against SARS-CoV amongHealthcare Workers
Among healthcare workers working with SARS patients, the prevalence of IgG against SARS-CoV was88.9% for those who contracted SARS and 1.4%for those who did not.By contrast, the seroprevalence was 0.5% for healthcare workers working in the non-SARS hospital
In conclusion, this study shows that a high proportionof healthcare workers who have contracted SARS have
IgG against SARS-CoV in their serum samples after theyhave fully recovered. Inapparent infection with SARS is uncommon.. The low seropositivity against SARS amonghealthcare workers who have not been exposed to SARS patients suggests a lack of immunity in this group and inthe general population where the number of SARS casesis comparatively small.
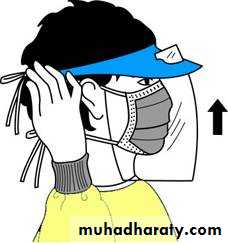
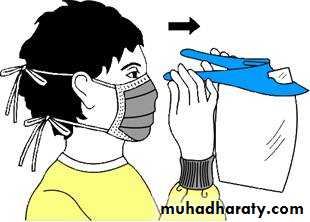
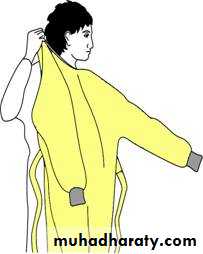
Types of PPE( Personal Protective Equipment) Used in Healthcare Settings
• Gloves – protect hands• Gowns/aprons – protect skin and/or clothing
• Masks and respirators– protect mouth/nose
• Respirators – protect respiratory tract from airborne infectious agents
• Goggles – protect eyes
• Face shields – protect face, mouth, nose, and eyes
Avian influenza in birds
Avian influenza is an infection caused by avian (bird) influenza (flu) viruses.These influenza viruses occur naturally among birds. Currently, there are two different groups (or clades) of H5N1 viruses circulating among poultry (clade 1, and clade 2 viruses). At least three subgroups or subclades of clade 2 H5N1 viruses have infected humans to date: subclades 2.1, 2.2, and 2.3 viruses.
Wild birds worldwide carry the viruses in their intestines, but usually do not get sick from them. However, avian influenza is very contagious among birds and can make some domesticated birds, including chickens, ducks, and turkeys, very sick and kill them.
Infected birds shed influenza virus in their saliva, nasal secretions, and feces
Infection with avian influenza viruses in domestic poultry causes two main formsThe “low pathogenic” form may go undetected and usually causes only mild symptoms (such as ruffled feathers and a drop in egg production).
the highly pathogenic form spreads more rapidly through flocks of poultry. This form may cause disease that affects multiple internal organs and has a mortality rate that can reach 90-100% often within 48 hours.
Human infection with avian influenza viruses
Human influenza virus” usually refers to those subtypes that spread widely among humans. Although avian influenza A viruses usually do not infect humans, rare cases of human infection with avian influenza viruses have been reported since 1997. There are only three known A subtypes of influenza viruses (H1N1, H1N2, and H3N2) currently circulating among humans. It is likely that some genetic parts of current human influenza A viruses came from birds originally. Influenza A viruses are constantly changing, and they might adapt over time to infect and spread among humans.
There are many different subtypes of type A influenza viruses. These subtypes differ because of changes in certain proteins on the surface of the influenza A virus (hemagglutinin [HA] and neuraminidase [NA] proteins). There are 16 known HA subtypes and 9 known NA subtypes of influenza A viruses. Many different combinations of HA and NA proteins are possible .
Usually, “avian influenza virus” refers to influenza A viruses found chiefly in birds, but infections with these viruses can occur in humans.
The risk from avian influenza is generally low to most people, because the viruses do not usually infect humans. However, confirmed cases of human infection from several subtypes of avian influenza infection have been reported since 1997.
Most cases of avian influenza infection in humans have resulted from contact with infected poultry (e.g., domesticated chicken, ducks, and turkeys) or surfaces contaminated with secretion/excretions from infected birds.
The spread of avian influenza viruses from one ill person to another has been reported very rarely, and has been limited, inefficient and unsustained
During an outbreak of avian influenza among poultry, there is a possible risk to people who have contact with infected birds or surfaces that have been contaminated with secretions or excretions from infected birds.
Symptoms of avian influenza in humans have ranged from typical human influenza-like symptoms (e.g., fever, cough, sore throat, and muscle aches) to eye infections, pneumonia, severe respiratory diseases (such as acute respiratory distress), and other severe and life-threatening complications. The symptoms of avian influenza may depend on which virus caused the infection.
Avian Influenza A (H5N1)
Influenza A (H5N1) virus – also called “H5N1 virus” – is an influenza A virus subtype that occurs mainly in birds, is highly contagious among birds, and can be deadly to them. H5N1 virus does not usually infect people, but infections with these viruses have occurred in humans. Most of these cases have resulted from people having direct or close contact with H5N1-infected poultry or H5N1-contaminated surfaces.Human health risks during the H5N1 outbreak
Of the few avian influenza viruses that have crossed the species barrier to infect humans, H5N1 has caused the largest number of detected cases of severe disease and death in humans. However, it is possible that those cases in the most severely ill people are more likely to be diagnosed and reported, while milder cases go unreported..
Of the human cases associated with the ongoing H5N1 outbreaks in poultry and wild birds in Asia and parts of Europe, the Near East and Africa, more than half of those people reported infected with the virus have died. Most cases have occurred in previously healthy children and young adults and have resulted from direct or close contact with H5N1-infected poultry or H5N1-contaminated surfaces.
In general, H5N1 remains a very rare disease in people. The H5N1 virus does not infect humans easily, and if a person is infected, it is very difficult for the virus to spread to another person
Most recently, in June 2006, WHO reported evidence of human-to-human spread in Indonesia. In this situation, 8 people in one family were infected.
Treatment and vaccination for H5N1 virus in humans
The H5N1 virus that has caused human illness and death in Asia is resistant to amantadine and rimantadine, two antiviral medications commonly used for influenza. Two other antiviral medications, oseltamavir and zanamavir, would probably work to treat influenza caused by H5N1 virus, but additional studies still need to be done to demonstrate their effectiveness.Nonetheless, because all influenza viruses have the ability to change, scientists are concerned that H5N1 virus one day could be able to infect humans and spread easily from one person to another .
Because these viruses do not commonly infect humans, there is little or no immune protection against them in the human population. If H5N1 virus were to gain the capacity to spread easily from person to person, an influenza pandemic (worldwide outbreak of disease) could begin.
Research suggests that currently circulating strains of H5N1 viruses are becoming more capable of causing disease (pathogenic) in animals than were earlier H5N1 viruses
One study found that ducks infected with H5N1 virus are now shedding more virus for longer periods without showing symptoms of illness. This finding has implications for the role of ducks in transmitting disease to other birds and possibly to humans as well. Additionally, other findings have documented H5N1 virus infection among pigs in China and Vietnam; H5N1 virus infection of cats
infection of dogs (isolation of H5N1 virus from a domestic dog in Thailand); and isolation of H5N1 viruses from tigers and leopards at zoos .
Human H5N1 Cases
)WHO) has reported human cases of avian influenza A (H5N1) in Asia, Africa, the Pacific, Europe and the Near East. Indonesia and Vietnam have reported the highest number of H5N1 cases to date. Overall mortality in reported H5N1 cases is approximately 60%. The majority of cases have occurred among children and adults aged less than 40 years old. Mortality was highest in cases aged 10-19 years old. Studies have documented the most significant risk factors for human H5N1 infection to be direct contact with sick or dead poultry or wild birds, or visiting a live poultry market.Questions and Answers About Avian Influenza (Bird Flu) and Avian Influenza A (H5N1) Virus
How is avian influenza detected in humansdiagnosed by collecting a swab from the nose or throat during the first few days of illness. This swab is then sent to a laboratory, where they will either look for avian influenza virus using a molecular test, or they will try to grow the virus. Growing avian influenza viruses should only be done in laboratories with high levels of protection
It may still be possible to diagnose avian influenza by looking for evidence of the body's response to the virus. This is not always an option because it requires two blood specimens (one taken during the first few days of illness and another taken some weeks later), and it can take several weeks to verify the results
Does seasonal influenza vaccine protect against avian influenza infection in people
No. Seasonal influenza vaccine does not provide protection against avian influenza .
There currently is no scientific evidence that people have been infected with bird flu by eating safely handled and properly cooked poultry or eggs
Even if poultry and eggs were to be contaminated with the virus, proper cooking would kill it.
So to stay safe, the advice is the same for protecting against any infection from poultry:
Wash your hands with soap and warm water for at least 20 seconds before and after handling raw poultry and eggs.
Clean cutting boards and other utensils with soap and hot water to keep raw poultry from contaminating other foods.
Use a food thermometer to make sure you cook poultry to a temperature of at least 165 degrees Fahrenheit Consumers may wish to cook poultry to a higher temperature for personal preference.
Cook eggs until whites and yolks are firm
Is there a vaccine to protect people from some strains of the H5N1 virus
Yes. On April 17, 2007, the U.S. Food and Drug Administration (FDA) announced its approval of the first vaccine to prevent human infection with one strain of the avian influenza (bird flu) H5N1 virus. The vaccine, produced by sanofi pasteur, Inc., has been purchased by the federal government for the U.S. Strategic National Stockpile; it will be distributed by public-health officials if needed. This vaccine will not be made commercially available to the general public. Other H5N1 vaccines are being developed by other companies against different H5N1 strainsSince the H5N1 virus is not a pandemic virus since it does not transmit efficiently from person to person, the H5N1 vaccine is being held in stockpiles rather than being used by the general public . This vaccine aids H5N1 preparedness efforts in case an H5N1 pandemic virus were to emerge.
A clinical study was conducted to collect safety information and information on recipient's immune responses and to determine the appropriate vaccine dose. A total of 103 healthy adults received a 90 microgram dose of the vaccine by injection followed by another 90 microgram dose 28 days later. In addition, there were approximately 300 healthy adults who received the vaccine at doses lower than 90 micrograms and a total of 48 who received a placebo injection.
The vaccine was generally well tolerated, with the most common side effects reported as pain at the injection site, headache, general ill feeling and muscle pain. The study showed that 45 percent of individuals who received the 90 microgram, two-dose regimen developed antibodies at a level that is expected to reduce the risk of getting influenza. Although the level of antibodies seen in the remaining individuals did not reach that level, current scientific information on other influenza vaccines suggests that less than optimal antibody levels may still have the potential to help reduce disease severity and influenza-related hospitalizations and deaths. Additional information on this H5N1 influenza vaccine is being collected on safety and effectiveness in other age groups and will be available to FDA in the near future.
Does CDC recommend travel restrictions to areas with known H5N1 outbreaks
CDC does not recommend any travel restrictions to affected countries at this time. However, CDC currently advises that travelers to countries with known outbreaks of H5N1 influenza avoid poultry farms, contact with animals in live food markets, and any surfaces that appear to be contaminated with feces from poultry or other animals. there is a risk to handling feather products from countries experiencing outbreaks of H5N1 influenza. unless they have been processed to render them noninfectious .Cans dogs be infected with avian influenza
While dogs are not usually susceptible to avian influenza viruses, the avian influenza A (H5N1) virus that emerged in Asia in 2003 has been documented to infect other carnivore species (e.g. cats, tigers, leopards, stone martens). This has raised concern that this strain of avian influenza A (H5N1) virus may be capable of infecting dogs.There is no evidence to date that cats can spread H5N1 to humans. No cases of avian influenza in humans have been linked to exposure to sick cats, and no outbreaks among populations of cats have been reported. All of the influenza A (H5N1) infections in cats reported to date appear to have been associated with outbreaks in domestic or wild birds and acquired through ingestion of raw infected meat.
What changes are needed for H5N1 or another avian influenza virus to cause a pandemic
Three conditions must be met for a pandemic to start:
1) a new influenza virus subtype must emerge for which there is little or no human immunity;
2) it must infect humans and causes illness;
3) it must spread easily and sustainably (continue without interruption) among humans. The H5N1 virus in Asia and Europe meets the first two conditions: it is a new virus for humans (H5N1 viruses have never circulated widely among people), and it has infected more than 190 humans, killing over half of them.
However, the third condition, the establishment of efficient and sustained human-to-human transmission of the virus, has not occurred. For this to take place, the H5N1 virus would need to improve its transmissibility among humans. This could occur either by “reassortment” or adaptive mutation.
Reassortment occurs when genetic material is exchanged between human and avian viruses during co-infection (infection with both viruses at the same time) of a human or another mammal. The result could be a fully transmissible pandemic virus—that is, a virus that can spread easily and directly between humans. A more gradual process is adaptive mutation, where the capability of a virus to bind to human cells increases during infections of humans.
Interim Recommendations for Infection Control in Health-Care Facilities Caring for Patients with Known or Suspected Avian Influenza
All patients who present to a health-care setting with fever and respiratory symptoms should be managed according to recommendations for Respiratory Hygiene and Cough Etiquette and questioned regarding their recent travel history.
Patients with a history of travel within 10 days to a country with avian influenza activity and are hospitalized with a severe febrile respiratory illness, or are otherwise under evaluation for avian influenza, should be managed using isolation precautions identical to those recommended for patients with known Severe Acute Respiratory Syndrome (SARS). These include:
Standard Precautions
Pay careful attention to hand hygiene before and after all patient contact or contact with items potentially contaminated with respiratory secretions.Contact Precautions
Use gloves and gown for all patient contact.
Use dedicated equipment such as stethoscopes, disposable blood pressure cuffs, disposable thermometers, etc.
Eye protection (i.e., goggles or face shieldsWear when within 3 feet of the patient.
Airborne Precautions
Place the patient in an airborne isolation room (AIR). Such rooms should have monitored negative air pressure in relation to corridor, with 6 to 12 air changes per hour (ACH), and exhaust air directly outside or have recirculated air filtered by a high efficiency particulate air (HEPA) filter. Use a fit-tested respirator.
Vaccination of Health-Care Workers against Human Influenza
Health-care workers involved in the care of patients with documented or suspected avian influenza should be vaccinated with the most recent seasonal human influenza vaccine. In addition to providing protection against the predominant circulating influenza strain, this measure is intended to reduce the likelihood of a health-care worker’s being co-infected with human and avian strains, where genetic rearrangement could take place, leading to the emergence of potential pandemic strain.What is the difference between isolation and quarantine
Isolation refers to the separation of persons who have a specific infectious illness from those who are healthy and the restriction of their movement to stop the spread of that illness.Quarantine refers to the separation and restriction of movement of persons who, while not yet ill, have been exposed to an infectious agent and therefore may become infectious. Both isolation and quarantine are public health strategies that have proven effective in stopping the spread of infectious diseases.
What is the difference between pandemic influenza and annual seasonal influenza
An influenza pandemic is a global outbreak of disease that occurs when a new influenza A virus appears or "emerges" in the human population, causes serious illness, and then spreads easily from person to person worldwide. Pandemics are different from seasonal outbreaks or "epidemics" of influenza. Seasonal outbreaks are caused by subtypes of influenza viruses that are already in existence among people, whereas pandemic outbreaks are caused by new subtypes or by subtypes that have never circulated (spread) among people or that have not circulated among people for a long time. Past influenza pandemics have led to high levels of illness, death, social disruption, and economic loss.Bird flu can cross the placenta in pregnant women
The avian flu virus H5N1 tends to target the lungs, causing overwhelming and often fatal alveolar damage in infected humans. Researchers using sensitive molecular techniques have also found traces of the virus in other organs, most recently in the brain, intestines, and lymph nodes of two Chinese patients. One of them, a 24 year old woman, was four months pregnant when she died. At autopsy, the researchers found evidence of the virus in the baby's lungs, circulation, and liver. Vertical transmission from the mother is the likeliest explanation, and it sets H5N1 apart from common human flu viruses, which are thought to be harmless to the fetus.The presence of H5N1 in the intestine fits well with the diarrhoea that often accompanies the infection, says a linked comment (p 1106). Others have already reported viral RNA in faecal samples from infected people, with obvious implications for controlling spread of the disease.
It now seems clear than H5N1 spreads far beyond the lungs in humans and may even cross the blood-brain barrier. We still don't know why the infection is so lethal, but it may be something to do with our immune response. The immunologically naive fetus in this report was infected but had no organ damage.
علمني المطركيف اغسل همومي واحزانيوكيف اجدد حياتي كما تغسل قطرات المطراوراق الشجروتعيد لها الحياة