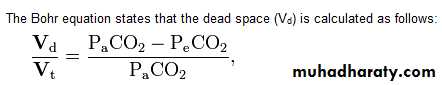Management of COPD Exacerbations Reviewed
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
COPD is now the fourth leading cause of death for both men and women in Canada.
Severe acute exacerbations of (COPD) are devastating, lifethreatening events; the 30-day mortality is greater than that with acute myocardial infarction (26% vs. 7.8%). Acute exacerbations of COPD dramatically change the course of the disease, sincethey are associated with a rapid decline in lung
function and worsening quality of life. They also
represent a substantial economic burden to society.
Prevention of exacerbations remains a primary
goal of management but is difficult because
the cause of acute exacerbations of COPD
remains largely unknown.
nejm.org august 25, 2011
Recent studies have shown that, when used
properly, inhaled glucocorticoids, long-acting
beta2-agonists, long-acting anticholinergic agents,
or phosphodiesterase-4 inhibitors may reduce the
incidence of acute exacerbations in selected subgroups of patients with COPD.
In the past,
long-term administration of antibiotics was used
to prevent acute exacerbations, but meta-analyses
of the studies of antibiotic administration
showed no significant benefit.
However, the potential for newer macrolide antibiotics to prevent acute exacerbations of COPD has been studied,
with the rationale that such agents have both
immunomodulatory and antiinflammatory properties in addition to their antibacterial properties.
In this issue of the Journal, Albert et al.5 report
the results of a large, prospective, parallel-group,placebo-controlled, randomized trial on the use
of azithromycin (at a dose of 250 mg per day for
1 year) to prevent acute exacerbations of COPD.
The results showed that azithromycin was associated with a significant decrease in the frequency of acute exacerbations of COPD and with
an improvement of the quality of life.
Study Participants
Eligible participants were at least 40 years of age, had a clinical diagnosis of COPD (defined as having a smoking history of at least 10 pack-years, a ratio of postbronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity of <70%, and a postbronchodilator FEV1 of <80% of the predicted value), were either using continuous supplemental oxygen or had received systemic glucocorticoids within the previous year, had gone to an emergency room or had been hospitalized for an acute exacerbation of COPD, and had not had an acute exacerbation of COPD for at least 4 weeks before enrollment.
Exclusion criteria were asthma, a resting heart rate greater than 100 beats per minute, a prolonged corrected QT (QTc) interval (>450 msec), the use of medications that prolong the QTc interval or are associated with torsades de pointes (with the exception of amiodarone), and hearing impairment documented by audiometric testing.
There was a price that had to be paid. There
was an excess rate of hearing decrements of approximately 5% in the patients receiving azithromycin.More important, there was an increased prevalence of macrolide-resistant bacteria colonizing the airway, although this was not associated with an increased incidence of pneumonia, a finding that is in agreement with previous reports involving fewer patients, the effect of this change is not known.Studies involving patients with cystic fibrosis
and bronchiectasis have shown no significant effectof the long-term use of azithromycin on resistance
patterns in the community or among close
household contacts. In addition, these studies
showed that the side effects of azithromycin were
fewer when a reduced regimen (three times per
week instead of daily) was used. These regimens
should be explored in patients with COPD.
In my opinion, the findings of Albert and coworkers
— that the interval between the studyonset and the first acute exacerbation of COPD
was almost twice as long in the patients receiving
long-term azithromycin treatment as in the patients
receiving placebo and that patients in the
azithromycin group had a significant improvement
in the quality of life — tip the scales toward
the benefits of azithromycin treatment.
However, if azithromycin is going to be used in
patients who are known to have frequent exacerbations of COPD, then the local antibiotic resistance patterns should be closely monitored.
It also makes sense to ask whether, in such patients,
subsequent exacerbations should be treated
empirically with a different class of antibiotics.
On balance, however, the long-term use of
azithromycin to prevent acute exacerbations of
COPD would not seem to be at odds with the
classical advice of Hippocrates, “Ωφελ′εειν ου
Βλ′απτειν” — “Do good, not harm.”
nejm.org august 25, 2011
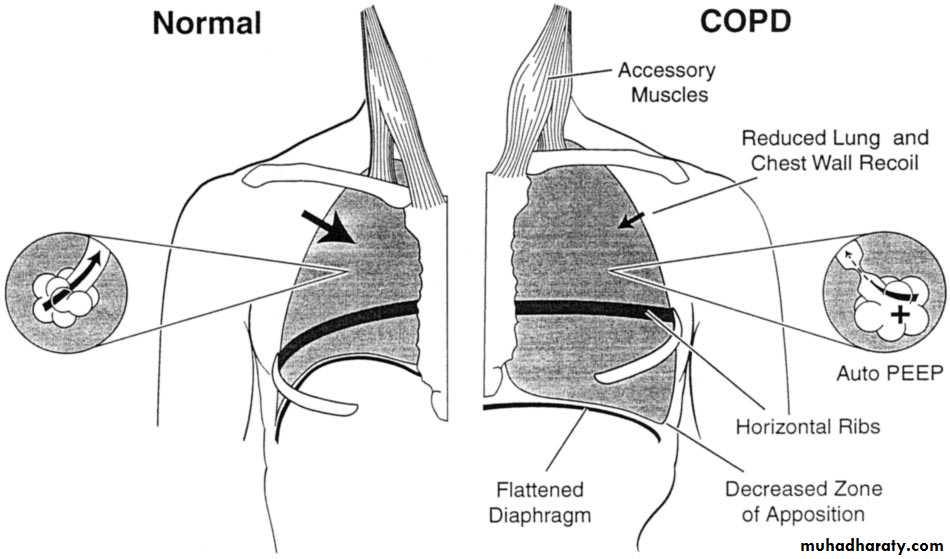
Increasing breathlessness is a feature of the inexorable progression of chronic obstructive pulmonary disease, especially in patients who continue to smoke. Other common comorbidities need to be considered—especially heart failure and lung cancer. People with moderate COPD lose up to 60 ml per year. Although severity is assessed on the basis of post-bronchodilator FEV1, lung function does not correlate well with functional disability, and it is important to make specific enquiry about symptoms such as breathlessness and impact on activities.
BMJ 2009;338:b70
Consider other causes for her increasing breathlessness. Smoking is a risk factor for lung cancer and ischaemic heart disease, and these comorbidities are common in people with COPD. A reduced FEV1 is an independent risk factor for lung cancer, especially in women.Only a third of patients with severe COPD will die of respiratory causes: heart failure and cancer are each responsible for about a quarter of deaths.
BMJ 2009;338:b70
Inhaled steroids do not reduce the rate of decline of lung function in COPD. They can, however, reduce the number of exacerbations from 1.32 per year to 0.99 per year, though the effect is significant only in patients with moderate or severe COPD (FEV1 <50% of predicted). trials using high dose inhaled steroids in COPD have confirmed an increase in both local effects (sore mouth, hoarseness) and systemic effects (especially bruising of the skin and loss of bone density). A recently recognised concern is the excess risk of pneumonia in patients taking inhaled steroids for COPD. Nearly 20% of the patients taking inhaled steroids in the TORCH trial had pneumonia, compared with 12% in the placebo group.
The mainstay of symptom relief for COPD is long acting bronchodilators, which are indicated to ameliorate symptoms. As patient is becoming increasingly breathless it would be appropriate to offer a trial of the long acting anticholinergic tiotropium in addition to regular treatment with salmeterol. In the recent UPLIFT trial, participants using tiotropium had better lung function throughout the trial and improved quality of life, and they experienced fewer exacerbations than the control group, although the rate of decline of lung function was not affected.
Dyspnoea should be assessed with a validated
instrument such as the MRC (Medical ResearchCouncil) dyspnoea score. This defines five grades
of breathlessness related to normal activities and
patients are asked to select the level that best
describes how breathlessness affects them. The
five levels are:
1 Not troubled by breathlessness except on strenuous exercise
2 Short of breath when hurrying or walking up a slight hill
3 Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace
4 Stops for breath after walking about 100 metres or after a few minutes on level ground
5 Too breathless to leave the house, or breathless when dressing or undressing.
Pulmonary rehabilitation(PR), which has been shown to improve exercise tolerance and enhances patients’ sense of control over their condition. Depression is common in patients with COPD and can contribute to a downward spiral of increasing symptoms and inability to cope. and nutrition. PR has been shown to improve
exercise capacity and quality of life for COPD, the patient performs aerobic, strength and breathing exercises.
OBSTRUCTIVE
AsthmaChronic obstructive lung disease
(chronic bronchitis, emphysema)
Bronchiectasis
Cystic fibrosis
Bronchiolitis
RESTRICTIVE—PARENCHYMA
Sarcoidosis
Idiopathic pulmonary fibrosis
Pneumoconiosis
Drug- or radiation-induced
interstitial lung disease
RESTRICTIVE—EXTRAPARENCHYMAL
Neuromuscular
Diaphragmatic weakness/paralysis
Myasthenia gravis
Guillain-Barre´ syndrome
Muscular dystrophies
Cervical spine injury
Chest wall
Kyphoscoliosis
Obesity
Ankylosing spondylitis
Common Respiratory Diseases by Diagnostic Categories
Two major patterns of abnormal ventilatory function are restrictive and obstructive
In obstructive pattern:
• Hallmark is decrease in expiratory flow rate, i.e., FEV1.• Ratio FEV1/FVC is reduced.
• TLC is normal or increased.
• RV is elevated due to trapping of air during expiration.
In restrictive disease:
Hallmark is decrease in TLC.
The diagnosis is confirmed by spirometry.
The presence of a postbronchodilator FEV1< 80% of the predicted value in combination with an FEV1/FVC < 70% confirms the presence of airflow limitation that is not fully reversible.COPD Interventions
The goals of effective management of a COPD exacerbation are acute symptom relief as well as a reduced risk for subsequent exacerbations. Although COPD exacerbations are a key factor underlying the high mortality rates among patients with this condition, several interventions have been shown in randomized controlled trials to be effective.COPD is characterised by poorly reversible airflow obstruction and progressive symptoms. Exacerbation of COPD is a clinical diagnosis of exclusion and there are many causes of worsening symptoms in a patient with underlying COPD that should be considered. The frequency of exacerbations varies between patients, and the best predictor is a patient’s history of exacerbations. Patients who have frequent exacerbations have a more rapid decline in lung function, poorer quality of life, and greater mortality. Preventing exacerbations is therefore a key goal of COPD management.
Increasing the dosage of inhaled short-acting bronchodilators is recommended as the first step in outpatient treatment. In particular, the combination of ipratropium and albuterol is helpful for reducing dyspnea.
Patients with purulent sputum are likely to benefit from oral corticosteroids, and moderately or severely ill patients are likely to benefit from antibiotic therapy, lowers the risk for treatment failure and death.
Local patterns of antibiotic resistance and a patient-specific history of recent antibiotic use should guide initial antibiotic choice. Evidence is limited that broad-spectrum antibiotics (eg, amoxicillin/clavulanate, macrolides, second- or third-generation cephalosporins, or quinolones) are more effective than narrow-spectrum antibiotics (eg, amoxicillin, ampicillin, trimethoprim/sulfamethoxazole, doxycycline, or tetracycline).
For exacerbations in hospitalized patients, treatment with regular doses of short-acting bronchodilators, continuous supplemental oxygen, antibiotics, and systemic corticosteroids is recommended.
Patients with worsening acidosis or hypoxemia should receive noninvasive positive pressure ventilation or invasive mechanical ventilation.
Key Clinical Recommendations
Noninvasive positive pressure ventilation improves respiratory acidosis while reducing respiratory rate, breathlessness, need for intubation, mortality rate, and length of hospital stay (evidence, A).Inhaled bronchodilators (beta-agonists, alone or in combination with anticholinergic agents) reduce dyspnea and improve exercise tolerance (level of evidence, A).
In patients with COPD, short courses of systemic corticosteroids prolong the time to subsequent exacerbation, reduce the rate of treatment failure, reduce length of hospitalization, and improve forced expiratory volume in 1 second and hypoxemia (level of evidence, A).
Compared with high-dosage corticosteroid regimens, low-dosage regimens are not inferior in reducing the risk for treatment failure in patients with COPD (level of evidence, B).
Compared with intravenous prednisolone, oral prednisolone is equivalent in lowering the risk for treatment failure in patients with COPD (level of evidence, B).
Oral corticosteroids are bioavailable, inexpensive, and convenient, and are therefore recommended in patients who can safely swallow and absorb them (level of evidence, B).
In patients with COPD, smoking cessation lowers the mortality rate and likelihood of subsequent exacerbations (level of evidence, A).
In severely ill patients, long-term oxygen therapy reduces the risk for hospitalization and duration of hospitalization (level of evidence, B).
To qualify for discharge, a patient should have stable clinical symptoms and a stable or improving arterial partial pressure of oxygen of more than 60 mm Hg for at least 12 hours [and] not require albuterol more often than every four hours.
If the patient is stable and can use a metered dose inhaler, there is no benefit to using nebulized bronchodilators.
Patient education may improve the response to future exacerbations; suggested topics include a general overview of COPD, available medical treatments, nutrition, advance directives, and advice about when to seek medical help.
Although criteria have been established for the diagnosis of COPD, there is no validated diagnostic test or biomarker for COPD exacerbations.
According to the American Thoracic Society and European Respiratory Society, an exacerbation can be defined as an acute change beyond normal variability from a patient's baseline dyspnea, cough, or sputum that mandates a change in treatment.
Clinical Context
In addition to these pulmonary symptoms, COPD exacerbation is typically accompanied by systemic inflammation causing extrapulmonary symptoms.
COPD exacerbations can be classified
Mild (can be controlled by increasing the dosage of regular medications),Moderate (requires treatment with systemic corticosteroids or antibiotics),
Severe (requires hospitalization or emergency department evaluation). Exacerbations contribute to the high mortality rate, but several interventions have been shown to be effective in randomized controlled trials.
Clinical Implications
The goals of effective management of COPD exacerbations are acute symptom relief and a lowered risk for future exacerbations. Short-acting bronchodilators, short courses of systemic corticosteroids, and/or antibiotics may be indicated, depending on the severity of the exacerbation.Noninvasive positive pressure ventilation or invasive mechanical ventilation is recommended for patients with worsening acidosis or hypoxemia. Other useful interventions may include smoking cessation, long-term oxygen therapy, and patient education.
A metered-dose inhaler (MDI) is a device that delivers a specific amount of medication to the lungs, in the form of a short burst of aerosolized medicine that is inhaled by the patient. It is the most commonly used delivery system for treating asthma, COPD and other respiratory diseases.
Transition to HFA propellants
In the United States starting from December 2008 inhalers containing chlorofluorocarbons, as a form of propellant to deliver the medication, will be discontinued for hydro-fluoroalkane-pressurized metered dose inhalers (HFA pMDI'sAs governed by the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer, all inhalers that contain CFCs are being discontinued.Disposable inhalers
A Dry powder inhaler (DPI) is a device that delivers medication to the lungs in the form of a dry powder. DPIs are commonly used to treat respiratory diseases such as asthma, bronchitis, emphysema and COPD although DPIs have also been used in the treatment of diabetes mellitus.DPIs are an alternative to the aerosol based inhalers commonly called metered-dose inhaler (or MDI). The DPIs may require some procedure to allow a measured dose of powder to be ready for the patient to take.
The medication is commonly held either in a capsule for manual loading or a proprietary form from inside the inhaler. Once loaded or actuated, the operator puts the mouthpiece of the inhaler into their mouth and takes a deep inhalation, holding their breath for 5-10 seconds. There are a variety of such devices. The dose that can be delivered is typically less than a few tens of milligrams in a single breath since larger powder doses may lead to provocation of cough.
Most DPIs rely on the force of patient inhalation to entrain powder from the device and subsequently break-up the powder into particles that are small enough to reach the lungs. For this reason, insufficient patient inhalation flow rates may lead to reduced dose delivery and incomplete deaggregation of the powder, leading to unsatisfactory device performance. Thus, most DPIs have a minimum inspiratory effort that is needed for proper use and it is for this reason that such DPIs are normally used only in older children and adults.
A jet nebulizer shown attached to a compressor
Nebulizers are commonly used for treatment of cystic fibrosis, asthma, COPD and other respiratory diseases.
nebulizer (spelled nebuliser in British English) is a device used to administer medication in the form of a mist inhaled into the lungs.
The common technical principle for all nebulizers is to either use oxygen, compressed air or ultrasonic power, as means to break up medical solutions/suspensions into small aerosol droplets, for direct inhalation from the mouthpiece of the device.
The definition of an aerosol is a "mixture of gas and liquid particles", and the best example of a natural occurring aerosol is "mist" (being formed when small vaporized water particles mixed with hot ambient air, are cooled down and condenses into a fine cloud of visible airborne water droplets).
When using a nebulizer for inhalation therapy with medicine to be administered directly to the lungs, it is important to note that inhaled aerosol droplets can only penetrate into the narrow branches of the lower airways if they have a small diameter of 1-5 micrometers. Otherwise they are only absorbed by the mouth cavity, where the effect is low.
An asthma spacer is an add-on device used to increase the ease of administering aerosolized medication from a "metered-dose inhaler" (MDI). The spacer adds space in the form of a tube called a “chamber” between the canister of medication and the patient’s mouth, allowing the patient to inhale the medication by breathing in slowly and deeply for ~5–10 breaths
Asthma spacer
Baby using inhaler and spacer.The spacer is a specially designed plastic or metal tube, The back end of the chamber is closed off by a back-piece. The MDI is inserted into the back-piece. The front part of the chamber is closed off by either a facemask that covers both the patient’s mouth and nose, or simply a mouthpiece that goes onto the patient’s mouth.
To administer the medication, the patient brings the facemask to the face (or the mouth-piece to the mouth) and depresses the metered-dose inhaler once, resulting in the release of one dose of medication. The medication from the MDI is then briefly suspended in the spacer’s chamber while the patient inhales the aerosolized medication by breathing in and out deeply at a slow rate of speed.
Some spacers are equipped with a whistle, which sounds as a warning when the patient is inhaling too quickly. The facemask on the spacer has valves which insure that the medication suspended in the chamber is inhaled by the patient, and that the exhaled breath exits the device through the exhalation valve mounted in the mask. When using a spacer without a facemask, the patient must inhale through their mouth and exhale through their nose.
Spacers with facemasks are used in toddlers and young children because that population is unable to coordinate inhaling through their mouths and exhaling through their noses. However, the facemasks are available in small, medium, and large sizes, and spacers with facemasks may also be used in the adult and elderly population.
The term spacer is often used to refer to any tube-like MDI add-on device. Some spacers utilize a collapsing bag design to provide visual feedback that successful inspiration is taking place.
Benefits of a spacer
In order to properly use an inhaler without a spacer, one has to coordinate a certain number of actions in a set order (pressing down on the inhaler, breathing in deeply as soon as the medication is released, holding your breath, exhaling), and not all patients are able to master this sequence. Use of a spacer avoids such timing issues.Spacers slow down the speed of the aerosol coming from the inhaler, meaning that less of the asthma drug impacts on the back of the mouth and somewhat more may get into the lungs. Because of this, less medication is needed for an effective dose to reach the lungs, and there are fewer side effects from corticosteroid residue in the mouth.
Valves on a spacer (which technically makes it a holding chamber) cause the patient to inhale the contents of the spacer, but exhalation goes out into the air. The problem of co-ordinating an inspiration with a press of an inhaler is avoided, making use easier for children under 5 and the elderly. It also makes asthma medication easier to deliver during an attack. So use of spacer is advised by many.
Spacer Disadvantages
A spacer can be bulky, limiting portability.Devices along the inhalation path such as a spacer may cause the medication to deposit prior to reaching the patient and the patient can receive less than the measured dose.
ß2-adrenoceptor agonists are probably the commonest prescribed medication in respiratory practice. They are used in the treatment of asthma and the reversible element of airways obstruction commonly found in chronic obstructive airways disease (COAD) ,with effects on smooth and skeletal muscle, which include bronchodilatation, relaxation of the uterus and tremor.
The two commonest ß2-agonists prescribed in the UK are salbutamol and terbutaline sulphate (Bricanyl®). Other less common drugs include fenoterol hydrobromide (Berotec®), rimiterol hydrobromide (Pulmadil®), pirbuterol (Exirel®), reproterol hydochloride (Bronchodil®) and tulobuterol hydrochloride (Brelomax®). There is no place for the use of orciprenaline and isoprenaline in current practice as they are not ß2 selective and therefore, they will not be discussed.
The maximal therapeutic effect is seen within 15 minutes of inhalation which suggests a local action within the lungs as the peak plasma concentration of the drug occurs after about 3 hours after inhalation.
Both salbutamol and terbutaline are highly ß2-receptor selective.also increase the heart rate, but this may be due to a reflex response following relaxation of vascular smooth muscle resulting in vasodilation rather than stimulation of ß1-receptors.
Both tremor and palpitations are more commonly seen when oral dosing or high dose nebulisers are used. Significant hypokalaemia may be seen, especially when parenteral therapy is used.
An asthmatic child with a troublesome cough
A 12 year old girl with an eight year history of asthma presented to her family doctor for the third time in four weeks with ongoing, troublesome, non-productive cough. The cough was described as “coming on in fits” and was disturbing her sleep. By day, the cough could be brought on by exercise or breathing in cold air but sometimes came on “out of the blue.” Once or twice a day, the cough would induce retching, and she had vomited on occasion.
BMJ February 2011
Over the past month she had been prescribed five days of prednisolone and a week’s worth of clarithromycin, and a long acting beta agonist had been added to her low dose inhaled steroid, all to no effect. She had no history of wheezing, and the only history of shortness of breath was during the coughing.
She had two hospital admissions for asthma at ages 6 and 7 and her attendance at the practice asthma clinic had been good. She also had eczema and mild hay fever. Her mother had asthma and was a regular smoker. Her immunisations were all up to date. Examination was normal aside from a right sided subconjunctival haematoma.
Questions
1 What is the most likely diagnosis?2 What investigations, if any, are appropriate?
3 What is the next management step?
4 How long will these troublesome symptoms persist?
Answers
1 What is the most likely diagnosis?Short answer
The most likely diagnosis is pertussis, otherwise known as whooping cough, which is caused by the Gram negative bacterium Bordetella pertussis.
Long answer
Infection with B pertussis is relatively common in the United Kingdom despite the national infant vaccination programme. Some individuals who are vaccinated will develop pertussis regardless, but there is evidence that they experience a less severe and slightly atypical illness.
Infections with B parapertussis and respiratory syncytial virus may also be associated with a paroxysmal cough.
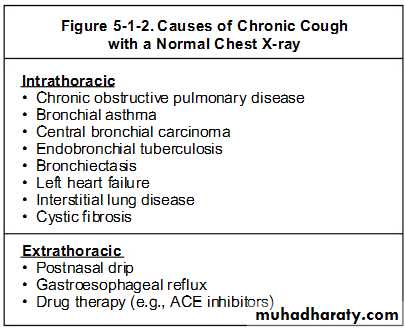
The definition of chronic cough in children is a cough lasting for more than eight weeks. Once serious conditions are excluded (such as cystic fibrosis, immune deficiency, congenital abnormality, and foreign body), the differential diagnosis includes pertussis and other rather poorly defined diagnoses such as viral bronchitis, postviral cough, cough variant asthma, and postnasal drip.
2 What investigations, if any, are appropriate?
Short answerThe diagnosis of pertussis can be made clinically but can be confirmed by serological testing, even in vaccinated individuals. PCR can be used to identify the bacterium in the acute phase.
Long answer
ELISA using serum is the most commonly available laboratory test used to confirm the diagnosis. Pertussis can be diagnosed in the acute phase by testing nasal secretions using PCR, although most cases present after many weeks (that is, in the chronic phase), by which time the bacteria have been cleared.B pertussis can be hard to grow in vitro, and a positive culture from nasal secretions is often not achieved.
B pertussis infection can be associated with a highly elevated lymphocyte count, so a blood count can assist in making the diagnosis, particularly in infants.
3 What is the next management step?
Short answerIn this case, nothing. Do not escalate asthma treatment. Macrolide antibiotics are indicated for eradication where symptoms are present for less than three weeks. Cases where pertussis immunisation is incomplete should “catch up” with vaccinations once recovered.
Long answer
Once the diagnosis of pertussis is confirmed in a child, management is supportive. Antibiotics do not alter the course of the infection once paroxysms are established. Eradication with a macrolide antibiotic is indicated to reduce transmission, but this is only thought to be effective where symptoms have been present for less than three weeks.Traditionally, a 10 day course of erythromycin was prescribed to eradicate infection; however, a seven day course of clarithromycin or a three to five day course of azithromycin are not inferior in terms of eradication and are associated with a third fewer cases of side effects.
Antibiotic prophylaxis should be offered to home contacts, particularly infants, and has been shown to reduce transfer of infection between family members. If not previously fully vaccinated, the index case should also be offered vaccination when fully recovered; that is, three infant vaccinations and one booster after the age of 5 years.
4 How long will these troublesome symptoms persist?
Short answerThe Chinese call pertussis “the cough of 100 days,” although the paroxysms can last for up to six months.
Long answer
The natural history of pertussis is well described.The initial prodromal “catarrhal” phase is characterised by nasal discharge and non-specific cough for a week.
The “paroxysmal” phase has a duration of approximately two months and is characterised by paroxysms or uncontrollable series of coughs during which the individual cannot inhale between coughs. At the end of the paroxysm, the patient’s face can be red or even apparently cyanosed owing to facial venous congestion. There may be vomiting or retching and then often a sharp inspiration from residual volume to total lung capacity (the “whoop”).
The third “convalescent” phase can last for another two months, and paroxysms may briefly recur in the event of an interval upper respiratory tract infection.
Acute complications are mostly limited to infants, in whom the infection can be life threatening, and include weight loss, apnoeas, pneumonia, and circulatory failure.
The most common chronic complication is bronchiectasis.
BMJ February 2011
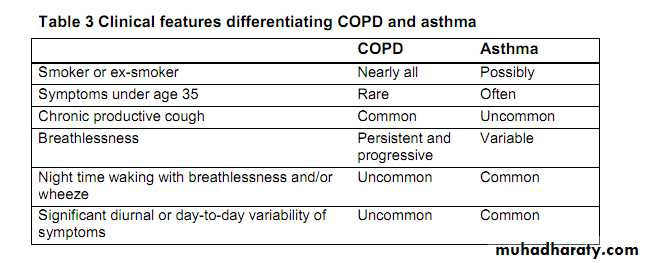
Differences between the pathologic mechanisms of asthma and COPD dictate the implementation of distinctively different treatment methods.
Asthma is primarily a chronic inflammatory disease involving episodes of reversible bronchoconstriction. Because inflammation is the main pathologic mechanism, anti- inflammatory agents (specifically inhaled corticosteroids) are first-line therapy in asthma. To prevent and counteract reversible episodes of bronchospasm, beta-2 adrenergic inhalers directly dilate the bronchioles.
In contrast, bronchoconstriction in COPD is progressive, largely cholinergic mediated, and only partially reversible.
Because bronchoconstriction is a major pathologic mechanism in COPD, bronchodilators (specifically anticholinergic inhalers) are first-line pharmacologic therapy.
Mechanisms of "Rescue" vs. "Maintenance" Inhalers
Short-acting bronchodilators are used as rescue medications for immediate relief of acute bronchospasm in asthma or COPD. These beta-2 adrenergic agonists exert a rapid bronchodilator effect and have a short duration.Long-acting beta-2 adrenergic bronchodilators are used as maintenance medications taken daily on a scheduled basis to prevent acute bronchospastic events.
Anticholinergic bronchodilator inhalers, also used as maintenance therapy, are first-line medications in managing COPD .These inhalers block cholinergic stimulation of the bronchioles, thereby inhibiting bronchoconstriction. Anticholinergic inhalers are used on a scheduled, daily basis for preventing bronchoconstriction, not for acute episodes of bronchospasm
Inhaled corticosteroids are long-acting maintenance medications taken daily for asthma. They have not been shown consistently to improve airway resistance in COPD, and their use is controversial in this disease group. They are to be taken on a scheduled, daily basis, not for acute bronchospasm. Corticosteroids are often found in combination with long-acting beta-2 adrenergic bronchodilators in inhaler devices
A common therapeutic regimen for a patient with persistent asthma or COPD consists of scheduled daily use of maintenance inhaler medications with rescue inhaler use as needed. In persistent asthma, this regimen consists of a daily inhaled long-acting bronchodilator and corticosteroid, with a short-acting bronchodilator (rescue) inhaler used as needed for acute bronchospastic episodes.
Another combination maintenance inhaler consists of an anticholinergic medication and a short-acting beta-2 adrenergic agonist. These have proven beneficial in COPD due to the synergistic effect of anticholinergic inhibition of bronchoconstriction and the beta-2 adrenergic agonist stimulation of bronchodilation
Cromolyn, a unique anti-inflammatory, is used for long-term management and prevention of acute bronchospastic episodes. Cromolyn stabilizes bronchiole mast cells and inhibits release of inflammatory mediators. Used as maintenance treatment, cromolyn can inhibit bronchospasm incited by exercise, aspirin, cold air, sulfur dioxide, toluene diisocyanate, and environmental pollutants. It also should be used shortly before anticipated exposure to bronchospasm- inciting factors such as exercise.
Inhaler pulmonary medications categorized :
Rescue inhaler* Short-acting beta-2 adrenergic bronchodilators
Maintenance inhaler treatment
• Long-acting beta-2 adrenergic bronchodilators
• Anticholinergic bronchodilators
• Corticosteroid inhalers
Combination inhaler treatment
• Corticosteroid plus short-acting beta-2 adrenergic bronchodilator
• Anticholinergic bronchodilator plus short-acting beta-2 adrenergic bronchodilator
Cromolyn inhaler treatment
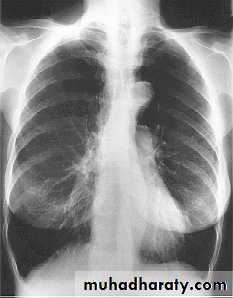
• Chest X-ray
• The presence emphysema can be suspected on routine chest radiography but this is not a sensitive technique for diagnosis.
• Large volume lungs with
• a narrow mediastinum and
• flat diaphragms are the typical appearances. In addition, the presence of bullae and irregular distribution of the lung vasculature may be present.
• In more advanced disease, the presence of pulmonary hypertension may be suspected by the prominence of hilar vasculature. The chest X-ray is not a very good indicator of the severity of disease and will not be able to identify patients with COPD without significant emphysema.
• However, the chest X-ray is useful to look for complications during acute exacerbations and to exclude other pathology such as lung cancer.
Chronic obstructive pulmonary disease (COPD), also known as chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic airflow limitation (CAL) and chronic obstructive respiratory disease (CORD), refers to chronic bronchitis and emphysema, a pair of commonly co-existing diseases of the lungs in which the airways become narrowed. This leads to a limitation of the flow of air to and from the lungs causing shortness of breath.
COPD is defined by its characteristically low airflow on lung function tests. In contrast to asthma, this limitation is poorly reversible and usually gets progressively worse over time. COPD is caused by noxious particles or gas, most commonly from tobacco smoking, which triggers an abnormal inflammatory response in the lung.
Efficiency of mouth to patient insufflation
Normal atmospheric air contains approximately 21% oxygen when created in. After gaseous exchange has taken place in the lungs, with waste products (notably carbon dioxide) moved from the bloodstream to the lungs, the air being exhaled by humans normally contains around 17% oxygen.This means that the human body utilises only around 19% of the oxygen inhaled, leaving over 80% of the oxygen in the exhalatory breath.
This means that there is more than enough residual oxygen to be used in the lungs of the patient, which then crosses the cell membrane to form oxyhemoglobin
The efficiency of artificial respiration can be greatly increased by the simultaneous use of oxygen therapy. The amount of oxygen available to the patient in mouth to mouth is around 16%. If this is done through a pocket mask with an oxygen flow, this increases to 40% oxygen. If a Bag Valve Mask or mechanical respirator is used with an oxygen supply, this rises to 99% oxygen. The greater the oxygen concentration, the more efficient the gaseous exchange will be in the lungs.
Oxygen
β-Blockers for Chronic Obstructive Pulmonary Disease?
β-blockers were associated with lower mortality and fewer acute exacerbations in patients with COPD.
Dogma around use of β-blocker medications in patients with (COPD) has been to avoid using them, because they might precipitate acute exacerbations.
However, COPD could be a risk factor in atherosclerotic disease progression (by causing systemic inflammation), and
β-blockers lengthen survival in patients with coronary artery disease and heart failure.
Additionally, meta-analysis data demonstrate that β-blockers are safe in patients with stable COPD.
Researchers in the Netherlands followed a cohort of 2230 COPD patients for a mean of 7 years to determine whether long-term β-blocker use lengthened survival and lowered risk for COPD exacerbations. Compared with patients who did not receive β-blockers, those who did were about 30% less likely to die from any cause or to experience COPD exacerbations. Subanalysis of patients without coexisting coronary disease or heart failure yielded similar results.
Comment: Potential mechanisms for beneficial effects of β-blockers in COPD patients include an anti-inflammatory effect and improvement of undiagnosed coexistent heart disease. Regardless of the mechanism by which β-blockers affect COPD patients, β-blocker use is associated with a mortality benefit and fewer COPD exacerbations.
This study paves the way for a randomized controlled trial designed to examine the efficacy and benefit of β-blockers in patients with COPD. These data might not prompt us to prescribe β-blockers to all COPD patients yet, but we can gain comfort in knowing that patients with COPD who possess other indications for β-blocker therapy are likely to attain substantial benefit.
Journal Watch Hospital Medicine July 12, 2010
Positive pressure ventilation
any of numerous types of mechanical ventilation in which gas is delivered into the airways and lungs under positive pressure, producing positive airway pressure during inspiration. It may be done via either an endotracheal tube or a nasal maskInhaled Long-Acting Muscarinic Antagonists (LAMAs) for COPD
Inhaled muscarinic antagonists work by inhibiting muscarinic receptors on the bronchial airways, which lead to muscle relaxation, bronchodilation and improved lung function. Until recently, only one inhaled muscarinic antagonist (ipratropium) has been available in the United States, both as a single agent and in combination with the short-acting beta2 agonist albuterol. This product is short acting and requires dosing four or more times per day.An inhaled LAMA (tiotropium, Spiriva®) suitable for once-a-day dosing has been available in Europe since 2002 and was launched in the United States in May 2004. Tiotropium produces a prolonged blockade of muscarinic M3 receptors. Although blocking the M3 receptor is important for bronchodilation, there is emerging evidence that other receptor sub-types may play a role in mediating bronchodilation.
In addition, after inhalation a significant amount of tiotropium reaches the systemic circulation, and, as a consequence, muscarinic M3 receptors at other sites in the body can be blocked for an extended time. We believe this systemic activity of tiotropium is the cause of bothersome side effects such as dry mouth and constipation, which have been seen more frequently with tiotropium (especially in elderly patients) than with short-acting muscarinic antagonists or with the long-acting beta2 agonist, salmeterol.
We are developing an inhaled LAMA designed to produce a prolonged blockade of the relevant receptor sub-types while also being highly lung-selective, which means that lower concentrations of drug should get into the systemic circulation. We believe this approach will result in improved tolerability over tiotropium at doses with comparable efficacy. At higher doses, a more lung-selective LAMA might offer improved efficacy versus tiotropium with comparable or improved tolerability.
Novartis reported on two Phase III studies demonstrating that treating COPD using its marketed once-daily OnBrez® Breezhaler® (indacaterol) in addition to tiotropium (Boehringer Ingelheim’s long-acting anti-muscarinic [LAMA] drug Spiriva® HandiHaler®) improves lung function to a significantly greater degree than tiotropium therapy alone.
Novartis claims Onbrez Breezhaler is the only marketed once-daily, long-acting beta-2 agonsit (LABA). “Previous studies have confirmed the efficacy of Onbrez Breezhaler as monotherapy, and these data show the potential for additional lung function benefits when two of the leading classes of treatment for COPD are combined
Onbrez Breezhaler was first approved in November 2009 in the EU, for the maintenance bronchodilator treatment of airflow obstruction in adult patients with COPD. The drug is currently approved in over 50 countries, and has been launched in 13 territories within Europe. FDA regulatory review of the marketing application in the U.S. is scheduled for March 2011. If approved, the treatment will be trademarked Arcapta® Neohaler.
Pharmacological therapy of chronic
obstructive pulmonary diseaseCOPD is a multicomponent disease that is highly preventable and treatable. The predominant presenting feature is one of progressive dyspnoea.This occurs as a result of airflow limitation due to a combination of damage to the airways and parenchyma. The other important aspects of the disease are the presence of an abnormal inflammatory
response and the presence of systemic manifestations.
Current literature suggests that smoking cessation, long-term oxygen therapy in hypoxaemic patients, non-invasive ventilation,and lung volume reduction surgery in selected patients improve survival.
The recent TORCH study3 of more than
6000 patientsshowed that the combination of salmeterol and fluticasone not only improved lung function and health status, but also reduced the relative mortality risk by 17.5% over the 3 years of the study
in comparison with placebo.
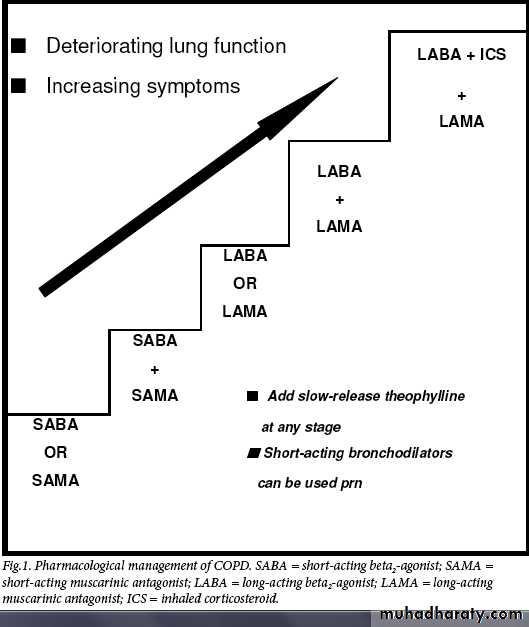
Bronchodilators
Bronchodilators are used as first-line agents for the symptomatic treatment of patients with COPD. Bronchodilators decrease airway smooth-muscle tone, thus improving lung emptying during expiration and hence decreasing lung hyperinflation. This reduces
dyspnoea both at rest and on exertion, with resultant improvement in effort tolerance.
Short-acting anticholinergics and beta2-agonists have been shown to improve pulmonary function, dyspnoea, and exercise performance in patients with COPD. Combining short-acting beta2-agonists and anticholinergics produces superior bronchodilation than either agent alone.
In patients with persistent symptoms it is preferable to use long acting bronchodilators. Long-acting beta2-agonists (LABAs) improve symptoms, increase exercise endurance and improve quality of life. The recent TORCH study3 has been reassuring with respect to the safety profile of salmeterol in COPD.
Treatment with tiotropium, a long-acting anticholinergic, has been associated with improvements in lung function, quality of life and exacerbations in a recently completed 4-year study in patients with COPD. This study (UPLIFT) was reassuring about the safety of the drug with long-term use in patients with COPD.
Theophylline is a nonspecific phosphodiesterase inhibitor and has bronchodilator effects at higher doses, where there is a higher risk of toxicity. It is recommended for patients who have difficulty using inhaler devices. The previously recommended therapeutic serum levels of 15 - 20 mg/dl are too close to the toxic level and a lower level of 8 - 13 mg/dl is therefore suggested as safer but still effective.
Specific phosphodiesterase E4 inhibitors such as cilomilast and roflumilast are currently undergoing clinical trials. They may have
anti-inflammatory and bronchodilator properties but preliminary results show a modest bronchodilator effect and some benefits on quality of life.
COPD is a progressive disease, and a stepwise increase in treatment is
recommended in most guidelines.Combination therapy produces better bronchodilation than individual agents. As the disease progresses, it is often necessary to use two or more bronchodilator agents from different classes to obtain symptomatic relief.
Inhaled corticosteroids (ICS) as monotherapy
The anti-inflammatory effects of corticosteroids in COPD are modest in comparison to asthma. Several studies have looked at the effect of ICS in slowing down the progressive decline in lung function in COPD. The results have been disappointing.There was minimal, if any, benefit in the rate of decline in lung function. However, there has been evidence of a reduction in exacerbation frequency and improvement in quality of life.
The combination of LABAs and ICS was superior in all endpoints studied compared with ICS alone. It is therefore suggested that in patients with COPD, ICS should not be used alone but rather in combination with a LABA.
Systemic corticosteroids
In stable COPD there is no role for chronicuse of systemic corticosteroids. No study so far has shown any benefit of chronic systemic use of these agents in patients with stable disease.
Long-term use of systemic corticosteroids is definitely associated with toxic effects such as hyperglycaemia,myopathy, hypertension and osteoporosis,all of which are more pronounced in the elderly.
Pharmacological therapy
Inhaled long-acting bronchodilators,e.g. arformoterol (for nebuliser use), and carmoterol, indacaterol, and new longactinganticholinergics
ICS/LABA combinations in once-daily dose
Antioxidant drugs such as Nacetylcysteine
Retinoids which have been shown to possibly reverse the changes of emphysema.
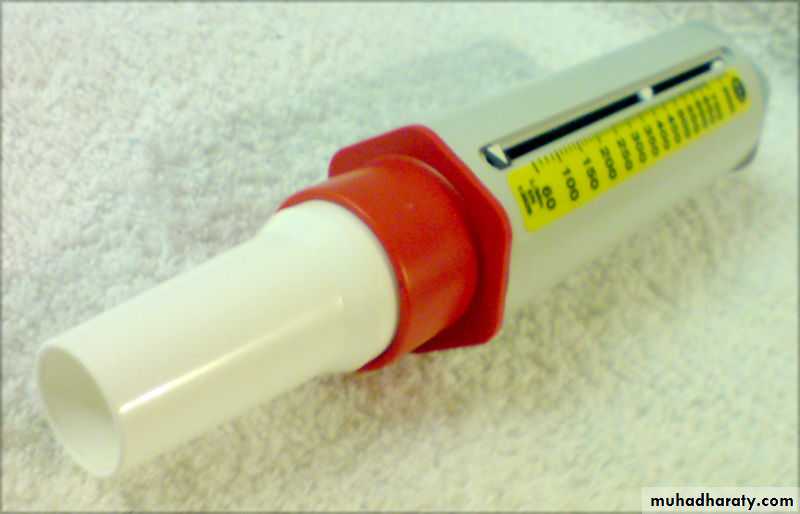
Spirometry (the measuring of breath) is the most common of the Pulmonary Function Tests (PFTs), measuring lung function, specifically the measurement of the amount (volume) and/or speed (flow) of air that can be inhaled and exhaled. Important tool used for generating pneumotachographs which are helpful in assessing conditions such as asthma, pulmonary fibrosis, cystic fibrosis, and COPD.
Device for spirometry. The patient places his or her lips around the blue mouthpiece. The teeth go between the nubs and the shield, and the lips go over the shield. A noseclip guarantees that breath will flow only through the mouth.
The spirometry test is performed using a device called a spirometer, which comes in several different varieties. Most spirometers display the following graphs, called spirograms:
a volume-time curve, showing volume (liters) along the Y-axis and time (seconds) along the X-axis
a flow-volume loop, which graphically depicts the rate of airflow on the Y-axis and the total volume inspired or expired on the X-axis
Procedure
FVC test varies slightly depending on the equipment used.Generally, the patient is asked to take the deepest breath they can, and then exhale into the sensor as hard as possible, for as long as possible, preferrably at least 6 seconds. It is sometimes directly followed by a rapid inhalation (inspiration), in particular when assessing possible upper airway obstruction.
Sometimes, the test will be preceded by a period of quiet breathing in and out from the sensor (tidal volume), or the rapid breath in (forced inspiratory part) will come before the forced exhalation.
During the test, soft nose clips may be used to prevent air escaping through the nose. Filter mouthpieces may be used to prevent the spread of microorganisms, particularly for inspiratory maneuvers.
Limitations of test
The maneuver is highly dependent on patient cooperation and effort, and is normally repeated at least three times to ensure reproducibility. Since results are dependent on patient cooperation, FEV1* and FVC can only be underestimated, never overestimated.(*FEV1 can be overestimated in people with some diseases - a softer blow can reduce the spasm or collapse of lung tissue to elevate the measureDue to the patient cooperation required, spirometry can only be used on children old enough to comprehend and follow the instructions given (6 years old or more), and only on patients who are able to understand and follow instructions - thus, this test is not suitable for patients who are unconscious, heavily sedated, or have limitations that would interfere with vigorous respiratory efforts.
Other types of lung function tests are available for infants and unconscious persons.
Another major limitation is the fact that many intermittent or mild asthmatics have normal spirometry between acute exacerbation, limiting spirometry's usefulness as a diagnostic. It is more useful as a monitoring tool: a sudden decrease in FEV1 or other spirometric measure in the same patient can signal worsening control, even if the raw value is still normal. Patients are encouraged to record their personal best measures.Related tests
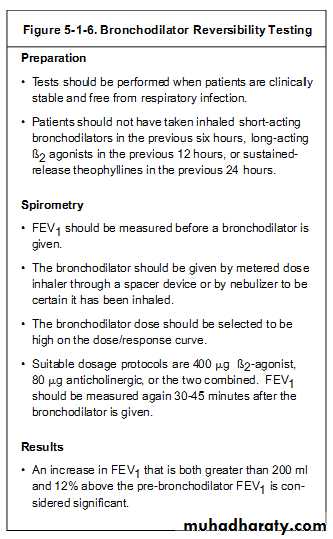
Spirometry can also be part of a bronchial challenge test, used to determine bronchial hyperresponsiveness to either rigorous exercise, inhalation of cold/dry air, or with a pharmaceutical agent such as methacholine or histamine.Sometimes, to assess the reversibility of a particular condition, a bronchodilator is administered before performing another round of tests for comparison. This is commonly referred to as a reversibility test, or a post bronchodilator test (Post BD), and is an important part in diagnosing asthma versus COPD.Other complementary lung functions tests include plethysmography and nitrogen washout.
Are Vital capacity (VC), Forced vital capacity (FVC), Forced expiratory volume (FEV) at timed intervals of 0.5, 1.0 (FEV1), 2.0, and 3.0 seconds, Forced expiratory flow 25–75% (FEF 25–75) and Maximal voluntary ventilation (MVV), also known as Maximum breathing capacity. Other tests may be performed in certain situations.parameters
Results are usually given in both raw data (litres, litres per second) and percent predicted - the test result as a percent of the "predicted values" for the patients of similar characteristics (height, age, sex, and sometimes race and weight).
The interpretation of the results can vary depending on the physician and the source of the predicted values. Generally speaking, results nearest to 100% predicted are the most normal, and results over 80% are often considered normal.
A bronchodilator is also given in certain circumstances and a pre/post graph comparison is done to assess the effectiveness of the bronchodilator.
Functional residual capacity (FRC) cannot be measured via spirometry, but it can be measured with a plethysmograph or dilution tests (for example, helium dilution test).
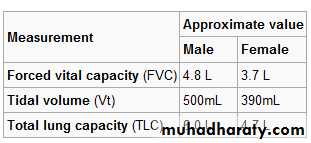
Forced Vital Capacity (FVC)
FVC is the volume of air that can forcibly be blown out as hard as possible, for as long as possible, preferrably at least 6 seconds. after full inspiration, measured in liters.Forced Expiratory Volume in 1 second (FEV1)
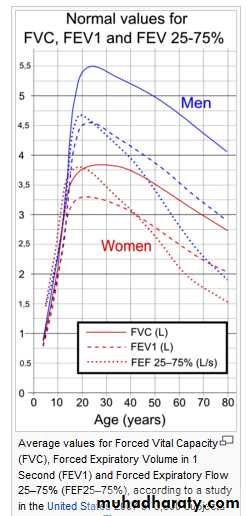
Average values for FEV1 in healthy people depend mainly on sex and age. Values of between 80% and 120% of the average value is considered normal.
FEV1/FVC ratio (FEV1%)
In healthy adults this should be approximately 75–80%. In obstructive diseases (asthma, COPD, chronic bronchitis, emphysema) FEV1 is diminished because of increased airway resistance to expiratory flow and the FVC may be decreased (for instance by premature closure of airway in expiration). This generates a reduced value (<80%, often ~45%).In restrictive diseases (such as pulmonary fibrosis) the FEV1 and FVC are both reduced proportionally and the value may be normal or even increased as a result of decreased lung compliance.
A derived value of FEV1% is
FEV1% predicted, which is defined as FEV1% of the patient divided by the average FEV1% in the population for any person of similar age, sex and body composition.
Forced Expiratory Flow (FEF)
Forced Expiratory Flow (FEF) is the flow (or speed) of air coming out of the lung during the middle portion of a forced expiration. It can be given at discrete times, generally defined by what fraction remains of the (FVC). The usual intervals are 25%, 50% and 75% (FEF25, FEF50 and FEF75), or 25% and 50% of FVC. It can also be given as a mean of the flow during an interval, also generally delimited by when specific fractions remain of FVC, usually 25–75% (FEF25–75%).Average ranges in the healthy population depend mainly on sex and age, with FEF25–75% .Values ranging from 50-60% and up to 130% of the average are considered normal.
MMEF or MEF stands for maximal (mid-expiratory flow ) and is the peak of expiratory flow as taken from the flow-volume curve and measured in liters per second. It should theoretically be identical to peak expiratory flow (PEF), which is, however, generally measured by a peak flow meter and given in liters per minute.
FEF 25–75% or 25–50% gives an indication of what is happening in the lower airways. It is a more sensitive parameter and not as reproducibles as the others. It is a useful serial measurement because it will be affected before FEV, so can act as an early warning sign of small airway disease. In small airway diseases such as asthma this value will be reduced, it could be more than 65% less than expected value.
Forced Inspiratory Flow 25–75% or 25–50%
Forced Inspiratory Flow 25–75% or 25–50% (FIF 25–75% or 25–50%) is similar to FEF 25–75% or 25–50% except the measurement is taken during inspiration.Peak Expiratory Flow (PEF) is the maximal flow achieved during the maximally forced expiration initiated at full inspiration, measured in liters per minute.
Tidal volume (TV)
Is the specific volume of air drawn into, and then expired out of, the lungs during normal tidal breathing.
Total Lung Capacity (TLC)
Is the maximum volume of air present in the lungs
Diffusion capacity (DLCO)
Is the carbon monoxide uptake from a single inspiration in a standard time (usually 10 sec). Since air consists of very minute or trace quantities of CO, 10 seconds is considered to be the standard time for inhalation, then rapidly blow it out .The exhaled gas is tested to determine how much of the tracer gas was absorbed during the breath. This will pick up diffusion impairments, for instance in pulmonary fibrosis.This must be corrected for anemia (because rapid CO diffusion is dependent on hemoglobin in RBC's; a low hemoglobin concentration, anemia, will reduce DLCO) and pulmonary hemorrhage (excess RBC's in the interstitium or alveoli can absorb CO and artificially increase the DLCO capacity).
Maximum Voluntary Ventilation (MVV)
Is a measure of the maximum amount of air that can be inhaled and exhaled within one minute. For the comfort of the patient this is done over a 15 second time period before being extrapolated to a value for one minute expressed as liters/minute. Average values for males and females are 140-180 and 80-120 liters per minute respectively.Static lung compliance (Cst)
When estimating static lung compliance, volume measurements by the spirometer needs to be complemented by pressure transducers in order to simultaneously measure the transpulmonary pressure. When having drawn a curve with the relations between changes in volume to changes in transpulmonary pressure, Cst is the slope of the curve during any given volume, or, mathematically, ΔV/ΔP.
Static lung compliance is perhaps the most sensitive parameter for the detection of abnormal pulmonary mechanics. It is considered normal if it is 60% to 140% of the average value in the population for any person of similar age, sex and body composition.
In those with acute respiratory failure on mechanical ventilation, "the static compliance of the total respiratory system is conventionally obtained by dividing the tidal volume by the difference between the "plateau" pressure measured at the airway opening (PaO) during an occlusion at end-inspiration and positive end-expiratory pressure (PEEP) set by the ventilator".
Others
Forced Expiratory Time (FET)Forced Expiratory Time (FET) measures the length of the expiration in seconds.Slow Vital capacity (SVC)Slow Vital capacity (SVC) is the maximum volume of air that can be exhaled slowly after slow maximum inhalation.
Maximal pressure (Pmax and Pi)Pmax is the asymptotically maximal pressure that can be developed by the respiratory muscles at any lung volume and Pi is the maximum inspiratory pressure that can be developed at specific lung volumes.This measurement also requires pressure transducers in addition. It is considered normal if it is 60% to 140% of the average value in the population for any person of similar age, sex and body composition.
A derived parameter is the coefficient of retraction (CR) which is Pmax/TLC <hedenstrom2009/>
Mean transit time (MTT)Mean transit time is the area under the flow-volume curve divided by the forced vital capacity.
A plethysmograph
Is an instrument for measuring changes in volume within an organ or whole body (usually resulting from fluctuations in the amount of blood or air it contains).Plethysmograph or "body box" used in lung measurements
Organs studied
LungsPulmonary plethysmographs are commonly used to measure the functional residual capacity (FRC) of the lungs—the volume in the lungs when the muscles of respiration are relaxed—and total lung capacity.
In a traditional plethysmograph, the test subject is placed inside a sealed chamber the size of a small telephone booth with a single mouthpiece. At the end of normal expiration, the mouthpiece is closed.
The patient is then asked to make an inspiratory effort.
As the patient tries to inhale (a maneuver which looks and feels like panting), the lungs expand, decreasing pressure within the lungs and increasing lung volume. This, in turn, increases the pressure within the box since it is a closed system and the volume of the box compartment has decreased to accommodate the new volume of the subject.
Boyle's Law is used to calculate the unknown volume within the lungs. First, the change in volume of the chest is computed. The initial pressure and volume of the box are set equal to the known pressure after expansion times the unknown new volume.
Once the new volume is found, the new volume minus the original volume is the change in volume in the box and also the change in volume in the chest. With this information, Boyle's Law is used again to determine the original volume of gas: the initial volume (unknown) times the initial pressure is equal to the final volume times the final pressure.
The difference between full and empty lungs can be used to assess diseases and airway passage restrictions. An obstructive disease will show increased FRC because some airways do not empty normally, while a restrictive disease will show decreased FRC.
Body plethysmography is particularly appropriate for patients who have air spaces which do not communicate with the bronchial tree; in such patients gas dilution would give an incorrectly low reading.
Newer lung plethysmograph devices have an option which does not require enclosure in a chamber.
Limbs
Some plethysmograph devices are attached to arms, legs or other extremities and used to determine circulatory capacity. In water plethysmography an extremity, e.g. an arm, is enclosed in a water-filled chamber where volume changes can be detected. Air plethysmography uses a similar principle but based on an air-filled long cuff, which is more convenient but less accurate.Another practical device is mercury-filled strain gauges used to continuously measure circumference of the extremity, e.g. at mid calf.
Impedance plethysmography is a non-invasive method used to detect venous thrombosis in these areas of the body.
Genitals
penile plethysmograph. This device is used to measure changes in blood flow in the penis. Although some researchers use this device to assess sexual arousal and sexual orientation, the data are usually not admissible in court cases in the United States. An approximate female equivalent to penile plethysmography is vaginal photoplethysmography, which optically measures blood flow in the vagina.A man undergoing whole body plethysmography
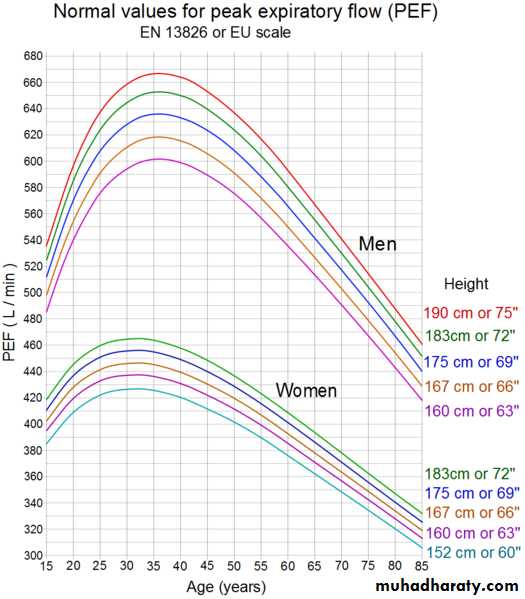
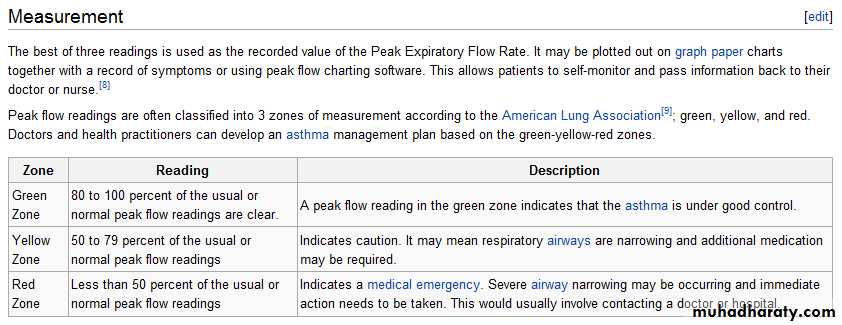
Peak expiratory flowThe peak expiratory flow (PEF), also called peak expiratory flow rate (PEFR) is a person's maximum speed of expiration, as measured with a peak flow meter, a small, hand-held device used to monitor a person's ability to breathe out air. It measures the airflow through the bronchi and thus the degree of obstruction in the airways.
A peak flow meter
European scale
Function
Peak flow readings are higher when patients are well, and lower when the airways are constricted. From changes in recorded values, patients and doctors may determine lung functionality, severity of asthma symptoms, and treatment options.First measure of precaution would be to check patient for signs and symptoms of asthmatic hypervolemia. This would indicate whether or not to even continue with the Peak Flow Meter procedure. Measurement of PEFR requires training to correctly use a meter and the normal expected value depends on a patient's sex, age and height. It is classically reduced in obstructive lung disorders such as asthma.
Due to the wide range of ‘normal' values and high degree of variability, peak flow is not the recommended test to identify asthma. However, it can be useful in some circumstances.
A small proportion of people with asthma may benefit from regular peak flow monitoring. When monitoring is recommended, it is usually done in addition to reviewing asthma symptoms and frequency of reliever medication use.
Nitrogen washout (or Fowler's method) is a test for measuring dead space in the lung during a respiratory cycle, as well as some parameters related to the closure of airways.
Procedure
A nitrogen washout can be performed with a single nitrogen breath, or multiple ones. Both tests use similar tools, both can estimate functional residual capacity and the degree of nonuniformity of gas distribution in the lungs, but the multiple-breath test more accurately measures absolute lung volumes.The following describes a single-breath nitrogen test:
A subject takes a breath of 100% oxygen and exhales through a one-way valve measuring nitrogen content and volume. A plot of the nitrogen concentration (as a % of total gas) vs. expired volume is obtained by increasing the nitrogen concentration from zero to the percentage of nitrogen in the alveoli.The nitrogen concentration is initially zero because the subject is exhaling the dead space oxygen they just breathed in (does not participate in alveolar exchange), and climbs as alveolar air mixes with the dead space air.
The dead space can be determined from this curve by drawing a vertical line down the curve such that the areas below the curve (left of the line) and above the curve (right of the line) area equal.
Most people with a normal distribution of airways resistances will reduce their expired end-tidal nitrogen concentrations to less than 2.5% within seven minutes. Individuals with high resistance in their airways can take longer than seven minutes to remove all the nitrogen.
Parameters
A nitrogen washout can obtain the following parameters:Closing volume (CV); the amount of air remaining in the lungs when the flow from the lower sections of the lungs becomes severely reduced or halts altogether during expiration as the small airways begin to close.
Closing capacity (CC), which equals CV + (TLC - VC), with VC taken from the curve acquired from the nitrogen washout test. As a reference, it should be 70% to 130% of what is the average value in the population, which, in turn, may vary with geographic location.
Mean slope of the alveolar plateau (phase III). It should be less than 175% of population average.
Also, from those values, additional parameters can be calculated:
CV/VC ratio
CC/TLC ratio
Both of the above should be less than 125% of population average.
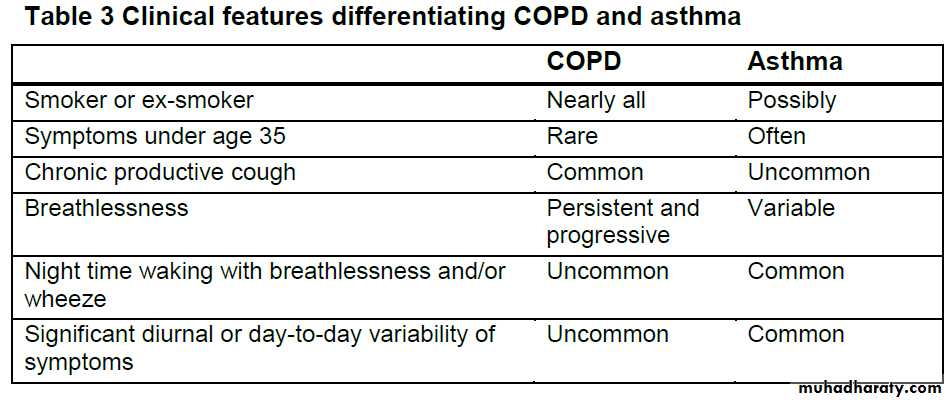
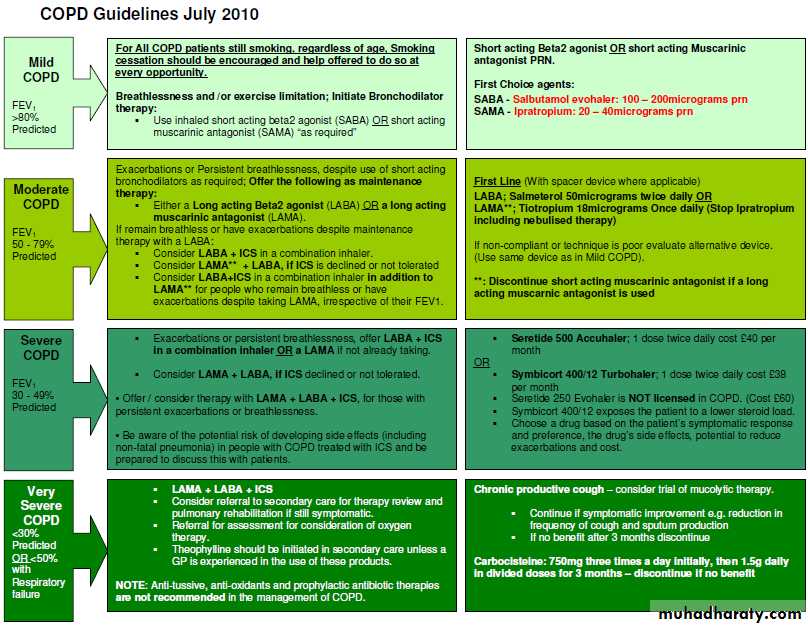
Consider a diagnosis of COPD in patients aged over 35 years who have a risk factor (generally smoking) and present with exertional breathlessness, chronic cough, regular sputum production, frequent winter "bronchitis" or wheeze.
Measure post-bronchodilator spirometry to confirm the diagnosis of COPD
BMJ 2010
Assessing severity of COPDContinuing advice to use a "fixed ratio" FEV1/FVC in spirometry rather than a lower limit of normal may overestimate COPD in older individuals and underestimate it in young adults, but remains more practical and easy to apply and avoids the need to use reference equations, which are not validated for some ethnic populations.
Clinical skills are paramount in interpreting spirometry, particularly in assessing patients with symptoms suggesting COPD and asymptomatic people identified by screening spirometry.
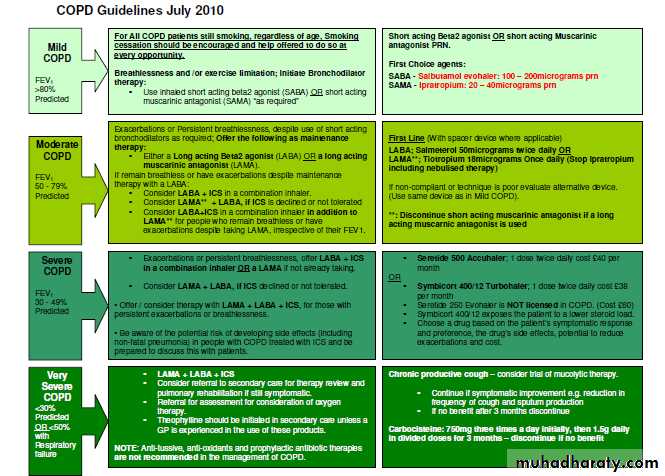
• Inhaled therapyThe updated guidance recommends long acting muscarinic antagonists rather than regular short acting muscarinic antagonists (SAMAs) in maintenance therapy on the basis of clinical and cost effectiveness evidence—a change from the previous guideline, in which both were options.
The use of an inhaled corticosteroid is recommended only in a combination inhaler with a long acting muscarinic antagonist.
The clinical and cost effectiveness evidence for the use of LABA+ICS over LABA was strong in people with severe or very severe airflow obstruction (FEV1 <50%).
Evidence was weaker in those with mild or moderate airflow obstruction (FEV1 50), leading to a weaker recommendation only to consider LABA+ICS treatment if symptoms persisted with LABA alone. This must, however, clearly be prescribed within the drug licence.
Limited evidence for addition of LABA+ICS in those who are still symptomatic despite taking LAMA alone led to a recommendation only to consider triple therapy in this situation.
The recommendation to offer LAMA in addition to LABA+ICS (triple therapy) to people with COPD who remain breathless or have exacerbations despite taking LABA+ICS, irrespective of their FEV1, was based on stronger clinical evidence.
Mucolytic drugsDo not routinely use mucolytic drugs to prevent exacerbations in people with stable COPD. The guidelines give clear guidance on avoiding possible inappropriate use of mucolytics in the absence of a robust evidence base, as the different study outcomes reflect differences in patient populations and background treatments.
Research recommendationsOn the basis of its review of evidence, the GDG has made the following research recommendations to improve NICE guidance and patient care in the future.
More information about why these were consideredimportant is available in the full guideline.
In people with COPD does pulmonary rehabilitation during hospital admission for exacerbation and/or in the early recovery period (within one month of an exacerbation) improve quality of life and reduceadmissions to hospital and exacerbations compared with a later (defined as after one month) pulmonary rehabilitation programme?
Could a simple multidimensional assessment be used to give a better indication of COPD outcomes than either FEV1 or other components measured alone in a wide range of patients with COPD, and would it be applicable in a primary care setting?
In people with COPD, does triple therapy (LABA, LAMA, and ICS) lead to better outcomes than single therapy (LAMA or LABA) or double therapy (LABA and ICS)?
In people with COPD, does mucolytic drug therapy prevent exacerbations in comparison with placebo and other therapies?
BMJ 2010
NICE (National Institute for Health and Clinical Excellence) clinical guidelines
are recommendations about the treatment and care of people with specific diseases and conditions in the NHS in England and Wales.This guidance represents the view of NICE, which was arrived at after careful consideration of the evidence available. Healthcare professionals are expected to take it fully into account when exercising their clinical judgement.Implementation of this guidance is the responsibility of local commissioners and/or providers. Commissioners and providers are reminded that it is their responsibility to implement the guidance, in their local context, in light of their duties to avoid unlawful discrimination and to have regard to promoting
equality of opportunity. Nothing in this guidance should be interpreted in a way that would be inconsistent with compliance with those duties.
Working definition of COPD
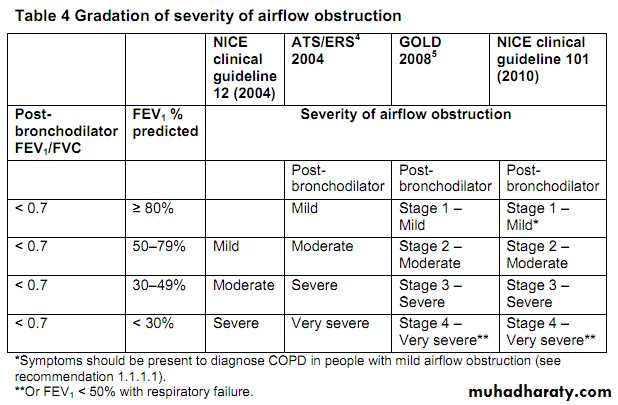
COPD is characterised by airflow obstruction that is not fully reversible. The airflow obstruction does not change markedly over several months and is usually progressive in the long term. COPD is predominantly caused by smoking. Other factors, particularly occupational exposures, may also contribute to the development of COPD. Exacerbations often occur, where there is a rapid and sustained worsening of symptoms .The following should be used as a definition of COPD:
Airflow obstruction is defined as a reduced FEV1/FVC ratio (where FEV1 is forced expired volume in 1 second and FVC is forced vital capacity), such that FEV1/FVC is less than 0.7.If FEV1 is ≥ 80% predicted normal a diagnosis of COPD should only be made in the presence of respiratory symptoms, for example breathlessness or cough.
The airflow obstruction is present because of a combination of airway and parenchymal damage. The damage is the result of chronic inflammation that differs from that seen in asthma and which is usually the result of tobacco smoke.
Significant airflow obstruction may be present before the person is aware of it.
There is no single diagnostic test for COPD. Making a diagnosis relies on
clinical judgement based on a combination of history, physical examinationand confirmation of the presence of airflow obstruction using spirometry.
Diagnose COPD
A diagnosis of COPD should be considered in patients over the age of 35 who have a risk factor (generally smoking) and who present with exertional breathlessness, chronic cough, regular sputum production, frequent winter ‘bronchitis’ or wheeze.
The presence of airflow obstruction should be confirmed by performing post-bronchodilator spirometry.
Stop smoking
Encouraging patients with COPD to stop smoking is one of the most important components of their management. All COPD patients still smoking, regardless of age, should be encouraged to stop, and offered help to do so, at every opportunity.Promote effective inhaled therapy
In people with stable COPD who remain breathless or have exacerbations despite use of short-acting bronchodilators as required, offer the followingas maintenance therapy:
if FEV1 ≥ 50% predicted: either long-acting beta2 agonist (LABA) or (LAMA)
if FEV1 < 50% predicted: either LABA with an inhaled corticosteroid (ICS) in a combination inhaler, or LAMA (longacting muscarinic antagonist)
Offer LAMA in addition to LABA+ICS to people with COPD who remain breathless or have exacerbations despite taking LABA+ICS, irrespective of their FEV1.
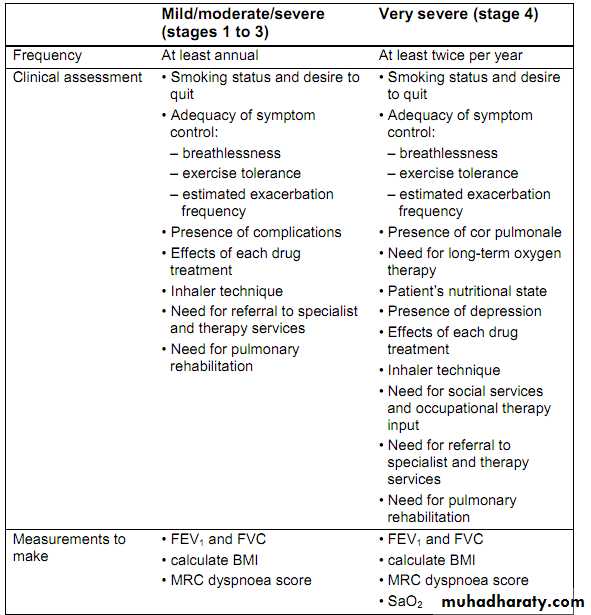
Provide pulmonary rehabilitation for all who need it
Pulmonary rehabilitation should be made available to all appropriate peoplewith COPD including those who have had a recent hospitalisation for an
acute exacerbation.
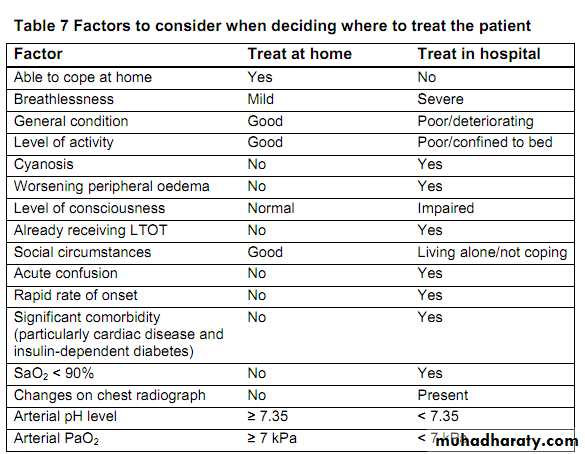
Use non-invasive ventilation
treatment of choice for persistent hypercapnic ventilatory failure during exacerbations notresponding to medical therapy. It should be delivered by staff trained in its application, experienced in its use and aware of its limitations.
When patients are started on NIV, there should be a clear plan covering what to do in the event of deterioration and ceilings of therapy should be agreed.
Manage exacerbations
Reduced by appropriate use of inhaled corticosteroids and bronchodilators, and vaccinations.
The impact of exacerbations should be minimised by:
giving self-management advice on responding promptly to the symptoms of an exacerbation starting appropriate treatment with oral steroids and/or antibiotics use of non-invasive ventilation when indicated.
Table 1 MRC dyspnoea scale
Grade Degree of breathlessness related to activities1 Not troubled by breathlessness except on strenuous exercise
2 Short of breath when hurrying or walking up a slight hill
3 Walks slower than contemporaries on level ground because of breathlessness, or has to stop for breath when walking at own pace
4 Stops for breath after walking about 100 metres or after a few minutes on level ground
5 Too breathless to leave the house, or breathless when dressing or undressing
To help resolve cases where diagnostic doubt remains, or both
COPD and asthma are present, the following findings should be used to help identify asthma:• a large (> 400 ml) response to bronchodilators
• a large (> 400 ml) response to 30 mg oral prednisolone daily for2 weeks
• serial peak flow measurements showing 20% or greater diurnal or day-to-day variability.
Clinically significant COPD is not present if the FEV1 and FEV1/FVC ratio return to normal with drug therapy.
Spirometry
Spirometry should be performed:• at the time of diagnosis
• to reconsider the diagnosis, if patients show an exceptionally
good response to treatment.
Measure post-bronchodilator spirometry to confirm the diagnosis of COPD.
Consider alternative diagnoses or investigations in:
older people without typical symptoms of COPD where the
FEV1/FVC ratio is < 0.7
younger people with symptoms of COPD where the FEV1/FVC
ratio is ≥ 0.7.
At the time of their initial diagnostic evaluation in addition to spirometry all patients should have:
a chest radiograph to exclude other pathologies
a full blood count to identify anaemia or polycythaemia
body mass index (BMI) calculated
Reversibility testing
Routine spirometric reversibility testing is not necessary as a part of the diagnostic process or to plan initial therapy with bronchodilators or corticosteroids. It may be unhelpful or misleading because:repeated FEV1 measurements can show small spontaneous fluctuations the results of a reversibility test performed on different occasions can be inconsistent and not reproducible over-reliance on a single reversibility test may be misleading unless the change in FEV1 is greater than 400 m l
Oral theophylline
Theophylline should only be used after a trial of short-acting bronchodilators and long-acting bronchodilators, or in patients who are unable to use inhaled therapy, as there is a need to monitor plasma levels and interactions. Particular caution needs to be taken with the use of theophylline in older people because of differences in pharmacokinetics, the increased likelihood of comorbidities and the use of othermedications.
The effectiveness of the treatment with theophylline should be assessed by improvements in symptoms, activities of daily living, exercise capacity and lung function.
The dose of theophylline prescribed should be reduced at the time of an exacerbation
if macrolide or fluroquinolone antibiotics or
other drugs known to interact) are prescribed.
Oral mucolytic therapy
Mucolytic drug therapy should be considered in patients with a chronic cough productive of sputum Mucolytic therapy should be continued if there is symptomatic improvement (for example, reduction in frequency of cough and Do not routinely use mucolytic drugs to prevent exacerbations in people with stable COPD.Oral anti-oxidant therapy
Treatment with alpha-tocopherol and beta-carotene supplements, alone or in combination, is not recommended.Anti-tussive therapy should not be used in the management of stable COPD.
There is insufficient evidence to recommend prophylactic antibiotic therapy in the management of stable COPD.Oxygen
Long-term oxygen therapy (LTOT) Clinicians should be aware that inappropriate oxygen therapy in people with COPD may cause respiratory depression. LTOT is indicated in patients with COPD who have a PaO2 <7.3 kPa when stable or a PaO2 >7.3 and less than 8 kPa when stable and one of: secondary polycythaemia, nocturnal hypoxaemia (oxygen saturation of arterial blood [SaO2] <90% for more than 30% of the time), peripheral oedema or pulmonary hypertension
To get the benefits of LTOT patients should breathe supplemental oxygen for at least 15 hours per day.
Greater benefits are seen in patients receiving oxygen for 20 hours per day.
The need for oxygen therapy should be assessed in:all patients with very severe airflow obstruction (FEV1 < 30% predicted) patients with
cyanosis,
polycythaemia
peripheral oedema
raised jugular venous pressure
oxygen saturations ≤ 92% breathing air.
Severe airflow obstruction (FEV1 30–49% predicted).
Ambulatory oxygen therapy
People who are already on LTOT who wish to continue with oxygen therapy outside the home, and who are prepared to use it, should have ambulatory oxygen prescribed. Ambulatory oxygen therapy should be considered in patients who have exercise desaturation, are shown to have an improvement in exercise capacity and/or dyspnoea with oxygen, and have themotivation to use oxygen.
Ambulatory oxygen therapy is not recommended in COPD if PaO2 is greater than 7.3 kPa and there is no exercise desaturation
Short-burst oxygen therapy
should only be considered for episodes of severe breathlessness in patients with COPD not relieved by other treatments.Short-burst oxygen therapy should only continue to be prescribed if an improvement in breathlessness following therapy has been
documented.
Non-invasive ventilation
Adequately treated patients with chronic hypercapnic respiratory failure who have required assisted ventilation (whether invasive or non-invasive) during an exacerbation or who are hypercapnic or acidotic on LTOT should be referred to a specialist centre for consideration of long-term NIV.
Management of pulmonary hypertension and cor pulmonale
This clinical syndrome of cor pulmonale includes patientswho have right heart failure secondary to lung disease and those in whom the primary pathology is retention of salt and water, leading to the development of peripheral oedema.
A diagnosis of cor pulmonale should be considered if patients have:
• peripheral oedema
• a raised venous pressure
• a systolic parasternal heave
• a loud pulmonary second heart sound.
It is recommended that the diagnosis of cor pulmonale is made clinically and that this process should involve excluding other
causes of peripheral oedema.
Treatment of cor pulmonale
Patients presenting with cor pulmonale should be assessed for the need for long-term oxygen therapy. Oedema associated with cor pulmonale can usually be controlled symptomatically with diuretic therapy.The following are not recommended for the treatment of cor pulmonale:
• angiotensin-converting enzyme inhibitors
• calcium channel blockers
• alpha-blockers
• digoxin (unless there is atrial fibrillation).
Pulmonary rehabilitation
defined as a multidisciplinary programme of care
for patients with chronic respiratory impairment that is individually tailored and designed to optimise each patient’s physical and social performance and
Autonomy.Pulmonary rehabilitation should be made available to all appropriate people with COPD including those who have had a recent hospitalisation for an acute exacerbation. Pulmonary rehabilitation should be offered to all patients who consider themselves functionally disabled by COPD
(usually MRC grade 3 and above).
Pulmonary rehabilitation is not suitable for
patients who are unable to walk, have unstable angina or who have had a recent myocardial infarction.Pulmonary rehabilitation programmes should include
multicomponent, multidisciplinary interventions, which are tailored to the individual patient’s needs. The rehabilitation process should incorporate a programme of physical training, disease education, nutritional, psychological and behavioural intervention. Patients should be made aware of the benefits of pulmonary rehabilitation and the commitment required to gain these.Vaccination and anti-viral therapy
Pneumococcal vaccination and an annual influenza vaccinationshould be offered to all patients with COPD
Lung surgery
Patients who are breathless, and have a single large bulla on a CT scan and an FEV1 less than 50% predicted should be referred for
consideration of bullectomy.
Patients with severe COPD who remain breathless with marked restrictions of their activities of daily living, despite maximal medical therapy (including rehabilitation), should be referred for consideration of lung volume reduction surgery if they meet all of the following criteria:
• FEV1 more than 20% predicted
• PaCO2 less than 7.3 kPa
• upper lobe predominant emphysema
• TLCO more than 20% predicted.
Patients with severe COPD who remain breathless with marked restrictions of their activities of daily living despite maximal medical therapy should be considered for referral for assessment for lung
transplantation bearing in mind comorbidities and local surgical protocols. Considerations include:
age
FEV1
PaCO2
homogeneously distributed emphysema on CT scan
elevated pulmonary artery pressures with progressive
deterioration.
Alpha-1 antitrypsin replacement therapy
Alpha-1 antitrypsin replacement therapy is not recommended for patients with alpha-1 antitrypsin deficiencyNutritional factors
BMI should be calculated in patients with COPD:the normal range for BMI is 20 to less than 25 if the BMI is abnormal (high or low), or changing over time, the patient should be referred for dietetic advice if the BMI is low patients should also be given nutritional supplements to increase their total calorific intake and be encouraged to take exercise to augment the effects of nutritionalsupplementation.
Definition of an exacerbation
Asustained worsening of the patient’s symptoms from their usual stable state which is beyond normal day-to-day variations, and is acute in onset. Commonly reported symptoms are worsening breathlessness, cough, increased sputum production and change in sputum colour. The change in these symptoms often necessitates a change in medication.sending sputum samples for culture is not recommended in routine practice pulse oximetry is of value if there are clinical features of a severe exacerbation. all patients with an exacerbation referred to hospital:
a chest radiograph should be obtained ,arterial blood gas tensions should be measured and the inspired ,oxygen concentration should be recorded an ECG should be recorded (to exclude comorbidities)
a full blood count should be performed and urea and electrolyte concentrations should be measured a theophylline level should be measured in patients on theophylline therapy at admission if sputum is purulent, a sample should be sent for microscopy
and culture blood cultures should be taken if the patient is pyrexial.
If a patient is hypercapnic or acidotic the nebuliser should be driven by compressed air, not oxygen (to avoid worsening hypercapnia). If oxygen therapy is needed it should be administered simultaneously by nasal cannulae. The driving gas for nebulised therapy should always be specified in the prescription.
In the absence of significant contraindications oral corticosteroids should be used, in conjunction with other therapies, in all patients admitted to hospital with an exacerbation of COPD.
In the absence of significant contraindications, oral corticosteroids should be considered in patients in the community who have an exacerbation with a significant increase in breathlessness which interferes with daily activities. Patients requiring corticosteroid therapy should be encouraged to present early to get maximum benefits .Prednisolone 30 mg orally should be prescribed for 7 to 14 days.It is recommended that a course of corticosteroid treatment should not be longer than 14 days as there is no advantage in prolonged Therapy.Osteoporosis prophylaxis should be considered in patients requiring frequent courses of oral corticosteroids.
Antibiotics should be used to treat exacerbations of COPD
associated with a history of more purulent sputum.Patients with exacerbations without more purulent sputum do notneed antibiotic therapy unless there is consolidation on a chest
radiograph or clinical signs of pneumonia.
Respiratory stimulants
It is recommended that doxapram is used only when non-invasive ventilation is either unavailable or considered inappropriate.In physiology, dead space is air that is inhaled by the body in breathing, but does not take part in gas exchange. Not all the air in each breath is able to be used for the exchange of oxygen and carbon dioxide. About a third of every resting breath has no change in O2 and CO2 levels. In adults, it is usually in the range of 150 mL.
Because of dead space, taking deep breaths more slowly (e.g. ten 500 mL breaths per minute) is more effective than taking shallow breaths quickly (e.g. twenty 250 mL breaths per minute). Although the amount of gas per minute is the same (5 L/min), a large proportion of the shallow breaths is dead space, and does not allow oxygen to get into the blood.
Dead space can be enlarged (and better envisaged) by breathing into a long tube. Even though one end of the tube is open to the air, when one inhales, it is mostly the carbon dioxide from expiration. Using a snorkel increases a diver's dead space in the airways.
Total dead space (also known as "physiological" dead space) can be divided into anatomical dead space and alveolar dead space.
Anatomical dead space
Anatomical dead space is the gas in the conducting areas of the respiratory system, such as the mouth and trachea, where air does not come into contact with the alveoli of the lungs.
Alveolar dead space
Caused by air contacting alveoli without bloodflow in their adjacent pulmonary capillaries, i.e. ventilation without perfusion. As a result, no gas exchange can occur. Alveolar dead space is negligible in healthy individuals, but can increase dramatically in some lung diseases.Overview
Anatomical and alveolar dead space can both be measured using the Bohr equation.Formally, Bohr's method is used to calculate the former. In practice, it is more commonly used to calculate the latter.The Bohr equation states that the dead space (Vd) is calculated as follows:
where Vd is dead space volume, Vt is tidal volume, PaCO2 is the partial pressure of carbon dioxide in the arterial blood, and PeCO2 is the partial pressure of carbon dioxide in the expired air.
When calculating the:
physiological dead space, a large plastic bag (that is, a Douglas bag) is used to collect all of the patients expired gas, and the total expired CO2 is measured as a fraction of total expired gas. This gives PeCO2, which is then substituted into the Bohr equation.
alveolar dead space, the end-tidal CO2 in capnography is used as a surrogate for the expired CO2. PetCO2 is then used instead of PeCO2 in the Bohr equation.
For patients with (COPD) or cardiogenic pulmonary edema, NPPV should be the first choice. It may also be used after surgery or in immunocompromised individuals.
In the absence of shock or acute coronary syndrome requiring urgent coronary revascularization, patients with cardiogenic pulmonary edema and respiratory failure should receive either NPPV or CPAP.
New Clinical Practice Guidelines Issued for Noninvasive Ventilation
Medscape Medical News © 2011
Patient undergoing noninvasive positive pressure ventilation.
Patients with a severe exacerbation of COPD, defined as a pH of less than 7.35 and relative hypercarbia, should have NPPV in addition to usual care.For patients with cardiogenic pulmonary edema, CPAP delivered by mask appears to be as effective as NPPV.
CPAP should not be used in patients who have acute lung injury.
In centers with extensive experience in the use of NPPV, a trial of early extubation to NPPV may be considered for patients with COPD.
In patients considered to be at low risk for respiratory failure, NPPV should not be used after planned extubation.
NPPV should not be routinely used in patients who do not have COPD and in those who have postextubation respiratory failure.
CPAP may be used in patients who have respiratory failure after abdominal surgery.
NPPV may be used in patients who have respiratory failure after lung resection surgery.
Patients with acute respiratory failure who are receiving NPPV should use an oronasal rather than a nasal mask.
• Mostly an acquired bronchopulmonary disorder with abnormal thickening of the bronchial wall and dilation of central and medium sized bronchi, due to a vicious circle of transmural infection and inflammation with mediator release. it is idiopathic in about 50% of adults and 25% of children.
Bronchiectasis
CauseNo (%) (n=150)
Idiopathic
80 (53)
Infection
44 (29)
Immune defect
12 (8)
Congenital
1 (<1)
Condition
Allergic bronchopulmonary aspergillosis
11 (7)Aspiration
6 (4)
Young’s syndrome
5 (3)
Cystic fibrosis
4 (3)
Rheumatoid arthritis
4 (3)
Ciliary dysfunction
3 (2)
Ulcerative colitis
2 (<1)
Panbronchiolitis
1 (<1)
• Causes of and conditions associated with bronchiectasis1
In the United States the prevalence of non-cystic fibrosis bronchiectasis among adults between 1999 and 2001 was estimated to be 51 per 100 000 population The prevalence was higher among women than men (71 v 32 per 100 000). In the United Kingdom, a full time, single handed practice of 2500 patients will have one or two patients with bronchiectasis, compared with 75 with asthma and 50 with chronic obstructive pulmonary disease.
prevalence
Why is it missed?
Symptoms of bronchiectasis (such as chronic cough and sputum production) may be mild, particularly at the beginning. These symptoms may resemble asthma, chronic obstructive pulmonary disease, rhinosinus diseases, tracheobronchial infection, and gastroesophageal reflux, which are much more common than bronchiectasis in an average UK general practice.Basal crackles may suggest heart failure. Spirometry may showcoexisting airway obstruction, leading to a misdiagnosis of chronic obstructive pulmonary disease instead of bronchiectasis. Chest x rays sometimes do not show mild bronchiectasis.
Why does it matter?
Patients with newly diagnosed bronchiectasis were found to have a poorer prognosis than those with asthma (matched for age and sex), but a better prognosis than those with chronic obstructive pulmonary disease. Over a follow-up period of 8.8 years, 25% of 372 Finnish patients with bronchiectasis died (mean age at the start of the follow-up was 56 years).• How is it diagnosed?
• Clinical featuresCough is present in 90% of the patients, and 76% produce sputum daily. Haemoptysis occurs in 51% of patients and tends to be recurrent. The usual abnormalities on chest examination are crackles (70%) and wheezes (34%).InvestigationsRequesting sputum cultures in patients with frequent respiratory infections may help to identify unusual infections that increase the suspicion of bronchiectasis. Chest x rays may show linear markings, crowding, cystic spaces, and honeycombing.In a small study the sensitivity for chest x rays to detect bronchiectasis was found to be 88%, with a specificity of 74%.
A normal chest x ray does not exclude bronchiectasis definitively. So, if there is a high clinical suspicion for bronchiectasis, a high resolution computed tomography scan of the chest should be considered. This scan has replaced contrast bronchography as optimal for diagnosis of bronchiectasis. Major features are airway dilation, lack of tapering (figure)thickening of the bronchial wall, mucopurulent plugs or debris, and cysts.
Thesensitivity of the scan is 82-97% and the specificity 99-100%.
Specific tests to identify underlying causes or contributing conditions depend on the clinical setting and the patient’s history and age. Tests might include a white blood cell count and differentiation, IgG, IgG subclasses, IgM, IgA, total IgE, α1 antitrypsin levels, rheumatoid factor, and aspergillus serology.High resolution computed tomography scan of chest of a young non-smoking woman with mild bronchiectasis. White arrows indicate airway dilation and lack of tapering
How is it managed?
The evidence base for management is contentious, but expert consensus advocates advising patients who smoke to quit. Treat acute exacerbations with short courses (<4 weeks) of antibiotics targeted to likely organisms such as Pseudomonas aeruginosa, Haemophilus influenza, or Mycobacterium avium-intracellulare; or on basis of the results of sputum culture.Consider prolonged use of antibiotics (>4 weeks) although the criteria fordoing so are not well established, we believe this might be beneficial in patients who often relapse (more than six times per year) or show progressive decline of lung function.
The effectiveness of continuous mucolytics, anti-inflammatory agents, and bronchodilators is not clear but can be considered on individual basis. Physical therapy techniques that improve bronchial clearance of mucus are widely used, yet there is little evidence to support or refute them.
Adding the anticholinergic agent tiotropium bromide, approved for the treatment of (COPD), to low-dose, inhaled glucocorticoid therapy is more effective at improving symptoms and lung function than doubling the corticosteroid dose in controlling asthma.
Also, the addition of tiotropium is as effective as the addition of the long-acting β-agonist (LABA) salmeterol to an inhaled corticosteroid, according to study results released by the US National Heart, Lung, and Blood Institute (NHLBI).
European Respiratory Society (ERS) 2010 Annual Congress
September 19, 2010. N Engl J Med
Although there was initially concern about cardiovascular risks with all anticholinergics, safety trials demonstrated that there is no increased cardiovascular risk associated with tiotropium.
However, data indicate that ipratropium is associated with a slight risk for excess cardiovascular mortality.
Both Spiriva (Tiotropium) and Ipratropium (Atrovent) are anticholinergic agents that are used for maintenance treatment of bronchospasm associated with COPD. Spiriva has an additional indication for reducing COPD exacerbations based on the outcomes of the large UPLIFT clinical trial. Both medications are not indicated for acute treatment of a bronchospasm and should never be used as rescue medications. The major difference between the two drugs is that Spiriva is a long acting medication and Ipratropium is a short acting drug. Spiriva is used once daily, while Ipratropium may be used every 4 to 6 hours.
In general Ipratropium is used as-needed for persistent or worsening symptoms. When regular use is needed, long-acting bronchodilator like Spiriva may be more effective and convenient than short-acting agent. Both medications share a similar adverse effect profiles. As anticholinergic drugs they may increase the risk of dry mouth and urinary retention. Both medications have a warning regarding the possible increase in intra-ocular pressure and increased risk of narrow angle glaucoma. It is recommended to avoid the contact between these medications and the eyes which may be harder to do with a nebulizer treatment.
Recently, some clinical studies raised the concern regarding he use of Ipratropium (Atrovent) and Spiriva and an increased risk of negative cardiovascular outcomes. In the case of Spiriva a large 4 year study (UPLIFT) conducted in 490 investigational centers in 37 countries did not show an increased risk of negative cardiovascular outcomes. There is some preliminary evidence that Ipratropium may increase risk of adverse cardiovascular effects, but more research with well designed clinical studies is needed to come to any definite conclusion.
Ipratropium bromide and tiotropium bromide are structural analogues of atropine which have minimal systemic absorption following inhalation because of their quaternary ammonium structure. These anticholinergic drugs are useful bronchodilators in chronic obstructive pulmonary disease. They are rarely indicated in asthma. Bronchodilators provide symptomatic relief and improve health-related quality of life in patients with chronic obstructive pulmonary disease, but they do not influence the decline in lung function. The only measure currently known to halt this decline is stopping cigarette smoking.
Animal studies show that cholinergic innervation is greatest in larger airways and diminishes peripherally. Studies in humans have shown that cholinergic bronchoconstriction occurs mainly in larger airways whereas bronchodilatation induced by beta adrenergic drugs occurs in both large and small airways. The resting bronchomotor tone in normal airways has a cholinergic component, because giving an anticholinergic drug such as atropine causes bronchodilatation while the inhalation of edrophonium, an acetylcholinesterase inhibitor, results in bronchoconstriction.
Muscarinic receptors
The effects of vagal stimulation in the lung are mediated via muscarinic receptors. mediate the mucus secretory response. Cholinergic agonists will stimulate mucus secretion from both submucosal glands and from goblet cells within the epithelium. These goblet cells are a major source of mucus in peripheral airways.The muscarinic receptors on airway smooth muscle belong to the M3 subtype and the presynaptic muscarinic receptors on vagal motor nerve fibres belong to the M2 subtype. These M2 receptors are called autoreceptors because their activation by acetylcholine inhibits further release of acetylcholine from the nerve terminals.
Anticholinergic bronchodilators
Atropine
Giving atropine, either systemically or as a nebulised solution, results in bronchodilatation. Inhaled doses of 2.5 mg atropine are associated with adverse effects such as dryness of the mouth, tachycardia, palpitations and blurred vision. With higher inhaled doses, systemic absorption can result in urinary retention (particularly in the elderly), headache and changes in mental status. Atropine is therefore no longer given as a nebulised solution.
Ipratropium bromide is a structural analogue of atropine, with a quaternary nitrogen structure. This structure reduces the ability of the molecule to cross cell membranes. There is, therefore, less systemic absorption with nebulised ipratropium than with nebulised atropine. Ipratropium blocks methacholine-induced bronchoconstriction, and induces bronchodilatation in patients with asthma and patients with (COPD). There are no measurable effects on sputum volume, sputum viscosity or mucociliary clearance with clinically recommended doses of ipratropium.
The maximal bronchodilatation with ipratropium, inhaled from a metered-dose inhaler, occurs with a dose of 40-80 microgram. Although some bronchodilatation is evident soon after inhalation the maximal response occurs 1.5-2.0 hours afterwards. The duration of significant bronchodilatation after a standard dose of ipratropium is 4-6 hours.
Ipratropium cannot be detected in the blood after an inhalation.. Long-term studies have shown no evidence of diminished responsiveness (tachyphylaxis) with regular therapy.
The main adverse effects of ipratropium relate to its anticholinergic activity. Up to 15% of patients will report transient dryness of the mouth and 'scratchiness' in the throat. In some studies up to 30% of patients have reported a bitter taste. These adverse effects rarely lead to patients discontinuing the drug if they perceive that it is helping them. Cardiovascular effects (tachycardia and increased cardiac output), are not seen with the usual doses of ipratropium.
The main clinical indication for ipratropium bromide is the symptomatic relief of breathlessness in patients with COPD. It is rarely required for the treatment of patients with asthma because proper treatment of asthmatic patients with inhaled corticosteroids and long-acting beta agonists provides good control for the majority of patients. The extent of bronchodilatation with ipratropium in patients with COPD is similar to that achieved with inhaled beta agonists. The choice between ipratropium and beta agonists for a patient with COPD is determined by the patient's tolerance of the drug, rather than its efficacy.
Tiotropium bromide is a structural analogue of ipratropium. In vitro studies have shown that tiotropium has a half-life on the M3 receptor of approximately 36 hours, whereas the receptor binding half-life of ipratropium is three hours. The duration of this binding to M3 receptors may explain why a single inhaled dose of tiotropium results in bronchodilatation which lasts for approximately 24 hours. Large-scale clinical trials have shown that tiotropium inhaled once daily increases the forced expiratory volume (FEV1)and quality of life in patients with COPD.
In comparative studies patients took tiotropium once daily, or ipratropium four times daily, for one year. Both drugs improved quality of life, but tiotropium resulted in a higher FEV1 at the end of the dose interval. Tiotropium also lengthened the time to first exacerbation and the time to first hospital admission due to an exacerbation of COPD. The number of patients who need to be treated with tiotropium for one year to prevent one exacerbation is nine, and 23 need to be treated to prevent one admission due to COPD.
There are no long-term studies of tiotropium in asthma so it is not indicated for patients with asthma.
Combination therapy
Anticholinergic drugs and beta adrenoceptor agonists produce bronchodilatation via separate mechanisms so there are theoretical reasons why they may be used in combination. Several studies have shown that the combination of ipratropium with a beta adrenoceptor agonist (either fenoterol or salbutamol) produces greater bronchodilatation than either drug alone.2 None of these studies has investigated whether a higher dose of the single drug(either ipratropium or beta agonist) would have achieved the same result as the combination. However, a higher dose of either drug would carry with it the greater risk of unwanted adverse effects.Both beta agonists and ipratropium are therefore frequently used in combination to treat inpatients with acute exacerbations of COPD. There is also a place for the combination of beta agonists and ipratropium in maintenance therapy for COPD, primarily to minimise the risk of adverse effects with higher doses of either ipratropium or the beta agonist.
The long acting anticholinergic, tiotropium, is most commonly delivered via the HandiHaler dry powder inhaler (18 µg/day) and more recently in some countries by a new propellant-free delivery system called the Respimat soft mist inhaler (2.5 µg two inhalations once a day). This last system is an effective alternative multi-dose delivery device for tiotropium.
BMJ 2010;340
Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials
BMJ 2011;342
Chronic obstructive pulmonary disease is ranked the fourth leading cause of death worldwide, with an estimated 3.02 million in 2004. Inhaled tiotropium bromide,a long acting anticholinergic drug, is approved for symptomatic benefit in patients with chronic obstructive pulmonary disease.
The treatment benefit of inhaled tiotropium is a reduction in both the symptoms of dyspnoea and the risk for exacerbations of chronic obstructive pulmonary disease.
Inhaled tiotropium is available in two formulations:
as a powder (Spiriva; Boehringer Ingelheim, Germany)delivered with a Handihaler device (BoehringerIngelheim) andin solution as a mist delivered with the Respimat Soft Mist Inhaler (Boehringer Ingelheim).
The mist inhaler is a propellant free device, which generates a fine, slow moving cloud for inhalation.
The delivered dose is independent of inspiratory effort and relatively unaffected by problems with breathing manoeuvre compared with other devices. It is recommended for use in patients who have poor manual dexterity and therefore have difficulty using the Handihaler.
Pharmacokinetic studies have shown
that compared with tiotropium 18 μg delivered bythe Handihaler, peak plasma concentrations with the
mist inhaler at doses of 5 μg and 10 μg were 35% and
threefold higher, respectively. The mist inhaler is
available in 55 countries (320 000 prescriptions compared with 3 million for tiotropium powder dispensed in England and Scotland in 2009), but this formulation has yet to gain regulatory approval in the United States.
Cardiovascular disease is the leading cause of morbidity
and mortality among patients with chronic
obstructive pulmonary disease. The cardiovascular
effects of inhaled anticholinergics have been evaluated
in recent studies. Ameta-analysis of 17 randomised controlled trials found a significantly increased risk of
major cardiovascular events (stroke, myocardial
infarction, and cardiovascular deaths, including sudden
death) with inhaled anticholinergics (relative risk
1.58, 95% confidence interval 1.21 to 2.06; P<0.001).
In this meta-analysis, the higher mortality rate in the group receiving inhaled anticholinergics did not reach significance (relative risk 1.26, 95% confidence interval 1.0 to 1.61; P=0.06). However, the UPLIFT (Understanding the Potential Long-Term Impacts on Function with Tiotropium) trial of about 6000 patients compared tiotropium powder with placebo15 and did not report any increase in mortality or cardiovascular events.
Some have considered data from that trial to be reassuring on the safety of tiotropium.
Patients receiving tiotropium with the mist inhaler are, however, potentially
exposed to higher concentrations and the powder and mist inhaler formulations are considered to be distinct products.Conclusions
Our meta-analysis explains safety concerns by regulatory agencies regarding the possibility of an increased risk of mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease.In accordance with guidance from the Medicines
and Healthcare Products Regulatory Agency, clinicians
should inform patients about the possibility of
this increased risk and exercise caution when prescribing tiotropium mist inhaler, particularly in patients with possible underlying cardiac disease.
BMJ 2011;342
In a 1-year, randomized, double-blind, double-dummy, parallel-group trial, we compared the effect of treatment with 18 μg of tiotropium once daily with that of 50 μg of salmeterol twice daily on the incidence of moderate or severe exacerbations in patients with moderate-to-very-severe COPD and a history of exacerbations in the preceding year. results show that, in patients with moderate-to-very-severe COPD, tiotropium is more effective than salmeterol in preventing exacerbations.NEJM March 24, 2011
New clinical practice guidelines describe the use of noninvasive positive-pressure ventilation (NPPV) and noninvasive continuous positive airway pressure (CPAP) in the acute care setting, according to a report published online February 14 2011 in the Canadian Medical Association Journal.
"Over the past two decades, the use of ...NPPV and of noninvasive ...CPAP by mask has increased tremendously among acutely ill patients.... Both methods of ventilation have been used to avoid endotracheal intubation in different patient populations with variable success."
Patient undergoing noninvasive positive pressure ventilation. National Institutes of Health
Specific Recommendations
new clinical practice guidelines include the following:For patients with (COPD) or cardiogenic pulmonary edema, NPPV should be the first choice. It may also be used after surgery or in immunocompromised individuals.
In the absence of shock or acute coronary syndrome requiring urgent coronary revascularization, patients with cardiogenic pulmonary edema and respiratory failure should receive either NPPV or CPAP.
Patients with a severe exacerbation of COPD, defined as a pH of less than 7.35 and relative hypercarbia, should have NPPV in addition to usual care.
For patients with cardiogenic pulmonary edema, CPAP delivered by mask appears to be as effective as NPPV.
CPAP should not be used in patients who have acute lung injury.
A trial of NPPV may be considered for patients with acute respiratory distress or hypoxemia, either postoperatively or in the presence of immunosuppression.
In centers with extensive experience in the use of NPPV, a trial of early extubation to NPPV may be considered for patients with COPD.
Helium-oxygen (heliox) should not be routinely used in patients receiving NPPV in the setting of a severe exacerbation of COPD.
NPPV should not be routinely used in patients who do not have COPD and in those who have postextubation respiratory failure.
CPAP may be used in patients who have respiratory failure after abdominal surgery.
NPPV may be used in patients who have respiratory failure after lung resection surgery.
Patients with acute respiratory failure who are receiving NPPV should use an oronasal rather than a nasal mask.
An ongoing trial will provide more certainty about the comparative safety of tiotroprium inhaler devices.
Cates C J BMJ 2011;342:bmj.d2970
• ©2011 by British Medical Journal Publishing GroupIn a 24-week placebo-controlled study and 20-week follow-up period, the investigators demonstrate that the anti-TNF agent infliximab is ineffective in the treatment of chronic obstructive pulmonary disease (COPD). Of most note, however, is the high incidence of new malignancies identified in patients exposed to infliximab, raising real concerns about the potential risks of TNF blockade in inducing, or accelerating malignant transformation
Frequent exacerbations in chronic obstructive pulmonary disease
A new clinical practice guideline recommends bronchodilator monotherapy for patients with chronic obstructive pulmonary disease and an FEV1 less than 60% predicted. The choice of a long-acting inhaled anticholinergic or long-acting inhaled beta-agonist should be based on patient preference, cost and adverse effects. The guideline on diagnosis and management of stable COPD was developed by the American College of Physicians (ACP), American College of Chest Physicians (ACCP), American Thoracic Society (ATS), and European Respiratory Society (ERS), and the recommendations are published in the August 2nd issue of the Annals of Internal Medicine.
For patients found to have mild-to-moderate COPD with an FEV1 of 60% to 80% predicted, there is low-quality evidence that inhaled bronchodilators may be helpful, the authors advise.
For those with an FEV1 less than 60% predicted, monotherapy with an inhaled bronchodilator is recommended, as mentioned. However, based on moderate-quality evidence, the guideline suggests that combination inhaled therapy with long-acting anticholinergics, long-acting beta-agonists, or corticosteroids may be used. Still, the panel notes, “it remains unclear when combination therapy is preferred over monotherapy.”
strong evidence recommend continuous oxygen therapy for patients with an FEV1 less than 50% and severe resting hypoxemia. Pulmonary rehabilitation should also be prescribed for this subgroup of patients.
Current literature suggests that
smoking cessation,long-term oxygen therapy in hypoxaemic patients, non-invasive ventilation,and
lung volume reduction surgery in selected patients improve survival.
COPD
Long-term oxygen therapy (LTOT)
Clinicians should be aware that inappropriate oxygen therapy in people with COPD may cause respiratory depression. LTOT is indicated in patients with COPD who have a PaO2 less than 7.3 kPa when stable or a PaO2 greater than 7.3 and less than 8 kPa when stable and one of: secondary polycythaemia, nocturnal hypoxaemia (oxygen saturation of arterial blood [SaO2] less than 90% for more than 30% of the time), peripheral oedema or pulmonary hypertension.To get the benefits of LTOT patients should breathe supplemental oxygen for at least 15 hours per day. Greater benefits are seen in patients receiving oxygen for 20 hours per day.
A diagnosis of cor pulmonale should be considered if patients have:
• peripheral oedema• a raised venous pressure
• a systolic parasternal heave
• a loud pulmonary second heart sound.
Treatment of cor pulmonale
Patients presenting with cor pulmonale should be assessed for the need for long-term oxygen therapy.Oedema can usually be controlled with diuretic.
The following are not recommended for the treatment of corpulmonale:
angiotensin-converting enzyme inhibitors
calcium channel blockers
alpha-blockers
digoxin (unless there is atrial fibrillation).
Pulmonary rehabilitation should be offered to all patients who consider themselves functionally disabled by COPD (usually MRC
grade 3 and above).
Pulmonary rehabilitation is not suitable for
patients who are unable to walk, have unstable angina or who have had a recent myocardial infarction.
Pulmonary rehabilitation programmes should include
multicomponent, multidisciplinary interventions, which are tailored to the individual patient’s needs. The rehabilitation process shouldincorporate a programme of
physical training, disease education,
nutritional, psychological and behavioural intervention.
Vaccination and anti-viral therapy
Pneumococcal vaccination and an annual influenza vaccination should be offered to all patients with COPD
Lung surgery
Patients who are breathless, and have a single large bulla on a CT scan and an FEV1 less than 50% predicted should be referred forconsideration of bullectomy.
Patients with severe COPD who remain breathless with marked restrictions of their activities of daily living, despite maximal medical
therapy (including rehabilitation), should be referred for consideration of
lung volume reduction surgery if they meet all of
the following criteria:
FEV1 more than 20% predicted
PaCO2 less than 7.3 kPa
upper lobe predominant emphysema
TLCO more than 20% predicted.
Patients with severe COPD who remain breathless with marked restrictions of their activities of daily living despite maximal medical therapy should be considered for referral for assessment for lung
transplantation bearing in mind comorbidities and local surgical protocols. Considerations include:
age
FEV1
PaCO2
homogeneously distributed emphysema on CT scan
elevated pulmonary artery pressures with progressive
deterioration.
Bronchodilators are used as first-line agents for the symptomatic treatment of patients with COPD. Bronchodilators decrease airway smooth-muscle tone, thus improving lung emptying during
expiration and hence decreasing lung hyperinflation. This reduces dyspnoea both at rest and on exertion, with resultant improvement
in effort tolerance.
Short-acting anticholinergics and beta2-agonists have been shown to improve pulmonary function, dyspnoea, and exercise performance in patients with COPD. Individual responses to different agents may vary. Combining short-acting beta2-agonists
and anticholinergics produces superior bronchodilation than either agent alone.
In patients with persistent symptoms it is preferable to use longacting bronchodilators. Long-acting beta2-agonists (LABAs) improve symptoms, increase exercise endurance and improve
quality of life.
The recent TORCH study has been reassuring with
respect to the safety profile of salmeterol in COPD.
Treatment with tiotropium, a long-acting anticholinergic, has been associated with improvements in lung function, quality of life and
exacerbations in a recently completed 4-year study in patients with COPD. This study (UPLIFT) was reassuring about the safety of the
drug with long-term use in patients with COPD.
Theophylline is a nonspecific phosphodiesterase inhibitor and has bronchodilator effects at higher doses, where there is a higher risk of toxicity. It is recommended for patients who have difficulty using inhaler devices. The previously recommended therapeutic serum levels of 15 - 20 mg/dl are too close to the toxic level and a lower level of 8 - 13 mg/dl is therefore suggested as safer but still effective.
Specific phosphodiesterase E4 inhibitors such as cilomilast and roflumilast are currently undergoing clinical trials.
They may have anti-inflammatory and bronchodilator properties but preliminary results show a modest bronchodilator effect and some benefits on
quality of life.
ICS/LABA combination
The TORCH study showed the benefits of the combination of ICS/LABA on FEV1,exacerbation rate, quality of life and survival compared with placebo and either of the monocomponents. There was an increased incidence of pneumonia (not confirmed on chest radiography or sputum culture) in patients receiving ICS but this was not associated with an increase in mortality.
The Optimal Therapy study compared the addition of placebo, salmeterol or the combination of salmeterol/fluticasone with tiotropium. Although exacerbation rate, the primary outcome, was similar in the three groups, lung function, health-related quality of life and hospitalisations were better in the group receiving tiotropium and salmeterol/fluticasone. In contrast to the TORCH study,no increased incidence of pneumonia was observed for the corticosteroid arm. There is an additive benefit for ICS and LABAs in COPD and this is an additional reason for using the ICS/LABA combination rather than ICS alone.
Corticosteroids
Inhaled corticosteroids (ICS) asmonotherapy
The anti-inflammatory effects of corticosteroids in COPD are modest in comparison to asthma. Several studies have looked at the effect of ICS in slowing down the progressive decline in lung function in COPD. The results have been disappointing. There was minimal, if any, benefit in the rate of decline in lung function. However, there has been evidence of a reduction in exacerbation frequency and improvement in quality of life.
In the TORCH study ICS (fluticasone) did not show clinically significant effects
on mortality compared with placebo, and although there were statistically significant improvements in health status, pulmonary function and exacerbation frequency, the clinical significance of these findings remains debatable. The combination of LABAs and ICS was superior in all endpoints studiedcompared with ICS alone. It is therefore suggested that in patients with COPD, ICS should not be used alone but rather in combination with a LABA.
Systemic corticosteroids
In stable COPD there is no role for chronic use of systemic corticosteroids. No study so far has shown any benefit of chronic systemic use of these agents in patients with stable disease. Long-term use of systemic corticosteroids is definitely associated
with toxic effects such as hyperglycaemia, myopathy, hypertension and osteoporosis,all of which are more pronounced in the elderly