Anti-rheumatic drug use and risk of serious infections
Dr: Hussein Mohammed JumaahCABM
16/3/2011
• The first is NSAIDs.COX-2 have been shown to be as effective as classic NSAIDs.
• second line of therapy involves use of low-dose oral glucocorticoids.• The third line are the DMARDs include methotrexate, sulfasalazine, hydroxychloroquine, gold salts, or D-penicillamine.
Rheumatoid Arthritis involves
Medical management offive general approaches
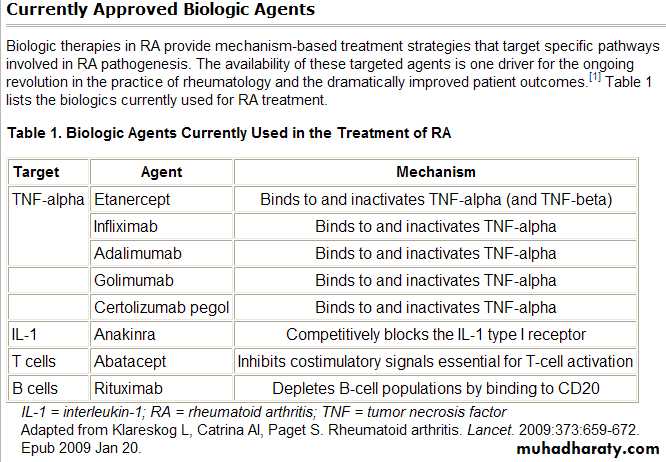
• 4. fourth group are the biologics, TNG-neutralizing agents (infliximab, etanercept, and adalimumab), IL-1-neutralizing agents (anakinra), those that deplete B cells (rituximab), and those that interfere with T cell activation (abatacept).
• 5. A fifth group immunosuppressive and cytotoxic ,leflunomide, cyclosporine, azathioprine, and cyclophosphamide.
RA is associated with significant morbidity and reduced survival. Suggestion of an increased risk of infections complicating RA has been reported for decades with particular concern regarding infections of the musculoskeletal system, soft-tissue and skin, as well as genitourinary and pulmonary infections.
The disease itself increases the risk for serious infections.In addition, both nonbiologic DMARDs and biologic agents may further increase the risk for infection.
• Skin/mucosal barriers
• Phagocytosis, eg neutrophils
• Cell-mediated immunity: T cells, B cells, spleen
• Humoral immunity: Antibodies made by B cells
• Complement
Five major host immune defects
Immunocompromised
Have alterations in phagocytic, cellular or humoral immunity that increase both the risk of infection and the ability to combat infection.Sources of infection
• Exogenous (environment):• a.Plants, animals, humans
• b.Nosocomial, donor organ, iv drug
• Endogenous:
• a.Colonization (skin, GI) then infection
• b.Latency then reactivation (herpes)
Immunosuppression and Infection
Fever is the principal and sometimes the only manifestation of serious infection in the immunocompromised patient. patients who are profoundly immunocompromised can (albeit rarely) have serious local or systemic infections in the absence of fever.Fever can also be suppressed or muted by immunosuppressive agents that may be part of the therapeutic regimen, especially steroids and nonsteroidal antiinflammatory agents. However, patients with infection usually have fever despite the use of these agents.
Fever is a manifestation of the release of proinflammatory cytokines (interleukin-1 , interleukin-1ß, interleukin-4, interleukin-6, and tumor necrosis factor ) from macrophages, lymphocytes, fibroblasts, epithelial cells, and endothelial cells as a consequence of infection or inflammation.
Signs of inflammation other than fever are often mild or absent and,
in patients undergoing cytotoxic chemotherapy, the side-effects of the treatment may mimic infection, eg. pain or mucositis.The use of GC is associated with increased susceptibility ,in a dose-dependent fashion,to viral, bacterial, fungal, and parasitic infections. The increased risk of infections is believed to occur through multiple alterations in host defenses including :
• altered cellular and humoral immunity.
• decreased phagocytosis and intracellular killing.
• and the inhibition of cytokine release.
Glucocorticoids
Most of these mechanisms, subside rapidly when treatment is interrupted, an observation that may explain the lower infectious risk with the use of
short acting GC and alternate day treatment.
Infections with atypical organisms and herpes zoster appear to occur more commonly in persons taking glucocorticoids .
Pneumocystis carinii infections may occur more frequently in patients using moderate doses of glucocorticoids .
The risk of infection increases with dose and duration of treatment, and tends to remain low in patients exposed to low doses, even with high cumulative dosages.
Studies evaluating the incidence of infections in RA patients taking low dose glucocorticoids have not shown significantly increased risks of infection.
However, in the current era of RA treatment, patients will likely be receiving other immuno- suppressive agents in addition to glucocorticoids, making it imperative that one maintain a high index of clinical suspicion for infection in patients with unusual symptoms.
In a meta-analysis of 71 trials involving over 2000 patients with different diseases and different dosages of GC, a relative risk of infection was found of 2.0. Five of these 71 trials involved patients with rheumatic diseases and showed no increased relative risk. In two studies specifically on RA the incidence of serious infections was found to be similar to that of placebo or only slightly increased.
Of the intensively reviewed four studies of low dose GC treatment in RA, both in the Utrecht and the WOSERACT trials, prednisone up to 10 mg/day was not associated with increased incidence of any kind of infections over the 2 years of the trials.
In patients treated with GC, physicians should anticipate the risk of infections with both usual and unusual organisms, realising that GC may blunt the classic clinical features and delay the diagnosis.
Under special clinical circumstances and in severely immunocompromised patients it may be wise to screen for latent infections, such as tuberculosis, or institute prophylactic chemotherapy.
Pneumocystis carinii
( jirovecii )Deserve special attention, as doses as low as
16 mg/day prednisone for 8 weeks have been associated with increased risk in one series. There is evidence in favour of PCP prophylaxis with trimethoprim/cotrimoxazole in patients receiving intense immunosuppression with high dose glucocorticoids and cyclophosphamide.
Methotrexate is one of the most popular and effective agent in RA and is used at low dose, ranging from7.5 to 20 mg/ week.
This low dose is associated with impaired immune function such as cytokine secretion (including TNFα), immunoglobulin function, T and B cell immunity as well as neutrophil function.
• Methotrexate
Some authors considered that MTX is
associated with an increased frequency of infection, but the results were not significant compared to a population of RA patients without MTX. However, some opportunistic infections including Pneumocystis jiroveci (carinii), Herpes zoster, Histoplasmosis or Aspergillosis,Tuberculosis have been reported in a limited number of patients .
Interstitial pneumonitis
Is a rare complication of methotrexate (<2%), but the clinician should be alert to symptoms of cough or shortness of breath that may herald the onset of this severe complication. Methotrexate pneumonitis may occur at any time during therapy and is not dose related. A baseline chest x-ray is useful for comparison.Patients with poor pulmonary reserve from other causes may be excluded from therapy over concerns of increased morbidity if methotrexate pneumonitis occurs.
A more chronic form of interstitial lung disease and fibrosis is also seen in patients with rheumatoid arthritis. This may be increased with methotrexate.
Hydroxychloroquine and chloroquine, did not appear to be associated with a greatly increased risk of infection.
Anti-malarial
Biologics
ROLE OF TNFα IN HOST DEFENCETNFα is a cytokine has a wide range of biologic effects such as cellular activation of cells playing a role in host defence:
It activates monocytes/macrophages, induces the chemotaxis and migration, increases the activities of polymorphonuclear cells ,induces T and B cell functions, activates cytotoxic T cell invasiveness. TNFα has also a major role in granuloma formation, thus explaining the influence of anti-TNFα agents in the development of granulomatous diseases such as tuberculosis ,and consequently, blocking TNFα leading to impaired defence mechanisms, notably those caused by intracellular organisms.
The infections which were described with TNFα inhibitors may have a benign course or may be a serious, life threatening disease, and may be localized or disseminated.
Tuberculosis is the most frequent opportunistic infection and the highest risk appears to be associated with infliximab. Thus all patients should be screened for latent TB before initiation of any TNF inhibitor.
TB commonly appeared within the first few months of use, frequently was disseminated or atypical in presentation, and resulted in high death rates. These cases were believed to occur because of reactivation of latent TB infection, leading to recommendations that candidates for these drugs undergo screening for latent TB infection and, if reactive to the tuberculin skin test, be given preventive therapy with isoniazid or rifampin before commencing anti-TNF-α.
The latest ACR guidelines
Updated statement on the use of biologics in rheumatic diseases are the most current evidence-based recommendations. Despite the reports of safety of anti-TNF medications in patients with chronic viral hepatitis, the guideline is to avoid biologic agents in chronic hepatitis B and C if there is evidence of significant liver injury .Biologic agents should also not be prescribed in the presence of active infections such as URTI with fever, nonhealed skin ulcer, life-threatening fungal infection or active herpes zoster viral infection.
In patients with active RA, anti-TNF therapy was not associated with increased risk of overall serious infection compared with DMARD treatment, after adjustment for baseline risk. In contrast, the rate of serious skin and soft tissue infections was increased, suggesting an important physiologic role of TNF in host defense in the skin and soft tissues beyond that in other tissues.
In a study of serious infections associated with TNFα inhibitors, the majority were pneumonias, followed by cellulitis and soft tissue infections, and renal/urinary tract infections.
The impact of TNF antagonists on vaccine therapy appears to depend on the vaccine being used. In general, TNF antagonists may reduce the immune response to vaccination, but the immune response is still strong enough for vaccination to be beneficial.
Vaccination is effective while on biological agents, although live vaccines should be avoided. Biologic therapy in the setting of HIV, HCV and HBV continues to be studied, but data are accumulating in support of a favorable safety profile.
The recent data on anti-tumor necrosis factor agents show encouraging results in relation to infections. The majority of the infections are minor, and opportunistic infections including tuberculosis are rare. The incidence of infections decreases with time on biologic therapy.
The challenges posed for clinicians involved with the care of candidates for anti-TNF-α therapy include :
• the essential benefit-to-risk calculus.
• pretreatment screening for latent TB infection.
• opportunities to prevent serious infections .
• surveillance for early detection of serious infections.
• the possibility of immune reconstitution inflammatory syndrome if anti-TNF-α treatment is stopped abruptly in the context of a serious infection.
The benefit-to-risk calculus is complex, because it embraces not only the relative efficacy but also the complications of non-TNF-α—based therapy. Clinicians cannot ignore the debilitating and morbid consequences of long-term high-dose corticosteroid therapy and the infectious, hepatic, and hematologic complications of traditional disease-modifying antirheumatic drugs, such as methotrexate or azathioprine.
A preliminary conclusion about the safety of TNFα inhibitors could be that these drugs are double-edged sword since they ameliorate clinical symptoms in inflammatory rheumatic diseases in one hand, but they increase the risk of infections in the other. However, the introduction of TNFα antagonists constitutes a major advance for these diseases.The overall safety of these agents appears good and comparable to certain DMARDs.
Diagnosis of infection
The wide-range of potential pathogens means that standard culture media may not cover all the possibilities and the microbiology department should be alerted to the patient’s clinical history and extent of immunosuppression. Initiation of treatment should not be delayed pending laboratory results, but treatment should be tailored once the results are obtained.Laboratory evaluation
• Blood samples should always be taken and both bacterial and fungal cultures should be assessed. Blood counts may also be useful to assess the degree of neutropenia. Cultures may also be obtained from:• Cerebrospinal fluid.
• Urine and stool samples.
• Nose and throat swabs or a sputum sample.
• viral study.
Principles for management
Generally, the treatment should target the pathogen most likely to be involved, depending upon the host condition and duration of immunosuppression. Resistant or opportunistic organisms should always be considered.
The core regimen should include:
• A combination of broad-spectrum antibiotics at high-doses to combat Gram-positive and Gram-negative aerobes, plus antifungal therapy from the outset of treatment to prevent secondary fungal infection• Administration via IV for rapid onset of action
• Consideration of local factors ie. underlying disease state, presence of an intravascular device, local bacterial ecology and known resistance patterns.