د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2011
MANAGEMENT OF DYSLIPIDAEMIAS
Heart 2006Atherosclerotic disease is the leading cause of death among men and women in Europe, the
United States, and worldwide. Previously considered a ‘‘western’’ disease, it is now clearthat various peoples of diverse ethnic backgrounds are vulnerable to atherosclerosis. The
epidemic of obesity, especially abdominal obesity, escalates the need for appropriate detection and
management of high risk individuals.
The overwhelming consumption of economic and medical
resources necessitates preventive measures in order to decrease the global burden of heart disease.The recent Interheart study, a very large case–control study of acute myocardial infarction in 52 countries, demonstrates that traditional risk factors for coronary heart disease (CHD) account for most of the risk worldwide (over 90%) and include abnormal lipids, smoking, diabetes,hypertension, abdominal obesity, psychosocial factors, diminished consumption of fruits and vegetables, lack of regular alcohol intake, and lack of regular physical activity.
The two most potent risk factors worldwide are smoking and dyslipidaemia.
The forefront of primary and secondary prevention of CHD is the management of dyslipidaemia. This article will review current strategies in managing dyslipidaemias, focusing on drug treatment and the adjunctive role of diet.
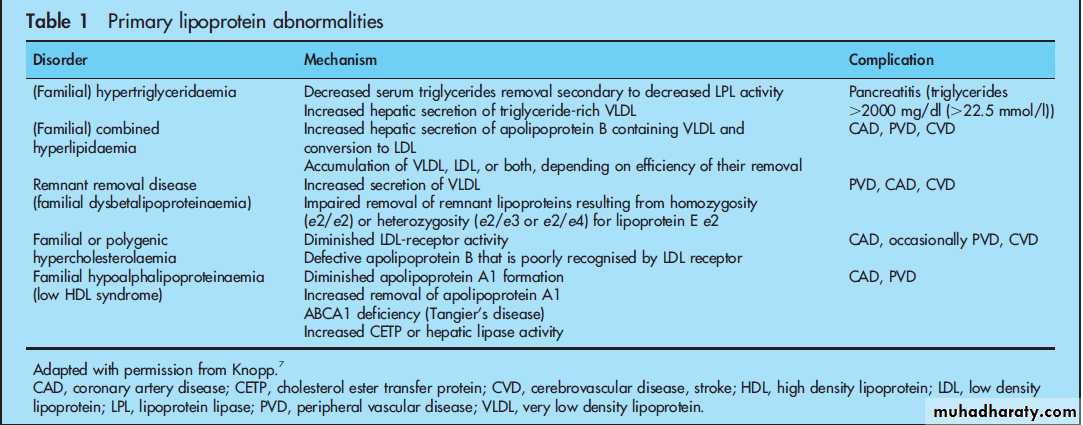
A lipid profile including total cholesterol, low density lipoprotein cholesterol (LDL), high density
lipoprotein cholesterol (HDL), and triglycerides should be checked in all adults beginning at age 20, but earlier if the patient is obese or there is a family history of premature atherosclerotic disease or primary lipoprotein abnormalities (table 1).
Elevated apo-B values may be a more accurate indicator of atherosclerotic risk than LDL alone. However, current guidelines base treatment on LDL values.
European guidelines advocate use of the SCORE model and risk charts.
while US guidelines advocate use of the Framingham risk model and implementation of NationalCholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) guidelines based on calculated risk. Multiple recent trials demonstrate the benefit of more aggressive lipid treatments, especially in very high risk groups such as those with established atherosclerotic disease and uncontrolled risk factors such as metabolic syndrome and diabetes.
The groups of people who derive the largest benefit from preventive strategies include those with
established atherosclerotic disease—CHD, peripheral vascular disease (PVD), cerebrovascular disease
and stroke (CVD)—and asymptomatic individuals who are at high risk of developing atherosclerotic
disease such as those with multiple risk factors resulting in a 10 year risk CVD mortality of>5% by the SCORE model, major elevation in one risk factor such as LDL>6 mmol/l (240 mg/dl), and those with diabetes mellitus which is considered a CHD equivalent.
European guidelines also stress that close relatives of patients with early onset atherosclerotic disease are high risk individuals.
Therapeutic lifestyle changes—including healthy eating habits, physical activity, and stopping or
never smoking—remain an essential aspect of therapy in all individuals regardless of risk. However,
lifestyle changes are typically not enough to reach target lipid concentrations.
National guidelines for determining target lipid concentrations are based on underlying risk. The
primary goal is LDL lowering because as LDL increases, CHD risk increases. The European SCORE
system assesses 10 year risk of total fatal vascular events and not just 10 year risk of CHD (includes
coronary death and non-fatal CHD events) like the US Framingham risk score. It incorporates data from pooled cohort studies from 12 European countries. It integrates sex, age, smoking, systolic blood pressure and either total cholesterol or the cholesterol/HDL ratio.
Unlike the Framingham risk score,the European SCORE system allows projection of risk to age 60, allowing young adults who may have low 10 year risks but relatively high lifetime risks to estimate near lifetime risk of having a fatal CVD event.
This approach is beneficial in a setting of societal increases in waistline and metabolic syndrome, especially for vulnerable populations such as South Asians.
The NCEP/ATP III guidelines identify the following groups as high risk: established atherosclerotic
disease (CHD, PVD, and CVD), diabetes, and those with 10 year Framingham risk >20%.
Risk is calculated based on Framinghamrisk score which incorporates age, total cholesterol, smoking status, HDL, and systolic blood pressure to determine the Framingham
10 year risk of CVD events. Important omissions in both SCORE and the Framingham risk algorithm are risk factors such as positive family history for CHD, impaired glucose tolerance, or hypertriglyceridaemia.
The target LDL value in high risk individuals is < 2.6 mmol/l (100 mg/dl) using SCORE or the Framingham risk algorithm.
Moderate risk individuals defined by the Framingham risk algorithm as having 10–20% 10 year risk for CHD events have atarget LDL of<3.4 mmol/l (130 mg/dl). Low risk individuals.
have <10% 10 year risk and the target LDL is
< 4.1 mmol/l (160 mg/dl).
Optimal LDL in very high risk individuals with established atherosclerotic disease and diabetes, ongoing uncontrolled risk factors, and recent
unstable angina may benefit from further LDL lowering to a target <1.8 mmol/l (70 mg/dl).
Once a patient’s risk is estimated then a decision to initiate treatment would be made based on estimated CHD risk, co-morbid conditions,
contraindications to therapy, and patient’s concerns.
Persons with multiple borderline abnormalities such as
young people with abdominal obesity and> 75th centile values of LDL, triglycerides, blood pressure, and/or waist circumference for age and sex will often have low 10 year risk scores by currently advocated risk assessments. Although they may not meet criteria for metabolic syndrome, if their risk factors are
not controlled there is an inevitable progression to metabolic syndrome and possibly future diabetes and heart disease, especially if there is a significant family history for these diseases.
These people should not be told they are low risk and
given false reassurance. It should be emphasised that they are metabolically abnormal and that they have multiple risk factors for heart disease. They should be aggressively counselled to modify diet by decreasing saturated fats and increasing fruits and vegetables, including one to two fatty fish meals per week,increasing physical activity, and stopping smoking.
Pharmacological treatment may be considered if behavioural changes do not improve risk profile. However, guidelines should be applied and consideration to long term costs and risk of
pharmacological treatment should also be considered before institution of treatment.
LIPID LOWERING TREATMENT
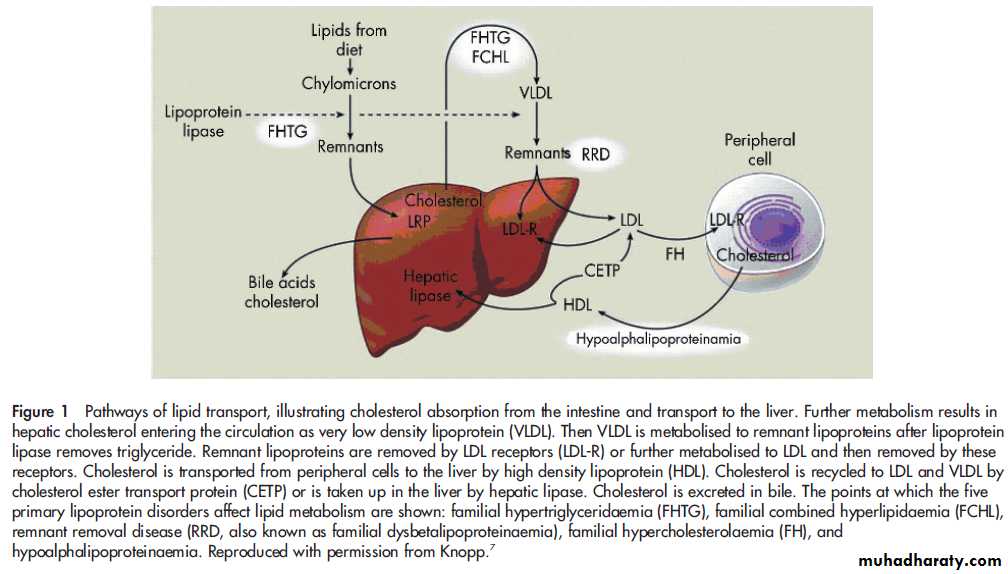
Lipid therapy is based on the understanding of lipidmetabolism.
Figure 1 outlines the pathways involved in lipid
transport. The effects of different drug treatments can be better understood by understanding these pathways.
Multiple clinical trials have shown benefit in decreasing CHD ‘‘hard’’ outcomes such as myocardial infarction and death with statin use (including atorvastatin, simvastatin, pravastatin, and lovastatin)
in both people with established CHD and those without
established CHD. These distinctions are termed primary and secondary prevention, respectively.
LDL lowering is still the primary goal in CVD prevention.
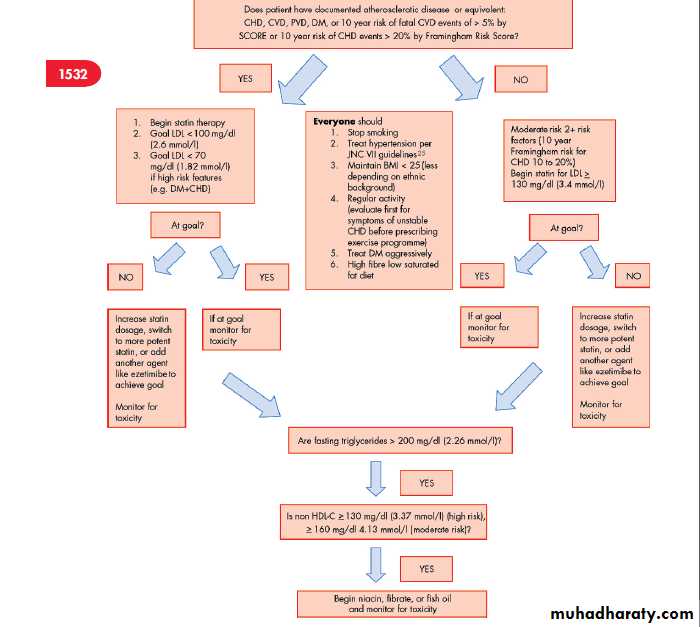
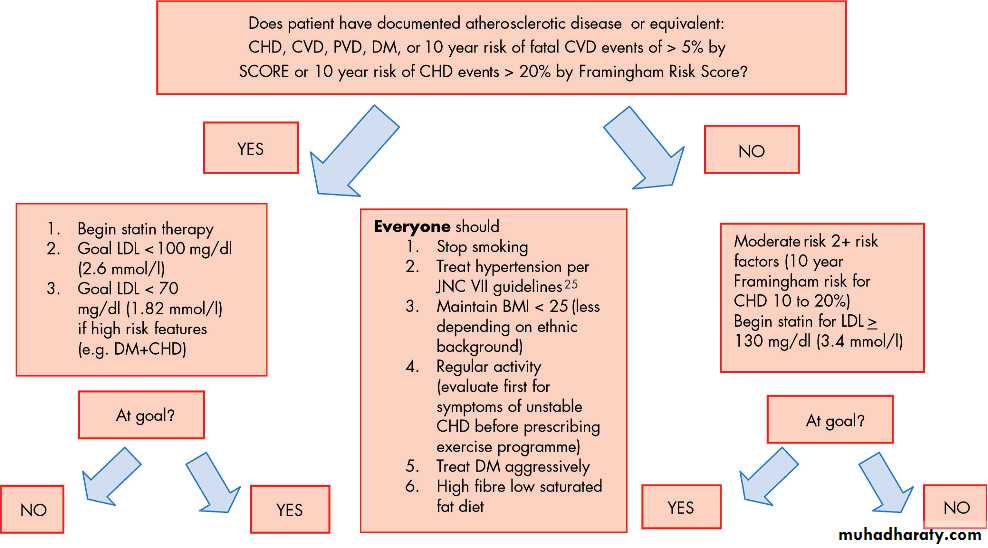
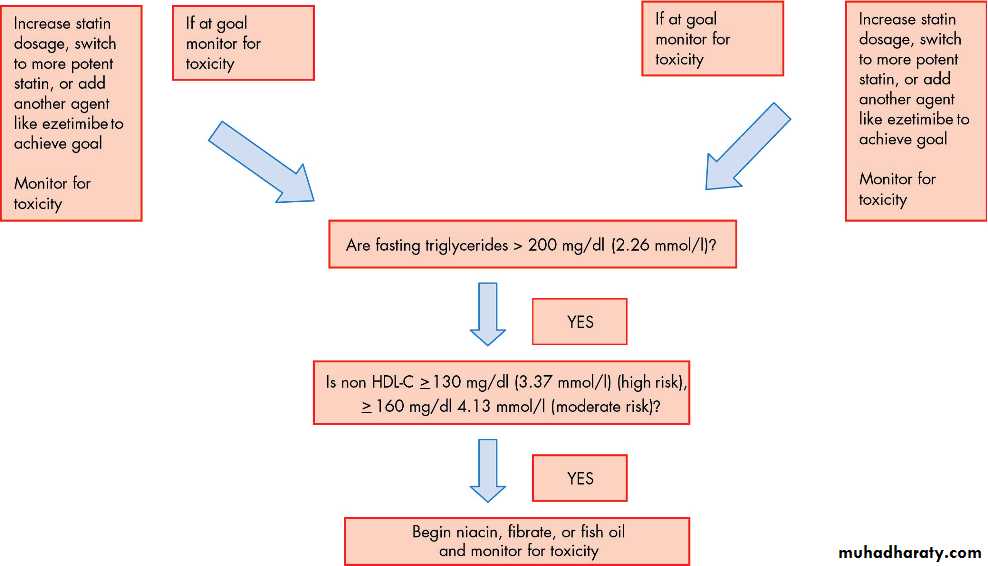
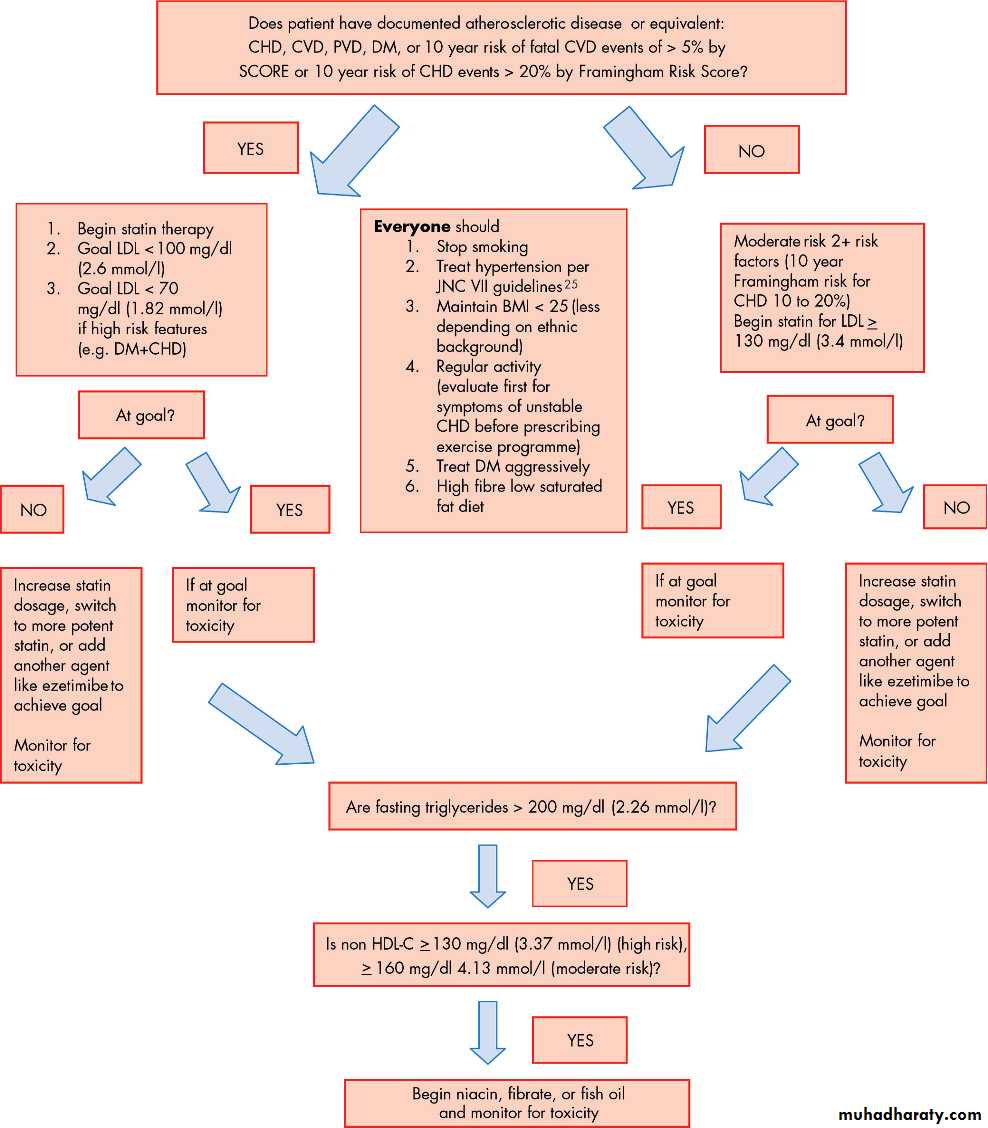
Figure 2 shows a flow chart that aids the practitioner in the decision making process in determining treatment in moderate and high risk individuals.
LDL LOWERING
LDL lowering treatments Statins are competitive inhibitors of HMG-CoA reductase, the key rate limiting enzyme involved in cholesterol synthesis. They decrease primarily LDL cholesterol concentrations by upregulating
LDL receptor activity and decreasing entry of LDL into the circulation.
also favourably decrease fibrinogen concentrations and viscosity, increase activation of endothelial nitric oxide synthase, decrease C-reactive protein (CRP) independent of LDL lowering effects, modestly decrease triglycerides, and modestly increase HDL.
Statins should be given in the evening because endogenous cholesterol synthesis is higher at
night. Different potencies of statins exist and determining drug choice and dosage is established primarily by the amount of LDL reduction needed, the safety profile of the drug, and concomitant
drug usage. Tissue penetration may be less with the
hydrophilic pravastatin and rosuvastatin.
All other statins are lipophilic.
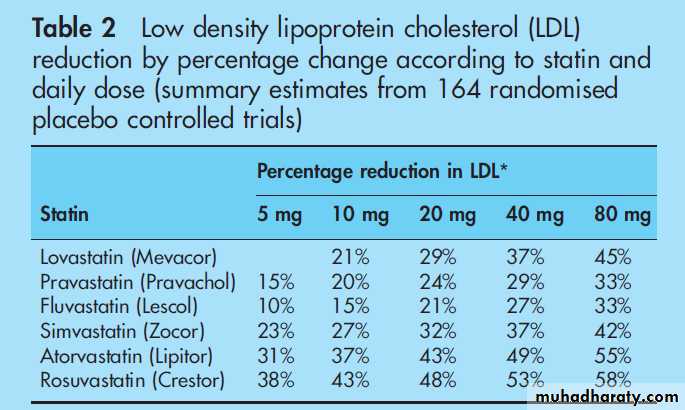
Table 2 summarises the expected reduction in LDL with various statins.
Monitoring for statin toxicity is very important. Patients need to be educated about possible symptoms that may indicate myositis or liver dysfunction.
These include new muscle aches which may signify myositis. Symptoms of fatigue, sluggishness, anorexia, nausea, and weight loss may indicate hepatitis. Right upper quadrant (RUQ) pain and jaundice are not common.
The major risks of statin use are myositis and rarely rhabdomyolysis and perturbations in liver
function tests (LFTs).
Liver failure is very rare with statin use alone.
The incidence of LFT elevations >3Xthe upper limit of normal is approximately 0.5–2%.
Elevation in LFTs is dose dependent but can be idiosyncratic.
Baseline LFTs should be checked in patients and repeated 4–6 weeks after initiation of statin, any significant change in statin dosage, or change to another statin, or after initiation of any medication that may cause change in the metabolism of statins by the liver.
A mild to moderate increase in LFTs (<3Xupper limit of normal) is not a contraindication to initiate or continue statin use, but LFTs should be followed very closely. In those with preexisting liver disease, consultation with the patient’s gastroenterologist/hepatologist may be prudent if statin (fibrate or niacin) treatment needs to be started.
If significant LFT elevation > 3Xupper limit of normal occurs then statin dose should be decreased or discontinued.
Risk factors for statin toxicity include older age, females, small frail frame, multisystem organ disease, multiple medications, steroid use, excessive alcohol intake, and drinking >1litre of grapefruit juice a day. Drugs that inhibit cytochrome P-450 3A4 or 2C9 can increase serum statin concentrations, increasing risk of toxicity.
Examples of these drugs include but are not limited to nicotinic acid, cyclosporine, azole antifungals,
macrolide antibiotics, HIV protease inhibitors, verapamil, amiodarone, and nefazadone. Fibrates such as gemfibrozil raise statin concentrations by inhibiting the glucuronidation of the statin drug. This is not an exhaustive list and the authors suggest pharmacological consultation with initiation of statins or other lipid lowering treatments in patients on medications
that may put them at risk for statin toxicity.
Myositis is another important concern. Patients often have joint or muscle complaints that are ongoing. Distinguishing new symptoms that may suggest myositis is difficult. Education of patients at onset of therapy will provide patients with a time reference so that accurate detection of symptoms is more feasible.
In addition to checking baseline LFTs, determining
baseline creatine phosphokinase (CPK) is helpful.
Elevations in CPK in asymptomatic individuals before initiation of statin treatment is common and may be a normal variant (African Americans often have elevated CPK) or may be secondary to hypothyroidism (check thyroid stimulating hormone) or possibly secondary to pre-existing myopathy of another aetiology such as dermatomyositis.
If patients develop symptoms indicative of myositis, CPK should be checked. Drug treatment should be discontinued if CPK elevation is >10X upper limits of normal. It is not clear what to do for lesser CPK
elevations and it is reasonable to decrease dosage to see if symptoms abate. If symptoms continue then cessation of drug is appropriate.
If CPK is not elevated from baseline but symptoms continue, aldolase may be a more specific test to identify muscle damage.
If no laboratory abnormalities are found but symptoms cannot be tolerated by the patient (afrequent occurrence) then treatment should be discontinued.
If toxicity develops, it is reasonable to rechallenge patients with adifferent statin.
Often rechallenging with a hydrophilic statin such as pravastatin or rosuvastatin at a low dose may prove beneficial.
Again, close monitoring of LFTs and/or CPK is
imperative if previous abnormalities existed.
Other adverse effects of statins include rash, peripheral neuropathy, insomnia, and difficulty sleeping or concentrating.
Statins are contraindicated in pregnancy and in breast feeding mothers as they are known to be teratogenic, with several different abnormalities reported such as limb dysplasias in the fetus. Physicians need to counsel women of child-bearing potential of this risk.
Statin extenders that decrease LDL cholesterol effectively include bile acid-binding resins and inhibitors of cholesterol absorption. Bile acid-binding resins bind bile acids in the intestine and disrupt the enterohepatic circulation of bile acids and thus increase the conversion of cholesterol into bile acids in the liver.
They are second line agents to statins.
Resins are not an effective alternative in persons withhypertriglyceridaemia as well since they increase cholesterol synthesis and increase VLDL triglyceride secretion into the circulation.
Available resins include cholestyramine, colsevelam
and colestipol. Important adverse events include
abdominal fullness, gas and constipation.
In children and patients with renal failure cholestyramine can cause hyperchloraemic
acidosis. Absorption of fat soluble vitamins maybe decreased. The resins can bind polar compounds such as warfarin, digoxin, thyroxin, thiazides, and statins.
Giving medications one hour before or four hours after resin administration may decrease this effect.
Another second line treatment for raised LDL is ezetimibe.
It is most often used in combination with statins foradditional LDL lowering. Ezetimibe may be used as monotherapy in people who do not tolerate statins, but long term reductions in atherosclerotic events are not yet demonstrated.
Ezetimibe inhibits absorption of cholesterol at the brush
border of the small intestine, decreasing cholesterol delivery to the liver. This effect results in decreased total cholesterol,LDL, apo B, and triglycerides, and modest increases of HDL.
ELEVATED TRIGLYCERIDES
The treatment of elevated triglycerides may be warranted.
Clearly triglyceride values>11.3 mmol/l (> 1000 mg/dl) require treatment to avoid pancreatitis.
Lower values may also warrant treatment, especially in those with combined hyperlipidaemia (associated with modest elevations of LDL that are small and dense and low HDL). This atherogenic profile results in increased risk of premature CHD.
The Copenhagen male study, an eight year prospective study, demonstrated that men
in the highest tertile of serum triglycerides had a significantly higher cumulative incidence and relative risk of CHD (2.2)compared with men in the lowest tertile. Treatment of
hypertriglyceridaemia with gemfibrozil has decreased coronary events in men with CHD.
Fenofibrate and bezafibrate treatment has also shown similar results. Fibrates, however, have not been shown to decrease all cause mortality.
Initial treatment of hypertriglyceridaemia involves decreasing caloric intake by decreasing fat and intake of simple sugars. In diabetic patients, glucose control can decrease triglycerides. If triglycerides remain elevated then treatment with fibrates should be considered.
Fibrates activate the nuclear transcription factor peroxisome proliferator-activated receptor a (PPAR-a) resulting in up-regulation of LDL cholesterol receptor and apolipoprotein A genes and downregulation of the expression of the apolipoprotein CII gene.
They increase fatty acid oxidation in the liver and decrease secretion of triglyceride-rich lipoproteins. Important adverse events with fibrates include gastrointestinal symptoms (especially gemfibrozil), erectile dysfunction (less with fenofibrate), myositis and hepatitis. Myositis risk is increased in patients with renal failure.
Fibrates also increase biliary cholesterol oncentrations and can cause gallstones.
Fish oil containing eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) also decreases serum triglycerides. The precise mechanism is not well understood.
Supplementation with 1.52 g/day of DHA in men and women with below average values of HDL raised LDL cholesterol but decreased triglycerides, triglyceride/HDL ratio, and the fraction of LDL cholesterol carried by small, dense particles.
The American Heart Association recommends 2–4 g of fish oil supplementation daily under physician supervision in people with significant elevation of triglycerides.
Adverse reactions of fish oil include LFT abnormalities and a theoretical increased risk of bleeding.
LOW HDL
Low HDL is often associated with combined hyperlipidaemia and metabolic syndrome but can occur in isolation. Regular aerobic exercise, weight loss (especially in abdominally obese individuals), moderate alcohol consumption, and smoking
cessation all have modest effects on improving HDL. Statins increase HDL by 10% on average.
Niacin increases HDL up to 20–35%.
Niacin increases HDL by inhibiting hepatic uptake of
apolipoprotein A-1 and increases plasma preb HDL cholesterol concentrations..
The Coronary Drug Project demonstrated asignificant reduction in the incidence of myocardial infarction
after five years of niacin treatment among men with a history of myocardial infarction, and death after 15 years of treatment. The side effects of niacin treatment include cutaneous flushing which is the most common reason for discontinuation.
Other side effects include dyspepsia, elevation of plasma glucose, and elevation of plasma uric acid concentrations.
Despite the modest elevation of glucose seen with niacin it can be used in diabetics as indicated. Flushing can be minimised by taking niacin with a
bedtime snack, 30 minutes after aspirin, or with an extended release formulation. It is prudent to start at low dose and escalate gradually.
Niacin hepatoxicity can be subtle and patients may complain of nausea and anorexia.
LFTs may be elevated and a clue may be unexpected lowering of LDL.
Cholesterol ester transfer protein (CETP) inhibitors are now being tested in clinical trials and are not currently available. Their primary action is transfer of cholesteryl esters from HDL to VLDL and LDL in exchange for triglyceride. HDL increases because of delayed catabolism of apolipoprotein A-1
and A-II and thus increases reverse cholesterol transport.
DIET
Determining the optimal diet in patients with dyslipidaemia is challenging. The ideal goal is to improve the metabolic profile in anyone withadyslipidaemia and induce weight loss in those
who are overweight. Traditional standards of BMI may not be as helpful in guiding appropriate weight loss, and waist circumference may be a better standard.
The metabolic syndrome
includes
abdominal obesity and two of the following abnormalities:
high triglycerides (≥ 150 mg/dl (1.69 mmol/l) or on
triglyceride treatment), low HDL ≤40 mg/dl (1.02 mmol/l) for men and ≤ 50 mg/dl (1.28 mmol/l) for women or on HDL treatment), elevated blood pressure (≥ 130/85 mm Hg or on antihypertensive treatment), and elevated fasting blood glucose
≥ 100 mg/dl (5.5 mmol/l) (includes diabetes).
The metabolic syndrome definition of abdominal obesity is traditionally ≥ 40 inches (100 cm) in men and ≥35 inches (88 cm) in women. However, these numbers may be too high when
applied to, for example, Asians, Hispanics, Native Americans,and South Asians. Thus the definition of abdominal obesity is population specific.
The typical low fat, high carbohydrate diet may not
ameliorate metabolic derangement, especially in those with insulin resistance and metabolic syndrome. In fact our group demonstrated that egg feeding resulted in a three- to fourfold greater LDL elevation in lean insulin sensitive individuals than in obese insulin resistant individuals, possibly suggesting impaired absorption of cholesterol in these individuals.Those with combined hyperlipidaemia, insulin resistance, and/or the metabolic syndrome may benefit from a higher amount of fat—possibly up to 35%—in their diet; importantly this fat should be primarily poly- and monounsaturated fat.
Everyone should avoid saturated fat, trans fats, and excess simple sugars.
Further research regarding the optimal diet in insulin resistance is needed.
A recent comparison of diets shows that low fat versus low carbohydrate diets result in similar weight loss patterns in the short term. Also CRP, total/HDL cholesterol, and insulin concentrations decreased
similarly. All people with dyslipidaemia should eat high fibre (aiming for >30 g per day) and vegetable intake.
Two fatty fish meals a week should be eaten and in those with documented CHD 1 g of omega-3 fatty acids per day should be taken as fatty fish meals or supplements.
Women who may conceive and pregnant women need to be careful with fatty fish intake due to risk of ingestion of heavy metals.
Omega-3 fatty acids may also delay parturition. They should consult their physician or nutritionist for further guidance.
SUMMARY
The primary goal in the treatment of dyslipidaemia is to avoid future morbidity and mortality from atherosclerotic diseases.Lifestyle changes including stopping smoking, improving diet, increasing physical activity, and decreasing weight are the first step. Pharmacological treatment is often needed and European and US guidelines exist to help the practitioner decide when to begin treatment and what goals to aim for.
Often a multidisciplinary approach may be needed involving the primary care physician, lipid expert, cardiologist, and nutritionist.
Finally, the patient must be educated about long term adverse consequences of dyslipidaemia, benefits of lifestyle changes, and potential benefits and adverse effects of drug treatment.
Figure 2 Determining treatment in moderate and high risk individuals for coronary heart disease. BMI, body mass index; CHD, coronary heart disease; CVD, cerebrovascular disease, stroke; DM, diabetes mellitus; HDL, high density lipoprotein; LDL, low density lipoprotein; PVD, peripheral vascular disease.