Update on mechanical circulatorysupport in heart failure
Heart 2012د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
Temporary mechanical support technology has
advanced, and the miniaturisation of these deviceshas permitted their use with less operative
morbidity and more rapid functional recovery
following operation. At present a broad range of
devices are available.
The most comprehensive mechanical support for both the systemic and the pulmonary circulation is still best provided by oxygenation for extremely ill patients with lung and heart failure,which remains a cumbersome and invasive but extremely effective form of short term mechanical support.
However, the development of devices such as the Impella and the Tandem-Heart has allowed less invasive forms of temporary support of the systemic circulation typically applied during high risk percutaneous intervention procedures, such as high risk coronary stenting and cardiogenic shock.
Larger, external pulsatile pumps such as the AbioMed 5000 and the more recent magnetically levitated centrifugal Centrimag pump are used to provide temporary support of either the left or right ventricle or both as a short term rescue strategy post-cardiotomy, or as a bridge to more long term cardiac replacement treatment or recovery.
Counterpulsation technology remains a mainstay
of acute care in patients with cardiogenicshock, both before and after surgical or percutaneous intervention. This technology has been
developed and miniaturised for potential long term
use in ambulatory patients, most notably the
Akpulsor (Cardiak, Ltd, Oxford, UK), C-Pulse
(Sunshine Heart Inc, New South Wales, Australia),
and CardioPlus (CardioPlus, Inc, Detroit, Michigan,
USA) devices.
None of these devices has been evaluated in a US Food and Drug Administration (FDA) or European CE approved trial. Finally, enhanced external counterpulsation treatment has been established as an effective therapy in intractable angina in non-revascularisable patients with coronary artery disease. The counterpulsation principle and marked left ventricular afterload reduction may also be helpful in congestive heart failurew10 w11 and this has been evaluated in the Prospective Evaluation of EECP (enhanced external counterpulsation) trial.
VENTRICULAR ASSIST DEVICES AS LONG TERM CARDIAC REPLACEMENT THERAPY
At present the gold standard of long term heartreplacement remains heart transplantation, but the
number of heart transplants performed is limited
by donor organ availability. Research in genetic
engineering and xeno-transplantation, using transgenic animals as donors, has progressed considerably but not yet to the stage of clinical trials.
Although much progress has been made in the
understanding of stem cell biology in heart failure,
the field is still in its infancy. Therefore, there has
been great interest in the development of left
ventricular assist devices (LVADs) as lifelong
support for end-stage chronic heart failure.
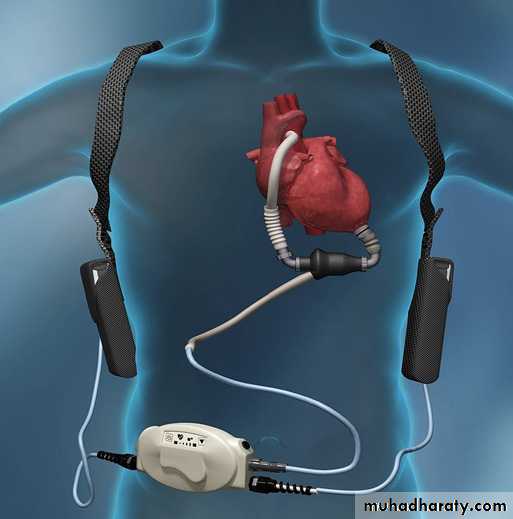
LEFT VENTRICULAR ASSIST DEVICES LVADs
have been in use as a bridge to heart transplantation for 20 years; the HeartMate XVE device, an electrically powered pulsatile pump, wasapproved for this purpose in 1994. The Randomised
Evaluation of Mechanical Assistance in
Treatment of Chronic Heart Failure (REMATCH)
study evaluated the long term benefit ofHeartMate
XVE placement compared with optimal medical
treatment in end-stage heart failure patients.
The rates of survival at 1 year were 52% in the device group and 25% in the medical treatment group (p¼0.002), and at 2 years the survival rates were 23% and 8% (p¼0.09), respectively. A 48% reduction in death from all causes was attributable to LVAD treatment compared with best medical treatment in this trial, and on this basis, the HeartMate XVE was approved for use as destination therapy in 2002.
Follow-up studies since REMATCH2 have shown
that uptake of chronic LVAD treatment has beenlimited because there is an unacceptably high incidence of device failure. In addition, Leitz’s work
shows that there continues to be a very high early
mortality with a continued decline in survival later.
Although REMATCH showed that LVAD implantation
improved survival compared to medical
treatment, both groups had an extremely high early
mortality and most were on inotropic support.
This underlines the importance of patient selection. In
this respect, Leitz and colleagues showed that using
a novel operative risk score encompassing severity
of heart failure, nutritional status, renal function,
and right ventricular (RV) function, the patients
with the lowest risk had the best early survival.
But, even in the sickest patients, LVAD treatment
offered a significant survival advantage, as shown
in a subsequent sub-study of the REMATCH
populationw16 and in a recent study with the
Novacor device (also a pulsatile device) in inotrope
dependent patients with end-stage heart failure.
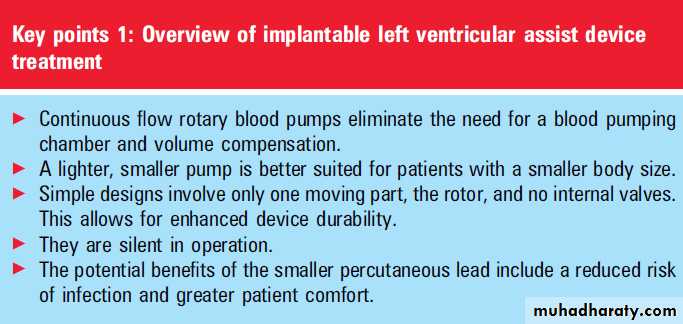
Unlike pulsatile pumps, a continuous flow pump
based on either axial or centrifugal motors can bemade smaller and more durable. They can also be
converted easily to a totally implantable system.
These types of axial flow devices have been in
development since 1988 and were first implanted in
clinical trials 10 years later (figures 1 and 2). The
advantage of these devices is their smaller size and
fewer moving parts, which should increase durability.
Concerns about non-physiological nonpulsatile output from these devices resulting in possible end-organ damage have been allayed by recent data showing their safety in relatively long term use as a bridge to transplantation when compared with pulsatile devices.
An important issue with axial flow devices is their requirement for anticoagulation and the risk of thrombosis and haemolysis.
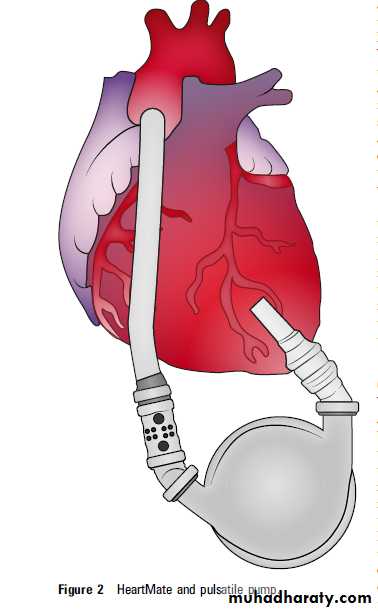
The most commonly used axial device, the HeartMate II, is already FDA approved for
bridge to transplantation. A totally implantableLVAD, the Lionheart device, was approved as
destination therapy in Europe. The absence of an
external drive-line was thought to significantly
reduce the risk of infection.w17 Unfortunately this
pump proved to have serious durability problems
with the blood sac which tended to rupture, and
this only came to light in patients after 1 year. It
has now been withdrawn from clinical practice.
In a recently reported randomised trial, Slaughter
and colleagues6 showed that a continuous flowLVAD, HeartMate ll, in patients with advanced
heart failure significantly improved the probability
of survival free from stroke and device failure at
2 years compared with a pulsatile device. The
primary composite end point was survival free
from disabling stroke and reoperation to repair or
replace the device.
This was achieved in more patients with continuous flow than with pulsatile flow devices (62 of 134 (46%) vs 7 of 66 (11%);
p<0.001; HR 0.38, 95% CI 0.27 to 0.54; p<0.001),
and patients with continuous flow devices had
superior actuarial survival rates at 2 years (58% vs
24%, p¼0.008). This is a significant achievement,
but the risk of stroke, infection and device malfunction remains a reality. In this study 59 of 134 patients (44%) receiving the continuous flow device had a disabling stroke or died within 2 years. While helpful and reliable, LVADs still represent a form of life support with a specific set of burdens and complications.
BIVENTRICULAR SUPPORT
The LVAD alone may be unsuitable for patientswith advanced congestive cardiac heart failure with concomitant RV failure. Very often, RV function improves after placement of an LVAD, when RV dysfunction has developed secondary to chronic pulmonary venous congestion, but occasionally persistent right heart failure only becomes apparent after LVAD implantation.
Specifically, in the setting of intrinsic RV myocardial dysfunction due to ischaemic heart disease or infiltrative disease, RV support may prove necessary, with or without additional LVAD support. Recently, risk factors have been identified that may help to better predict patients with ongoing RV failure after LVAD
implantation.
CURRENT DEVICES IN CLINICAL TRIALS
Cardiac assist devices that are already approved andbeing evaluated in clinical trials have been
implanted under the rather artificial designations of
either a bridge to transplantation or as destination
therapy (table 1). In reality, a significant number of
patients who were thought to be poor transplant
candidates initially became reasonably good candidates for cardiac transplantation when their
multisystem organ dysfunction improved with
effective haemodynamic support on a ventricular
assist device (VAD).
In addition, LVAD implantation as a bridge to cardiac transplantation permits effective exercise capacity and weight loss, improvement in end-organ perfusion, and even reversal of pre-existing medically unresponsive
pulmonary hypertension.
With increasing experience of VAD treatment,
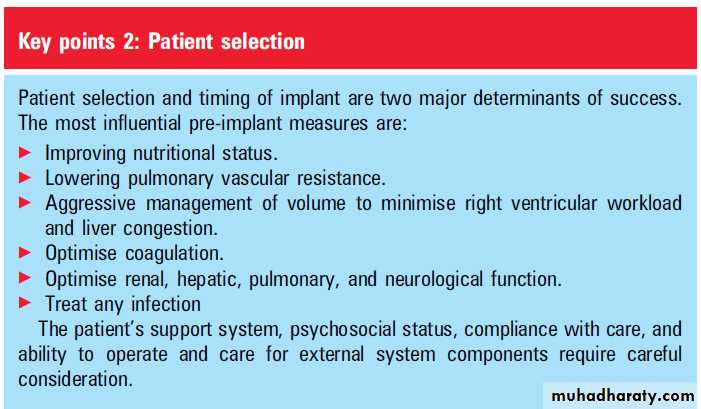
other interesting clinical and laboratory observations have been made. Myocytes at subcellular and cellular levels, as well as the heart as an organ, have displayed an ability to recover function. Birks and colleagues have reported on the very promising possibility of meaningful, clinical recovery.PATIENT SELECTION
The landmark findings of the post-REMATCHdata highlighted the importance of nutritional
parameters, haematological abnormalities, and
markers of RV failure and end-organ dysfunction in
determining mortality post-LVAD placement.
These findings shone a new light on the original REMATCH trial, in that much of the early
mortality could have been attributable to patientselection, as these patients were uniformly New
York Heart Association (NYHA) functional class IV,
with severely low cardiac indices (mean 1.9 l/min/
m2) and evidence of end-organ dysfunction (mean
serum creatinine 180 mmol/l).
In Leitz’s univariate analysis of the post-REMATCH data, highly
significant predictors for 90 day mortality post-LVAD placement were thrombocytopenia (<148 000/ml), low serum albumin (<3.3 g/dl) as a measure of nutritional deficiency, elevated aspartate aminotransferase (AST >45 U/ml) reflecting
liver congestion, and low haematocrit (<34%).
These findings have led to an increased awareness that the previous practice of LVAD implantation as a last resort in severely decompensated patients is not in their best interest, and that either LVADs should be considered earlier in the evolution of
advanced heart failure, when nutrition and endorgan
function are still optimal, or means should be taken to improve these factors preoperatively where possible.
Whether LVADs are implanted as destination
therapy or as a bridge to transplant, full commitmentfrom the patient and optimal support from
family or other caregivers is essential. In this
respect, the psychological and sociological milieu of
the patient is critical and requires detailed assessment by specialised staff before LVAD implantation, as is routinely true in the consideration of patients for cardiac transplantation.
COMPLICATIONS AFTER LVAD IMPLANTATION
The main complications specific to LVAD placementare related to driveline infection, postoperative
bleeding, and thromboembolism. Driveline infections
are common and serious if allowed to progress
to pump pocket infection, which can only be eradicated definitively by LVAD explantation. These issues underline the importance of patient and careprovider compliance with driveline exit site care.
Hopes that total implantability of assist devices and
the elimination of a driveline would reduce the risk
of infection may be realistic based on recent reports
of the Lionheart experience in Europe. Increased
perioperative mediastinal bleeding and spontaneous
haemorrhage (commonly gastrointestinal or
epistaxis and rarely intracranial) have been associated with LVAD implantation, more than what
would be expected based on the anticoagulation
regimen alone.
Some of the increased gastrointestinal bleeding may be attributable to the formation of arteriovenous malformations, which may be more common with continuous flow devices.
Recent data have shown that the increased bleeding
tendency overall may be largely attributable to
acquired platelet dysfunction due to high shear rates
and abnormal microaggregate formation, and in this regard resemble an acquired von Willebrand’s
disease.
The incidence of neurological events and
thromboembolism post-LVAD implantation is low(<20%) for both the pulsatile and non-pulsatile
devices, and for the HeartMate II, prolonged
periods of low or even no anticoagulation due to
bleeding concerns may be safe. RV failure
post-LVAD placement is associated with increased
perioperative mortality and morbidity but is difficult
to predict. Investigations are in progress to
define better means of assessing the need for RV
support post-LVAD implantation.
Other complications
seen frequently post-LVAD implantation are exudative pleural effusions. These effusions
occasionally interfere with the rehabilitation of the
patient and radiological guided drainage is effective
and safe.
BRIDGE TO RECOVERY
Reports regarding rates of recovery during pulsatileLVAD support are varied (table 2). The Columbia
group reported a 1% rate of sustained cardiac
recovery in 111 patients with ischaemic and nonischaemic aetiology. In contrast, the German Heart
Institute reported that 13% of patients with nonischaemic heart failure demonstrated sustained
recovery with a minimum follow-up of 36 months
after LVAD explantation.
The LVAD Working Group reported on a multicentre prospective study of 67 LVAD patients with both ischaemic and nonischaemic aetiology. Six per cent of the entire cohort and 7% of all non-ischaemic patients were able to undergo LVAD explantation.
There were no reports of the consistent use of pharmacological treatment during LVAD support, until the first Harefield recovery study. In this study, 15 LVAD patients received maximal doses of heart failure drugs, followed by high dose clenbuterol, a bagonist.
All patients had a non-ischaemic aetiology,and 80% had had heart failure for more than 6 months.
The authors reported that 75% of patients receiving clenbuterol could undergo LVAD explantation and 46% of all patients with nonischaemic heart failure could be managed in this way. More recently, Birks and colleagues have reported a similar experience with a continuous flow pump, HeartMate II. Thirty-three patients underwent LVAD implantation at Harefield during the 3 year study period.
Twenty-three patients (70%) with non-ischaemic cardiomyopathy were considered appropriate for the recovery protocol at the time of implantation, and 20 patients (61%) who survived LVAD implantation formed the studycohort. Using the same intensive recovery protocol as in their first study, the authors were able to demonstrate that 30% of all patients and 43% of all non-ischaemic patients could be managed to long lasting recovery.
Although this strategy appears very promising,
a number of issues remain to be resolved. Differentstrategies of continuous flow pump management
and the challenges of restarting heart failure drugs
after LVAD implantation need to be investigated.
Furthermore, the differential effects of the two
stages of drug managementdphase l: conventional
neurohormonal blockade; phase ll: clenbuterold
need to be further assessed.
THE TOTAL ARTIFICIAL HEART
The first successful implantation in an animalmodel took place in 1957; the subject, a dog,
survived just 90 min but this was a landmark
achievement. The first clinical implant occurred in
1969. The patient was successfully bridged to
transplant for 64 h but died of an overwhelming
pneumonia. Joyce and his team at the University of
Utah subsequently developed the Jarvik-7 Total
Artificial Heart (TAH), which was first implanted
in 1982. The patient survived 112 days.
Several subsequent implants took place in different centres, the longest survivor being 620 days. Due to the unacceptable morbidity and mortality as well as a very poor quality of life while on TAH support,the Jarvik-7 was no longer approved by the FDA
from 1990 onward. The updated version, the
CardioWest TAH, now known as the SynCardia
temporary TAH, was approved by the US FDA for
temporary use in patients with irreversible biventricular heart failure who are potential candidates for cardiac transplantation. This approval was granted on the basis of a multi-institutional study of 80 patients.
The survival rate has been 79% to the time of
transplantation; 86% of those survivors have livedfor 1 year after transplantation. Sixty-nine per cent
of the TAH recipients, compared with 37% of
a matched control group, have reached the endpoint
of successful post-transplantation survival (p<0.01). Stroke was seen in 10% of patients, but nearly all occurred at the time of implantation or explantation. The stroke rate during device support was <2%.w3
EXTENDING VAD TECHNOLOGY TO THE ‘LESS SICK’
In many respects, VAD technology has advancedwith a view towards engineering devices that are
small, totally implantable, and durable for years as
a long term cardiac replacement. Currently the
most promising features surfacing in technology
are third generation, magnetically levitated
impellor devices with fewer moving parts and
increased durability, and transcutaneous power
delivery, which is expected to reduce driveline
related device infection significantly.
The provision of chronic ventricular assist at an
appropriate stage of a patient’s heart failure before they deteriorate to the point of being moribund will be critical to an improved outcome, both in survival and quality of life. As new devices prove to be more patient friendly and durable, shifting the target population to a less ill group would be in the best interest of patients suffering from advanced heart failure.
Patients with less severe heart failure are also less likely to require a high output from these devices. The potential need for a device with an output of only up to 2-4 litres/min renders it conceivable to miniaturise the devices and also the route of access required for their implantation. In addition, their lower power requirements would facilitate the development of totally implantable power supply units.
Currently, many companies are developing technology for this application,
most notably Circulite Inc, whose Synergy devicerequires minimally invasive access for implantation,
delivers up to 4 litres/min of blood to the
aorta via the left subclavian artery, and has
a completely implantable power supply. Devices of
this kind with minimal morbidity related to
implantation have already been used successfully as
a bridge to transplantation.
They may potentially alter the course of the disease in advancing heart failure, and are the focus of clinical trials.
Cardiac assistance such as this in the otherwise
reasonably compensated patient may even allow
intrinsic myocardial recovery and reverse remodelling, as has been shown for larger LVADs.
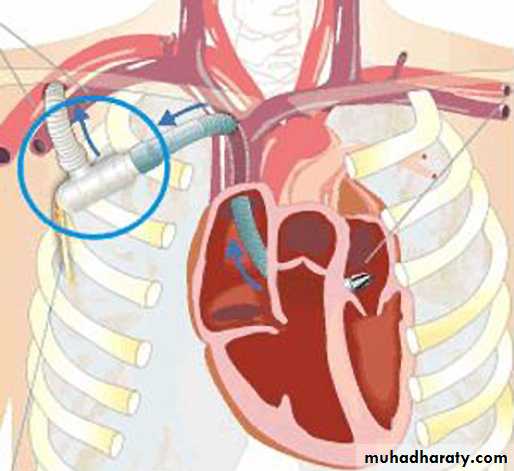
Meyns and colleagues have reported on long
term partial support with the Synergy PocketMicro-pump (CircuLite Inc, Saddle Brook, New
Jersey, USA) (figure 3). The operation uses a minimally
invasive incision below the right clavicle that
allows for pump outflow to the axillary artery and
provides pump inflow through the interatrial
groove into the left atrium.
They introduced the pump into 17 patients (14 men), aged 53 (69 years) with an ejection fraction of 2166%, mean arterial pressure 7367 mm Hg, pulmonary capillary wedge pressure 2966 mm Hg, and cardiac index 1.960.4 l/min/m2. The duration of support ranged from 6‒213 (median 81) days. Nine patients underwent follow-up right heart catheterisation at 10.666 weeks.
These patients showed significant increases in arterial pressure (6768 mm Hg vs 8069 mm Hg; p<0.01) and cardiac index (2.060.41 l/min/m2 vs 2.860.61 l/min/m2; p¼0.01), with large reductions in pulmonary capillary wedge pressure (3065 mm Hg vs 1865 mm Hg; p¼0.001).
A major concern with partial support has been
that pumps might be more liable to develop pumpthrombosis at low flow. The pump was modified
after stopping the clinical trial and performing
bench testing, and then a new pump was released
with enhanced washing within the rotor and a new
target international normalised ratio (INR). With
the new design and anticoagulation strategy, there
have had been no further episodes of pump
thrombosis with 29 implants over a period of
support of 14 months.
COST EFFECTIVENESS
Heart failure is associated with substantialmorbidity and mortality, leading to frequent
admissions to hospital and long term drug costs. As
a consequence it is a major cost to the NHS and increasingly the focus of policy initiatives. Clegg
and colleagues13 reported on the clinical outcome
and cost effectiveness of LVADs as a bridge to heart
transplantation.
They conducted a systematic review and an economic evaluation according to internationally recognised methods. They found that LVADs appear beneficial, improving survival, functional status and quality of life, but adverse events were a serious concern. The economic evaluation showed that LVADs had a cost per quality adjusted life year of £65 242 (€78 410, US$100 585).
They concluded that it is unlikely that they will be
cost effective unless costs decrease or the benefits of
their use increase.
A report from Duke Universityw35 examined
long term outcomes and costs of VADs among allMedicare claimants for the period 2000 to 2006.
Overall 1 year survival was 51.6% (N¼669 out of
1476) in the primary device group and 30.8%
(N¼424 out of 1467) in the post-cardiotomy
group. Among primary device patients, 815
(55.2%) were discharged to home with a device.
Of those, 450 (55.6%) were readmitted within
6 months and 504 (73.2%) were alive at 1 year.
Of the 493 (33.6%) post-cardiotomy patients
discharged to home with a device, 237 (48.3%)were readmitted within 6 months and 355 (76.6%)
were alive at 1 year. The authors concluded that
improving patient selection and reducing perioperative mortality will be critical for improving
overall patient outcomes.
CONCLUSION
LVADs have been shown to be efficacious as
a bridge to transplantation and as destination
therapy in advanced heart failure. The threshold
level of heart failure beyond which patients will
benefit from the insertion of an LVAD needs to be
determined.
Currently, LVADs are indicated in patients with advanced heart failure who cannot be
weaned from inotropic support and who havea cardiac index <2.0 l/min/m2, a systolic blood
pressure <80 mm Hg, and a pulmonary capillary
wedge pressure >20 mm Hg (Hunt 200114). As the
technology improves and as LVADs get smaller,
more efficient and safer, it is likely that this
threshold level will change such that patients with
less advanced heart failure may also benefit from an
LVAD (Birks 201015).
Figure 1 HeartMate II rotary axial impeller pump.