Management of chronic totalocclusion by percutaneous coronaryintervention
BMJ July 3, 2012د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
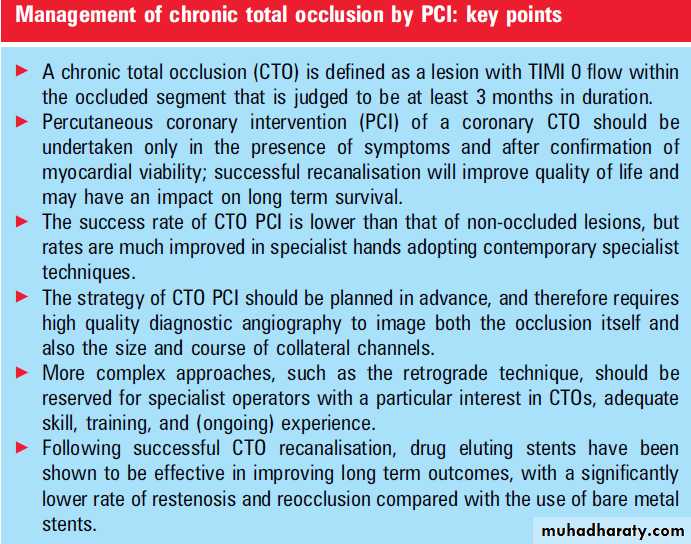
A chronic total occlusion (CTO) is defined as complete occlusion of the coronary vessel with TIMI 0 flow, present for an (estimated) duration of 3 months. In patients with significant coronary artery disease (defined as at least one major epicardial vessel with a stenosis 70%), a CTO is common and found in approximately half of such patients.
Importantly, the presence of a CTO has a major impact on management, and is a strong predictor against recommending percutaneous coronary intervention (PCI), with a preference for medical treatment or coronary artery bypass surgery (CABG). This relates to perceived difficulties in recanalisation; however, in recent years,
through advances in specialist equipment and
techniques, expert operators have significantly
improved recanalisation rates leading to a resurgence
of interest in CTO PCI. The purpose of this article is to review the available clinical data and discuss the contemporary management of CTOs by PCI.
WHY IS IT BENEFICIAL TO OPEN A CTO?
Multiple studies have demonstrated that successful
CTO PCI improves quality of life, through reducing
symptoms of angina, improving exercise capacity,
improving left ventricular function, and reducing
the need for subsequent CABG. Furthermore,
multiple registries have demonstrated that successful CTO PCI is associated with improved long term survival compared to patients with an unsuccessful PCI (table 1), with some data suggesting that this is particularly the case for left anterior descending artery occlusions.
The benefit in outcome is only partly explained by
adverse events directly associated with an unsuccessful procedure, and appears to relate to fewer events occurring at long term follow-up. Thepotential reasons for this are poorly defined but
possibly include a reduction in ischaemia driven
arrhythmia. Certainly patients undergoing primary
PCI for ST elevation myocardial infarction (STEMI)
have a significantly higher mortality rate if they have
a concurrent CTO in another major vessel. In
one series of 3277 STEMI patients, the presence of
a concurrent CTO was a strong and independent
predictor of 30 day mortality (HR 3.6, 95% CI 2.6
to 4.7; p<0.01).
Furthermore, in those patients alive at 30 days, the presence of a CTO (and not simply the presence of multivessel disease) remained a predictor of 5 year mortality (HR 1.9,
95% CI 1.4 to 2.8; p<0.01). Successful opening of aCTO with PCI may therefore increase patients’
tolerance to future cardiac events.
One of the criticisms of the current data is that there are no randomised studies that compare percutaneous revascularisation with optimal medical treatment in patients with ischaemia due to a CTO. The Open Artery Trial (OAT) is often (inappropriately) discussed in this context. This randomised study compared angioplasty versus conservative therapy in (asymptomatic) patients in the early period following myocardial infarction. It
demonstrated no advantage to opening the occluded artery; however, it must be appreciated that this was a different patient population not symptomatic patients with a CTO causing ischaemia.
Multiple studies have shown that complete revascularisation is a predictor of improved outcome;
however, in patients managed with PCI, the presence
of a CTO is an independent predictor of incomplete revascularisation. Hannan et al evaluated
11 294 patients treated in the era of drug eluting
stents (DES); 7795 (69.0%) were incompletely
revascularised, and had a higher mortality rate at
18 months (adjusted HR 1.23, 95% CI 1.04 to 1.45).
Patients with two unattempted diseased vessels including a CTO were at greatest risk
(adjusted survival HR 1.44, 95% CI 1.14 to 1.82; riskadjusted survival 94.9% vs 92.9%; p¼0.002). One
study specifically evaluated patients (n¼486) with
a CTO (n¼527 CTO lesions). Cardiac survival at
a median follow-up of 2.0 years was significantly
higher in those with complete revascularisation
compared to incomplete revascularisation (94.061.7
vs 83.863.6%; p<0.001)
RESULTS OF PCI FOR CTO: THE IMPACT OF DES
Historical studies of bare metal stents (BMS)
demonstrate that CTO lesions were associated
with a relatively increased risk of restenosis (and
reocclusion). This may relate to the complexity of
the disease per se, and also the requirement for
relatively long stents.
Several studies, including one randomised study of the sirolimus eluting (Cipher) stent,6 have demonstrated efficacy of DES at (long-term) follow-up (table 2). Two metaanalyses have been recently publishedand confirm safety, with no difference in terms of survival or the occurrence of myocardial infarction in those treated with DES and BMS. However, the use of DES was associated with a significantly lower rate of restenosis, reocclusion, and need for repeat revascularisation.
ASSESSMENT OF THE PATIENT
As with all elective PCI, it is important to assessthe patient adequately before undertaking the
procedure. Specifically, informed consent must
incorporate a discussion of the individual operator’s
success rate and a discussion of the risks and
alternative therapeutic options. Perhaps the most
important pre-procedural assessment relates to
ensuring the presence of myocardial viability in the
territory of the CTO.
Viability is presumed when left ventricular function is good; however, regional hypokinesia/akinesia indicates the need for additional investigation such as stress echocardiography, nuclear imaging, MRI, or positron emission tomography (PET). Both the recent US and European guidelines regarding CTO revascularisation have stressed the importance of performing PCI only in the presence of symptoms (despite good medical treatment), or considered, if asymptomatic, only if there is a large area of reversible ischaemia; this emphasises the importance of risk stratification with non-invasive imaging.The benefits of using DES mean that patients should be assessed to determine whether they are suitable for (1 year) dual antiplatelet therapy.
.
Specific to the PCI procedure, dual arterial access
may sometimes be required (when needed, this istypically bilateral femoral access, though with
increasing use of one or more radial arteries) and
this should be evaluated. In addition, CTO PCI
may involve relatively large volumes of contrast so
it is important to consider the baseline renal function
with a low threshold for renal protection
therapy including intravenous saline infusion.
ASSESSMENT OF THE LESION
It cannot be underestimated the importance ofperforming high quality diagnostic angiography in
order to evaluate better the chances of success and plan the interventional procedure. The proximal cap needs to be clearly imaged as to location, configuration, and relationship with any side branches.
It is essential to make long acquisition runs to understand the degree of filling of the distal
vessel, both when opacifying the target vessel as
well as when performing angiography of the
opposite vessel to clearly delineate the size and
extent of filling via retrograde collaterals. This
enables the interventionist to appreciate the length
of the occlusion and to plan PCI strategy.
As described below, specialist CTO operators may
approach the occlusion in a retrograde fashion via
the collaterals from another coronary vessel, so it is
important to make sufficient images to delineate
the size and course of these small vessels adequately. This may require taking extra diagnostic images for example, a patient with a proximal left anterior descending (LAD) artery occlusion may need an image made of the right coronary artery in a right anterior oblique (RAO) cranial projection to delineate septal collaterals filling the distal LAD.
By positioning the table appropriately, panning during acquisition runs should be avoided.
It is important to understand that the presence ofretrograde collaterals does not necessarily imply
myocardial viability of the CTO territory, and
when necessary, assessment of viability should be
made as described above.
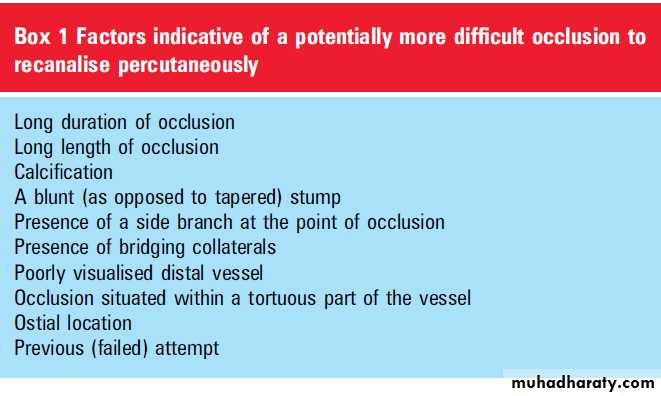
Features of a CTO that may indicate a more
difficult PCI are listed in box 1; however, none ofthese features should necessarily preclude an
attempt at percutaneous recanalisation. Studies
from expert operators in Japan suggest that in
contemporary practice, the most important
predictors of failed CTO recanalisation are severe
tortuosity and moderate-to-severe calcification.
CT angiography provides additional information
and is superior to conventional angiography in
assessing occlusion length, and in identifying the
severity and location of calcification.
This information may affect PCI strategy for example, heavy calcification at the proximal cap may indicate the need for a highly penetrative guidewire, or
indicate a low threshold to consider a dual (antegrade and retrograde) approach. Non-invasive coronary imaging should therefore be contemplated,
particularly if the patient is being considered for a second PCI attempt. In the future, patients may be initially evaluated with diagnostic multislice CT
(MSCT) angiography, and the three dimensional reconstruction images then linked to the images acquired in the cardiac catheterisation laboratory
during angioplasty to help guide recanalisation.
ANGIOPLASTY SUCCESS: IMPORTANCE OF
OPERATOR EXPERIENCEThe success rate of CTO PCI remains lower than
PCI for more straightforward lesions; audit returns
in the UK suggest that the success rate for CTO
recanalisation has remained remarkably stable over the last decade.
In 2008, 3445 patients with stable angina underwent PCI for a CTO, which was successful in 68.0% of cases. However, also during the last decade, colleagues in Japan have shown that expert operators, with the correct equipment and specialised techniques, can achieve success rates of >85%. Importantly, this high recanalisation rate can be achieved with relatively low
complication rates.
Adoption of these strategies by interventionalists
with an interest in CTO PCI has been shown tohave a similar degree of success both in Europe
and the USA.16 Expertise remains critical to
success; in one study of 636 consecutive patients
treated in two centres in the USA, the success rate
for two high volume specialist CTO operators was
75% compared with 59% for ‘standard’ operators.
Furthermore, the success rate of the two CTO operators who incorporated strategies including retrograde techniques improved to 90% over time, while that of the non-specialist operators remained stable.
Procedures performed by the expert operators
were shorter (mean procedure time 107 min vs
141 min, p<0.0001), and utilised less contrast
(342 ml vs 434 ml, p<0.0001). This underscores the
opinion of bodies such as the European CTO club
that only high volume operators, who have a higher
chance of success, should perform CTO PCI; this
reduces the need for second attempt procedures,
and thereby ensures better utilisation of resources.
EQUIPMENT
Specialist guidewires are commonly required forCTOs, with features such as hydrophilic coating to
make them slippery, having a stiff tip, or a tapered
distal tip (from 0.014 inch down to 0.009 inch).
The tip load is the amount of force required to bend
or buckle the tip of the wire, and is #1 g for floppy
wires (eg, workhorse wires for non-CTO angioplasty),
and z3 g for intermediate wires; specialist
stiff/tapered CTO guidewires have higher tip loads.
It is vital that operators understand the properties
of the wire that they are using. Soft, hydrophilic
wires ‘slip’ through but have relatively poor tactile
feedback and have a tendency to ‘follow the path of least resistance’ into the subintimal space. Stiff
wires, particularly when combined with a tapered
tip, have greater penetration power, so are particularly useful for old fibrotic or heavily calcified occlusions. All the specialist wires have a greater risk of perforation as compared with standard ‘workhorse’ wires.
Histology is interesting in that it provides evidence that 75% of CTO lesions, as evaluated on angiography, are in fact not completely blocked.
Neovascularisation is associated with microvessels
that frequently have a diameter of 100e200 mm,
though they may be as large as 500 mm. Some
microvessels run radially and can be recognised as
bridging collaterals; the potential disadvantage is
that via these channels, slippery wires may pass
into the subintimal space.
However, other microvessels run longitudinally and can therefore facilitate successful wire crossing. Coronary angiography has a resolution of approximately
100-200 mm so it can be appreciated that small
microchannels will not be visualised. To put the
size of these microchannels into context, the tip of
a 0.014 inch guidewire is approximately 360 mm,
and a 0.009 inch tip is 230 mm.
SPECIALIST TECHNIQUES
Angiography is not particularly good at predicting
how straightforward a CTO will be to recanalise.
Optimal guide catheter support should be chosen
from the outset; many specialist operators use $7F
as certain specialised CTO techniques cannot be
performed through 6F catheters. The use of penetrative
CTO wires should be guided by clear visualisation
of the distal vessel, commonly utilising
simultaneous injection into the contralateral artery
(therefore requiring two arterial access sites).
A microcatheter or over-the-wire balloon is positioned
proximal to the occlusion, and a specialistCTO wire with a very short (1e2 mm) tip is used
to try to negotiate across the occlusion. In
contemporary practice, many operators favour the
Fielder XT (Abbott Vascular) to see whether the
tapered tip (0.009 inch) can traverse through
microchannels. If unsuccessful, increasingly penetrative
wires may then be taken.
Parallel wires After a wire passes into the subintimal space, it is left in position to encourage a second wire, in parallel to the first, to find an alternative path into
the distal true lumen.
The two guidewires can be manipulated in turn in a ‘see-saw’ fashion until one is successful.
Anchor balloon The ‘anchor balloon’ technique increases backup support; a floppy wire is placed in a side branch, and a compliant balloon (vessel:balloon ratio of 1:1) is gently inflated to ‘fix’ the guiding catheter position.
This facilitates increased forward penetration
power with the CTO guidewire/balloon.
The retrograde approach
One of the key advances to increased success ratesis to approach the CTO in a retrograde fashion via
collaterals (figure 1). The technique has been
extensively described elsewhere17 and is best
reserved for suitably experienced/trained operators.
Both septal and epicardial collaterals may be
utilised; septal channels are preferred as there is
a small risk of perforation occurring, particularly if
the vessels are tortuous.
If perforation occurs from a septal collateral, this is usually self-limiting and without serious adverse consequences; however,
perforation of an epicardial vessel is far more likely to lead to tamponade and even emergency surgery.
Favourable anatomical features of a suitable
collateral include its size, course (relatively straight
as opposed to corkscrew), and lack of angulation
when joining the distal vessel.
Collaterals are usually traversed with a hydrophilic-coated wire with support from the specialist Corsair catheter (see below). Once in the main vessel distal to the CTO, the retrograde guidewire may then be
replaced with a more penetrative one, and the CTO
can then be approached simultaneously from both
the proximal and distal caps. If a Corsair will not
track into the distal vessel (or is not available), then
a very small over-the-wire balloon or microcatheter
may be used as an alternative.
The CART (controlled antegrade and retrograde
subintimal tracking) and (more commonly) reverse-CART techniques are used when the wires are
unable to progress and have passed into the
subintimal space. A balloon is inflated, usually
on the antegrade wire, to create a localised dissection
and enlarge the size of the subintimal space to
facilitate advancement of the other wire into the
same space, and then subsequently into the true
vessel lumen.
An additional technique to facilitate this approach is to form a loop or ‘knuckle’ on the distal end of a soft-tipped hydrophilic wire. This enlarges the subintimal space and also speeds up
wire progression as the ‘knuckle’ follows the path
of least resistance along the vessel adventitia
without perforating it.
The techniques described may be time consuming, and it is important to ensure adequate
anticoagulation with the activated clotting time
(ACT) monitored to remain >300 s. It is vital to
pay close attention to both the left and right
coronary arteries and ensure prompt treatment
should complications occur such as guide catheterinduced dissection.
Intravascular ultrasound
The adjunctive use of intravascular ultrasound(IVUS) may facilitate recanalisation. Used in suitable
anatomy, IVUS performed in a side branch can
identify precisely the site of the proximal CTO cap
to facilitate correct puncture. IVUS guidance may
also be useful to direct the guidewire puncture from
the subintimal space back into true lumen.
SPECIALIST EQUIPMENT
Developed in Japan, the Tornus and Corsair cathetershave made a significant impact on the practice
of specialist CTO interventionalists. Both have
a braided steel wire design with a tapered tip and
are used to enlarge the vessel by ‘screwing’ forwards. Following successful passage of a guidewire cross a CTO, the Tornus device may be able
to traverse the occlusion when it has not been
possible to pass even a small balloon. The Corsair
catheter is smaller and specifically designed to
traverse collateral channels during a retrograde
approach (figure 2).
It is able to track around even tortuous vessels and has become an invaluable part of the CTO interventionalists ‘toolbox’.
Over the years, several specialist devices have been tried, but most have not improved success rates compared to expert operators. The most promising new device is the Stingray CTO re-entry system (Bridgepoint Medical, Minneapolis, Minnesota, USA) which is designed to facilitate wire passage from the sub-intimal space back into the true lumen of the distal vessel beyond the occlusion. Preliminary evaluation of 147 patients with a failed antegrade attempt demonstrated a technical success rate of 77%, with a 4.8% 30 day adverse event rate.
Encouragingly, success rates increased to 86% in the second half of the study.
The device has been recently introduced to limitedCTO operators in the UK and is under evaluation.
RISKS, COMPLICATIONS, AND WHEN TO STOP
As with all PCI, there is a (small) risk of procedural
complications, with increased event rates following
unsuccessful PCI (table 3). However, in expert hands, CTO PCI can be performed at relatively low risk.
A study of 904 consecutive patients treated by
an experienced Japanese group demonstrated lowrates of procedural mortality (0.6%), urgent CABG
(0.2%), Q wave myocardial infarction (0.6%),
delayed tamponade (0.7%), and significant (type 2) perforation (0.6%).11 The low mortality rate is seen in other contemporary registries, with no deaths reported by Valenti et al and Thompson et al, and is supported by results of 1262 patients treated at the Mayo Clinic demonstrating reduced inhospital mortality over time (2.4% for those treated 1979e1989, 1.3% for 1990e1996, 0.4% for 1997eMarch 2003, 0% for April 2003July 2005; p¼0.009).
The occurrence of perforation and tamponade
remains a concern of CTO PCI due to the need forpenetrative wires. In one study of 152 patients
treated in Chinese hospitals without on-site
surgery, the success rate was 86.8%; however, of the
20 patients with an unsuccessful procedure, perforation
occurred in five (25%) and two died (10%).
The authors concluded that CTO PCI was unsafe if
performed in remote hospitals without CABG facilities.
It is vital that operators can identify and manage
complications promptly and effectively, and the
department must be stocked with appropriate
equipment such as pericardiocentesis kits, covered
stents and coils. After the procedure, whether
successful or unsuccessful, the patient must be
closely monitored and any concerns regarding
haemodynamic status should be investigated
promptly with echocardiography to exclude
delayed tamponade; it should not be assumed that
the patient has simply had a ‘vasovagal’ episode.
CTO procedures may be prolonged and it is
important to have in mind from the outset whenthe procedure should be halted. In the absence of
a procedural complication such as significant
perforation, factors such as radiation dose (especially
in overweight patients), contrast load, and
patient comfort must be taken into account.
CONCLUSIONS
PCI is indicated for patients with a CTO who haveongoing symptoms despite medical treatment, and
a successful procedure will improve quality of life.
Registry data suggest that successful revascularisation
may also incur a survival benefit as it improves
tolerance to future cardiac events. The success rate
of CTO PCI is highly dependent on operator expertise, and consideration should be made to refer patients with more complex lesions to specialist interventional cardiologists with a particular interest in CTOs.
It is important to plan PCI strategy in advance.
This requires high quality diagnostic angiography
to evaluate not only the occlusion itself but also
include long acquisitions to delineate clearly the
degree of retrograde filling and identify the presence, size, and course of collateral vessels.
Once recanalised, the use of DES improves the long term outcome.
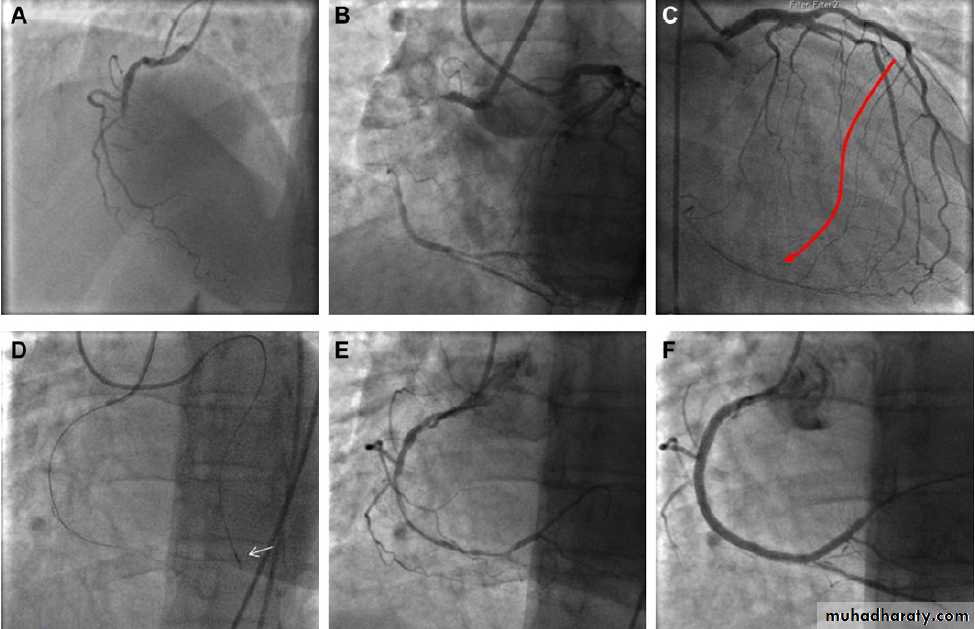
Figure 1 Example of a chronic total occlusion (CTO) of the right coronary artery (RCA) that was treated successfully through a retrograde approach.
The patient was a 56-year-old woman with single vessel disease and class II angina despite good medical treatment. The occlusion had previously
been attempted (unsuccessfully) from an antegrade approach. (A) Baseline image. (B) The length of the occlusion can be estimated from simultaneous
injections into both the left and right coronary arteries.
(C) A septal collateral vessel can be seen supplying blood to the distal RCA. This has several
promising features in that it is of relatively large calibre for such a vessel, has a relatively straight course, and joins the distal vessel without severe
angulation. (D) The collateral was wired with a Fielder FC wire supported by a Corsair catheter (arrow). (E) Using the retrograde wire as a guide, the
antegrade wire was able to be manipulated into the same channel as the retrograde wire and delivered into the distal RCA. The vessel was pre-dilated
in the usual manner and then stented. (F) Final result.