Guidelines for the Diagnosis andManagement of Heart Failure in Adults
© 2009 by the American College of Cardiology Foundation and the American Heart Associationد. حسين محمد جمعة
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
تموز 2010
The clinically overt DHF and SHF appear to be 2 separate syndromes with
distinctive morphologic and functional changes althoughsigns, symptoms, and prognosis are very similar.
In DHF, the left ventricle is not dilated and the ejection fraction is preserved.
In contrast in SHF, it is dilated and the ejection fraction is reduced.
The neurohormonal abnormalities in DHF and SHF appearto be similar.
The stimuli and the signals that ultimately produce these 2 different phenotypes of chronic heart failure remain, presently, largely unknown.
Heart failure is a syndrome manifesting as the inability of the heart to fill with or eject blood due to any structural or functional cardiac conditions. when the heart does not fully relax, so it does not fill properly with blood. This is called diastolic heart failure.
Heart failure may be caused by myocardial failure but may also occur in the presence of near-normal cardiac function under conditions of high demand. Heart failure always causes circulatory failure, but the converse is not necessarily the case because various noncardiac conditions (eg, hypovolemic shock, septic shock) can produce circulatory failure in the presence of normal, modestly impaired, or even supranormal cardiac function.
In terms of incidence, prevalence, morbidity, and mortality, the epidemiologic magnitude of heart failure (HF) is staggering. According to the American Heart Association, heart failure is a condition that affects nearly 5.7 million Americans of all ages and is responsible for more hospitalizations than all forms of cancer combined.
It is the number 1 cause for hospitalization among Medicare patients.
With improvement in survival of acute myocardial infarctions and a population that continues to age, heart failure will continue to increase in prominence as a major health problem in the United States.
Heart failure (HF) is a major and growing public health
Problem.The numberof HF deaths has increased steadily despite advances in
treatment, in part because of increasing numbers of patients with HF due to better treatment and “salvage” of patients with acute myocardial infarctions (MIs) Heart failure is primarily a condition of the elderly,
and thus the widely recognized “aging of the population”
also contributes to the increasing incidence of HF. The
incidence of HF approaches 10 per 1000 population after
age 65, and approximately 80% of patients hospitalized
with HF are more than 65 years old..
Pathophysiology
Regardless of the precipitating event, the common pathophysiologic state that perpetuates the progression of heart failure is extremely complex. Compensatory mechanisms exist on every level of organization from sub-cellular all the way through organ-to-organ interactions.Only when this network of adaptations becomes overwhelmed does heart failure ensue. In this section, we focus on those
adaptations that represent significant therapeutic targets in the treatment of heart failure.
Most important among these adaptations are the
(1) Frank-Starling mechanism, in which an increased preload helps to sustain cardiac performance;
(2) alterations in myocyte regeneration and death;
(3) myocardial hypertrophy with or without cardiac chamber dilatation, in which the mass of contractile tissue is augmented; (4) activation of neurohumoral systems, especially the release of norepinephrine by adrenergic cardiac nerves, which augments myocardial contractility and includes activation of the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system (SNS), and other neurohumoral adjustments that act to maintain arterial pressure and perfusion of vital organs.
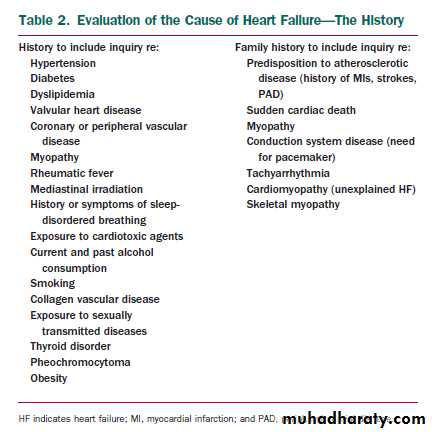
Stages of Heart Failure (UPDATED)
The HF writing committee previously developed a new approach to the classification of HF ,one that emphasized both the development and progression of the disease. In doing so, they identified 4 stages involved in the development of the HF syndrome. The first 2 stages (A and B) are clearly not HF but are an attempt to help healthcare providers with the early identification of patients who are at risk for developing HF.Stages A and B patients are best defined as those with risk factors that clearly predispose toward the development of HF.
Stage A
For example, patients with coronary artery disease, hypertension, or diabetes mellitus who do not yet demonstrate impaired left ventricular (LV) function, hypertrophy, or geometric chamber distortion .
Stage B
whereas patients who are asymptomatic but demonstrate LV hypertrophy (LVH) and/or impaired LV function would be designated as Stage B
Stage C then denotes patients with current or past symptoms of HF associated with underlying structural heart disease (the bulk of patients with HF), and
Stage D designates patients with truly refractory HF who might be eligible for specialized, advanced treatment strategies, such as mechanical circulatory support, procedures to facilitate fluid removal, continuous inotropic infusions, or cardiac transplantation or other innovative or experimental surgical procedures, or for end-of-life care.
This classification recognizes that there are established
risk factors and structural prerequisites for the development of HF and that therapeutic interventions introduced even before the appearance of LV dysfunction or symptoms can reduce the population morbidity and mortality of HF.
This classification system is intended to complement but in no way to replace the New York Heart Association (NYHA) functional classification, which primarily gauges the severity of symptoms in patients who are in Stage C or Stage D.
According to this new staging approach, patients would only be expected to either not advance at all or to advance from one stage to the next, unless progression of the disease was slowed or stopped by treatment, and
spontaneous reversal of this progression would be considered unusual.
For instance,
although symptoms (NYHA functional class) might vary widely over time (in response to therapy or to progression of disease) in a patient who has already developed the clinical syndrome of HF (Stage C), the patient could never return to Stage B (never had HF), and therapies recommended for Stage C will be appropriate even if this patient is in NYHA class I.This new classification scheme adds a useful dimension to our thinking about HF that is similar to that achieved by staging or risk assessment systems for other disorders (e.g., those used in the approach to cancer).
Definition of Heart Failure
Heart failure is a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.The cardinal manifestations of HF are dyspnea and fatigue,
which may limit exercise tolerance, and fluid retention, which may lead to pulmonary congestion and peripheral edema. Both abnormalities can impair the functional capacity and quality of life of affected individuals, but they do not necessarily dominate the clinical picture at the same time.
Some patients have exercise intolerance but little evidence of fluid retention, whereas others complain primarily of edema and report few symptoms of dyspnea or fatigue.
Because not all patients have volume overload at the time of initial or subsequent evaluation, the term “heart failure” is preferred over the older term “congestive heart failure.”
The clinical syndrome of HF may result from disorders of
the pericardium, myocardium, endocardium, or great vessels,but the majority of patients with HF have symptoms
due to an impairment of LV myocardial function. Heart
failure may be associated with a wide spectrum of LV
functional abnormalities, which may range from patients
with normal LV size and preserved EF to those with severe dilatation and/or markedly reduced EF.
In most patients,abnormalities of systolic and diastolic dysfunction coexist,regardless of EF.
Coronary artery disease, hypertension, and dilated cardiomyopathy are the causes of HF in a substantial proportion of patients in the Western world. As many as 30% of patients with dilated cardiomyopathy may have a genetic cause .Valvular heart disease is still a common cause of HF. In fact, nearly any form of heart disease may ultimately lead to the HF syndrome.
It should be emphasized that HF is not equivalent to
cardiomyopathy or to LV dysfunction; these latter termsdescribe possible structural or functional reasons for the
development of HF. Instead, HF is defined as a clinical
syndrome that is characterized by specific
symptoms (dyspnea and fatigue) in the medical history and signs (edema,rales) on the physical examination.
There is no single diagnostic test for HF because it is largely a clinical diagnosis that is based on a careful history and physical examination.
Heart Failure as a Symptomatic Disorder
The approach that is most commonly used to quantify the degree of functional limitation imposed by HF is one first developed by the NYHA. This system assigns patients to 1 of 4 functional classes, depending on thedegree of effort needed to elicit symptoms: patients may have symptoms of HF at rest (class IV), on less-than-ordinary exertion (class III), on ordinary exertion (class II), or only at levels of exertion that would limit normal individuals (class I) .
the severity of symptoms characteristically
fluctuates even in the absence of changes in medications,.
The mechanisms responsible for the exercise intolerance
of patients with chronic HF have not been defined clearly.Although HF is generally regarded as a hemodynamic
disorder, many studies have indicated that
there is a poor relation between measures of cardiac performance and the symptoms produced by the disease. Patients with a very low EF may be asymptomatic, whereas patients with preserved LVEF may have severe disability.
The apparent discordance between EF and the degree of functional impairment is not well understood but may be explained in part by alterations in ventricular distensibility,valvular regurgitation, pericardial restraint, cardiac rhythm,conduction abnormalities, and right ventricular function.
In addition, in ambulatory patients, many noncardiac
factors may contribute substantially to exercise intolerance.These factors include but are not limited to changes in
peripheral vascular function, skeletal muscle physiology,
pulmonary dynamics, neurohormonal and reflex autonomic activity, and renal sodium handling. The existence of these noncardiac factors may explain why the hemodynamic improvement produced by therapeutic agents in patients with chronic HF may not be immediately or necessarily translated into clinical improvement.
Heart Failure as a Progressive Disorder
Left ventricular dysfunction begins with some injury to, orstress on, the myocardium and is generally a progressive
process, even in the absence of a new identifiable insult to
the heart. The principal manifestation of such progression is
a change in the geometry and structure of the LV, such that
the chamber dilates and/or hypertrophies and becomes
more spherical—a process referred to as
cardiac remodeling.
This change in chamber size and structure not only increases the hemodynamic stresses on the walls of the failing heart and depresses its mechanical performance but may also increase regurgitant flow through the mitral valve.
Cardiac remodeling generally precedes
the development of symptoms (occasionally by months or even years), continues after the appearance of symptoms,and contributes substantially to worsening of symptoms despite treatment.
Progression of coronary artery disease,diabetes mellitus, hypertension, or the onset of atrial fibrillation may also contribute to the progression of HF.
there is substantial evidence that the activation of endogenous neurohormonal systems plays an important role in cardiac remodeling and thereby in the progression of HF. Patients with HF have elevated circulating or tissue levels of norepinephrine, angiotensin II, aldosterone, endothelin,vasopressin, and cytokines, which can act (alone or in concert) to adversely affect the structure and function of the heart.
These neurohormonal factors not only increase the hemodynamic stresses on the ventricle by causing sodium retention and peripheral vasoconstriction but may also exert direct toxic effects on cardiac cells and stimulate myocardial fibrosis, which can further alter the rchitecture and impair the performance of the failing heart. Neurohormonal activation also has direct deleterious effects on the myocytes and interstitium, altering the performance and phenotype of these cells.
Initial and Serial Clinical Assessment of
Patients Presenting With Heart FailureCLASS I
1. A thorough history and physical examination should be obtained/performed in patients presenting with HF to identify cardiac and noncardiac disorders or behaviors that might cause or accelerate the development or progression of HF. (Evidence: C)
2. A careful history of current and past use of alcohol, illicit drugs,current or past standard or “alternative therapies,” and chemotherapy drugs should be obtained from patients presenting with HF. (Level of Evidence: C)
3. In patients presenting with HF, initial assessment should be made of the patient’s ability to perform routine and desired activities of daily living. (Level of Evidence: C)
4. Initial examination of patients presenting with HF should include assessment of the patient’s volume status, orthostatic blood pressure changes, measurement of weight and height, and
calculation of body mass index. (Evidence: C)
5. Initial laboratory evaluation of patients presenting with HF should include complete blood count, urinalysis, serum electrolytes
(including calcium and magnesium), blood urea nitrogen, serum creatinine, fasting blood glucose (glycohemoglobin), lipid profile, liver function tests, and thyroid-stimulating hormone. Evidence: C
6. Twelve-lead electrocardiogram and chest radiograph (posterioranterior and lateral) should be performed initially in all patients presenting with HF. (Level of Evidence: C)
7. Two-dimensional echocardiography with Doppler should be performed during initial evaluation of patients presenting with HF to assess LVEF, left ventricular size, wall thickness, and valve function. Radionuclide ventriculography can be performed to assess LVEF and volumes. (Level of Evidence: C)
8. Coronary arteriography should be performed in patients presenting with HF who have angina or significant ischemia unless the patient is not eligible for revascularization of any kind . (Level of Evidence: B)
CLASS IIb
1. The value of serial measurements of BNP to guide therapy for
patients with HF is not well established. (Level of Evidence: C)
Identification of Patients
In general, patients with LV dysfunction or HF present to the healthcare provider in 1 of 3 ways:1. With a syndrome of decreased exercise tolerance.
Most patients with HF seek medical attention with complaints of a reduction in their effort tolerance due to dyspnea and/or fatigue. These symptoms, which may occur at rest or during exercise, may be attributed inappropriately by the patient and/or healthcare provider to aging, other physiological abnormalities (e.g., deconditioning), or other medical disorders (e.g., pulmonary disease).
Therefore, in a patient whose exercise capacity is limited by dyspnea or fatigue, the healthcare provider must determine whether the principal cause is HF or another abnormality. Elucidation of the precise reason for exercise intolerance can be difficult because several disorders may coexist in the same patient. A clear distinction can sometimes be made only by measurements of gas exchange or blood oxygen saturation or by invasive hemodynamic measurements during graded levels of exercise.
2. With a syndrome of fluid retention.
Patients may present with complaints of leg or abdominal swelling as their primary (or only) symptom. In these patients, the impairment of exercise tolerance may occur so gradually that it may not be noted unless the patient is questioned carefully and specifically about a change in activities of daily living.3. With no symptoms or symptoms of another cardiac or noncardiac disorder.
During their evaluation for adisorder other than HF (e.g., abnormal heart sounds or abnormal electrocardiogram or chest x-ray, hypertension or hypotension, diabetes mellitus, an acute myocardialinfarction (MI), an arrhythmia, or a pulmonary or systemic thromboembolic event), patients may be found to have evidence of cardiac enlargement or dysfunction.
A variety of approaches
have been used to quantify the degree of functional limitation imposed by HF. The most widely used scale is the NYHA functional classification ,but this system is subject to considerable interobserver variability and is insensitive to important changes in exercise capacity. These limitations may be overcome by formal tests of exercise tolerance. Measurement of the distance that apatient can walk in 6 minutes may have prognostic significance and may help to assess the level of functional impairment in the very sick, but serial changes in walking distance may not parallel changes in clinical status.Identification of a Structural and Functional Abnormality
A complete history and physical examination are the first steps in evaluating the structural abnormality or cause responsible for the development of HF.
The single most useful diagnostic test in the evaluation of patients with HF is the comprehensive 2-dimensional echocardiogram coupled with Doppler flow studies to determine whether abnormalities of myocardium, heart valves, or pericardium are present and which chambers are involved.
Three fundamental questions must be addressed:
1)Is the LV ejection fraction (EF) preserved or reduced?2) Is the structure of the LV normal or abnormal?
3) Are there other structural abnormalities such as valvular, pericardial, or right ventricular abnormalities that could account for the clinical presentation?
Other tests may be used to provide information regarding
the nature and severity of the cardiac abnormality. Radionuclide ventriculography can provide highly accurate measurements of LV function and right ventricular EF,but it is unable to directly assess valvular abnormalities or cardiac hypertrophy.
Magnetic resonance imaging or computed tomography may be useful in evaluating chamber size and ventricular mass, detecting right ventricular dysplasia, or recognizing the presence of pericardial disease, as well as in assessing cardiac function and wall motion
Magnetic resonance imaging may also be used to identify myocardial viability and scar tissue. Chest radiography can be used to estimate the degree of cardiac enlargement and pulmonary congestion or to detect the presence of pulmonary disease. A 12-lead electrocardiogram may demonstrate evidence of prior MI, LV hypertrophy, cardiac conduction abnormality (e.g., left bundle-branch block), or a cardiac arrhythmia.
However, because of their low sensitivity and specificity, neither the chest x-ray nor the electrocardiogram should form the primary basis for determining the specific cardiac abnormality responsible for the development of HF.
Evaluation of the Cause of Heart Failure
LABORATORY TESTING
Laboratory testing may reveal the presence of disorders or conditions that can lead to or exacerbate HF. The initial evaluation of patients with HF should include a complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), glycohemoglobin, and blood lipids,as well as tests of both renal and hepatic function, a chest radiograph, and a 12-lead electrocardiogram. Thyroid function tests (especially thyroid-stimulating hormone) should be measured, because both hyperthyroidism and hypothyroidism can be a primary or contributory cause of HF.Afasting transferrin saturation is useful to screen for hemochromatosis;
several mutated alleles for this disorder are common in individuals of Northern European descent, and affected patients may show improvement in LV function after treatment with phlebotomy and chelating agents.MRI of the heart or liver may be needed to confirm the presence of iron overload. Screening for human immunodeficiency virus (HIV) is reasonable and should be considered for all high-risk patients.
Several recent assays have been developed for natriuretic peptides (BNP and NT-proBNP). Several of the natriuretic peptides are synthesized by and released from the heart.
Elevated plasma BNP levels have been associated with
• reduced LVEF .
• LV hypertrophy, elevated LV filling pressures,
• acute MI and ischemia,
• pulmonary embolism and chronic obstructive pulmonary disease.
• renal failure
Natriuretic peptides are sensitive to other biological factors, such as age, sex, weight, and renal function . Elevated levels lend support to a diagnosis of abnormal ventricular function or hemodynamics causing symptomatic HF .Trials with these diagnostic markers suggest use in the urgent-care setting, where they have been used in combination with clinical evaluation to differentiate dyspnea due to HF from dyspnea of other causes ,and suggest that its use may reduce both the time to hospital discharge and the cost of treatment .
BNP levels tend to be less elevated in HF with preserved EF than in HF with low EF
and are lower in obese patients .Levels of natriuretic peptides may be elevated meaningfully in women and in people over 60 years of age who do not have HF, and thus these levels should be interpreted cautiously in such individuals when distinguishing between cardiac and noncardiac causes of dyspnea. Elevated natriuretic peptide levels may lend weight to a suspected diagnosis of HF or trigger consideration of HF when the diagnosis is unknown but should not be used in isolation to confirm or exclude the presence of HFEVALUATION OF THE POSSIBILITY OF CORONARY ARTERY DISEASE
Coronary artery disease is believed to be the underlying cause in approximately two thirds of patients with HF and low EF and also contributes to the progression of HF through mechanisms that include endothelial dysfunction, ischemia, and infarction.
PATIENTS WITH CORONARY ARTERY DISEASE AND ANGINA.
Coronary artery bypass grafting has been shown to improve symptoms and survival in patients with modestly reduced EF (variably defined in clinical trials) and angina.EVALUATION OF THE POSSIBILITY OF MYOCARDIAL DISEASE
One half of patients with HF and low EF have normal ornear-normal coronary arteries on coronary angiography, and
myocardial disorders are responsible for the development of
cardiomyopathy in most such individuals .Most patients
with a cardiomyopathy have no identifiable causativefactor (i.e., idiopathic dilated cardiomyopathy), but in some
patients, the cardiomyopathy is related to a systemic disorder
(e.g., hypertension, diabetes mellitus, hyperthyroidism,
hemochromatosis, or hypocalcemia), exposure to a cardiotoxic agent (alcohol, cocaine, methamphetamine, anthracycline, or trastuzumab), or the presence of myocardial inflammation or infiltration.
Endomyocardial biopsy can be used to make a diagnosis of sarcoidosis and amyloidosis, but changes characteristic of these disorders are often missed on histological evaluation, and there is no conclusive evidence that treatment can favorably affect the course of these diseases.Examples of cases in which a biopsy might be helpful usually occur in a setting in which the cause of the cardiomyopathy is already suspected because of other supportive data. Tissue obtained by biopsy can be used to make the diagnosis of hemochromatosis, endocardial fibroelastosis, and Loeffler’s syndrome in patients in whom these disorders are suspected on clinical grounds.Biopsy tissue may also be used to assess the risk of continued anthracycline therapy in patients with cancer, especially when combined with imaging of ventricular function
Biopsies can confirm the presence of cardiac disorders that often might weigh against eligibility for heart transplantation (e.g., amyloidosis). Finally, the biopsy can be used to identify patients with giant-cell myocarditis, who generally progress rapidly to death and are unresponsive to treatment and who thus may be considered for mechanical circulatory support or immediate heart transplantation . However, endomyocardial biopsy is not indicated in the routine evaluation of cardiomyopathy. Although the risk of
a serious complication is less than 1% in centers experienced in this technique, biopsies should be performed only when there is a strong reason to believe that the results will have a meaningful effect on subsequent therapeutic decisions or prognosis and only by operators experienced in its performance.
Assessment of Functional Capacity
During the initial and subsequent visits, healthcare providers should inquire about the type, severity, and duration of symptoms that occur during activities of daily living and that may impair the patient’s functional capacity. Questions regarding the ability to perform specific tasks may provide greater insight than general inquiries about what symptoms the patient is experiencing, because many patients curtail their activities to limit discomfort.
Patients with modest limitations of activity should be asked about their participation in sports or their ability to perform strenuous exercise, whereas patients with substantial limitations of activity should be asked about their ability to get dressed without stopping, take a shower or bath, climb stairs, or perform specific routine household chores. A useful approach is to ask patients to describe activities that they would like to do but can no longer perform, because changes in the ability to perform specific tasks are generally related to important changes in clinical status or course. Ideally, these inquiries should be coupled with direct observations of the patient during a walk around the clinic or up the stairs.
A variety of approaches have been used to quantify the degree of functional limitation imposed by HF. The most widely used scale is the NYHA functional classification ,but this system is subject to considerable interobserver variability and is insensitive to important changes in exercise capacity. These limitations may be overcome by formal tests of exercise tolerance.
Measurement of the distance that a patient can walk in 6 minutes may have prognostic significance and may help to assess the level of functional impairment in the very sick, but serial changes in walking distance may not parallel changes in clinical status. Maximal exercise testing, with measurement of peak oxygen uptake,has been used to identify appropriate candidates for cardiac transplantation, to determine disability, and to assist in the formulation of an exercise prescription, but its role in the general management of patients with HF has not been defined.
Assessment of Volume Status
It is critically important for healthcare providers to evaluate the fluid or volume status of patients with HF during the initial visit and each follow-up examination. This assessment plays a pivotal role in determining the need for diuretic therapy and in detecting sodium excesses or deficiencies that may limit efficacy and decrease the tolerability of drugs used to treat HF.The physical examination is the primary step in evaluating the presence and severity of fluid retention in patients with HF. At each visit, healthcare providers should record the patient’s body weight and sitting and standing blood pressures and determine the degree of jugular venous distension and its response to abdominal pressure, the presence and severity of organ congestion (pulmonary rales and hepatomegaly), and the magnitude of peripheral edema in the legs, abdomen, presacral area, and scrotum, as well as ascites in the abdomen.
The most reliable sign of volume overload is jugular venous distention .Right-sided filling pressures are elevated in the basal state or with abdominal compression (hepatojugular reflux) in many patients with chronically elevated left-sided filling pressures . Most patients with peripheral edema should also be considered to have volume overload, but the possibility of noncardiac causes for edema may limit the utility of this sign in some patients.
In contrast, most patients with chronic HF do not have rales. This is true even in patients with end-stage disease who have markedly elevated left-sided filling pressures.
The presence of rales generally reflects the rapidity of onset of HF rather than the degree of volume overload. Indeed, many patients with chronic HF have elevated intravascular volume in the absence of peripheral edema or rales.
The majority of patients with clinical evidence of volume overload do not exhibit hypoperfusion, even though cardiac performance may be severely depressed. Clinical signs of hypoperfusion become most apparent when cardiac output declines markedly or abruptly. Clues that suggest the presence of such a marked reduction in cardiac output include
narrow pulse pressure, cool extremities, altered mentation, Cheyne-Stokes respiration, resting tachycardia,and a disproportionate elevation of blood urea nitrogen relative to serum creatinine. Renal dysfunction in HF is poorly understood and appears to be mediated by interactions between the heart and kidney beyond those primarily due to depressed cardiac output
Laboratory Assessment
Serial measurement of serum potassium, because hypokalemia is a common adverse effect of treatment with diuretics and may cause fatal arrhythmias and increase the risk of digitalis toxicity, whereas hyperkalemia may complicate therapy with angiotensin-converting enzyme (ACE) inhibitors, (ARBs), and aldosterone antagonists. Worsening renal function may require adjustment of the doses of diuretics, renin-angiotensin-aldosterone system antagonists, digoxin,and noncardiac medications. Development of hyponatremia or anemia may be a sign of disease progression and is associated with impaired survival.
Serum BNP levels have been shown to parallel the clinical severity of HF as assessed by NYHA functional class in broad populations. Levels are higher in hospitalized patients and tend to decrease during aggressive therapy for decompensation..Indeed, there is an increasing body of evidence demonstrating the power of the addition of BNP (or NT-proBNP) levels in the assessment of prognosis in a variety of cardiovascular disorders. However, it cannot be assumed that BNP levels can be used effectively as targets for adjustment of therapy in individual patients.
Many patients taking optimal doses of medications continue to show markedly elevated levels of BNP, and some patients demonstrate BNP levels within the normal range despite advanced HF. The use of BNP measurements to guide the titration of drug doses has not been shown conclusively to improve outcomes more effectively than achievement of the target doses of drugs shown in clinical trials to prolong life .Ongoing trials will help to determine the role of serial BNP (or other natriuretic peptides)measurements in both diagnosis and management of HF.
Brain natriuretic peptide
Also known aspeptide; proBNP Formal name: B-type natriuretic peptide; N-terminal pro b-type natriuretic peptide (BNP), now known as B-type natriuretic peptide (also BNP) or GC-B, is a 32 amino acid polypeptide secreted by the ventricles of the heart in response to excessive stretching of heart muscle cells (cardiomyocytes). BNP is named as such because it was originally identified in extracts of porcine brain, although in humans it is produced mainly in the cardiac ventricles.BNP is co-secreted along with a 76 amino acid N-terminal fragment (NT-proBNP) which is biologically inactive. BNP binds to and activates the atrial natriuretic factor receptors NPRA, and to a lesser extent NPRB, in a fashion similar to atrial natriuretic peptide (ANP) but with 10-fold lower affinity. The biological half-life of BNP, however, is twice as long as that of ANP, and that of NT-proBNP is even longer, making these peptides better targets than ANP for diagnostic blood testing.
Clinical significance
Both BNP and NT-proBNP levels in the blood are used for screening, diagnosis of acute congestive heart failure (CHF) and may be useful to establish prognosis in heart failure, as both markers are typically higher in patients with worse outcome. The plasma concentrations of both BNP and NT-proBNP are also typically increased in patients with asymptomatic or symptomatic left ventricular dysfunction.There is no level of BNP that perfectly separates patients with and without heart failure.BNP accurately reflects current ventricular status The half-life of NT-ProBNP is 1 to 2 hours vs. 20 minutes for BNP.
BNP > 100 pg per milliliter
sensitivity = 90%
specificity = 76%
BNP > 50 pg per milliliter
sensitivity = 97%
specificity = 62%
BNP can be elevated in renal failure BNP is cleared by binding to natriuretic peptide receptors (NPRs) and neutral endopeptidase (NEP) Less than 5% of BNP is cleared renally. NTproBNP is the inactive molecule resulting from cleavage of the prohormone Pro-BNP and is SOLELY reliant on the kidney for excretion. The achilles heel of the NT proBNP molecule is the overlap in kidney disease in the heart failure patient population.
The BNP test is used as an aid in the diagnosis and assessment of severity of congestive heart failure.
The BNP test is also used for the risk stratification of patients with acute coronary syndromes.
When interpreting an elevated BNP level, it is useful to remember that values may be elevated due to factors other than heart failure.
Higher levels are often seen in obese patients and those with renal disease, in the absence of heart failure.
BNP is also one of the reasons why people will feel the need to urinate after getting into the bathtub or pool. The increased pressure on the body drives more fluid back into systemic circulation which in turn leads to a slight increase in preload. The left ventricle, and to a small degree the left atrium, secrete BNP in response. The natriuretic effect of BNP leads to an increase in urine production.
• Atrial natriuretic peptide (ANP)
• Also known as atrial natriuretic factor (ANF) or atriopeptin, a 28-amino acid polypeptide hormone which is involved in the homeostatic control of body water, sodium, and adiposity. Atrial natriuretic peptide (ANP) is released by atrial myocytes – cells in the atria of the heart – in response to signals of raised blood pressure and acts to reduce the water, sodium, and adipose loads on the circulatory system, thereby returning blood pressure to more normal levels.Elevated levels of ANP are found during hypervolemic states (elevated blood volume) and congestive heart failure. Children with congenital heart disorders causing heart failure have high levels of ANP. These levels fall after successful surgery to correct the abnormality. A second natriuretic peptide, called brain-type natriuretic peptide (BNP), is a 32-amino acid peptide that is synthesized within the ventricles (as well as in the brain where it was first identified).
The physiologic actions of BNP are similar to ANP and include decrease in systemic vascular resistance and central venous pressure as well as an increase in natriuresis.
Thus, the net effect of BNP and ANP is a decrease in blood volume and a decrease in cardiac output.
Natriuretic peptide precursor C
Natriuretic peptide precursor C, also known as NPPC, is a protein that in humans is encoded by the NPPC gene.The precursor NPPC protein is cleaved to the 22 amino acid peptide C-type natriuretic peptide (CNP).These peptides possess potent natriuretic, diuretic, and vasodilating activities and are implicated in body fluid homeostasis and blood pressure control.
Unlike ANP and BNP, CNP does not have direct natriuretic activity. This is because CNP is a selective agonist for the B-type natriuretic receptor (NPRB) whereas ANP and BNP are selective for NPRA
B-Type Natriuretic Peptide–Guided Heart Failure Therapy
The use of plasma levels of B-type natriuretic peptides (BNPs) to guide treatment of patients with chronic heart failure (HF) has been investigated in a number of randomized controlled trials (RCTs). However, the benefits of this treatment approach have been uncertain. We therefore performed a meta-analysis to examine the overall effect of BNP-guided drug therapy on cardiovascular outcomes in patients with chronic HF. Eight RCTs with a total of 1726 patients and with a mean duration of 16 months (range, 3-24 months) were included in the meta-analysis.
Arch Intern Med.March 22, 2010
Conclusions
B-type natriuretic peptide–guided therapy reduces all-cause mortality in patients with chronic HF compared with usual clinical care, especially in patients younger than 75 years. A component of this survival benefit may be due to increased use of agents proven to decrease mortality in chronic HF. However, there does not seem to be a reduction in all-cause hospitalization or an increase in survival free of hospitalization using this approach.Serial chest radiographs are not recommended in the
management of chronic HF. Although the cardiothoracicratio is commonly believed to reflect the cardiac dilatation that is characteristic of HF, enlargement of the cardiac silhouette primarily reflects changes in right ventricular volume rather than LV function, because the right ventricle forms most of the border of dilated hearts on radiographs. Similarly, changes in the radiographic assessment of pulmonary vascular congestion are too insensitive to detect any but the most extreme changes in fluid status
In long standing biventricular chronic heart failure, chest radiographs may only show cardiomegaly without alveolar edema or pleural effusions due to adaptive lung mechanism with increased arterial vasoconstriction and lymphatic drainage.
Assessment of Prognosis
Multivariate analysis of clinical variables has helped to identify the most significant predictors of survival, and prognostic models have been developed and validated .Decreasing LVEF, worsening NYHA functional status,degree of hyponatremia, decreasing peak exercise oxygen uptake, decreasing hematocrit, widened QRS on 12-lead electrocardiogram, chronic hypotension, resting tachycardia, renal insufficiency, intolerance to conventional therapy, and refractory volume overload are all generally recognized key prognostic parameters, although the actual prognostic models incorporating them are not widely used in clinical practice .Although elevated circulating levels of neurohormonal factors have also been associated with high mortality rates, the routine assessment of neurohormones such as norepinephrine or endothelin is neither feasible nor helpful in clinical management. Likewise, elevated BNP (or NT-proBNP) levels predict higher risk of HF and other events after MI, whereas marked elevation in BNP levels during hospitalization for HF may predict rehospitalization and death. Nonetheless, the BNP measurement has not been clearly shown to supplement careful clinical assessment for management.
Therapy
Patients at High Risk for Developing
Heart Failure (Stage A)
Recommendations
CLASS I
hypertension should be controlled
blood sugar should be controlled
lipid disorders should be treated
avoid behaviors that may increase the risk of HF (e.g., smoking,
excessive alcohol consumption, and illicit drug use). Ventricular rate should be controlled or sinus rhythm restored Thyroid disorders should be treated
CLASS IIa
1. Angiotensin converting enzyme inhibitors can be useful to prevent HF in patients at high risk for developing HF who have ahistory of atherosclerotic vascular disease, diabetes mellitus, orhypertension with associated cardiovascular risk factors. (Level of Evidence: A)
2. Angiotensin II receptor blockers can be useful to prevent HF in patients at high risk for developing HF who have a history of atherosclerotic vascular disease, diabetes mellitus, or hypertension with associated cardiovascular risk factors. (Level of EvidenceC)
Control of Risk
TREATMENT OF HYPERTENSIONIn the Framingham Heart Study, hypertension accounted for 39%
of HF cases in men and 59% in women .In addition,the benefits of treating hypertension in patients who have had a prior MI (Stage B) are even more dramatic, with an 81% reduction in the incidence of HF .Diuretic-based antihypertensive therapy has repeatedly been shown to prevent HF in a wide range of target populations .ACE inhibitors (ACEIs) and betablockers
are also effective in the prevention of HF.
whereas calcium antagonists and alpha-blockers are less effective in preventing HF syndrome among patients with diabetes or other cardiovascular complications ,ACEIs have been most notable with respect to a reduction in the onset of HF and new-onset diabetes. Likewise, compared with placebo, the ARBs losartan and irbesartan significantly reduced the incidence of HF in patients with type 2 diabetes mellitus and nephropathy. Ultimately, an appropriate antihypertensive regimen frequently consists of several drugs used in combination.
TREATMENT OF DIABETES
Obesity and insulin resistance are important risk factors for the development of HF. Long-term treatment with several ACEIs or ARBs has been shown to decrease the risk of renal disease in diabetic patients and prolonged therapy with the ACEIramipril has been shown to lower the likelihood of cardiovascular death, MI, and HF .Likewise, the use of ARBs in patients with diabetes mellitus and hypertension or LVH has been shown to reduce the incidence of first hospitalization for HF, in addition to having other beneficial effects on renal function.
MANAGEMENT OF THE METABOLIC SYNDROME
The clustering of cardiovascular risk factors in individual patients, termed the metabolic syndrome or syndrome X, includes any 3 of the following criteria:• Abdominal diposity,
• Hypertriglyceridemia,
• Low high-density lipoprotein,
• Hypertension,
• Fasting hyperglycemia.
MANAGEMENT OF ATHEROSCLEROTIC DISEASE
Patients with known atherosclerotic disease (e.g., of thecoronary, cerebral, or peripheral blood vessels) are likely to develop HF, and healthcare providers should seek to control vascular risk factors in such patients according to recommended guidelines
CONTROL OF CONDITIONS THAT MAY CAUSE CARDIAC INJURY
patients should be strongly advised about the hazards of smoking, as well as the use of alcohol, cocaine, amphetamines, and other Several interventions used in the treatment of cancer can injure the heart and lead to the development of HF, even in patients with no other cardiovascular risk factors. Such treatments include ionizing radiation that involves the mediastinum and chemotherapeutic agents such as anthracyclines, immunotherapy such as trastuzumab, or high-dose cyclophosphamide .Patients who take trastuzumab in combination with anthracyclines are at particular risk of HF. Heart failure may occur years after initial exposure to anthracyclines or mediastinal radiotherapy. Use illicit drugs.
Patients With Cardiac Structural Abnormalities
or Remodeling Who Have Not Developed HeartFailure Symptoms (Stage B)
Recommendations
CLASS I
• All Class I recommendations for Stage A should apply to patients with cardiac structural abnormalities who have not developed HF. (Levels of Evidence: A, B, and C as appropriate)
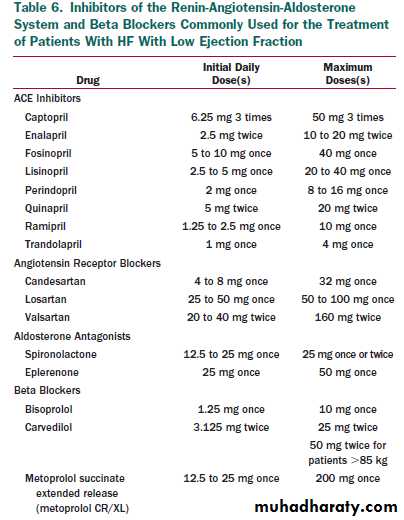
• Beta blockers and ACEIs should be used in all patients with arecent or remote history of MI regardless of EF or presence of HF (see Table 3). (Level of Evidence: A)
3. Beta blockers are indicated in all patients without a history of MI who have a reduced LVEF with no HF symptoms (see Table 3 and text). (Level of Evidence: C)
4. Angiotensin converting enzyme inhibitors should be used in patients with a reduced EF and no symptoms of HF, even if they have not experienced MI. (Level of Evidence: A)
5. An ARB should be administered to post-MI patients without HF who are intolerant of ACEIs and have a low LVEF. (Level of Evidence: B)
6. Patients who have not developed HF symptoms should be treated according to contemporary guidelines after an acute MI.
7. Coronary revascularization should be recommended in appropriate patients without symptoms of HF in accordance with contemporary guidelines . (Evidence: A)
8. Valve replacement or repair should be recommended for patients with hemodynamically significant valvular stenosis or regurgitation and no symptoms of HF in accordance with contemporary guidelines. (Level of Evidence: B).
CLASS IIa
1. Angiotensin converting enzyme inhibitors or ARBs can be beneficial in patients with hypertension and LVH and no symptoms of HF. (Level of Evidence: B)
2. Angiotensin II receptor blockers can be beneficial in patients with low EF and no symptoms of HF who are intolerant of ACEIs. (Level of Evidence: C)
3. Placement of an ICD is reasonable in patients with ischemic cardiomyopathy who are at least 40 days post-MI, have an LVEF of 30% or less, are NYHA functional class I on chronic optimal
medical therapy, and have reasonable expectation of survival with a good functional status for more than 1 year. (Level of Evidence: B)
CLASS III
1. Digoxin should not be used in patients with low EF, sinus rhythm, and no history of HF symptoms, because in this population, the risk of harm is not balanced by any known benefit. (Level of Evidence: C)2. Use of nutritional supplements to treat structural heart disease or to prevent the development of symptoms of HF is not recommended.(Level of Evidence: C)
3. Calcium channel blockers with negative inotropic effects may be harmful in asymptomatic patients with low LVEF and no symptoms of HF after MI (see text in Stage C). (Level of Evidence: C)
The aldosterone antagonist eplerenone has been shown to reduce morbidity and mortality in a population of patients with low EF and HF after MI that has already been treated with ACEIs and beta blockers.
Prevention of Cardiovascular Events
PATIENTS WITH AN ACUTE MYOCARDIAL INFARCTIONThe infusion of a fibrinolytic agent or the use of percutaneous coronary intervention can decrease the risk of developing HF, and these interventions can reduce the risk of death, especially in patients with a prior myocardial injury , also benefit from the administration of both a beta blocker and either an ACEI or ARB, which can decrease the risk of reinfarction or death when initiated within days after the ischemic event, especially in patients whose course is complicated by HF .
Combined neurohormonal blockade (beta blocker and ACEI or ARB) produces additive benefits .
PATIENTS WITH A HISTORY OF MI BUT NORMAL LEFT VENTRICULAR EJECTION FRACTION
Both hypertension and hyperlipidemia should be treated vigorously in patients with a history of MI, because the benefits of treating these coronary risk factors are particularly marked in patients with a prior ischemic event .Patients with a recent MI should also receive treatment with ACEIs and beta blockers ,which have been shown to reduce the risk of death when initiated days or weeks after an ischemic cardiac event. Evidence from 2 large-scale studies indicates that prolonged therapy with an ACEI can also reduce the risk of a major cardiovascular event, even when treatment is initiated months or years after MIPATIENTS WITH CHRONIC REDUCTION OF LEFT VENTRICULAR EJECTION FRACTION BUT NO SYMPTOMS
Long-term treatment with an ACEI has been shown to delay the onset of HF symptoms and decrease the risk of death and hospitalization for HF in asymptomatic patients with reduced LVEF, whether due to a remote ischemic injury or to a nonischemic cardiomyopathy . Although a recent trial investigated patients with low EF and HF at the time of MI, there are no studies that specifically address use of ARBs in symptomatic patients with reduced LVEF.
Given results of studies in symptomatic patients with low EF, ARBs may be an appropriate alternative, particularly in patients who cannot tolerate an ACEI. Furthermore, although controlled clinical trials are lacking, the use of beta blockers in patients with a low EF and no symptoms (especially those with coronary artery disease) is also recommended .
The use of ICD therapy in patients with chronic reduction
of LVEF but no symptoms has been evaluated in one large trial including only patients with ischemic cardiomyopathy.The trials assessing ICD for primary prophylaxis in nonischemic cardiomyopathy have not included functional class I patients and the efficacy of ICDs in this population as a whole is unknown .The trial involving patients with ischemic cardiomyopathy included a subset of asymptomatic patients post-MI with LVEF 30% or less, and there was demonstrated benefit of ICD placement (MADIT-II) in that subset.The use of calcium channel blockers with negative inotropic effects is not recommended in asymptomatic patients with EF less than 40% after MI
PATIENTS WITH SEVERE VALVULAR DISEASE BUT NO SYMPTOMS
Valve replacement or repair surgery should be considered for patients with severe aortic or mitral valve stenosis or regurgitation, even when ventricular function is impaired Long-term treatment with a systemic vasodilator drug may be considered for those with severe aortic regurgitation who are deemed to be poor candidates for surgery.Several studies have suggested that prolonged therapy with hydralazine and nifedipine in patients with severe aortic regurgitation and preserved LV function might act to minimize structural changes in the ventricle and thereby possibly delay the need for surgical intervention; however, these drugs are often poorly tolerated in this setting, and no trial has shown that these vasodilators can reduce the risk of HF or death .There are no long-term studies of vasodilator therapy in patients with severe asymptomatic mitral regurgitation.
Early Detection of Heart Failure
As noted, the symptoms and signs of HF are often difficultto identify because they are frequently confused with other disorders or are attributed to aging, obesity, or lack of conditioning. Limitations of exercise tolerance can occur so gradually that patients may adapt their lifestyles (consciously or subconsciously) to minimize symptoms and thus fail to report them to healthcare providers.
Hence, patients at risk should be advised to inform their healthcare providers about limitations of exercise tolerance or unexplained fatigue, and healthcare providers should intensify their vigilance for the
signs and symptoms of HF in such individuals.
Use of 1 of the 3 beta blockers proven to reduce mortality (i.e.,
bisoprolol, carvedilol, and sustained release metoprolol succinate)
is recommended for all stable patients with current or prior symptoms of HF and reduced LVEF, unless contraindicated .(Evidence: A)
An implantable cardioverter-defibrillator is recommended as secondary prevention to prolong survival in patients with current or prior symptoms of HF and reduced LVEF who have a history of cardiac arrest, ventricular fibrillation, or hemodynamically destabilizing ventricular tachycardia (Level of Evidence: A)
Implantable cardioverter-defibrillator therapy is recommended for primary prevention of sudden cardiac death to reduce total mortality in patients with non-ischemic dilated cardiomyopathy or ischemic heart disease at least 40 days post-MI, a LVEF less than or equal to 35%, and NYHA functional class II or III symptoms while receiving chronic optimal medical therapy, and who have reasonable expectation of survival with a good functional status for more than 1 year (Evidence: A)
Patients with LVEF of less than or equal to 35%, sinus rhythm, and NYHA functional class III ambulatory class IV symptoms despite recommended optimal medical therapy and who have cardiac dyssynchrony, which is currently defined as a QRS duration greater than or equal to 0.12 seconds, should receive cardiac resynchronization therapy, with or without an ICD, unless contraindicated .(Evidence: A)
Addition of an aldosterone antagonist is recommended in selected patients with moderately severe to severe symptoms of HF and reduced LVEF who can be carefully monitored for preserved renal function and normal potassium concentration. Creatinine should be 2.5 mg per dL or less in men or 2.0 mg per dL or less in women and potassium should be less than 5.0 mEq per liter. Under circumstances where monitoring for hyperkalemia or renal dysfunction is not anticipated to be feasible, the risks may outweigh the benefits of aldosterone antagonists .(Evidence: B)
The combination of hydralazine and nitrates is recommended to improve outcomes for patients self-described as African-Americans, with moderate-severe symptoms on optimal therapy with ACEIs, beta blockers, and diuretics (Evidence: B)
GENERAL MEASURES
Measures listed as Class I recommendations for patients in stage A or B are also appropriate for patients with current or prior symptoms of HF. In addition, moderate sodium restriction, along with daily measurement of weight, is indicated to permit effective use of lower and safer doses of diuretic drugs, even if overt sodium retention can be controlled by the use of diuretics.Immunization with influenza and pneumococcal vaccines may reduce the risk of a respiratory infection. Although most patients should not participate in heavy labor or exhaustive sports, physical activity should be encouraged (except during periods of acute exacerbation of the signs and symptoms of HF, or in patients with suspected myocarditis), because restriction of activity promotes physical
deconditioning, which may adversely affect clinical status and contribute to the exercise intolerance of patients with HF
Three classes of drugs can exacerbate the syndrome of HF
and should be avoided in most patients:1) Antiarrhythmic agents can exert important cardiodepressant
and proarrhythmic effects. Of available agents, only amiodarone and dofetilide have been shown not to adversely affect survival.
2) Calcium channel blockers can lead to worsening HF and have been associated with an increased risk of cardiovascular events Of available calcium channel blockers,only the vasoselective ones have been shown not to adversely affect survival .
3) Nonsteroidal anti-inflammatory drugs can cause sodium retention and peripheral vasoconstriction and can attenuate the efficacy and enhance the toxicity of diuretics and ACEIs .
Calcium channel blockers
may be used to treat diastolic heart failure. Diastolic heart failure happens when your heart has a hard time filling with blood.
Calcium channel blockers may help your heart fill with blood more easily by slowing your heart rate and lowering your blood pressure. When your heart beats more slowly, it has more time to fill between each heartbeat. Calcium channel blockers may also help your heart muscle to relax, which can help your heart fill with blood. Lower blood pressure may help treat diastolic heart failure because your heart does not have to work as hard to pump blood.
Calcium entry through L-type calcium channels is essential for contraction of both arterial smooth muscle and the myocardium, and is important in cardiac conduction.
First-generation calcium entry blockers lack or have a modest degree of vascular selectivity and inhibit cardiac function at doses producing therapeutic arterial dilatation. Such agents may cause deterioration in patients with left ventricular dysfunction, and their combination with a beta-adrenergic blocker may adversely affect cardiac contractility and conduction.
Development of newer agents has focused on obtaining a higher degree of vascular selectivity.
Felodipine is a highly vascular selective calcium entry blocker, with a vascular selectivity ratio greater than 100, as shown experimentally.
Isradipine and nicardipine are also vascularly selective calcium entry blockers. Hemodynamic studies in patients with hypertension, coronary artery disease, congestive heart failure, or in patients receiving beta-adrenergic blockade, show that felodipine can produce profound arteriolar dilatation without the negative effects of left ventricular systolic performance. Furthermore, felodipine alone or when added to a beta-adrenergic blocker does not interfere with cardiac conduction.
The primary mechanism that accounts for the efficacy of dihydropyridine calcium entry blockers in hypertension and angina pectoris is arterial dilation,
whereas nondihydropyridines may also derive part of their effect from inhibition of cardiac performance. As some of these patients, most commonly the elderly, have concomitant left ventricular dysfunction, it should be advantageous to avoid myocardial depression in the treatment of their primary disease.
Preliminary studies in patients with heart failure indicate that felodipine and amlopidine may improve hemodynamics, reduce neurohormonal activation, and increase exercise tolerance, but final conclusions must await the randomized clinical trials now underway
The data do not support the use of dihydropyridines when primarily given as treatment for CHF. The results, however, suggest that these drugs can be safely given to patients with left ventricular dysfunction or CHF who need additional treatment for angina pectoris or hypertension.
Patients with HF should be monitored carefully for changes in serum potassium, and every effort should be made to prevent the occurrence of either hypokalemia or hyperkalemia, both of which may adversely affect cardiac excitability and conduction and may lead to sudden death .many experts believe that serum potassium concentrations should be targeted in the 4.0 to 5.0 mEq per liter range.
Of the general measures that should be used in patients with HF, possibly the most effective yet least used is close observation and follow-up. Nonadherence with diet and medications can rapidly and profoundly affect the clinical status of patients, and increases in body weight and minor changes in symptoms commonly precede by several days the occurrence of major clinical episodes that require emergency care or hospitalization.
Patient education and close supervision, which includes surveillance by the patient and his or her family, can reduce the likelihood of nonadherence and lead to the detection of changes in body weight or clinical status early enough to allow the patient or a healthcare provider an opportunity to institute treatments that can prevent clinical deterioration. Supervision need not be performed by a physician and may ideally be accomplished by a nurse or physician’s assistant with special training in the care of patients with HF. Such an approach has been reported to have significant clinical benefits .
Recommendations Concerning Aldosterone Antagonists.
Is recommended in carefully selected patients with moderately severe or severe HF symptoms and recent decompensation or with LV dysfunction early after MI. These recommendations are based on the strong data demonstrating reduced death and rehospitalization in2 clinical trial populations . In the trial of patients after MI, there was a significant interaction between serum creatinine and benefit of eplerenone. The average serum creatinine of enrolled patients was 1.1 mg per dL, above which there was no demonstrable benefit for survival.
To minimize the risk of life-threatening hyperkalemia in patients with low LVEF and symptoms of HF, patients should have initial serum creatinine less than 2.0 to 2.5 mg per dL without recent worsening and serum potassium less than 5.0 mEq per dL without a history of severe hyperkalemia.
In view of the consistency of evidence for patients with low LVEF early after MI and patients with recent decompensation and severe symptoms, it may be reasonable to consider addition of aldosterone antagonists to loop diuretics for some patients with mild to moderate symptoms of HF; however, the writing committee strongly believes that there are insufficient data or experience to provide aspecific or strong recommendation. Because the safety and efficacy of aldosterone antagonist therapy have not been shown in the absence of loop diuretic therapy, it is not currently recommended that such therapy be given without other concomitant diuretic therapy in chronic HF.
DRUGS RECOMMENDED FOR ROUTINE USE
Most patients with HF should be routinely managed with acombination of 3 types of drugs: a diuretic, an ACEI or an ARB, and a beta blocker .The value of these drugs has been established by the results of numerous large-scale clinical trials, and the evidence supporting a central role for their use is compelling and persuasive. Patients with evidence
of fluid retention should take a diuretic until aeuvolemic state is achieved, and diuretic therapy should be continued to prevent the recurrence of fluid retention.
Even if the patient has responded favorably to the diuretic, treatment with both an ACEI and a beta blocker should be initiated and maintained in patients who can tolerate them because they have been shown to favorably influence the long-term prognosis of HF.
Therapy with digoxin as a fourth agent may be initiated at any time to reduce symptoms,prevent hospitalization, control rhythm, and enhance exercise tolerance.
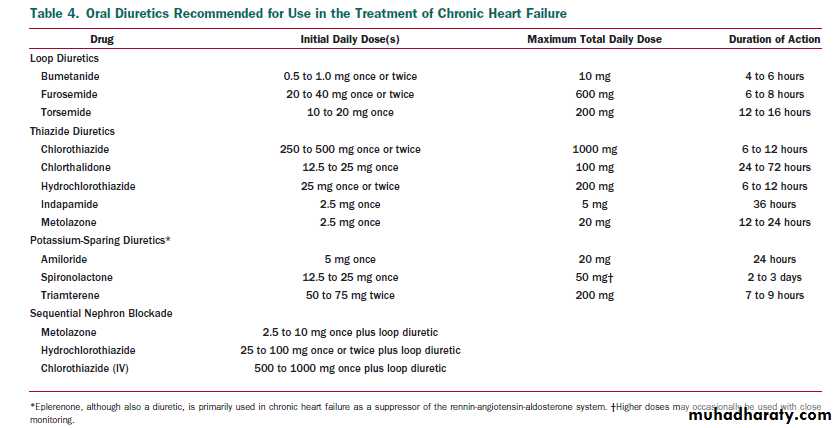
DIURETICS.
Diuretics interfere with the sodium retention of HF by inhibiting the reabsorption of sodium or chloride at specific sites in the renal tubules. Bumetanide,furosemide, and torsemide act at the loop of Henle (thus,they are called loop diuretics),whereas thiazides, metolazone, and potassium-sparing agents (e.g., spironolactone) act in the distal portion of the tubule .These 2 classes of diuretics differ in their pharmacological actions.
The loop diuretics increase sodium excretion up to 20% to 25% of the filtered load of sodium, enhance free water clearance, and maintain their efficacy unless renal function is severely impaired. In contrast, the thiazide diuretics increase the fractional excretion of sodium to only 5% to 10% of the filtered load, tend to decrease free water clearance, and lose their effectiveness in patients with impaired renal function (creatinine clearance less than 40 mL per min).
Consequently,the loop diuretics have emerged as the preferred diuretic agents for use in most patients with HF; however, thiazide diuretics may be preferred in hypertensive HF patients with mild fluid retention because they confer more persistent antihypertensive effects.
Effect of Diuretics in the Management of HF. Controlled trials have demonstrated the ability of diuretic drugs to increase urinary sodium excretion and decrease physical signs of fluid retention in patients with HF .In these shortterm studies, diuretic therapy has led to a reduction in jugular venous pressures, pulmonary congestion, peripheral edema, and body weight, all of which were observed within days of initiation of therapy.
In intermediate-term studies,
diuretics have been shown to improve cardiac function,symptoms, and exercise tolerance in patients with HF.There have been no long-term studies of diuretic therapy in HF, and thus, their effects on morbidity and mortality are not known.
When using diuretics in patients with HF, healthcare
providers should keep several points in mind:1) Diuretics produce symptomatic benefits more rapidly than any other drug for HF. They can relieve pulmonary and peripheral edema within hours or days, whereas the clinical effects of digitalis, ACEIs, or beta blockers may require weeks or months to become apparent ,
2) Diuretics are the only drugs used for the treatment of HF that can adequately control the fluid retention of HF. Although both digitalis and low doses of ACEIs can enhance urinary sodium excretion ,few patients with HF and a history of fluid retention can maintain sodium balance without the use of diuretic drugs. Attempts to substitute ACEIs for diuretics can lead to pulmonary and peripheral congestion .
3) Diuretics should not be used alone in the treatment of Stage C HF. Even when diuretics are successful in controlling symptoms and fluid retention, diuretics alone are unable to maintain the clinical stability of patients with HF for long periods of time .The risk of clinical decompensation can be reduced, however, when diuretics are combined with an ACEI and a beta blocker .
4) Appropriate use of diuretics is a key element in the success of other drugs used for the treatment of HF. The use of inappropriately low doses of diuretics will result in fluid retention, which can diminish the response to ACEIs and increase the risk of treatment with beta blockers .
PRACTICAL USE OF DIURETIC THERAPY.
Diuretics should be prescribed to all patients who haveevidence of, and to most patients with a prior history of,fluid retention. Diuretics should generally be combined with an ACEI and a beta blocker. Few patients with HF will be able to maintain dry weight without the use of diuretics.
PRACTICAL USE OF DIURETIC THERAPY.
Initiation and maintenance. The most commonly used loop diuretic for the
treatment of HF is furosemide, but some patients respond favorably to other agents in this category (such as torsemide) because of superior absorption and longer duration of action .In outpatients with HF, therapy is commonly initiated with low doses of a diuretic, and the dose is increased until urine output increases and weight decreases, generally by 0.5 to 1.0 kg daily.
Further increases in the dose or frequency (i.e., twice-daily dosing) of diuretic administration may be required to maintain an active diuresis and sustain the loss of weight. The ultimate goal of diuretic treatment is to eliminate clinical evidence of fluid retention,such as jugular venous pressure elevation and peripheral edema.
Diuretics are generally combined with moderate dietary sodium restriction (3 to 4 g daily).
If electrolyte imbalances are seen, these should be treated aggressively and the diuresis continued. If hypotension or azotemia is observed before the goals of treatment are achieved, the physician may elect to slow the rapidity of diuresis, but diuresis should nevertheless be maintained until fluid retention is eliminated, even if this strategy results in mild or moderate decreases in blood pressure or renal function, as long as the patient remains asymptomatic.
The response to a diuretic is dependent on the concentration of the drug and the time course of its entry into the urine .Patients with mild HF respond favorably to
low doses because they absorb diuretics rapidly from the
bowel and deliver these drugs rapidly to the renal tubules.
However, as HF advances, the absorption of the drug may
be delayed by bowel edema or intestinal hypoperfusion, and
the delivery of the drug and the response to a given
intratubular concentration may be impaired by a decline in
renal perfusion and function .Consequently, the
clinical progression of HF is characterized by the need for
increasing doses of diuretics.
Patients may become
unresponsive to high doses of diuretic drugs if1.they consume large amounts of dietary sodium,
2.are taking agents that can block the effects of diuretics (e.g., nonsteroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors) or
3.have a significant impairment of renal function or perfusion
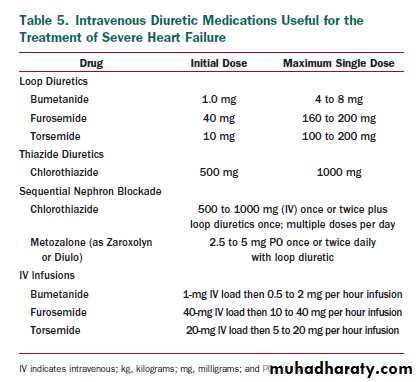
Diuretic resistance can generally be overcome by the
intravenous administration of diuretics (including the use of
continuous infusions)the use of 2 or more diuretics in
combination (e.g., furosemide and metolazone)
or the use of diuretics together with drugs that increase renal
blood flow (e.g., positive inotropic agents)
The principal adverse effects of diuretics
electrolyte and fluid depletion, as well as hypotension and azotemia.Diuretics may also cause rashes and hearing difficulties, but these are generally idiosyncratic or are seen with the use of very large doses, respectively.
Diuretics can cause the depletion of important cations
(potassium and magnesium), which can predispose patients to serious cardiac arrhythmias, particularly in the presence of digitalis therapy .
The risk of electrolyte depletion is
markedly enhanced when 2 diuretics are used in combination.The loss of electrolytes is related to enhanced delivery
of sodium to distal sites in the renal tubules and the
exchange of sodium for other cations, a process that is
potentiated by activation of the renin-angiotensin aldosterone system .Potassium deficits can be corrected by the short-term use of potassium supplements or, if severe, by the addition of magnesium supplements
Excessive use of diuretics can decrease blood pressure and impair renal function and exercise tolerance ,
but hypotension and azotemia may also occur as a result of worsening HF, which may be exacerbated by attempts to reduce the dose of diuretics.
If there are no signs of fluid retention, hypotension and azotemia are likely to be related to volume depletion and may resolve after a reduction in diuretic dose.
The signs of fluid retention, hypotension and azotemia, are likely to reflect worsening HF and a decline in effective peripheral perfusion.
INHIBITORS OF THE RENIN-ANGIOTENSIN ALDOSTERONE SYSTEM.
can take place at multiple sites: at the level of the enzyme that converts angiotensin I to angiotensin II (ACEIs), at the angiotensin receptor (ARBs), or at the receptor for aldosterone, which is under control of both the renin angiotensin system and other systemic and local influences (aldosterone antagonists). Angiotensin converting enzyme inhibitors are the best-studied class of agents in HF, with multiple mechanisms of benefit for both HF,coronary disease, and other atherosclerotic vascular disease, as well as diabetic nephropathy.
During chronic therapy with ACEIs, the renin-angiotensin system demonstrates partial “escape” from inhibition with “normalization” of angiotensin levels, in part owing to alternative local pathways for production of angiotensin. This leaves the potential for benefit from additional therapy with ARBs and with the aldosterone antagonists.
Angiotensin Converting Enzyme Inhibitors in the Management of Heart Failure. It is not clear whether the effects of ACEIs can be explained solely by the suppression of angiotensin II production, because ACE inhibition not only interferes with the renin-angiotensin system but also enhances the action of kinins and augments kinin-mediated prostaglandin production .In experimental models of HF, ACEIs modify cardiac remodeling more favorably than ARBs, and this advantage of ACEIs is abolished by the coadministration of a kinin receptor blocker .
Analysis of this collective experience indicates that
ACEIs can alleviate symptoms, improve clinical status, andenhance the overall sense of well-being of patients with HF.In addition,ACEIs can reduce the risk of death and the combined risk of death or hospitalization.
These benefits of ACE inhibition were seen in
patients with mild, moderate, or severe symptoms and in patients with or without coronary artery disease.
Angiotensin converting enzyme inhibitors should not be
prescribed without diuretics in patients with a current or recent history of fluid retention, because diuretics are needed to maintain sodium balance and prevent the development of peripheral and pulmonary edema .Angiotensin converting enzyme inhibitors are often preferred over ARBs or direct-acting vasodilators because of the greater experience and weight of evidence in support of their effectiveness.
Patients should not be given an ACEI if
they have experiencedlife-threatening adverse reactions angioedema Or
anuric renal failure) during previous exposure to the drug or if they are pregnant.
caution if they have very low systemic blood pressures (systolic blood pressure less than 80 mm Hg), creatinine (greater than 3 mg perdL), bilateral renal artery stenosis, or elevated levels of serumpotassium (greater than 5.5 mEq per liter).
should not be initiated in
hypotensive patients who are at immediate risk of cardiogenic shock.
Treatment with an ACEI
should be initiated at low doses ,followed by gradual increments in dose if lower doses have been well tolerated. Renal function and serum potassium should be assessed within 1 to 2 weeks of initiation of therapy and periodically thereafter, especially in patients with preexisting hypotension, hyponatremia, diabetes mellitus, or azotemia or in those taking potassium supplements.
Because fluid retention can blunt the therapeutic effects and fluid depletion can potentiate the adverse effects of ACE ,healthcare providers should ensure that patients are being given appropriate doses of diuretics before and during treatment with these drugs.
Most patients (85% to 90%) with HF can tolerate short and long-term therapy with these drugs
Higher doses of an ACEI were better than low doses in reducing the risk of hospitalization,
but they showed similar effects on symptoms and mortality . Clinicians should attempt to use doses that have been shown to reduce the risk of cardiovascular events in clinical trials. If these target doses of an ACEI cannot be used or are poorly tolerated, intermediate doses should be used with the expectation that there are likely to be only small differences in efficacy between low and high doses.
More importantly, clinicians should not delay the institution of beta blockers in patients because of a failure to reach target ACEI doses. Once the drug has been titrated to the appropriate dose, patients can generally be maintained on long-term therapy with an ACEI with little difficulty. Although symptoms may improve in some patients within the first 48 hours of therapy with an ACEI, the clinical responses to these drugs are generally delayed and may require several weeks, months, or more to become apparent .
Even if symptoms do not improve, long-term treatment with an ACEI should be maintained to reduce the risk of death or hospitalization. Abrupt withdrawal of treatment with an ACEI can lead to clinical deterioration and should be avoided in the absence of lifethreatening complications (e.g., angioedema).
Every effort should be made to minimize the occurrence of sodium retention or depletion during long-term treatment with an ACEI, because changes in salt and water balance can exaggerate or attenuate the cardiovascular and renal effects of treatment .
Fluid retention can minimize the symptomatic benefits of ACE inhibition,whereas fluid loss increases the risk of hypotension and azotemia.
Nonsteroidal anti-inflammatory drugs can block the favorable effects and enhance the adverse effects of ACEIs in patients with HF and should be avoided
Clinical experience in patients who are hemodynamically or clinically unstable suggests that the hypotensive effects of ACE inhibition may attenuate the natriuretic response to diuretics and antagonize the pressor response to intravenous vasoconstrictors .As a result, in such patients (particularly those who are responding poorly to diuretic drugs), it may be prudent to interrupt treatment with the ACEI temporarily until the clinical status of the patient stabilizes.
Retrospective analyses of large-scale clinical trials have
suggested that aspirin might interfere with the benefits of ACE inhibition in patients with HF by inhibiting kinin Mediated prostaglandin synthesis. In short-term hemodynamic and maximal-exercise studies, aspirin can attenuate the hemodynamic actions of ACEIs in patients with HF ,an effect not seen with nonaspirin antiplatelet agents (e.g., clopidogrel)PRACTICAL USE OF ACEIS.
Risks of treatment.
Most of the
adverse reactions of ACEIs can be attributed to the 2
principal pharmacological actions of these drugs:
those related to
angiotensin suppression and those related to
kinin potentiation.
Other types of side effects may also occur (e.g.,
rash and taste disturbances).
Adverse effects related to angiotensin suppression.
1. HYPOTENSIONThe most common adverse effects of ACE inhibition in patients with HF are hypotension and dizziness. hypotension is generally a concern only if it is accompanied by postural symptoms, worsening renal function, blurred vision, or syncope. Hypotension is seen most frequently during the first few days of initiation of increments in therapy, particularly in patients with hypovolemia, a recent marked diuresis, or severe hyponatremia .
Should symptomatic hypotension occur with the first doses, it may not recur with repeated administration of the same doses of the drug. However, it is prudent under such circumstances to reduce the activation of and dependence on the renin-angiotensin system by reducing the dose of diuretics, liberalizing salt intake, or both, provided the patient does not have significant fluid retention.
The doses of other hypotensive agents (especially vasodilators) can be reduced or staggered so their peak effect does not coincide with that of the ACEI. Most patients who experience early symptomatic hypotension remain excellent candidates for longterm ACE inhibition if appropriate measures are taken to minimize recurrent hypotensive reactions.-
2. WORSENING RENAL FUNCTION
reduced renal perfusion (such as HF), glomerular filtration is critically dependent on angiotensin-mediated efferent arteriolar vasoconstriction ,and ACE inhibition may cause functional renal insufficiency . the risk of azotemia is highest in patients who are most dependent on the renin-angiotensin system for support of renal homeostasis (i.e., class IV hyponatremicpatients) .A significant increase in serum creatinine (e.g., greater than 0.3 mg per dL) with the use of ACEIs is observed in 15% to 30% of patients with severe HF.
The risks are substantially greater if patients have bilateral renal artery stenosis or are taking
nonsteroidal anti-inflammatory drugs .Renal function usually improves after a reduction in the dose of concomitantly administered diuretics, and thus, these patients can generally be managed without the need to withdraw treatment with the ACEI . However, if the dose of diuretic cannot be reduced because the patient has fluid retention, the physician and patient may need to tolerate mild to moderate degrees of azotemia to maintain therapy with the ACEI.
3. POTASSIUM RETENTION
Hyperkalemia can occur during ACE inhibition in patients with HF and may be sufficiently severe to cause cardiac conduction disturbances. In general, hyperkalemia is seen in patients whose renal function deteriorates or who are taking oral potassium supplements or potassium-sparing diuretics,or aldosterone antagonists, especially if they have diabetes mellitus.4. Adverse effects related to kinin potentiation.
1. COUGHCough related to the use of ACEIs is the most common reason for the withdrawal of long-term treatment with thesedrugs .the frequency of cough is approximately 5% to 10% in white patients of European descent and rises to nearly 50% in Chinese patients .It is characteristically nonproductive, is accompanied by a persistent and annoying “tickle” in the back of the throat, usually appears within the first months of therapy, disappears within 1 to 2 weeks of discontinuing treatment, and recurs within days of rechallenge.
Other causes of cough, especially pulmonary congestion,should always be considered, and the ACEI should be implicated only after these have been excluded. Demonstration that the cough disappears after drug withdrawal and recurs after rechallenge with another ACEI strongly suggests that ACE inhibition is the cause of the cough.
In anumber of studies of ACEI cough, it was found that this symptom did not recur with rechallenge and probably was acoincidental finding. Because of the long-term benefits of ACEIs, physicians should encourage patients to continue taking these drugs if the cough is not severe. Only if the cough proves to be persistent and troublesome should the physician consider withdrawal of the ACEI and the use of alternative medications (e.g., an ARB).
2. ANGIOEDEMA
Angioedema occurs in fewer than 1% of patents taking an ACEI but is more frequent in blacks. Because its occurrence may be life-threatening, the clinical suspicion of this reaction justifies subsequent avoidance of all ACEIs for the lifetime of the patient . Angiotensin converting enzyme inhibitors should not be initiated in any patient with a history of angioedema.Although ARBs may be considered
as alternative therapy for patients who have developed angioedema while taking an ACEI, there are a number of patients who have also developed angioedema with ARBs and extreme caution is advised when substituting an ARB in a patient who has had angioedema associated with ACEI use.Angiotensin Receptor Blockers. Agents that block
these receptors were developed on the rationale that1)angiotensin II production continues in the presence of ACE inhibition, driven through alternative enzyme pathways, and
2) interference with the renin-angiotensin system without inhibition of kininase would produce all of the benefits of ACEIs while minimizing the risk of their adverse reactions .
However, it is now known that some of the benefits may be related to the accumulation of kinins) rather than to the suppression of angiotensin II formation, whereas some of the side effects of ACEIs in HF are related to the suppression of angiotensin II formation ..
Several ARBs (e.g., candesartan, eprosartan, irbesartan, losartan, telmisartan, olmesartan, and valsartan) are available for clinical use. Experience with these drugs in controlled clinical trials of patients with HF is considerably less than that with ACEIs. Nevertheless, in several placebo controlled studies, long-term therapy with ARBs produced hemodynamic, neurohormonal, and clinical effects consistent with those expected after interference with the renin angiotensin system .
In patients with evidence of LV dysfunction early after MI, a recent trial demonstrated
that ARBs had a benefit that was not inferior to that of ACEIs without an advantage in terms of tolerability .However, the addition of an ARB to an ACEI did not improve outcomes and resulted in more side effects.
Although angioedema is much less frequent with
ARBs, there are cases of patients who developed angioedema to both ACEIs and later to ARBs . There is little information available about the addition of ARBs to therapy with both ACEIs and aldosterone antagonists, but risks of renal dysfunction and hyperkalemia would be further increased. Until further information is available, the routine combined use of all 3 inhibitors of the reninangiotensin system cannot be recommended.Patients with systolic blood
• pressure below 80 mm Hg,• low serum sodium,
• diabetes mellitus, and
• impaired renal function
merit particular surveillance during therapy with inhibitors
of the renin angiotensin-aldosterone system. Titration
is generally achieved by doubling doses. For stable patients,it is reasonable to add therapy with beta-blocking agents before full target doses of either ACEIs or ARBs are reached.
Aldosterone Antagonists. Although short-term
therapy with both ACEIs and ARBs can lower circulating levels of aldosterone, such suppression may not be sustained during long-term treatment . The lack of long-term suppression may be important, because experimental data suggest that aldosterone exerts adverse effects on the structure and function of the heart, independently of and in addition to the deleterious effects produced by angiotensin II. Spironolactone is the most widely used aldosterone antagonist.In a large-scale, long-term trial (256), low doses of spironolactone (starting at 12.5 mg daily) were added to ACEI therapy for patients with NYHA functional class IV HF symptoms or class III symptoms and recent hospitalization.The risk of death was reduced from 46% to 35% (30% relative risk reduction) over 2 years, with a 35% reduction in HF hospitalization and an improvement in functional class. Initial creatinine levels were below 2.0 mg per dL in the dose-ranging pilot trial and below 2.5 mg per dL in the main trial. Potassium replacements were stopped at trial entry, and serum potassium and renal function were followed very closely.
A recent trial investigated the newer aldosterone antagonist eplerenone in patients with LVEF less than or equal to 40% and clinical evidence of HF or diabetes mellitus within 14 days of MI. Mortality was decreased from 13.6% to 11.8% at 1 year. Hyperkalemia occurred in 5.5% of patients treated with eplerenone compared with 3.9% of those given placebo overall and in up to 10.1% versus 4.6% of patients with estimated creatinine clearance less than 50 mL per minute
Serum creatinine levels often underestimate renal
dysfunction, particularly in the elderly, in whom estimated creatinine clearance less than 50 mL per minute should trigger a reduction of the initial dose of spironolactone to 12.5 mg daily or of eplerenone to 25 mg daily, andaldosterone antagonists should not be given when clearance is less than 30 mL per minute .
Patients chronically requiring high doses of diuretics without potassium replacement should be evaluated closely, because potassium handling
may be impaired.
Gynecomastia
or other antiandrogen effects that can occurduring therapy with spironolactone are not generally seen with the newer aldosterone antagonist eplerenone.
Patients should be instructed specifically
to stop the aldosterone antagonist during an episode of diarrhea(volume depletion)or while loop diuretic therapy is interrupted.
BETA-ADRENERGIC RECEPTOR BLOCKERS
BETA-ADRENERGIC RECEPTOR BLOCKERS.Beta blockers act principally to
inhibit the adverse effects of the sympathetic nervous system in patients with HF, and these effects far outweigh their well-known negative inotropic effects.
Whereas cardiac adrenergic drive initially supports the performance of the failing heart, long-term activation of the sympathetic nervous system exerts deleterious effects that can be antagonized by the use of beta blockers.
Sympathetic activation
can increase ventricular volumes and pressure by causing peripheral vasoconstriction and byimpairing sodium excretion by the kidneys
Norepinephrine can also induce cardiac hypertrophy,
provoke arrhythmias by increasing the automaticity
of cardiac cell, development of hypokalemia,
increase heart rate and potentiate the actions of other neurohormonal systems. Finally, by stimulating growth and oxidative stress in terminally differentiated cells, norepinephrine can trigger programmed cell death or apoptosis .These deleterious effects are mediated through actions on alpha-1–,beta-1–, and beta-2–adrenergic receptors .
Three beta blockers have been shown to be effective in
reducing the risk of death in patients with chronic HF:bisoprolol and sustained-release metoprolol (succinate) ,which selectively block beta-1–receptors, and carvedilol ,which blocks alpha-1–, beta-1–, and beta-2–receptors.
long-term treatment with beta blockers can
lessen the symptoms of HF,improve the clinical status of patients,and overall sense of well-being
In addition,like ACEIs, beta blockers can reduce the risk of death and the combined risk of death or hospitalization.
These benefits of beta blockers were seen in
patients with or without coronary artery disease and in
patients with or without diabetes mellitus, as well as in
women and black patients. The favorable effects of beta
blockers were also observed in patients already taking
ACEIs, which suggests that combined blockade of the 2
neurohormonal systems can produce additive effects.
PRACTICAL USE OF BETA BLOCKERS.
Beta blockers should be prescribed to all patients with stable .HF due to reduced LVEF unless they have a contraindication to their use or have been shown to be unable to tolerate treatment with these drugs.Because of the favorable effects of beta blockers on survival and disease progression, treatment
with a beta blocker should be initiated as soon as LV
dysfunction is diagnosed.
Even when symptoms are mild or have responded to other therapies, beta-blocker therapy is important and should not be delayed until symptoms return
or disease progression is documented during treatment with other drugs. Therefore, even if patients do not benefit symptomatically because they have little disability, they should receive treatment with a beta-blocker to reduce the risk of disease progression, future clinical deterioration, and sudden death .
Patients need not be taking high doses of ACEIs before being considered for treatment with a beta blocker, because most patients enrolled in the beta-blocker trials were not taking high doses of ACEIs. Furthermore, in patients taking a low dose of an ACEI, the addition of a betablocker
produces a greater improvement in symptoms and
reduction in the risk of death than an increase in the dose of the ACEI, even to the target doses used in clinical trials.
In patients with current or recent history of fluid
retention, beta blockers should not be prescribed without diuretics, because diuretics are needed to maintain sodium and fluid balance and prevent the exacerbation of fluid retention that can accompany the initiation of beta-blocker Therapy.
Which patients are sufficiently stable to be considered for
treatment with a beta blocker? Regardless of the severity ofsymptoms, patients should not be hospitalized in an intensive
care unit, should have no or minimal evidence of fluid overload or volume depletion, and should not have required recent treatment with an intravenous positive inotropic .
agent. Those excluded from treatment for these reasons
should first receive intensified treatment with other drugs
for HF (e.g., diuretics) and then be re-evaluated for betablockade after clinical stability has been achieved. Betablockers
may be considered in patients who have reactive
airway disease or asymptomatic bradycardia but should be
used with great caution or not at all in patients with
persistent symptoms of either condition.
Once the target dose has been achieved, patients can
generally continue long-term therapy with a beta blocker with little difficulty. Patients should be advised that clinical responses to the drug are generally delayed and may require 2 to 3 months to become apparent . Even if symptoms do not improve, long-term treatment should be maintained to reduce the risk of major clinical events. Abrupt withdrawal of treatment with a beta blocker can lead to clinical deterioration and should be avoided.How should clinical deterioration be managed in patients who have been taking a beta blocker for long periods of time (more than 3 months)?
Because long-term treatment with a beta blocker reduces the risk of worsening HF, discontinuation of long-term treatment with these drugs after an episode of worsening HF will not diminish and may in fact increase the subsequent risk of clinical decompensation.Consequently, if patients develop fluid retention, with or without mild symptoms, it is reasonable to continue the beta blocker while the dose of diuretic is increased .
However, if the deterioration in clinical status is characterized by hypoperfusion or requires the use of intravenous positive inotropic drugs, it may be prudent to halt or significantly reduce treatment with beta blockers temporarily until the status of the patient stabilizes. In such patients, positive inotropic agents whose effects are mediated independently of the beta receptor (e.g., a phosphodiesterase inhibitor such as milrinone) may be preferred. Once stabilized, the beta blocker should be reintroduced to reduce the subsequent risk of clinical deterioration.
PRACTICAL USE OF BETA BLOCKERS.
Initiation of treatment with a beta blocker has produced4 types of adverse reactions that require attention and management,
1. FLUID RETENTION AND WORSENING HF
Initiation of therapy with a beta blocker can cause fluid retention ,which is usually asymptomatic and is detected primarily by an increase in body weight but which may become sufficiently marked to cause worsening symptoms of HF .Patients with fluid retention before treatment are at greatest risk of fluid retention during treatment, and thus, physicians should ensure that patients are not volume overloaded before a beta blocker is initiated.
Furthermore, physicians should monitor patients closely for increases in weight and for worsening signs and symptoms of HF and should augment the dose of diuretic if weight increases whether or not other signs or symptoms of worsening HF are present.
2. FATIGUE
Treatment with a beta blocker can be accompanied byfeelings of general fatigue or weakness. In many cases, the
sense of lassitude resolves spontaneously within several
weeks without treatment, but in some patients, it may be
severe enough to limit increments in dose or require the
withdrawal of treatment. Complaints of fatigue can generally be managed by a reduction in the dose of the beta blocker (or the accompanying diuretic),
but treatment should be discontinued if the syndrome of weakness is accompanied by evidence of peripheral hypoperfusion.Reinitiation at a later time or with a different effective beta blocker may be successful.
3. BRADYCARDIA AND HEART BLOCK
generally asymptomatic and requires no treatment; however, if the bradycardia is accompanied by dizziness or lightheadedness or if second or third-degree heart block occurs, physicians should decrease the dose of the beta blocker. Physicians should also consider the possibility of drug interactions, because other drugs can cause bradycardia or heart block and may be discontinued. The role of pacemaker therapy with or without cardiac resynchronization therapy (CRT) to permit the use of beta-blocker therapy is entirely unknown.4. HYPOTENSION
Beta blockers, especially those that also block alpha-1–receptors, can produce hypotension, which is usually asymptomatic but may produce dizziness, lightheadedness, or blurred vision .For beta blockers that also block alpha receptors, such as carvedilol, these vasodilatory side effects are generally seen within 24 to 48 hours of the first dose or the first increments in dose and usually subside with repeated dosing without any change in dose.
Physicians may minimize the risk of hypotension by administering the beta blocker and ACEI at different times during the day. If this is ineffective, the occurrence of hypotension may require atemporary reduction in the dose of the ACEI. Hypotensive symptoms may also resolve after a decrease in the dose of diuretics in patients who are volume depleted, but in the absence of such depletion, relaxation of diuretic therapy may increase the risk or consequences of fluid retention .If hypotension is accompanied by other clinical evidence of hypoperfusion, beta-blocker therapy should be decreased or discontinued pending further patient evaluation.
DIGITALIS.
inhibit (Na-K ATPase) adenosine triphosphatase (ATPase) enzyme in cardiac cells results in an increase in the contractile state of the heart, and for many decades, the benefits of digitalis in HF were ascribed exclusively to this positive inotropic action. However, recent evidence suggests that the benefits of digitalis may be related in part to enzyme inhibition in noncardiac tissues.Inhibition of Na-K ATPase in vagal afferent fibers acts to sensitize cardiac baroreceptors, which in turn reduces sympathetic outflow from the central nervous system .In addition, by inhibiting Na-K ATPase in the kidney, digitalis reduces the renal tubular reabsorption of sodium ,the resulting increase in the delivery of sodium to the distal tubules leads to the suppression of renin secretion from the kidneys .These observations have led to the hypothesis that digitalis acts in HF primarily by attenuating the activation of neurohormonal systems and not as a positive inotropic drug .
EFFECT OF DIGITALIS IN THE TREATMENT OF HF.
Several placebo-controlled trials have shown that treatment with digoxin for 1 to 3 months can improve symptoms, quality of life, and exercise tolerance in patients with mild to moderate HF .These benefits have been seen regardless of the underlying rhythm (normal sinus rhythm or atrial fibrillation), cause of HF (ischemic or nonischemic cardiomyopathy), or concomitant therapy (with or without ACEIs). In a long-term trial that enrolled patients who primarily had Class II or III symptoms, treatment with digoxin for 2 to 5 years had no effect on mortality but modestly reduced the combined risk of death and hospitalizationPRACTICAL USE OF DIGITALIS IN HF.
Physicians may consider adding digoxin in patients withpersistent symptoms of HF during therapy with diuretics,
an ACEI (or ARB), and a beta blocker .Digoxin may also be added to the initial regimen in patients with severe symptoms who have not yet responded symptomatically during treatment with diuretics, an ACEI, and beta blockers. Alternatively, treatment with digoxin may be delayed until the patient’s response to ACEIs and beta blockers has been defined and be used only in patients who remain symptomatic despite therapy with the neurohormonal antagonists.
Digoxin is prescribed routinely in patients with HF and chronic atrial fibrillation, but beta blockers are usually more effective when added to digoxin in controlling the ventricular response, particularly during exercise .
Because beta blockers improve survival and may be effective in controlling rate alone, digoxin should be considered as an adjunctive agent for rate control.
Digoxin is not indicated as primary therapy for the stabilization of patients with an acute exacerbation of HF symptoms, including fluid retention or hypotension. Such patients should first receive appropriate treatment for HF (usually with intravenous medications); therapy with digoxin may be initiated after stabilization as part of an effort to establish a long-term treatment strategy.
Patients should not be given digoxin if they have significant sinus or atrioventricular block, unless the block has been addressed with a permanent pacemaker. The drug should be used cautiously in patients taking other drugs that
can depress sinus or atrioventricular nodal function or affect
digoxin levels (e.g., amiodarone or a beta blocker), even though such patients usually tolerate digoxin without difficulty.
PRACTICAL USE OF DIGITALIS IN HF.
digoxin is the most commonly used, and it is the only glycoside that has been evaluated in placebo-controlled trials. There is little reason to prescribe other cardiac glycosides for the management of HF.Therapy commonly initiated and maintained at a dose of 0.125 to 0.25 mg daily. Low doses (0.125 mg daily or every other day) if the patient is more than 70 years old, has impaired renal function, or has a low lean body mass . Higher doses (e.g., digoxin 0.375 to 0.50 mg daily) are rarely used or needed in patients with HF.
There is no reason to use loading doses of digoxin to initiate therapy in patients with HF.
The principal adverse reactions
occur primarily when digoxin is administered in large doses, but large doses may not be needed to produce clinical benefits .The major side effects include cardiacarrhythmias (e.g., ectopic and re-entrant cardiac rhythms and heart block), gastrointestinal symptoms (e.g., anorexia, nausea, and vomiting), and neurological complaints (e.g., visual disturbances, disorientation, and confusion).
Overt digitalis toxicity is commonly associated with serum digoxin levels greater than 2 ng per mL. However, toxicity may occur with lower digoxin levels, especially if
hypokalemia,hypomagnesemia, or hypothyroidism coexists .
The concomitant use of clarithromycin, erythromycin, amiodarone,itraconazole, cyclosporine, verapamil, or quinidine can increase serum digoxin concentrations and may increase the likelihood of digitalis toxicity .The dose of digoxin should be reduced if treatment with these drugs is initiated.
Spironolactone does not inhibit the disposition of digoxin ,cross-reactivity of some digoxin antibodies
with spironolactone confounded earlier attempts to assess the effect of spironolactone on digoxin clearance.
a low lean body mass and
impaired renal function can also elevate serum digoxin levels, which may explain the increased risk of digitalis toxicity in elderly patients.
Of note, one analysis suggested that women may not benefit from digoxin therapy and may be at increased risk for death with such therapy
Digoxin should be used with caution or not used at all in post-MI patients, particularly if they have ongoing ischemia
VENTRICULAR ARRHYTHMIAS AND PREVENTION OF SUDDEN DEATH
Patients with LV dilation and reduced LVEF frequently manifest ventricular tachyarrhythmias, both nonsustained ventricular tachycardia (VT) and sustained VT. The cardiac mortality of patients with all types of ventricular tachyarrhythmias is high. The high mortality results from progressive HF, as well as from sudden death.Sudden death is often equated with a primary arrhythmic event, but multiple causes of sudden death have been documented and include ischemic events such as acute MI ,electrolyte disturbances, pulmonary or systemic emboli, or other vascular events. Although ventricular tachyarrhythmias are the most common rhythms associated with unexpected sudden death, bradycardia and other pulseless supraventricular rhythms are common in patients with advanced HF .
Sudden death can be decreased meaningfully
by the therapies that decrease disease progression, as discussed elsewhere in these guidelines. For instance, clinical trials with beta blockers have shown a reduction in sudden death,as well as in all-cause mortality, in both postinfarction patients and patients with HF regardless of cause. Aldosterone antagonists decrease sudden death and overall mortality in HF early after MI and in advanced HF .Sudden unexpected death can be decreased further by the use of implanted devices that terminate sustained arrhythmias .Even when specific antiarrhythmic therapy is necessary to diminish recurrent ventricular tachyarrhythmias
and device firings, the frequency and tolerance of arrhythmias may be improved with appropriate therapy for HF In some cases, definitive therapy of myocardial ischemia or other reversible factors may prevent recurrence of tachyarrhythmia, particularly polymorphic VT, ventricular fibrillation, and nonsustained VT. Nonetheless, implantable defibrillators should be recommended in all patients who have had a life threatening tachyarrhythmia and have an otherwise good prognosis.
• Secondary Prevention of Sudden Death.
Patients with previous cardiac arrest or documented sustained ventricular arrhythmias have a high risk of recurrent events. Implantation of an ICD has been shown to reduce mortality in cardiac arrest survivors. An ICD is indicated for secondary prevention of death from ventricular tachyarrhythmias in patients with otherwise good clinical function and prognosis, for whom prolongation of survival is a goal. Patients with chronic HF and a low EF who experience syncope of unclear origin have a high rate of subsequent sudden death and should also be considered for placement of an ICD .However, when ventricular tachyarrhythmias occur in a patient with aprogressive and irreversible downward spiral of clinical HF decompensation, placement of an ICD is not indicated to prevent recurrence of sudden death, because death is likely imminent regardless of mode. An exception may exist for the small minority of patients for whom definitive therapy such as cardiac transplantation is planned.
2. Primary Prevention of Sudden Death.
Patients with low EF without prior history of cardiac arrest, spontaneous VT, orinducible VT (positive programmed electrical stimulation study) have a risk of sudden death that is lower than for those who have experienced previous events, but it remains significant. Within this group, it has not yet been possible to identify those patients at highest risk, especially in the absence of prior MI.
Approximately 50% to 70% of patients with low EF and symptomatic HF have episodes of nonsustained VT on routine ambulatory electrocardiographic monitoring; however, it is not clear whether the occurrence of complex ventricular arrhythmias in these patients with HF contributes to the high frequency of sudden death or,alternatively, simply reflects the underlying disease process . Antiarrhythmic drugs to suppress premature ventricular depolarizations and nonsustained ventricular arrhythmias have not improved survival ,although nonsustained VT may play a role in triggering ventricular tachyarrhythmias.
Furthermore, most antiarrhythmic drugs have negative inotropic effects and can increase the risk of serious arrhythmia; these adverse cardiovascular effects are particularly pronounced in patients with low EF This risk is especially high with the use of Class IA agents (quinidine and procainamide), Class IC agents (flecainide and propafenone), and some Class III agents (dsotalol), which have increased mortality in post-MI trials
The role of ICDs in the primary prevention of sudden death in patients without prior history of symptomatic arrhythmias has been explored recently in a number of trials.If sustained ventricular tachyarrhythmias can be induced in the electrophysiology laboratory in patients with previous MI or chronic ischemic heart disease, the risk of sudden death in these patients is in the range of 5% to 6% per year and can be improved by ICD implantation .The role of ICD implantation for the primary prevention of sudden death in patients with HF and low EF and no history of spontaneous or inducible VT has been addressed by several large trials that used only readily available clinical data as entry criteria .
The first of these demonstrated that ICDs, compared with standard medical therapy, decreased the occurrence of total mortality for patients with EF of 30% or less after remote MI .Absolute mortality was decreased in the ICD arm by 5.6%, a relative decrease of 31% over 20 months. In a second trial, a survival benefit was not demonstrated with devices implanted within 6 to 40 days after an acute MI in patients who at that time had an EF less than 35% and abnormal heart rate variability.
Comorbidities common in the elderly population, such as prior stroke, chronic pulmonary disease, and crippling arthritic conditions, as well as nursing home residence, should be factored into discussions regarding ICD. Atrial fibrillation, a common trigger for inappropriate shocks, is
more prevalent in the elderly population. The gap between community and trial populations is particularly important for a device therapy that may prolong survival but has no positive impact on function or quality of life. Some patients may suffer a diminished quality of life because of device site complications, such as bleeding, hematoma, or infections,or after ICD discharges, particularly those that are inappropriate.
Consideration of ICD implantation is thus recommended in patients with EF less than or equal to 35% and mild to moderate symptoms of HF and in whom survival with good functional capacity is otherwise anticipated to extend beyond 1 year. Because medical therapy may substantially improve EF, consideration of ICD implants should follow documentation of sustained reduction of EF despite a course of beta blockers and ACEIs or ARBs;however, ICDs are not warranted in patients with refractory symptoms of HF (Stage D) or in patients with concomitant diseases that would shorten their life expectancy independent of HF.
Before implantation, patients should be fully informed of their cardiac prognosis, including the risk of both sudden and nonsudden mortality; the efficacy, safety,and risks of an ICD; and the morbidity associated with an ICD shock.
Patients and families should clearly understand that the ICD does not improve clinical function or delay HF progression. Most important, the possible reasons and process for potential future deactivation of defibrillator features should be discussed long before functional capacity or outlook for survival is severely reduced.
ISOSORBIDE DINITRATE.
one of the first vasodilator agents reported to be useful for chronic therapy of HF. may decrease symptoms of dyspnea at night and during exercise and may improve exercise tolerance in patients who have persistent limitations despite optimization of other therapies.Most experience relates to the oral dinitrate and more recently the mononitrate preparations, with little information available about topical nitrate therapy in this population.
Recent evidence suggests that nitrates can inhibit abnormal myocardial and vascular growth and
may thereby attenuate the process of ventricular remodeling and improve symptoms.
The only common side effects of nitrate therapy are
headaches and hypotension.
nitrates predominantly are potent venodilators that also have effects on arterial tone when used alone, particularly when systemic vascular resistance is severely elevated. Because they act through cyclic guanosine monophosphate,there is a theoretical reason that they may be titrated up to facilitate weaning of intravenous infusions that act through the same pathway.
Nitrate tolerance.
This appears to be minimized by prescriptionof a “nitrate-free interval” of at least 10 hours
and bycombination with ACEIs or hydralazine.
HYDRALAZINE.
arterial vasodilator with relatively little effect on venous tone and cardiac filling pressures. The rationale for its combined use with nitrates was to achieve both venous and arterial vasodilation .
In addition to its direct vascular actions, hydralazine in theory may interfere with the biochemical and molecular mechanisms responsible for the progression of HF and the development of nitrate tolerance . There are limited data regarding the use of hydralazine alone in HF.
The combination of hydralazine and isosorbide
dinitrate should not be used for the treatment of HF in patients who have no prior use of an ACEI and should not be substituted for ACEI in patients who are tolerating ACEIs without difficulty.CARDIAC RESYNCHRONIZATION THERAPY (CRT)
Approximately one-third of patients with low EF and class III to IV symptoms of HF manifest a QRS duration greater than 0.12 seconds .This electrocardiographic representation of abnormal cardiac conduction has been used to identify patients with dyssynchronous ventricular contraction. While imperfect, no other consensus definition of cardiac dyssynchrony exists as yet, although several echocardiographic measures appear promising.This approach to HF therapy, commonly called cardiac resynchronization therapy (CRT), may enhance ventricular contraction and reduce the degree of secondary mitral regurgitation.
In addition, the short-term use of CRT has been associated with improvements
in cardiac function and hemodynamics without an accompanying increase in oxygen use as well as adaptive changes in the biochemistry of the failing heart .
The mechanical consequences of dyssynchrony include
1.suboptimal ventricular filling,
2.prolonged duration of mitral regurgitation, and
3.paradoxical septal wall motion .Ventricular dyssynchrony has also been
4.associated with increased mortality
Dyssynchronous contraction can be addressed by electrically activating the right and left ventricles in a synchronized manner with a biventricular pacemaker device.
To date, more than 4000 HF patients with ventricular
dyssynchrony have been evaluated in randomized controlled trials of optimal medical therapy alone versus optimal medical therapy plus CRT with or without an ICD. CRT, when added to optimal medical therapy in persistently symptomatic patients, has resulted in significant improvements inquality of life,
functional class,
exercise capacity (by peak oxygen uptake) and exercise distance during a6-minute walk test,
and EF in patients randomized to CRT or to the combination of CRT and ICD .
In a meta-analysis of several CRT trials, HF hospitalizations were reduced by 32% and all-cause mortality by 25% The effect on mortality in this meta-analysis became apparent after approximately 3 months of therapy .
Thus, there is strong evidence to support the use of
CRTto improve
symptoms,
exercise capacity,
quality of life,
LVEF, and survival
and to decrease hospitalizations in
patients with persistently symptomatic HF undergoing
optimal medical therapy who have cardiac dyssynchrony (as
evidenced by a prolonged QRS duration). The use of an
ICD in combination with CRT should be based on the
indications for ICD therapy .
CRT peri-implant morbidity and mortality.
There were 13 deaths in 3113 patients
(0.4%). From a pooled assessment of 3475 patients in 17
studies, the success rate of implantation was approximately
90% (215). Device-related problems during the first 6
months after implantation reported in 13 studies included
lead malfunction or dislodgement in 8.5%, pacemaker
problems in 6.7%, and infection in 1.4% of cases. These
morbidity and mortality data are derived from trials that
used expert centers.
EXERCISE TRAINING. In the past, patients with HF
were advised to avoid physical exertion in the hope that bed rest might minimize symptoms and in the belief that physical activity might accelerate the progression of LV dysfunction ,however, it is now understood that a reduction in physical activity (produced by the symptoms of HF or prescribed by physicians treating HF) leads to astate of physical deconditioning that contributes to the symptoms and exercise intolerance of patients with chronic HF .Limitations of activity not only may impair exercise capacity but also may produce adverse psychological effects and impair peripheral vasodilatory responses .
These findings have led to the hypothesis that exercise training might improve the clinical status of patients with chronic HF.
DRUGS AND INTERVENTIONS UNDER ACTIVE INVESTIGATION
Investigational drug therapies currently in phase III evaluation for the treatment of chronic HF includevasopressin receptor antagonists, intermittent nesiritide infusions, and
oral phosphodiesterase III inhibitors.
newer devices and technologies, such as implantable hemodynamic monitors and
internal cardiac support devices,
external counterpulsation, treatment for sleep disordered breathing,
myocardial growth factors and
stem cell transplantation, and devices to achieve intravascular volume reduction, as well as novel surgical approaches,including
surgical ventricular restoration, are under active investigation.
TECHNIQUES FOR RESPIRATORY SUPPORT.
Patients with HF frequently exhibit abnormal respiratory patterns,including Cheyne-Stokes breathing and sleep-disordered breathing . The use of nocturnal oxygen and devices that provide continuous positive airway pressure has been reported to produce symptomatic improvement .Although there is no direct evidence that treatment of sleep-disturbed breathing prevents incident HF, treatment of established LV dysfunction with continuous positive airway pressure breathing has been shown to improve LV structure and function in patients with either obstructive or central sleep apnea disturbed breathing syndrome .Additional studies are in progress to evaluate the efficacy of these interventions. It is hoped that such studies will provide information about the efficacy and safety of this approach and help identify patients most likely to benefit from treatment.
EXTERNAL COUNTERPULSATION
The technique involves the use of a device with inflatable cuffs that surround the lower limbs and inflate and deflate in synchronization with the cardiac cycle. The device is designed to reduce loading conditions in systole while increasing coronary perfusion pressures in diastole .External counterpulsation has been shown to reduce the frequency and severity of anginal attacks in patients with symptomatic coronary artery disease .A possible mechanism of action for this observed clinical effect may be
an improvement in endothelial function of the coronary vascular bed .Early trials of this therapy in patients with HF and low EF have been encouraging, and arandomized trial has been completed recently .Until more data are available, routine use of this therapy cannot be recommended for the management of patients with symptomatic reduced LVEF.VASOPRESSIN RECEPTOR ANTAGONISTS.
is a peptide hormone with significant cardiovascular and renal effects. These effects are mediated through at least 2 receptor subtypes: the V1A receptor, which is found on vascular smooth muscle cells and in the myocardium, and the V2 receptors, which are found in the kidney. Vasopressin levels are often elevated in patients with HF and LV dysfunction, and they appear to be associated with adverse outcomes in the setting of low EF after MI .Early studies with 2 different vasopressin receptor antagonists have shown favorable changes in hemodynamics and urine output without a significant change in blood pressure or heart rate. The drugs appear to reduce body weight and edema, and they normalized serum sodium in patients with hyponatremia, but the duration and significance of these clinical effects are not clear .Currently, longerterm clinical trials are under way to determine the role, if any, of these vasopressin antagonists in patients with chronic HF
IMPLANTABLE HEMODYNAMIC MONITORS.
Several implantable systems are in development for the chronic,remote, outpatient monitoring of ventricular filling pressures and other hemodynamic and clinical variables in HF patients. One such system has completed phase I and II study and is currently being evaluated in a phase III randomized outcomes trial. The hypothesis underlying this approach suggests that changes in therapy to optimize LV filling pressure may improve outcomes in HF patientsCARDIAC SUPPORT DEVICES.
There is developing experience with surgical devices that are designed to alter physical stresses on the LV; theoretically, the devices may improve performance or attenuate further ventricular dilatation.One such device now being evaluated clinically is acardiac wrapping device made from a bidirectional woven polyester that allows for shortening but resists circumferential expansion beyond the limits of the wrap . Clinical trials in Europe and the United States are currently under way to evaluate the safety and efficacy of this device in patients.
SURGICAL APPROACHES UNDER INVESTIGATION.
A number of surgical approaches have emerged as potentially beneficial in patients with ischemic HF. The goals of such procedures generally include revascularization, reduction in “geometric” or functional mitral regurgitation, and restoration of a more normal LV geometry and function. In this context, the so-called surgical ventricular restoration procedure is one of the most extensively studied and applied techniques for reshaping or excluding anteroapical and septal regions of asynergy.The National Heart, Lung, and Blood Institute’s multicenter, international, randomized STICH (Surgical Treatment for Ischemic Heart Failure) trial began enrolling patients with coronary artery disease and HF in the spring of 2002. The goal of this study is to determine whether abenefit over medical therapy can be found for coronary revascularization and whether this benefit can be enhanced by ventricular restoration surgery.
NESIRITIDE
Natriuretic peptides are novel compoundsthat promote diuresis and natriuresis, have vasodilatory properties, lead to an indirect increase in cardiac output, and suppress neurohormonal activation; they have been approved for use in the management of acute HF In this setting, nesiritide has been shown to improve symptoms of acute HF, but the effect on morbidity and mortality has not been clear from available .
clinical trials are currently under investigation as adjunctive therapy, administered on an intermittent outpatient basis,for advanced chronic HF. Unless a definitive study does demonstrate safety and efficacy, intermittent or continuous outpatient infusion of nesiritide and other natriuretic peptides is not recommended.
DRUGS AND INTERVENTIONS OF UNPROVED VALUE AND NOT RECOMMENDED
NUTRITIONAL SUPPLEMENTS AND HORMONAL THERAPIES.Patients with HF, particularly those treated with diuretics, may become deficient in vitamins and micronutrients. Several nutritional supplements (e.g., coenzyme Q10, carnitine, taurine, and antioxidants) and hormonal therapies (e.g., growth hormone or thyroid hormone) have been proposed for the treatment of HF.
Aside from replenishment of documented deficiencies, randomized trials have failed to demonstrate benefit for routine vitamin, nutritional, or hormonal supplementation .
Most patients with HF due to reduced LVEF respond favorably to pharmacological and nonpharmacological treatments and enjoy a good quality of life and enhanced survival; however, some patients do not improve or experience rapid recurrence of symptoms despite optimal medical therapy. Such patients characteristically have symptoms at rest or on minimal exertion, including profound fatigue; cannot perform most activities of daily living; frequently have evidence of cardiac cachexia; and typically require repeated and/or prolonged hospitalizations for intensive management. These individuals represent the most advanced stage of HF and should be
considered for specialized treatment strategies,
such as mechanical circulatory support,continuous intravenous positive inotropic therapy,referral for cardiac transplantation, or hospice care Before a patient is considered to have refractory HF,
physicians should confirm the accuracy of the diagnosis,identify any contributing conditions, and ensure that all
conventional medical strategies have been optimally employed.
INTERMITTENT INTRAVENOUS POSITIVE INOTROPIC THERAPY Although can improve cardiac performance during short- and longterm therapy ,long-term oral therapy with these drugs has not improved symptoms or clinical status) and has been associated with a significant increase in mortality, especially in patients with advanced HF .Despite these data, some physicians have proposed that the regularly scheduled intermittent use of intravenous positive inotropic drugs (e.g., dobutamine or milrinone) in a supervised outpatient setting might be ssociated with some clinical benefits . However, there has been little experience with intermittent home infusions of positive inotropic agents in controlled clinical trials.
Most trials have been small and short in duration and thus have not been able to provide reliable information about the effect of treatment on the risk of serious cardiac events. Much, if not all, of the benefit seen in these uncontrolled reports may have been related to the increased surveillance of the patient’s status and intensification of concomitant therapy and not to the use of positive inotropic agents. Only 1 placebo-controlled trial of intermittent intravenous positive inotropic therapy has been published ,and its findings are consistent with the results of long-term studies with continuous oral positive inotropic therapy in HF (e.g., with milrinone), which showed little efficacy and were terminated early because of an increased risk of death.
Given the lack of evidence to support their efficacy and concerns about their toxicity, intermittent infusions of positive inotropic agents (whether at home, in an outpatient clinic, or in a short-stay unit) should not be used in the long-term treatment of HF, even in its advanced stages.
Patients With Heart Failure and Normal
Left Ventricular Ejection FractionPatients With Heart Failure and Normal
Left Ventricular Ejection FractionRecommendations
CLASS I
1. Physicians should control systolic and diastolic hypertension in patients with HF and normal LVEF, in accordance with published guidelines. (Evidence: A)
2. Physicians should control ventricular rate in patients with HF and normal LVEF and atrial fibrillation. (Level of Evidence: C)
3. Physicians should use diuretics to control pulmonary congestion and peripheral edema in patients with HF and normal LVEF. (Level of Evidence: C)
CLASS IIa
1. Coronary revascularization is reasonable in patients with HF and normal LVEF and coronary artery disease in whom symptomatic or demonstrable myocardial ischemia is judged to be having an adverse effect on cardiac function. (Level of Evidence: C)
CLASS IIb
1. Restoration and maintenance of sinus rhythm in patients with atrial fibrillation and HF and normal LVEF might be useful to improve symptoms. (Evidence: C)
2. The use of beta-adrenergic blocking agents, ACEIs, ARBs, or calcium antagonists in patients with HF and normal LVEF and controlled hypertension might be effective to minimize symptoms of HF. (Evidence: C)
The usefulness of digitalis to minimize symptoms of HF in
patients with HF and normal LVEF isnot well established.
(Level of Evidence: C)
IDENTIFICATION OF PATIENTS
For many years, the syndrome of HF was considered to besynonymous with diminished contractility of the LV, or
reduced LVEF. Over the past few years, however, there has
been a growing appreciation that a large number of patients
with HF have a relatively normal EF, or preserved EF. The
pathophysiology of this type of HF has been reviewed in
Depth,and a large, randomized study that enrolled
patients with HF and normal EF has been completed .
Currently, a number of investigators are seeking to clarify
the epidemiology, clinical characteristics, and prognosis of
patients with HF and a normal LVEF
It is estimated that as many as 20% to 60% of patients with HF have a relatively (or near) normal LVEF and, in the absence of valvular disease, are believed to have reduced ventricular compliance as a major contributor to the clinical syndrome. Some investigators have found that in a significant number of patients, a tendency to fluid retention and reduced vascular compliance, rather than myocardial stiffness,represent the principal abnormalities .
Regardless, abnormal renal sodium handling and arterial stiffness, in addition to myocardial stiffness, are likely to play important pathophysiologic roles in many patients. Diastole is that period in the cardiac cycle during which the myocardium loses its ability to generate force and shorten and
returns to an unstressed length and force, and diastolic dysfunction occurs when these events are prolonged, slowed, or are incomplete .
It should also be recognized that diastolic function is abnormal in patients with HF and reduced LVEF, as well as those with preserved LVEF.
Several recognized myocardial disorders are associated with HF and a normal LVEF,
including restrictive cardiomyopathy,
obstructive and nonobstructive hypertrophic cardiomyopathy,
and infiltrative cardiomyopathies.
The vast majority of patients with HF and relatively preserved LVEF have a history of hypertension, and many, if not most, of these patients have evidence of LVH on echocardiography.
However, some patients who present with HF and relatively preserved LVEF have no identifiable myocardial pathology.Because these patients usually present with symptoms typical of HF, they should be classified as Stage C. Indeed, most patients will have some detectable structural abnormality of the heart, including LVH, atrial dilation, mitral annular calcification, aortic sclerosis, or myocardial scar.
Heart failure associated with relatively preserved LVEF is most prevalent among elderly women, most of whom have hypertension, diabetes mellitus, or both and often coronary artery disease or atrial fibrillation as well .This observation may be related to the fact that aging has a greater impact on ventricular filling characteristics than on EF .Aging is associated with decreases in the elastic properties of the heart and great vessels, which leads to an increase in systolic blood pressure and an increase in myocardial stiffness. The rate of ventricular filling decreases in part because of structural changes in the heart (due to fibrosis) and because of a decline in relaxation and compliance. These deleterious effects on diastolic function are exacerbated by a decrease in beta-adrenergic receptor density and a decline in peripheral vasodilator capacity, both of which are characteristic of elderly patients. In addition, elderly patients commonly have associated disorders (e.g.,coronary artery disease, diabetes mellitus, aortic stenosis,atrial fibrillation, or obesity), which can adversely affect the diastolic properties of the heart or decrease the time available for ventricular filling. There may also be sexspecific responses to hypertension and diabetes mellitus that make women more susceptible than men to the cumulative effects of aging on diastolic function
A number of recent investigations have focused on the
differences between HF with preserved EF and that with low LVEF .Myocardial infarction or other evidence of atherosclerotic disease appears to be less common in HF with normal LVEF, but hypertension is at least as common in this subgroup. The morbidity and mortality associated with HF and a relatively preserved LVEF may be nearly as profound as that with low LVEF; frequent and repeated hospitalizations characterize the patient with HF and a normal LVEF .Most, but not all, series of patients with HF and relatively preserved LVEF have shown better survival than is seen in patients with HF and reduced LVEF.
however, these comparisons are difficult to interpret, because it is difficult to be certain that such series do not contain at least some patients in whom the diagnosis of HF is erroneous.
DIAGNOSIS
symptoms and signs of HF in a patient who is shown to have a normal LVEF and no valvular bnormalities (aortic stenosis or mitral regurgitation, for example) on echocardiography. Every effort should be made to exclude other possible explanations or disorders that may present in a similar manner.Noninvasive methods (especially those that rely on
Doppler echocardiography) have been developed to assist in the diagnosis of HF with normal LVEF, but these testshave significant limitations, because cardiac filling patterns are readily altered by nonspecific and transient changes in loading conditions in the heart and by aging, changes in heart rate, or the presence of mitral regurgitation .The analysis of BNP levels in association with echocardiographic filling patterns can improve diagnostic accuracy. For example, a normal BNP level along with completely normal diastolic end-filling parameters makes HF much less likely; however, HF does remain a strictly clinical diagnosis.
PRINCIPLES OF TREATMENT
In contrast to the treatment of HF due to reduced LVEF, few clinical trials are available to guide the management of patients with HF and relatively preserved LVEF. Although controlled studies have been performed with digitalis,ACEIs, ARBs, beta blockers, and calcium channel blockers in patients with HF who had a relatively preserved LVEF, for the most part, these trials have been small or have produced inconclusive results .Nevertheless,many patients with HF and normal LVEF are treated with these drugs because of the presence of comorbid conditions (i.e., atrial fibrillation, hypertension, diabetes mellitus, and coronary artery disease). A large, randomized trial recently completed included patients with HF and normal LVEF, which demonstrates that studies in such patients can be accomplished .In that trial, the addition of candesartan to the treatment regimen for patients with symptomatic HF and relatively preserved LVEF significantly reduced morbidity but did not reach the primary endpoint.
In the absence of other controlled clinical trials, the management of these patients is based on the control of physiological factors (blood pressure, heart rate, blood volume, and myocardial ischemia) that are known to exert important effects on ventricular relaxation . Likewise,diseases that are known to cause HF with normal LVEF should be treated, such as coronary artery disease, hypertension, or aortic stenosis. Clinically, it seems reasonable to target symptom reduction, principally by reducing cardiac filling pressures at rest and during exertion.
Recommendations regarding the use of anticoagulation and antiarrhythmic agents apply to all patients with HF, irrespective of LVEF.
POTENTIAL TREATMENT STRATEGIES.
Hypertension exerts a deleterious effect on ventricular function by causing both structural and functional changes in the heart. Increases in systolic blood pressure have been shown to slow myocardial ,and the resulting hypertrophy may adversely affect passive chamber stiffness. Physicians should make every effort to control both systolic and diastolic hypertension with effective antihypertensive therapy in accordance with published guidelines .Consideration should at least be given to achieving target levels of blood pressure lower than those recommended for patients with uncomplicated hypertension (e.g., less than 130 mm Hg systolic and less than 80 mm Hg diastolic) .
Because myocardial ischemia can impair ventricular relaxation, coronary revascularization should be considered in patients with coronary artery disease in whom symptomatic or demonstrable myocardial ischemia is believed to be exerting a deleterious effect on cardiac function .
Because tachycardia can shorten the time available for ventricular filling and coronary perfusion, drugs that slow the heart rate or the ventricular response to atrial arrhythmias (e.g., beta blockers, digoxin, and some calcium channel blockers) can provide symptomatic relief in patients with HF and normal LVEF.
Similarly, patients with HF and preserved LVEF may be particularly sensitive to loss of atrial kick, which supports a potential benefit for restoration of sinus rhythm in patients with atrial fibrillation.
The benefits of restoring sinus rhythm in these individuals are less clear, and the large trials of rhythm versus rate control in atrial fibrillation published recently have excluded patients with HF. Moreover, the presence of systolic or diastolic dysfunction may diminish the efficacy and enhance the toxicity of drugs used to achieve and maintain sinus rhythm.
Circulating blood volume is a major determinant of ventricular filling pressure, and the use of diuretics may improve breathlessness in patients with HF and normal LVEF as well as those with reduced LVEF. Other possible agents used to reduce diastolic filling pressures are nitrates or agents that block neurohumoral activation. Hypotension may be a significant problem in this population, especially in the very elderly, because they can be quite sensitive to preload reduction.
Patients With Refractory End-Stage Heart Failure
Patients With Refractory End-Stage Heart FailureRecommendations
CLASS I
1. Meticulous identification and control of fluid retention is recommended (Level of Evidence: B)
2. Referral for cardiac transplantation in potentially eligible patients
is recommended for patients with refractory end-stage HF ;
3. Referral of patients with refractory end-stage HF to a HF program
with expertise in the management of refractory HF is useful
4. Options for end-of-life care should be discussed with the patient
and family when severe symptoms in patients with refractory
end-stage HF persist despite application of all recommended
therapies. (Level of Evidence: C)
5. Patients with refractory end-stage HF and implantable defibrillators should receive information about the option to inactivate the defibrillator. (Level of Evidence: C)
CLASS IIa
1. Consideration of an LV assist device as permanent or “destination”
therapy is reasonable in highly selected patients with refractory end-stage HF and an estimated 1-year mortality over 50% with medical therapy (Evidence: B)
CLASS IIb
1. Pulmonary artery catheter placement may be reasonable to guide therapy in patients with refractory end-stage HF and persistently severe symptoms
2. The effectiveness of mitral valve repair or replacement is not well
established for severe secondary mitral regurgitation in refractory
end-stage HF (Level of Evidence: C)
3. Continuous intravenous infusion of a positive inotropic agent may be considered for palliation of symptoms in patients with refractory end-stage HF
CLASS III
1. Partial left ventriculectomy is not recommended in patients with nonischemic cardiomyopathy and refractory end-stage HF. (Level of Evidence: C)
2. Routine intermittent infusions of vasoactive and positive inotropic agents are not recommended for patients with refractory end-stage HF (Evidence: A)
Management of Fluid Status
Many patients with advanced HF have symptoms that are related to the retention of salt and water and thus will respond favorably to interventions designed to restore sodium balance. Hence, a critical step in the successful management of end-stage HF is the recognition and meticulous control of fluid retention. In most patients with chronic HF, volume overload can be treated adequately with low doses of a loop diuretic combined with moderate dietary sodium restriction; however, as HF advances, the accompanying decline in renal perfusion can limit the ability of the kidneys to respond to diuretic therapy .In such patients, the control of fluid retention may require progressive increments in the dose of a loop diuretic and frequently the addition of asecond diuretic that has a complementary mode of action (e.g., metolazone).If the patient continues to exhibit evidence of volume overload despite these measures, hospitalization is generally required for further adjustment of therapy possibly including intravenous dopamine or dobutamine. This strategy can elicit a marked increase in urine volume, but such a diuresis is frequently accompanied by worsening azotemia, especially if patients are also being treated with an ACEI.
Provided that renal function stabilizes, small or moderate elevations of blood urea nitrogen and serum creatinine should not lead to efforts to minimize the intensity of therapy; however, if the degree of renal dysfunction is severe or if the edema becomes resistant to treatment, ultrafiltration or hemofiltration may be needed to achieve adequate control of fluid retention . The use of such mechanical methods of fluid removal can produce meaningful clinical benefits in patients with diuretic-resistant HF and may restore responsiveness to conventional doses of loop diuretics.
In general, patients should not be discharged from the hospital until a stable and effective diuretic regimen is established, and ideally, not until euvolemia is achieved.
Patients who are sent home before these goals are reached
are at high risk of recurrence of fluid retention and early readmission ,because unresolved edema may itself attenuate the response to diuretics .Once euvolemia is achieved, the patient’s dry weight can be defined and used as a continuing target for the adjustment of diuretic doses. Many patients are able to modify their own diuretic regimen in response to changes in weight that exceed apredefined range. The restriction of dietary sodium (to 2 g daily or less) can greatly assist in the maintenance of volume balance.
Patients with persistent or recurrent fluid retention despite sodium restriction and high-dose diuretic use may benefit from review of fluid intake and restriction to 2 liters daily. The ongoing control of fluid retention may be enhanced by enrollment in an HF program, which can provide the close surveillance and education needed for the early recognition and treatment of volume overload
Utilization of Neurohormonal Inhibitors
Controlled trials suggest that patients with advanced HF respond favorably to treatment with both ACEIs and beta blockers in a manner similar to those with mild to moderate disease . However, because neurohormonal mechanisms play an important role in the support of circulatory homeostasis as HF progresses, neurohormonal antagonism may be less well tolerated by patients with severe symptoms than by patients with mild symptoms.Patients who are at the end stage of their disease are at particular risk of developing hypotension and renal insufficiency after the administration of an ACEI and of experiencing worsening HF after treatment with a beta blocker. As a result, patients with refractory HF may tolerate only small doses of these neurohormonal antagonists or may not
tolerate them at all.
Consequently, physicians should exercise great care when considering the use of both ACEIs and beta blockers in patients with refractory HF.
Treatment with either type of drug should not be initiated in patients who have systolic blood pressures less than 80 mm Hg or who have signs of peripheral hypoperfusion.
In addition, patients should not be started on a beta blocker if they have significant fluid retention or if they recently required treatment with an intravenous positive inotropic agent.
Treatment with an ACEI or beta blocker should be initiated in very low doses,and patients should be monitored closely for signs or symptoms of intolerance. If low doses are tolerated, further dosage increments may be considered but may not be tolerated. However, clinical trials with lisinopril and carvedilol suggest that even low doses of these drugs may provide important benefits
Alternative pharmacological treatments may be considered for patients who cannot tolerate ACEIs or betablockers. A combination of nitrates and hydralazine has been reported to have favorable effects on survival in patients with mild to moderate symptoms who were not taking an ACEI or a beta blocker ,but the utility of this vasodilator combination in patients with end-stage disease who are being given these neurohormonal antagonists remains unknown. In addition, many patients experience headaches or gastrointestinal distress with these direct acting vasodilators, which can prevent patients from undergoing long-term treatment.
Spironolactone has been reported to prolong life and reduce the risk of hospitalization for HF in patients with advanced disease ,however,the evidence supporting the use of the drug has been derived in patients who have preserved renal function, and the drug can produce dangerous hyperkalemia in patients with impaired renal function. Finally, although ARBs are frequently considered as alternatives to ACEIs because of the low incidence of cough and angioedema with these medications, it is not clear that ARBs are as effective as ACEIs, and they are as likely as ACEIs to produce hypotension or renal insufficiency
Intravenous Peripheral Vasodilators and Positive Inotropic Agents
Patients with refractory HF are hospitalized frequently for clinical deterioration, and during such admissions, they commonly receive infusions of both positive inotropic agents (dobutamine, dopamine, or milrinone) and vasodilator drugs (nitroglycerin, nitroprusside, or nesiritide) in an effort to improve cardiac performance, facilitate diuresis, and promote clinical stability.Some physicians have advocated the placement of pulmonary artery catheters in patients with refractory HF, with the goal of obtaining hemodynamic measurements that might be used to guide the selection and titration of therapeutic agents .
However, the logic of this approach has been questioned,because many useful drugs for HF produce benefits by mechanisms that cannot be evaluated by measuring their short-term hemodynamic effects . Regardless of whether invasive hemodynamic monitoring is used, once the clinical status of the patient has stabilized, every effort should be made to devise an oral regimen that can maintain symptomatic improvement and reduce the subsequent risk of deterioration. Assessment of the adequacy and tolerability of orally based strategies may necessitate observation in the hospital for at least 48 hours after the infusions are discontinued
Patients who cannot be weaned from intravenous to oral therapy despite repeated attempts may require placement of an indwelling intravenous catheter to allow for the continuous infusion of dobutamine or milrinone or, as has been used more recently, nesiritide. Such a strategy is commonly used in patients who are awaiting cardiac transplantation, but it may also be used in the outpatient setting in patients who otherwise cannot be discharged from the hospital.
The decision to continue intravenous infusions at home should not be made until all alternative attempts to achieve stability have failed repeatedly, because such an approach can present a major burden to the family and health services and may ultimately increase the risk of death. However, continuous intravenous support may provide palliation of symptoms as part of an overall plan to allow the patient to die with comfort at home .
Intermittent outpatient infusions of either vasoactive drugs such as nesiritide or positive inotropic drugs have not shown to improve symptoms or survival in patients with advanced HF
Mechanical and Surgical Strategies
Cardiac transplantation is currently the only established surgical approach to the treatment of refractory HF, but it is available to fewer than 2500 patients in the United States each year.Current indications for cardiac transplantation focus on the identification of patients with
1.severe functional impairment or dependence on intravenous inotropic agents.Less common indications for cardiac transplantation include recurrent
2. life-threatening ventricular arrhythmias or angina that is refractory to all currently available treatments .
Alternative surgical and mechanical approaches for the
treatment of end-stage HF are under development. Clinicalimprovement has been reported after
mitral valve repair or replacement in patients who have a clinically important degree of mitral regurgitation that is secondary to LV dilatation .
However, no controlled studies have evaluated the effects of this procedure on ventricular function, clinical status, or survival.
One recent single-center report of a nonrandomized
series of patients considered appropriate candidates for mitral valve repair did not demonstrate a survival advantage.
Although both cardiomyoplasty and left ventriculectomy
(Batista procedure) at one time generated considerable
excitement as potential surgical approaches to the treatment of refractory HF .these procedures failed to result in clinical improvement and were associated with a high risk of death .A variant of the aneurysmectomy procedure is now being developed for the management of patients with ischemic cardiomyopathy ,but its role in the management of HF remains to be defined. None of the current surgical reconstruction techniques offer “rescue therapy” to patients with critical hemodynamic compromise.
The use of mechanical circulatory assist devices in endstage HF is an area of intense investigation. Extracorporeal devices can be used for short-term circulatory support in patients who are expected to recover from a major cardiac insult (e.g., myocardial ischemia, postcardiotomy shock, or fulminant myocarditis). Left ventricular assist devices provide similar degrees of hemodynamic support; many are implantable and thus allow for long-term support, patient ambulation, and hospital discharge . Most clinical experience with these devices has been derived from their use in patients being “bridged” to transplant.
The completion of the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial investigated the use of these devices as permanent or “destination” therapy in selected non–transplant-eligible patients.
This trial enrolled 129 patients, for whom 2-year survival was 23% in the 68 patients treated with the device and 8% in the 61 patients who received medical therapy Device-related adverse events were numerous and included bleeding, infection, thromboembolic events, and device failure. This trial established the efficacy of device therapy for end-stage HF. Improvements in newer generations of devices will hopefully permit even further prolongation of survival. Presently, destination device therapy is anticipated to benefit those patients predicted to have a 1-year survival of less than 50%. One such group could be the population of non–transplant-eligible patients requiring continuous intravenous inotropic infusions
Some reports have suggested that prolonged mechanical decompression of the failing heart may occasionally be followed by sufficient recovery of myocardial function to allow explantation of the device .Improvements in ventricular mechanics, myocardial energetics, histology, and cell signaling have been reported with LV assist device support. However, the frequency and duration of myocardial recovery have been variable ,and sufficient recovery to permit device explantation is rare except in a few patients with acute onset of HF and the absence of coronary artery disease.
Coupling of device therapy with cell transplantation and a variety of angiogenesis or myocardial growth factors are approaches planned for future investigation. Many patients with HF are members of subpopulations who are likely to exhibit unique responses that accelerate the development or progression of HF or complicate the management of HF.
The Hospitalized Patient
RecommendationsCLASS I
1. The diagnosis of HF is primarily based on signs and symptoms. Clinicians should determine the following:
a. adequacy of systemic perfusion;
b. volume status;
c. the contribution of precipitating factors and/or comorbidities;
d. if the heart failure is new onset or an exacerbation of chronic disease; and
e. whether it is associated with preserved ejection fraction.Chest radiographs, electrocardiogram, and echocardiography are key tests in this assessment.
2. Concentrations of B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) should be measured in patients being evaluated for dyspnea in which the contribution of HF is not known. Final diagnosis requires interpreting these results in the context of all available clinical data and ought not to be considered a stand-alone test .
3. Acute coronary syndrome precipitating HF hospitalization should be promptly identified by electrocardiogram and cardiac troponin testing, and treated as appropriate to the overall condition and
prognosis of the patient. (Level of Evidence: C)
4. It is recommended that the following common potential precipitating factors for acute HF be identified as recognition of these comorbidities is critical to guide therapy:
a. acute coronary syndromes/coronary ischemia;
b. severe hypertension;
c. atrial and ventricular arrhythmias;
d. infections;
e. pulmonary emboli;
f. renal failure; and
g. medical or dietary noncompliance. (Level of Evidence: C)
5. Oxygen therapy should be administered to relieve symptoms related to hypoxemia. (Evidence: C)
6. Whether the diagnosis of HF is new or chronic, patients who present with rapid decompensation and hypoperfusion associated with decreasing urine output and other manifestations of shock are critically ill and rapid intervention should be used to improve systemic perfusion. (Level of Evidence: C)
7. Patients admitted with HF and with evidence of significant fluid overload should be treated with intravenous loop diuretics. Therapy should begin in the emergency department or outpatient clinic without delay, as early intervention may be associated with better outcomes for patients hospitalized with decompensated HF (Level of Evidence: B) If patients are already receiving loop diuretic therapy, the initial intravenous dose should equal or exceed their chronic oral daily dose. Urine output and signs and symptoms of congestion should be serially assessed, and diuretic dose should be titrated accordingly to relieve symptoms and to reduce extracellular fluid volume excess. (Level C)
8. Effect of HF treatment should be monitored with careful measurement of fluid intake and output; vital signs; body weight, determined at the same time each day; clinical signs (supine and standing) and symptoms of systemic perfusion and congestion. Daily serum electrolytes, urea nitrogen, and creatinine concentrations should be measured during the use of IV diuretics or active titration of HF medications. (Level of Evidence: C)
9. When diuresis is inadequate to relieve congestion, as evidenced by clinical evaluation, the diuretic regimen should be intensified using either: a. higher doses of loop diuretics; b. addition of a second diuretic (such as metolazone, spironolactone, or intravenous chlorothiazide); or c. continuous infusion of a loop diuretic. (Level of Evidence: C)
10. In patients with clinical evidence of hypotension associated with hypoperfusion and obvious evidence of elevated cardiac filling pressures (e.g., elevated jugular venous pressure; elevated pulmonary artery wedge pressure), intravenous inotropic or vasopressor drugs should be administered to maintain systemic perfusion and preserve end-organ performance while more definitive therapy is considered. (Level of Evidence: C)
11. Invasive hemodynamic monitoring should be performed to guide therapy in patients who are in respiratory distress or with clinical evidence of impaired perfusion in whom the adequacy or excess of intracardiac filling pressures cannot be determined from clinical assessment. (Level of Evidence: C)
12. Medications should be reconciled in every patient and adjusted as appropriate on admission to and discharge from the hospital.(Level of Evidence: C)
13. In patients with reduced ejection fraction experiencing a symptomatic exacerbation of HF requiring hospitalization during chronic maintenance treatment with oral therapies known to improve outcomes, particularly ACEIs or ARBs and beta-blocker therapy, it is recommended that these therapies be continued in most patients in the absence of hemodynamic instability or contraindications. (Level of Evidence: C)
14. In patients hospitalized with HF with reduced ejection fraction not treated with oral therapies known to improve outcomes, particularly ACEIs or ARBs and beta-blocker therapy, initiation of these therapies is recommended in stable patients prior to hospital discharge (Evidence: B)
15. Initiation of beta-blocker therapy is recommended after optimization of volume status and successful discontinuation of intravenous diuretics, vasodilators, and inotropic agents. Beta-blocker therapy should be initiated at a low dose and only in stable patients. Particular caution should be used when initiating beta blockers in patients who have required inotropes during their hospital course (Level of Evidence: B )
16. In all patients hospitalized with HF, both with preserved and low EF, transition should be made from intravenous to oral diuretic therapy with careful attention to oral diuretic dosing and monitoring of electrolytes. With all medication changes, the patient should be monitored for supine and upright hypotension, and worsening renal function and HF signs/ symptoms. (Level of Evidence: C)
17. Comprehensive written discharge instructions for all patients with a hospitalization for HF and their caregivers is strongly recommended, with special emphasis on the following 6 aspects of care: diet; discharge medications, with a special focus on adherence, persistence, and uptitration to recommended doses of ACEI/ARB and beta-blocker medication; activity level; follow-up appointments; daily weight monitoring; and what to do if HF symptoms worsen. (Level of Evidence: C)
18. Postdischarge systems of care, if available, should be used to facilitate the transition to effective outpatient care for patients hospitalized with HF .(Evidence: B)
CLASS IIa
1. When patients present with acute HF and known or suspected acute myocardial ischemia due to occlusive coronary disease, especially when there are signs and symptoms of inadequate systemic perfusion, urgent cardiac catheterization and revascularization is reasonable where it is likely to prolong meaningful survival. (Level of Evidence: C)2. In patients with evidence of severely symptomatic fluid overload in the absence of systemic hypotension, vasodilators such as intravenous nitroglycerin, nitroprusside or nesiritide can be beneficial when added to diuretics and/or in those who do not respond to diuretics alone. (Level of Evidence: C)
3. Invasive hemodynamic monitoring can be useful for carefully selected patients with acute HF who have persistent symptoms despite empiric adjustment of standard therapies, and
a. whose fluid status, perfusion, or systemic or pulmonary vascular resistances are uncertain;
b. whose systolic pressure remains low, or is associated with symptoms, despite initial therapy;
c. whose renal function is worsening with therapy;
d. who require parenteral vasoactive agents; or
e. who may need consideration for advanced device therapy or transplantation. (Level of Evidence: C)
4. Ultrafiltration is reasonable for patients with refractory congestion not responding to medical therapy
CLASS IIb
1. Intravenous inotropic drugs such as dopamine, dobutamine ormilrinone might be reasonable for those patients presenting with
documented severe systolic dysfunction, low blood pressure and
evidence of low cardiac output, with or without congestion, to
maintain systemic perfusion and preserve end-organ performance.
CLASS III
1. Use of parenteral inotropes in normotensive patients with acute decompensated HF without evidence of decreased organ perfusion is not recommended .(Level of Evidence: B)
2. Routine use of invasive hemodynamic monitoring in normotensive patients with acute decompensated HF and congestion with symptomatic response to diuretics and vasodilators is not recommended .(Level of Evidence: B)
A patient may develop acute or progressive symptoms of HF and require hospitalization. In general, there are 3 clinical profiles that describe the hospitalized patient with HF:
1) the patient with volume overload, manifested by pulmonary and/or systemic congestion, frequently precipitated by an acute increase in chronic hypertension;
2) the patient with profound depression of cardiac output manifested by hypotension, renal insufficiency, and/or a shock syndrome, and
3) the patient with signs and symptoms of both fluid overload and shock. Irrespective of the presenting clinical picture, there have been a confusing variety of terms in the literature used to describe these patients, including acute HF syndrome, acute decompensated HF, or cardiogenic shock. However different these 3 groups of patients may be in outcome, they can all be characterized as having a change in HF signs and symptoms resulting in a need for
urgent therapy. Patients with HF and preserved LVEF are just as likely to be admitted to hospital as those with HF and low LVEF
Admission with HF is often triggered by
aconcomitant cardiovascular event such as a symptomatic tachyarrhythmia, unstable coronary syndrome, or a cerebrovascular event; often the admission is related to medical or dietary noncompliance. The threshold for admission may also be lowered when HF exacerbation is accompanied with anoncardiac condition such as pneumonia or newly diagnosed anemia.Indeed, it is important to note that concurrent conditions and comorbidities such as coronary artery disease, hypertension, valvular heart disease, arrhythmias, renal dysfunction, diabetes, thromboembolism, and anemia are often present, more so than has usually been described in clinical trials, and may precipitate or contribute to the pathophysiology of the syndrome. Unfortunately, the precipitating event leading to hospitalization is not always readily apparent.
Common Factors That Precipitate Hospitalization for Heart Failure
• Noncompliance with medical regimen, sodium and/or fluid restriction• Acute myocardial ischemia
• Uncorrected high blood pressure
• Atrial fibrillation and other arrhythmias
• Recent addition of negative inotropic drugs (e.g., verapamil,nifedipine, diltiazem, beta blockers)
• Pulmonary embolus
• Nonsteroidal anti-inflammatory drugs
• Excessive alcohol or illicit drug use
• Endocrine abnormalities (e.g., diabetes mellitus, hyperthyroidism,hypothyroidism)
• Concurrent infections (e.g., pneumonia, viral illnesses)
The prognosis after an index hospitalization for HF is
ominous, with a 50% rate of readmission at 6 months and a 25% to 35% incidence of death at 12 months .Indeed, many HF trials now incorporate the need for
hospitalization as an important endpoint with which to
evaluate a new therapy; government agencies and insurance companies are increasingly interested in understanding the frequency of repeat HF hospitalizations. Thus, it is important to outline what should occur in the hospital for the HF patient requiring therapy.
Diagnostic Strategies
The diagnosis of HF in the hospitalized patient should be based primarily on signs and symptoms, Initial Evaluation of Patients. Clinicians need to determine as accurately and as quickly as possible
1) the volume status of the patient,
2) the adequacy of circulatory support or perfusion, and
3) the role or presence of precipitating factors and/or comorbidities.
In the patient with previously established HF, efforts should likewise be directed toward understanding what has caused the apparent acute worsening of clinical symptoms. Many of the steps in this investigation are identical to those used in the initial evaluation of HF .When the diagnosis of HF is uncertain, determination of plasma BNP or NT-proBNP concentration should be considered in patients being evaluated for dyspnea who have signs and symptoms compatible with HF. The natriuretic peptide concentration should not be interpreted in isolation but in the context of all available clinical data bearing on the diagnosis of HF.
Treatment in the Hospital
DIURETICS: THE PATIENT WITH VOLUME OVERLOADPatients admitted with evidence of significant fluid overload should initially be treated with loop diuretics, usually given intravenously. Therapy for this compelling presentation of HF should begin in the emergency department and should be initiated without delay. Early intervention has been associated with better outcomes for patients hospitalized with decompensated HF .
After admission to the hospital, patients should be carefully monitored in accordance with the severity of their symptoms and the results of initial findings on the physical examination and laboratory assessment. Careful and frequent serial evaluation of the patient is important primarily to assess volume status and adequacy of circulatory support. Laboratory parameters are likewise necessary to judge efficacy of treatment Monitoring of daily weight, supine and standing vital signs, fluid input, and output is a necessary part of daily management; assessment of daily electrolytes and renal function should be done while intravenous diuretics or active HF medication titration is being undertaken.
Intravenous loop diuretics have the potential to reduce glomerular filtration rate (GFR), further worsen neurohumoral activation, and produce electrolyte disturbances.Thus, although the use of diuretics may result in the effective relief of symptoms, their impact on mortality has not been well studied. Diuretics should be administered at doses sufficient to produce a rate of diuresis that will optimize volume status and relieve signs and symptoms of congestion without inducing an excessively rapid reduction in intravascular volume, which could result in hypotension,renal dysfunction, or both.
Therefore, strictly limiting sodium intake and dosing the diuretic multiple times per day will enhance effectiveness of the diuresis .Some patients may present with congestion and moderate to
severe renal dysfunction. The response to diuretics may be significantly blunted, requiring higher initial doses. In many cases, reduction of fluid overload may improve not only congestion but also renal dysfunction, particularly if significant venous congestion is reduced
Clinical experience suggests it is difficult to determine whether congestion has been adequately treated in many patients, and registry data have confirmed that patients are frequently discharged after a net weight loss of only a few pounds. Although patients may rapidly improve symptomatically,they may remain hemodynamically compromised.
Unfortunately, the routine use of serial natriuretic peptide measurement (BNP or NT-proBNP) or even a Swan-Ganz catheter to monitor hemodynamics has not been shown to be helpful in improving the outcomes of the hospitalized
patient with HF. Nevertheless, careful evaluation of all physical findings, laboratory parameters, weight change, and net fluid change should be considered before discharge planning is commenced.
When a patient with congestion fails to respond to initial doses of intravenous diuretics, several options may be considered. Efforts should be taken to make certain that, indeed, congestion persists and that another hemodynamic
profile or perhaps another disease process is not evident.This is particularly important for the patient with progressive renal insufficiency. If there is substantial doubt about the fluid status of the patient, HF experts suggest that it is an appropriate time for a formal hemodynamic assessment of ventricular filling pressures and cardiac output, typically done with a right heart catheterization.
If volume overload is confirmed, the dose of the loop diuretic should be initially increased to ensure that adequate drug levels reach the kidney. If this is inadequate, a second type of diuretic,typically a thiazide (metolazone or intravenous chlorothiazide) or spironolactone, can be added to improve diuretic responsiveness.
As a third strategy, continuous infusion of the loop diuretic may be considered. By continuous delivery of the diuretic to the nephron, rebound resorption occurring during the time blood levels of diuretic are low is avoided and ototoxicity risk may actually be reduced .If all diuretic strategies are unsuccessful, ultrafiltration or another renal replacement strategy may be reasonable. Ultrafiltration moves water and small- to medium-weight solutes across a semipermeable membrane to reduce volume overload. Because the electrolyte concentration is similar to plasma, relatively more sodium can be removed than by diuretics .
VASODILATORS
There are a number of clinical scenarios whereby the addition of vasodilators to the HF regimen of the hospitalized patient might be appropriate. For patients with adequate blood pressure and ongoing congestion not sufficiently responsive to diuretics and standard oral therapy (e.g.,maintenance of prior HF medications, if applicable), intravenous vasodilators such as nitroprusside, nitroglycerin, or nesiritide may be added to the treatment regimen.Regardless of the agent used, the clinician should make certain that intravascular volume is, in fact, expanded and that the patient’s blood pressure can tolerate the addition of the vasodilating drug. Intravenous nitroglycerin, primarily through venodilation effects, lowers preload and may help to more rapidly reduce pulmonary congestion. Patients with HF and hypertension, coronary ischemia, or significant mitral regurgitation are often cited as ideal candidates for the use of intravenous nitroglycerin.
However, tachyphylaxis (reduced responsiveness )to nitroglycerin may develop rather quickly and up to 20% of those with HF may develop resistance to even high doses . Sodium nitroprusside is a balanced preload-reducing venodilator and After
load-reducing arteriodilator that also dilates the pulmonary vasculature. Data demonstrating efficacy are limited, and invasive hemodynamic blood pressure monitoring is typically required.
Nitroprusside has the potential for producing marked hypotension and is usually used in the intensive care setting as well; longer infusions of the drug have been associated with thiocyanate toxicity, particularly in the setting of renal insufficiency. Nitroprusside is potentially of value in severely congested patients with hypertension or severe mitral valve regurgitation complicating LV dysfunction.
Nesiritide (human BNP) reduces LV filling output, and sodium excretion.
The severity of dyspnea is reduced more rapidly compared to diuretics alone. Because nesiritide has a longer effective half-life than nitroglycerin or nitroprusside, side effects such as hypotension may persist longer. Conservative dosing of the drug (i.e., no bolus) and use of only the recommended doses may reduce complications. Adverse renal consequences with nesiritide have been suggested; careful monitoring of renal function is mandatory.The effects of nesiritide on mortality remain uncertain and active clinical investigation is ongoing.Drug Interactions
Coadministration of nesiritide with angiotensin converting enzyme (ACE) inhibitors may cause unsafe drops in blood pressure.Nesiritide can react physically with many other drugs that are given by injection. These interactions are characterized by changes in the physical properties of nesiritide or the drug with which it is given. These changes can reduce its effectiveness, or cause toxicity.
Drugs that can interact physically with nesiritide include the following:
bumetanide (Bumex)
enalaprilat
ethacrynate (Edecrin)
furosemide(Lasix)
hydralazine (Apresoline)
heparin
insulin
In general, these drugs are not given at the same time as nesiritide.
How Nesiritide Works
Nesiritide stimulates an enzyme in the cells that line blood vessels and other tissues in the body. It acts like a BNP hormone. Nesiritide binds to natriuretic peptide receptors on vascular smooth muscle and endothelial cells, through which it triggers guanylate cyclase dependent signal transduction resulting in increase of intracellular concentrations of cGMP. This leads to smooth muscle cell relaxation causing arterial and venous dilatation.In the kidneys, it causes excretion of sodium and water. It relaxes muscles in blood vessel walls, allowing them to dilate (vasodilation) venous and arterial vasodilation . Finally, it inhibits the release of renin and aldosterone, which are two hormones that promote fluid and sodium retention. Overall, these effects reduce fluid retention and blood pressure, as well as allow the heart to work more efficiently.
Nesiritide is a synthetic version of a naturally-occurring hormone called B-type natriuretic peptide. As such, it dramatically increases excretion of sodium in the urine, a phenomenon known as natriuresis. prescribed across the US for the treatment of acutely decompensated heart failure patients with dyspnea at rest or with minimal activity (such as talking, eating or bathing). Use of the drug in less-severely ill patients declined after reports of an increased risk of kidney damage and death.
Side Effects
Cardiovascularhypotension
arrhythmia
chest pain (angina)
central nervous system
headache
insomnia
dizziness
anxiety
confusion
abnormal sensations
tremor
nausea and vomiting
abdominal pain
back pain
leg cramps
INOTROPES
Patients presenting with either predominantly low output syndrome (e.g., symptomatic hypotension) or combined congestion and low output may be considered for intravenous inotropes such as dopamine, dobutamine, and milrinone.
These agents may help relieve symptoms due to poor perfusion and preserve end-organ function in patients with severe systolic dysfunction and dilated cardiomyopathy.Inotropic agents are of greatest value in patients with relative hypotension and intolerance or no response to vasodilators and diuretics.
clinicians should not use a specific blood pressure value that might or might not mean hypotension, to dictate the use of inotropic agents. Rather, a depressed blood pressure associated with signs of poor cardiac output or hypoperfusion (e.g., cold clammy skin,cool extremities, decreased urine output, altered mentation) should prompt a consideration for more aggressive intravenous therapy.
Dobutamine
requires the beta-receptor for its inotropic effects, while milrinone does not. This may be asignificant consideration for patients already maintained on beta-blocking drugs. Furthermore, milrinone has vasodilating properties for both the systemic circulation and the pulmonary circulation. Despite these considerations, there is no evidence of benefit for routine use of inotropic support in patients presenting with acute HF due to congestion only .Indeed, data from several studies suggest an increase in adverse outcomes when inotropes are used.Thus, inotropes should be confined to carefully selected patients with low blood pressure and reduced cardiac output who can have blood pressure and heart rhythm monitored closely.
Milrinone
phosphodiesterase 3 inhibitor. It potentiates the effect of cyclic adenosine monophosphate (cAMP).enhances relaxation of the left ventricle by increasing Ca2+-ATPase activity on the cardiac sarcoplasmic reticulum. This increases calcium ion uptake.It has positive inotropic, vasodilating and minimal chronotropic effects. It is used in the management of heart failure only when conventional treatment with vasodilators and diuretics has proven insufficient. This is due to the potentially fatal adverse effects of milrinone, including ventricular arrhythmias
Whereas beneficial hemodynamic effects are shown (at least short-term), several studies have shown no or a negative effect on mortality rates of hospitalized patients receiving milrinone.
One negative side to the use of milrinone is the short half-life (1 to 2 hours).
This can result in a prolonged weaning and possible adverse outcomes from stopping this medication rapidly.
Dopamine is not just a precursor of noradrenaline and adrenaline but a neurotransmitter, as well. is a catecholamine neurotransmitter .In the brain, functions as a neurotransmitter, activating the five types of dopamine receptors—D1, D2, D3, D4, and D5. Dopamine is produced in several areas of the brain, including the substantia nigra and the ventral tegmental area, hypothalamus. Its main function as a hormone is to inhibit the release of prolactin from the anterior lobe of the pituitary.
Dopamine can be supplied as a medication that acts on the sympathetic nervous system, producing effects such as increased heart rate and blood pressure. However, because dopamine cannot cross the blood-brain barrier, dopamine given as a drug does not directly affect the central nervous system. To increase the amount of dopamine in the brains of patients with diseases such as Parkinson's disease and dopa-responsive dystonia,
L-DOPA, which is the precursor of dopamine, can be given because it can cross the blood-brain barrier. Dopamine is biosynthesized in the body (mainly by nervous tissue and the medulla of the adrenal glands
Routine invasive hemodynamic monitoring is not indicated for most patients hospitalized with symptoms of worsening HF. Recent evaluations of the use of right heart catheterization to improve outcomes have been essentially neutral with regard to overall benefit .However, hemodynamic monitoring should be strongly considered in patients whose volume and filling pressures are uncertain or who are refractory to initial therapy, particularly in those whose filling pressures and cardiac output are unclear.
Patients with clinically significant hypotension (systolic blood pressure typically less than 90 mm Hg or symptomatic low systolic blood pressure) and/or worsening renal function during initial therapy might also benefit. Patients being considered for cardiac transplantation or placement of amechanical circulatory support device are also candidates for complete right heart catheterization, a necessary part of the initial evaluation .
Invasive hemodynamic monitoring should be performed in patients with
1) presumed cardiogenic shock2) severe clinical decompensation in which therapy is limited by uncertainty regarding relative contributions
of elevated filling pressures, hypoperfusion.
3) apparent dependence on intravenous inotropic infusions after initial clinical improvement; or
4) persistent severe symptoms despite adjustment of recommended therapies.
OTHER CONSIDERATIONS
Other treatment or diagnostic strategies may be necessary for individual patients after stabilization, particularly related to the underlying cause of the acute event. The patient hospitalized with HF is at increased risk for thromboembolic complications and deep venous thrombosis and should receive prophylactic anticoagulation with either intravenous unfractionated heparin or subcutaneous preparations of unfractionated or low-molecular-weight heparin, unless contraindicated.As the hospitalized patient becomes more clinically stable and volume status normalizes, oral HF therapy should be initiated or reintroduced. Particular caution should be used when initiating beta blockers in patients who have required inotropes during their hospital course or when initiating ACEIs in those patients who have experienced marked azotemia. During additional hospital days, the patient should be fully transitioned off all intravenous therapy, and oral therapy should be adjusted and maximized.
Treatment of
Special PopulationsBecause HF is frequently accompanied by
erectile dysfunction,men may express interest in the use of a phosphodiesterase type 5 inhibitor (e.g., sildenafil) as a means of enhancing sexual performance. Few patients with HF were enrolled in controlled trials with sildenafil, and thus, the efficacy and safety of this drug in patients with HF are not known. Nevertheless, recent studies suggest that sildenafil may produce hemodynamic benefits in patients with coronary artery disease and may act to improve some of the peripheral vascular abnormalities that characterize patients with HF .
Although patients with HF appear to tolerate short-term administration of the drug without difficulty, sildenafil should not be given to patients taking nitrates, who may experience profound hypotension due to its ability to potentiate the systemic vasodilator effects of drugs that increase intracellular levels of cyclic guanosine monophosphate.
Ethnic Considerations
Heart failure is a major public health problem in blacks. Heart failure is more common in the black population, affecting approximately 3% of all black adults. This reflects a 50% higher incidence of HF in the black population than is seen in the general population.Black patients develop symptoms of HF at an earlier average age than nonblacks, possibly because black patients are more likely to have hypertension and diabetes mellitus than nonblacks and because they more frequently exhibit sodium retention, ventricular hypertrophy, and vascular injury.
Once the diagnosis is made, HF progresses more
rapidly in black than in white patients, as evidenced by ahigher risk of initial and recurrent hospitalizations .This risk cannot be explained by the presence of epicardial coronary artery disease or documented MI, both of which are less common in black than in nonblack patients with HF. The data are not clear as to whether a definitive increase in mortality risk exists .The literature is mixed on whether blacks with HF more frequently receive suboptimal inpatient care for their HF However, deficiencies in cardiovascular risk factor evaluation and disease detection and treatment as well as in access to quality outpatient care may contribute to the increased incidence and morbidity of blacks with HF.
Elderly Patients
Heart failure is particularly common in elderly patients. The prevalence of HF rises from 2% to 3% at age 65 to more than 80% in persons over 80 years of age ,and HF is the most common reason for hospitalization in elderly patients .The high prevalence of HF in the elderly may be associated with age-related changes in ventricular function (particularly diastolic function) and to the cumulative effects of hypertension and other chronic risk factors .In addition, risk factors for HF (e.g.,hypertension, diabetes mellitus, and hyperlipidemia) are generally not treated aggressively in the elderly, yet elderly patients commonly take medications that can exacerbate the syndrome of HF (e.g., nonsteroidal anti-inflammatory drugs)
Heart failure in elderly patients is inadequately recognized
and treated .Both patients and physicians frequently attribute the symptoms of HF to aging, and noninvasive cardiac imaging commonly fails to reveal impaired systolic function because HF with a preserved LVEF is frequently found in the elderly. In addition, some reports suggest that elderly patients may have diminished responses to diuretics, ACEIs, and positive inotropic agents compared with younger patients and may experience ahigher risk of adverse effects attributable to treatment .Uncertainties regarding the relation of risk to benefit are exacerbated by the fact that very old individuals
are poorly represented in large-scale clinical trials designed to evaluate the efficacy and safety of new treatments for HF.
Some multidisciplinary HF programs have been successful in decreasing the rate of readmission and associated morbidity in elderly patients . Managed care organizations continue to struggle to find improved ways to implement these pathways.
Patients With Heart Failure Who Have
Concomitant DisordersRecommendations
CLASS I
1. All other recommendations should apply to patients with concomitant disorders unless there are specific exceptions. (Level of Evidence: C)
2. Physicians should control systolic and diastolic hypertension and diabetes mellitus in patients with HF in accordance with recommended
guidelines. (Level of Evidence: C)
3. Physicians should use nitrates and beta blockers for the treatment of angina in patients with HF. (Level of Evidence: B)
4. Physicians should recommend coronary revascularization according to recommended guidelines in patients who have both HF and angina. Evidence: A
5. Physicians should prescribe anticoagulants in patients with HF who have paroxysmal or persistent atrial fibrillation or a previous thromboembolic event. (Level of Evidence: A)
6. Physicians should control the ventricular response rate in patients with HF and atrial fibrillation with a beta blocker (or amiodarone, if the beta blocker is contraindicated or not tolerated).(Evidence: A)
7. Patients with coronary artery disease and HF should be treated in accordance with recommended guidelines for chronic stable angina. (Evidence: C)
8. Physicians should prescribe antiplatelet agents for prevention of MI and death in patients with HF who have underlying coronary artery disease. (Evidence: B)
CLASS IIa
1. It is reasonable to prescribe digitalis to control the ventricular response rate in patients with HF and atrial fibrillation. (Level of Evidence: A)2. It is reasonable to prescribe amiodarone to decrease recurrence of atrial arrhythmias and to decrease recurrence of ICD discharge
for ventricular arrhythmias. (Evidence: C)
CLASS IIb
1. The usefulness of current strategies to restore and maintain sinus rhythm in patients with HF and atrial fibrillation is not well established. (Evidence: C)
2. The usefulness of anticoagulation is not well established in patients with HF who do not have atrial fibrillation or a previous thromboembolic event. (Level of Evidence: B)
3. The benefit of enhancing erythropoiesis in patients with HF and anemia is not established. (Level of Evidence: C)
CLASS III
1. Class I or III antiarrhythmic drugs are not recommended in patients with HF for the prevention of ventricular arrhythmias.(Level of Evidence: A)
2. The use of antiarrhythmic medication is not indicated as primary treatment for asymptomatic ventricular arrhythmias or to improve
survival in patients with HF. (Level of Evidence: A)
Patients with reduced LVEF frequently have associated cardiovascular and noncardiovascular disorders, the course or treatment of which may exacerbate the syndrome of HF. In many patients, appropriate management of these concomitant illnesses may produce symptomatic and prognostic benefits that may be as important as the treatment of the HF condition itself.
Cardiovascular Disorders
Hypertension, Hyperlipidemia, andDiabetes Mellitus
Approximately two thirds of patients with HF have a past or current history of hypertension, and approximately one third have diabetes mellitus .Both disorders can contribute to the development of systolic or diastolic dysfunction either directly or by contributing (together with hyperlipidemia) to the development of coronary artery disease .Long-term treatment of both hypertension and hyperlipidemia decreases the risk of developing HF .
In a large-scale trial, the administration of a lipid-lowering agent to patients with hypercholesterolemia and a history of MI reduced all-cause mortality and the risk of developing HF. In 2 large-scale multicenter studies, the treatment of hypertension reduced both the risk of death and the risk of HF; this was true regardless of whether the elevation of bloodpressure was primarily systolic or diastolic .The benefits of lowering blood pressure may be particularly marked in patients with diabetes mellitus
Heart failure may complicate the management of both hypertension and diabetes mellitus.
Some antihypertensive agents should be avoided in patients with HF because of their ability to depress cardiac function or to lead to salt and water retention. In addition, HF itself is associated withresistance to the actions of insulin ,and the resulting hyperinsulinemia may promote both cardiac and vascular hypertrophy and thus may hasten the progression of HF.
These mechanisms may compound the deleterious effects of accelerated atherosclerosis and altered energy metabolism on cardiac function and may help to explain why diabetic patients with HF have a worse prognosis than their nondiabetic counterparts .
Thiazolidinediones have been associated with increased peripheral edema and symptomatic HF in patients with underlying risk factors or known cardiovascular disease. The risk of developing edema with thiazolidinediones is dose related and is higher in diabetic patients who are taking concomitant insulin therapy. However, the incidence of thiazolidinedione-related fluid retention is low in patients with NYHA functional class I to II symptoms, in whom these drugs can be administered safely with careful monitoring for fluid retention.
Initiation of these drugs is not recommended in patients with NYHA functional class III to IV symptoms of HF. Clinical experience has shown that one side effect of newer oral agents of the thiazolidinedione class is weight gain, which is due in part to fluid retention. This effect may have the potential to precipitate or exacerbate HF in patients with reduced cardiac reserve. Thiazolidinediones probably should be used with caution in such patients
Recommendations Concerning Management. Little is known
about the benefits of treating hypertension, hypercholesterolemia,or diabetes mellitus in patients with established reduced LVEF and symptoms of HF. The lack of such data is noteworthy, both because the progression of HF is frequently associated with decreases in blood pressure (due to deterioration of cardiac performance) and decreases in serum lipids (due to development of cardiac cachexia)and because the benefits of drugs used to lower blood pressure or blood lipids may be seen only during prolonged periods of treatment (i.e., those that exceed the expected life span of many patients with HF) .Nevertheless, it is prudent to manage hypertension, hypercholesterolemia, and diabetes mellitus in patients with HF as if the patients did not have HF. This may be particularly true in patients with HF and preserved LVEF, whose symptoms may respond particularly well to treatments that lower blood pressure .Renal artery stenosis should be considered in patients with hypertension and HF, because renal artery stenting can treat both conditions.
Drugs that can both control blood pressure and treat HF should be preferred in patients with both conditions; this includes the use of diuretics, ACEIs, and beta blockers. In contrast, physicians should avoid the use of most calcium channel blockers, because of their cardiodepressant effects,or potent direct-acting vasodilators such as minoxidil, because of their sodium-retaining effects.
The drugs routinely used in the management of HF in nondiabetic patients should be administered to those with diabetes mellitus. Angiotensin converting enzyme inhibitors and beta blockers prevent the progression of HF in diabetic and nondiabetic patients .Physicians should
not avoid the use of beta blockers in diabetic patients despite fears that these drugs may mask symptoms of hypoglycemia produced by antidiabetic therapy or may exacerbate glucose intolerance or insulin resistance.
Coronary Artery Disease
Approximately two thirds of patients with HF have underlying coronary artery disease, which may limit exercise tolerance by causing angina pectoris or may lead to further myocardial injury by causing an MI. Therefore, physicians should manage both the symptomatic and prognostic consequences of the patient’s underlying coronary artery disease in accordance with contemporary guidelines.Recommendations Concerning Management of Patients With Angina Pectoris.
In general, patients who have both angina pectoris and HF should be given drugs that relieve angina along with drugs that are appropriate in the management of HF .Both nitrates and beta blockers can improve anginal symptoms and may produce hemodynamic and clinical benefits in patients with reduced LVEF, and thus, they are preferred if these conditions coexist .Yet, the combination of the 2 drugs may produce little improvement in anginal pain unless fluid retention is adequately controlled with diuretics.
It is therefore noteworthy that the decrease in ventricular volume and pressures produced by diuretics may exert independent antianginal effects.
Some have suggested that the systemic and coronary vasodilator actions of calcium channel blockers might improve cardiac performance and relieve myocardial ischemia, but these theoretical advantages have not been translated into clinical benefits in controlled clinical trials in HF.
These drugs have not improved symptoms of HF or enhanced exercise tolerance ,and shortand long-term treatment with these drugs (even the use of sustained-release or vasoselective preparations) has increased
the risk of worsening HF and death in patients with LV dysfunction .
Therefore, most calcium channel blockers should be avoided in patients with HF, even when used for the treatment of angina or hypertension.
Of available agents,
only amlodipine has been shown not to adversely affect survival, although experience with the drug exists largely in patients who are not taking beta blockers .
In patients with both HF and angina pectoris, strong consideration should be given to the use of coronary revascularization.
Coronary revascularization can relieve symptoms of myocardial ischemia ,and coronary artery bypass surgery has been shown to lessen angina and reduce the risk of death in patients who have multivessel disease, reduced LVEF, and stable angina .
Recommendations Concerning Management of Patients Without Angina.
In patients with a prior MI but without HF or angina, 4 types of interventions have been used to reduce the risk of reinfarction and death:neurohormonal antagonists such as ACEIs and beta blockers drugs to address dyslipidemia, such as statins; antiplatelet drugs such as aspirin and clopidogrel ,and coronary revascularization .In patients who have had an MI and who have HF but not angina, the use of ACEIs and beta blockers can also decrease the risk of reinfarction and death ,but it is less clear whether such patients benefit from the use of aspirin or revascularization.
Aspirin has been shown to reduce the risk of major ischemic events in patients without HF.
The role of aspirin in patients with HF has not been established ,and concerns have been raised that it may attenuate the hemodynamic and survival benefits of ACEIs .For these reasons, the role of aspirin in preventing ischemic events in patients with chronic HF is controversial .Alternative antiplatelet agents (e.g. clopidogrel) may not interact adversely with ACEIs and may have superior effects in preventing clinical events ,but their ability to favorably affect outcomes in HF has not been demonstrated.
Some physicians recommend the use of coronary revascularization in patients with HF and coronary artery disease who do not have symptoms of angina. Advocates of this approach have suggested that surgical reperfusion can improve cardiac function and relieve symptoms of HF in patients with myocardium that appears on imaging to be viable but not contracting normally and may also reduce the risk of a fatal coronary occlusion in patients with established multivessel disease .Despite these theoretical possibilities,
however, coronary revascularization has not been shown to improve cardiac function or symptoms or to prevent reinfarction or death in patients with HF and no angina
Supraventricular Arrhythmias
The most common treatable atrial arrhythmia is atrial fibrillation, which affects 10% to 30% of patients with chronic HF and is associated with a reduction in exercise capacity and a worse long-term prognosis.Supraventricular tachyarrhythmias
may exert adverse effects via 4 different mechanisms:1) the loss of atrial enhancement of ventricular filling may compromise cardiac output;
2) the rapid heart rate may increase demand and decrease coronary perfusion (by shortening ventricular filling time);
3) the rapidity of ventricular response may diminish both cardiac contraction (by aggravating abnormalities of the force-frequency relation) and cardiac relaxation and
4) the stasis of blood in the fibrillating atria may predispose patients to pulmonary or systemic emboli.
In most patients with an ischemic or nonischemic dilated cardiomyopathy, the rapidity of ventricular response is more important than the loss of atrial support, because restoration of sinus rhythm does not result in predictable clinical benefits Rapid supraventricular arrhythmias may actually cause a cardiomyopathy (even in patients without an underlying contractile abnormality) or may exacerbate a cardiomyopathy caused by another disorder .Hence, the control of ventricular rate and the prevention of thromboembolic events are essential elements of treatment of HF in patients with an underlying supraventricular arrhythmia .
The agent previously used in clinical practice to slow the ventricular response in patients with HF and atrial fibrillation is digoxin, but the cardiac glycoside
slows atrioventricular conduction more effectively at rest than during exercise Hence, digitalis does not block the excessive exercise-induced tachycardia that may limit the functional capacity of patients with HF .Beta blockers are more effective than digoxin during exercise and are preferred because of their favorable effects on the natural history of HF .
The combination of digoxin and beta blockers may be more effective than beta blockers alone for rate control. Although both verapamil and diltiazem can also suppress the ventricular response during exercise, they can depress myocardial function and increase the risk of worsening HF, especially in patients with HF and low EF, in whom these drugs should be avoided .If beta blockers are ineffective or contraindicated in patients with atrial fibrillation and HF, amiodarone may be a useful alternative.
Atrioventricular nodal ablation may be needed if tachycardia persists despite pharmacological therapy .
Catheter ablation
for pulmonary vein isolation has been most effective in patients without structural heart disease; the benefit for patients with established HF is not known .
Regardless of the intervention used, every effort should be made to reduce the ventricular response to less than 80 to 90 bpm at rest and less than 110 to 130 bpm during moderate exercise. Anticoagulation should be maintained in all patients with HF and a history of atrial fibrillation, regardless of whether sinus rhythm is achieved, because of the high rate of silent recurrence of atrial fibrillation with its attendant embolic risk, unless a contraindication exists.
Should patients with HF and atrial fibrillation be converted to and maintained in sinus rhythm?
The efficacy and safety of restoring and maintaining sinus rhythm in patients with atrial fibrillation were evaluated in a total of 5032 patients in 4 separate trials .Both strategies for the management of atrial fibrillation, either to restore and maintain sinus rhythm by electrical or pharmacologic conversion, or to control ventricular rate in atrial fibrillation,have been shown to have equivalent outcomes.
These results were confirmed in 2007 with the conclusion of a large trial of patients with both atrial fibrillation and HF .Most patients revert to atrial fibrillation within a short time unless they are treated with a Class I or III antiarrhythmic drug .However, patients with HF are not likely to respond favorably to Class I drugs and may be
particularly predisposed to their cardiodepressant and proarrhythmic effects . which can increase the risk of death .
Class III antiarrhythmic agents (e.g., sotalol, dofetilide, and amiodarone) can maintain sinus rhythm in some patients, but treatment with these drugs is associated with an increased risk of organ toxicity (amiodarone) and proarrhythmia (dofetilide) . Most patients who had thromboembolic events, regardless of the strategy used, were in atrial fibrillation at the time of the event and either were not undergoing anticoagulation therapy
or were undergoing therapy at subtherapeutic levels. Thus, it is reasonable to treat HF patients with atrial fibrillation with a strategy of either scrupulous rate control or an attempt at rhythm control.
Prevention of Thromboembolic Events
Patients with chronic HF are at increased risk of thromboembolic events due to stasis of blood in dilated hypokinetic cardiac chambers and in peripheral blood vessels and perhaps due to increased activity of procoagulant factors.However, in large-scale studies, the risk of thromboembolism in clinically stable patients has been low (1% to 3% per year), even in those with very depressed EFs and echocardiographic evidence of intracardiac thrombi . These rates are sufficiently low to limit the detectable benefit of anticoagulation in these patients.In several retrospective analyses, the risk of thromboembolic events was not lower in patients with HF taking warfarin than in patients not treated with antithrombotic drugs .The use of warfarin was associated with a reduction in major cardiovascular events and death in patients with HF in one retrospective analysis but not inanother.A randomized trial comparing the outcome of patients with HF and low EF assigned to aspirin, warfarin, or clopidogrel was completed recently.Unfortunately, low enrollment in the trial precluded definitive conclusions about efficacy, but no therapy appeared to be superior..
Recommendations Concerning Management.
Despite the lack of supportive data, some physicians prescribe anticoagulants to all patients with markedly depressed EFs and dilated hearts .Others would advocate the use of warfarin in patients who are known to harbor a cardiac thrombus ,even though many thrombi detected by echocardiography do not embolize and many embolic events are probably related to thrombi that are not visualized .Anticoagulation with warfarin is most justified in patients with HF who have experienced a previous embolic event or who have paroxysmal or persistent atrial fibrillation .
Anticoagulation should also be considered
in patients with underlying disorders that may be associated with an increased thromboembolic risk (e.g., amyloidosis or LV noncompaction) and
in patients with familial dilated cardiomyopathy and ahistory of thromboembolism in first-degree relatives.
Noncardiovascular Disorders
Patients With Renal InsufficiencyPatients with HF frequently have impaired renal function as a result of poor renal perfusion, intrinsic renal disease, or drugs used to treat HF. Patients with renal hypoperfusion or intrinsic renal disease show an impaired response to diuretics and ACEIs and are at increased risk of adverse effects during treatment with digitalis .Renal function may worsen during treatment with diuretics or ACEIs although the changes produced by these drugs are frequently short-lived, generally asymptomatic, and reversible.
Persistent or progressive renal functional impairment often reflects deterioration of the underlying renal disease process and is associated with a poor prognosis .The symptoms of HF in patients with end-stage renal disease may be exacerbated by an increase in loading conditions produced both by anemia and by fistulas implanted to permit dialysis. In addition, toxic metabolites and abnormalities of phosphate, thyroid, and parathyroid metabolism associated with chronic renal insufficiency can depress myocardial function.
Despite the potential for these adverse interactions, most patients with HF tolerate mild to moderate degrees of functional renal impairment without difficulty. In these individuals, changes in blood urea nitrogen and serum creatinine are generally clinically insignificant and can usually
be managed without the withdrawal of drugs needed to slow the progression of HF.
However, if the serum creatinine
increases to more than 3 mg per dL, the presence ofrenal insufficiency can severely limit the efficacy and enhance the toxicity of established treatments .In patients with a serum creatinine greater than 5 mg per dL,hemofiltration or dialysis may be needed to control fluid retention, minimize the risk of uremia, and allow the patient to respond to and tolerate the drugs routinely used for the management of HF.
Patients With Pulmonary Disease
Because dyspnea is the key symptom in both HF and pulmonary disease, it is important to distinguish the 2 diseases and to quantify the relative contribution of cardiac and pulmonary components to the disability of the patient when these disorders coexist.Exercise testing with simultaneous gas exchange or blood gas measurements may be helpful in this regard, particularly when used in conjunction with right heart catheterization .
Some drugs used to treat HF can produce or exacerbate pulmonary symptoms. Angiotensin converting enzyme inhibitors can cause a persistent nonproductive cough that can be confused with a respiratory infection, and conversely, ACEIs may be inappropriately stopped in patients with pulmonary causes of cough. Therefore, physicians should seek a pulmonary cause in all patients with HF who complain of cough, whether or not they are taking an ACEI.
The cough should be attributed to the ACEI only if respiratory disorders have been excluded and the cough disappears after cessation of ACEI therapy and recurs after reinstitution of treatment. Because the ACEI-related cough does not represent any serious pathology, many patients can be encouraged to tolerate it in view of the important beneficial effects of ACEIs.
Beta blockers can aggravate bronchospastic symptoms in
patients with asthma; however, many patients with asymptomatic or mild reactive airways disease tolerate betablockers well. Also, most patients with chronic obstructive pulmonary disease do not have a bronchospastic component to their illness and remain reasonable candidates for betablockade .Of note, both metoprolol tartrate and bisoprolol may lose their beta-1 selectivity when prescribed in doses that have been associated with an improvement in survival in patients with HF.
Patients With Cancer
Patients with cancer are particularly predisposed to the development of HF as a result of the cardiotoxic effects of many cancer chemotherapeutic agents, especially the anthracyclines ,high-dose cyclophosphamide ,and trastuzumab .Trastuzumab is a monoclonal antibody recently approved for therapy of metastatic breast cancer that has a significant potential to cause HF,especially when combined with anthracyclines.Mediastinal radiation can also cause acute and chronic injury to the pericardium, myocardium, cardiac valves, and coronary arteries, particularly when used in conjunction with cardiotoxic chemotherapy .Patients undergoing potentially cardiotoxic treatments for cancer should be monitored closely for the development
of cardiac dysfunction. Heart failure may appear many years after anthracycline exposure, particularly in association with another stress, such as tachycardia. Although noninvasive assessments of LV function and endomyocardial biopsy have been advocated by some investigators ,many cases escape early detection despite close surveillance.
Dexrazoxane may confer some cardioprotection in patients undergoing anthracycline-based chemotherapy and may allow for higher doses of the chemotherapy to be given Heart failure due to chemotherapeutic agents is managed similarly to HF due to other causes, although it is not clear whether patients with cancer respond similarly to patients with other causes of HF.
Nevertheless, because most patients with anthracycline-induced cardiomyopathy have striking degrees of tachycardia, many experts believe that beta blockers play a particularly important role in the management of these patients. Although once thought to progress inexorably, HF related to chemotherapy often improves in response to therapy, even when it appears late after exposure.
Patients With Thyroid Disease
Patients with both hyperthyroidism and hypothyroidism are prone to develop HF. Special vigilance is required for patients who are taking amiodarone, who may developeither hyperthyroidism or hypothyroidism. New atrial fibrillation or exacerbation of ventricular arrhythmias should trigger reevaluation of thyroid status.
Patients With Hepatitis C and Human Immunodeficiency Virus
Hepatitis C viral infection can be a cause of cardiomyopathy and myocarditis. It appears that the virus can cause both dilated cardiomyopathy and hypertrophic cardiomyopathy
The relatively high prevalence of this virus in Japanese populations compared with those in North America and Europe suggests that there may be a genetic predisposition to this type of viral myocarditis .
Asmall study showed that hepatitis C virus myocarditis might respond favorably to immunosuppressive therapy with prednisone and azathioprine .Preliminary data also suggest that this type of myocarditis might respond well to interferon therapy ,although there is concern that interferon can also depress myocardial function.
Human immunodeficiency virus has been recognized as a probable occasional cause of dilated cardiomyopathy. The presence of reduced LVEF in patients with HIV infection appears to correlate with decreased survival .Reduced LVEF is often seen in association with a significantly reduced CD4 count, although progression of cardiomyopathy does not appear to be related to falling CD4 levels.Drug therapy for HIV with zidovudine has also been implicated as a cause of cardiomyopathy, possibly through its effect on cardiac myocyte mitochondrial function .
Heart failure in patients with HIV infection may also be caused or exacerbated by pericardial effusion or pulmonary hypertension.
Interferon-alpha therapy for HIV-related Kaposi’s sarcoma has also been associated with reversible reduction in LVEF. Because of the occurrence of complex opportunistic infections, autoimmune responses to the viral infection, and drug cardiotoxicity, it is difficult to determine how therapies influence the development and control of cardiomyopathy with HIV.
Patients With Anemia
Anemia is seldom the cause of HF in the absence of underlying cardiac disease. To be the sole cause of highoutput HF, anemia must be severe (e.g., hemoglobin levels less than 5 g per deciliter). On the other hand, patients with HF frequently have anemia for a variety of reasons. Theseverity of anemia may contribute to the increasing severity of HF. Several studies have demonstrated worse outcomes in patients with HF and anemia .It is unclear whether anemia is the cause of decreased survival or a result of more severe disease.Several small studies have suggested benefit from use of erythropoietin and iron for treatment of mild anemia in HF . There is concern, however, that thromboembolic events may be increased. This therapy is undergoing further investigation.
End-of-Life Considerations
RecommendationsCLASS I
1. Ongoing patient and family education regarding prognosis for functional capacity and survival is recommended for patients with HF at the end of life.
2. Patient and family education about options for formulating and implementing advance directives and the role of palliative and hospice care services with reevaluation for changing clinical status is recommended for patients with HF at the end of life.(Evidence: C)
3. Discussion is recommended regarding the option of inactivating ICDs for patients with HF at the end of life. (Level of Evidence: C)
4. It is important to ensure continuity of medical care between inpatient and outpatient settings for patients with HF at the end of life. (Evidence: C)
5. Components of hospice care that are appropriate to the relief of suffering, including opiates, are recommended and do not preclude the options for use of inotropes and intravenous diuretics for symptom palliation for patients with HF at the end of life. (Level of Evidence: C)
6. All professionals working with HF patients should examine current end-of-life processes and work toward improvement in approaches to palliation and end-of-life care. (Level of Evidence: C)
CLASS III
1. Aggressive procedures performed within the final days of life (including intubation and implantation of a cardioverterdefibrillatorin patients with NYHA functional class IV symptoms who are not anticipated to experience clinical improvement from available treatments) are not appropriate. (Level of Evidence: C)
Roles of Generalist Physicians and Cardiologists
Insufficient evidence exists to allow for recommendations about the most appropriate roles for generalist physicians and cardiologists in the care of patients with HF. Several studies indicate that primary care physicians as a group have less knowledge about HF and adhere to guidelines less closely than cardiologists .Some studies have noted better patient outcomes in patients cared for by cardiologists than in those cared for by generalist physicians ,whereas another study reported that cardiologistsdeliver more costly care that is accompanied by a trend toward improved survival .
Despite these observations, primary care physicians with knowledge and experience in HF should be able to care for most patients with uncomplicated HF. By contrast, patients who remain symptomatic despite basic medical therapy may benefit from care directed by consulting physicians who have special expertise and training in the care of patients with HF.
Do generalist physicians and cardiologists provide similar levels of care for the noncardiac comorbid conditions frequently present in patients with HF? What is the optimal time for referral to a specialist? What is the most effective system of co management of patients by generalists and cardiologists? What is the most cost-effective entry point into a disease-management program? Regardless of the ultimate answers to these questions, all physicians and other healthcare providers must advocate and follow care practices that have been shown to improve patient outcomes.
If aphysician is not comfortable following a specific recommendation (e.g., the use of beta blockers), then the physician should refer the patient to someone with expertise in HF. Acollaborative model in which generalist and specialist physicians work together to optimize the care of patients with HF is likely to be most fruitful.
As heart failure advances, there is a relative decline in the counterregulatory effects of endogenous vasodilators, including nitric oxide (NO), prostaglandins (PGs), bradykinin (BK), atrial natriuretic peptide (ANP), and B-type natriuretic peptide (BNP). This occurs simultaneously with the increase in vasoconstrictor substances from the RAAS and adrenergic systems. This fosters further increases in vasoconstriction and thus preload and afterload, leading to cellular proliferation, adverse myocardial remodeling, and antinatriuresis with total body fluid excess and worsening congestive heart failure symptoms.
Endogenous vasodilators
Nitric oxide (NO),
Prostaglandins (PGs),
Bradykinin (BK),
Atrial natriuretic peptide (ANP),
and B-type natriuretic peptide (BNP).
Endogenous vasoconstrictor substances from the RAAS and adrenergic systems. substances from the RAAS and adrenergic systems.
ANP and BNP are endogenously generated peptides activated in response to atrial and ventricular volume/pressure expansion. ANP and BNP are released from the atria and ventricles, respectively, and both promote vasodilation and natriuresis. Their hemodynamic effects are mediated by decreases in ventricular filling pressures, owing to reductions in cardiac preload and afterload.
BNP, in particular, produces selective afferent arteriolar vasodilation and inhibits sodium reabsorption in the proximal convoluted tubule. BNP inhibits renin and aldosterone release and, therefore, adrenergic activation as well. Both ANP and BNP are elevated in chronic heart failure. BNP, in particular, has potentially important diagnostic, therapeutic, and prognostic implications.
Despite recent advances in the management of patients with heart failure, morbidity and mortality rates remain high, with an
estimated 5-year mortality rate of 50%.
Hypoxemia that occurs in decompensated heart failure, which may be severe, can result in diffuse end-organ damage including myocardial ischemia or myocardial infarction and hypoxic brain injury. Respiratory failure with hypercapnic respiratory acidosis may occur in severe decompensated heart failure, requiring mechanical ventilation if medical therapy is delayed or unsuccessful. Endotracheal intubation and mechanical ventilation are associated with their own risks, including aspiration (during the intubation process), mucosal trauma (more common with nasotracheal intubation than orotracheal intubation), and barotrauma.
In patients with heart failure, the risk of cardiac sudden death from ventricular tachycardia (VT) or ventricular fibrillation (VF) is considerable, and the degree of risk is correlated with the degree of decompensation and the degree of LV dysfunction. Recognition of the role of ventricular arrhythmias and advances in their treatment have resulted in decreased mortality rates in individuals with heart failure. Progressive renal insufficiency is common in patients with long-standing heart failure as well as acutely decompensated heart failure.
Furthermore, renal function is at least as powerful an adverse prognostic factor as most clinical variables, including ejection fraction and New York Heart Association (NYHA) function class. Although renal dysfunction predicts all-cause mortality, it is most predictive of death from progressive heart failure, which suggests that it is a manifestation of and/or exacerbating factor for left ventricular dysfunction.11
Liver dysfunction due to passive hepatic congestion is particularly common in patients with right-sided heart failure with elevated right ventricular (RV) pressure that is transmitted back into the portal vein. Mild jaundice, mild abnormalities in coagulation, and derangements in liver metabolism of medications, some of which are used in the treatment of heart failure, may result from this liver dysfunction.
Toxic levels of medications such as warfarin, theophylline, phenytoin, and digoxin can result from delayed liver metabolic clearance of these drugs in the presence of decompensated heart failure, thereby leading to potentially fatal bleeding, cardiac dysrhythmias, and neurologic abnormalities.
heart failure (HF) now accounts for 7% of all deaths from cardiovascular disease. Hypertension (HTN) increases the risk of development of HF and it precedes it in 75% of cases.
HTN increases the risk of HF two- to threefold. HF patients are nearly evenly divided between those with reduced left ventricular (LV) function or systolic dysfunction and those with preserved LV systolic function or diastolic dysfunction.The latter group is associated with essential hypertension (HTN) more often.
Management of Hypertension in Chronic Heart Failure
The management of HTN in patients with CHF is challenging. Drugs such as β-blockers, angiotensin-converting enzyme inhibitiors, angiotensin receptor blockers, aldosterone receptor blockers, hydralazine and nitrates, which have shown mortality benefit in CHF and exert ntihypertensive effects, should be used as first-line agents to control HTN in CHF.
Long-term treatment of both systolic and diastolic HTN reduces the risk of developing HF by 50%. The goal of the Heart Failure Society of America for patients with renal insufficiency
(> 1 g/day of proteinuria) and those with a high risk of developing HF is 125/75 mmHg, and
130/85 mmHg in patients with less than 1 g/day of proteinuria.
The The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) recommendations, based on the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) data, recommend that a thiazide diuretic, such as hydrochlorthiazide or chlorthalidone, should be first-line therapy for HTN in patients without diabetes, angina, ischemic heart disease, HF or chronic kidney disease, which is fortuitous given the low cost of these drugs.
Hydrochlorothiazide can normalize blood pressure in up to 46% of patients with mild HTN.Thiazides can reduce the incidence of HF in patients with HTN and prevent HTN-related mortality and morbidity, but fail to prolong survival if patients already have HF.
In hypertensive patients with diabetes, ACE inhibitors are preferred as they slow the progression of diabetic renal disease, prevent recurrent events, such as myocardial infarction in vascular patients, and may prevent the development of HF.
β-adrenergic receptor blockers are discouraged for the treatment of HTN as they have been associated with increased stroke, especially in the elderly, and do not reduce all-cause mortality or cardiovascular morbidity and mortality. Although these findings were observed in studies predominantly using atenolol, the European Society of Hypertension.
Most patients require more than one agent for optimal blood pressure control. Guidelines recommend starting two drugs for blood pressures
20 mmHg or more above the desired goal.
Treatment of HTN with α-adrenergic blockade is generally restricted to patients with urinary tract obstruction or benign prostatic hyperplasia, since these drugs may increase the incidence of HF. In the ALLHAT study, secondary end points (major cardiovascular disease events, mostly driven by the occurrence of HF) were 25% higher in the doxazosin than the chlorthalidone arm, and hospitalization for HF was twice as likely.
β-adrenergic Receptor Blockade
Owing to their favorable effects on survival and disease progression, the Heart Failure Society highly recommends the use of β-adrenergic receptor blockers for patients whose ejection fraction is less than 40%, unless there is a contraindication or intolerance to the drug. The administration of β-blockers should be started as soon as possible after the diagnosis systolic dysfunction and may be used in patients with compensated class II, III or IV HF.European Society of Cardiology and NICE discourage β-blocker use for HTN unless there are compelling reasons to do otherwise.
Nevertheless, β-adrenergic blockade is preferred in hypertensive patients with previous myocardial infarction, in whom they reduce cardiovascular mortality and provide benefits beyond those attributable to blood pressure lowering alone.
Carvedilol, sustained-release metoprolol and bisoprolol have been shown to improve overall and event-free survival of patients with mild-to-advanced HF and, thus, are approved for treating HF in the USA.
Carvedilol is distinguished by its blocking of β2- and α1-adrenergic receptors, in addition to β1-receptors, and by its antioxidant properties.
The administration of β-receptor blockers with intrinsic sympathomimetic activity, such as pindolol and acebutolol, should be avoided.
β-receptor blockers are especially appropriate in HF patients with HTN. Unlike their performance in patients with HTN only, β-blockers improve overall and event-free survival in HF patients.Improvement in survival with β-blockade and ACE inhibition appears to be additive and, in seeking their blood pressure goal, hypertensive patients with HF should start β-blocker therapy without waiting to maximize the dose of ACE inhibitor. …
β-blockers significantly reduced mortality at 1 and 2 years compared with placebo. The study estimated that, during the first year, β-blockade saved 3.8 lives per 100 treated patients and reduced hospitalizations by four per 100 treated patients. Since the improvement appears to be dose related, the aim in stable HF patients with HTN is to eventually maximize both β-blocker therapy and ACE-inhibition (or angiotensin receptor blockade).
β-adrenergic Receptor Blockade
Patients with HFNEF respond well to β-adrenergic receptor blockade, and the Heart Failure Society recommends these medications for treating HTN with HF.In addition to lowering blood pressure, these agentsincrease diastolic filling time and, thus, enhance ventricular filling and coronary perfusion. They also decrease the ventricular rate response to atrial arrhythmias and induce regression of hypertrophy.Carvedilol may reduce the symptoms and repeat hospitalizations of these patients.In a large study of over 4000 patients, initiation of β-blockers at discharge in patients with HFNEF was not associated with a significant decrease in 1-year mortality or rehospitalization.
Less-ideal Agents
Some drugs are not recommended for HF patients. Calcium channel blockade does not improve symptoms and can worsen CHF and the risk of death in these patients. Amlodipine and felodipine neither improve nor worsen the survival of HF patients and, generally, would not be chosen before other drugs of known survival benefit. However, the American College of Cardiology (ACC), American Heart Association (AHA) and Heart Failure Society accept using amlodipine in HF patients with HTN because it may be safe and well tolerated.
Limited trials have reported that reducing central sympathetic outflow (clonidine) and blocking peripheral α-receptors (prazosin) can benefit CHF patients.These agents are not recommended for CHF at this time. Potent vasodilators (minoxidil) are avoided in HF patients because they promote sodium retention .
Aldosterone Antagonists
Aldosterone can adversely affect the heart's structure and function.Blocking its effects can improve survival of patients with moderate-to-severe HF symptoms with recent decompensation,and recent myocardial infarction with LV dysfunction.Aldosterone-blocking agents may also reduce arrhythmic death in patients with mild-to-moderate HF.The Randomized Aldactone Evaluation Study (RALES) trial found that when added to an ACE inhibitor and a loop diuretic, spironolactone significantly reduced mortality at 24 months in patients with class IV HF, and in those with class III HF who had had class IV HF within 6 months.The Epleronone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial showed that eplerenone was associated with a 15% reduction in overall mortality at 16 months if started within 2 weeks of a myocardial infarction in patients with a low ejection fraction and evidence of HF and/or diabetes.Both spironolactone or epleronone therapy significantly decreased both systolic and diastolic arterial pressure compared with placebo and can be of additional aid in the management of HTN in these HF population.
Diuretics
Although diuretics are the mainstay of treating acutely decompensated HF, no long-term, randomized clinical trial has shown that they reduce morbidity or mortality, despite their relief of pulmonary and systemic venous congestion. Loop diuretics (e.g., furosemide, bumetenide, torsemide and ethacrynic acid) are used most commonly in HF patients, followed by thiazide and thiazide-like diuretics (e.g., chlorthalidone, hydrochlorothiazide, indapamide and metolazone), which are usually less effective in this setting.Treating volume overload with a loop diuretic reduces intracardiac filling pressure and cardiac output and, in hypertensive patients, may decrease systolic and diastolic blood pressures by as much as 15.8 and 8.2 mmHg, respectively.Even if this fall in output is tolerated symptomatically, a consequent rise in blood urea nitrogen (BUN) may represent tissue hypoperfusion that warrants a reduction in dose.
Thiazides can reduce the incidence of HF in patients with HTN and prevent HTN-related mortality and morbidity but fail to prolong survival if patients already have HF. Recent guidelines suggest using thiazide diuretics in hypertensive HF patients with mild fluid retention because of their persistent antihypertensive action.
The Heart Failure Society suggests adding chlorothiazide or metolazone to a loop diuretic if the latter is unsuccessful in reduce fluid retention alone. One may fully block the distal convoluted tubule by administering a diuretic that affects the distal tubule, followed by a loop diuretic. This may increase the natriuresis in chronic renal failure patients, in whom thiazides alone are much less effective. However, this combination may not significantly reduce blood pressure.
Hydralazine & Nitrates
Combining isosorbide dinitrate and hydralazine with standard therapy is especially effective in prolonging the survival of African-American patients with moderate-to-severe HF,and may provide good blood pressure control in those already taking an ACE inhibitor, β-blocker and, perhaps, also an aldosterone antagonist. The African-American Heart Failure Trial (AHeFT) showed that a fixed-dose combination of isosorbide dinitrate and hydralazine improved the survival of African-American patients with class III or IV HF by 43% .The combination's reduction of blood pressure may have helped improve survival. Since this regimen calls for multiple daily doses, is less efficient in reducing mortality and blood pressure and causes more side effects than ACE inhibition alone and, therefore, it is usually added to ACE inhibitor and β-blocker therapy.
α-receptor blockers
that can increase mortality in HF should be avoided.Management of HTN in Patients with Reduced Ejection Fraction
Management of Hypertension in Chronic Heart FailurePatients with HF may present either with reduced or normal ejection fraction. Despite the difference in ejection fraction, both groups have similar symptoms and signs. HFREF has been well studied but little is known about the benefits of treating coexisting HTN because blood pressure usually decreases as cardiac function declines and many patients do not live to see the benefits of long-term antihypertensive therapy.HTN further worsens the loading conditions of the failing ventricle, and small increases in afterload can produce large decreases in stroke volume.
A large body of data indicates that ACE inhibitors
decrease the morbidity of patients with LV dysfunction,reduce hospitalization rates andreduce the risk of death by 1-4 years in patients at any stage of HF, including those who are asymptomatic and those who develop HF early after a myocardial infarction, making these agents first-line therapy for all categories of HF.ACE inhibition also alleviates symptoms, improves clinical status and enhances the sense of wellbeing in patients with HF.
Mechanisms responsible for these beneficial effects include limitation of cardiac hypertrophy and fibrosis, reduction of ventricular wall stress and decreased efferent sympathetic traffic from the brain.
ARBs are an acceptable alternative. but a more recent meta-analysis suggested that, when used together, these drugs may markedly increase adverse effects, such as renal dysfunction, hyperkalemia and symptomatic hypotension.Thus, such combination therapy is not recommended at this time except in rare circumstances of refractory HTN.
Renin-angiotensin Inhibitors
Angiotensin receptor blockade has been well studied in patients. The CHARM-Preserved trial showed that candesartan reduced hospitalizations of patients with class II-IV CHF whose ejection fraction was greater than 40%, although it did not significantly reduce cardiovascular death. In another study, losartan improved exercise tolerance and quality of life.LV hypertrophy may regress to a greater degree with angiotensin receptor blockade than ACE inhibition.A meta-analysis examining the efficacy of antihypertensive drugs in reversing LV hypertrophy in patients with HTN illustrated that angiotensin receptor blockade and ACE inhibition decreased LV mass index by 13 and 10%, respectively.Such regression may improve diastolic function; however, another large randomized trial showed that valsartan had no such effect.
The Irbesartan in Heart Failure with Preserved Systolic Function (I-PRESERVE) trial showed no significant decrease in cardiovascular mortality or morbidity measured by death, hospitalization and quality of life in patients with HFNEF who were randomly treated with an ARB-irbesartan or placebo. There was a significant increase in serum creatinine and hyperkalemia in the ARB arm though these did not translate to significant clinically adverse events.
The AHA/ACC guidelines recommend ACE inhibition, but no studies have clearly demonstrated these medicines benefit patients with HFNEF. In the OPTIMIZE-HF Registry, ACE inhibition did not improve the mortality or readmission rates of these patients.However, it should be considered for HFNEF patients who have symptomatic coronary disease, or diabetes and another risk factor. It can reduce symptoms, ventricular mass, and myocardial stiffness.These drugs should be titrated carefully to avoid hypotension, since the poor diastolic function of these patients may make them very preload dependent.
ARBs have greater but conflicting evidence for use in patients with preserved ejection fraction.Thiazides are the first-line drugs in patients with HTN at risk for HF. However, in patients with established HF, loop diuretics are prescribed more often and a thiazide may be added to promote diuresis by sequentially blocking sodium transport in renal tubules in cases where volume overload persists, despite loop diuretics.
Conflicting data exist in the use of β-blockers for patients with HTN without HF and in patients with HFNEF.
Extensive data support the use of β-blockers in patients with HFREF, especially carvedilol and metoprolol.
Calcium channel blockers are widely used to treat HTN in patients at risk for HF..however, they are not considered in the management of HTN in HFREF.A dihydropyridine group of calcium channel blockers could be used to control HTN only once other medications have failed, since they exert a neutral effect on mortality in HFREF patients. Potentially, they could be beneficial in patients with HFNEF. Aldosterone receptor blockers significantly decreased blood pressure in patients with HTN at risk for HF requiring three or more antihypertensive medications.
In patients with HFREF, aldosterone receptor blockers decrease both HTN and mortality.
Calcium Channel Blockade
Unlike HF patients with reduced systolic function, HFNEF patients are likely to benefit from calcium channel blockade. Amlodipine may be especially helpful in HFNEF patients with HTN and limiting angina, in whom the drug may decrease both blood pressure and the number of ischemic episodes.Again, as with any agent that suddenly reduces preload in patients with diastolic dysfunction, dose titration must proceed carefully.The Heart Failure Society highly recommends nondihydropyridine agents (e.g., verapamil and diltiazem) for HFNEF patients with atrial fibrillation that cannot tolerate β-receptor blockade or whose heart rates do not decrease sufficiently in response to it.Verapamil may be particularly helpful because it can improve myocardial relaxation and compliance
Several agents currently under investigation include
NebivololA third-generation, highly selective β1-receptor blocker that
also promotes endothelial nitric oxide production.HF patients with HTN tolerate this drug well. In patients with symptomatic HF and reduced systolic function, its hemodynamic effects are similar to carvedilol. A large trial showed that nebivolol reduced the composite end point of mortality and hospitalization in HF patients.
Europe has approved the drug for mild-to-moderate, uncomplicated HTN and mild-to-moderate HF and, in the USA, the drug is now under FDA review.
In the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors With Heart Failure (SENIORS) trial, nebivolol
significantly reduced the composite end point of death and cardiovascular hospitalizations in patients aged at least 70 years whose ejection fractions were reduced or preserved.
It is promoted as an effective and well-tolerated first-line antihypertensive that combines highly selective beta blockade with vasodilation associated with nitric oxide modulation. The clinical importance of this mechanism in patients receiving chronic oral therapy and the extent of the contribution of this mechanism to the drug’s antihypertensive activity remain Unclear. The most frequent adverse events (incidence between 1-10%) were headache, dizziness, tiredness and paraesthesia. Other adverse events reported by at least 1% of patients were: diarrhoea, constipation, nausea,dyspnoea and oedema
Aliskiren, a direct renin inhibitor, is another novel agent whose dose-dependent reductions in blood pressure are comparable to an ARB, but aliskiren blocks the renin-angiotensin-aldosterone system more completely than other downstream inhibitors and prevents the increase in plasma renin that follows
diuretic therapy, ACE inhibition, calcium channel and angiotensin receptor blockade. The US FDA-approved aliskiren for HTN in 2007.It can effectively treat hypertensive African-American patients who typically do not respond well to β-receptor blockade.
The agent may also help HF patients and, although not approved for HF, it appears promising. In the Aliskiren Observation of Heart Failure Treatment (ALOFT) study, favorable neurohormonal changes occurred in HF patients after combining aliskiren with ACE inhibition or angiotensin receptor blockade and a β-receptor blocker.
Aliskiren binds to the S3bp binding pocket of renin, essential for its activity. Binding to this pocket prevents the conversion of angiotensinogen to angiotensin I. Aliskiren is also available as combination therapy with hydrochlorothiazide.
Renin is the first enzyme in the renin-angiotensin-aldosterone system which plays a role in blood pressure control. Renin cleaves angiotensinogen to angiotensin I, which is in turn converted by (ACE) to angiotensin II. Angiotensin II has both direct and indirect effects on blood pressure. It directly causes arterial smooth muscle to contract, leading to vasoconstriction and increased blood pressure. Angiotensin II also stimulates the production of aldosterone from the adrenal cortex, which causes the tubules of the kidneys to increase reabsorption of sodium, with water following thereby increasing plasma volume and blood pressure.
Many drugs control blood pressure by interfering with angiotensin or aldosterone. However, when these drugs are used chronically, the body increases renin production, which drives blood pressure up again. Therefore, doctors have been looking for a drug to inhibit renin directly. Aliskiren is the first drug to do so.
Aliskiren may have renoprotective effects that are independent of its blood pressure−lowering effect in patients with hypertension, type 2 diabetes, and nephropathy who are receiving the recommended renoprotective treatment. According to the AVOID study, researchers found that treatment with 300 mg of aliskiren daily, as compared with placebo, reduced the mean urinary albumin-to-creatinine ratio by 20% (95% confidence interval, 9 to 30; P<0.001), with a reduction of 50% or more in 24.7% of the patients who received aliskiren as compared with 12.5% of those who received placebo (P<0.001).
Furthermore, the AVOID trial shows that treatment with 300 mg of aliskiren daily reduces albuminuria in patients with hypertension, type 2 diabetes, and proteinuria who are receiving the recommended maximal renoprotective treatment with losartan and optimal antihypertensive therapy. Therefore, direct renin inhibition will have a critical role in strategic renoprotective pharmacotherapy, in conjunction with dual blockade of the renin−angiotensin−aldosterone system with the use of ACE inhibitors and angiotensin II–receptor blockers, very high doses of angiotensin II−receptor blockers, and aldosterone blockade.[8
Angioedema
Hyperkalemia (particularly when used with ACE inhibitors in diabetic patients)
Hypotension (particularly in volume-depleted patients)
Diarrhea and other GI symptoms
Rash, elevated uric acid, gout, and renal stones.
Rarely: allergic swelling of the face, lips or tongue and difficulty breathing
Adverse effects
Contraindications
Pregnancy: other drugs such as ACE inhibitors, also acting on the renin-angiotensin system have been associated with fetal malformations and neonatal death[9Breast feeding: during animal studies, the drug has been found present in milk.[10]Aliskiren has not yet been evaluated in patients with significantly impaired renal function
Drug Interactions
Aliskiren is a minor substrate of CYP3A4 and, more important, P-glycoprotein:Reduces furosemide blood concentration.
Atorvastatin may increase blood concentration, however no dose adjustment needed.
Possible interaction with ciclosporin ( the concomitant use of ciclosporin and aliskiren is contraindicated).
Caution should be exercised when aliskiren is administered with ketoconazole or other moderate P-gp inhibitors (itraconazole, clarithromycin, telithromycin, erythromycin, amiodarone).
In short-term studies it was effective in lowering blood pressure either alone or in combination with valsartan and hydrochlorothiazide and had a low incidence of serious adverse effects. Aliskiren is more expensive than most other antihypertensive agents and no long-term clinical outcome data are available.
The recommended dose is 150mg once daily, which may be increased to 300 mg daily if blood pressure (BP) remains uncontrolled. Aliskiren was well tolerated, with low discontinuation rates, in the study populations. The incidence of hyperkalaemia (serum potassium > 5.5 mmol/L) was low when aliskiren was used as monotherapy (2%). In combination with valsartan, hyperkalaemia was more frequent(4%). Nevertheless, routine biochemical monitoring of electrolytes and renal function would be advisable.
Diarrhoea was the only adverse effect occurring significantly more frequently with aliskiren than placebo, and this was only in asubgroup receiving 600 mg aliskiren. Angioedema has been reported rarely. There is no information on the use of this agent in patients with severely impaired renal function. As with other agents affecting the renin-angiotensin pathway, aliskiren is contraindicated in pregnancy and should be used with caution in women of childbearing age.
At present there is no convincing evidence to support the choice of aliskiren above other, more established agents. It has not been studied in combination with a number of these established agents,and its efficacy in severe hypertension is unknown. The benefits, if any, of inhibiting the renin angiotensin pathway at an earlier stage than with ACE inhibitors and angiotensin receptor blockers are currently unclear.
There are no long-term clinical outcome data with this agent which is expensive, compared to other available antihypertensive drugs. Many hypertensive patients require more than one agent to control BP and aliskiren may have a role as an add-on therapy in those uncontrolled using traditional treatment algorithms.
Drug
Contraindications to useACE-I/ARB
Contraindicated if life-threatening adverse reactions (e.g., angioedema or anuric renal failure on previous exposures .Pregnancy.use with caution if markedly increased serum levels of creatinine (> 3 mg/dl), bilateral renal artery stenosis or elevated levels of serum potassium (> 5.5 mmol/l)
β-blocker
Caution should be used if β-blockers are initiated in patients with marked bradycardia (< 55 beats/min), diabetes with recurrent hypoglycemia, asthma or resting limb ischemia. Not recommended in patients with asthma with active bronchospasm .Do not initiate in patients hospitalized in an intensive-care unit or those who have required recent treatment with an intravenous positive inotropic agent
Spironolactone
Not recommended when creatinine is > 2.5 mg/dl (or creatinine clearance < 30 ml/min) or serum potassium is > 5.0 mmol/l
Contraindications to Use of Major Antihypertensive Drugs Used in Heart Failure
Digitalis
Mechanism of actionThe positive inotropic effect of digitalis has 2 components.
1-Direct inhibition of membrane-bound sodium- and potassium-activated adenosine triphosphatase (Na+/K+ -ATPase), which leads to an increase in the intracellular concentration of calcium
2-Associated increase in a slow inward calcium current (iCa) during the action potential (AP) (contributes to the plateau of the AP.) .
Digitalis glycosides bind specifically to Na+/K+ -ATPase, inhibit its enzymatic activity, and impair active transport of extruding sodium and transport of potassium into the fibers (3:2 ratio). As a result, intracellular sodium ([Na+]i) gradually increases, and a gradual, small decrease in intracellular potassium ([K+]i) occurs.
decreased impulse discharge/generation from the SA node and decrease impulse propagation through the AV node.
In general, cardiac glycosides slow conduction and increase the refractory period in specialized cardiac conducting tissue by stimulating vagal tone.
Digitalis has parasympathetic properties, which include hypersensitization of carotid sinus baroreceptors and stimulation of central vagal nuclei.
Alterations in cardiac rate and rhythm occurring in digitalis toxicity may simulate almost every known type of dysrhythmia. Although no dysrhythmia is pathognomonic for digoxin toxicity, toxicity should be suspected when evidence of increased automaticity and depressed conduction is noted. Underlying these dysrhythmias is a complex influence of digitalis on the electrophysiologic properties of the heart as already discussed, as well as via the cumulative results of the direct, vagotonic, and antiadrenergic actions of digitalis.
The effects of digoxin vary with the dose and differ depending on the type of cardiac tissue involved. The atria and ventricles exhibit increased automaticity and excitability, resulting in extrasystoles and tachydysrhythmias. Conduction velocity is reduced in both myocardial and nodal tissue, resulting in increased PR interval and atrioventricular (AV) block accompanied by decrease in QT interval.
In addition to these effects, the direct effect of digitalis on repolarization often is reflected in the ECG by ST segment and T-wave forces opposite in direction to the major QRS forces. The initial electrophysiologic manifestation of digitalis effects and toxicity usually is mediated by increased vagal tone. Early in acute intoxication, depression of sinoatrial (SA) or AV nodal function may be reversed by atropine. Subsequent manifestations are the result of direct and vagomimetic actions of the drug on the heart and are not reversed by atropine.
The most common precipitating cause of digitalis intoxication is depletion of potassium stores, which occurs often in patients with heart failure as a result of diuretic therapy and secondary hyperaldosteronism.
Other causes include the following:
Advanced age
Myocardial infarction or ischemia
Hypothyroidism
Hypercalcemia
Renal insufficiency
Also consider drug interactions. Drugs that have been reported to potentiate digoxin toxicity include the following:
Quinidine
Erythromycin
Verapamil, diltiazem, nifedipine
Captopril
Anticholinergic drugs
Ibuprofen
Amiodarone
Clarithromycin
Epidemiology
Digoxin Toxicity is common (Incidence 7-20%)
Risk Factors
Hypokalemia
Hypomagnesemia
Hypercalcemia
Medication use interfering with Digoxin excretion
Quinidine
Verapamil
Amiodarone
Digoxin Toxicity
Symptoms
Anorexia
Nausea
Vomiting
Somnolence
Muscle Weakness
Diarrhea
Yellow vision (xanthopsia)
Labs
Serum Digoxin Level over 2.5 mg/mlDoes not always correlate with toxicity
Toxicity may occur at low levels and not at high ones
Electrocardiogram
Dysrhythmia
Premature beats
Bigeminy
Paroxysmal Atrial Tachycardia with 2:1 AV Block
Atrial Fibrillation
Nodal rhythm
Ventricular Tachycardia
T Wave inversion
ST Depression
PR interval increased
Management
A.Stop Digoxin
B.Correct Hypokalemia
Use caution if Heart Block is present
C.Treat associated arrhythmias appropriately
D.Avoid potentially harmful interventions
1.Catecholamines
2.Electrical Cardioversion
a.Safe if Digoxin Level is under 2 ng/ml
b.Use lowest possible energy if needed (10-20 J)
E.Digibind (40 mg/vial)
1.Indications
a.Massive Digoxin overdose
b.Refractory Digitalis toxicity
2.Calculate vials needed based on Digoxin level
3.Vials = (Digoxin Level in ng/ml) x (WtKg)/100
Contraindication of Digoxin
1. Wolff Parkinson white (WPW)2. In diastolic failure
3. Heart block
4. Previous history of stroke’s Adams syndrome
5. Obstructive cardiac myopathy
• Digitoxin
• Digoxin
• Less polar and more lipid soluble
• More polar and less lipid soluble
• Easily crosses BBB
• Does not cross BBB
• Produces CNS symptom
• Does not produce CNS symptom
• ½ life is 5 days
• ½ life is 1½ days
• Heart : plasma ratio is 7:1
• Heart : plasma ratio is 30:1
• Mostly metabolized in the liver, so it’s excretion is independent of renal function
• More than 80% excreted unchanged via urine, rest is removed by non-renal routes like biliary excretion and hepatic metabolism
• Digitalization requires (4x5) 20 days
• Digitalization requires (4x1½) 20 days
• Differences between Digitoxin and Digoxin
Digitalis may be given as tablets or capsules
Therapeutic index is very low so, low safety marginWide distribution to various tissues because of lipid solubility
{Advantage—reaches the target site very quickly, Disadvantage—goes to other tissues}
Digoxin mainly eliminated by the kidney (may be given to patients with liver diseases)
Digitoxin is mainly eliminated by hepatic metabolism (may be given to patients with renal failure)
Diagnosing diastolic heart failure
How to always diagnose DHF accurately is uncertain but a correct diagnosis is important. Some guidelines propose that 3 requirements be met to make the call:1.symptoms or signs of CHF
2.normal systolic function
3.abnormal diastolic function
There are some situations where symptoms alone may lead to a misdiagnosis. This is especially true in older patients who get short of breath mainly because they have very poor physical fitness. It can also happen with non-heart-related shortness of breath such as with lung disease. So diagnosing DHF requires some signs or symptoms of CHF like lung congestion, edema, raised jugular vein, etc. You do have to rule out problems like mitral valve disease and and lung disease.
These criteria are too tight and that number 3 should be eliminated. Ninety percent of patients who fit the first 2 will indeed turn out to have diastolic heart failure confirmed by cath. The problem arises because measuring diastolic function with noninvasive tests like echo is difficult and confusing.
DHF cannot usually be distinguished from SHF by patient history, physical exam, x-ray, and EKG alone. Diagnosis requires an estimate of LV size and EF. These measurements can be made using echo, MUGA, or cath. Really, DHF diagnosis is a matter of ruling out other possible causes in patients seeming to have heart failure but who have normal heart size and EF.Since echo does have limits for spotting DHF and cath is invasive, one trial studied whether the BNP blood test could help spot diastolic heart failure. It turns out that a quick BNP test can help a doctor diagnose DHF.
By slowing heart rate, you give the ventricle more time to relax, lowering filling pressures. Also, many DHF patients have ischemia. By slowing the heart rate, you reduce the heart's need for oxygen and that helps balance supply and demand of blood and oxygen
Treating heart failure in patients with an EF of 30% requires that beta-blocker dose be raised carefully, starting with tiny doses and raising it slowly over several months. In the DHF patient, that very slow rise in dose is not usually necessary.
Another example is calcium channel blockers. These drugs should not be used in systolic heart failure patients at all, but they may help DHF patients. As another example, diuretic use needs to be much more cautious in the DHF patient since drying them out too much can easily cause too-low blood pressure.
So even though the same drugs may be used to treat SHF and DHF, there are important differences. It is important for doctors to be aware of the differences when treating CHF patients.
Prognosis of patients with DHF is better than for SHF. Annual mortality for DHF is about 5 to 8%, while it is about 10 to 15% for SHF.