Angina pectoris in patients withnormal coronary angiograms:current pathophysiologicalconcepts and therapeutic options
Heart 2012
د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
The presence of angina pectoris (AP) in patients
with either normal coronary angiograms or withnon-obstructive coronary artery disease (CAD) is
not only a frequent clinical finding but also a clinical
and therapeutic challenge. Only recently, Patel
et al evaluated the diagnostic yield of coronary
Angiography regarding the presence or absence of
obstructive CAD among almost 400 000 patients
with suspected CAD.
Although the majority (70%) of these patients was suffering from chest pain symptoms, only 37.6% of them demonstrated obstructive CAD by invasive coronary angiography
(defined as diameter stenosis >50% of the left main coronary artery or >70% of a major epicardial vessel).
This surprising finding raises the following question:
how should we explain the presence of AP symptoms (typical enough to motivate coronary angiography) in patients without obstructive CAD? Moreover, we need to consider the followingclinical issues:(1) non-obstructed coronary arteries are also found in a sizeable subgroup of 10% of patients who undergo urgent coronary angiography due to angina accompanied by troponin elevation,and these patients represent a high risk group with a worse prognosis even in the absence of obstructive lesion; and
(2) the presence of myocardial ischaemia in the absence of obstructive CAD still predicts cardiovascular outcome and is associated with higher rates of anginal hospitalisation, repeat catheterisation, and greater treatment costs.
Therefore, further evaluation of the underlying
pathophysiology and potential treatment options
in patients presenting with AP in the absence of
obstructive CAD is a clinically highly relevant issue.
In the following review, different (sometimes
overlapping) pathophysiologies causing symptomsof AP in the absence of obstructive CAD are
discussed, and current diagnostic as well as therapeutic
options are illustrated.
THE PATIENT WITH ‘HYPERTENSIVE HEARTDISEASE’
Obviously, hypertension is a frequent and importantcardiovascular risk factor. As recently summarised by
Raman et al, hypertension is a predisposing factor
not only for the development of heart failure
symptoms, atrial fibrillation, and ventricular
arrhythmias, but also for ischaemic heart disease and
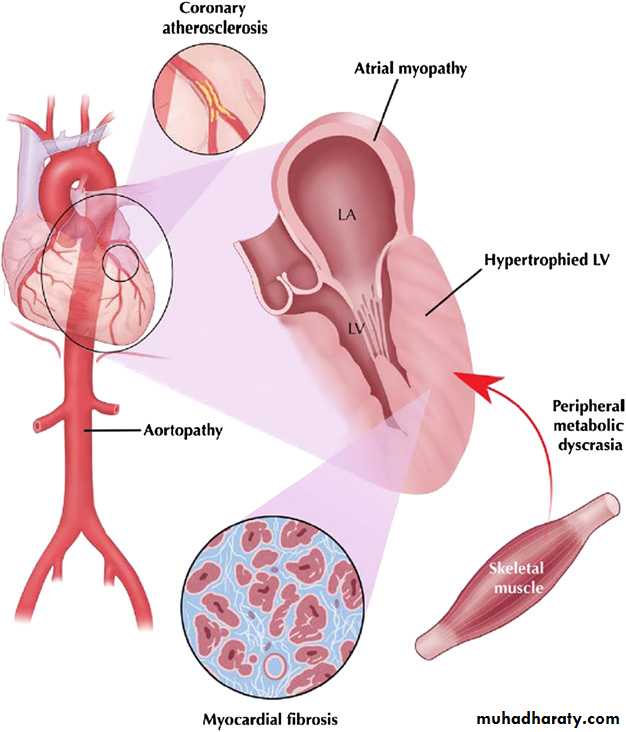
the risk of myocardial infarction.w5 Structural alterations in patients with hypertensive heart disease comprise cardiomyocyte hypertrophy, expansion of interstitial and perivascular fibrosis by progressive collagen accumulation, and increased arterial stiffness (figure 1).
These alterations are not only associated with left ventricular hypertrophy and an increase in left ventricular mass, but also with a decrease in intramyocardial capillary density and arteriolar wall thickening. As a result of these structural alterations, both epicardial CAD as well as microvascular disease may occur in patients with hypertensive heart disease and cause symptoms of AP.
The association between hypertension and microvascular dysfunction (resulting in symptoms of AP and dyspnoea in addition to a reduced coronary flow reserve) is well established.
Previous studies have revealed those aforementioned
structural changes of the microvasculature
as well as functional abnormalities such as endothelial
dysfunction with decreased nitric oxide production. Furthermore, previous nuclear imaging based studies showed a low specificity (as low as36%) for the detection of CAD in hypertensive patients, owing to scintigraphic defects caused by microvascular dysfunction in the absence of significant epicardial stenosis.
Therefore, it is believed that those aforementioned structural and functional alterations in hypertensive patients cause a coronary vasomotor dysfunction, which in turn may cause symptoms of AP as well as
myocardial ischaemia in the absence of obstructive
CAD. Recently, Escaned et al demonstrated
that both arteriolar obliteration and capillary rarefaction have an independent influence on microcirculatory haemodynamics (figure 2).
They clearly proved the link between microvascular structural changes and functional impairment, which in turn may result in clinical symptoms and pathological
non-invasive stress test results in the absence of
significant epicardial stenosis.
Interestingly, most available imaging modalitiesd
apart from stress echocardiographydthat assess the haemodynamic significance of ‘epicardial’ CAD have
a moderate to low specificity for detection of
obstructive epicardial CAD raising the possibility that these techniques may detect true perfusion abnormalities in the context of angina without visible coronary stenosis.
Only in the case of stress echocardiography has a satisfactory diagnostic specificity (80-91%) for the detection of obstructive CAD been demonstrated and attributed to the absence of wall motion abnormalities in patients with myocardial ischaemia not caused by epicardial stenosis.
Coronary vasomotility and/or microvascular disorders have been discussed as possible explanations for the presence of myocardial ischaemia in the absence of obstructive CAD.
In the last few years, cardiovascular magnetic resonance (CMR) imaging with adenosine-stress first-pass perfusion (perfusion-CMR) has been shown to be a sensitive non-invasive method for the detection of myocardial ischaemia caused by obstructive CAD. However, pathological perfusion-CMR results also do not allow a perfusion defect due to significant epicardial stenosis to be differentiated from one due to a coronary vasomotility disorder (figure 3).
Therefore, patients with hypertension were even excluded from studies that assessed the diagnostic accuracy of perfusion-CMR in suspected CAD in order to keep the specificity high.
Many patients with hypertension nevertheless undergo myocardial perfusion imaging
(by nuclear techniques or increasingly CMR) intwo common clinical scenarios:
1. They present with typical AP or angina-like
symptoms (usually dyspnoea upon exertion or
atypical angina) or
2. They are asymptomatic but either have ST
depression during exercise stress testing or are
felt to be at very high risk for the development
of epicardial CAD because of additional atherosclerotic risk factors.
Patients with positive tests usually undergo
coronary angiography in order to definitely verify orrule out potentially hazardous obstructive epicardial
CAD. A large number of those patients will not
have relevant epicardial disease. These patients
are commonly reassured that they are ‘healthy’.
Such a statement can be surprising and even
shocking for the patient, who may suffer from
severe typical or atypical AP symptoms.
Therefore, the clinician should not be content with
a normal or near normal coronary angiogram, but
consider a coronary vasomotility disorder as
underlying disease for symptoms of AP and/or
myocardial ischaemia. On the other hand, what
may look like a coronary stenosis may not always
be the cause of the patient’s symptoms, which is
becoming increasingly obvious with the increasing
use of fractional flow reserve measurements before
coronary interventions.
This indicates that epicardial and microvascular disease may coexist.Hence, the task of the clinician is becoming more challenging when dealing with patients complaining of AP.
In patients without severe epicardial diseasedfor
example, patients with hypertensive heart diseased
clinical studies have suggested an improvement of
structural and functional alterations as well as
a relief in clinical symptoms on treatment with ACE
inhibitors and/or calcium antagonists.
THE PATIENT WITH ‘MICROVASCULAR DISEASE’
Abnormalities in the structure and function of themicrovasculature occur not only in cases of hypertensive heart disease but also in many other clinical and pathological conditions.
The main epicardial coronary arteries run on the surface of the myocardium and bifurcate into smaller arteries and arterioles, thereby forming a tree-like network while spirally penetrating into the mid- and
subendocardium where they empty into a capillary
(non-tree-like) network with interconnections.
These capillaries drain into post-capillary venules
which are directed from the endocardium to the
epicardium and finally form larger veins. Large
coronary arteries (diameter >500 mm) are called
conduit vessels because they contribute <5% to
total coronary resistance, while prearterioles
(diameter 100-500 mm) and arterioles (diameter
<100 mm) cause the major flow resistance (resistance
vessels). Hence, a dysfunction of smallresistance vessels (pre-arterioles and arterioles with a diameter <500 mm)dwhich are not visible at coronary angiographydhas been suggested to be
responsible for microvascular disease.
We mention microvascular dysfunction throughout this paper. Thus, it may be appropriate to briefly review the normal function of this important part of the coronary tree. Under normal conditions, arterioles dilate or constrict in response
to surrounding myocardial metabolic conditions to
match flow appropriate to myocardial oxygen
demands.
Hence, dysfunction of the microvasculature may occur as a consequence of disturbances in the complex signalling pathways in endothelial as well as smooth muscle cells, but also as a consequence of abnormal production of molecules necessary for normal signalling.
It should be emphasised that direct in vivo
visualisation of the microvasculature is still notpossible in humans. However, the function of the
microvasculature can be assessed by employing
invasive and non-invasive methods.
Coronary flow can be quantitated using intracoronary
Doppler registration, whereas positron emission
tomography or perfusion-CMR measure myocardial
blood flow (figure 4).
For example, coronary flow measurements both at rest and during maximal hyperaemia (eg, using adenosine) with an intracoronary Doppler wire permit assessment of coronary flow reserve, which is usually
impaired in patients with microvascular disease.
Accordingly, the (auto-)regulation and modulation
of coronary blood flow in response to different stimuli (such as physical exercise, mental pressure or sensation of cold) is disturbed in patients with microvascular disease, which in turn may cause symptoms of either AP and/or dyspnoea.
Particular attention should be paid not only to those patients presenting with typical AP, but also to those patients presenting with recurrent unexplained (chronic) dyspnoea in the presence of normal left ventricular systolic function;
symptoms in such patients may also reflect
microvascular disease and be caused by increased
left ventricular stiffness, resulting in increased left
ventricular filling pressures and diastolic dysfunction.
Recently, Camici and Crea suggested a clinical
classification of microvascular diseases into fourgroups:
The first group encompasses patients with
traditional coronary risk factors (smoking,
hypertension, hyperlipidaemia, and diabetes) in
the absence of obstructive CAD and myocardial
diseases. These traditional cardiovascular risk
factors may lead to microvascular disease and
subclinical coronary atherosclerosis. In these
patients, microvascular disease can be identified,
for example, by non-invasively demonstrating
a globally reduced coronary flow reserve.
Microvascular function may improve by instituting
treatments aimed at reducing the burden of risk
factors.
The second group comprises patients with myocardial diseases such as primary cardiomyopathies (eg, dilated or hypertrophic cardiomyopathy) as well as secondary cardiomyopathies (eg, diabetic or valvular), in whom adverse remodelling of intramural coronary arterioles is
occurring. The underlying mechanisms causing
microvascular disease in this group encompass
expansion of interstitial and perivascular fibrosis,
capillary rarefaction, and increased arterial stiffness.
The pathophysiological overlap between the first group and this group may be illustrated by focusing on patients with diabetes: the presence of diabetes may not only lead to functional abnormalities such as coronary endothelial dysfunction, thereby causing an impaired coronary flow reserve, but may also result in
diabetic cardiomyopathy which is characterised
by structural changes such as interstitial and
perivascular fibrosis associated with severe
microvascular diseasew21 (figure 5).
Whether medical treatment may improve the massive
disturbance of microvascular function in thesepatients is unclear.
The third group encompasses patients with obstructive CAD. Not only patients with angina but normal coronary arteries, but also those with impressive and even obstructive plaque formation by coronary angiography,
may suffer from microvascular disease.
Obviously, coronary atherosclerosis is a diffuse
disease process affecting not only a single
coronary artery but rather the whole coronary
tree. Accordingly, impaired coronary flow reserve
in addition to impaired glucose metabolism as
signs of microvascular disease were documented
in ‘normal’ arteries and ‘normal’ (remote) myocardial segments, respectively, not directly affected by the infarcted myocardium in patients with single vessel disease.
Microvascular disease and microvascular spasm may also be the cause of increased interstitial fibrosis
found in the remote myocardium of such patients.
Hence, a critical epicardial stenosis and/or a plaque rupture with subsequent coronary obstruction may represent a late stage in the development of coronary atherosclerosis (figure 6). In patients suffering from combined epicardial and microvascular disease, symptoms of AP may persist even after treating a critical stenosis and/or obstructed coronary segment,
since diffuse microvascular disease still exists.
In this group, appropriate medical treatment may
improve or abolish the symptoms due tomicrovascular disease.
The last group is denoted ‘iatrogenic coronary
microvascular dysfunction’ and encompasses
patients with coronary revascularisationdfor
example, distal embolisation. Obviously, the
washout of thrombotic tissue from the epicardial
area of coronary obstruction to the distal
microvascular area during a coronary intervention
results, on the one hand, in an unobstructed
epicardial coronary artery, but on the other hand
will lead to diminished coronary flow due to
diffuse microvascular embolisation.
Hence, in this group, even though pharmacologic treatment may restore coronary flow, the change in
clinical outcome will be primarily based on the
resulting perfusion and status of the microvasculature.
Taken together, microvascular disease is far more
common than, for example, obstructive CAD, since
(1) different cardiovascular risk factors, and (2)
different coronary and myocardial diseases may
cause microvascular dysfunction. In fact, in
patients fulfilling the strictest definition of cardiac
syndrome X microvascular disease seems to be an
independent disease entity.
Therapy should aim at treating and/or eliminating the underlying disease. However, since microvascular disease may have severely advanced before the first clinical symptoms occur, successful therapy of microvascular disease is often difficultdand much more challenging than treating the immediate underlying disease such as hypertension or diabetes.
Calcium antagonists and nitrates are the most
commonly used agents in patients with AP symptoms.However, the use of nitrates may be disappointing,
particularly in patients with microvascular disease, as neither coronary blood flow nor subendomyocardial flow will increase following intracoronary glyceryl trinitrate application in some patients with non-obstructive CAD.
This disappointing finding is explained by the observation of some groups that GTN dilates larger conduit vessels, whereas smaller resistance regulating arterioles remain unaffected because these vessels lack the necessary GTN converting enzymes at least in some animal models.
Hence, treatment of anginal symptoms in patients with mainly microvascular disease may be disappointing since these patients often do not react to GTN and hence do not benefit from GTN treatment.
However, about 50% of patients improve with
nitrates such as pentaerythrityl tetranitrate, which
suggests some heterogeneity in the underlying
pathological substrate in the microvasculature.
THE PATIENT WITH ‘CARDIAC SYNDROME X’
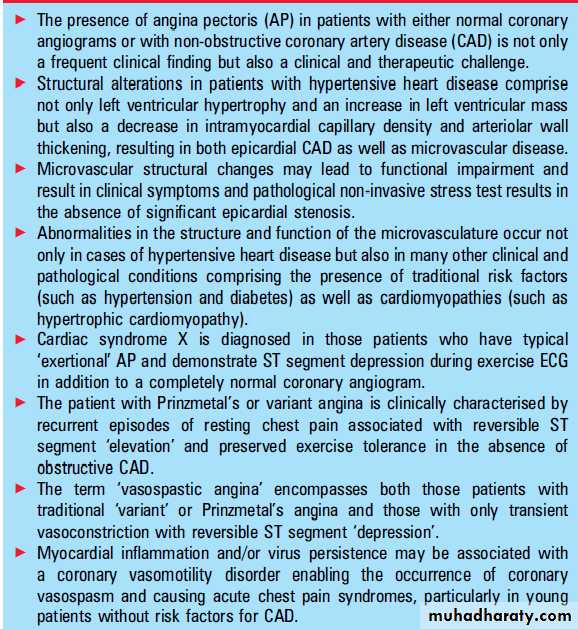
In principal, cardiac syndrome X (CSX) is diagnosed
in those patients who have typical ‘exertional’ AP
and demonstrate ST segment depression during
exercise ECG in addition to a completely normal
coronary angiogram, but who do not have cardiovascular risk factors for CAD such as hypertension
or hypercholesterolaemia.
However, today the term CSX is also used for those patients demonstrating exertional AP and myocardial
ischaemia during exercise ‘with’ cardiovascular risk
factors. Cannon et al were the first to suspect microvascular dysfunction as the underlying cause for
chest pain in patients with CSX in 1988.10 Since
that time, numerous studies have been published
addressing the underlying pathophysiology in
patients with CSX.
As recentlyreviewed, endothelial dysfunction and/
or microvascular dysfunction frequently occur in‘normal’ people who just have coronary risk
factors, but there is usually no associated
ischaemia, ST segment depression or chest pain in
the majority of these people. Therefore, an
important question still to be answered is whether
microvascular dysfunction may indeed lead to
ischaemia, ST segment depression, and most
importantly chest pain in patients with CSX.
For example, Panting et al demonstrated subendocardial
perfusion defects in patients with CSX
based on perfusion-CMR studies.5 However,
Vermeltfoort et al could not reproduce these findings
using a similar approach.w31 More recently,
Monaco et al found a significant impairment of
cardiac uptake of iodine-123-meta-odobenzylguanidine (MIBG) on myocardial scintigraphy, indicating abnormal function of cardiac adrenergic nerve fibres and also abnormalities in coronary microvascular function in patients with CSX.
Most likely, these discrepant findings are due to
the fact that the effects of microvascular disease interms of causing objective ischaemia and chest
pain are importantly modulated by the extent of
the disease (rarefaction and anatomic/functional
narrowing of the microvessels) and the pain
perception of the patient.
Like other forms of microvascular disease, CSX is
more frequent in female than male patients.
This may be the result of risk factor clustering, vascular
inflammation and remodelling, and hormonalalterations. Therapeutically, a recent pilot study
(randomised, double blind, placebo controlled,
crossover trial) found that anginal symptoms in
women with angina, no obstructive CAD, and
>10% ischaemic myocardium on adenosine stress
CMR imaging, were significantly improved by
ranolazine compared with placebo.
THE PATIENT WITH ‘VARIANT ANGINA’ OR
‘PRINZMETAL’S ANGINA’
In his landmark paper of 1959, Prinzmetal et al
described “another type of angina pectoris which
appears to be a separate entity, and has not been set apart from the heterogeneous group of anginal
syndromes. It does not show the two major characteristics of the classic form and, in addition, has
other important clinical and experimental differences.
In this variant type of angina the pain comes
on with the subject at rest or during ordinary
activity during the day or night. It is not brought
on by effort.
During an attack, the STsegments are transiently and often remarkably elevated and there are reciprocal ST depressions in the standard leads”.
Importantly, none of those patients studied by Prinzmetal and colleagues underwent coronary angiography, and in no patient was the
angiographic morphology of spasm demonstrated.
Nevertheless, Prinzmetal et al postulated an
increase of ‘tonus’ at the site of a subcritical
stenosis as a prerequisite for Prinzmetal’s or variant
angina, the hallmark of which is the association
with ST segment elevation.
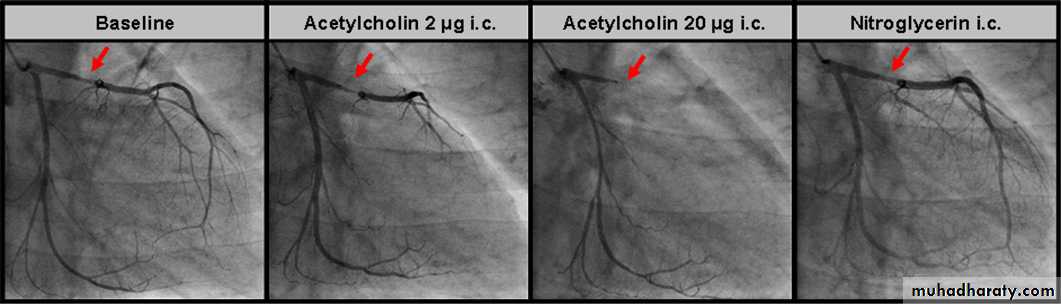
Today, the patient with Prinzmetal’s or variant angina is clinically characterised by recurrent episodes of resting chest pain associated with reversible ST segment ‘elevation’ and preserved exercise tolerance in the absence of obstructive CAD. Angiography during an
ischaemic episode or invasive provocative testing
with acetylcholine or ergonovine should typically
demonstrate a subtotal/total occlusive spasm of
a major epicardial coronary artery (figure 7).
The mechanism of angina in patients with Prinzmetal’s
angina more likely differs from the mechanism ofother forms of angina that occur in the much larger
group of patients demonstrating ST segment
depression, both during the angina attack as well as
during provocative testing. In these patients
acetylcholine testing does not show focal occlusion
but does show distal diffuse epicardial spasm or no
significant epicardial vasomotion. Thus, Prinzmetal’s
angina is rare and only represents one extreme
aspect of a continuous spectrum of vasospastic
myocardial ischaemia.
Patients with resting angina not accompanied by
ST segment elevation and preserved exercise tolerancewill be more common, as the periodically
increased vasomotor tone will only rarely result in
total occlusion of a major epicardial coronary artery
but will result more commonly in significant
transient vasoconstriction (without total occlusion).
Hence, the term ‘vasospastic angina’ encompasses
both those patients with traditional ‘variant’
or Prinzmetal’s angina but also those with only
transient vasoconstriction with reversible ST
segment ‘depression’.
The exact subcellular mechanisms responsible for coronary spasm still remain to be elucidated, although interesting data have been obtained from animal models.
A coronary vasomotility disorder may be caused by severe endothelial dysfunction due to a decreased
bioavailability of the vasodilator nitric oxide (NO),
as this pathophysiology has previously been
suggested as a possible mechanism for coronary
vasospasm. However, this hypothesis of endothelial
dysfunction is competing with the view of
coronary smooth muscle cell hyperreactivity as the
underlying cause for coronary vasospasm.
Since patients suffering from vasospastic angina
will often show normal coronary arteries or nonobstructive CAD during coronary angiography, theclinician ignoring the potential diagnosis of coronary
vasospasm may misleadingly either attribute
the patient’s symptoms to a non-cardiac or
psychosomatic origin or even perform stenting of
a moderate, non-critical stenosis in an effort to
treat the patient’s problem.
Since coronary vasospasm may be an occasional occurrence and may not occur during a normal 24 h Holter ECG,Prinzmetal himself noted that establishing the
diagnosis of vasospastic angina by recording an
ECG during an acute attack might not be an easy
task.17 Consequently, current guidelines recommend
(among others) intracoronary provocative
testing to identify coronary spasm in patients with
normal findings or non-obstructive lesions on
coronary angiography presenting with the clinical
picture of coronary vasospasm.
Patients with variant or vasospastic angina should be treated symptomatically with calcium antagonists and nitrates. Moreover, they should receive a statin independent of their cholesterol value since additional statin therapy has been shown to decrease the number of patients with coronary vasospasm by 30% after 6 months of treatment. Whether ß-blocker therapy in these
patients is beneficial or rather detrimental is still
discussed controversially. While early clinical data
suggest against the use non-specific ß-blocking
agents such as propranolol, due to an increase in the
frequency of AP symptoms. Preclinical data support the use of ß1-specific ß-blockers such as metoprolol in case of coronary vasospasm.
THE YOUNG PATIENT WITH ‘ACUTE MYOCARDITIS’
MIMICKING ACUTE MYOCARDIAL INFARCTIONYoung patients, particularly males, with acute chest pain syndrome in whom CAD is very unlikely on the basis of their risk profile are a frequent clinical challenge in the emergency room. Such patients may even present with ST segment elevations in the resting ECG and elevated cardiac enzymes in their blood analysis, suggesting acute
ST elevation myocardial infarction. In such a scenario, acute coronary obstruction (for example, by spontaneous coronary dissection) as well as acute aortic dissection need to be first ruled out, for example, by invasive angiography and CT, respectively.
After obstructive CAD and aortic dissectionhave been excluded, the most important differential diagnosis in troponin positive patients is acute myocarditis.
Men are twice as likely as women to present
with clinical signs of acute myocarditis. Virus
genomes indicating myocarditis are commonly
found in patients clinically presenting with
a picture mimicking acute myocardial infarction,
but demonstrating normal coronary anatomy.
What is the cause of the patient’s chest pain and
ECG changes? Initially, it was thought that these
findings represented the effects of myocardial
damage caused by the inflammation and the virus.
Although it has been shown that peripheral and
coronary endothelial function is impaired in patients with myocardial virus persistence, and that this impairment is even more pronounced in the case of both myocardial virus persistence and inflammation, this does not explain the clinical symptom of resting chest pain in subjects with acute myocarditis.
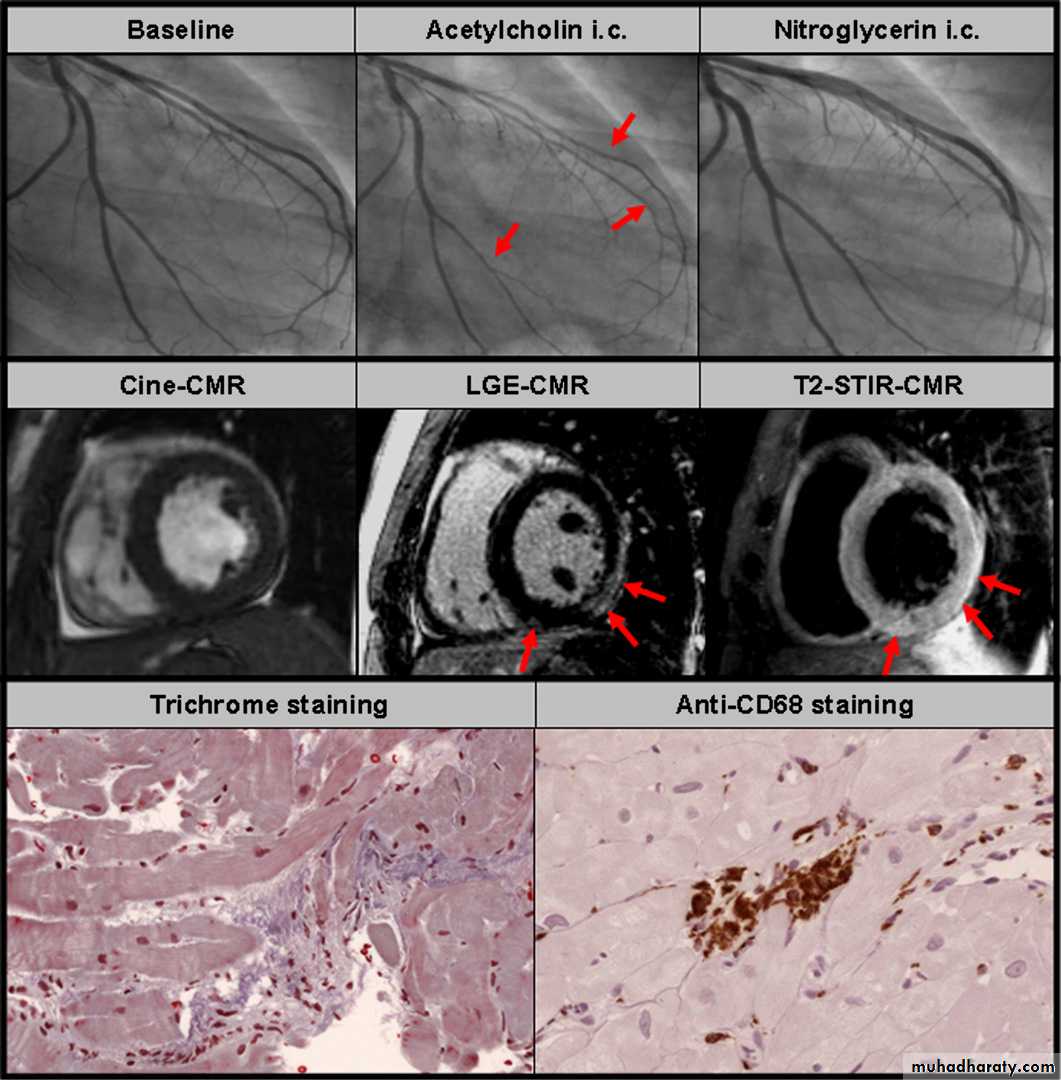
Recently, we were able to demonstrate that coronary vasospasm may explain the chest pain symptoms in patients with acute myocarditis, although other effects of viral inflammation may contribute to the clinical picture
(figure 8). Hence, myocardial inflammation or virus persistence, or both, may be associated with
or even cause a coronary vasomotility disorder,
enabling the occurrence of coronary vasospasm and
causing acute chest pain syndromes, particularly in
young patients with no risk factors for CAD.
Presently, there is no targeted specific therapy for
acute myocarditis complicated by coronary vasospasm, and current recommendations comprisemainly anti-anginal therapy with calcium antagonists
and nitrates in those patients with documented coronary vasospasm.
However, this type of acute vasospasm is a self-limiting disease and resting chest pain spontaneously subsides usually after a few days.
CONCLUSIONS
AP is a frequent clinical finding in patients withoutobstructive CAD. It may be caused by coronary
vasomotility disorders which comprise epicardial as
well as microvascular dysfunction. Traditional risk
factors (such as hypertension and diabetes) as well
as cardiomyopathies (such as hypertrophic cardiomyopathy) may be associated with functional as
well as structural changes such as endothelial
dysfunction, interstitial and perivascular fibrosis,
capillary rarefaction, and increased arterial stiffness.
These functional and structural changes are major
predisposing factors for the occurrence of both
epicardial coronary vasospasm and microvascular
disease, and cause the occurrence of AP in the
absence of obstructive CAD. Targeted therapy is
primarily aimed at eliminating the underlying risk
factor and/or disease.
However, treatment may be challenging, particularly if microvascular disease is the major problem.
Figure 1 Hypertensive heart disease involves disparate elements, ranging from
aortopathy to myocardial remodelling and even peripheral energy utilisation that interactto produce sequelae such as heart failure, arrhythmias, and ischaemic events. LA, left
atrium; LV, left ventricle. Reprinted with permission from Raman et al.
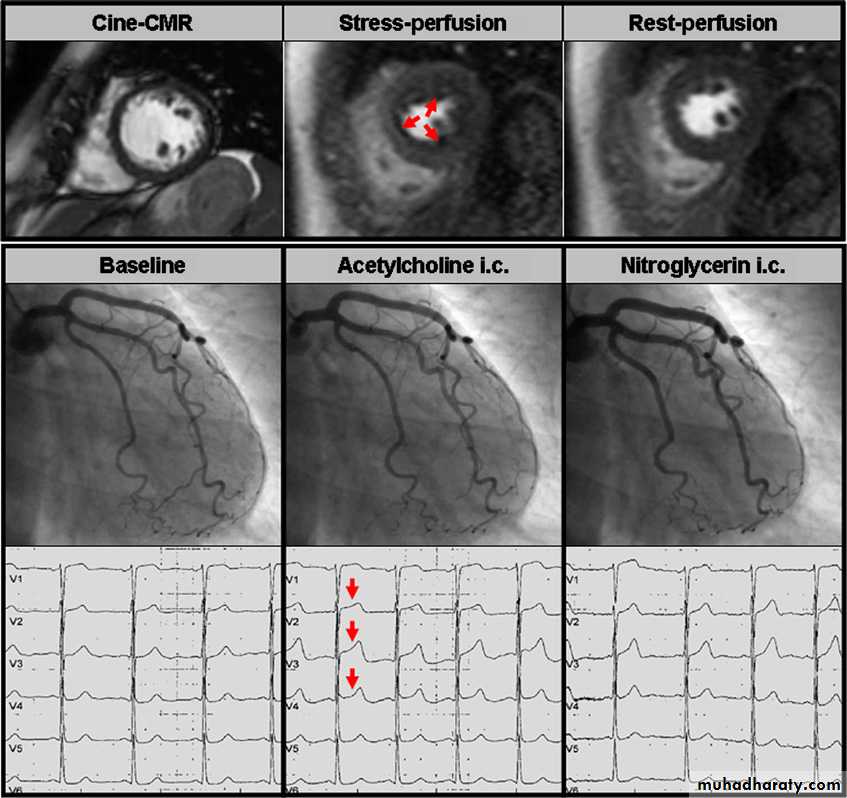
Figure 3 Upper panel: Cine, stress-perfusion, and rest-perfusion cardiac magnetic resonance (CMR) images of
a patient with unstable angina pectoris. A large subendocardial perfusion defect extending from the anteroseptal
segment to the inferoseptal segment was documented during adenosine stress (red arrows). Mid and lower panel: The
left coronary artery (as well as the right coronary artery, not shown) of this patient did not show any significant stenosis
at baseline. During intracoronary acetylcholine infusion there was no significant epicardial vasoconstriction; however,
the patient felt the same chest pain as she did at home and demonstrated ST segment elevation in leads V1eV3. After
infusion of glyceryl trinitrate the patient’s chest pain and the ECG changes disappeared while there was only a mild
epicardial vasodilation. Hence, microvascular disease was diagnosed in this patient.
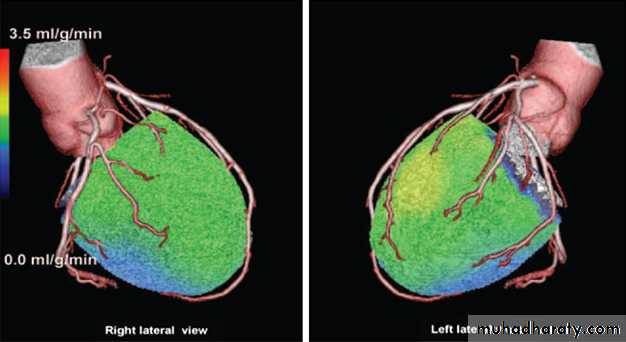
Figure 4 Positron emission tomography/CT hybrid images of a 63-year-old man with
suspected coronary artery disease, atypical chest pain, and 2 mm ST depression on theECG at the exercise test. In hybrid images, stress myocardial perfusion was reduced in
most regions (green and blue). However, both coronary CT angiography and invasive
coronary angiography showed normal coronary arteries, indicating possible microvascular disease.
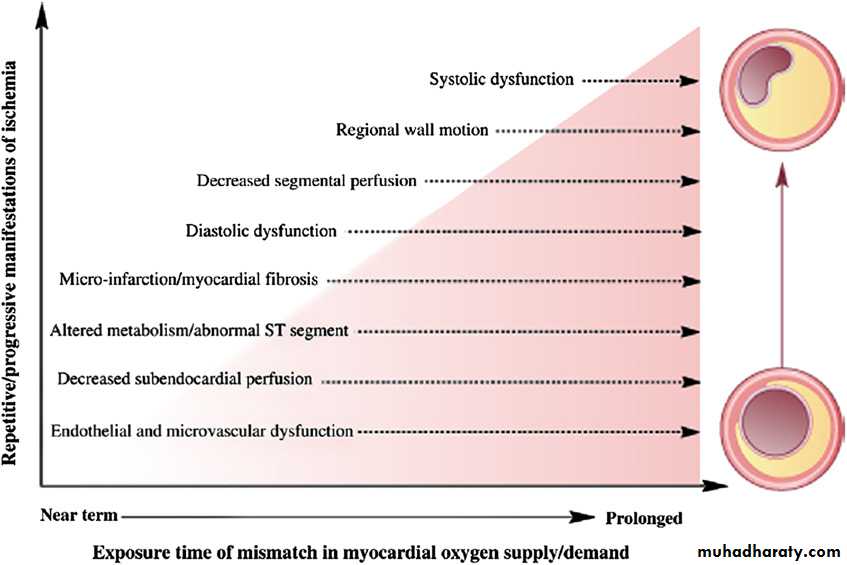
Figure 6 Cascade of mechanisms and manifestations of ischaemia having an impact on ischaemic heart disease risk
in women. Reprinted with permission from Shaw et al
Figure 7 Coronary angiograms of the left coronary artery at baseline, during increasing doses of acetylcholine infusion and after glyceryl trinitrate
(GTN) infusion in a patient with atypical angina. At baseline, minor non-obstructive atherosclerotic coronary wall irregularities are seen in the proximal
left anterior descending artery (LAD, red arrow). Angiography during invasive provocative testing with acetylcholine demonstrated a subtotal/total
occlusive spasm of the proximal LAD. After infusion of GTN, the LAD spasm and the patient’s chest pain quickly disappeared.
Figure 8 Young male patient with acute myocarditis presenting with a clinical picture of acute coronary syndrome. Upper panel: Baseline coronary
angiograms did not show any significant stenosis (only left coronary artery is shown). During intracoronary acetylcholine infusion diffuse epicardial
coronary vasospasm occurred (red arrows) in the left anterior descending artery (LAD) and the left circumflex artery (LCX) and the patient felt the same chest pain as he did at home. After intracoronary glyceryl trinitrate infusion the chest pain as well as the coronary vasospasm disappeared. Mid panel: Cine cardiac magnetic resonance (CMR) images revealed a normal systolic function. However, late gadolinium enhancement (LGE) and T2 weighted oedema CMR images were suggestive of acute myocarditis with myocardial damage in the subepicardium of the left ventricular free wall (red arrows). Lower panel: Endomyocardial biopsies were taken from the left ventricular free wall and trichrome staining revealed accumulation of inflammatory cells and essential structural abnormalities indicative of myocarditis. Immunohistochemical staining with anti-CD68 antibodies proved the accumulation of macrophages and confirmed the diagnosis of acute myocarditis (courtesy of Professor K Klingel and Professor R Kandolf from the University of Tu¨bingen).
Angina pectoris in patients with normal coronary angiograms: key points