15-16
د. بسام
-
كليه طب نينوى
1
Porphyrins
Porphyrins (Porphins) are cyclic tetrapyrol compounds formed by the linkage
of four pyrrole rings through methenyl bridges ((
HC
)).
In
the reduced
porphyrins
(
Porphyrinogens) the linkage of four pyrrole rings (tetrapyrol)
through methylene bridges
((
CH
2
))
The characteristic property of porphyrins is the formation of complexes with
the metal ion bound to nitrogen atoms of the pyrrole rings.
e.g. Heme (iron porphyrin).
Proteins which contain heme ((hemoproteins)) are widely distributed e.g.
Hemoglobin, Myoglobin, Cytochromes, Catalase & Tryptophan pyrrolase.
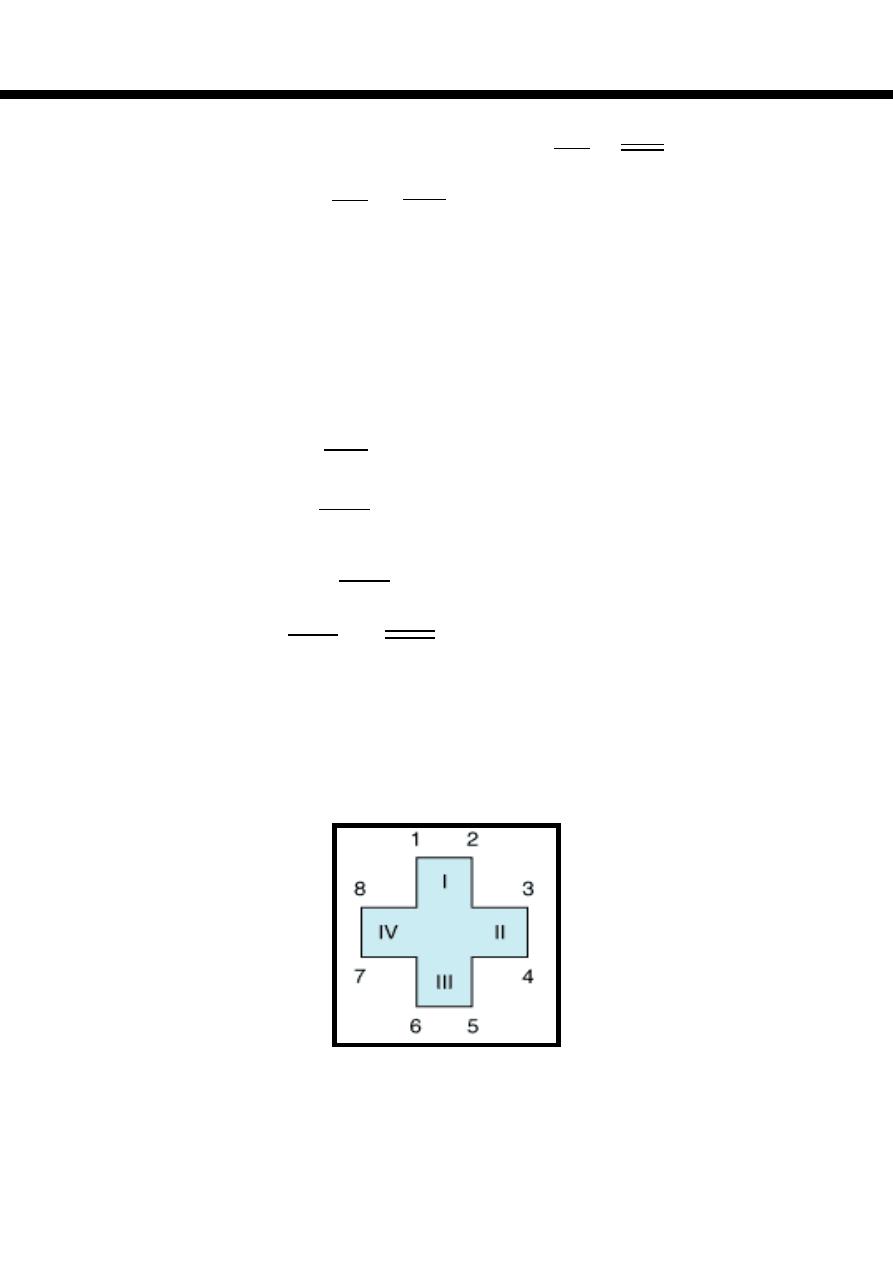
Natural porphyrins have substituent side chains on the eight hydrogen atoms
numbered on the pyrrole rings.
These side chains are:
1-Methyl-group (M)… ((
CH
3
))
2-Acetate-group (A)… ((
CH
2
COOH
))
3-Propionate-group (P)… ((
CH
2
CH
2
COOH
))
4-Vinyl-group (V)… ((
CH
CH
2
))
Porphyrins with asymmetric arrangement of the side chains are classified as
type III porphyrins while those with symmetric arrangement of the side chains
are classified as type I porphyrins.
Only types I & III are present in nature & type III series is more important
because it includes heme.

15-16
د. بسام
-
كليه طب نينوى
2
Heme Biosynthesis
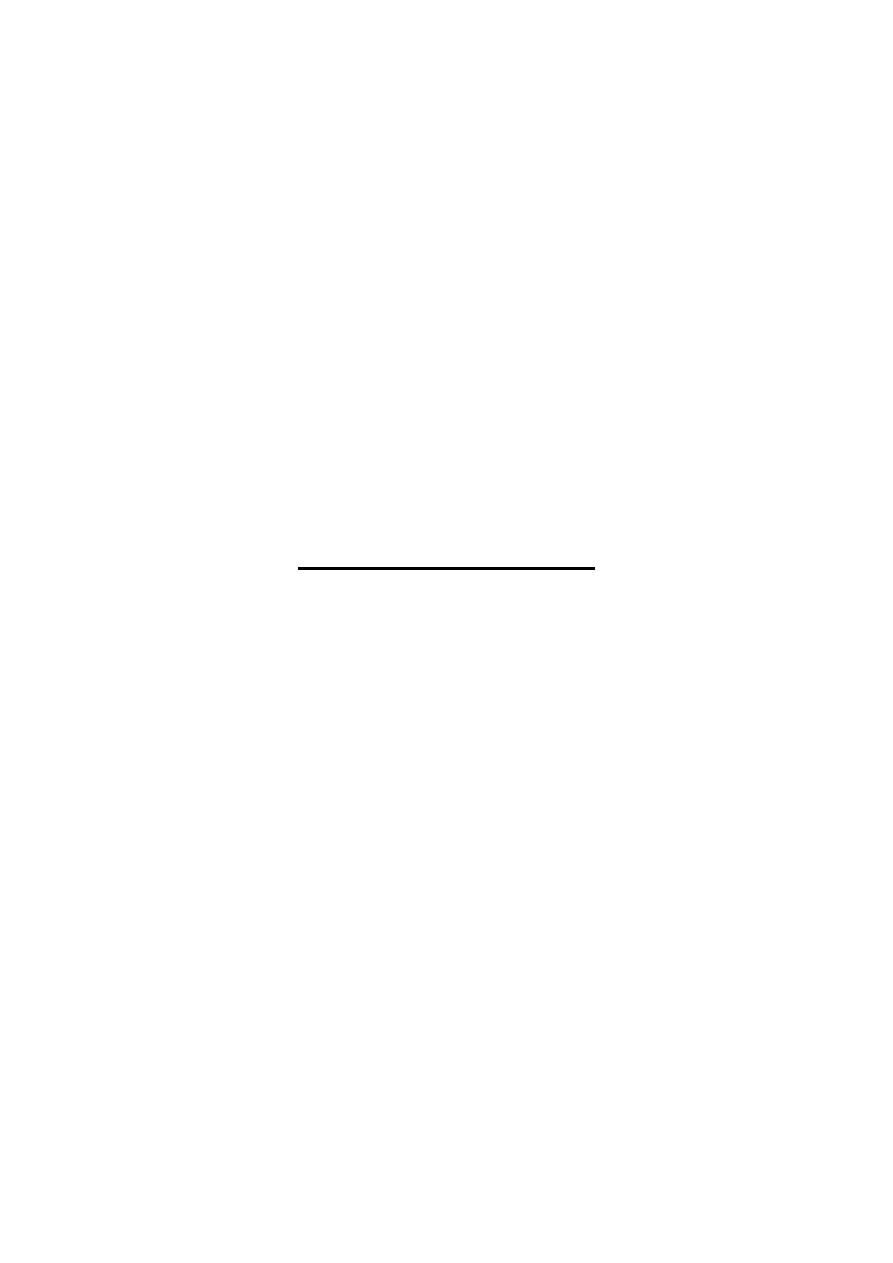
Heme biosynthesis occurs through the following steps:
1-The starting reaction is the condensation between succinyl-CoA ((derived from
citric acid cycle in the mitochondria)) & glycine forming δ-aminolevulinate
(ALA), this reaction is a rate limiting reaction in the hepatic heme synthesis, it
occurs in mitochondria & is catalyzed by ALA synthase(Aminolevulinate
synthase) enzyme in the presence of pyridoxal phosphate as a cofactor.
2-In the cytoplasm condensation reaction between two molecules of ALA is
catalyzed by ALA dehydratase enzyme to form two molecules of water & one
molecule of porphobilinogen (PBG) which is a precursor of pyrrole.
ALA dehydratase is inhibited by lead as occurred in lead poisoning.
3-Condensation of four molecules of PBG with loss of four molecules of
ammonia lead to formation of linear tetrapyrrole called hydroxymethylbilane
(HMB), this reaction is catalyzed by uroporphyrinogen I synthase (PBG
deaminase, HMB synthase)
enzyme.
HMB cyclizes spontaneously to form
uroporphyrinogen I or:
4- HMB converted to uroporphyrinogen III by the action of uroporphyrinogen
III synthase enzyme, under normal conditions the uroporphyrinogen formed is
almost exclusively the III isomer.
Both of these uroporphyrinogens (I & III) contain A & P side chains & are
colorless compounds, however, they readily reoxidized to their respective
colored porphyrins (as in all porphyrinogens) by light with loss of six hydrogen
atoms.
5-Uroporphyrinogen I & III are converted into coproporphyrinogen I & III by
decarboxylation (loss of four CO
2
) of all of the acetate (A) groups to be
converted into methyl (M) groups, this reaction is catalyzed by
uroporphyrinogen decarboxylase enzyme.
15-16
د. بسام
-
كليه طب نينوى
3
6-Coproporphyrinogen III then enters the mitochondria to be converted into
protoporphyrinogen III by oxidative decarboxylation of two (P) side chains into
(V) side chains, this reaction is catalyzed by coproporphyrinogen oxidase
enzyme, this enzyme acts only on coproporphyrinogen III, therefore,
coproporphyrinogen I is not further progressed.
7-Protoporphyrinogen III is oxidized into Protoporphyrin III ((loss of six
hydrogen atoms), this reaction is catalyzed by protoporphyrinogen oxidase
enzyme & it occurs in the mitochondria, however, this conversion can be induced
by light in vitro.
8-The final step is the formation of heme by the incorporation of ferrous iron into
protoporphyrin III in a reaction catalyzed by ferrochelatase (heme synthase)
enzyme, this reaction occurs in the mitochondria.
Heme synthesis occurs in most human cells with the exception of mature RBC
because it does not contain mitochondria, however, about 85% of heme synthesis
occurs in erythroid precursors cells in the bone marrow to form hemoglobin &
the majority of the remainder in the hepatocytes to form other hemoproteins
mainly cytochrome P450.
ALA Synthase {ALAS}
Two main forms:
1-Hepatic((ALAS 1)).. It is a regulatory (rate limiting) enzyme in the hepatic
heme synthesis, heme acts as a negative regulator for this enzyme.
Many drugs are porphyrogenic as barbiturates which can result in a marked
increase of ALAS 1 activity by utilizing cytochrome P450 which in turn reduces
the intracellular heme concentration lead to the activation of ALAS 1.
Other factors can lead to marked decrease of ALAS 1 activity as:
A-Excess glucose which cause reduction of fat burn in which toxins are stored,
therefore, excess glucose reduces toxin release so that the consumption of
cytochrome P450 is reduced.
B- Hematin (oxidized form of heme) leads to increase of intracellular heme
concentration result in marked decrease of ALAS 1 activity.
2-Erythroid((ALAS 2)).. Unlike ((ALAS 1)), it is not affected by drugs & does
not undergoes feedback regulation by heme.
15-16
د. بسام
-
كليه طب نينوى
4
15-16
د. بسام
-
كليه طب نينوى
5
Properties of Porphyrins
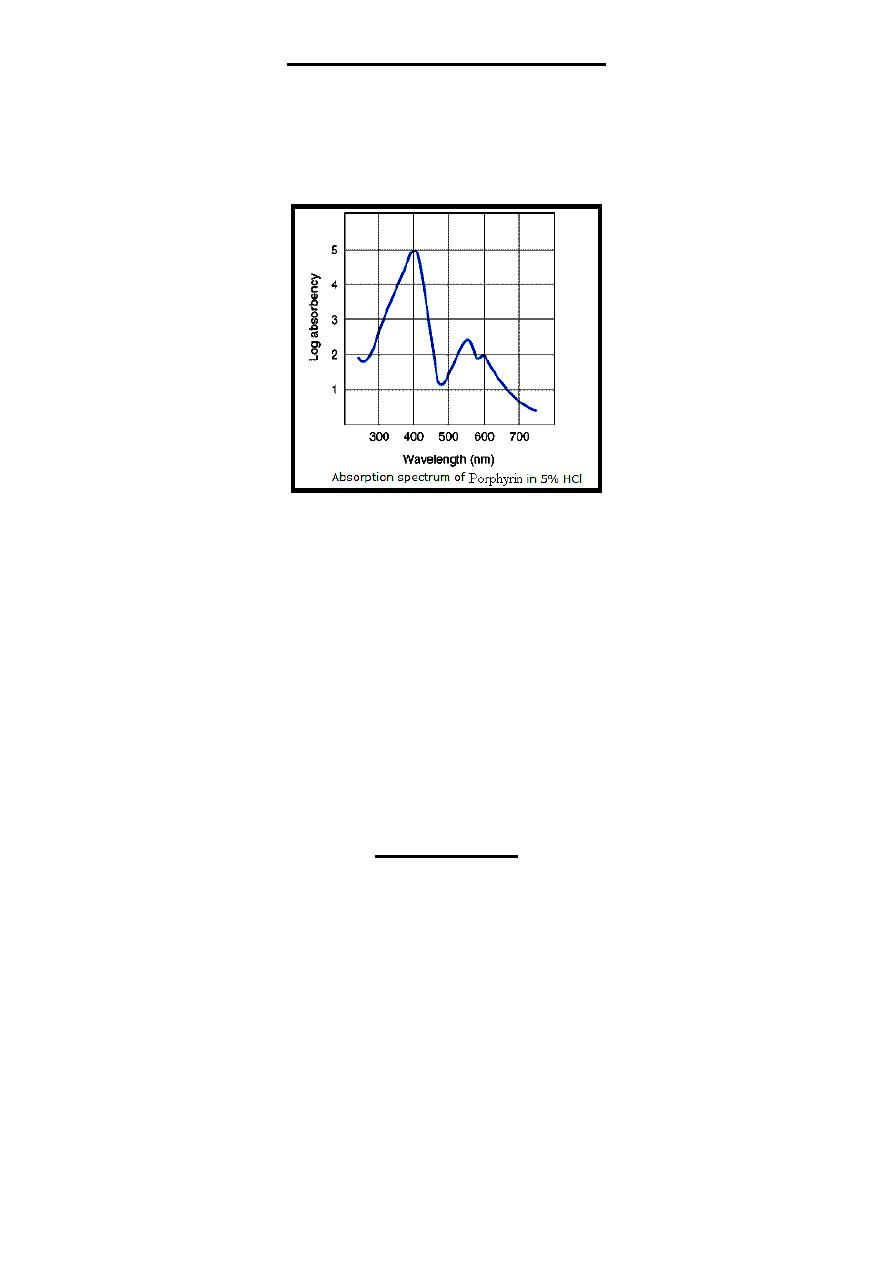
1-The various porphyrinogens are colorless, where as the various porphyrins are
colored because it contains double bonds joining pyrrole rings which is absent in
porphyrinogens, therefore, all porphyrins ((in 5% HCl)) but not porphyrinogens
have a significant sharp absorbance band near 400 nm wavelength, this band is
called Soret band ((discovered by the French physicist; Charles Soret))
2-Porphyrins when illuminated by ultraviolet light, they emit a strong red
fluorescence because it contains double bonds joining pyrrole rings while
porphyrinogens (because of absence of double bonds joining pyrrole rings) they
have no fluorescence.
3-Coproporphyrins, uroporphyrins & protoporphyrins are of clinical importance
because they are excreted in increased amounts in the porphyrias, these
compounds when present in feces and/or urine; can be extracted & quantified
using spectrophotometric methods.
Generally uroporphyrins are present in urine; coproporphyrins are present in
urine & feces while protoporphyrins are present in feces.
4-ALA & PBG can measured in urine by colorimetric method.
Porphyrias
Porphyrias are group of disorders due to abnormalities of the heme biosynthesis
pathway, they can be genetic or acquired.
They are not prevalent; however, their importance lies in the differential
diagnosis of certain clinical presentations as abdominal pain, neuropsychiatric &
dermatological findings.
Seven types of genetic porphyrias were found according to enzyme involved:
Generally porphyria is inherited as autosomal dominant except congenital
erythropoietic porphyria is inherited as autosomal recessive.

15-16
د. بسام
-
كليه طب نينوى
6
Clinical Features of Porphyria
The clinical features of porphyria can result from a deficiency of metabolic
products beyond the enzymatic block and/or from accumulation of metabolites
before the block, the main clinical features are some or all of the followings:
1- Abdominal pain.
2- Neuropsychiatric presentations.
2- Photosensitivity: When porphyrinogens are accumulated in the skin, it forms
oxygen free radicals lead to photosensitivity.
Classifications of Porphyrias
*I-According to organs that are mostly affected:
A-Erythropoietic porphyrias: heme synthesis in the bone marrow was affected
as in congenital erythropoietic porphyria & protoporphyria.
B-Hepatic porphyrias: heme synthesis in the liver was affected as in ALA
dehydratase deficient porphyria, acute intermittent porphyria, porphyria cutanea
tarda, hereditary coproporphyria & variegates porphyria.
*II-According to clinical presentations:
A-Acute porphyrias: Patient has abdominal pain & neuropsychiatric symptoms.
B-Cutaneous porphyrias: Patient has photosensitivity.
C-Latent porphyrias: Patient is relatively symptoms-free between the attacks.
*III-According to cause:
A-Genetic porphyrias: Inherited as autosomal dominant except congenital
erythropoietic porphyria which inherited as autosomal recessive.
B-Acquired porphyrias: as in lead poisoning can affect ALA dehydratase.
15-16
د. بسام
-
كليه طب نينوى
7
Diagnosis of Porphyrias
1-Clinical & family history.
2-Physical examination.
3-Laboratory tests include:
A-Measure enzyme activity in blood.
B-Urine test for uroporphyrins & coproporphyrins.
C-Stool test for protoporphyrins.
D-Urine test for ALA & PBG.
Treatment of Porphyrias
1-Gene therapy.
2-Avoid porphyrogenic drugs as barbiturates.
3- Administration of excess glucose & hematin that cause block of ALAS1.
4-Administration of β-carotene which is antioxidant to reduce oxygen free
radicals formation, therefore, reduces photosensitivity.
5-Use of sunscreen that filter out visible light preventing the exposure of light
with wave length 400 nm.
6-Sever pain is treated by analgesia.
Heme Catabolism
Normally, senescent red blood cells & heme from other sources are engulfed
by the cells of the reticuloendothelial system.
-The globins is recycled or converted into amino acids.
-Heme is converted into biliverdin ((green pigment)) with the release of ferric
iron & CO ((only reaction in the body that is known to produce CO)).
Finally by the action of biliverdin reductase biliverdin was converted into
bilirubin ((yellow pigment)) which transported to liver for further metabolism.
The daily bilirubin formation in human adult is about 250-350 mg, derived
mainly from hemoglobin.
Bilirubin
Transport of Bilirubin
Bilirubin is mildly soluble in aqueous media (as blood), however, its solubility
is increased by binding to serum albumin.
Each molecule of albumin has one high-affinity site & one low-affinity site for
bilirubin binding. In 100 mL of blood, approximately 20-25 mg of bilirubin can
be tightly bound to albumin at its high-affinity site, bilirubin in excess of this
quantity can be bound only loosely on low-affinity site & thus can easily be
detached & diffuse into tissues specially CNS causing kernicterus.
15-16
د. بسام
-
كليه طب نينوى
8
A number of drugs such as antibiotics compete with bilirubin for the high-affinity
binding site on albumin. Thus, these compounds can displace bilirubin from
albumin & so it has significant clinical effects.
Metabolism of Bilirubin
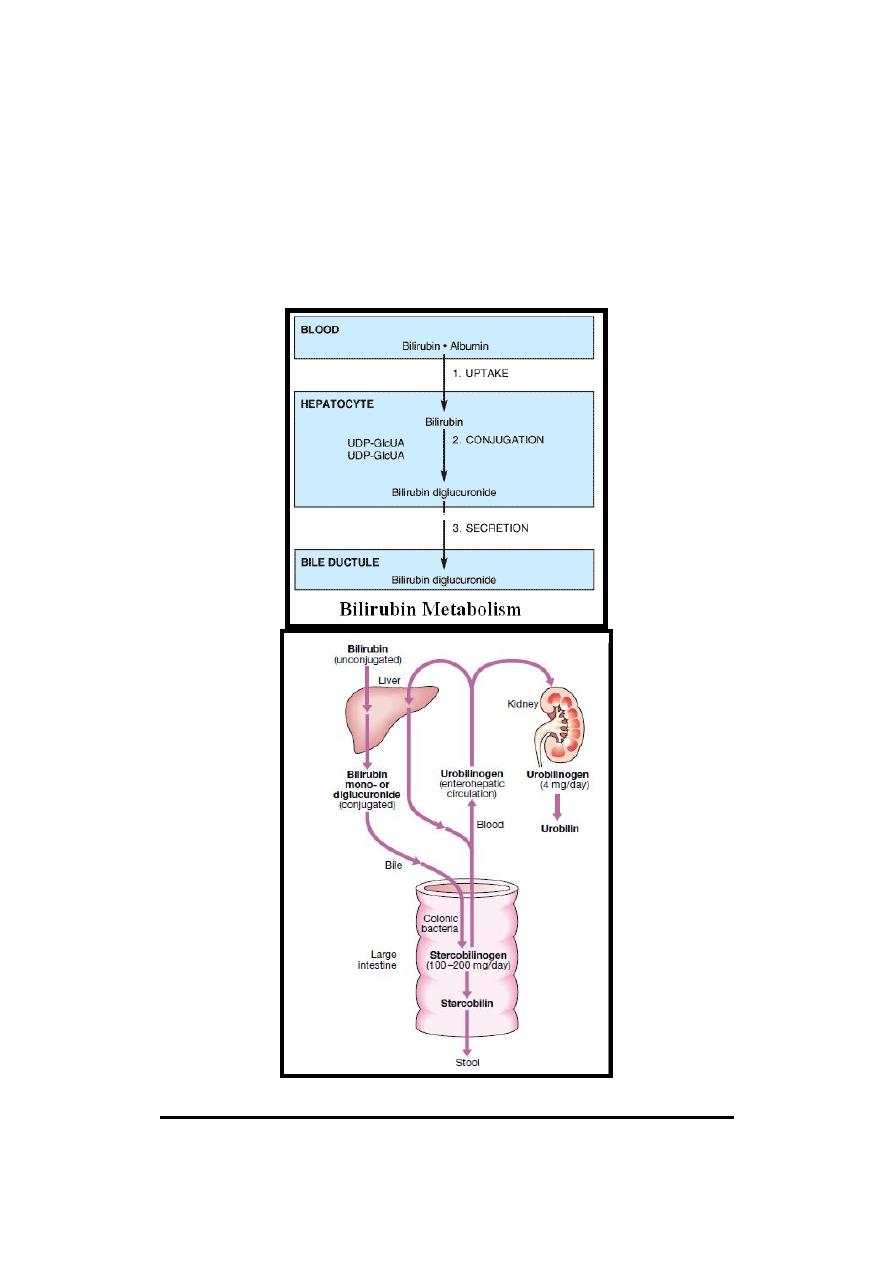
The metabolism of bilirubin occurs through the following sequential steps:
(1) Uptake of Bilirubin.
(2) Conjugation of Bilirubin.
(3) Secretion of Bilirubin into Bile.
(1) Uptake of Bilirubin
In the liver, bilirubin is removed from albumin & taken up by hepatocytes.
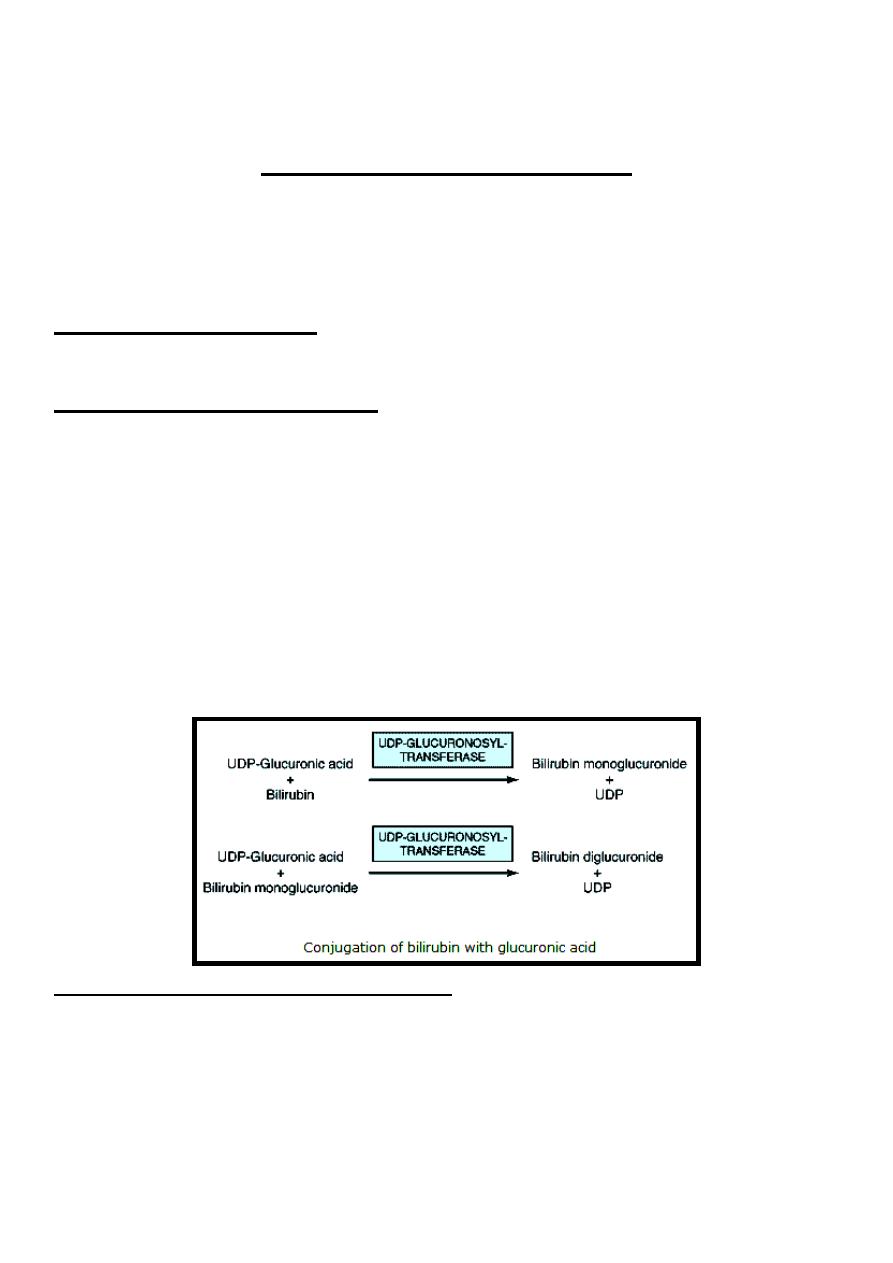
(2) Conjugation of Bilirubin
Bilirubin is nonpolar & would persist in liver cells if not rendered water-
soluble. Hepatocytes convert bilirubin to a polar form, which is readily excreted
in the bile, by adding glucuronic acid molecules to it, this process is called
conjugation
that
catalyzed
by
a
specific
enzyme
called
UDP-
glucuronosyltransferase (uridine diphosphate-glucuronosyltransferase) or
called
Bilirubin-UGT
(bilirubin-uridine
diphosphate
glucuronosyltransferase), this enzyme transfer glucuronate from UDP-
glucuronic acid to bilirubin forming bilirubin monoglucuronide which is an
intermediate & is subsequently converted to the bilirubin diglucuronide.
UDP-glucuronosyltransferase enzyme activity can be induced by a number of
clinically useful drugs as phenobarbital.
(3) Secretion of Bilirubin into Bile
Secretion of conjugated bilirubin into the bile is an active process & it is
probably the rate-limiting for hepatic bilirubin metabolism.
The hepatic transport of conjugated bilirubin into the bile is induced by the
same drugs that are capable of inducing the conjugation of bilirubin.
15-16
د. بسام
-
كليه طب نينوى
9
As the conjugated bilirubin reaches the terminal ileum & large intestine, it
converted by intestinal bacterial flora to a group of colorless tetrapyrrolic
compounds called urobilinogen.
In terminal ileum & large intestine a small fraction of urobilinogen is
reabsorbed & reexcreted by liver to constitute the enterohepatic urobilinogen
cycle while only very trace amount of urobilinogen appears in urine.
Normally, most of the colorless urobilinogen in the colon (in GIT urobilinogen
is called stercobilinogen) are oxidized to a colored compound known as urobilin
(in GIT urobilin is called stercobilin) & are excreted in the feces giving the
brown color of stool.
Reference Ranges of Serum Bilirubin
In adult, the reference ranges of serum bilirubin are:
Total Bilirubin = 0.3-1 mg/dL (5.1-17 µmol/L)
15-16
د. بسام
-
كليه طب نينوى
10
Unconjugated (Indirect) Bilirubin = 0.2-0.7 mg/dL (3.4 -12 µmol/L)
Conjugated (Direct) Bilirubin = 0.1-0.3 mg/dL (1.7 - 5.1 µmol/L)
However, in newborn, the reference ranges is usually higher than adult.
Hyperbilirubinemia
Hyperbilirubinemia exists when serum total bilirubin concentration exceeds the
upper limit of its reference range [1 mg/dL (17µmol/L)] & when it reaches a
certain concentration (approximately 2.5-3 mg/dL); it diffuses into the tissues,
which then become yellow. This condition is called jaundice or icterus.
Hyperbilirubinemia may be due to:-
1- Overproduction of bilirubin above the capacity of the normal liver to
metabolize it (Pre-hepatic hyperbilirubinemia) as in hemolysis.
2- Failure of a damaged liver to conjugate bilirubin produced in normal amounts
(Hepatic hyperbilirubinemia).
3- Obstruction of the excretory ducts of the liver that preventing the excretion of
bilirubin (Post-hepatic hyperbilirubinemia).
Depending on the type of bilirubin present in plasma whether unconjugated
(indirect) and/or conjugated (direct), hyperbilirubinemia may be classified as:
1- Unconjugated Hyperbilirubinemia (Retention Hyperbilirubinemia):
Due
to overproduction of unconjugated bilirubin lead to:
A- Elevated level of serum unconjugated bilirubin (Indirect bilirubin) above its
upper reference limit (in adult; 0.7 mg/dL).
B- Since unconjugated bilirubin is hydrophobic, it can cross the blood-brain
barrier into CNS (especially when serum unconjugated bilirubin level reach 20-
25 mg/dL) causing encephalopathy, this condition called kernicterus.
C- Since unconjugated bilirubin is hydrophobic, it does not appear in urine.
Accordingly, acholuric jaundice (absence of bilirubin in the urine) occurs.
D- The increased production of bilirubin leads to increased production of
urobilinogen which appears in the urine in large amounts (excess urobilinoginuria)
while normally there are very traces amount of urobilinogen in the urine.
2-Conjugated Hyperbilirubinemia (Regurgitation Hyperbilirubinemia): Due
to reflux of conjugated bilirubin into the bloodstream lead to:
A- Elevated level of serum conjugated bilirubin (Direct bilirubin) above its upper
reference limit (in adult; 0.3 mg/dL).
B- Since conjugated bilirubin is hydrophilic, it cannot cross the blood-brain
barrier so that there is no development of kernicterus.
C- Since conjugated bilirubin is hydrophilic & cannot be excreted (due to biliary
obstruction). It thus regurgitates from the liver & appears in urine. Accordingly,
choluric jaundice (presence of bilirubin in the urine) occurs, clinically the color
of choluric urine is described as a tea-color.
D- Since bilirubin has no access to the intestine (due to biliary obstruction) where
it can be converted to urobilinogen, therefore, no urobilinogen (stercobilinogen)

15-16
د. بسام
-
كليه طب نينوى
11
is found in urine & feces, therefore, no urobilin (stercobilin) in the stool, the stool
is clinically described as pale-stool.
3- Unconjugated & conjugated hyperbilirubinemia: Due to defect in both
conjugation & secretion of bilirubin as occur mainly in hepatitis.
Examples of Unconjugated Hyperbilirubinemia
:
1)- Neonatal (Physiologic) Jaundice.
2)- Hemolytic Anemias.
3) - Type I Crigler-Najjar Syndrome.
4)- Type II Crigler-Najjar Syndrome.
5)-Gilbert Syndrome.
6)-Toxic Hyperbilirubinemia.
Examples of Conjugated Hyperbilirubinemia:
1)-Obstruction of Biliary Tree (Cholestatic Jaundice).
2)-Dubin-Johnson Syndrome.
3)-Rotor Syndrome.
Neonatal (Physiologic) Jaundice
This transient condition is the most common cause of unconjugated
hyperbilirubinemia in neonate. It results from an accelerated hemolysis around
the time of birth & an immature hepatic system for bilirubin metabolism. Since
the increased amount of bilirubin is unconjugated, it's capable of penetrating the
blood-brain barrier when its concentration in plasma exceeds that which can be
tightly bound by albumin (20–25 mg/dL) causing kernicterus.
Treatment
1- Phenobarbital administration because it induce bilirubin-metabolizing system.
2- Exposure to blue light (phototherapy) promotes hepatic excretion of
unconjugated bilirubin.
3- Exchange blood transfusion when plasma bilirubin exceeds that which can be
tightly bound by albumin (20–25 mg/dL) to avoid kernicterus.
