THE METABOLIC RESPONSE TO INJURY
By Dr. Housam SousanHomeostasis
The stability of the “milieu intérieur”i.e. body systems act to maintain internal constancy
The more severe the injury, the greater the response
Physiological changesMetabolic changes
Immunological changes/sequelae.
Elective surgery of intermediate severity
Transient and modest rise intemperature,
heart rate,
respiratory rate,
energy expenditure and
peripheral white cell count
Major trauma/Sepsis
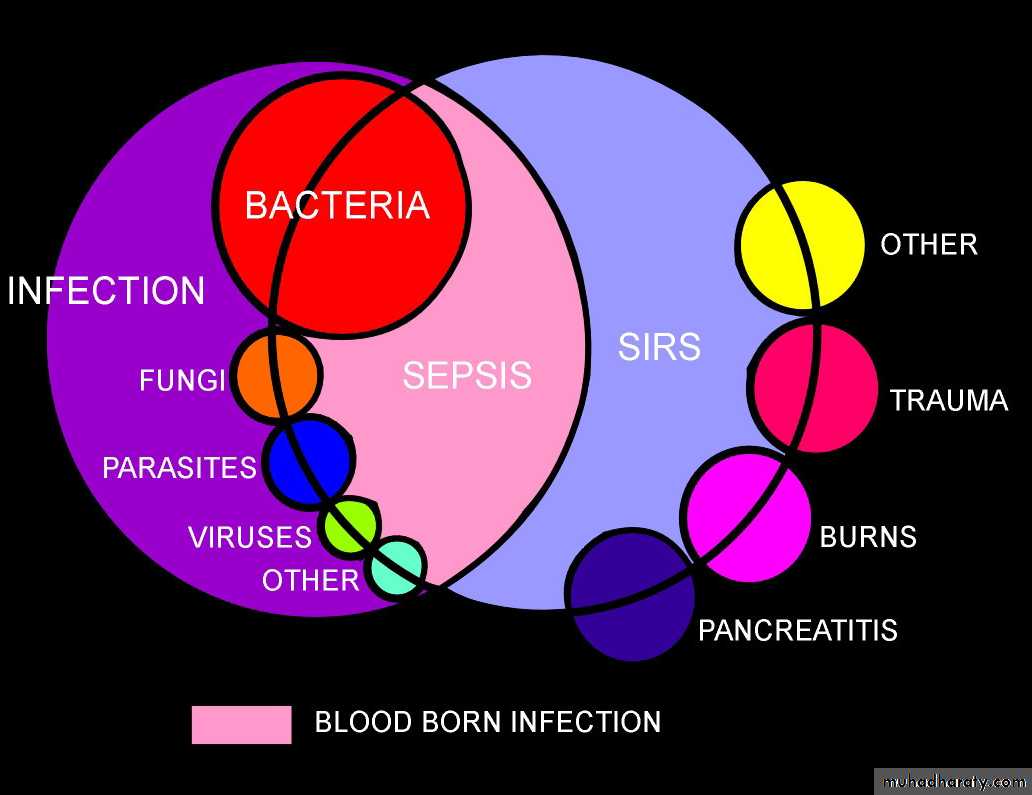
Systemic inflammatory response syndrome (SIRS),
hypermetabolism,
marked catabolism,
shock
multiple organ dysfunction (MODS).
Copyright© medicalstudies.in
• Fever of more than 38°C (100.4°F) or less than 36°C (96.8°F)
• Heart rate of more than 90 beats per minute• Respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension (PaCO2) of less than 32mm Hg
• Abnormal white blood cell count (>12,000/µL or < 4,000/µL or >10% immature [band] forms)
We define SIRS if 2 or more of the following variables are present
SIRS is nonspecific and can be caused by ischemia, inflammation, trauma, infection, or several insults combined. Thus, SIRS is not always related to infection.Infection is defined as
Microbial phenomenon characterized by an inflammatory response to the microorganisms or
The invasion of normally sterile tissue by those organisms."
Bacteremia is the presence of bacteria within the bloodstream, but this condition does not always lead to SIRS or sepsis.
Sepsis is the systemic response to infection and is defined as the presence of SIRS in addition to a documented or presumed infection.
Severe sepsis meets the aforementioned criteria and is associated with Multiorgan dysfunction, hypoperfusion, or hypotension.
Sepsis-induced hypotension is defined as "the presence of a systolic blood pressure of less than 90 mm Hg or a reduction of more than 40 mm Hg from baseline in the absence of other causes of hypotension.
Patients meet the criteria for Septic shock if they have persistent hypotension and perfusion abnormalities despite adequate fluid resuscitation.
MODS is a state of physiologic derangements in which organ function is not capable of maintaining homeostasis.
Stage I
Following an insult, local cytokine is produced with the goal of inciting an inflammatory response, thereby promoting wound repair and recruitment of the reticular endothelial systemStage II
Small quantities of local cytokines are released into the circulation to improve the local response. This leads to growth factor stimulation and the recruitment of macrophages and platelets. This acute phase response is typically well controlled by a decrease in the proinflammatory mediators and by the release of endogenous antagonists; the goal is homeostasis.Stage III
If homeostasis is not restored, a significant systemic reaction occurs. The cytokine release leads to destruction rather than protection. A consequence of this is the activation of numerous humoral cascades and the activation of the reticular endothelial system and subsequent loss of circulatory integrity. This leads to end-organ dysfunction.
Respiratory failure, acute respiratory distress syndrome (ARDS), and nosocomial pneumonia
Renal failureGastrointestinal (GI) bleeding and stress gastritis
Anemia
DVT
Intravenous catheter–related bacteremia
Electrolyte abnormalities
Hyperglycemia
Disseminated intravesicular coagulation (DIC)
MEDIATORS OF THE METABOLIC RESPONSE TO INJURY
Neuroendocrine pathwaysafferent nociceptive neurones,
the spinal cord,
thalamus,
hypothalamus and
pituitary
Neuroendocrine response
Hypothalamus Corticotrophin-releasing factor (CRF) anterior pituitary ACTH adrenal cortisol
Neuroendocrine response
Hypothalamic activation of the sympathetic nervous system causesrelease of adrenaline
release of glucagon.
Neuroendocrine response
Insulin release and sensitivity( resistance).Hypersecretion of prolactin and growth hormone (GH).
Inactivation of peripheral thyroid(T3) hormones.
Inactivation of gonadal function.
1. neuroendocrine response
Nervous System
Endocrine System
Copyright© medicalstudies.in
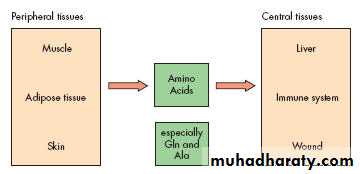
Starvation & Trauma
Skeletal muscle
Amino acidsAdipose tissue
Protein breakdown
Triglyceride
Glycerol & FFAGlycerol
FFA
Glucose
Lactate from tissues
Amino
Acids
Glucose Synthesis
Liver
Sepsis
Skeletal muscle
Amino acidsAdipose tissue
Protein breakdown
Triglyceride
Glycerol & FFAGlycerol
FFA
Glucose
Lactate from tissues
Amino
Acids
Glucose Synthesis
Liver
Glycogen
Ketone BodiesKetone
BodiesInsulin βcells decrease
TRH hypothalamus decreaseTSH anterior pituitary decrease
T3, T4 Thyroid decrease
GhRH hypothalamus decrease
Hormone Secretion site Change
-Endorphine anterior pituitary increaseADH hypothalamus increase
Aldosterone Adrenal cortex increase
Glucagon cells increase
Prolactin anterior pituitary increase
Growth hormone anterior pituitary acute↑, chronic↓
other hormonal responses to stress
Stress
Neuroendocrine response
Finally, hyperglycaemia may aggravate the inflammatory response viasubstrate overflow in the mitochondria,
causing the formation of excess free oxygen radicals and also
Altering gene expression to enhance cytokine production.
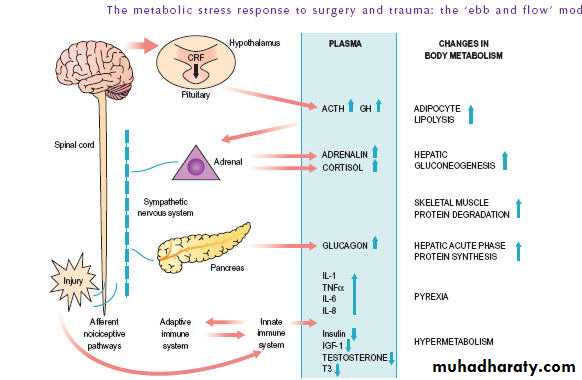
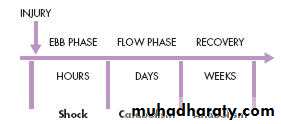
THE METABOLIC STRESS RESPONSE TOSURGERY AND TRAUMA: THE ‘EBB ANDFLOW’MODEL
The Ebb phase is characterised byhypovolaemia,
decreased basal metabolic rate,
reduced cardiac output,
hypothermia and
lactic acidosis
THE ‘EBB AND FLOW’ MODEL
Physiological response to injury.The natural response to injury includes:
■ Immobility/rest
■ Anorexia
■ Catabolism
The changes are designed to aid survival in case of moderate injury in the absence of medical intervention.
Ebb phase
The main physiological role of the ebb phase is to conserve both
1-Circulating volume and
2-Energy stores
for recovery and repair
The predominanthormones regulating the ebb phase
CatecholaminesCortisol
Aldosterone
Following resuscitation, the ebb phase evolves into a hypermetabolic flow phase, which corresponds to the SIRS
Flow phase characterized by
tissue oedemavasodilatation and
increased capillary leakage,
increased basal metabolic rate (hypermetabolism),
increased cardiac output,
raised body temperature,
leucocytosis,
increased oxygen consumption and
increased gluconeogenesis.
Hypermetabolism
Trauma patients demonstrate energy expenditures approximately 15–25% above predicted healthy resting valuesCentral thermodysregulation (caused by the pro-inflammatory cytokine cascade
Increased sympathetic activity,
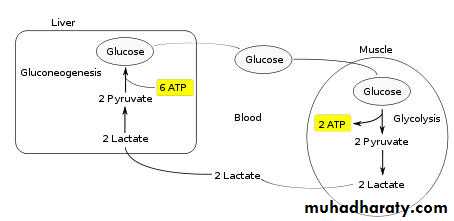
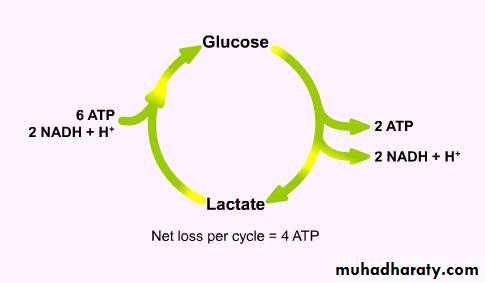
Ischaemic areas produce lactate,
which must be metabolised by the adenosine triphosphate (ATP)-consuming hepatic Cori cycle;
Copyright© medicalstudies.in
Copyright© medicalstudies.in
Several features of standard intensive care counteract the hypermetabolic driving forces of the stress response
including
1-Bed rest,
2-Paralysis,
3-Ventilation and
4-External temperature regulation
Alterations in skeletal muscle protein metabolism
Normally - synthesis equals breakdown and muscle bulk remains constant.Physiological stimuli
Feeding - extracellular amino acid concentration
Exercise
Paradoxically, during exercise, skeletal muscle protein synthesis is depressed, but it increases again during rest and feeding.
Alterations in skeletal muscle protein metabolism
Catabolic phase of the stress response,Muscle protein degradation
Major site peripheral skeletal muscle
Nitrogen losses also occur in
Respiratory muscles (predisposing the patient to hypoventilation and chest infections)
gut (reducing gut motility).
Cardiac muscle appears to be mostly spared
Alterations in skeletal muscle protein metabolism
Extreme conditions of catabolism (e.g. Major sepsis)
Urinary nitrogen losses can reach 14–20 g day
This is equivalent to the loss of 500 g of skeletal muscle per day
Muscle catabolism cannot be inhibited fully by providing artificial nutritional support as long as the stress response continues
Alterations in skeletal muscle protein metabolism
The predominant mechanism involved in the wasting of skeletal muscle is the ATP-dependent ubiquitin–proteasome pathwayalthough the lysosomal cathepsins and the calcium– calpain pathway play facilitatory and accessory roles.
Skeletal muscle wasting
Provides amino acids for protein synthesis in central organs/tissuesIs mediated at a molecular level mainly by activation of the ubiquitin–proteasome pathway
Can result in immobility and contribute to hypostatic pneumonia and death if prolonged and excessive
Alterations in hepatic protein metabolism:
The acute phase protein response (APPR)The liver and skeletal muscle together account for > 50% of daily body protein turnover.
Skeletal muscle has a large mass but a low turnover rate (1–2% /day),
whereas the liver has a relatively small mass (1.5 kg) but a much higher protein turnover rate (10–20% /day).
The acute phase protein response (APPR)
Hepatic protein synthesis is divided roughly 50:50 between renewal of structural proteins and synthesis of export proteins.
Albumin is the major export protein produced by the liver and is renewed at the rate of about 10% /day.
The acute phase protein response (APPR)
Albumin TER (transcapillary escape rate) may be increased threefold following major injury/sepsis.short-term changes in albumin concentration are most probably due to increased vascular permeability.
cytokines, in particular IL-6, promote the hepatic synthesis of positive acute phase proteins, e.g. fibrinogen and C-reactive protein (CRP).
The hepatic acute phase response represents a reprioritisation of body protein metabolism towards the liver and is characterised by:
Positive reactants (e.g. CRP): plasma concentration
Negative reactants (e.g. albumin): plasma concentration
Insulin resistance
Following surgery or trauma, postoperative hyperglycaemia develops as a result of increased glucose production combined with decreased glucose uptake in peripheral tissues.Decreased glucose uptake is a result of insulin resistance which is transiently induced within the stressed patient.
Insulin resistance
The degree of insulin resistance is proportional to the magnitude of the injurious process.Following routine upper abdominal surgery, insulin resistance may persist for approximately 2 weeks.
Insulin resistance
patients with insulin resistance behave in a similar manner to individuals with type II diabetes mellitus and are at
Increased risk of sepsis,
Deteriorating renal function,
Polyneuropathy and
Death.
Insulin resistance
Mainstay managementIntravenous insulin infusion
Intensive approach
conservative approach
insulin may also exert minor, organ specific effects
CHANGES IN BODY COMPOSITION FOLLOWING INJURY
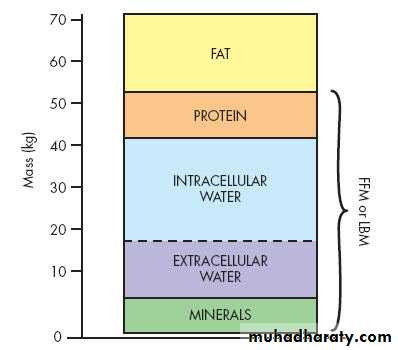
70-kg male can be considered to consist ofFat (13 kg)
fat-free mass (or lean body mass: 57 kg)
Protein (12 kg),
Skeletal muscle (4 kg)
non-skeletal muscle (8 kg)
Water (42 kg) and
Intracellular (28 l)
extracellular (14 l)
Minerals (3 kg) bony skeleton.
Copyright© medicalstudies.in
CHANGES IN BODY COMPOSITION FOLLOWING INJURY
The main labile energy reserve in the body is fat, and the main labile protein reserve is skeletal muscle.While fat mass can be reduced without major detriment to function,
loss of protein mass results not only in skeletal muscle wasting, but also depletion of visceral protein status.
CHANGES IN BODY COMPOSITION FOLLOWING INJURY
Each 1 g of nitrogen is contained within 6.25 g of protein, which is contained in approximately 36 g of wet weight tissue.The loss of 1g of nitrogen in urine is equivalent to the breakdown of 36 g of wet weight lean tissue.
Protein turnover in the whole body is of the order of 150–200 g /day.
A normal human ingests about 70–100 g protein /day, which is metabolised and excreted in urine as ammonia and urea ( approximately 14 g N /day).
CHANGES IN BODY COMPOSITION FOLLOWING INJURY
During total starvation, urinary loss of nitrogen is rapidly attenuated by a series of adaptive changes.Loss of body weight follows a similar course, thus accounting for the survival of hunger strikers for a period of 50–60 days.
Following major injury, and particularly in the presence of on-going septic complications, this adaptive change fails to occur, and there is a state of ‘autocannibalism’, resulting in continuing urinary nitrogen losses of 10–20 g N /day (equivalent to 500 g of wet weight lean tissue/ day).
As with total starvation, once loss of body protein mass has reached 30–40% of the total, survival is unlikely.
Copyright© medicalstudies.in
CHANGES IN BODY COMPOSITION FOLLOWING INJURY
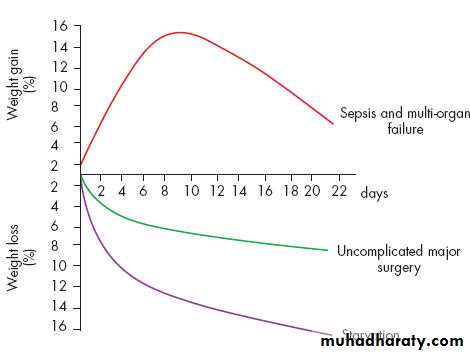
Critically ill patients admitted to the ICU with severe sepsis or major blunt trauma undergo massive changes in body compositionBody weight increases immediately on resuscitation with an expansion of extracellular water by 6–10 Lsl within 24 hours.
total body protein will diminish by 15% in the next 10 days, and body weight will reach negative balance as the expansion of the extracellular space resolves.
This can be achieved
by blocking the neuroendocrine stress response withepidural analgesia and providing
early enteral feeding.
Moreover, the early fluid retention phase can be avoided by careful intraoperative management of fluid balance, with avoidance of excessive administration of intravenous saline
In marked contrast, it is now possible to maintain body weight and nitrogen equilibrium following major elective surgery
AVOIDABLE FACTORS THAT COMPOUND THE RESPONSE TO INJURY
■ Continuing haemorrhage■ Hpoxia
■ Hypothermia
■ Tissue oedema
■ Tissue underperfusion (Ischaemia)
■ Starvation
■ Immobility
Volume loss
During simple haemorrhage,
Pressor receptors in the carotid artery and aortic arch, and
volume receptors in the wall of the left atrium,
Initiate afferent nerve input to the central nervous system (CNS),
resulting in the release of both aldosterone and antidiuretic hormone (ADH).
Pain can also stimulate ADH release.
ADH acts directly on the kidney to cause fluid retention.
Decreased pulse pressure stimulates the juxtaglomerular apparatus in the kidney and directly activates the renin–angiotensin system, which in turn increases aldosterone release
Aldosterone causes the renal tubule to reabsorb sodium (and onsequently also conserve water).
ACTH release also augments the aldosterone response.
The net effects of ADH and aldosterone result in the natural oliguria observed after surgery and
conservation of sodium and water in the extracellular space.
The tendency towards water and salt retention is exacerbated by resuscitation with saline-rich fluids.
Salt and water retention can result in not only peripheral oedema, but also visceral oedema(e.g. stomach).
Such visceral oedema has been associated with
reduced gastric emptying, delayed resumption of food intake and prolonged hospital stay.
Careful limitation of intraoperative administration of colloids and crystalloids, so that there is no net weight gain following elective surgery has been proven to reduce postoperative complications and length of stay.
Hypothermia
Results in increased elaboration of adrenal steroids and catecholamines.
Even mild hypothermia results in a 2-3 fold increase in postoperative cardiac arrhythmias and increased catabolism.
Maintaining normothermia by an upper body forced-air heating cover reduces
wound infections, cardiac complications and bleeding and transfusion requirements.
Tissue oedema
During systemic inflammation, fluid, plasma proteins, leucocytes, macrophages and electrolytes leave the vascular space and accumulate in the tissues.This can diminish the alveolar diffusion of oxygen and may lead to reduced renal function.
Increased capillary leak is mediated by a wide variety of mediators including cytokines, prostanoids, bradykinin and nitric oxide.
Systemic inflammation and tissue underperfusion
The vascular endothelium controls vasomotor tone and microvascular flow, and regulates trafficking of nutrients and biologically active molecules.When endothelial activation is excessive, compromised microcirculation and subsequent cellular hypoxia contribute to the risk of organ failure.
Maintaining normoglycaemia with insulin infusion during critical illness has been proposed to protect the endothelium, probably in part, via inhibition of excessive iNOS-induced NO release, and thereby contribute to the prevention of organ failure and death.
Administration of activated protein C to critically ill patients has been shown to reduce organ failure and death and is thought to act, in part, via preservation of the microcirculation in vital organs.
Starvation
Cerebral energy metabolism (100 g of glucose /day)in the first 24 hours by mobilising glycogen stores
thereafter by hepatic gluconeogenesis from amino acids, glycerol and lactate.
The energy metabolism of other tissues is sustained by mobilising fat from adipose tissue.
Such fat mobilisation is mainly dependent on a fall in circulating insulin levels.
Starvation
Liver converting free fatty acids into ketone bodies, which can serve as a substitute for glucose for cerebral energy metabolism.
Provision of at least 2 litres of intravenous 5% dextrose as intravenous fluids for surgical patients who are fasted provides 100 g of glucose /day and has a significant protein-sparing effect.
Immobility
Immobility has long been recognised as a potent stimulus for inducing muscle wasting.Inactivity impairs the normal meal-derived amino acid stimulation of protein synthesis in skeletal muscle.
Avoidance of unnecessary bed rest and active early mobilisation are essential measures to avoid muscle wasting as a consequence of immobility.
CONCEPTS BEHIND OPTIMALPERIOPERATIVE CARE
Avoidingunmodulated exposure to stress,
Prolonged fasting and
excessive administration of intravenous (saline) fluids.
β-blockers and statins have recently been shown to improve long-term survival after major surgery.
Epidural analgesia to reduce pain,
block the cortisol stress response and
attenuate postoperative insulin resistance may, via effects on the body’s protein economy, favourably affects many of the patient-centred outcomes that are important to postoperative recovery
A proactive approach to prevent unnecessary aspects of the surgical stress response
■ Minimal access techniques■ Blockade of afferent painful stimuli (e.g. epidural analgesia)
■ Minimal periods of starvation
■ Early mobilisation