Copyright © 2000-2004 by Mark Brandt, Ph.D.
28
Prostaglandin biosynthesis and functions
Introduction
Prostaglandins and related molecules are called eicosanoids as a class. The term
eicosanoid is derived from “eicosa” meaning “twenty”, referring to the 20 carbons in
most of the molecules. The eicosanoids are used as signaling molecules. They
generally act locally, either affecting cell that makes them or nearby cells; in most
cases, eicosanoids are not systemic hormones, because of their short half-lives.
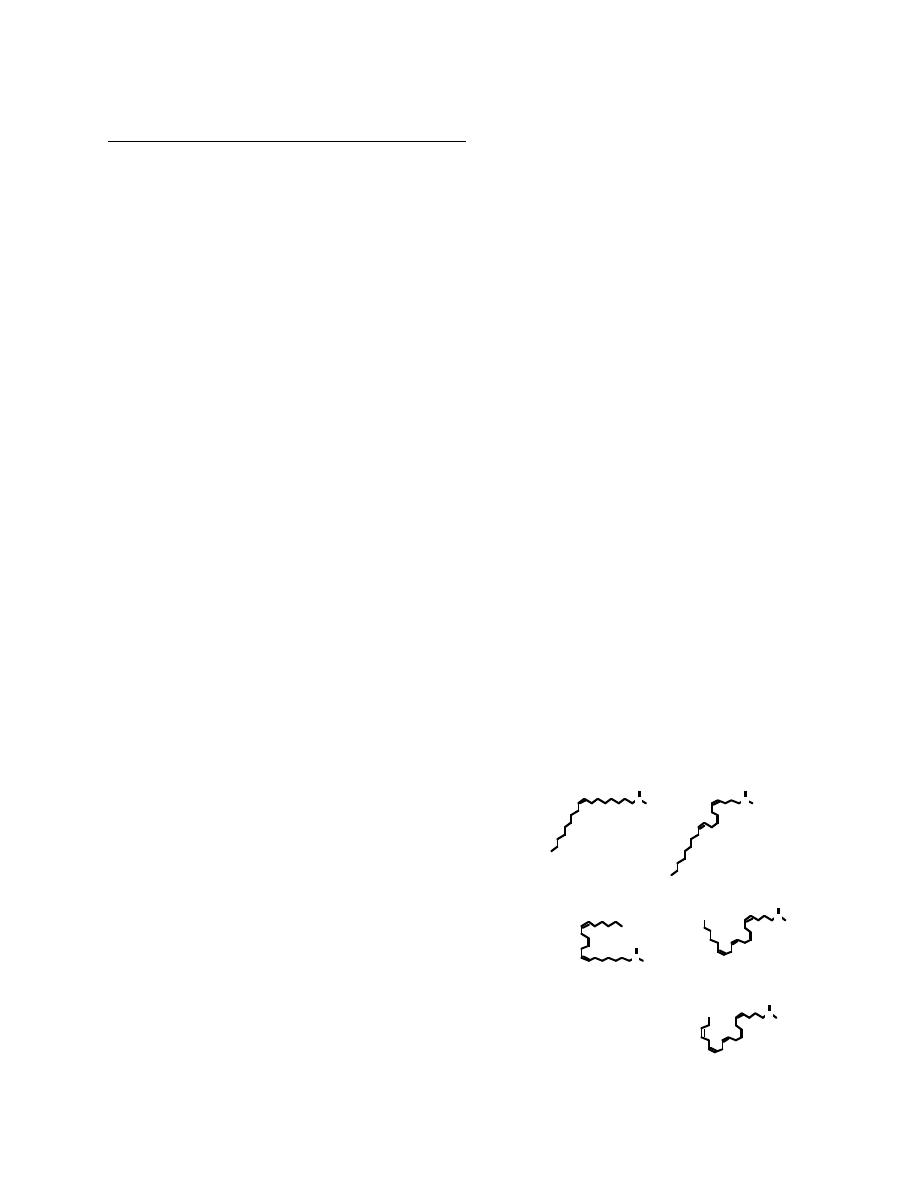
Most prostaglandins are synthesized from arachidonic acid (20:4 ∆
5,8,11,14
). These
are called “Series 2” products, because most have two double bonds. However, the
triene fatty acid 20:3 ∆
8,11,14
can also be used; the products have one fewer double
bond than the arachidonic acid derivatives and are called Series 1 products.
Both of these potential precursor molecules are ω
6
fatty acids. In the absence of ω
6
fatty acids, the organism may attempt to produce eicosanoids from ω
9
fatty acids.
These ω
9
-derivative compounds, regardless of the number of double bonds, are
inactive.
In contrast, 20:5 ∆
5,8,11,14,17
, a fatty acid produced from diets high in seafood fatty
acids (such as the typical Eskimo diet) is also a substrate for prostaglandin
synthesis; the products from this compound have one more double bond than the
series two products. The properties of the different series are somewhat different.
Eskimos have a low incidence of heart disease in spite of an extremely high fat diet;
one likely contributing factor is the higher degree of unsaturation in the fatty acid
prostaglandin precursors and in the prostaglandins.
Reminder of ω nomenclature
Polyunsaturated fatty acids all have double bonds three carbons apart. This allows
the first or the last carbon present as a double bond to be used in identifying the
compound. It is possible therefore to count from the methyl-group end of the fatty
acid; the Greek letter ω (the last letter in the Greek alphabet) is used to refer to the
position of the double bond counting from the terminal methyl group.
Humans can synthesize ω
9
fatty acids such as
oleic acid and its 20:3 ∆
5,8,11
derivative.
However, this is ordinarily a minor pathway, and
the 20:3 ∆5,8,11 cannot be used to make
functional prostaglandins.
OH
Oleic acid
(18:1
∆9
)
C
O
OH
5,8,11-Eicosatrienoic acid
(20:3
∆5,8,11
)
O
C
Two ω
6
fatty acids, 20:3 ∆
8,11,14
, and arachidonic
acid (20:4 ∆
5,8,11,14
) are substrates for most
prostaglandin biosynthesis (producing the series
one and series two products, respectively.
OH
8,11,14-Eicosatrienoic acid
(20:3
∆8,11,14
)
O
C
OH
Arachidonic acid
5,8,11,14-Eicosatetraenoic acid
(20:4
∆5,8,11,14
)
O
C
In addition, the 20:5 ∆
5,8,11,14,17
fatty acid
mentioned above, an ω
3
fatty acid, can also be
used for prostaglandin biosynthesis.
OH
5,8,11,14,17-Eicosapentaenoic acid
(20:5
∆5,8,11,14,17
)
O
C
Copyright © 2000-2004 by Mark Brandt, Ph.D.
29
Synthesis
Prostaglandin biosynthesis has two control points.
Phospholipase A
2
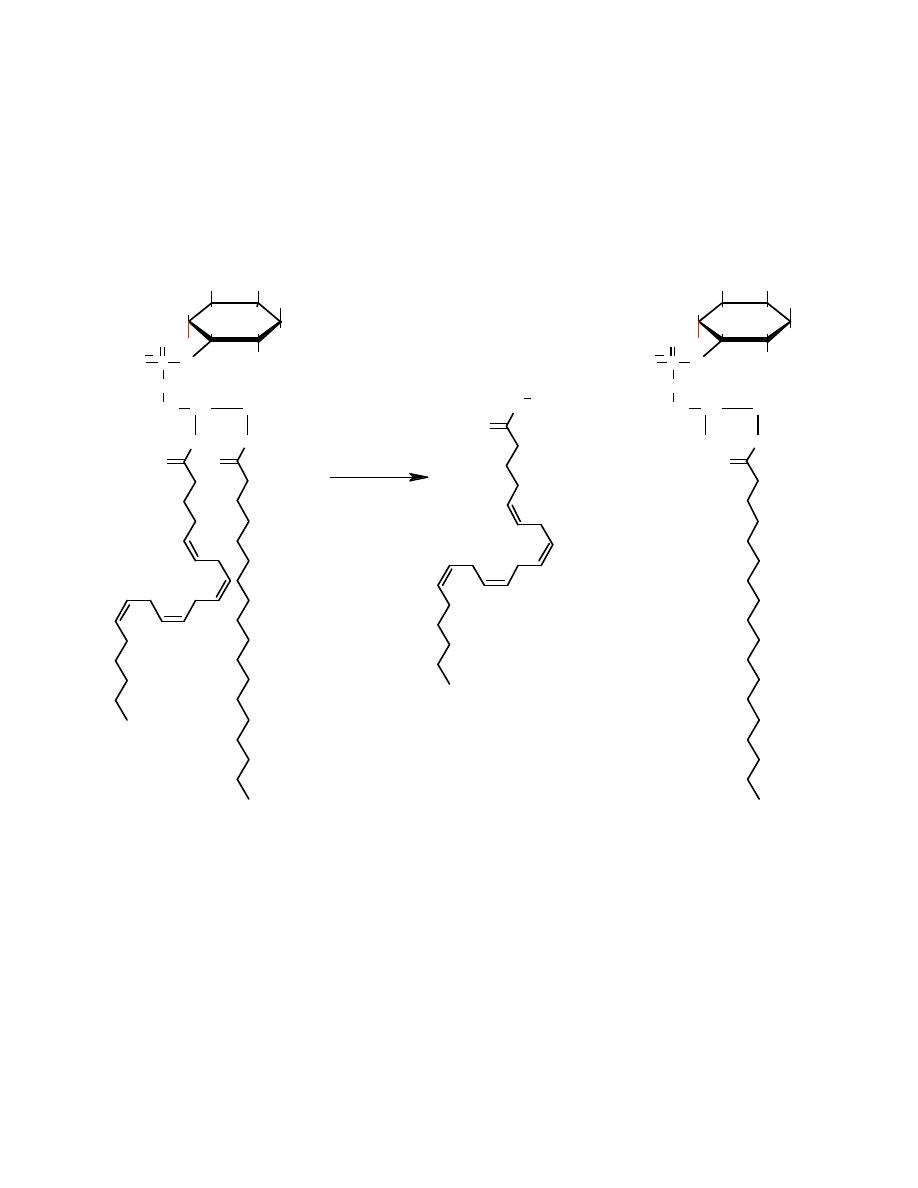
The starting material for prostaglandin biosynthesis is a fatty acid. The fatty acid
used is nearly always derived from the 2-position of a membrane phospholipid
(usually phosphatidylinositol).
1
6
5
4
3
2
H
O
H
O
H
H
H
O
H
H
O
H
O
H
O
H
H
O
O
C
H
C
H
2
O
O
C
H
2
O
P
O
O
O
O
1
6
5
4
3
2
H
O
H
O
H
H
H
O
H
H
O
H
O
H
O
H
H
O
H
C
H
C
H
2
O
O
C
H
2
O
P
O
O
Phosphatidyl
inositol
Phospholipase A2
+
Arachidonic
acid
Release of the fatty acid from the phospholipid is the first control point in
the prostaglandin biosynthetic pathway. One function of glucocorticoids is
inhibition of phospholipase A
2
and therefore of eicosanoid synthesis.
COX and lipoxygenase
The second control point is the enzyme responsible for converting the fatty acid to
the first molecule in the relevant pathway. Two enzymes are primarily involved in
eicosanoid biosynthesis. Prostaglandin synthase and 5-lipoxygenase.
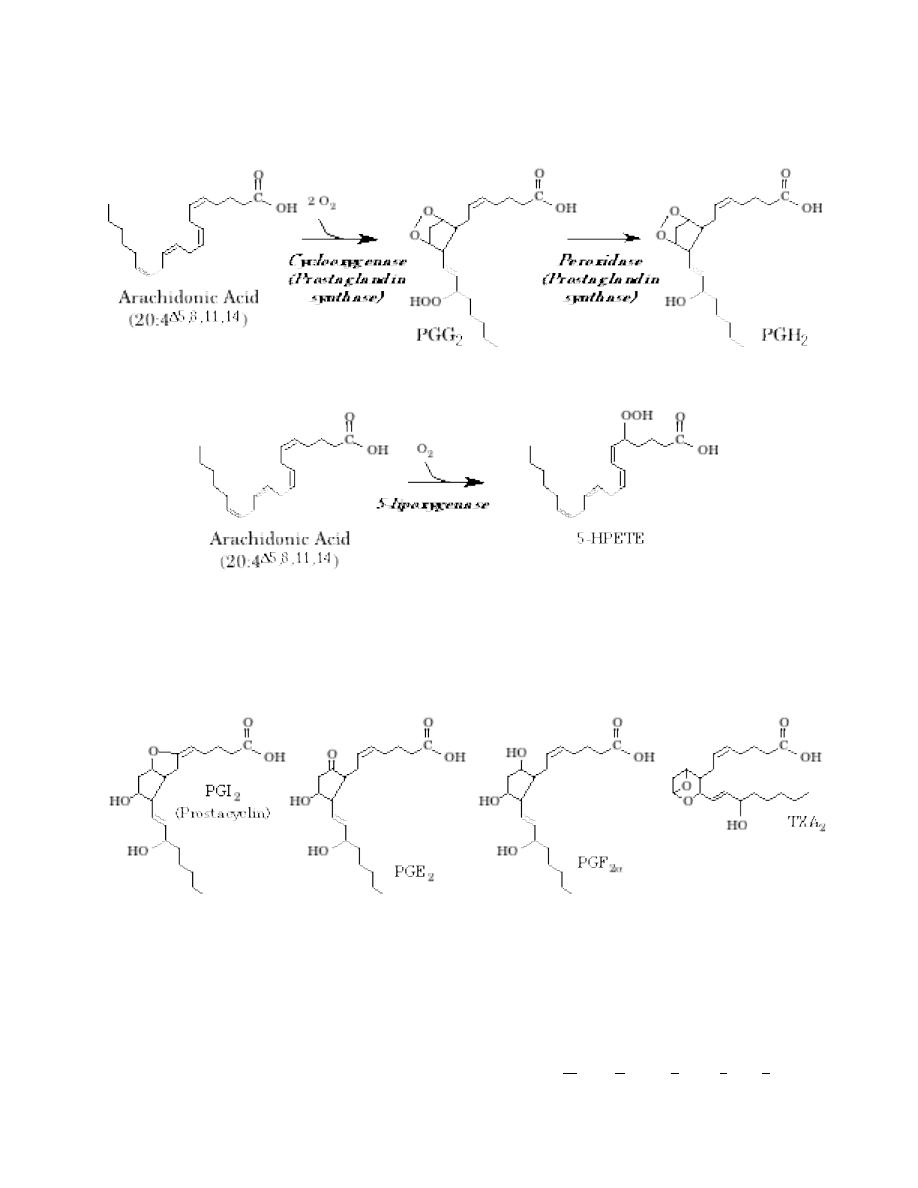
Prostaglandin synthase is a complex enzyme that catalyzes the first two steps in
the prostaglandin synthesis pathway. It is often called cyclooxygenase (referring
to the first of the two reactions it mediates); cyclooxygenase is abbreviated COX.
Copyright © 2000-2004 by Mark Brandt, Ph.D.
30
The two reactions catalyzed by COX are shown below:
5-Lipoxygenase is one type of lipoxygenase; 5-Lipoxygenase catalyzes the first step
in one of the more important pathways.
Physiological Eicosanoids
Prostaglandins and Thromboxanes
The product of the COX reactions can then be converted to the physiologically active
compounds. A number of biologically active compounds are known to exist. Some of
the more important ones are shown below.
In the abbreviations, “PG” = “prostaglandin” and “TX” = “thromboxane”. The letters
(e.g., the “I” in “PGI
2
”) indicate the structure and substituents of the ring, while the
number refers to the number of double bonds present. The structures shown above
are series 2 compounds, with two double bonds; series one compounds such as PGE
1
lack the double bond closest to the carboxylate.
Leukotrienes
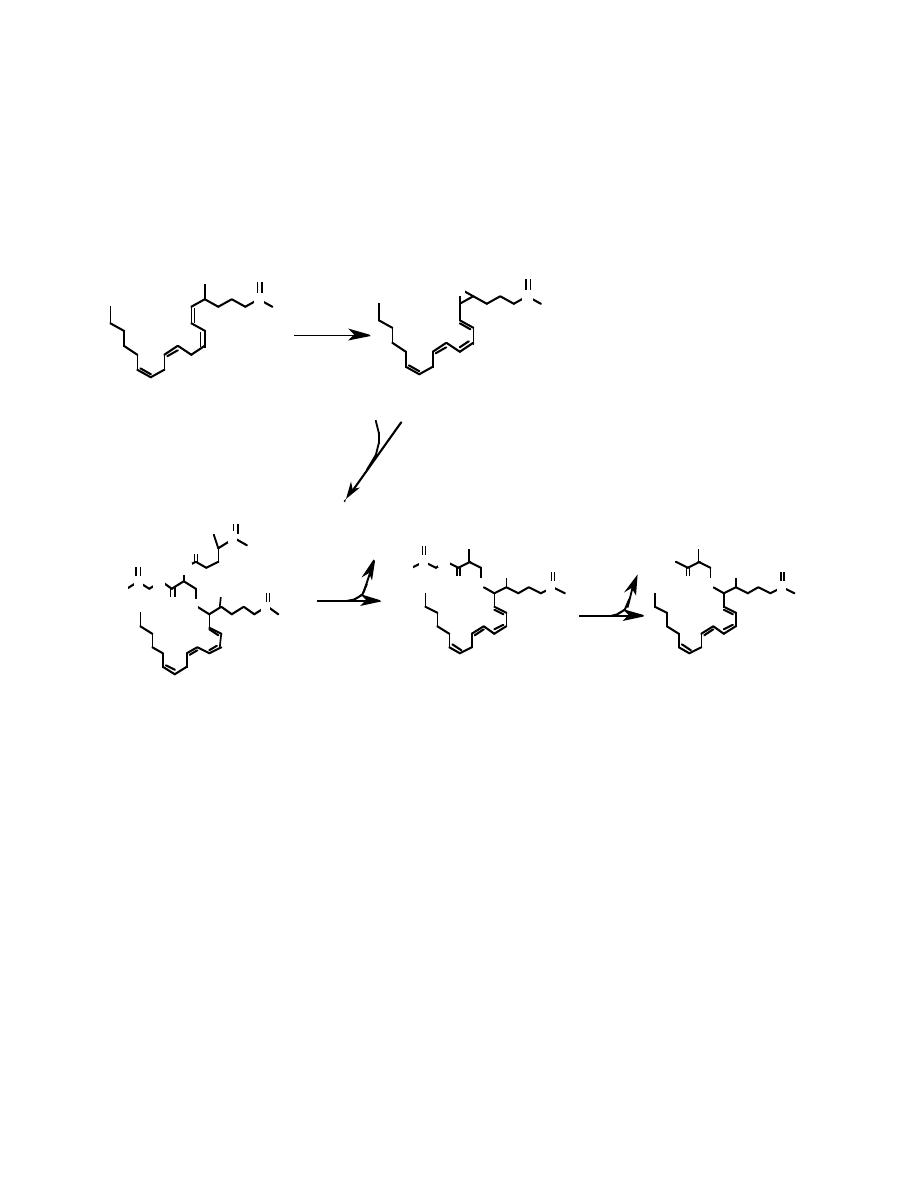
The product of the 5-lipoxygenase reaction, HPETE (= Hydroperoxyeicosatetraenoic
Copyright © 2000-2004 by Mark Brandt, Ph.D.
31
acid) is usually converted to leukotrienes. (Note: the word leukotriene implies three
double bonds; however, leukotriene derivatives of arachidonic acid have four double
bonds.)
Leukotrienes C
4
, D
4
, and E
4
are usually present as a mixture of the three
compounds. This mixture is known as the Slow Reacting Substance of Anaphylaxis,
and is a powerful inflammatory agent that is responsible for some forms of allergic
reactions.
OH
5-HPETE
O
C
OOH
OH
Leukotriene A
4
O
C
O
Glutathione-
S-transferase
Glutathione
OH
Leukotriene C
4
O
C
OH
H
N
O
O
C
HO
S
HN
O
H
2
N
OH
O
C
OH
Leukotriene D
4
O
C
OH
H
N
O
O
C
HO
S
NH
2
Gl u tam i c ac i d
γ
-Glutamyl
transferase
OH
Leukotriene E
4
O
C
OH
O
HO
S
NH
2
Gl yc i n e
Cysteinyl-
glycine
dipeptidase
Mechanism of action
Physiological functions of prostaglandins
Prostaglandins are rapidly degraded, and have such short half-lives that their
functions are usually considered to be limited to actions on nearby cells.
Prostaglandins seem to act via two separate mechanisms. S e c r e t e d
prostaglandins bind to specific cell surface G-protein coupled receptors, and
generally increase cAMP levels. Prostaglandins may also bind to nuclear
receptors and alter gene transcription.
Prostaglandin action is incompletely understood.
Known actions include:
Induction of inflammation
Mediation of pain signals
Induction of fever
Smooth muscle contraction (including uterus) – (especially PGF
2α
)
Smooth muscle relaxation -- especially PGE series
Protection of stomach lining
Simulation of platelet aggregation (thromboxanes)
Copyright © 2000-2004 by Mark Brandt, Ph.D.
32
Inhibition of platelet aggregation (prostacyclin)
COX-1, COX-2, and COX-3
Humans, and most other mammals have two genes for cyclooxygenase.
The products of the genes, COX-1 and COX-2, are structurally quite similar, with
only subtle differences. The catalyze the same reactions, although COX-2 works
with a wider range of substrates. COX-1 is constitutively expressed in nearly all
tissues. In contrast, COX-2 is inducible, especially by inflammatory stimuli.
Some evidence suggests that COX-1 is responsible for generating the prostaglandins
required for protection of the gastrointestinal tract, while COX-2 is responsible for
the increased prostaglandin synthesis associated with inflammation, fever, and
pain responses. This has led to attempts to find specific inhibitors of COX-2. On the
other hand, some evidence suggests that the roles of the two isozymes may not be
quite that clearly defined.
A new isozyme, COX-3 was discovered in 2002; it is thought to be a intron-splice
variant of COX-1. It has a similar sequence, but not identical amino acid sequence
to that of COX-1, but has some functional differences. The role of COX-3 is the
subject of considerable interest, but much remains to be learned about the role of all
of the isozymes.
Inflammation
The inflammatory response involves the migration of immune system cells into a
damaged tissue. In some cases, this is beneficial (especially for fighting infection); in
many cases, however, the inflammatory response actually increases the damage to
the tissue. This is true for asthma, several forms of arthritis, and for muscle and
connective tissue damage associated with sprains and similar injuries; in addition,
there is evidence that inflammation may be a step on the pathway toward certain
cancers (especially colon cancer).
Inflammation can be treated with two major classes of antiinflammatory drugs:
steroids, and non-steroids. The steroids are compounds with glucocorticoid activity,
and include the physiological glucocorticoid, cortisol, and synthetic glucocorticoid
analogs such dexamethasone.
O
O
O
H
O
H
O
H
O
O
O
H
O
H
O
H
C
H
3
F
Cortisol
Dexamethasone
Copyright © 2000-2004 by Mark Brandt, Ph.D.
33
Glucocorticoids inhibit inflammatory responses by several mechanisms, and are
more powerful drugs than NSAIDs. One mechanism is phospholipase A
2
inhibition;
this inhibits both prostaglandin and leukotriene synthesis, and therefore has
a stronger effect than COX inhibition alone. In addition, glucocorticoids have other
effects, unrelated to eicosanoid pathways.
The non-steroidal compounds are called NSAIDs (Non-Steroidal Anti-Inflammatory
Drugs). The NSAIDs are COX inhibitors; some of the most widely used drugs,
including aspirin, ibuprofen, and naproxen fall into this class.
O
H
O
O
O
O
H
O
Ibuprofen
[α-methyl-4-(2-methylpropyl)benzene-acetic acid]
[2-(4-isobutylphenyl)propionic acid]
Aspirin
[2-acetoxybenzoic acid]
[salicylic acid acetate]
O
C
H
3
O
H
O
C
H
3
Naproxen
[2-(6-methoxynaphthyl)
propionic acid]
Most currently available NSAID compounds, such as aspirin, ibuprofen, and
naproxen are inhibitors of both COX isozymes. Aspirin covalently modifies the
enzymes; this abolishes cyclooxygenase activity (although it leaves peroxidase
activity intact). In contrast, ibuprofen and naproxen are reversible inhibitors of
COX.
Acetaminophen is often classed with the NSAIDs. Although the structure of
acetaminophen is similar to the NSAIDs mentioned above, and although
acetaminophen inhibits some prostaglandin-mediated responses, probably via
specific inhibtion of COX-3, it does not inhibit COX-1 or COX-2, and does not have
anti-inflammatory actions. It is therefore not an NSAID. The actual mechanism of
acetaminophen action remains controversial.
N
H
O
O
H
Acetaminophen
[N-(4-hydroxyphenyl)acetamide]
[p-hydroxyacetanilide]
[p-acetaminophenol]
COX inhibition and the stomach
Indomethacin, a high affinity inhibitor of COX (and in some individuals, aspirin,
and to a lesser extent ibuprofen) induces ulceration; some anti-ulcer drugs appear to
function by increasing prostaglandin synthesis.
Copyright © 2000-2004 by Mark Brandt, Ph.D.
34
COX inhibition and the kidney
Normal kidneys do not appear to require prostaglandins. However, kidneys in
individuals with chronic liver, heart, or kidney disease do require prostaglandin
biosynthesis in the kidney. In these individuals, COX inhibitors can severely
damage the kidney.
Prostaglandins and pregnancy
Prostaglandins are required for normal implantation of the fertilized oocyte. In
addition, prostaglandins are involved in initiation of labor. Prostaglandins are used
for labor induction (and for RU-486 induced abortions); COX-inhibitors (probably
via COX-2) delay onset of labor. COX-2 seems to be required for ovulation.
Prostaglandins and fever and pain
Prostaglandins appear to form a major part of the signaling pathway in fever
induction. COX inhibitors are thought to exert their anti-pyretic actions by
interrupting this pathway. Prostaglandins appear to be involved in some pain
pathways; inhibition of COX (probably COX-2) is thus analgesic.
COX-2 inhibitors
The current hypotheses regarding prostaglandin action suggest that inhibitors
specific for COX-2 should have many useful effects, including anti-inflammatory
actions, analgesic effects, and anti-pyretic effects, without altering platelet function
or damaging the gastrointestinal tract. The first generation compounds were
discovered by searching for effective compounds with minimal stomach irritation;
new compounds are in trials based on direct assays on COX-1 and COX-2, and on
analyses of the crystal structures of the two isozymes.
Aspirin and indomethacin both have higher affinity for COX-1 and COX-3 than
COX-2 (although both compounds bind to all three enzymes). Indomethacin is about
100-fold more potent than aspirin, and is rarely used as a drug as a result of its
toxic effects.
N
S
O
O
N
H
2
N
C
F
3
C
H
3
S
O
O
C
H
3
O
O
N
Cl
O
C
H
3
O
O
H
O
C
H
3
Rofecoxib
Celecoxib
Indomethacin
COX-2 specific inhibitors such as celecoxib and refecoxib have not been nearly as
heavily tested as aspirin (aspirin is consumed at the rate of several thousand tons
each year!); some unknown side effects of the COX-2 inhibitors may therefore exist.
For example, some evidence indicates that COX-2 mediated prostaglandin synthesis
is important in wound healing; in addition, little testing has been done on the

Copyright © 2000-2004 by Mark Brandt, Ph.D.
35
effects of these compounds on fertility or on fetal development. Studies using mice
with COX-2 gene deletions suggest that COX-2 products are important for ovulation
and for early development. Early studies with COX-2 inhibitors have suggested a
greatly reduced incidence of stomach damage. However, aspirin induces stomach
damage only in a small subset of individuals; it is therefore possible that the studies
on the COX-2 inhibitors have not been large enough to detect the potentially
significant side-effects.
Aspirin and heart disease
Platelet aggregation is regulated by eicosanoids (among a number of other stimuli).
Thromboxane A
2
is produced in platelets and stimulates aggregation. Prostacyclin
(PGI
2
) is synthesized in the vascular endothelium, and inhibits aggregation. Aspirin
irreversibly inhibits cyclooxygenase in both platelets and endothelial cells; however
the endothelial cells can synthesize new enzyme, while the platelets, which lack
protein biosynthetic machinery, cannot. Platelets normally circulate for 8-10 days;
aspirin therefore has a significant antithrombosis effect. Clinical studies have found
strong evidence suggesting that ~75 mg/day of aspirin (a small fraction of the
normal 325 mg aspirin tablet) reduces risk of heart disease and stroke by reducing
blood clot formation.
Note: aspirin increases clotting time, but is not a true anti-coagulant. COX-1
knockout mice exhibit changes in their platelets associated with aspirin
administration, but do not the exhibit symptoms of severe anti-coagulation that are
observed with warfarin administration; warfarin (an indirect inhibitor of synthesis
of some clotting factors via interference with the Vitamin K cycle) induces life-
threatening internal and external hemorrhages.
COX inhibition and cancer
Colon cancer is a major life-threatening cancer. Aspirin has been shown to have an
apparent protective effect against colon cancer; some evidence suggests that
inhibition of colon tumor induction is due to inhibition of COX-2. Breast and
stomach cancer growth may also be inhibited by COX inhibitors.
COX and Alzheimer’s disease
The brain damage associated with Alzheimer’s disease appears to be largely
mediated by inflammatory responses; some epidemiological data have suggested a
reduced incidence of Alzheimer’s disease in individuals taking COX inhibitors.

Copyright © 2000-2004 by Mark Brandt, Ph.D.
36
Summary
Eicosanoids are important signaling molecules. Eicosanoids are synthesized from
twenty-carbon polyunsaturated fatty acids that most animals cannot synthesize
from acetyl-CoA. The precursors for these molecules are therefore called essential
fatty acids.
Synthesis of any of the eicosanoid signaling molecules is controlled by two enzymes.
The first enzyme, phospholipase A
2
, is required for the synthesis of all of these
molecules. The second enzyme depends on the type of molecule. Cyclooxygenase is
the main regulated enzyme for prostaglandin and thromboxane synthesis, while
leukotriene synthesis is regulated by 5-lipoxygenase.
Eicosanoids have a wide variety of actions, including mediating some pain
pathways, many types of inflammation, and fever responses.
Phospholipase A
2
is inhibited by glucocorticoids. Cyclooxygenase is inhibited by
aspirin and a number of other widely used drugs.
