Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
SECRETION AND ABSORPTION IN
THE SMALL INTESTINE
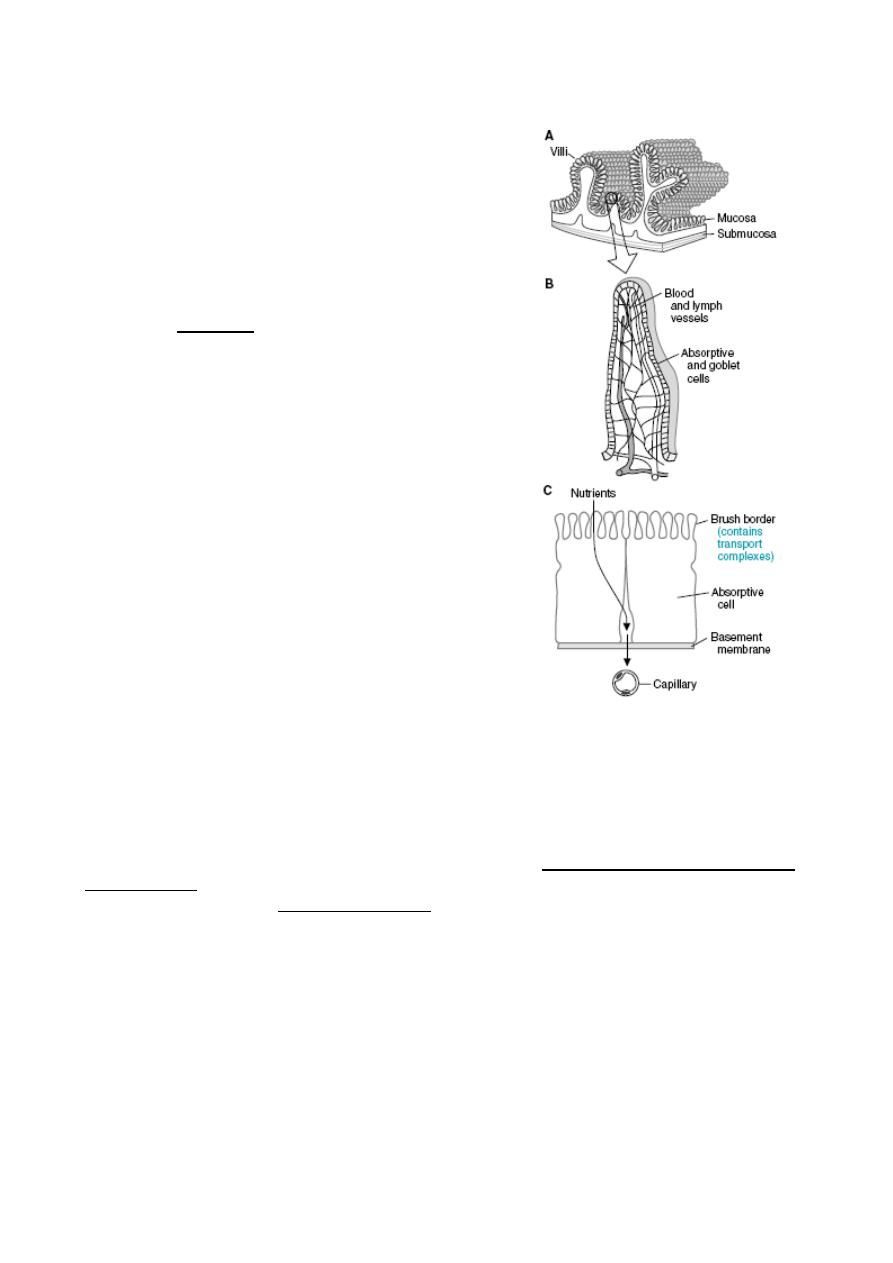
The small intestine is the portal for absorption of virtually all nutrients into blood.
Accomplishing this transport requires breaking down large supramolecular aggregates into
small molecules that can be transported across the epithelium.
By the time ingesta reaches the small intestine, foodstuffs have been mechanically broken
down and reduced to a liquid by mastication and grinding in the stomach. Once within the
small intestine, these macromolecular aggregates are exposed to pancreatic enzymes and
bile, which enables digestion to molecules capable or almost capable of being absorbed.
The final stages of digestion occur on the surface of the small intestinal epithelium.
The net effect of passage through the small intestine is absorption of most of the water
and electrolytes (sodium, chloride, potassium) and essentially all dietary organic molecules
(including glucose, amino acids and fatty acids). Through these activities, the small
intestine not only provides nutrients to the body, but plays a critical role in water and
acid-base balance.
Secretion in the Small Intestine
Large quantities of water are secreted into the lumen of the small intestine during the
digestive process. Almost all of this water is also reabsorbed in the small intestine.
Regardless of whether it is being secreted or absorbed, water flows across the mucosa in
response to osmotic gradients. In the case of secretion, two distinct processes establish
an osmotic gradient that pulls water into the lumen of the intestine:
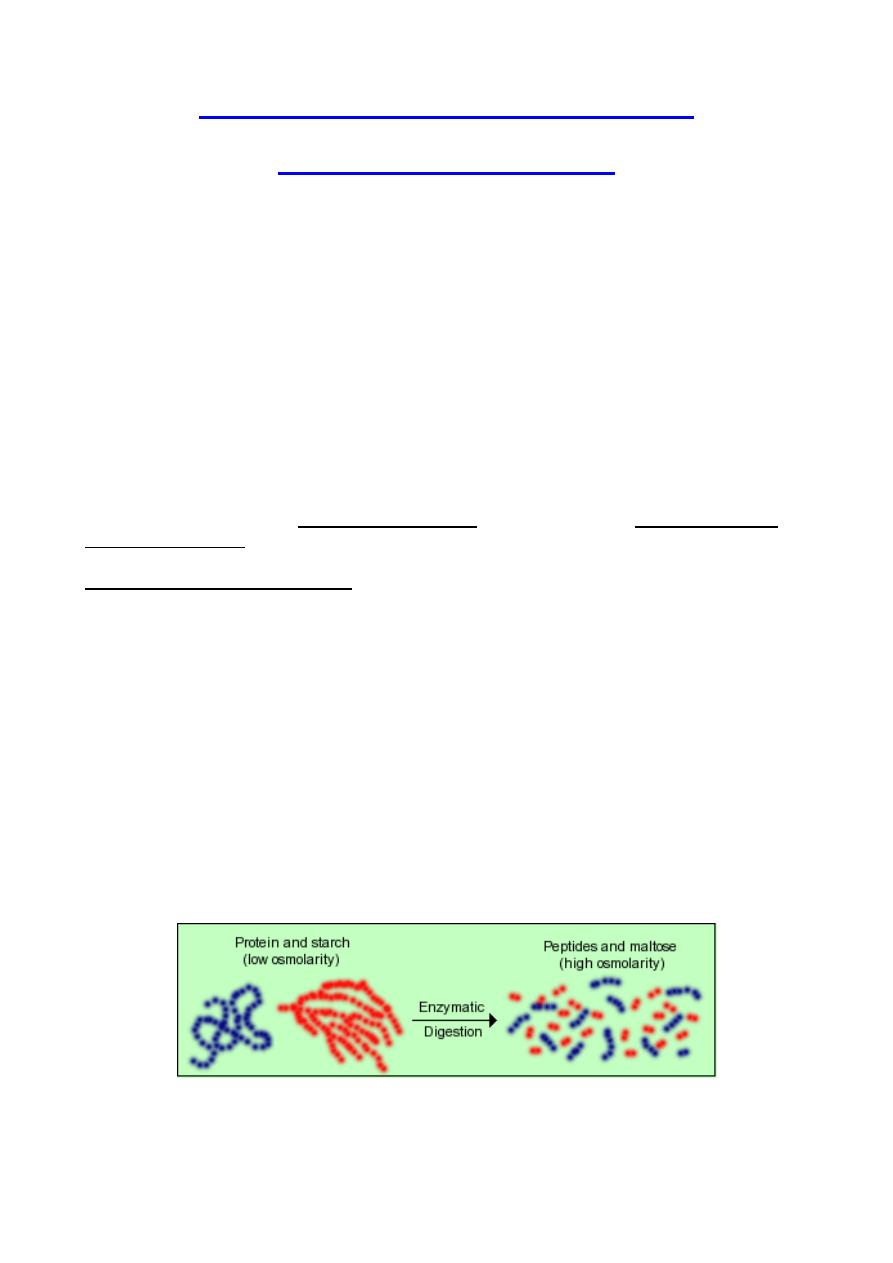
1. Increases in luminal osmotic pressure resulting from influx and digestion of
foodstuffs: The chyme that floods into the intestine from the stomach
typically is not hyperosmotic, but as its macromolecular components are
digested, osmolarlity of that solution increases dramatically.
Starch, for example, is a huge molecule that contributes only a small amount to osmotic
pressure, but as it is digested, thousands of molecules of maltose are generated, each of
which is as osmotically active as the original starch molecule.
Thus, as digestion proceeds lumenal osmolarity increases dramatically and water is pulled
into the lumen. Then, as the osmotically active molecules (maltose, glucose, amino acids)
are absorbed, osmolarity of the intestinal contents decreases and water can be absorbed.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
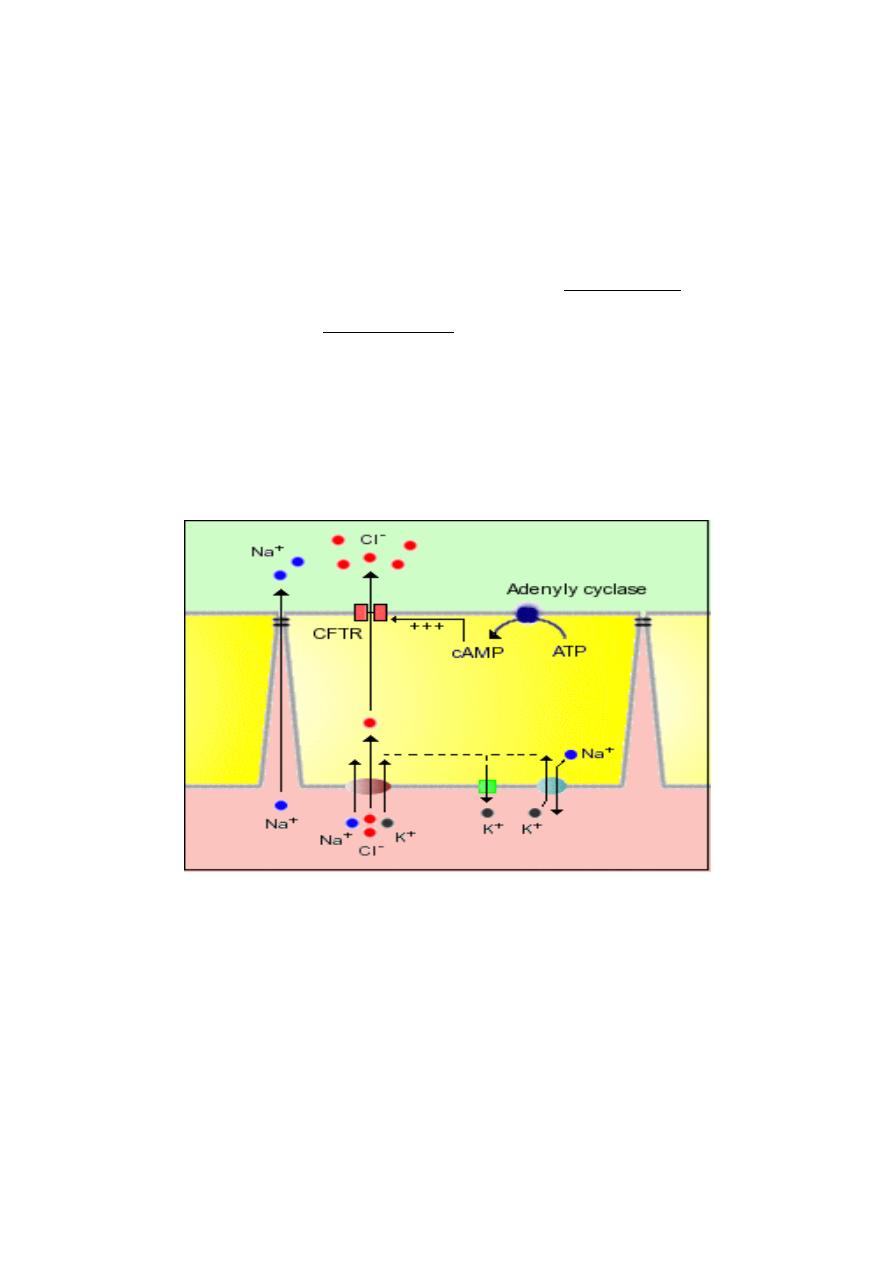
2. Crypt cells actively secrete electrolytes, leading to water secretion: The apical
or lumenal membrane of crypt epithelial cells contain an ion channel of
immense medical significance - a cyclic AMP-dependent chloride channel
known also as the cystic fibrosis transmembrane conductance regulator or
CFTR. Mutations in the gene for this ion channel result in the disease cystic
fibrosis. This channel is responsible for secretion of water by the following
steps:
1. Chloride ions enter the crypt epithelial cell by cotransport with sodium and
potassium; sodium is pumped back out via sodium pumps, and
potassium is exported via a number of channels.
2. Activation of adenylyl cyclase by a number of so-called secretagogues
leads to generation of cyclic AMP.
3. Elevated intracellular concentrations of cAMP in crypt cells activate the
CFTR, resulting in secretion of chloride ions into the lumen.
4. Accumulation of negatively-charged chloride anions in the crypt creates
an electric potential that attracts sodium, pulling it into the lumen,
apparently across tight junctions - the net result is secretion of NaCl.
5. Secretion of NaCl into the crypt creates an osmotic gradient across the
tight junction and water is drawn into the lumen.
CLINICAL CORRELATION
Cystic fibrosis
Abnormal activation of the cAMP-dependent chloride channel (CFTR) in crypt cells has
resulted in the deaths of millions upon millions of people. Several types of bacteria
produce toxins that strongly, often permanently, activate the adenylate cyclase in crypt
enterocytes. This leads to elevated levels of cAMP, causing the chloride channels to
essentially become stuck in the "open" position". The result is massive secretion of water
that is manifest as severe diarrhea. Cholera toxin, produced by cholera bacteria, is the
best known example of this phenomenon, but several other bacteria produce toxins that
act similarly.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
Absorption in the Small Intestine: General Mechanisms
Virtually all nutrients from the diet are absorbed into blood across the mucosa of the small
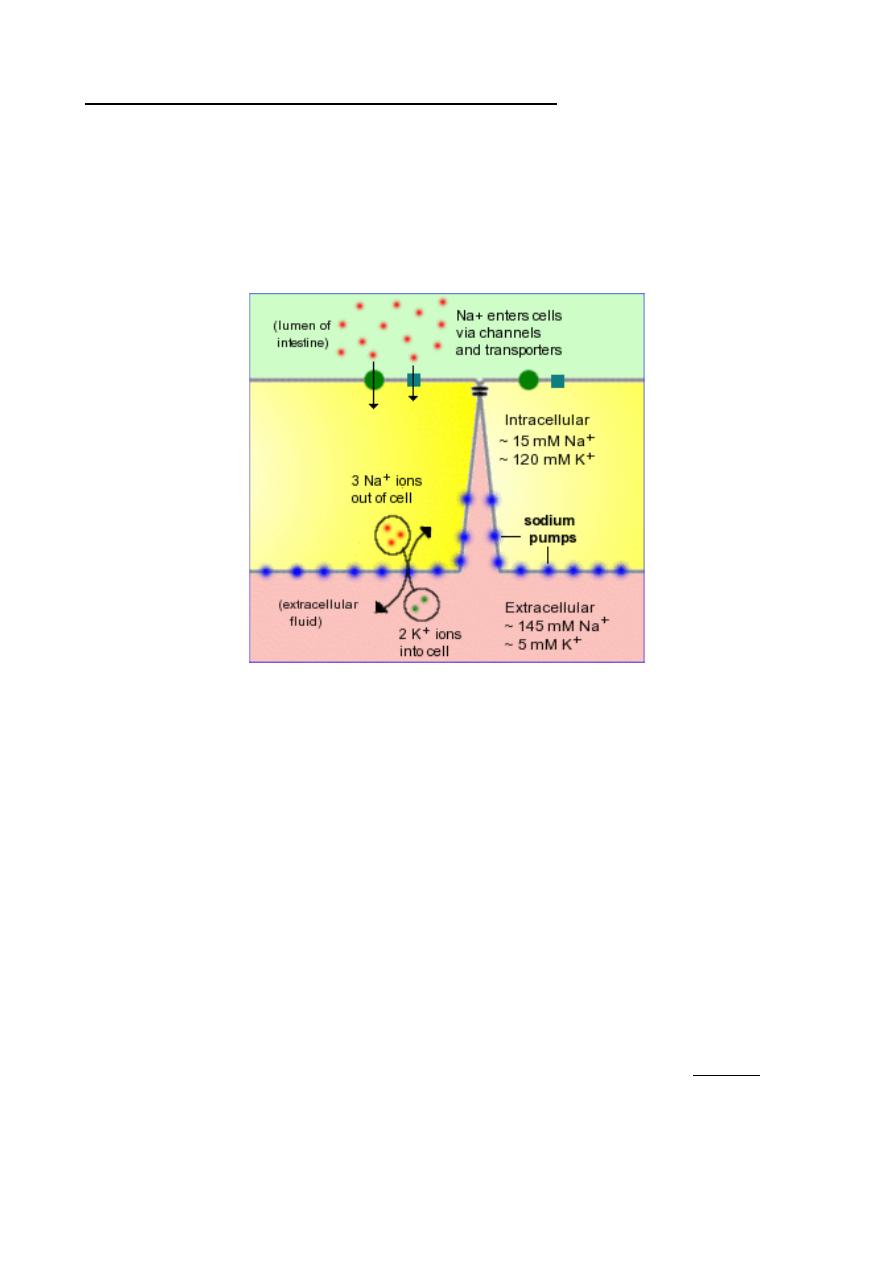
intestine.To remain viable, all cells are required to maintain a low intracellular
concentration of sodium. In polarized epithelial cells like enterocytes, low intracellular
sodium is maintained by a large number of Na
+
/K
+
ATPases – (sodium pumps) -
embedded in the basolateral membrane. These pumps export 3 sodium ions from the cell
in exchange for 2 potassium ions, thus establishing a gradient of both charge and sodium
concentration across the basolateral membrane.
Aside from the electrochemical gradient of sodium, several other concepts are required to
understand absorption in the small intestine. Also, dietary sources of protein, carbohydrate
and fat must all undergo the final stages of chemical digestion just prior to absorption of,
for example, amino acids, glucose and fatty acids.
•
Water and electrolytes
•
Carbohydrates, after digestion to monosaccharides
•
Proteins, after digestion to small peptides and amino acids
•
Neutral fat, after digestion to monoglyceride and free fatty acids
1) Absorption of Water and Electrolytes
The small intestine must absorb massive quantities of water. A normal person takes in
roughly 1 to 2 liters of dietary fluid every day. On top of that, another 6 to 7 liters of fluid is
received by the small intestine daily as secretions from salivary glands, stomach,
pancreas, liver and the small intestine itself.
By the time the ingesta enters the large intestine, approximately 80% of this fluid has been
absorbed. Net movement of water across cell membranes always occurs by osmosis, and
the fundamental concept needed to understand absorption in the small gut is that
absorption of water is absolutely dependent on absorption of solutes, particularly sodium:

Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
•
Sodium is absorbed into the cell by several mechanisms, but chief among them is
by co-transport with glucose and amino acids - this means that efficient sodium
absorption is dependent on absorption of these organic solutes.
•
Absorbed sodium is rapidly exported from the cell via sodium pumps - when a lot of
sodium is entering the cell, a lot of sodium is pumped out of the cell, which
establishes a high osmolarity in the small intercellular spaces between adjacent
enterocytes.
•
Water diffuses in response to the osmotic gradient established by sodium - in this
case into the intercellular space. It seems that the bulk of the water absorption is
transcellular, but some also diffuses through the tight junctions.
•
Water, as well as sodium, then diffuses into capillary blood within the villus.
Water is thus absorbed into the intercellular space by diffusion down an osmotic gradient.
However, looking at the process as a whole, transport of water from lumen to blood is
often against an osmotic gradient - this is important because it means that the intestine
can absorb water into blood even when the osmolarity in the lumen is higher than
osmolarity of blood.
2) Absorption of Monosaccharides
Monosaccharides, are only rarely found in normal diets. Rather, they are derived by
enzymatic digestion of more complex carbohydrates within the digestive tube.
Particularly important dietary carbohydrates include starch and disaccharides such as
lactose and sucrose. None of these molecules can be absorbed for the simple reason that
they cannot cross cell membranes unaided and, unlike the situation for monosaccharides,
there are no transporters to carry them across.
Brush Border Hydrolases Generate Monosaccharides
Polysaccharides and disaccharides must be digested to monosaccharides prior to
absorption and the key players in these processes are the brush border hydrolases, which
include maltase, lactase and sucrase. Dietary lactose and sucrose are "ready" for
digestion by their respective brush border enzymes. Starch, as discussed previously, is
first digested to maltose by amylase in pancreatic secretions and, saliva.
Dietary lactose and sucrose, and maltose derived from digestion of starch, diffuse in the
small intestinal lumen and come in contact with the surface of absorptive epithelial cells
covering the villi where they engage with brush border hydrolases:
•
maltase cleaves maltose into two molecules of glucose
•
lactase cleaves lactose into a glucose and a galactose
•
sucrase cleaves sucrose into a glucose and a fructose
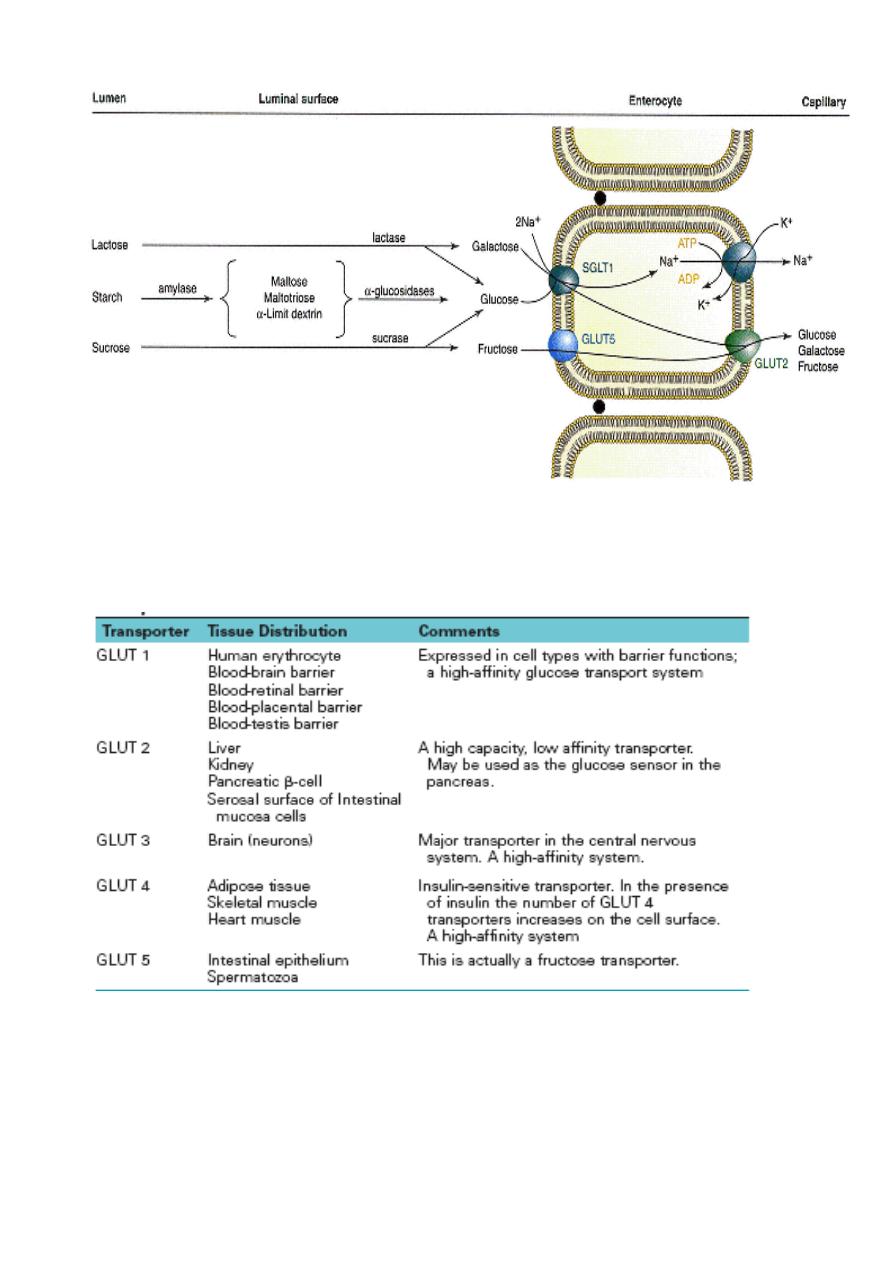
Glucose and galactose are taken into the enterocyte by cotransport with sodium using the
same transporter. Fructose enters the cell from the intestinal lumen via facilitated diffusion
through another transporter.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
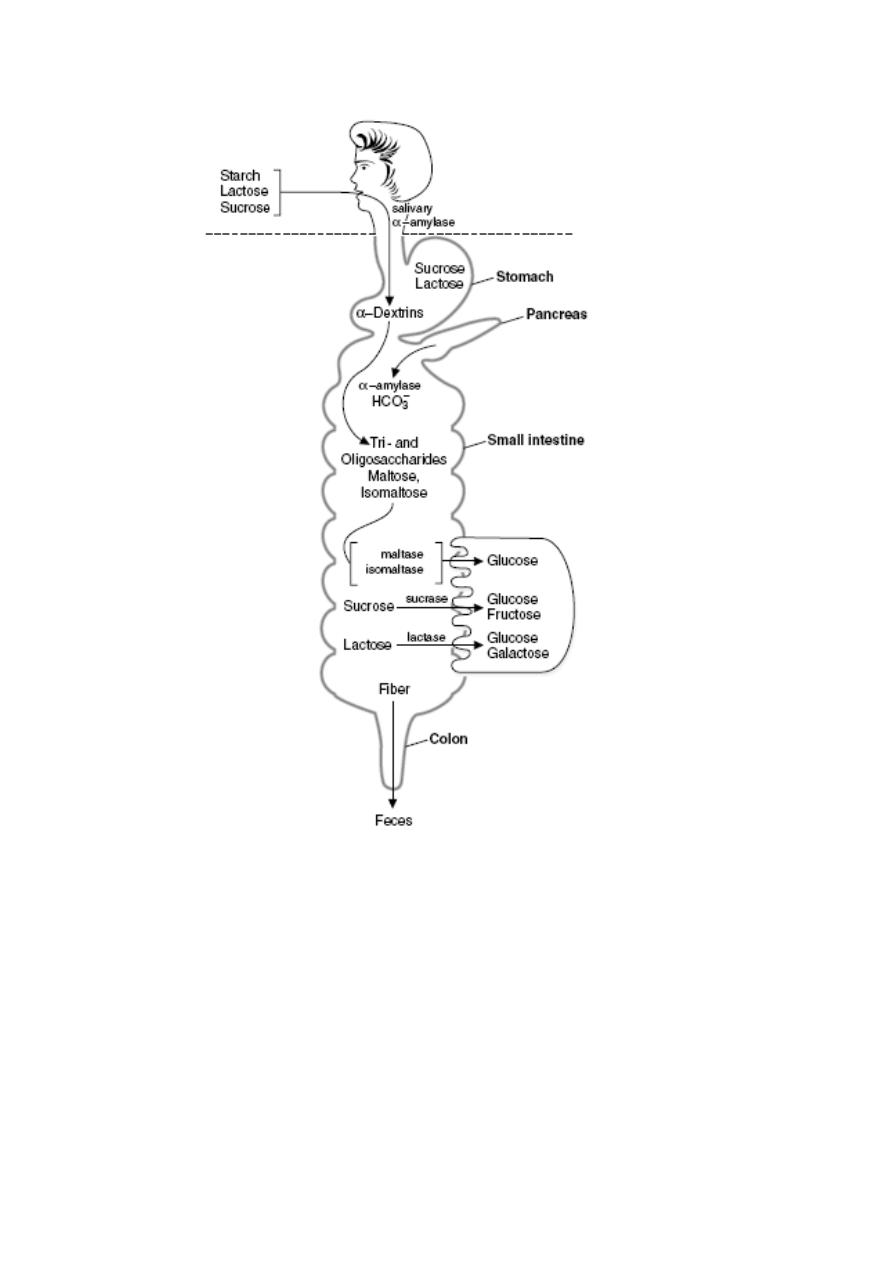
Overview of carbohydrate digestion. Digestion of the carbohydrates occurs first,
followed by absorption of monosaccharides. Subsequent metabolic reactions occur after
the sugars are absorbed.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
Absorption of Glucose and other
Monosaccharides:
Transport across the Intestinal Epithelium
Absorption of glucose entails transport from the
intestinal lumen, across the epithelium and into
blood. The transporter that carries glucose and
galactose into the enterocyte is the sodium-
dependent hexose transporter, known more
formally as SGLUT-1. As the name indicates, this
molecule transports both glucose and sodium ion
into the cell and in fact, will not transport either
alone.
The essence of transport by the sodium-
dependent hexose transporter involves a series of
conformational changes induced by binding and
release of sodium and glucose, and can be
summarized as follows:
1. the transporter is initially oriented facing
into the lumen - at this point it is capable of
binding sodium, but not glucose
2. sodium binds, inducing a conformational
change that opens the glucose-binding
pocket
3. glucose binds and the transporter reorients
in the membrane such that the pockets
holding sodium and glucose are moved
inside the cell
4. sodium dissociates into the cytoplasm, causing glucose binding to destabilize
5. glucose dissociates into the cytoplasm and the unloaded transporter reorients back
to its original, outward-facing position
Fructose is not co-transported with sodium. Rather it enters the enterocyte by another
hexose transporter (GLUT5).
Once inside the enterocyte, glucose and sodium must be exported from the cell into blood.
Sodium is rapidly shuttled out in exchange for potassium by the battery of sodium pumps
on the basolateral membrane, this process maintains the electrochemical gradient across
the epithelium. The massive transport of sodium out of the cell establishes the osmotic
gradient responsible for absorption of water.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
Na_-dependent and facilitative transporters in the intestinal epithelial cells. Both glucose and fructose are
transported by the facilitated glucose transporters on the luminal and serosal sides of the absorptive cells.
Glucose and galactose are transported by the Na_-glucose cotransporters on the luminal (mucosal) side of
the absorptive cells.
Properties of the GLUT 1-GLUT 5 Isoforms of the Glucose Transport Proteins
Genetic techniques have identified additional GLUT transporters (GLUT 7-12), but the role of these
transporters has not yet been fully described
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
CLINICAL CORRELATION
Disaccharidase Deficiency
Intestinal disaccharidase deficiencies are encountered relatively frequently in humans. Deficiency can
be present in one enzyme or several enzymes for a variety of reasons (genetic defect, physiological
decline with age, or the result of "injuries" to the mucosa). Of the disaccharidases, lactase is the most
common enzyme with an absolute or relative deficiency, which is experienced as milk intolerance. The
consequences of an inability to hydrolyze lactose in the upper small intestine are inability to absorb
lactose and bacterial fermentation of ingested lactose in the lower small intestine. Bacterial
fermentation results in the production of gas (distension of gut and flatulence) and osmotically active
solutes that draw water into the intestinal lumen (diarrhea). The lactose in yogurt has already been
partially hydrolyzed during the fermentation process of making yogurt. Thus individuals with lactase
deficiency can often tolerate yogurt better than unfermented dairy products. The enzyme lactase is
commercially available to pretreat milk so that the lactose is hydrolyzed
.
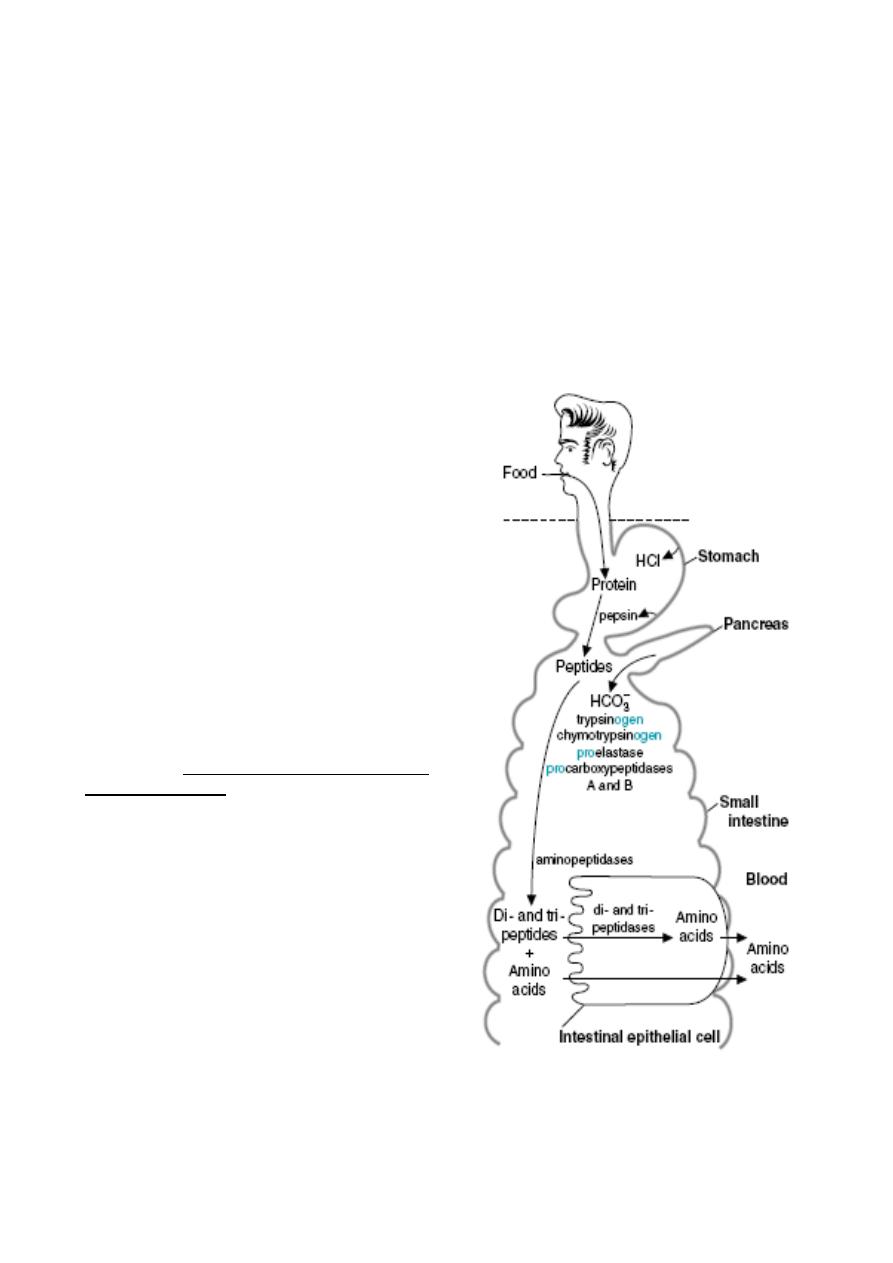
3) Absorption of Amino Acids and
Peptides
Dietary proteins are, with very few
exceptions, not absorbed. Rather, they
must be digested into amino acids or di-
and tripeptides first, through the action of
gastric and pancreatic proteases. The
brush border of the small intestine is
equipped with a family of peptidases. Like
lactase and maltase, these peptidases are
integral membrane proteins rather than
soluble enzymes. They function to further
the hydrolysis of lumenal peptides,
converting them to free amino acids and
very small peptides. These endproducts of
digestion, formed on the surface of the
enterocyte, are ready for absorption.
a) Absorption of Amino Acids
The mechanism by which amino acids are
absorbed is conceptually identical to that of
monosaccharides. The lumenal plasma
membrane of the absorptive cell bears at
least four sodium-dependent amino acid
transporters - one each for acidic, basic,
neutral and amino acids. These
transporters bind amino acids only after
binding sodium. The fully loaded transporter
then undergoes a conformational change
that dumps sodium and the amino acid into
the cytoplasm, followed by its reorientation
back to the original form.
Thus, absorption of amino acids is also
absolutely dependent on the
electrochemical gradient of sodium across
the epithelium. Further, absorption of amino acids, like that of monosaccharides,
contributes to generating the osmotic gradient that drives water absorption.
The basolateral membrane of the enterocyte contains additional transporters which export
amino acids from the cell into blood. These are not dependent on sodium gradients.

Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
CLINICAL CORRELATION
1.
Neutral Amino Aciduria (Hartnup Disease)
Transport functions, like enzymatic functions, are subject to modification by mutations. An example
of a genetic lesion in epithelial amino acid transport is Hartnup disease, named after the family in
which the disease entity resulting from the defect was first recognized. The disease is
characterized by the inability of renal and intestinal epithelial cells to absorb neutral amino acids
from the lumen. In the kidney, in which plasma amino acids reach the lumen of the proximal tubule
through the ultrafiltrate, the inability to reabsorb amino acids manifests itself as excretion of amino
acids in the urine (amino aciduria). The intestinal defect results in malabsorption of free amino
acids from the diet. Therefore the clinical symptoms of patients with this disease are mainly those
due to essential amino acid and nicotinamide deficiencies. The pellagra-like features are explained
by a deficiency of tryptophan, which serves as precursor for nicotinamide. Investigations of patients
with Hartnup disease revealed the existence of intestinal transport systems for di- or tripeptides,
which are different from the ones for free amino acids. The genetic lesion does not affect transport
of peptides, which remains as a pathway for absorption of protein digestion products
Q:
Why do patients with cystinuria and Hartnup disease have a hyperaminoaciduria without an
associated hyperaminoacidemia?
A:
Patients with cystinuria and Hartnup disease have defective transport proteins in both the
intestine and the kidney. These patients do not absorb the affected amino acids at a normal rate
from the digestive products in the intestinal lumen. They also do not readily resorb these amino
acids from the glomerular filtrate into the blood. Therefore, they do not have a hyperaminoacidemia
(a high concentration in the blood). Normally, only a few percent of the amino acids that enter the
glomerular filtrate are excreted in the urine; most are resorbed. In these diseases, much larger
amounts of the affected amino acids are excreted in the urine, resulting in a hyperaminoaciduria.
2.
Kwashiorkor,
A common problem of children in Third World countries, is caused by a deficiency of protein in a
diet that is adequate in calories. Children with kwashiorkor suffer from muscle wasting and a
decreased concentration of plasma proteins, particularly albumin. The result is an increase in
interstitial fluid that causes edema and a distended abdomen that make the children appear
“plump”. The muscle wasting is caused by the lack of essential amino acids in the diet; existing
proteins must be broken down to produce these amino acids for new protein synthesis. These
problems may be compounded by a decreased ability to produce digestive enzymes and new
intestinal epithelial cells because of a decreased availability of amino acids for the synthesis of new
proteins.
b) Absorption of Peptides
There is virtually no absorption of peptides longer than four amino acids. However, there is
abundant absorption of di- and tripeptides in the small intestine. These small peptides are
absorbed into the small intestinal epithelial cell by cotransport with H
+
ions via a transporter called
PepT1.
Once inside the enterocyte, the vast bulk of absorbed di- and tripeptides are digested into amino
acids by cytoplasmic peptidases and exported from the cell into blood. Only a very small number of
these small peptides enter blood intact.
c) Absorption of Intact Proteins
Absorption of intact proteins occurs only in a few circumstances. Normal" enterocytes do not have
transporters to carry proteins across the plasma membrane and they certainly cannot permeate
tight junctions.
One important exception is that for a very few days after birth, neonates have the ability to absorb
intact proteins. This ability, which is rapidly lost, is of immense importance because it allows the
newborn animal to acquire passive immunity by absorbing immunoglobulins in colostral milk. The
small intestine rapidly loses the capacity to absorb intact proteins.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
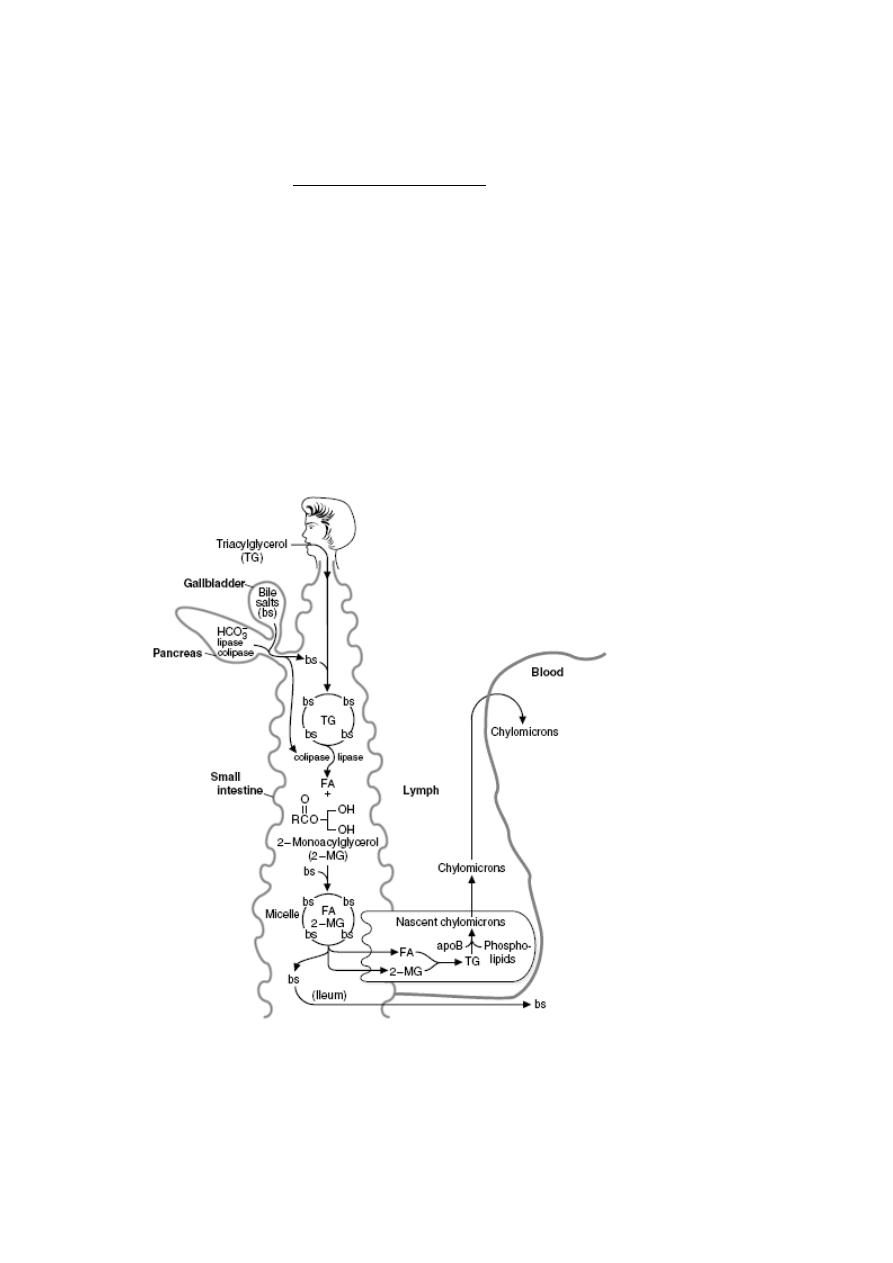
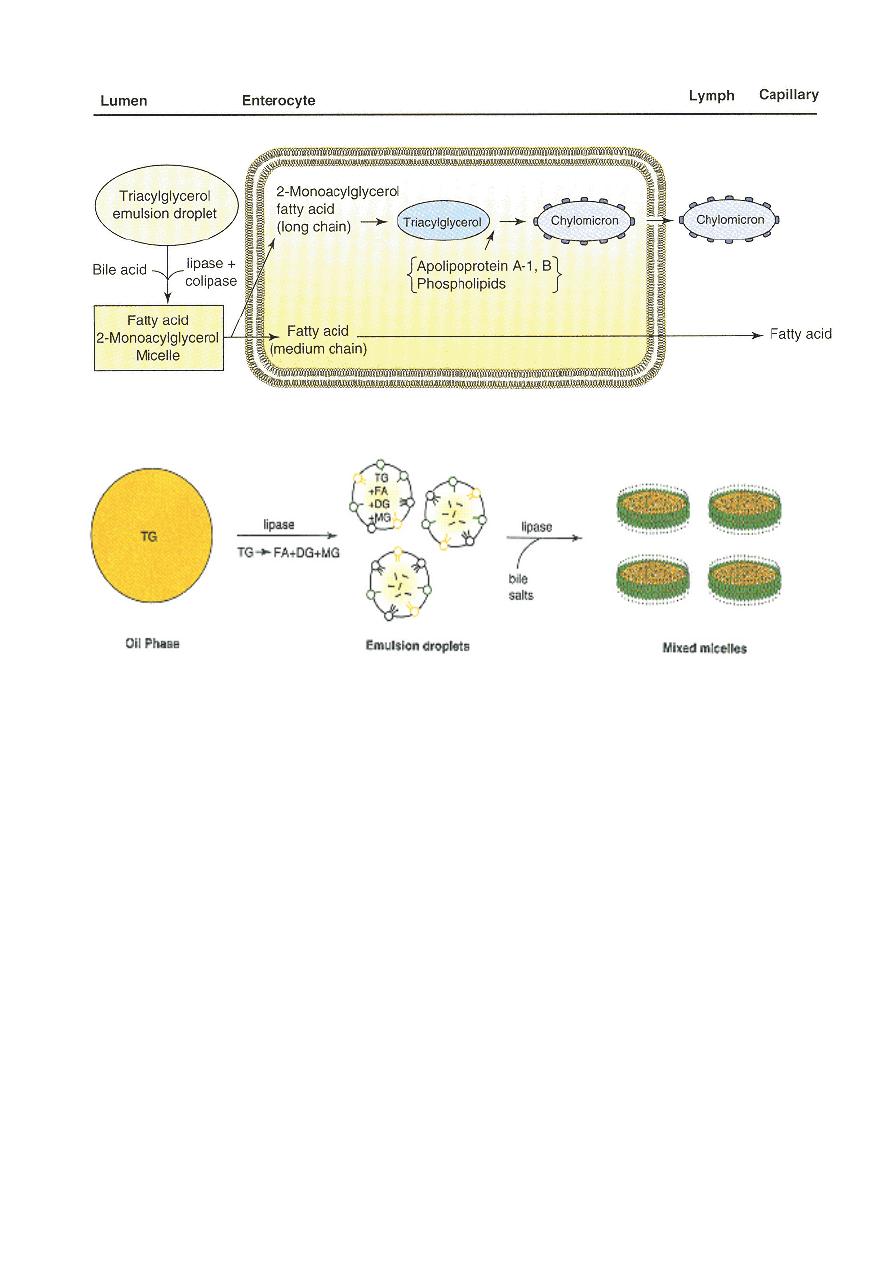
4) Absorption of Lipids
The bulk of dietary lipid is neutral fat or triglyceride, composed of a glycerol backbone with
each carbon linked to a fatty acid. Additionally, most foodstuffs contain phospholipids,
sterols like cholesterol and many minor lipids, including fat-soluble vitamins. In order for
the triglyceride to be absorbed, two processes must occur:
•
Large aggregates of dietary triglyceride, which are virtually insoluble in an aqueous
environment, must be broken down physically and held in suspension - a process
called emulsification.
•
Triglyceride molecules must be enzymatically digested to yield monoglyceride and
fatty acids, both of which can efficiently diffuse into the enterocyte
The key players in these two transformations are bile salts and pancreatic lipase, both of which are
mixed with chyme and act in the lumen of the small intestine.
Digestion of triacylglycerols in the intestinal lumen. TG _ triacylglycerol; bs _ bile
salts; FA _ fatty acid; 2-MG _ 2-monoacylglycerol
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
Digestion and absorption of lipids
Changes in physical state during triacylglycerol digestion.
Abbreviations: TG, triacylglycerol; DG, diacylglycerol; MG, monoacylglycerol; FA, fatty acid
CLINICAL CORRELATION
A-
β
-Lipoproteinemia
A-b-lipoproteinemia is an autosomal recessive disorder characterized by the absence of all lipoproteins
containing apo-
β
-lipoprotein, that is, chylomicrons, very low density lipoproteins (VLDLs), and low density
lipoproteins (LDLs). Serum cholesterol is extremely low. This defect is associated with severe malabsorption
of triacylglycerol and lipid-soluble vitamins (especially tocopherol and vitamin E) and accumulation of apo B
in enterocytes and hepatocytes. The defect does not appear to involve the gene for apo B, but rather one of
several proteins involved in processing of apo B in liver and intestinal mucosa, or in assembly and secretion
of triacylglycerol-rich lipoproteins, that is, chylomicrons and VLDLs from these tissues, respectively.
Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
5) Absorption of Minerals and Metals
The vast bulk of mineral absorption occurs in the small intestine. The best-studied
mechanisms of absorption are clearly for calcium
and iron, deficiencies of which are significant health
problems throughout the world.
a) Calcium
The quantity of calcium absorbed in the intestine is
controlled by how much calcium has been in the diet
during recent periods of time. Calcium is absorbed
by two distinct mechanisms:
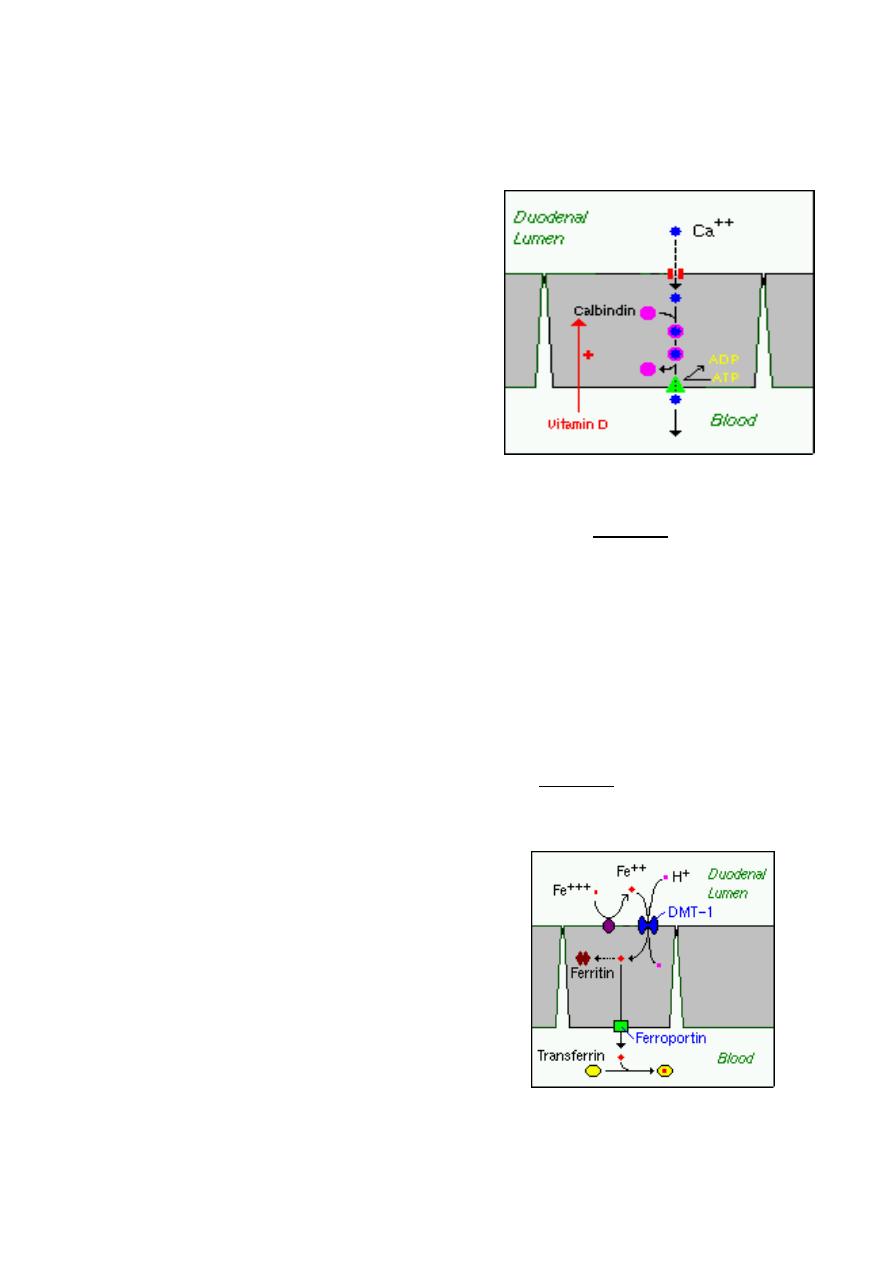
1. Active, transcellular absorption occurs only in the
duodenum when calcium intake has been low. This
process involves import of calcium into the enterocyte, transport across the cell, and
export into extracellular fluid and blood. The rate limiting step in transcellular calcium
absorption is transport across the epithelial cell, which is greatly enhanced by the carrier
protein calbindin, the synthesis of which is totally dependent on vitamin D.
2. Passive, paracellular absorption occurs in the jejunum and ileum, and, to a much lesser
extent, in the colon when dietary calcium levels have been moderate or high. In this case,
ionized calcium diffuses through tight junctions into the basolateral spaces around
enterocytes, and hence into blood. Such transport depends on having higher
concentrations of free calcium in the intestinal lumen than in blood.
b) Phosphorus
Phosphorus is predominantly absorbed as inorganic phosphate in the upper small
intestine. Phosphate is transported into the epithelial cells by cotransport with sodium, and
expression of this (or these) transporters is enhanced by vitamin D.
c) Iron
Iron homeostasis is regulated at the level of intestinal
absorption, and it is important that adequate but not
excessive quantities of iron be absorbed from the diet.
Inadequate absorption can lead to iron-deficiency
disorders such as anemia. On the other hand,
excessive iron is toxic because mammals do not have a
physiologic pathway for its elimination.
Iron is absorbed by villus enterocytes in the proximal
duodenum. Efficient absorption requires an acidic
environment.
Ferric iron (Fe+++) in the duodenal lumen is reduced to its ferrous form through the action
of a brush border ferrireductase. Iron is then co transported with a proton into the

Lecture 5
Tuesday 2/10/2012
Prof. Dr. H.D.El-Yassin
2012
enterocyte via the divalent metal transporter DMT-1. This transporter is not specific for
iron, and also transports many divalent metal ions.
Once inside the enterocyte, iron follows one of two major pathways:
•
Iron abundance states: iron within the enterocyte is trapped by incorporation into
ferritin and hence, not transported into blood. When the enterocyte dies and is
shed, this iron is lost.
•
Iron limiting states: iron is exported out of the enterocyte via a transporter
(ferroportin) located in the basolateral membrane. It then binds to the iron-carrier
transferrin for transport throughout the body.
d) Copper
There appear to be two processes responsible for copper absorption:
i) a rapid, low capacity system and
ii) a slower, high capacity system, which may be similar to the two processes seen
with calcium absorption.
Many of the molecular details of copper absorption remain to be elucidated. Inactivating
mutations in the gene encoding an intracellular copper ATPase have been shown
responsible for the failure of intestinal copper absorption in Menkes disease.
A number of dietary factors have been shown to influence copper absorption. For
example, excessive dietary intake of either zinc or molybdenum can induce secondary
copper deficiency states.
e) Zinc
Zinc homeostasis is largely regulated by its uptake and loss through the small intestine.
Although a number of zinc transporters and binding proteins have been identified in villus
epithelial cells, a detailed picture of the molecules involved in zinc absorption is not yet in
hand.
A number of nutritional factors have been identified that modulate zinc absorption. Certain
animal proteins in the diet enhance zinc absorption. Phytates from dietary plant material
(including cereal grains, corn, rice) chelate zinc and inhibit its absorption. Subsistence on
phytate-rich diets is thought responsible for a considerable fraction of human zinc
deficiencies.
