Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
I
I
n
n
a
a
c
c
t
t
i
i
v
v
a
a
t
t
i
i
o
o
n
n
a
a
n
n
d
d
D
D
e
e
t
t
o
o
x
x
i
i
f
f
i
i
c
c
a
a
t
t
i
i
o
o
n
n
o
o
f
f
X
X
e
e
n
n
o
o
b
b
i
i
o
o
t
t
i
i
c
c
s
s
a
a
n
n
d
d
M
M
e
e
t
t
a
a
b
b
o
o
l
l
i
i
t
t
e
e
s
s
i
i
n
n
t
t
h
h
e
e
L
L
i
i
v
v
e
e
r
r
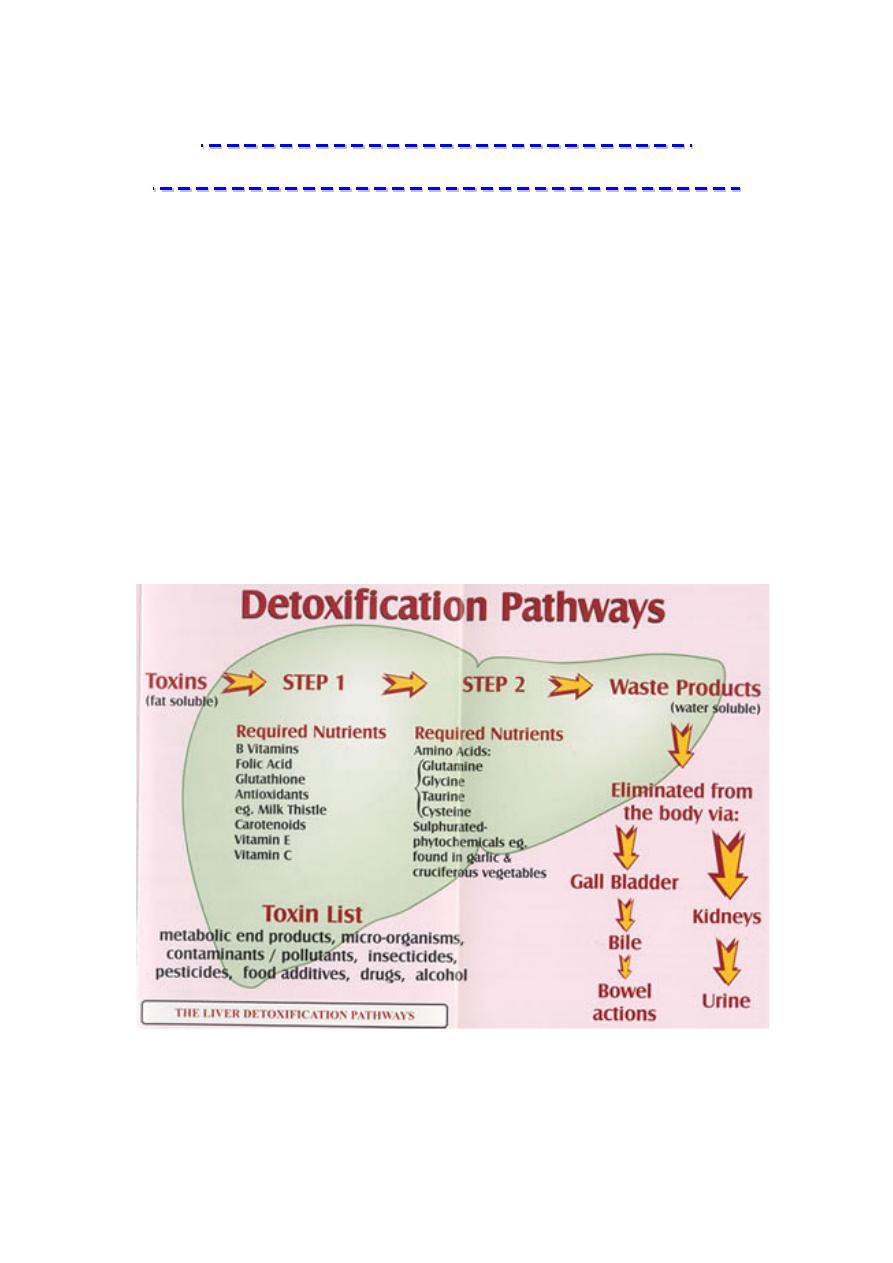
The liver is one of the most important organs in the body when it
comes to detoxifying or getting rid of foreign substances or toxins,
especially from the gut.
The liver detoxifies harmful substances by a complex series of
chemical reactions. The role of these various enzyme activities in the
liver is to convert fat soluble toxins into water soluble substances that
can be excreted in the urine or the bile depending on the particular
characteristics of the end product. Many of the toxic chemicals that
enter the body are fat-soluble, which means they dissolve only in
fatty or oily solutions and not in water. This makes them difficult for
the body to excrete. Fat soluble chemicals have a high affinity for fat
tissues and cell membranes, which are composed of fatty acids and
proteins. In these fatty tissues of the body, toxins may be stored for
years, being released during times of exercise, stress or fasting.
The liver plays several roles in detoxification: it filters the blood to
remove large toxins, synthesizes and secretes bile full of cholesterol
and other fat-soluble toxins, and enzymatically disassembles
unwanted chemicals.

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
The enzymatic process usually occurs in two steps
referred to as: phase I and phase II.
Xenobiotics are compounds that have no nutrient value (cannot
be used by the body for energy requirements) and are potentially toxic.
They are present as natural components of foods or they may be
introduced into foods as additives or through processing.
Pharmacologic and recreational drugs are also xenobiotic compounds.
The liver is the principal site in the body for the degradation of these
compounds. Because many of these substances are lipophilic, they are
oxidized, hydroxylated, or hydrolyzed by enzymes in phase I reactions.
Phase I reactions introduce or expose hydroxyl groups or other reactive
sites that can be used for conjugation reactions (the phase II reactions).
The conjugation reactions add a negatively charged group such as
glycine or sulfate to the molecule. Many xenobiotic compounds will be
transformed
through several different pathways.
Phase I either directly neutralizes a toxin, or modifies the toxic chemical to form activated intermediates
which are then neutralized by one of more of the several phase II enzyme systems
.
The conjugation and inactivation pathways are similar to those used by
the liver to inactivate many of its own metabolic waste products. These
pathways are intimately related to the biosynthetic cascades that exist
in the liver. The liver can synthesize the precursors that are required for
conjugation and inactivation reactions from other compounds. For
example, sulfation is used by the liver to clear steroid hormones from
the circulation. The sulfate used for this purpose can be obtained from
the degradation of cysteine or methionine. The liver, kidney, and
intestine are the major sites in the body for biotransformation of
xenobiotic compounds. Many xenobiotic compounds contain aromatic
rings (such as benzopyrene in tobacco smoke) or heterocyclic ring
structures (such as the nitrogen-containing rings of nicotine or
pyridoxine) that we are unable to degrade or recycle into useful
components. These structures are hydrophobic, causing the molecules
to be retained in adipose tissue unless they are sequestered by the
liver, kidney, or intestine for biotransformation reactions. Sometimes,
however, the phase I and II reactions backfire, and harmless
hydrophobic molecules are converted to toxins or potent chemical
carcinogens.
Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
The level of exposure to environmental carcinogens varies widely, as does
the efficiency of the detoxification enzymes, particularly phase II. High levels
of exposure to carcinogens coupled with slow detoxification enzymes
significantly increases susceptibility to cancer
.
Phase I Detoxification
CYTOCHROME P450 AND XENOBIOTIC METABOLISM
The cytochrome P450 enzyme family contains at least 100 to 150
different isozymes. The human enzymes are generally divided into six
major subfamilies, and each of these is further subdivided. For example,
in the naming of the principal enzyme involved in the oxidation of
ethanol to acetaldehyde, CYP2E1, the CYP denotes the cytochrome
P450 family, the 2 denotes the subfamily, the E denotes ethanol, and
the 1 denotes the specific isozyme.
The cytochrome P450–dependent monooxygenase enzymes are
determinants in oxidative, peroxidative, and reductive degradation of
exogenous (chemicals, carcinogens, and pollutants, etc.) and
endogenous (steroids, prostaglandins retinoids, etc.) substances. The
key enzymatic constituents of this system are the flavo-protein NADPH-
cytochrome P450 oxidoreductase and cytochrome P450.
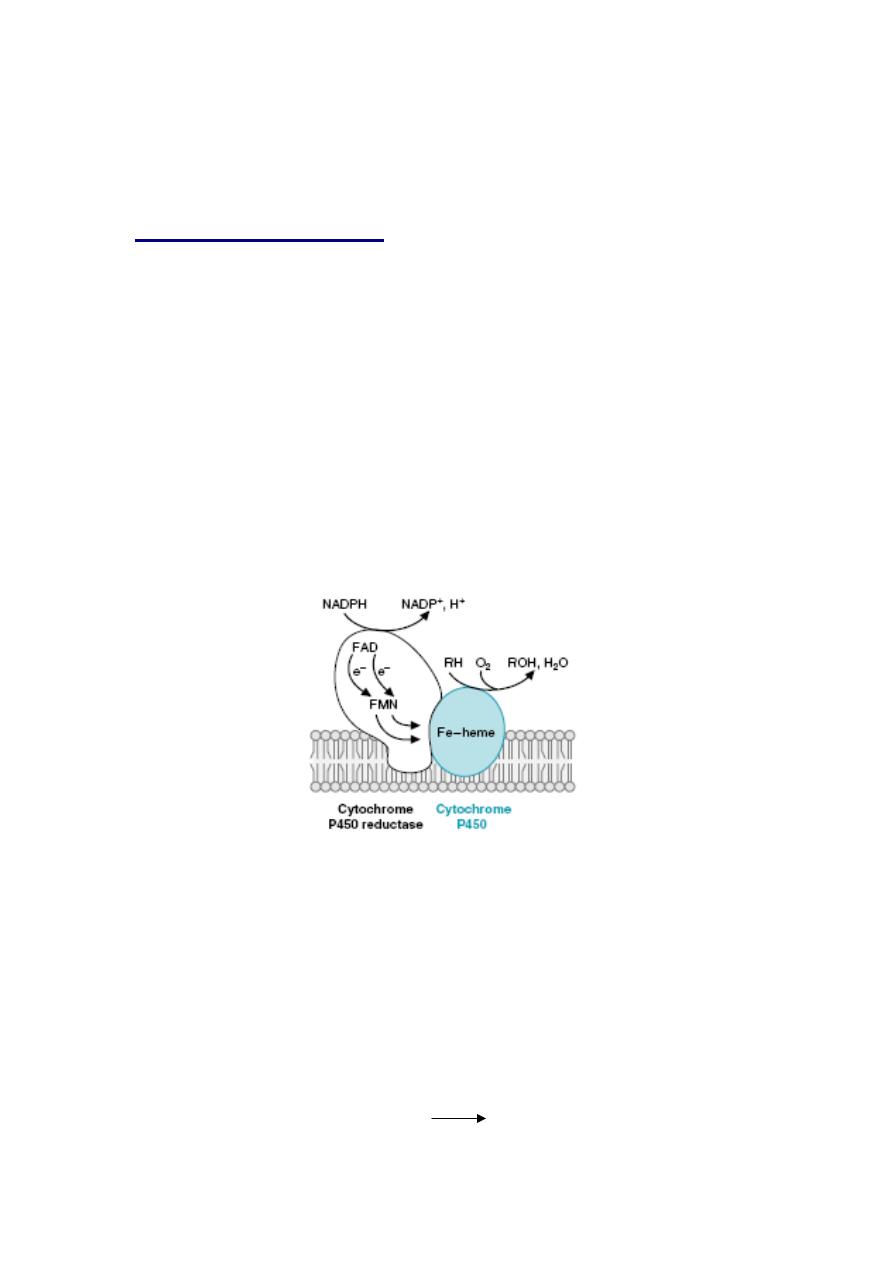
General structure of the P450 enzymes. O
2
binds to the P450 Fe-heme in the active site
and is activated to a reactive form by accepting electrons. The electrons are donated
by the cytochrome P450 reductase, which contains an FAD plus an FMN or Fe-S center
to facilitate the transfer of single electrons from NADPH to O2. The P450 enzymes
involved in steroidogenesis have a somewhat different structure. For CYP2E1, RH is
ethanol (CH3CH2OH), and ROH is acetaldehyde (CH3COH).
Monooxygenase incorporate one atom from molecular oxygen into a substrate
(creating a hydroxyl group), with the other atom being reduced to water. In the
cytochrome P450 monooxygenase system NADPH provides the reducing
equivalents required by the series of reactions. This system performs different
functions in two separate locations in cells.The overall reaction catalyzed by a
cytochrome P450 enzyme is:
R-H + O
2
+ NADPH + H
+
R-OH + H
2
O + NADP
+
where R may be a steroid, drug or other chemical.

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
The cytochrome P450 isozymes all have certain features in common:
1. They all contain cytochrome P450, oxidize the substrate, and
reduce oxygen.
2. They all have a flavin-containing reductase subunit that uses
NADPH, and not NADH, as a substrate.
3. They are all found in the smooth endoplasmic reticulum and are
referred to as microsomal enzymes (for example, CYP2E1 is also
referred to as the microsomal ethanol oxidizing system, MEOS).
4. They are all bound to the lipid portion of the membrane, probably
to phosphatidylcholine.
5. They are all inducible by the presence of their own best substrate
and somewhat less inducible by the substrates for other P450
isozymes.
6. They all generate a reactive free radical compound as an
intermediate in the reaction.
Excessive amounts of toxic chemicals such as pesticides can disrupt
the P-450 enzyme system by causing hyper activity or what is called
'induction' of this pathway. This will result in high levels of damaging
free radicals being produced. Substances that may cause hyperactivity
of the P- 450 enzymes: Caffeine, Alcohol, Dioxin, Saturated fats,
Organophosphorus pesticides, Paint fumes, Sulfonamides, Exhaust
fumes, Barbiturates.
Transforming a toxin to a more chemically reactive form makes it more
easily metabolized by the phase II enzymes.
If the phase II detoxification systems are not working adequately, these
intermediates can cause substantial damage, including the initiation of
carcinogenic processes. Each enzyme works best in detoxifying certain
types of chemicals, but with considerable overlap in activity among the
enzymes.
The activity of the various cytochrome P450 enzymes varies
significantly from one individual to another, based on genetics, the
individual's level of exposure to chemical toxins, and his or her
nutritional status. Since the activity of cytochrome P450 varies so much,
so does an individual's risk for various diseases. This variability of
cytochrome P450 enzymes is seen in the variability of people's ability to
detoxify the carcinogens found in cigarette smoke and helps to explain
why some people can smoke with only modest damage to their lungs,
while others develop lung cancer after only a few decades of smoking.
A significant side-effect of phase I detoxification is the production of free
radicals as the toxins are transformed--for each molecule of toxin
metabolized by phase I, one molecule of free radical is generated.

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
Without adequate free radical defenses, every time the liver neutralizes
a toxin exposure, it is damaged by the free radicals produced.
The most important antioxidant for neutralizing the free radicals
produced in phase I is glutathione. In the process of neutralizing free
radicals, however, glutathione (GSH) is oxidized to glutathione disulfide
(GSSG). Glutathione is required for one of the key phase II
detoxification processes. When high levels of toxin exposure produce
so many free radicals from phase I detoxification that the glutathione is
depleted, the phase II processes dependent upon glutathione stop,
producing oxidative stress or liver damage. The toxins transformed into
activated intermediates by phase I are substantially more reactive than
the phase I toxins were. Unless quickly removed from the body by
phase II detoxification mechanisms, they can cause widespread
problems, especially carcinogenesis. Therefore, the rate at which phase
I produces activated intermediates must be balanced by the rate at
which phase II finishes their processing. People with a very active
phase I detoxification system coupled with slow or inactive phase II
enzymes are termed pathological detoxifiers. These people suffer
unusually severe toxic reactions to environmental poisons.
An efficient liver detoxification system is vital to health and in order to
support this process it is essential that many key nutrients are included
in the diet. Vitamins and minerals – particularly the B vitamins – play a
major role, acting as cofactors for many enzyme systems including
those of liver detoxification. Depletion of vitamin C may also impair the
detoxification process; vitamin C also prevents free radical formation.
Vitamin E and selenium are cofactors for glutathione peroxidase activity
as well as being powerful antioxidants. Other nutrients which play vital
roles in the Phase II pathway include amino acids glycine, cysteine,
glutamine, methionine, taurine, glutamic acid and aspartic acid.
Grapefruit juice, which contains naringenin, slows down Phase I
enzyme activity.
As with all enzymes, the cytochrome P450s require several nutrients to
function, such as copper, magnesium, zinc and vitamin C.

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
Phase II Detoxification
This is called the conjugation pathway, whereby the liver cells add
another substance (eg. cysteine, glycine or a sulphur molecule) to a
toxic chemical or drug. This makes the toxin or drug water-soluble, so it
can then be excreted from the body via watery fluids such as bile or
urine. Individual xenobiotics and metabolites usually follow one or two
distinct pathways.
There are essentially six phase II detoxification pathways:
1. Glutathione conjugation
2. Amino acid conjugation
3. Methylation
4. Sulfation
5. Acetylation
6. Glucuronidation
1. Glutathione conjugation
A primary phase II detoxification route is
conjugation with glutathione
(γ-
glutamylcysteinylglycine), (a tripeptide
composed of three amino acids--cysteine,
glutamic acid, and glycine).
Glutathione conjugation produces water-soluble
mercaptates which are excreted via the kidneys.
The elimination of fat-soluble compounds,
especially heavy metals like mercury and lead,
is dependent upon adequate levels of
glutathione, which in turn is dependent upon adequate levels of
methionine and cysteine. When increased levels of toxic compounds
are present, more methionine is utilized for cysteine and glutathione
synthesis. Methionine and cysteine have a protective effect on
glutathione and prevent depletion during toxic overload. This, in turn,
protects the liver from the damaging effects of toxic compounds and
promotes their elimination.
If the availability of methionine is reduced, not only will the capability of
the liver to detoxify be impaired, but there will also be less glutathione
available to complex with foreign substances.
Studies have demonstrated that a deficiency of methionine can, in itself,
cause liver cancer without the presence of a carcinogen, and also that
the deficiency of methionine can permit a heavy metal to cause toxic
effects.
Glutathione is also an important antioxidant. This combination of
detoxification and free radical protection, results in glutathione being
one of the most important anticarcinogens and antioxidants in our cells,

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
which means that a deficiency is cause of serious liver dysfunction and
damage. Exposure to high levels of toxins depletes glutathione faster
than it can be produced or absorbed from the diet. This results in
increased susceptibility to toxin-induced diseases, such as cancer,
especially if phase I detoxification system is highly active.
A deficiency can be induced either by diseases that increase the need
for glutathione, deficiencies of the nutrients needed for synthesis, or
diseases that inhibit its formation. Glutathione is available through two
routes: diet and synthesis. Dietary glutathione (found in fresh fruits and
vegetables, cooked fish, and meat) is absorbed well by the intestines
and does not appear to be affected by the digestive processes. Dietary
glutathione in foods appears to be efficiently absorbed into the blood.
2. Amino acid conjugation
Several amino acids (glyucine, taurine, glutamine, arginine, and
ornithine) are used to combine with and neutralize toxins. Of these,
glycine is the most commonly utilized in phase II amino acid
detoxification.
Patients suffering from hepatitis, alcoholic liver disorders, carcinomas,
chronic arthritis, hypothyroidism, toxemia of pregnancy, and excessive
chemical exposure are commonly found to have a poorly functioning
amino acid conjugation system.
Even in normal adults, a wide variation exists in the activity of the
glycine conjugation pathway. This is due not only to genetic variation,
but also to the availability of glycine in the liver. Glycine, and the other
amino acids used for conjugation, become deficient on a low-protein
diet and when chronic exposure to toxins results in depletion.
3. Methylation
Methylation involves conjugating methyl groups to toxins.
Most of the methyl groups used for detoxification comes from S-
adenosylmethionine (SAM). SAM is synthesized from the amino acid
methionine, a process which requires the nutrients choline, the active
form of B
12
--methyl cobalamin, and the active form of folic acid --5-
methyltetrahydrofolate. Methionine is a major source of numerous
sulfur-containing compounds, including the amino acids cysteine and
taurine.
4. Sulfation
Sulfation is the conjugation of toxins with sulfur-containing compounds.
The sulfation system is important for detoxifying several drugs, food
additives, and, especially, toxins from intestinal bacteria and the
environment. In addition to environmental toxins, sulfation is also used
to detoxify some normal body chemicals and is the main pathway for
the elimination of steroid and thyroid hormones. Since sulfation is also
the primary route for the elimination of neurotransmitters, dysfunction in

Lecture 4
Sunday 30/9/2012
Prof. Dr.H.D.El-Yasin
this system may contribute to the development of some nervous system
disorders.
Many factors influence the activity of sulfate conjugation. For example,
a diet low in methionine and cysteine has been shown to reduce
sulfation.
5. Acetylation
Conjugation of toxins with acetyl-CoA is the primary method by which
the body eliminates sulfa drugs. This system appears to be especially
sensitive to genetic variation, with those having a poor acetylation
system being far more susceptible to sulfa drugs and other antibiotics.
While not much is known about how to directly improve the activity of
this system, it is known that acetylation is dependent on thiamine,
pantothenic acid, and vitamin C.
6. Glucuronidation
Glucuronidation, the combining of glucuronic acid with toxins, in Phase
II can be reversed by Beta glucuronidase enzymes produced by
pathological bacteria and cause toxins to be reabsorbed increasing
toxicity. Many of the commonly prescribed drugs are detoxified through
this pathway. It also helps to detoxify aspirin, menthol, vanillin (synthetic
vanilla), food additives such as benzoates, and some hormones.
Sulfoxidation
Sulfoxidation is the process by which the sulfur-containing molecules in
drugs and foods are metabolized. It is also the process by which the
body eliminates the sulfite food additives used to preserve many foods
and drugs. Normally, the enzyme sulfite oxidase (molybdenum
dependentenzyme) metabolizes sulfites to safer sulfates, which are
then excreted in the urine. Those with a poorly functioning sulfoxidation
system, however, have an increased ratio of sulfite to sulfate in their
urine. Those with a poorly functioning sulfoxidation detoxification
pathway are more sensitive to sulfur-containing drugs and foods
containing sulfur or sulfite additives.
