MACROLIDES
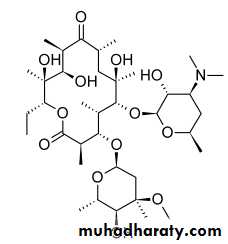
The macrolide antibiotics have three common chemical characteristics:(a) a large lactone ring (which prompted the
name macrolide),
(b) a ketone group, and
(c) a glycosidically linked amino sugar
Usually, the lactone ring has 12, 14, or 16 atoms in it, and it is often unsaturated, with an olefinic group conjugated with the ketone function
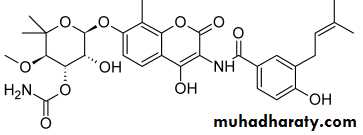
Erythromycin
Because of the dimethyl amino group on the sugar moiety, the macrolides are bases that form salts with pKa values between 6.0 and 9.0.
This feature has been used to make clinically useful salts.
The free bases are only slightly soluble in water but dissolve in somewhat polar organic solvents.
They are stable in aqueous solutions at or below room temperature but are inactivated by acids, bases, and heat.
Mechanism of Action and Resistance
It binds selectively to a specific site on the 50S ribosomal subunit to prevent the translocation step of bacterial protein synthesis
nonspecific resistance erythromycin among many species of Gram-negative bacilli largely related to the inability of the antibiotic to penetrate the cell walls of these organisms
In fact, the sensitivities of members of the Enterobacteriaceae family are pH dependent, with MICs decreasing as a function of increasing pH
protoplasts from Gram-negative bacilli, which lack cell walls, are sensitive to erythromycin
Specific resistance mechanism to the macrolide antibiotics occurs in erythromycin-resistant strains of S. aureus
Spectrum of Activity
The spectrum of antibacterial activity of the more potent macrolides, such as erythromycin, resembles that of penicillinIn contrast to penicillin, macrolides are also effective against Mycoplasma, Chlamydia, Campylobacter, and Legionella spp.
Their activity against most species of Gram-negative bacilli is generally low
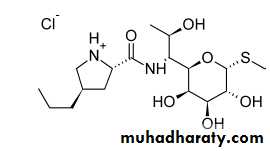
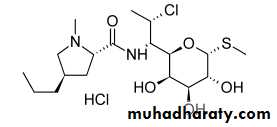
LINCOMYCINS
The lincomycins are sulfur-containing antibiotics isolated from Streptomyces lincolnensisExtensive efforts to modify the lincomycin structure to improve its antibacterial and pharmacological properties resulted in the preparation of the 7-chloro-7-deoxy derivative clindamycin
Its appears to have the greater antibacterial potency and better pharmacokinetic properties than lincomycin
They are primarily active against Grampositive bacteria, particularly the cocci
A pattern of bacterial resistance and cross-resistance to lincomycins similar to that observed with the macrolides has been emerging
POLYPEPTIDES
Among the most powerful bactericidal antibiotics are those that possess a polypeptide structureTheir clinical use has been limited by their undesirable side reactions, particularly renal toxicity
Another limitation is the lack of systemic activity of most peptides following oral administration
Polypeptide antibiotics variously possess several interesting and often unique characteristics:
(a) they frequently consist of several structurally similar but chemically distinct entities isolated from a single source;
(b) most of them are cyclic, with a few exceptions (e.g., the gramicidins);
(c) they frequently contain D-amino acid and/or“unnatural” amino acids not found in higher plants or animals; and
(d) many of them contain non–amino acid moieties, such as heterocycles, fatty acids, sugars, etc
Polypeptide antibiotics may be acidic, basic, zwitterionic, or neutral depending on the number of free carboxyl and amino or guanidino groups in their structures
Antibiotics of the polypeptide class differ widely in their mechanisms of action and antimicrobial properties.
Bacitracin and vancomycin interfere with bacterial cell wall synthesis and are effective only against Gram-positive bacteria
Neither antibiotic apparently can penetrate the outer
envelope of Gram-negative bacteria.
Both the gramicidins and the polymyxins interfere with cell membrane functions in bacteria
The gramicidins are effective primarily against Gram-positive bacteria, whereas the polymyxins are effective only against Gram-negative species
Polymyxins are highly basic compounds that penetrate the outer membrane of Gram-negative bacteria through porin channels to act on the inner cell membrane
UNCLASSIFIED ANTIBIOTICS
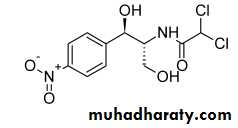
Chloramphenicol;Chloramphenicol possesses two chiral carbon atoms in the acylamidopropanediol chain.
Biological activity resides almost exclusively in the D-threo isomer; the L-threo and the D- and L-erythro isomers are virtually inactive
Chloramphenicol is very stable in the bulk state and in solid dosage forms.
In solution, however, it slowly undergoes various hydrolytic and light-induced reactions
Because it is bitter, this antibiotic is administered orally either in capsules or as the palmitate ester.Chloramphenicol palmitate is insoluble in water and may be suspended in aqueous vehicles for liquid dosage forms
Sterile chloramphenicol sodium succinate has been used to prepare aqueous solutions for intravenous injection.
Novobiocin Sodium
In the search for new antibiotics, three different research groups independently isolated novobiocin, streptonivicin (Albamycin) from Streptomyces spp.Chemically, novobiocin has a unique structure among antibiotics, though, like several others, it possesses a glycosidic sugar moiety.
The sugar in novobiocin, devoid of its carbamate ester, has been named noviose .
The aglycon moiety has been termed novobiocic acid.
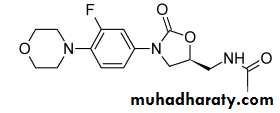
Linezolid
Linezolid (Zyvox) is an oxazolidinedione-type antibacterial agent that inhibits bacterial protein synthesisLinezolid binds to the 30S and 70S ribosomal subunits and prevents initiation complexes involving these subunits
Linezolid possesses a wide spectrum of activity against Gram-positive organisms, including MRSA, penicillin-resistant pneumococci
The indications for linezolid are for complicated and uncomplicated skin and soft-tissue infections, community and hospital-acquired pneumonia, and drug-resistant Grampositive infection
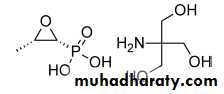
Fosfomycin Tromethamine
Fosfomycin tromethamine (Monurol) is a phosphonic acid epoxide derivative that was initially isolated from fermentations of Streptomyces sppFosfomycin is a broad-spectrum, bactericidal antibacterial that inhibits the growth of E. coli, S. aureus, and Serratia, Klebsiella, Citrobacter, Enterococcus, and Enterobacter spp. at a concentration less than 64 mg/L.
Currently fosfomycin is recommended as single-dose therapy for uncomplicated urinary tract infections.
It possesses in vitro efficacy similar to that of norfloxacin and trimethoprim-sulfamethoxazole
NEWER ANTIBIOTICS
Tigecycline
Tigecycline (Tygacil) is a first-in-class (a glycylcycline) intravenous antibiotic that was designed to circumvent many important bacterial resistance mechanisms.
It is not affected by resistance mechanisms such as ribosomal protection, efflux pumps, target site modifications, or DNA gyrase mutations.
Tigecycline binds to the 30S ribosomal subunit and blocks peptide synthesis.
The glycylcyclines bind to the ribosome with five times the affinity of common tetracyclines
NEW DIRECTIONS IN ANTIBIOTICDISCOVERY
Multidrug resistant bacteria have become a major public health crisis because existing antibiotics are no longer effective in many cases.Antibiotics like vancomycin that have traditionally been drugs of last resort are becoming the first line of treatment of resistant infections.
in recent times very few novel antibiotics have been reported, and the development of new compounds by the pharmaceutical industry has been slow
It is essential to discover antibiotics that act through the disruption of a novel target
the research of scientists at Merck, who conducted of specialized metabolites against FabF, an enzyme that is involved in bacterial fatty acid biosynthesis
In nature there are two distinct types of fatty acid biosynthesis pathways. Type 1 is referred to as the associated system, whereas type 2 is referred to as the dissociated system
Associated systems are found in higher organisms.
These are composed of a large multidomain protein that is capable of catalyzing all of the steps of fatty acid biosynthesis.
Dissociated systems are found in plants and bacteria In these systems a set of discrete enzymes each catalyze a single step in the biosynthetic pathway, Hence, type 2 biosynthesis represents a good target for novel antibiotics
Moreover, two enzymes of the dissociated pathway, FabH and FabF/B, are well-conserved across many bacterial strains.
This fact goes hand in hand with broad spectrum activity
These screenings led to the discovery of a new antibiotic hitting a new target, platensimycin
In vitro, platensimycin compares favorably with linezolid.
No cross-resistance to MRSA, vancomycin-intermediate S. aureus, and vancomycin-resistant enterococci has been observed