HORMONAL FACTORS IN PREGNANCY
Lecture 714-3-2016
In pregnancy, the placenta forms especially large quantities of human chorionic gonadotropin, estrogens, progesterone, and human chorionic somatomammotropin, the first three of which, and probably the fourth as well, are all essential to a normal pregnancy.
1-HUMAN CHORIONIC GONADOTROPIN CAUSES PERSISTENCE OF
THE CORPUS LUTEUM AND PREVENTS MENSTRUATIONMenstruation normally occurs in a nonpregnant woman about 14 days after ovulation, at which time most of the endometrium of the uterus sloughs away from the uterine
wall and is expelled to the exterior. If this should happen after an ovum has implanted, the pregnancy would terminate. However, this sloughing is prevented by the secretion of human chorionic gonadotropin by the newly developing embryonic tissues.
Coincidental with the development of the trophoblast cells from the early fertilized ovum, the hormone human chorionic gonadotropin is secreted by the syncytial trophoblast
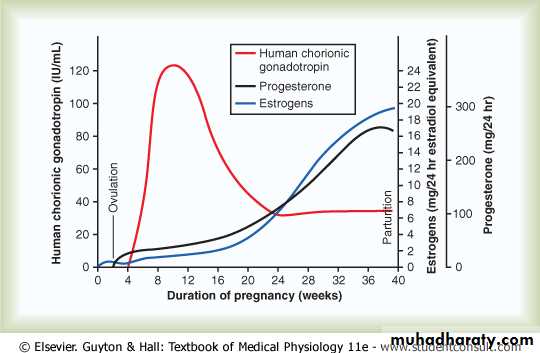
cells into the fluids of the mother, as shown in Figure 83-7. The secretion of this hormone can first be measured in the blood 8 to 9 days after ovulation, shortly
after the blastocyst implants in the endometrium. Then the rate of secretion rises rapidly to reach a maximum at about 10 to 12 weeks of pregnancy and decreases back to
a lower value by 16 to 20 weeks. It continues at this level for the remainder of the pregnancy.
Function of Human Chorionic Gonadotropin. Human chorionic gonadotropin is a glycoprotein having a molecular weight of about 39,000 and much the same molecular
structure and function as luteinizing hormone secreted by the pituitary gland. By far, the most important function of human chorionic gonadotropin is to prevent involution
of the corpus luteum at the end of the monthly female sexual cycle. Instead, it causes the corpus luteum to secrete even larger quantities of its sex hormones—progesterone and estrogens—for the next few months. These sex hormones prevent menstruation and cause the endometrium to continue to grow and store large amounts of nutrients rather than being shed in the menstruum. As a result, the decidua-like cells that develop in the endometrium during the normal female sexual cycle become actual decidual cells—greatly swollen and nutritious—at about the time that the blastocyst implants.
Under the influence of human chorionic gonadotropin, the corpus luteum in the mother’s ovary grows to about twice its initial size by a month or so after pregnancy begins. Its continued secretion of estrogens and progesterone maintains the decidual nature of the uterine endometrium, which is necessary for the early development
of the fetus.
If the corpus luteum is removed before approximately the seventh week of pregnancy, spontaneous abortion almost always occurs, sometimes even up to the 12th week. After that time, the placenta secretes sufficient quantities of progesterone and estrogens to maintain pregnancy for the remainder of the gestation period. The corpus luteum involutes slowly after the 13th to 17th week of gestation.
Figure 82-7 Rates of secretion of estrogens and progesterone, and concentration of human chorionic gonadotropin at different stages of pregnancy
Human Chorionic Gonadotropin Stimulates the Male Fetal Testes to Produce Testosterone. Human chorionic gonadotropin also exerts an interstitial cell– stimulating effect on the testes of the male fetus, resulting in the production of testosterone in male fetuses until the time of birth. This small secretion of testosterone during gestation is what causes the fetus to grow male sex organs instead of female organs. Near the end of pregnancy, the testosterone secreted by the fetal testes also causes the testes to descend into the scrotum.
2-SECRETION OF ESTROGENS BY THE PLACENTA
The placenta, like the corpus luteum, secretes both estrogens and progesterone. Histochemical and physiological studies show that these two hormones, like most otherplacental hormones, are secreted by the syncytial trophoblast cells of the placenta.
Figure 83-7 shows that toward the end of pregnancy, the daily production of placental estrogens increases to about 30 times the mother’s normal level of production. However, the secretion of estrogens by the placenta is quite different from secretion by the
ovaries. Most important, the estrogens secreted by the placenta are not synthesized de novo from basic substrates in the placenta. Instead, they are formed almost entirely from androgenic steroid compounds, dehydroepiandrosterone and 16 hydroxydehydroepiandrosterone, which are formed both in the mother’s adrenal glands
and in the adrenal glands of the fetus. These weak androgens are transported by the blood to the placenta and converted by the trophoblast cells into estradiol, estrone, and estriol. (The cortices of the fetal adrenal glands are extremely large, and about 80 percent consists of a so-called fetal zone, the primary function of which seems to be to secrete dehydroepiandrosterone during pregnancy.)
Function of Estrogen in Pregnancy. In Chapter 82, we pointed out that estrogens exert mainly a proliferative function on most reproductive and associated organs of
the mother. During pregnancy, the extreme quantities of estrogens cause (1) enlargement of the mother’s uterus, (2) enlargement of the mother’s breasts and growth of the
breast ductal structure, and (3) enlargement of the mother’s female external genitalia.
The estrogens also relax the pelvic ligaments of the mother, so the sacroiliac joints become relatively limber and the symphysis pubis becomes elastic. These changes
allow easier passage of the fetus through the birth canal. There is much reason to believe that estrogens also affect many general aspects of fetal development during pregnancy,
for example, by affecting the rate of cell reproduction in the early embryo.
3-SECRETION OF PROGESTERONE BY THE PLACENTA
Progesterone is also essential for a successful pregnancy; in fact, it is just as important as estrogen. In addition to being secreted in moderate quantities by the corpus luteum at the beginning of pregnancy, progesterone is secreted later in tremendous quantities by the placenta, as shown in Figure 83-7.
The following special effects of progesterone are essential
for the normal progression of pregnancy:
1. Progesterone causes decidual cells to develop in the uterine endometrium. These cells play an important role in the nutrition of the early embryo.
2. Progesterone decreases the contractility of the pregnant uterus, thus preventing uterine contractions from causing spontaneous abortion.
3. Progesterone contributes to the development of the conceptus even before implantation because it specifically increases the secretions of the mother’s fallopian tubes and uterus to provide appropriate nutritive matter for the developing morula (the spherical mass of 16 to 32 blastomeres formed before the blastula) and blastocyst. There is also reason to believe that progesterone affects cell cleavage in the early developing embryo.
4. The progesterone secreted during pregnancy helps estrogen prepare the mother’s breasts for lactation, which is discussed later in this chapter.
4-HUMAN CHORIONIC SOMATOMAMMOTROPIN
Human chorionic somatomammotropin, a protein hormone with a molecular weight of about 22,000, begins to be secreted by the placenta at about the fifth week of pregnancy. Secretion of this hormone increases progressively throughout the remainder of pregnancy in direct proportion to the weight of the placenta. Although the functions of chorionic somatomammotropin are uncertain, it is secreted in quantities several times greater thanthat of all the other pregnancy hormones combined. It has several possible important effects.
First, when administered to several types of animals, human chorionic somatomammotropin causes at least partial development of the animal’s breasts and in some instances causes lactation. Because this was the first function of the hormone that was discovered, it was first named human placental lactogen and was believed to
have functions similar to those of prolactin. However, attempts to use it to promote lactation in humans have not been successful.
Second, this hormone has weak actions similar to those of growth hormone, causing the formation of protein tissues in the same way that growth hormone does. It also has a chemical structure similar to that of growth hormone, but 100 times as much human
chorionic somatomammotropin as growth hormone is required to promote growth.
Third, human chorionic somatomammotropin causes decreased insulin sensitivity and decreased utilization of glucose in the mother, thereby making larger quantities of
glucose available to the fetus. Because glucose is the major substrate used by the fetus to energize its growth, the possible importance of such a hormonal effect is obvious.
Further, the hormone promotes the release of free fatty acids from the fat stores of the mother, thus providing this alternative source of energy for the mother’s metabolism
during pregnancy. Therefore, it appears that human chorionic somatomammotropin is a general metabolic hormone that has specific nutritional implications for both the mother and the fetus.
Other Hormonal Factors in Pregnancy
Almost all the nonsexual endocrine glands of the mother also react markedly to pregnancy. This reaction results mainly from the increased metabolic load on the mother but also, to some extent, from the effects of placental hormones on the pituitary and other glands. The following effects are some of the most notable.
5-Pituitary Secretion. The anterior pituitary gland of the mother enlarges at least 50 percent during pregnancy and increases its production of corticotropin, thyrotropin, and prolactin. Conversely, pituitary secretion of follicle stimulating hormone and luteinizing hormone is almost
totally suppressed as a result of the inhibitory effects of estrogens and progesterone from the placenta.
6-Increased Corticosteroid Secretion. The rate of adrenocortical secretion of the glucocorticoids is moderately increased throughout pregnancy. It is possible that these glucocorticoids help mobilize amino acids from the mother’s tissues so these amino acids can be used for the synthesis of tissues in the fetus.
Pregnant women usually have about a twofold increase in aldosterone secretion, reaching a peak at the end of gestation. This increase, along with the actions of estrogens, causes a tendency for even a normal pregnant woman to reabsorb excess sodium from her renal tubules and,
therefore, to retain fluid, which occasionally leads to pregnancy-induced hypertension.
7-Increased Thyroid Gland Secretion. The mother’s thyroid gland ordinarily enlarges up to 50 percent during pregnancy and increases its production of thyroxine a corresponding amount. The increased thyroxine production is caused at least partly by a thyrotropic effect of human
chorionic gonadotropin secreted by the placenta and by small quantities of a specific thyroid-stimulating hormone, human chorionic thyrotropin, also secreted by the placenta.
8-Increased Parathyroid Gland Secretion. The mother’s parathyroid glands usually enlarge during pregnancy; this enlargement especially occurs if the mother’s diet is deficient
in calcium. Enlargement of these glands causes calcium absorption from the mother’s bones, thereby maintaining normal calcium ion concentration in the mother’s extracellular fluid even while the fetus removes calcium to ossify its own bones. This secretion of parathyroid
hormone is even more intensified during lactation after the baby’s birth because the growing baby requires many times more calcium than does the fetus.
9-Secretion of “Relaxin” by the Ovaries and Placenta. Another substance besides the estrogens and progesterone, a hormone called relaxin, is secreted by the corpus luteum of the ovary and by placental tissues. Its secretion is increased by a stimulating effect of human chorionic
gonadotropin at the same time that the corpus luteum and the placenta secrete large quantities of estrogens and progesterone.
Relaxin is a 48–amino acid polypeptide with a molecular weight of about 9000. This hormone, when injected, causes relaxation of the ligaments of the symphysis pubis in the estrous rat and guinea pig. This effect is weak or possibly even absent in pregnant women. Instead, this role
is probably played mainly by the estrogens, which also cause relaxation of the pelvic ligaments. It has also been claimed that relaxin softens the cervix of the pregnant woman at the time of delivery. Relaxin is also thought to serve as a vasodilator, contributing to increased blood flow
in various tissues, including the kidneys, and increasing venous return and cardiac output in pregnancy.
Response of the Mother’s Body to Pregnancy
Most apparent among the many reactions of the mother to the fetus and to the higher levels of hormones of pregnancy is the increased size of the various sexual organs. For instance, the uterus increases from about 50 grams to 1100 grams, and the breasts approximately double in size. At the same time, the vagina enlarges and the introitus opens more widely. Also, the various hormones can cause marked changes in a pregnant woman’s appearance, sometimes
resulting in the development of edema, acne, and masculine or acromegalic features.
Weight Gain in the Pregnant Woman
The average weight gain during pregnancy is about 25 to 35 pounds, with most of this gain occurring during the last two trimesters. Of this added weight, about 8 pounds is fetus and 4 pounds is amniotic fluid, placenta, and fetal membranes. The uterus increases about 3 pounds and the breasts another 2 pounds, still leaving an average weight increase of 8 to 18 pounds. About 5 pounds of this added weight is extra fluid in the blood and extracellular fluid, andthe remaining 3 to 13 pounds is generally fat accumulation. The extra fluid is excreted in the urine during the first few days after birth—that is, after loss of the fluid-retaining
hormones from the placenta.
During pregnancy, a woman often has a greatly increased desire for food, partly as a result of removal of food substrates from the mother’s blood by the fetus and partly because of hormonal factors. Without appropriate prenatal control of diet, the mother’s weight gain can be as great as 75 pounds instead of the usual 25 to 35 pounds.
Metabolism During Pregnancy
As a consequence of the increased secretion of many hormones during pregnancy, including thyroxine, adrenocortical hormones, and the sex hormones, the basal metabolic rate of the pregnant woman increases about 15 percent during the latter half of pregnancy. As a result, shefrequently has sensations of becoming overheated. Also, owing to the extra load she is carrying, greater amounts of energy than normal must be expended for muscle activity.
Nutrition During Pregnancy
By far the greatest growth of the fetus occurs during the last trimester of pregnancy; its weight almost doubles during the last 2 months of pregnancy. Ordinarily, the mother does not absorb sufficient protein, calcium, phosphates, and iron from her diet during the last months ofpregnancy to supply these extra needs of the fetus. However, in anticipation of these extra needs, the mother’s body has already been storing these substances—some in the placenta, but most in the normal storage depots of the mother.
If appropriate nutritional elements are not present in a pregnant woman’s diet, several maternal deficiencies can occur, especially in calcium, phosphates, iron, and the vitamins. For example, the fetus needs about 375 milligrams of iron to form its blood, and the mother needs an additional 600 milligrams to form her own extra blood. The normal store of nonhemoglobin iron in the mother at the outset of pregnancy is often only 100 milligrams and almost never
more than 700 milligrams. Therefore, without sufficient iron in her food, a pregnant woman usually develops hypochromic anemia. Also, it is especially important that she receive vitamin D, because although the total quantity of calcium used by the fetus is small, calcium is normally
poorly absorbed by the mother’s gastrointestinal tract without vitamin D. Finally, shortly before birth of the baby, vitamin K is often added to the mother’s diet so the baby will have sufficient prothrombin to prevent hemorrhage, particularly brain hemorrhage, caused by the birth process.
Changes in the Maternal Circulatory System During Pregnancy
Blood Flow Through the Placenta and Maternal Cardiac Output Increase During Pregnancy. About 625 milliliters of blood flows through the maternal circulation of the placenta
each minute during the last month of pregnancy. This flow, plus the general increase in the mother’s metabolism, increases the mother’s cardiac output to 30 to 40 percent above normal by the 27th week of pregnancy; then, for unexplained reasons, the cardiac output falls to only a little
above normal during the last 8 weeks of pregnancy, despite the high uterine blood flow, indicating that blood flow in some other tissue(s) may be reduced.
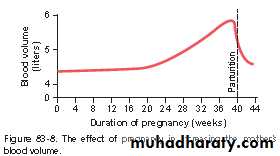
Maternal Blood Volume Increases During Pregnancy. The maternal blood volume shortly before term is about 30 percent above normal. This increase occurs mainly during the latter half of pregnancy, as shown by the curve of Figure 83-8. The cause of the increased volume is likely
due, at least in part, to aldosterone and estrogens, which are greatly increased in pregnancy, and to increased fluid retention by the kidneys. In addition, the bone marrow becomes increasingly active and produces extra red blood cells to go with the excess fluid volume. Therefore, at the
time of the birth of the baby, the mother has about 1 to 2 liters of extra blood in her circulatory system. Only about one fourth of this amount is normally lost through bleeding during delivery of the baby, thereby allowing a considerable safety factor for the mother.
Maternal Respiration Increases During Pregnancy. Because of the increased basal metabolic rate of a pregnant woman and because of her greater size, the total amount of oxygen used by the mother shortly before the birth of the baby is about 20 percent above normal, and a commensurate amount of carbon dioxide is formed. These effects cause the mother’s minute ventilation to increase. It is also believed that the high levels of progesterone during
pregnancy increase the minute ventilation even more, because progesterone increases the sensitivity of the respiratory center to carbon dioxide. The net result is an increase in minute ventilation of about 50 percent and a decrease in arterial PCO2 to several millimeters of mercury below that in a nonpregnant woman. Simultaneously, the growing uterus presses upward against the abdominal contents, which press upward against the diaphragm, so the total excursion of the diaphragm is decreased. Consequently, the respiratory rate is increased to maintain the extra ventilation.
Maternal Kidney Function During Pregnancy
The rate of urine formation by a pregnant woman is usually slightly increased because of increased fluid intake and increased load of excretory products. In addition, severalspecial alterations of kidney function occur.
First, the renal tubules’ reabsorptive capacity for sodium, chloride, and water is increased as much as 50 percent as a consequence of increased production of salt and water retaining
hormones, especially steroid hormones by the placenta and adrenal cortex.
Second, the renal blood flow and glomerular filtration rate increase up to 50 percent during normal pregnancy as a result of renal vasodilation. Although the mechanisms that cause renal vasodilation in pregnancy are still unclear, some studies suggest that increased levels of nitric oxide or the ovarian hormone relaxin may contribute to these changes. The increased glomerular filtration rate likely occurs, at least in part, as a compensation for increased tubular reabsorption of salt and water. Thus, the normal pregnant woman ordinarily accumulates only about 5
pounds of extra water and salt.
Amniotic Fluid and Its Formation
Normally, the volume of amniotic fluid (the fluid inside the uterus in which the fetus floats) is between 500 milliliters and 1 liter, but it can be only a few milliliters or as much as several liters. Isotope studies of the rate of formation of amniotic fluid show that, on average, the water in amniotic fluid is replaced once every 3 hours and the electrolytes sodium and potassium are replaced an average of once every 15 hours. A large portion of the fluid is derived from
renal excretion by the fetus. Likewise, a certain amount of absorption occurs by way of the gastrointestinal tract and lungs of the fetus. However, even after in utero death of a fetus, some turnover of the amniotic fluid still occurs, which indicates that some of the fluid is formed and
absorbed directly through the amniotic membranes.
Preeclampsia and Eclampsia
About 5 percent of all pregnant women experience pregnancy-induced hypertension, that is, a rapid rise in arterial blood pressure to hypertensive levels during the last few months of pregnancy that is also associated with leakage of large amounts of protein into the urine. Thiscondition is called preeclampsia or toxemia of pregnancy. It is often characterized by excess salt and water retention by the mother’s kidneys and by weight gain and the development of edema and hypertension in the mother. In addition, function of the vascular endothelium is impaired and
arterial spasm occurs in many parts of the mother’s body, most significantly in the kidneys, brain, and liver. Both the renal blood flow and the glomerular filtration rate are decreased, which is exactly opposite to the changes that occur in the normal pregnant woman. The renal effects also include thickened glomerular tufts that contain a protein deposit in the basement membranes.
Various attempts have been made to prove that preeclampsia is caused by excessive secretion of placental or adrenal hormones, but proof of a hormonal basis is still lacking. Another theory is that preeclampsia results from some type of autoimmunity or allergy in the mother caused
by the presence of the fetus. In support of this theory, the acute symptoms usually disappear within a few days after birth of the baby.
Evidence also indicates that preeclampsia is initiated by insufficient blood supply to the placenta, resulting in the placenta’s release of substances that cause widespread dysfunction of the maternal vascular endothelium. During normal placental development, the trophoblasts invade
the arterioles of the uterine endometrium and completely remodel the maternal arterioles into large blood vessels with low resistance to blood flow. In women with preeclampsia,
the maternal arterioles fail to undergo these adaptive changes, for reasons that are still unclear, and blood supply to the placenta is insufficient. This insufficient blood supply, in turn, causes the placenta to release various substances that enter the mother’s circulation and cause
impaired vascular endothelial function, decreased blood flow to the kidneys, excess salt and water retention, and increased blood pressure.
Although the factors that link reduced placental blood supply with maternal endothelial dysfunction are still uncertain, some experimental studies suggest a role for increased levels of inflammatory cytokines such as tumor necrosis factor-α and interleukin-6. Placental factors that
impede angiogenesis (blood vessel growth) have also been shown to contribute to increased inflammatory cytokines and preeclampsia. For example, the antiangiogenic proteins soluble fms-related tyrosine kinase 1 (s-Flt1) and soluble endoglin are increased in the blood of women with preeclampsia. These substances are released by the placenta into the maternal circulation in response to ischemia and hypoxia of the placenta. Soluble endoglin and s-Flt1 have multiple effects that may impair function of the maternal vascular endothelium and result in hypertension, proteinuria, and the other systemic manifestations of preeclampsia. However, the precise role of the various factors released from the ischemic placenta in causing the multiple cardiovascular and renal abnormalities in women with preeclampsia is still uncertain.
Eclampsia is an extreme degree of preeclampsia characterized by vascular spasm throughout the body; clonic seizures in the mother, sometimes followed by coma; greatly decreased kidney output; malfunction of the liver; often extreme hypertension; and a generalized toxic condition of the body. It usually occurs shortly before the birth of the baby. Without treatment, a high percentage of mothers with eclampsia die. However, with optimal and immediate use of rapidly acting vasodilating drugs to reduce the arterial pressure to normal, followed by immediate termination of pregnancy—by cesarean section if necessary—the mortality even in mothers with eclampsia has been reduced to 1 percent or less.