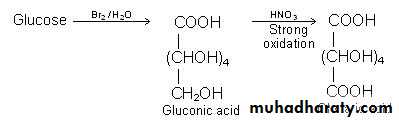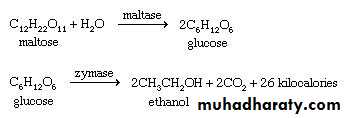The Chemist’s View of Carbohydrates
Carbohydrates are made of carbon, hydrogen and oxygen atoms.Carbohydrates (glycans) have the following basic composition:
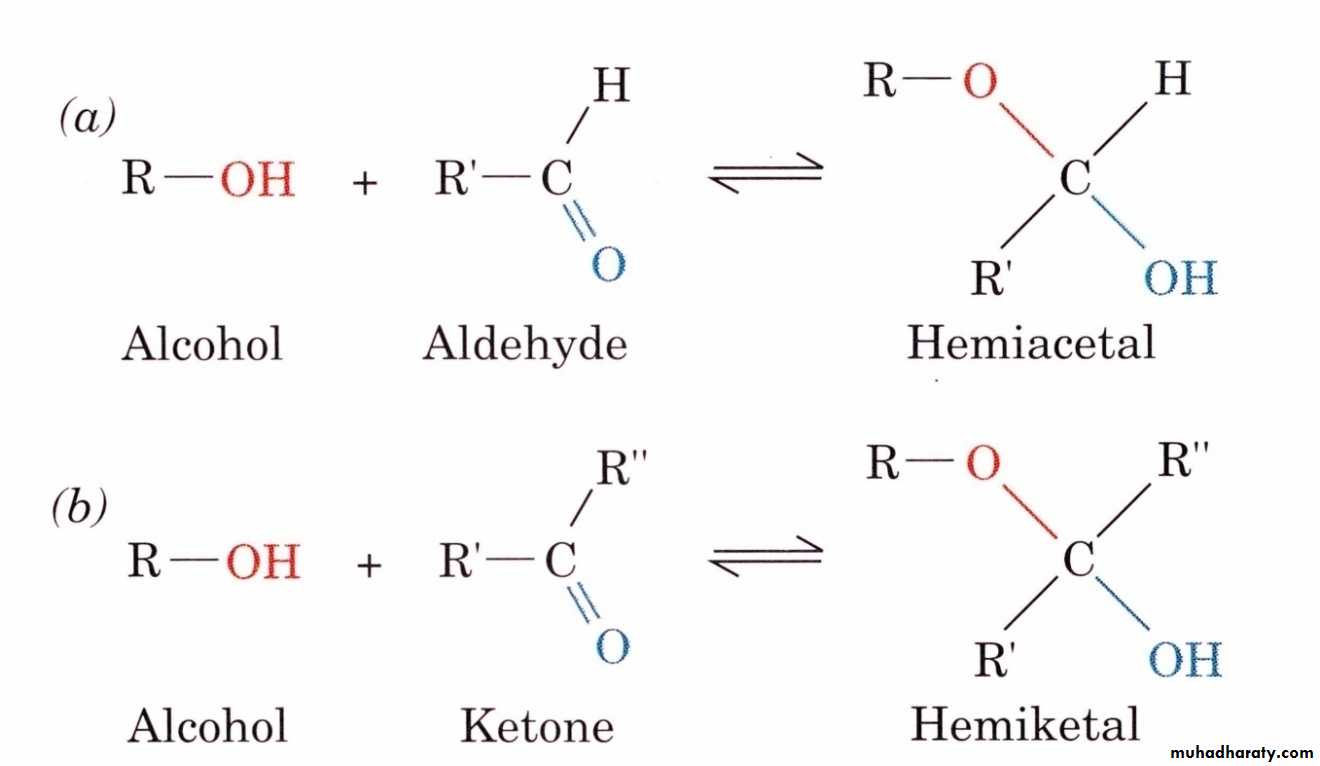
At the molecular level, most carbohydrates are polyhydroxyaldehydes, polyhydroxyketones, or compounds that yield either of these after hydrolysis. Therefore, the chemistry of carbohydrates is essentially the chemistry of hydroxyl groups and carbonyl groups, and of the acetal bonds formed between these two functional groups.
Classification of Carbohydrates.
A- Number of carbohydrate units
1- Monosaccharides (simple carbohydrates) : one carbohydrate unit .
.2- Disaccharides (complex carbohydrates) : two carbohydrate units
. 3- Trisaccharides : three carbohydrate units
. 4- Polysaccharides : many carbohydrate units
B- Position of carbonyl group
at C1, carbonyl is an aldehyde : aldose .
at any other carbon, carbonyl is a ketone : ketose .
C- Number of carbons
three carbons : triose ‚
four carbons : tetrose ‚
five carbons : pentose ‚
six carbons : hexose ‚
seven carbons : heptose ‚ etc .
D- Cyclic form
Monosaccharides
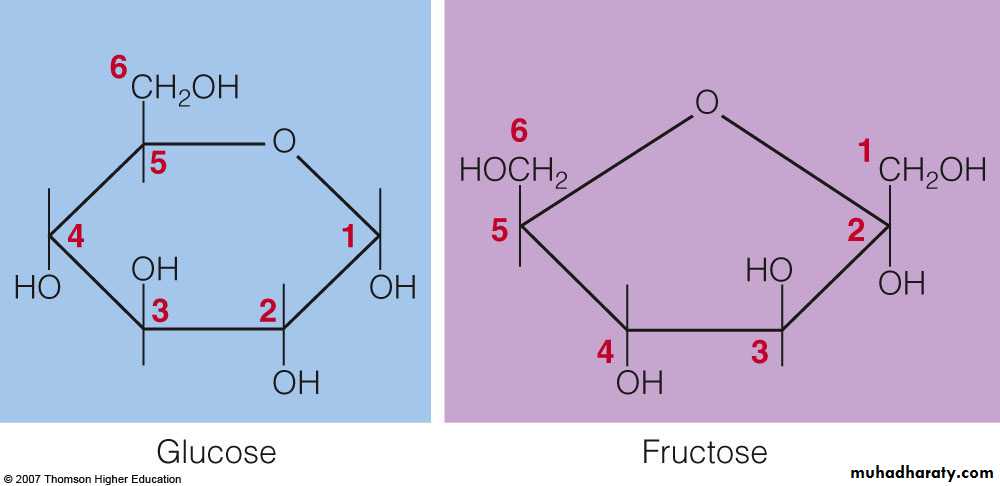
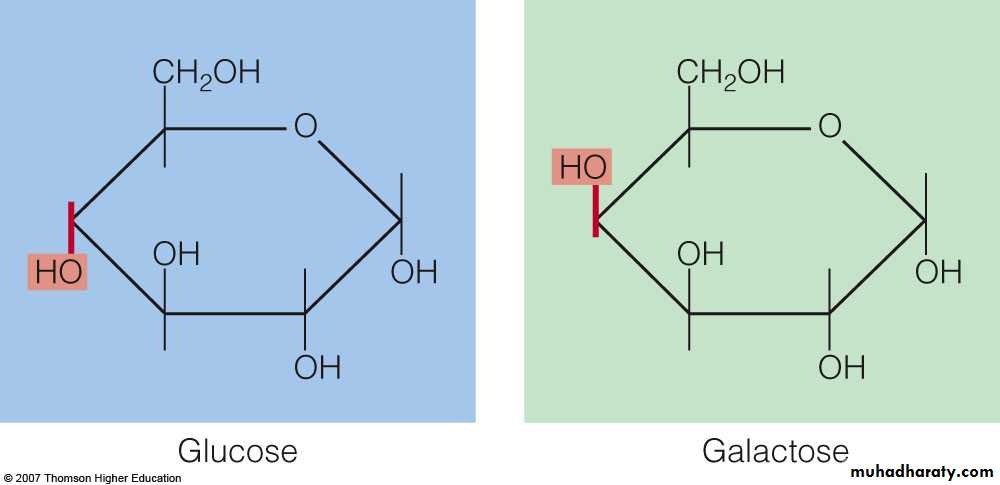
Monosaccharrides : are single sugars (most are hexoses). 1- Glucose : serves as the essential energy source, and is commonly known as blood sugar or dextrose.
2- Fructose : is the sweetest, occurs naturally in honey and fruits, and is added to many foods in the form of high-fructose corn syrup.
3- Galactose : rarely occurs naturally as a single sugar.
Disaccharides
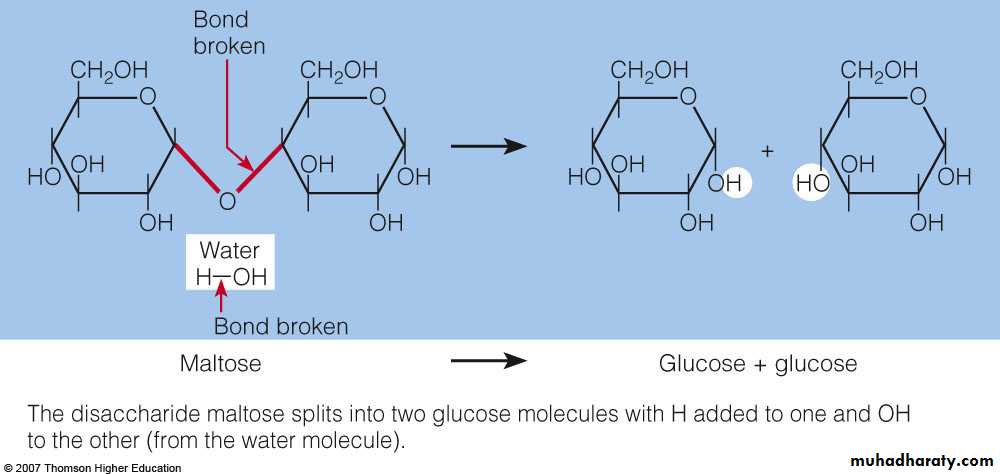
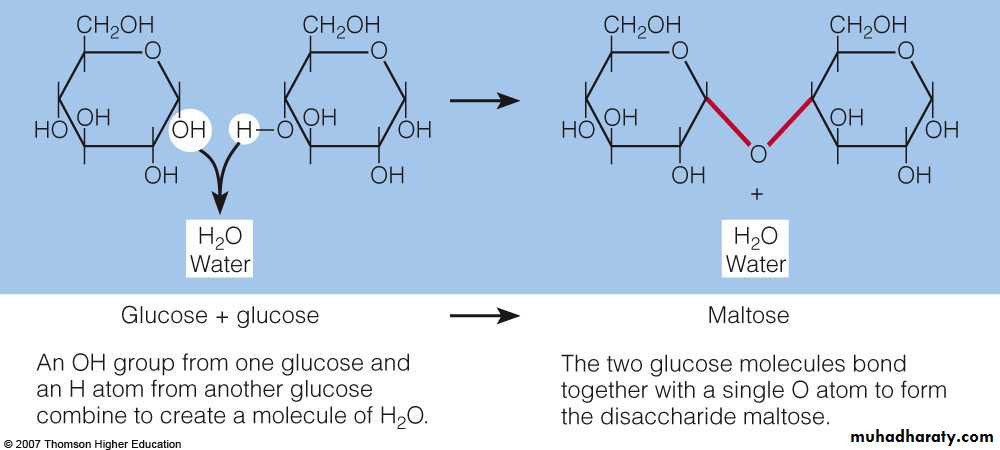
Disaccharides : are pairs of monosaccharides, one of which is always glucose Condensation reactions link monosaccharides together. Hydrolysis reactions split molecules and commonly occur during digestion.Maltose : consists of two glucose units. It is produced during the germination of seeds and fermentation.
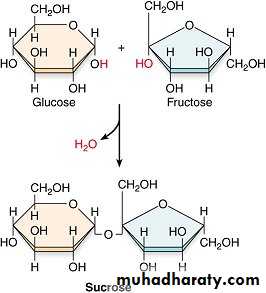
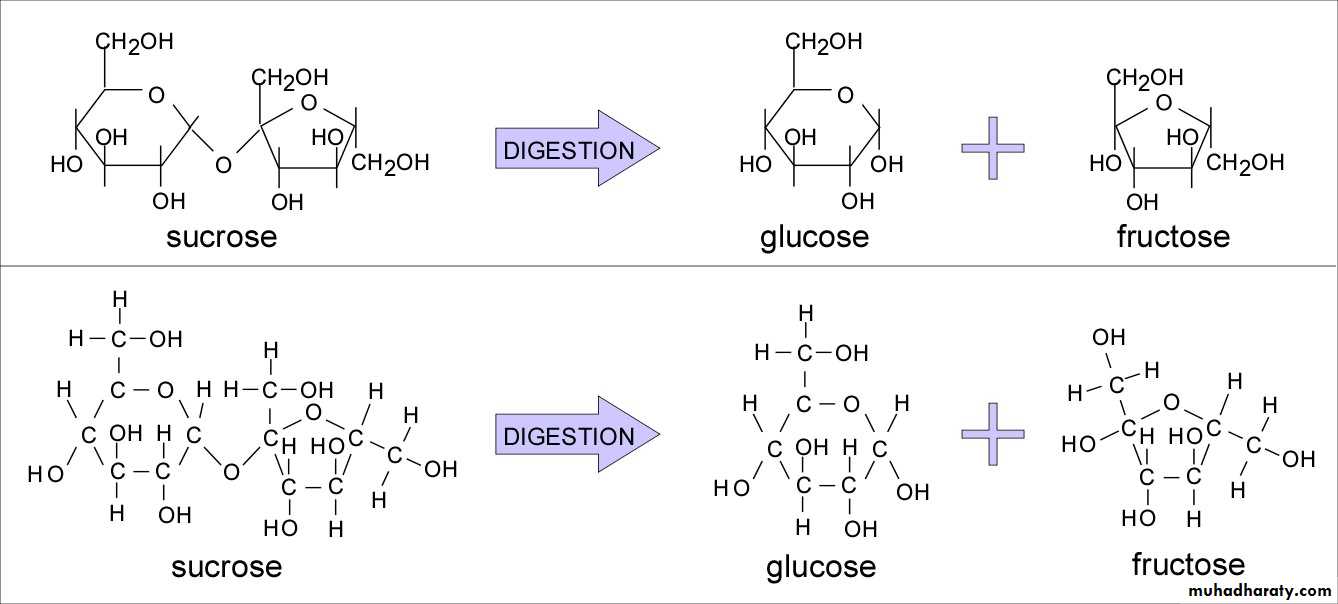
Sucrose : is fructose and glucose combined. It is refined from sugarcane and sugar beets, tastes sweet, and is readily available.
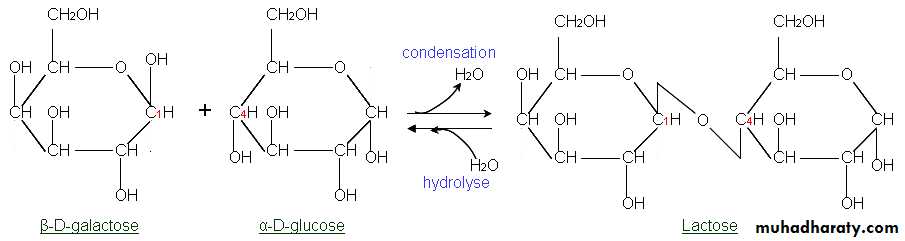
Lactose : is galactose and glucose combined. It is found in milk and milk products.
The Complex Carbohydrates
Few (oligosaccharides) or many (polysaccharides) glucose units bound / linked together in straight or branched chains.1- Glycogen
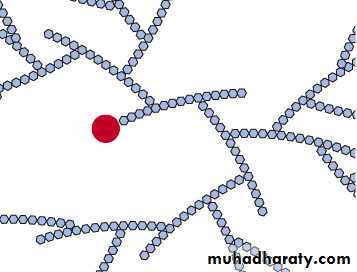
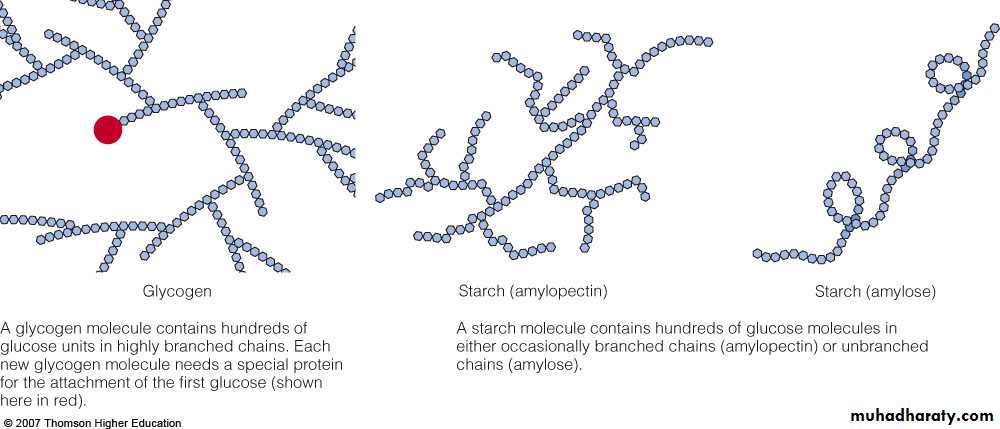
Storage form of glucose in the body Provides a rapid release of energy when needed . Starches Storage form of glucose in plants Found in grains, tubers, and legumes . A glycogen molecule contains hundreds of glucose units in highly branched chains. Each new glycogen molecule needs a special protein for the attachment of the first glucose (shown here in red).
Glycogen
Dietary fibers provide structure in plants, are very diverse, and cannot be broken down by human enzymes . Soluble fibers are viscous and can be digested by intestinal bacteria ( this property is also known as fermentability ) . These fibers are found in fruits and vegetables. Insoluble fibers are nonviscous and are not digested by intestinal bacteria . These fibers are found in grains and vegetables .
Sugar Nomenclature
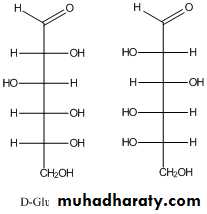
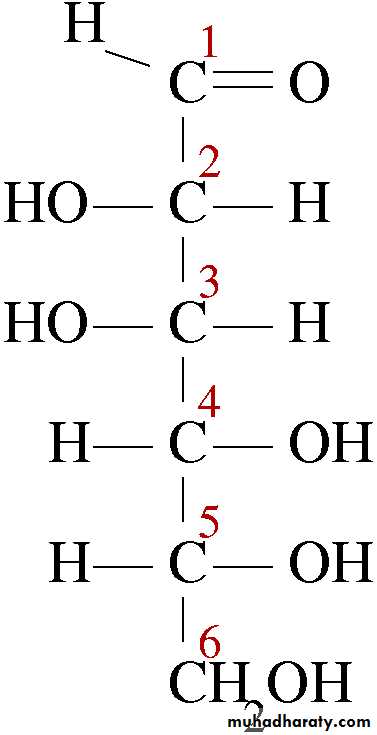
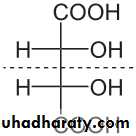
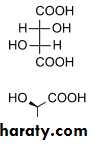
For sugars with more than one chiral center, D or L refers to the asymmetric C farthest from the aldehyde or keto group. Most naturally occurring sugars are D isomers . D & L sugars are mirror images of one another. They have the same name, e.g., D-glucose & L-glucose. Other stereoisomers have unique names,
e.g., glucose, mannose, galactose, etc. The number of stereoisomers is 2n, where n is the number of asymmetric centers. The 6 - C aldoses have 4 asymmetric centers. Thus there are 16 stereoisomers (8 D-sugars and 8 L-sugars).
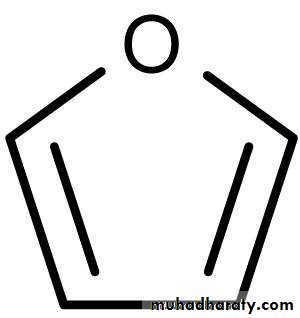
Furan Pyran
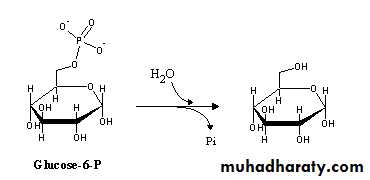
Cyclic Forms of Carbohydrates : Pyranose Forms .Pentoses and hexoses can cyclize as the ketone or aldehyde reacts
with a distal OH . Glucose forms an intra - molecular hemiacetal , as the C1 aldehyde & C5 OH react , to form a 6 - member pyranose ring , named after pyran . These representations of the cyclic sugars are called Haworth projections .
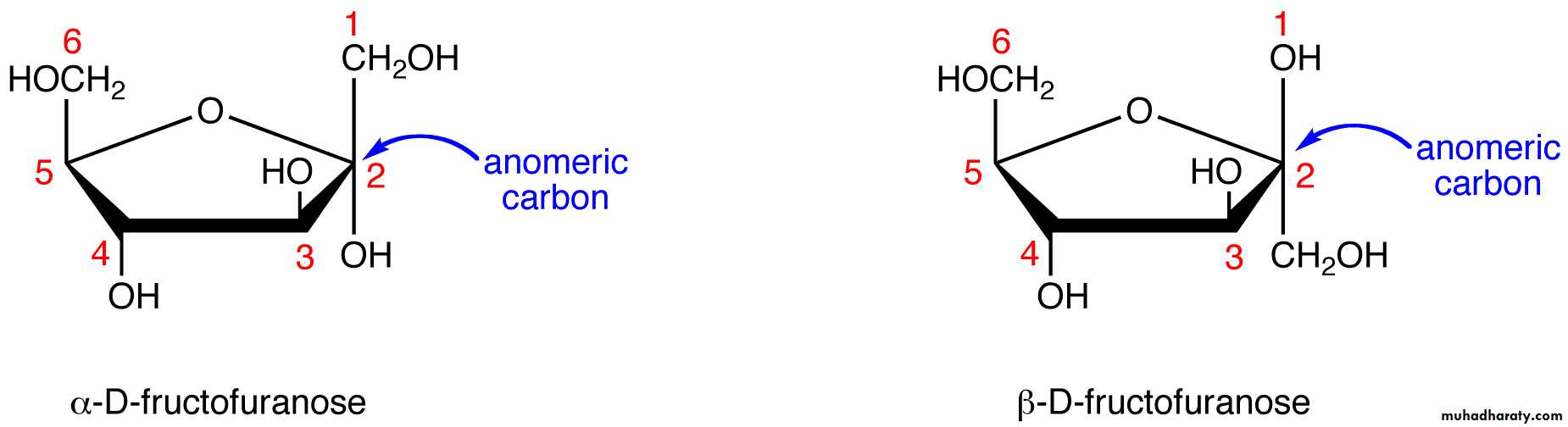
Fructose forms either a 6 - member pyranose ring, by reaction of the C2 keto group with the OH on C6, or a 5-member furanose ring, by reaction of the C2 keto group with the OH on C5.
Cyclization of glucose produces a new asymmetric center at C1.
Haworth projections represent the cyclic sugars as having essentially planar rings, with the OH at the anomeric C1:a (OH below the ring)
b (OH above the ring).
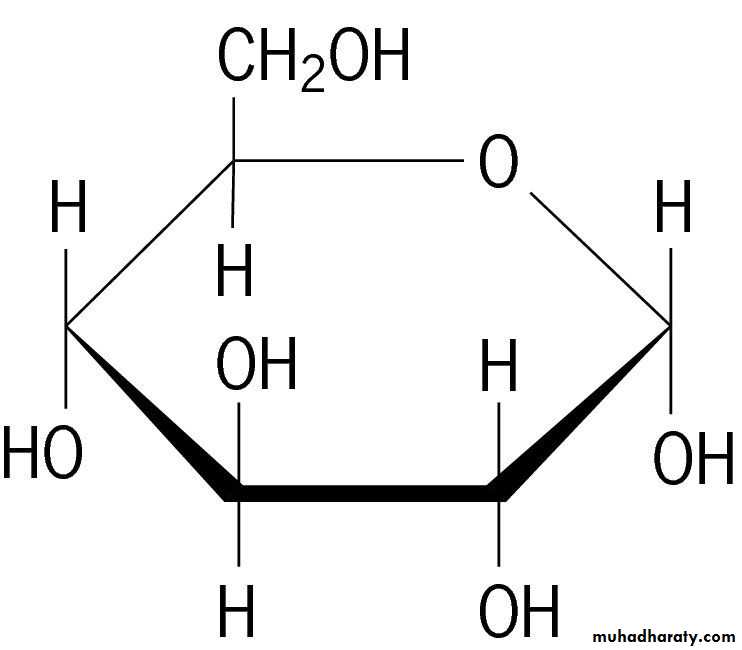
Because of the tetrahedral nature of carbon bonds, pyranose sugars actually assume a "chair" or "boat" configuration, depending on the sugar.
The representation above reflects the chair configuration of the glucopyranose ring more accurately than the Haworth projection.
Reactions of Monosaccharides and Properties
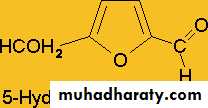
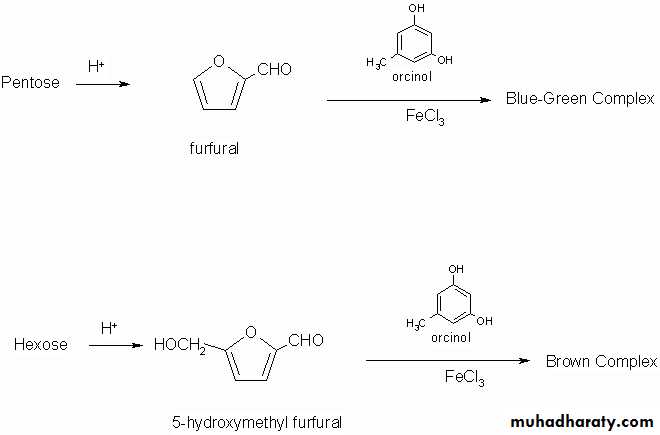
1-Action of acids:Monosaccharides on treatment with strong concentrated sulphuric acid undergoes dehydration to give furfural or furfural derivatives which on condensation with α – naphthol yield a violet or purple colored complex. Pentoses yield furfural whereas hexoses yield 5- hydroxyl furfural .
+ 3H2O
Conc. H2SO4 -------------˃+ D- Ribose Furfural
+ Conc. H2SO4 ------˃
+ 3H2O
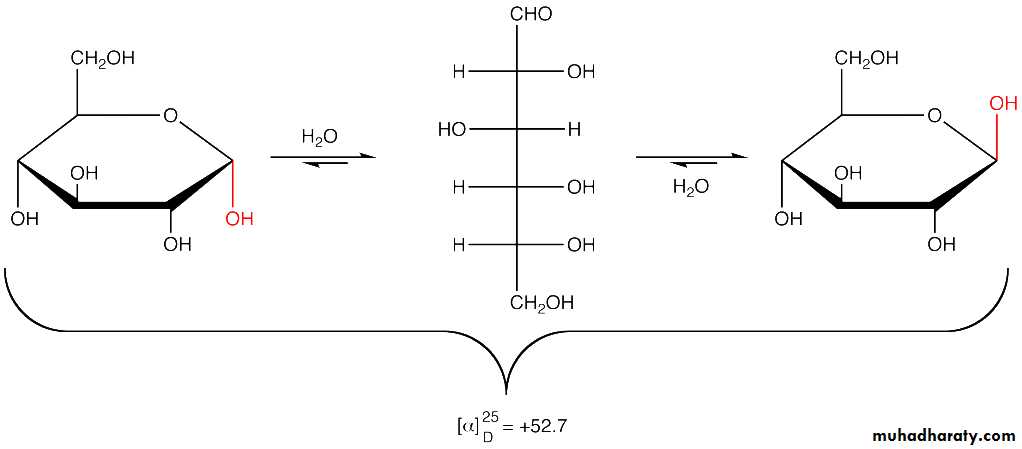
2- Mutarotation
Mutarotation is defined as the change in specific rotation of optically active solution without any change in other properties . When glucose is dissolved in water , the optical rotation of the solution gradually changes and attains an equilibrium value . The change in optical rotation is called mutarotation Mutarotation occurs due to the cyclization of open chain form of glucose into α or β form with equal probability . This α and β cyclic form of glucose have different optical rotations. They differ in configuration about the anomeric carbon ( C1) but have the same configuration at C2, C3, C4, and C5 asymmetric carbons. These cyclic forms are in equilibrium with open chain structure in aqueous solution .such a change from a single form to an equilibrium mixture that includes its other form is called mutarotation.3- Reducing property
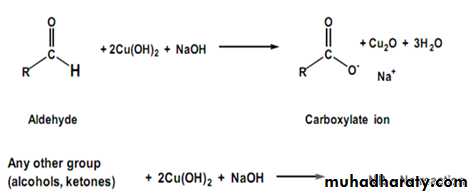
Monosaccharides by virtue free aldehydic or ketonic group in their structure , i.e., presence of free anomeric carbon atom, reduces certain heavy metallic cation, e.g., Cu+2 ions in alkaline solution at high temperature .So all the reducing sugars will give Benedicts qualitative test and Fehling test positive . The reaction is as follows :
CuSO4 ↔ Cu++ + SO4--
Reducing sugars + Na2CO3 ----------------> Enediol form of reducing sugar Cu+2 + Enediols + high temp.--------------> Cu+ + Mixture of sugar acids Cu+ + OH- + Δ --------------> CuOH
2CuOH + Δ -------------------> Cu2O ( cuprous oxide ) + H2O
Benedict՚s qualitative reagent contains cupric sulphate , sodium carbonate and sodium citrate whereas Fehling solution contains cupric sulphate , sodium hydroxide and sodium potassium tartrate ( Rochelle salt ) . Benedict՚s qualitative reagent is preferred above Fehling solution because it is stable .
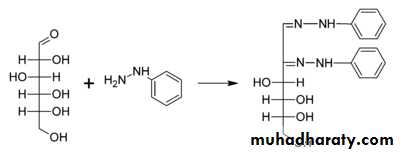
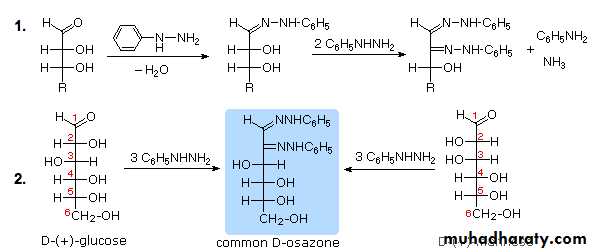
4- Osazone Formation
It involves two reactions . Firstly glucose with phenylhydrazine gives glucosephenylhydrazone by elimination of a water molecule from the functional group. The next step involves reaction of one mole of glucosephenylhydrazone with two moles of phenylhydrazine (excess). First phenylhydrazine is involved in oxidizing the alpha carbon to a carbonyl group, and the second phenylhydrazine involves in removal of one water molecule with the formyl group of that oxidized carbon and forming the similar carbon nitrogen bond . The alpha carbon is attacked here because its more reactive than the others . Osazones are highly coloured and crystalline compounds and can be easily detected . Glucose gives broomstick or needle shaped crystals with this whereas maltose gives sunflower shaped crystals .5- Action of dilute alkali
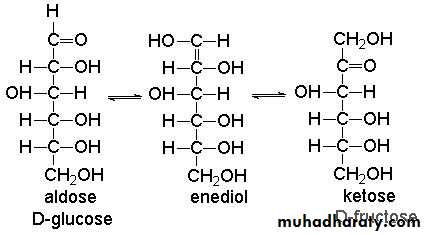
Monosaccharides on treatment with dilute alkali undergo a variety of molecular transformation through enediol formation .
The enediols of sugars are good reducing agents and form the basis of reducing action of sugars in alkaline medium . When glucose is treated with dilute alkali for several hours, the resulting mixture obtained contains both fructose and mannose in addition to glucose . A similar mixture of same sugars is optained with any of the other tow sugars . Mannose
║
Enediol = fructose = glucose
mannose
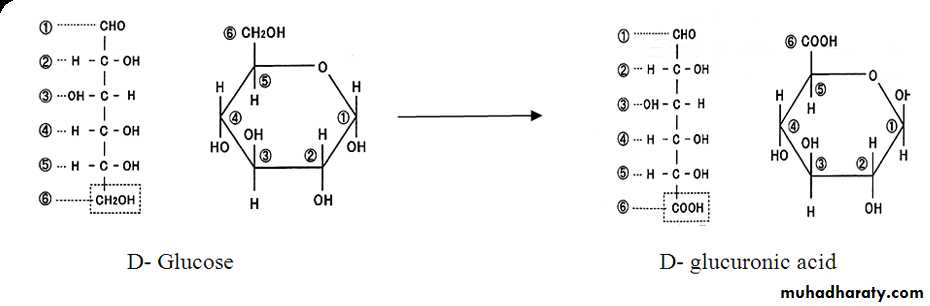
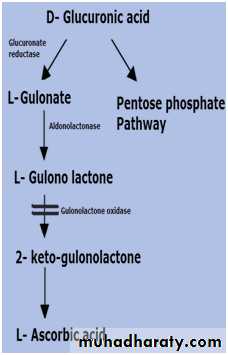
║6- Oxidation
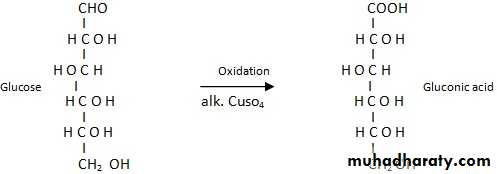
Aldoses are oxidized under variety of conditions to the following :1- Aldonic acid : Whereby the first carbon atom (C-1) is oxidized to carboxyl group only . The rest of the molecule structure remains unaffected .
2- Uronic acid : Whereby the terminal carbon atom is oxidized to carboxyl group only .
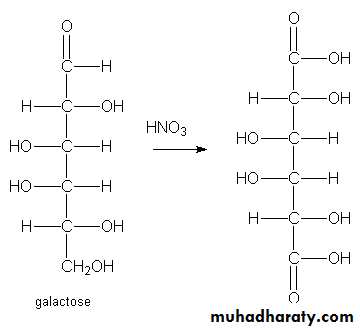
3- Aldaric acid or saccharic acid : Whereby both the first carbon atom, i.e., aldehydic group and the terminal carbon atom , i.e., primary alcoholic group are oxidized to carboxyl group .
7 – Fermentation
Fermentation is the process of breakdown of complex organic substances into smaller substances with the help of Glucose is fermented to ethyl alcohol and carbon dioxide by yeast . Hence this process is called alcoholic fermentation as alcohol is produced .Lactobacilli
C6H12O6 --------------> 2CH2CH.OH.COOHgalactose Sterptococcoi lactic acid
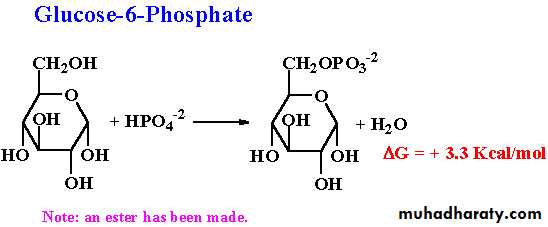
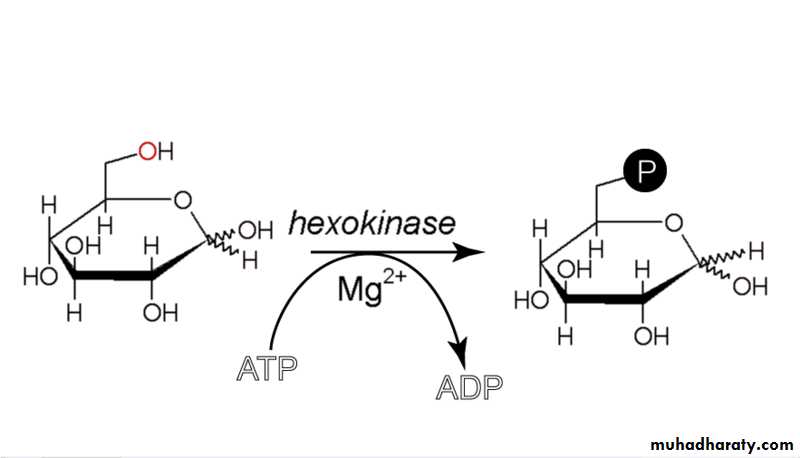
8- Ester formation
Monosaccharides interact with phosphoric acid to give phosphoric sugars, and this plays an important role in the metabolic processes of carbohydrates
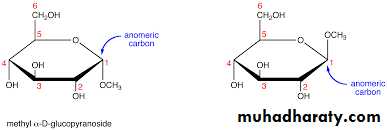
9- Glycoside or acetal formation
Glycosides are sugar derivatives in which hydrogen of the hydroxyl group of hemiacetal or hemiketal form of the sugar is replaced by an organic moiety. A molecule of water is eliminated when this reaction takes place . Glycosides are not reducing sugars and do not show mutarotation . I f the organic moiety is derived from another monosaccharide , the product formed is disaccharide. If the organic moiety is a noncarbohydrate, then it is called a glycone. A glycone: The noncarbohydrate portion of the glycoside is called the a glycone or a glucone . Glycosides do not reduce alkaline copper sulphate because sugar group is combined , i.e., aldehyde group is converted to an acetal group .Gcosides = Carbohydrate + Carbohydrate part or noncarbohydrate part (a glycone).
D - glucose
+CH3OH
↓
Types of glycosidic bonds in carbohydrate
( 1:4 ) - α - D - Glucosidic linkage in maltose .( 1:4 ) - β - D - Glucosidic linkage in lactose .
α ( 1 ) → β ( 2 ) - D - Glucosidic linkage in sucrose .
α ( 1 → 6 ) - D - glycosidic linkage in isomaltose ( a disaccharide is derived from the branch point of starch ) .
α ( 1 , 4 ) and α ( 1 , 6 ) glycosidic linkages in starch .
β ( 1 , 4) glycosidic inkage in cellulose.
Α ( 1 , 4 ) and α ( 1 , 6 ) glycosidic linkage in glycogen .
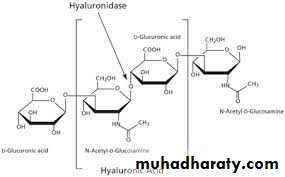
β ( 1,4 ) and β ( 1,3 ) glycosidic linkage in hyaluronic acid .
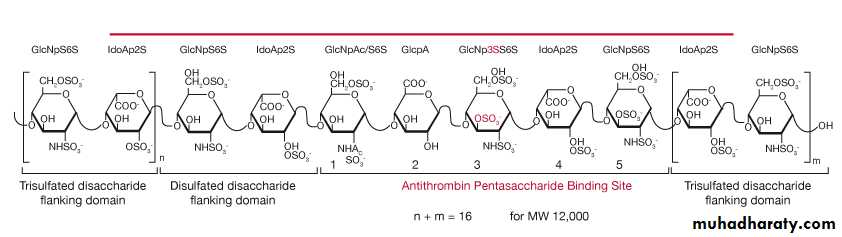
α ( 1,4 ) glycosidic linkage in heparin .
Poly saccharides :
Polysaccharides have two important biological functions :
1- As storage form of fuel (i.e. glycogen of animal origin and starch of plant origin ).
2- As structural components .
Polysaccharides can be divided into two groups :
-Homopolysaccharides .
-Heteropolysaccharides .
Homopolysaccharides .
Starch
Native starch is a mixture of two polysaccharides .
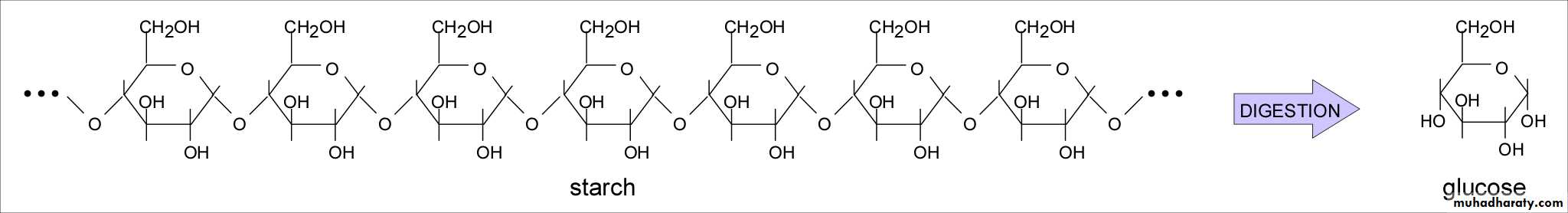
a- Amylose : is a linear unbranched molecule in which D- glucose units are linked by a ( 1 → 4 ) glycosidic linkages . It is water soluble and gives blue color with iodine .
b- Amylopectins : is a branched chain molecule in which D – glucose units in addition to α- ( 1→4 ) linkages are branched by α- ( 1→6 ) glycosidic linkages . This branching occurs on an average of 24 to 30 D- glucose units. It is water insoluble and gives violet color with iodine . Starch is a non reducing polysaccharide, on hydrolysis with dilute mineral acids, i.e. with hydrochloric acid gives glucose only .
Starch
2- CelluloseCellulose is a linear polymer of D – glucose units joined together by β (1,4 ) glycosidic linkages. On partial hydrolysis, cellulose yields β – 1,4 disaccharide cellobiose instead of maltose. Cellobiose is a disaccharide with the formula [HOCH2CHO(CHOH)3]2O. is a reducing sugar, consists of two β-glucose molecules linked by a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage and one hemiacetal linkage, which give rise to strong inter- and intra-molecular hydrogen bonds. It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose rich materials such as cotton, or paper.
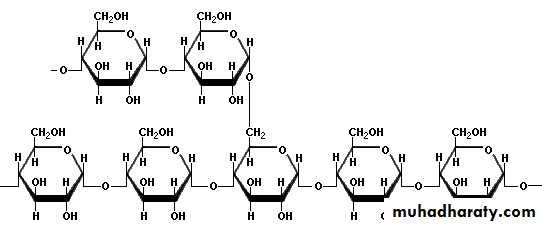
3- Glycogen:
Glycogen is the carbohydrate reserve of the body . Glycogen is also called animal starch, because it serves as nutritional reservoir in animal tissues. Glycogen is a highly branched chain molecule in which glucose unit in addition to linear α (1,4) linkages are also linked by α (1,6) at the branched point . This branching repeats after every 8 -10 glucose units. Glycogen is water soluble and has no reducing property. It gives red color with iodine.
4- Dextrins
They are the partial hydrolytic products of starch by α-amylase, β –amylase and acids. All dextrins have free sugar group and accordingly reduced alkaline copper sulphate solution.
b- Heteropolysaccharides
1- Hyaluronic acid
2- Heparin
heparin
3-Chondroitin sulphates.
4-Sialic Acids.
mesotartaric acid
D - glyceraldehyde
- glyceraldehyde (+)l - glyceraldehyde
- glyceraldehyde(−)
Chemistry of Lipids
Lipids (fats)
Lipids : are one of the large biological molecules (Carbohydrates, fats, proteins and nucleic acids) , substances such as a fat, oil or wax that dissolves in alcohol , non polar solvents like ether, chloroform, benzene, etc., but not in water . Associated with them are various fat soluble , non - lipid substances which includes carotenoid pigments (are organic pigments that are found in the chloroplasts and chromoplasts of plants and some other photosynthetic organisms, including some bacteria and some fungi. Carotenoids can be produced from fats and other basic organic metabolic building blocks by all these organisms), and certain vitamins, i.e., vitamins A, D, E and K. Lipids contain carbon, hydrogen and oxygen atoms and they are an important part of living cells which widely distributed throughout both plant and animal kingdom and are essential constituents of cell membrane. Fats are said to be protein sparing because their availability in the diet reduces the need to burn proteins for energy. Lipids consist about 5% of the organic materials used in the structure of a living cell, and there are about 40-50 type of lipid molecules in the cell. The brain cells and especially nerve tissues are rich in complex lipid compounds. Some lipids contain ionic groups as phosphates or cholines but the largest part of the lipid molecule is non polar. The essential building units of lipids are mostly consist of fatty acids, glycerol, sphingosine, and steroids.
Lipids biological functions
Lipids have several important biological functions:
1- They serve as the reservoir of energy because of their :
a- High energy content. The calorific value is 9 Kcal / gm as compared to carbohydrates which have calorific value of 4 Kcal / gm. b- Storage in concentrated form in water free state in the tissues as compared to carbohydrates which are highly hydrated and cannot be stored in such concentrated form.
2- As structural components of cell membranes.
3- As transport forms of various metabolic fuel.
4- As protective coating on the surface of many organs such as kidney, against injury.
5- To facilitate the absorption of the fat soluble vitamins A, D, E and K.
Dietry fat can be divided into two types :
a- Visible fat or fat consumed as such, e.g. butter, oil, ghee.
b- Invisible fat or fat present as part of other foods items, e.g. egg, fish, meat, cereal, nuts, etc.
Classification of lipids
1- Neutral lipids ( glycerides ) ( ester compounds for fatty acids with glycerol ).
2- Phosphoglycerides (phospholipids):
(ester phosphate for glyceride compounds it may also contains nitrogen compound).
3- Sphingolipids (sphingosine , fatty acid, phosphate group, nitrogen compound).
4- Glycolipids (compounds contain fatty acid, alcohol, sugar).
5- Lipoproteins (compounds contain lipids and proteins).
6- Waxes (ester compounds for fatty acids, mono hydroxyl alcohols).
7- Steroids (derivatives of cyclic alcohol compounds).
8- Terpens (derivatives for polymers contain condensed isoprene units.
Fatty acids
a- Fatty acids in nature as such are not very abundant but are present as ester.
b- Fatty acids are derivatives of lipid because they interfere in the formation of various types of lipids.
c- Fatty acids are represented as general formula R— COOH.
General points about fatty acids:
1- They are monocarboxylic acids.
2- Number of carbon atoms are even, though odd number fatty acids exist but are very rare.
3- They may be saturated or may be unsaturated.
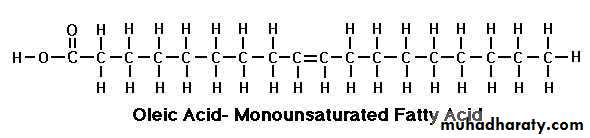
If unsaturated they can be monounsaturated acid or polyunsaturated acid. Mammals and plants contain both mono saturated and poly unsaturated fatty acids whereas all the fatty acids containing double bonds that are present in bacteria are monounsaturated. Plant and fish fats contain more polyunsaturated fatty acids than animal fats. The double bonds in a polyunsaturated fatty acid are neither adjacent nor conjugated since this would make the structure to easily oxidisable when exposed to environment oxygen. 1- The most common among the saturated fatty acids are palmitic acid (C16), stearic acid (C18) and among the unsaturated fatty acid, oleic acid (C18).
2- Unsaturated fatty acids have lower melting point than saturated fatty acids of same chain length. 3- Fatty acids with odd number of carbon atoms occur in trace amounts in terrestrial and marine animals.
4- Fatty acids with one to eight carbons are liquids at room temperature while those with more carbon atoms are solids. 5- The presence of double bond in the molecule gives rise to geometric isomerism. All naturally occurring unsaturated long chain fatty acids are found in cis isomer. 6- Most plant fats are liquid since they contain a large proportions of unsaturated fatty acids with melting points. 7- Animal fats, on the other hand, contain a high proportion of palmitic and stearic acids, and are solid or semi – solid at room temperature. Milk fat is unusual in containing a high proportion of shorter chain (C4 – C14) fatty acids.
The most common fatty acids in neutral fats are :
Formula
No. of atoms
CH3 — (CH2)2 — COOH
CH3 — (CH2)4 — COOH
CH3 — (CH2)10 — COOH
CH3 — (CH2)14 — COOH
CH3 — (CH2)16 — COOH
CH3 — (CH2)7 —CH═CH—(CH2)7 —COOH
4
6
12
16
18
18
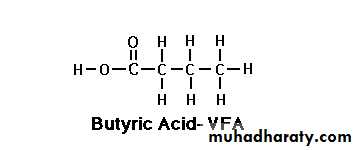
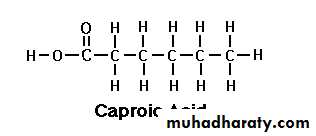
Butyric acid Caproic acid
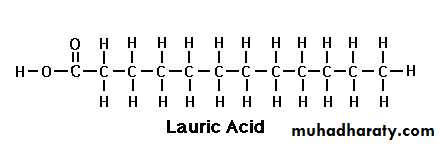
Lauric acid
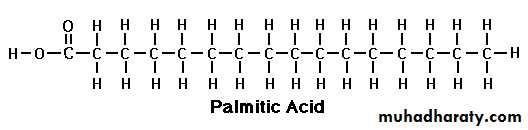
Palmitic
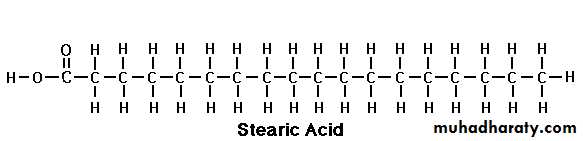
Stearic acid
Oleic acid
Naturally occurring straight chain saturated fatty acid
Systematic name
Type of chain
Common name
No. of C atoms
n- Ethanoic acid
n- Propanoic acid
n- Butanoic acid
n- Octanoic acid
n- Decanoic acid
n- Dodecanoic acid
n- Tetradecanoic acid
n- Hexadecanoic acid
n- Octadecanoic acid
n- Eicosanoic acid
Short chain
Short chain
Short chain
Medium chain
Medium chain
Long chain
Long chain
Long chain
Long chain
Long chain
Acetic acid
Propionic acid
Butyric acid
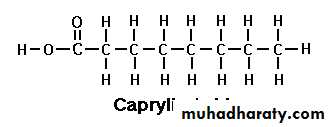
Caprylic acid
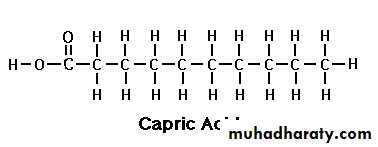
Capric acid
Lauric acid
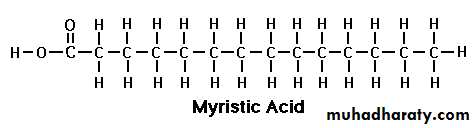
Myristic acid
Palmitic acid
Stearic acid
Arachidic acid
2
3
4
8
10
12
14
16
18
20
Structures
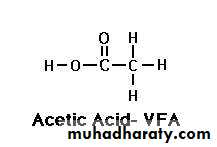
The first three fatty acids are known as the volatile fatty acids VFA's Acetic Acid (C 2:0):
Propionic Acid (C 3:0):
Butyric Acid (C 4:0):
Caproic Acid (C 6:0):
Caprylic Acid (C 8:0):
Capric Acid (C 10:0):
Lauric Acid (C 12:0):
Myristic Acid (C 14:0):
Palmitic Acid (C 16:0):
Stearic Acid (C 18:0):
Oleic Acid (C 18:1)
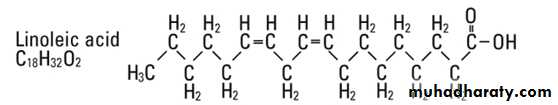
Linoleic Acid (C 18:2):
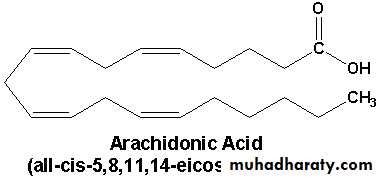
Arachidonic acid
Essential Fatty Acids
They are also called polyunsaturated fatty acids. They are not synthesized in the body and hence, have to be provided in the diet.They deficiency of essential fatty acids in humans gives rise to dry, scaly skin, hair loss, poor wound healing, failure of growth and increase in metabolic rate, These essential fatty acids requirement is about 1% of the caloric intake be in the form of essential fatty acids.
Two of the essential fatty acids, linoleic and linolenic acids are not synthesized in the mammal but are synthesized by plants. Essential fatty acids are necessary in the biosynthesis of prostaglandins. Prostaglandins are hormone like compounds which in small amounts have profound effect.
Essential fatty acids are :
Dietary source
Position of double bonds from carboxyl end
No. of double bonds
No. of carbon atoms
Fatty acid
Vegetable oil Vegetable oil
Vegetable oil Fish oil
9,12
9,12,15
5,8,11,14
5,8,11,14,17
2
3
4
5
18
18
20
20
1- Linoleic acid
2- Linolenic acid
3- Arachidonic acid
4- Timnodonic acid
Important fatty acids in mammalian tissues
Position of double bonds
Double bonds
No. of carbon atoms
Common name
-
-
-
9
-
9
9,12
9,12,15
5,8,11,14
0
0
0
1
0
1
2
3
4
2
12
14
16
18
18
18
18
20
Acetic acid
Lauric acid
Myristic acid
Palmitic acid
Stearic acid
Oleic acid
Linoleic acid
Linolenic acid
Arachidonic acid
Prostaglandins :
1- Prostaglandins are the derivatives of prostanoic acid which are the cyclic derivatives of unsaturated fatty acids having twenty carbon atoms.
2- Prostaglandins are synthesized from essential fatty acids such as linoleic acid, linolenic acid and arachidonic acid.
3- Five type of rings are found in the naturally occurring Prostaglandins.
4- The Prostaglandins which are widely distributed in the body are :
PGE1, PGE2, PGE3, PGF1α, PGF2α and PGF3α.
5- Linolenic acid is the precursor to PGE3 and PGF1α, Arachidonic acid is the precursor to PGE2 and PGF2α.
6- Prostaglandins are synthesized and released by all mammalian cells and tissues except RBC.
7- All Prostaglandins are not stored in cells but are synthesized and released immediately.
Biological function of prostaglandins:
1- They lower blood pressure.2- They are used in the induction of labor, termination of pregnancy and prevention of conception (PGE2).
3- They are used in treatment of gastric ulcer (PGE).
4- They are used to prevent inflammation.
5- They are used in asthma.
6- They are used in congenital heart disease.
7- They inhibit platelet aggregation and promote clotting process.
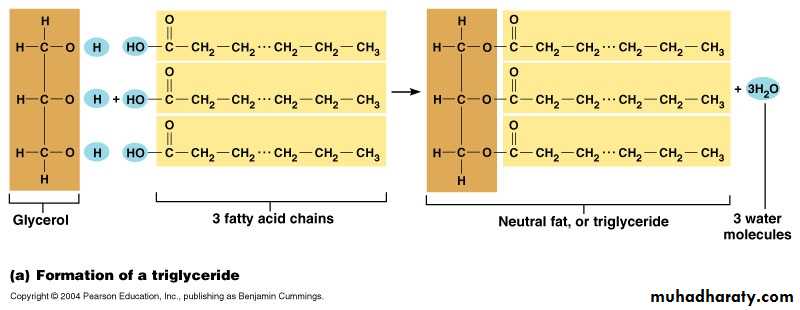
1- Neutral lipids :
Neutral lipids considered as simplest type of lipids, which are compounds of glycerol and fatty acids ester, they also called triacylglycerols or triglycerides when the three OH groups in glycerol esterifies with three fatty acids. If the fatty acids from stearic acid, the fat called tristearin while from palmitic acid it called tripalmitin.
grease and oil
Neutral lipids include grease (fat) and oil, stored in adipose tissue, mostly, the fats are solid in room temperature (25cᵒ), because of containing large amount of saturated fatty acids. While oils are liquid because of containing large amount of unsaturated fatty acids.
Important reactions of neutral lipids
1- Acrolein formation:
When glycerol is heated with potassium bisulphate or concentrated H2SO4, dehydration occurs and aldehyde Acrolein formed which has characteristic unpleasant odor. This test responds to glycerol free or linked as an ester.
2- Hydrogenation
Unsaturated fats can be hydrogenated by the addition of hydrogen across the double bonds of the fatty acids in the presence of nickel as catalyst to give fully saturated fats. The above process is called Hardening of oils whereby vegetable oils are hydrogenated to produce commercial cooking fats.3- Saponification:
Hydrolysis of a fat by alkali is called Saponification. The products of hydrolysis are glycerol and alkali salts of fatty acids, which are called soaps. Soaps are polarized molecules which formed groups in the water called micelles. CH2OCOR—CHOCOR—CH2OCOR +3NaOH→ CH2OH—CHOH—CH2OH+3RCOONa Fat Glycerol soaps4- Rancidity (Peroxidation):
1- Rancidity is a chemical change resulting in unpleasant odor and taste on storage when fats are exposed to light, heat, air and moisture.
2- Rancidity is more rapid at high temperature.
3- Rancidity may be due to hydrolytic or oxidative change taking place at the double bonds of the unsaturated fatty acids resulting in short chain aldehydes or ketones which have unpleasant odor.
4- The addition of certain substances, called antioxidants such as ascorbic acid and vitamin E prevents rancidity whereas addition of peroxidants like copper, lead and nickel quickens rancidity.
5- The oxidation of unsaturated bonds in fatty acids when are exposed to oxygen in the environment is referred to as either auto oxidation or peroxidation.
6- Rancid fats are those that contain an appreciable amount of peroxidized fatty acid.
7- Antioxidants are generally added to many food fats to improve their storage quantities.
2-Compound lipids
1- Phosphoglycerides (phospholipids):
They are also known as phosphatides. Phospholipids act as a detergent and increase the solubility of other lipids. They are present in all cells as well as in the plasma. Phospholipids include the following groups:
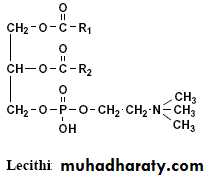
a- Phosphatidyl cholines (Lecithins):
1- When choline or trimethyl ethanol amine esterified with phosphoric acid side of phosphatidic acid, the phosphatidyl cholines produces also called lecithins.
2- Lecithin contains saturated fatty acid residue at the α– position and unsaturated fatty acid residue at the β– position of the glycerol. 3- Lecithins on hydrolysis give glycerol, fatty acid, phosphoric acid and choline.
4- Lecithin compounds play an essential role in reducing the surface tension of the cells in the lung alveoli, without it, shortness of breath process happens.
b – Phosphatidyl ethanolamine (Cephalins)
Cephalins differ from lecithins with respect to base attached to phosphoric acid.
1- If the base is ethanol amine then it is called phosphatidyl ethanolamine or ethanolamine cephalin.
2- If the base is amino acid serine then it is called phosphatidyl serine which is also called serine cephalin.
Cephalins on hydrolysis yield glycerol, fatty acids, phosphoric acids, ethanol amine or serine.
2- Cerebrosides or glycolipids:
Glycolipids are carbohydrate - glyceride derivatives containing sugar, sphingosine and a fatty acid. These compounds do not contain phosphoric acid. If the sugar component is galactose, the lipid is termed galactolipid. The term cerebroside is used because it is found in large quantities in brain tissues particularly in white matter. On hydrolysis cerebrosides give sphingosine, a fatty acid and galactose. Cerebrosides are differentiated on the basis of fatty acid present.
3- Gangliosides:
They are found in nerve tissues. They contain carbohydrates, N- acetyl galactosamine and N-acetyl neuraminic acid.
4- Sulfatides (Sulpholipids):
They are cerebrosides having a sulphate group attached to the galactosyl residue.
Derived lipid:
Lecithins are hydrolyzed by certain enzymes, phospholipases or lecithinases. The nature of hydrolysis depends upon the type of phospholipase used.1- Phospholipase A: present in snake venom (cobra) hydrolyzes fatty acid in α or 1- position of glycerol in the lecithin to form lysolecithins.
2- Phospholipase B: hydrolyzes the remaining fatty acid of lysolecithin present at β or 2- position to form glyceryl phosphoryl choline.
3- Phospholipase C: hydrolyzes phosphorylcholine from lecithins to form diglycerides.
4- Phospholipase D: hydrolyzes choline from phosphatidyl ethanolamine (cephalin) form phosphatidyl serines.
These are two classes of non saponifiable lipids:
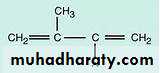
1- Terpenes: They are linear or cyclic compounds formed by condensation of two or more isoprene units.
Isoprene
Other important terpenoid compounds are:a- Tocopherol (vitamin E)
b- Coenzyme Q (also called ubiquinone)
c- Vitamin K (naphthoquinone)
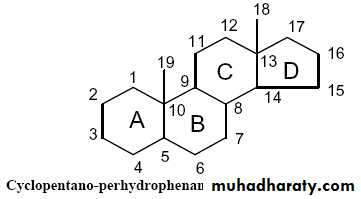
2- Steroids:
1- Steroids are the derivatives of cyclopentano-perhydro-phenanthrene ring (consists of four fused rings).
2- Steroids are steroidal alcohol. The most important member of the group is cholesterol.
Some of the biologically important steroids are:
1- Ergosterol: UV radiation causes rupture of ring B to produce vitamin D.2- Bile acids: In lipid metabolism.
3- Adrenal cortex steroids: Corticosterone and cortisol.
4- Female hormones: Progesterone and estrogen.
5- Male sex hormones: Testosterone and androsterone.
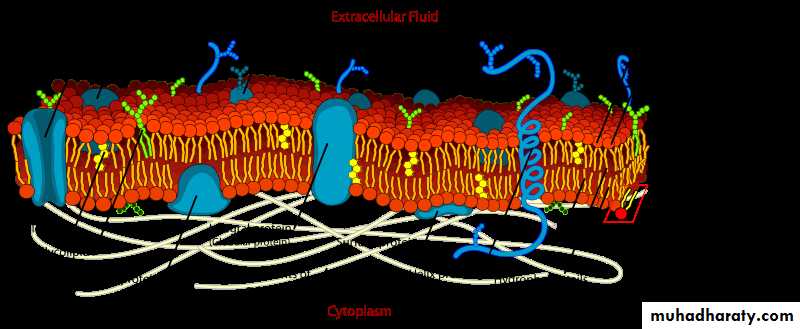
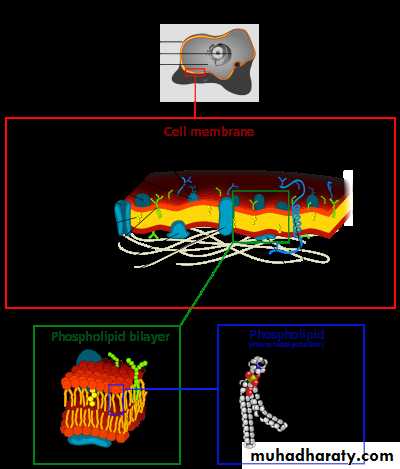
Mammalian cell membrane composition:
1- The cell membrane (also known as the plasma membrane or cytoplasmic membrane) is a biological membrane that separates the interior of all cells from the outside environment.
2- The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells.
3- The basic function of the cell membrane is to protect the cell from its surroundings. It consists of the phospholipids bilayer with embedded proteins.
4- Cell membranes are involved in a variety of cellular processes such as:
a- cell adhesion,
b- ion conductivity and cell signaling,
c- serve as the attachment surface for several extracellular structures, including the cell wall, glycocalyx, and intracellular cytoskeleton.
Glycocalyx: A thin layer of material that covers the surface of many (especially the free surfaces), or all cells. It contains polysaccharide mucous acid. It can be responsible for the selective permeability of the cell wall.
d- Cell membranes can be artificially reassembled Composition
1- Cell membranes contain a variety of biological molecules, notably lipids and proteins.
2- Material is incorporated into the membrane, or deleted from it, by a variety of mechanisms:
a- Fusion of intracellular vesicles with the membrane (exocytosis) not only excretes the contents of the vesicle but also incorporates the vesicle membrane's components into the cell membrane.
b- The membrane may form blebs around extracellular material that pinch off to become vesicles (endocytosis).
c- If a membrane is continuous with a tubular structure made of membrane material, then material from the tube can be drawn into the membrane continuously.
3- Although the concentration of membrane components in the aqueous phase is low (stable membrane components have low solubility in water), there is an exchange of molecules between the lipid and aqueous phases.
Lipids:
Examples of the major membrane phospholipids and glycolipids:
1- phosphatidylcholine (PtdCho),
2- phosphatidylethanolamine (PtdEtn),
3- phosphatidylinositol (PtdIns),
4- phosphatidylserine (PtdSer).
The cell membrane consists of three classes of amphipathic lipids:
1- phospholipids,
2- glycolipids, and
3- sterols.
The amount of each depends upon the type of cell, but in the majority of cases phospholipids are the most abundant. In RBC studies, 30% of the plasma membrane is lipid.
The fatty chains in phospholipids and glycolipids usually contain an even number of carbon atoms, typically between 16 and 20. The 16- and 18-carbon fatty acids are the most common. Fatty acids may be saturated or unsaturated, with the configuration of the double bonds nearly always "cis". The length and the degree of unsaturation of fatty acid chains have a profound effect on membrane fluidity as unsaturated lipids create a kink, preventing the fatty acids from packing together as tightly, thus decreasing the melting temperature (increasing the fluidity) of the membrane. Under physiological conditions phospholipid molecules in the cell membrane are in the liquid crystalline state. It means the lipid molecules are free to diffuse and exhibit rapid lateral diffusion along the layer in which they are present. However, the exchange of phospholipid molecules between intracellular and extracellular leaflets of the bilayer is a very slow process. A fraction of the lipid in direct contact with integral membrane proteins, which is tightly bound to the protein surface is called annular lipid shell; it behaves as a part of protein complex.
In animal cells cholesterol is normally found dispersed in varying degrees throughout cell membranes, in the irregular spaces between the hydrophobic tails of the membrane lipids, where it confers a stiffening and strengthening effect on the membrane.
Carbohydrates
Plasma membranes also contain carbohydrates, predominantly glycoproteins, but with some glycolipids (cerebrosides and gangliosides). For the most part, no glycosylation occurs on membranes within the cell; rather generally glycosylation occurs on the extracellular surface of the plasma membrane. The glycocalyx is an important feature in all cells, especially epithelia with microvilli. Recent data suggest the glycocalyx participates in cell adhesion, lymphocyte homing, and many others. The penultimate sugar is galactose and the terminal sugar is sialic acid, as the sugar backbone is modified in the Golgi apparatus. Sialic acid carries a negative charge, providing an external barrier to charged particles.
Proteins:
The cell membrane has large content of proteins, typically around 50% of membrane volume. These proteins are important for cell because they are responsible for various biological activities.
The cell membrane, being exposed to the outside environment, is an important site of cell–cell communication. As such, a large variety of protein receptors and identification proteins, such as antigens, are present on the surface of the membrane.
Functions of membrane proteins can also include:
1- cell–cell contact,
2- surface recognition,
3- cytoskeleton contact,
4- signaling,
5- enzymatic activity, or transporting substances across the membrane.
Chemistry of amino acids and proteins
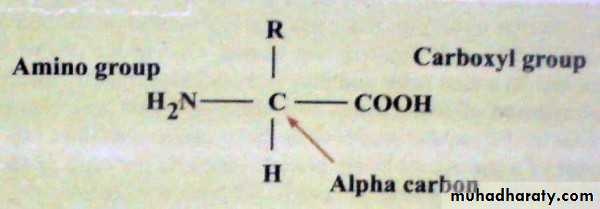
Chemistry of amino acids:Amino acid, any of a group of organic molecules that consist of a basic amino group (−NH2), an acidic carboxyl group (−COOH), and an organic R group (or side chain) that is unique to each amino acid. The term amino acid is short for “α-amino [alpha-amino] carboxylic acid.” Each molecule contains a central carbon (C) atom, termed the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally satisfied by a hydrogen (H) atom and the R group. The formula of a general amino acid is:
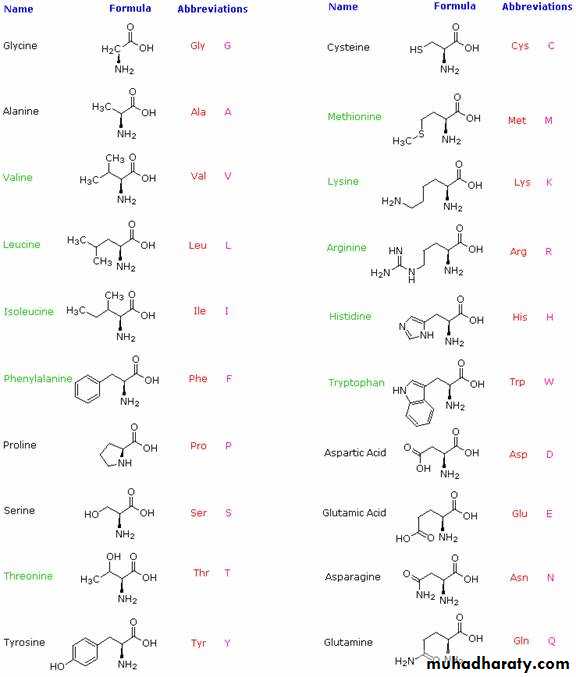
Structure of amino acids: The structures of the most important amino acids are:
Physical properties of amino acids:
1- Solubility: Amino acids are soluble in water, acids, alkalies, but sparingly soluble in organic solvents and insoluble in ether.2- Color: Amino acids are colorless, white and solids.
3- State: Amino acids are solid crystalline compounds (crystals).
4- Optical activity: All amino acids ( except glycine) are optically active.
5- Melting points: Amino acids have high melting points.
6- Amphoteric (react as acidic and basic), (NH2 and COOH group).
Due to presence of basic and acidic groups in the same molecule, they may be regarded as salts and hence, most of them either possess higher melting point or melt with decomposition.
Chemical properties of amino acids:2 reactive groups
A – COOH Reactions:
1- Ester with alcohol
Amino acids react with alcohol to form ester. Esterification of the carboxylic acid is usually conducted under acidic conditions.
COO COOH COOR
| HCl | ROH |
NH3 — C — H —————> ClNH3 — C — H ————> ClNH3 — C — H + H2O
| | |
R R R
2- With NH3 (Amidation).
Aspartic acid ————> AspargineAspartic acid
↓Aspargine
3- Reduction by LiAlH4Lithium aluminium hydride , commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. LiAlH4 R— CH — (NH2) —COOH —————> R— CH — (NH2) CH2 OH
Amino alcohol
4- Decarboxylation
B – NH2 Reaction:1- Salt formation with acids
H H
| HCl |
R — C — COOH —————> R — C — COOH
| |
NH2 NHCl
2- Acylation: Reaction with strong acids
(Acid anhydride + NaOH)
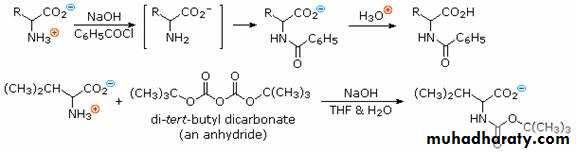
In order to convert the amine function of an amino acid into an amide, the pH of the solution must be raised to 10 or higher so that free amine nucleophiles are present in the reaction system. Carboxylic acids are all converted to carboxylate anions at such a high pH, and do not interfere with amine acylation reactions.
3- Methylation and benzoylation: important, detoxification process.
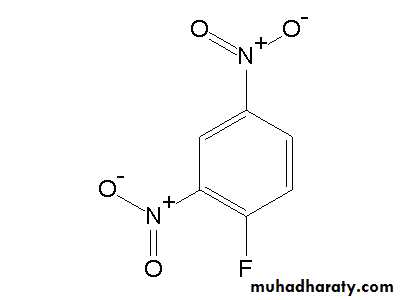
4- Reaction with Sanger՚ s reagent FDNB (1-floro-2,4-di nitro benzene).FDNB
Amino acid + FDNB ————> Di nitro phenyl amino acidIs a test for free amino acid.
5- With nitrous acids HNO2
α- amino acids are deaminated to the corresponding α- hydroxy acids with nitrous acid. Each amino group yields one molecule of nitrogen which can be measured accurately.
H H
| |
R — C — COOH + HNO2————> R — C — COOH + N2 + H2O
| |
NH2 OH
N2= measure of free NH2 group in amino acids, peptides and proteins.
6- Oxidative deamination (Removal of NH2) ——> Oxo acid.
H
|
R— C — COOH————> R— C— COOH———> R— C — COOH + NH3
| || ||
NH2 NH O
Oxo acid (keto acid)
7- Ninhydrin Reaction
In addition to these common reactions of amines and carboxylic acids, common alpha - amino acids, except proline, undergo a unique reaction with the tri ketohydrindene hydrate known as ninhydrin. (Quantitative measurement of free α- amino group) .
Ninhydrin Hydrindentin
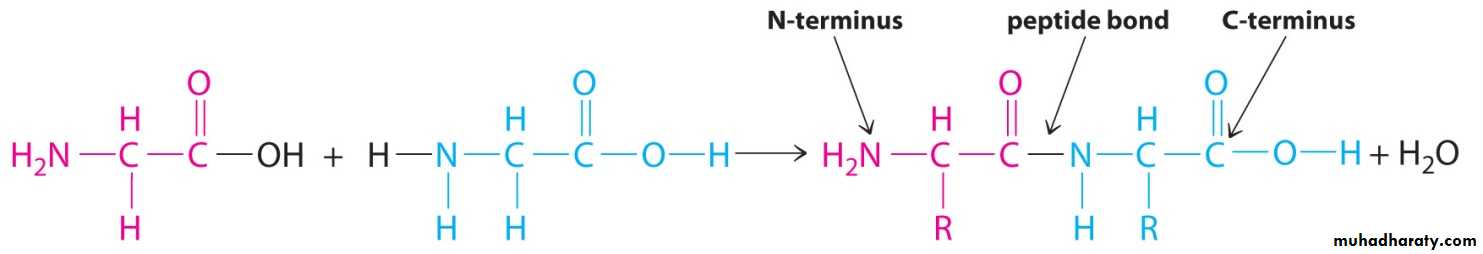
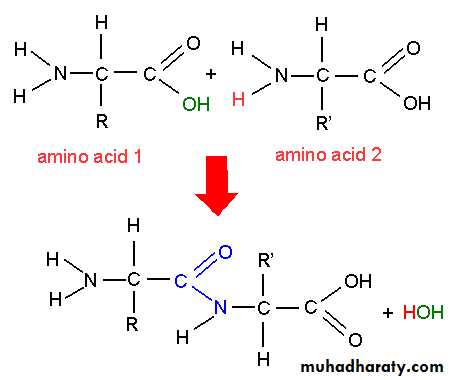
Proteins:Proteins are defined as compounds of high molecular weight made up of α- amino acids linked to one another by peptide linkages. Proteins contain 20 amino acids present in characteristic proportions and linked in a specific sequence in each protein. Proteins are linear polymers consisting of L- α-amino acids. The amino acids are joined together by peptide bonds. The peptide bond is formed by the anion of carboxyl group of one amino acids with amino group of other amino acid with an elimination of water molecule.
The polymeric Nature of Protein
1- Peptides: A short chain of residues with a defined sequence.
- No max number of residues in a peptide.
- Its physical properties are those expected from the sum of its amino acid resides.
- No fixed 3D conformation.
2- Polypeptide: A longer chain with a defined sequence and
Length.
3- Polyamino acids: Nonspecific polymerization of one or a few amino acids
4- Protein:
– Polypeptides that occur naturally
– Have a definite 3D structure under physiological conditions
Peptide bonding
A covalent bond that links amino acids together in protein.
(α- amino group of amino acid with α- COO group of another amino acid) .
Polypeptide: many repeated peptide bonds. Biological active protein contain 1 or more polypeptide chains.
Protein Function
Make up about 15% of the cell and have many functions in it:1- Catalysis: enzymes.
2- Structure: muscle proteins. ( Structural proteins form the basis of the cells, which come together to form organs, muscle tissue, bones, skin, hair and nails. They help organize the cells into separate tissues and they can protect the body as well; for example, specialized proteins tightly connect one skin cell to another to create a cohesive barrier against the outside environment. When tissues become damaged due to injury or illness, structural proteins repair wounds and can generate new cellular components. 3- Movement: myosin, actin. (The bulk of the muscle tissue is made of protein, which provides not only structural integrity but also the ability to move. Muscle fibers consist of the proteins actin and myosin, organized in a manner that allows them to slide back and forth to shorten or lengthen a muscle, leading to movement. The proteins involved in nerve impulses are also part of stimulating muscle contraction).
4- Defense: antibody. (Immunity: The immune system is rich in proteins. The white blood cells synthesize several immune molecules, including antibodies and chemokines, to help protect the body against infection and inflammation. In concert with biological messengers, which can warn of foreign invaders or injury, the immune system has the ability to rapidly ramp up production of immune proteins to respond to this type of physiological threat to the well - being.
5- Communication and regulation: enzymes, hormones.
(Proteins serve as messengers in many ways. Enzymes are biological catalysts, molecules that speed up a reaction without themselves becoming involved, and they can orchestrate physiological functions ranging from energy production to new protein synthesis to activating another protein, depending on your body’s needs. Hormones are molecules that can influence the behavior of cells or tissues; for instance, insulin stimulates glucose uptake. Receptor proteins can also stimulate the activity of other cellular components by binding molecules and eliciting a change within a cell).
6- Transport and storage: globins, Mb, ferritin.( Protein can function as both transport and storage molecules. For instance, hemoglobin is a protein that carries oxygen to the tissues throughout the body, and specialized transporter proteins allow the cells of the small intestine to absorb digested nutrients from the gut into the blood stream. Myoglobin stores small amounts of oxygen in the muscles, while, in the liver, the protein ferritin binds iron as a backup source of this mineral.
7- Energy: Although not the body’s preferred fuel source, protein can provide energy to the cells. Each gram you eat can supply 5.5 calories to help power your physiological functions. 8- Stress Response: hormones.
Classification of protein structure
Proteins exhibit four levels of organization.
1- Primary structure: Refers to amino acid sequence.
2- Secondary structure: Refers to folding of polypeptide chain into specific coiled structure which is repetitive in one direction.
3- Tertiary structure: Refers to arrangement and interrelationship of twisted chain into a three dimensional structure.
4- Quaternary structure: Refers to the association of different monomeric subunit into a composite polymeric protein.
Chemistry of nucleic acid