1
Fifth stage
Medicine
Lec-8
د.بشار
15/12/2015
Stroke
Overview of Stroke
Strokes are a heterogeneous group of disorders involving sudden, focal interruption
of cerebral blood flow that causes neurologic deficit. Strokes can be ischemic (80%),
typically resulting from thrombosis or embolism, or hemorrhagic (20%), resulting
from vascular rupture (eg, subarachnoid or intracerebral hemorrhage). Transient
stroke symptoms (typically lasting < 1 h) without evidence of acute cerebral
infarction (based on diffusion-weighted MRI) are termed a transient ischemic attack
(TIA). In the US, stroke is the 4th most common cause of death and the most
common cause of neurologic disability in adults.
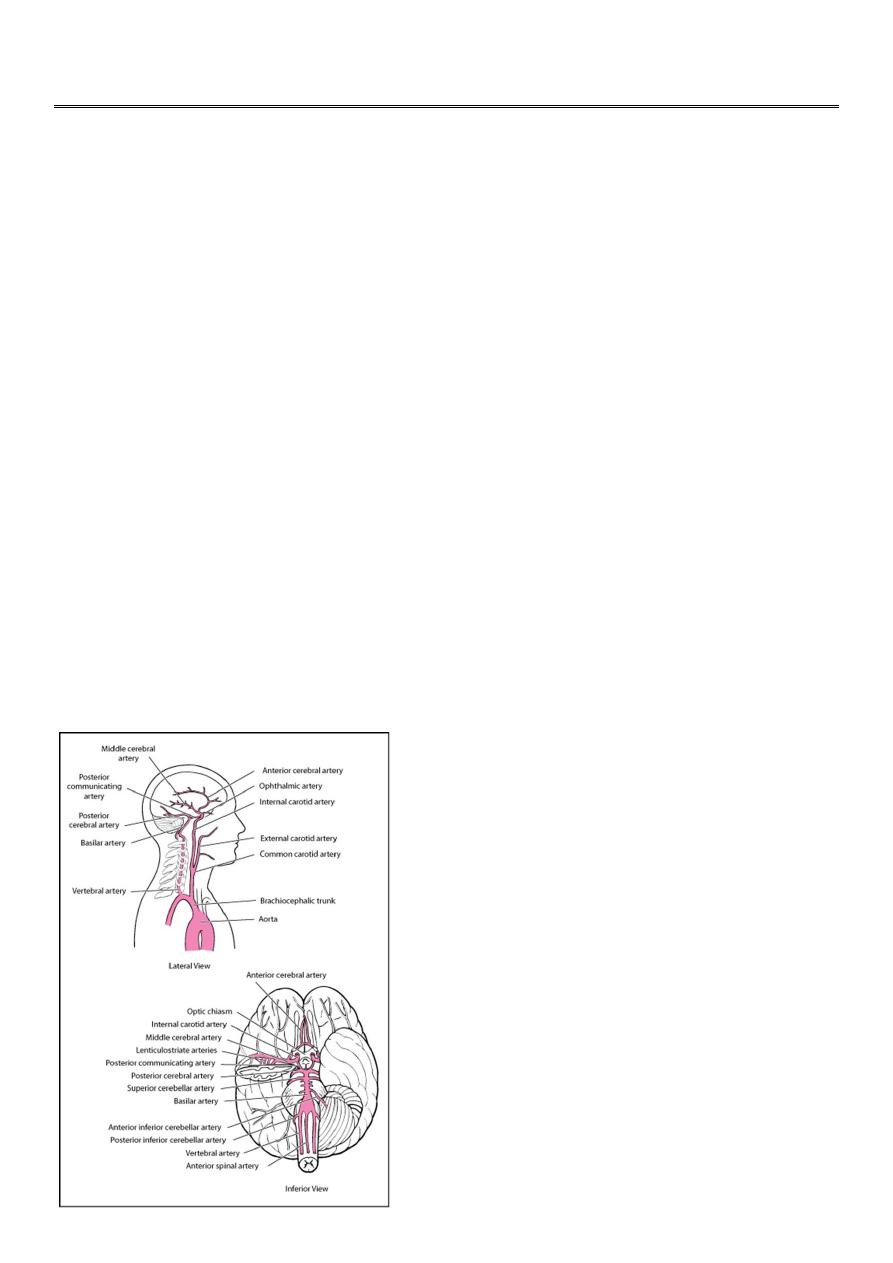
Strokes involve the arteries of the brain (see Arteries of the brain. ), either the
anterior circulation (branches of the internal carotid artery) or the posterior
circulation (branches of the vertebral and basilar arteries).
The anterior cerebral artery supplies the medial portions of the frontal and parietal
lobes and corpus callosum. The middle cerebral artery supplies large portions of the
frontal, parietal, and temporal lobe surfaces. Branches of the anterior and middle
cerebral arteries (lenticulostriate arteries) supply the basal ganglia and anterior limb
of the internal capsule.

2
The vertebral and basilar arteries supply the brain stem, cerebellum, posterior
cerebral cortex, and medial temporal lobe. The posterior cerebral arteries bifurcate
from the basilar artery to supply the medial temporal (including the hippocampus)
and occipital lobes, thalamus, and mammillary and geniculate bodies.
Anterior circulation and posterior circulation communicate in the circle of Willis.
Ischemic Stroke
Ischemic stroke is sudden neurologic deficits that result from focal cerebral ischemia associated with
permanent brain infarction (eg, positive results on diffusion-weighted MRI). Common causes are (from most
to least common) atherothrombotic occlusion of large arteries; cerebral embolism (embolic infarction);
nonthrombotic occlusion of small, deep cerebral arteries (lacunar infarction); and proximal arterial stenosis
with hypotension that decreases cerebral blood flow in arterial watershed zones (hemodynamic stroke).
Etiology
The following are the modifiable risk factors that contribute the most to increased risk of ischemic stroke:
Hypertension
Cigarette smoking
Dyslipidemia
Diabetes
Abdominal obesity
Alcoholism
Lack of physical activity
High-risk diet (eg, high in saturated fats, trans fats, and calories)
Psychosocial stress (eg, depression)
Heart disorders (particularly disorders that predispose to emboli, such as acute MI, infective
endocarditis, and atrial fibrillation)
Use of certain drugs (eg, cocaine, amphetamines)
Hypercoagulability
Vasculitis
Unmodifiable risk factors include the following:
Prior stroke
Older age
Family history of stroke
Male sex
Ischemia usually results from thrombi or emboli.
Thrombosis:
Atherothrombotic occlusion of large arteries (thrombus superimposed on an atherosclerotic artery) is the
most common cause of ischemic stroke.

3
Atheromas, particularly if ulcerated, predispose to thrombi. Atheromas can occur in any major cerebral
artery and are common at areas of turbulent flow, particularly at the carotid bifurcation.
Partial or complete
thrombotic occlusion occurs most often at the main trunk of the middle cerebral artery and its branches but
is also common in the large arteries at the base of the brain, in deep perforating arteries, and in small
cortical branches.
The basilar artery and the segment of the internal carotid artery between the cavernous
sinus and supraclinoid process are often occluded.
Less common causes of thrombosis include vascular inflammation secondary to disorders such as acute or
chronic meningitis, vasculitic disorders, and syphilis; dissection of intracranial arteries or the aorta;
hypercoagulability disorders (eg, antiphospholipid syndrome, hyperhomocysteinemia); hyperviscosity
disorders (eg, polycythemia, thrombocytosis, hemoglobinopathies, plasma cell disorders); and rare
disorders (eg, fibromuscular dysplasia, moyamoya disease).Older oral contraceptive formulations increase
risk of thrombosis. In children, sickle cell disease is a common cause of ischemic stroke.
Embolism:
Emboli may lodge anywhere in the cerebral arterial tree.
Emboli may originate as cardiac thrombi, especially in the following conditions:
Atrial fibrillation
Rheumatic heart disease (usually mitral stenosis)
Post-MI
Vegetations on heart valves in bacterial or marantic endocarditis
Prosthetic heart valves
Other sources include clots that form after open-heart surgery and atheromas in neck arteries or in the
aortic arch. Rarely, emboli consist of fat (from fractured long bones), air (in decompression sickness), or
venous clots that pass from the right to the left side of the heart through a patent foramen ovale with shunt
(paradoxical emboli). Emboli may dislodge spontaneously or after invasive cardiovascular procedures (eg,
catheterization).
Lacunar infarcts:
Ischemic stroke can also result from lacunar infarcts. These small (
≤ 1.5 cm) infarcts result from
nonatherothrombotic obstruction of small, perforating arteries that supply deep cortical structures; the usual
cause is lipohyalinosis (degeneration of the media of small arteries and replacement by lipids and
collagen). Whether emboli cause lacunar infarcts is controversial. Lacunar infarcts tend to occur in elderly
patients with diabetes or poorly controlled hypertension.
Other causes:
Any factor that impairs systemic perfusion (eg, carbon monoxide toxicity, severe anemia or hypoxia,
polycythemia, hypotension) increases risk of all types of ischemic strokes. A stroke may occur along the
borders between territories of arteries (watershed areas); in such areas, blood supply is normally low,
particularly if patients have hypotension and/or if major cerebral arteries are stenotic.
Less commonly, ischemic stroke results from vasospasm (eg, during migraine, after subarachnoid
hemorrhage, after use of sympathomimetic drugs such as cocaine or amphetamines) or venous sinus
thrombosis (eg, during intracranial infection, postoperatively, peripartum, secondary to a hypercoagulability
disorder).

4
Pathophysiology
Inadequate blood flow in a single brain artery can often be compensated for by an efficient collateral
system, particularly between the carotid and vertebral arteries via anastomoses at the circle of Willis and, to
a lesser extent, between major arteries supplying the cerebral hemispheres. However, normal variations in
the circle of Willis and in the caliber of various collateral vessels, atherosclerosis, and other acquired
arterial lesions can interfere with collateral flow, increasing the chance that blockage of one artery will
cause brain ischemia.
Some neurons die when perfusion is < 5% of normal for > 5 min; however, the extent of damage depends
on the severity of ischemia. If it is mild, damage proceeds slowly; thus, even if perfusion is 40% of normal,
3 to 6 h may elapse before brain tissue is completely lost. However, if severe ischemia (ie, decrease in
perfusion) persists > 15 to 30 min, all of the affected tissue dies (infarction). Damage occurs more rapidly
during hyperthermia and more slowly during hypothermia. If tissues are ischemic but not yet irreversibly
damaged, promptly restoring blood flow may reduce or reverse injury. For example, intervention may be
able to salvage the moderately ischemic areas (penumbras) that often surround areas of severe ischemia
(these areas exist because of collateral flow).
Mechanisms of ischemic injury include edema, microvascular thrombosis, programmed cell death
(apoptosis), and infarction with cell necrosis. Inflammatory mediators (eg, IL-1B, tumor necrosis factor-
α)
contribute to edema and microvascular thrombosis. Edema, if severe or extensive, can increase intracranial
pressure. Many factors may contribute to necrotic cell death; they include loss of ATP stores, loss of ionic
homeostasis (including intracellular Ca accumulation), lipid peroxidative damage to cell membranes by free
radicals (an iron-mediated process), excitatory neurotoxins (eg, glutamate), and intracellular acidosis due to
accumulation of lactate.
Symptoms and Signs
Symptoms and signs depend on the part of brain affected. Patterns of neurologic deficits often suggest the
affected artery (
),
Deficits may become maximal within several minutes of onset, typically in embolic stroke. Less often,
deficits evolve slowly, usually over 24 to 48 h (called evolving stroke or stroke in evolution), typically in
atherothrombotic stroke. In most evolving strokes, unilateral neurologic dysfunction (often beginning in one
arm, then spreading ipsilaterally) extends without causing headache, pain, or fever. Progression is usually
stepwise, interrupted by periods of stability.
Embolic strokes often occur during the day; headache may precede neurologic deficits. Thrombi tend to
occur during the night and thus are first noticed on awakening. Lacunar infarcts may produce one of the
classic lacunar syndromes (eg, pure motor hemiparesis, pure sensory hemianesthesia, ataxic hemiparesis,
dysarthria
–clumsy hand syndrome); signs of cortical dysfunction (eg, aphasia) are absent. Multiple lacunar
infarcts may result in multi-infarct dementia.
A seizure may occur at stroke onset, more often with embolic than thrombotic stroke. Seizures may also
occur months to years later; late seizures result from scarring or hemosiderin deposition at the site of
ischemia.

5
Deterioration during the first 48 to 72 h after onset of symptoms, particularly progressively impaired
consciousness, results more often from cerebral edema than from extension of the infarct. Unless the
infarct is large or extensive, function commonly improves within the first few days; further improvement
occurs gradually for up to 1 yr.
Diagnosis
Primarily clinical evaluation
Neuroimaging and bedside glucose testing
Evaluation to identify the cause
Diagnosis is suggested by sudden neurologic deficits referable to a specific arterial territory. Ischemic
stroke must be distinguished from other causes of similar focal deficits (eg, hypoglycemia; postictal [Todd]
paralysis; hemorrhagic stroke; rarely, migraine), sometimes called stroke mimics. Headache, coma or
stupor, and vomiting are more likely with hemorrhagic stroke.
Differentiating clinically between the types of stroke is imprecise; however, some clues based on symptom
progression, time of onset, and type of deficit can help.
Although diagnosis is clinical, neuroimaging and bedside glucose testing are mandatory. CT is done first to
exclude intracerebral hemorrhage, subdural or epidural hematoma, and a rapidly growing, bleeding, or
suddenly symptomatic tumor. CT evidence of even a large anterior circulation ischemic stroke may be
subtle during the first few hours; changes may include effacement of sulci or the insular cortical ribbon, loss
of the gray-white junction between cortex and white matter, and a dense middle cerebral artery sign. Within
6 to 12 h of ischemia, medium-sized to large infarcts start to become visible as hypodensities; small infarcts
(eg, lacunar infarcts) may be visible only with MRI. Diffusion-weighted MRI (highly sensitive for early
ischemia) can be done immediately after initial CT neuroimaging.
Distinction between lacunar, embolic, and thrombotic stroke based on history, examination, and
neuroimaging is not always reliable, so tests to identify common or treatable causes and risk factors for all
of these types of strokes are routinely done.
Patients should be evaluated for the following categories of
causes and risk factors:
Cardiac (eg, atrial fibrillation, potential structural sources of emboli)
Vascular (eg, critical arterial stenosis)
Blood (eg, hypercoagulability)
For cardiac causes, testing typically includes ECG, telemetry or Holter monitoring, serum troponin, and
transthoracic or transesophageal echocardiography.
For vascular causes, testing may include magnetic resonance angiography (MRA), CT angiography
(CTA), carotid and transcranial duplex ultrasonography, and conventional angiography.
The choice and
sequence of testing is individualized, based on clinical findings. MRA, CTA, and carotid ultrasonography all
show the anterior circulation; however, MRA and CTA provide better images of the posterior circulation
than carotid ultrasonography. MRA is generally preferred to CTA if patients can remain still during MRA (to
avoid motion artifact).

6
For blood-related causes (eg, thrombotic disorders), blood tests are done to assess their contribution and
that of other causes. Routine testing typically includes CBC, platelet count, PT/PTT, fasting blood glucose,
and lipid profile.
Depending on which causes are clinically suspected, additional tests may include measurement of
homocysteine, testing for thrombotic disorders (antiphospholipid antibodies, protein S, protein C,
antithrombin III, factor V Leiden), testing for rheumatic disorders (eg, antinuclear antibodies, rheumatoid
factor, ESR), syphilis serologic testing, Hb electrophoresis, and a urine drug screen for cocaine and
amphetamines.
A cause cannot be identified for some strokes (cryptogenic strokes).
Prognosis
Stroke severity and progression are often assessed using standardized measures such as the National
Institutes of Health (NIH) Stroke Scale (the score on this scale correlates with extent of functional
impairment and prognosis. During the first days, progression and outcome can be difficult to predict. Older
age, impaired consciousness, aphasia, and brain stem signs suggest a poor prognosis. Early improvement
and younger age suggest a favorable prognosis.
About 50% of patients with moderate or severe hemiplegia and most with milder deficits have a clear
sensorium and eventually can take care of their basic needs and walk adequately. Complete neurologic
recovery occurs in about 10%. Use of the affected limb is usually limited, and most deficits that remain after
12 mo are permanent. Subsequent strokes often occur, and each tends to worsen neurologic function.
About 20% of patients die in the hospital; mortality rate increases with age.
Treatment
General stroke treatments
Acute antihypertensive therapy only in certain circumstances
Antiplatelet therapy
Occasionally for acute treatment, reperfusion (eg, recombinant tissue plasminogen activator [tPA] or
thrombolysis-in-situ)
Sometimes anticoagulation
Long-term control of risk factors
Sometimes carotid endarterectomy or stenting
Acute:
Patients with acute ischemic strokes are usually hospitalized. Supportive measures may be needed during
initial evaluation and stabilization.
Perfusion of an ischemic brain area may require a high BP because autoregulation is lost; thus, BP should
not be decreased except in the following cases:
BP is > 220 mm Hg systolic or > 120 mm Hg diastolic on 2 successive readings >15 min apart.
There are signs of other end-organ damage (eg, aortic dissection, acute MI, pulmonary edema,
hypertensive encephalopathy, retinal hemorrhages, acute renal failure).
Use of recombinant tPA is likely.
To lower BP, clinicians can give nicardipine
2.5 mg/h IV initially; dose is increased by 2.5 mg/h q 5 min to a
maximum of 15 mg/h as needed to decrease systolic BP by 10 to 15%. Alternatively, IV labetalol
20 mg IV

7
can be given over 2 min; if response is inadequate, 40 to 80 mg can be given every 10 min up to a total
dose of 300 mg.
Patients with presumed thrombi or emboli may be treated with tPA, thrombolysis-in-situ, antiplatelet drugs,
and/or anticoagulants. Most patients are not candidates for thrombolytic therapy; they should be given an
antiplatelet drug (usually aspirin
325 mg po) when they are admitted to the hospital.
Contraindications to
antiplatelet drugs include aspirin- or NSAID-induced asthma or urticaria, other hypersensitivity to aspirin or
to tartrazine, acute GI bleeding, G6PD deficiency, and use of warfarin
Recombinant tPA is used for patients with acute ischemic stroke up to 4.5 h after symptom onset if they
have no contraindications to tPA. Although tPA can cause fatal or other symptomatic brain hemorrhage,
patients treated with tPA strictly according to protocols still have a higher likelihood of functional neurologic
recovery.
Only physicians experienced in stroke management should use tPA to treat patients with acute
stroke; inexperienced physicians are more likely to violate protocols, resulting in more brain hemorrhages
and deaths. When tPA is given incorrectly (eg, when given despite the presence of exclusion criteria), risk
of hemorrhage due to tPA is high mainly for patients who have had stroke; risk is low for patients who have
had a stroke mimic. If experienced physicians are not available on site, consultation with an expert at a
stroke center (including video evaluation of the patient [telemedicine]), if possible, may enable these
physicians to use tPA. Because most poor outcomes result from failure to strictly adhere to the protocol, a
checklist of inclusion and exclusion criteria should be used.
tPA must be given within 4.5 h of symptom onset
—a difficult requirement. Because the precise time of
symptom onset may not be known, clinicians must start timing from the moment the patient was last
observed to be well.
Before treatment with tPA, brain hemorrhage must be excluded by CT, systolic BP must be < 185 mm Hg,
and diastolic BP must be < 110 mm Hg. Dose of tPA is 0.9 mg/kg IV (maximum dose 90 mg); 10% is given
by rapid IV injection, and the remainder by constant infusion over 60 min. Vital signs are closely monitored
for 24 h after treatment, and BP is maintained below 185 mm Hg systolic and 110 mm Hg diastolic. Any
bleeding complications are aggressively managed. Anticoagulants and antiplatelet drugs are not used
within 24 h of treatment with tPA.
Thrombolysis-in-situ (angiographically directed intra-arterial thrombolysis) of a thrombus or embolus can
sometimes be used for major strokes if symptoms began >3 h but < 6 h ago, particularly for strokes due to
large occlusions in the middle cerebral artery. Clots in the basilar artery may be intra-arterially lysed up to
12 h after stroke onset, sometimes even later depending on the clinical circumstances. This treatment,
although standard of care in some large stroke centers, is often unavailable in other hospitals.
Mechanical thrombectomy (angiographically directed intra-arterial removal of a thrombus or embolus by a
device) is often used as rescue treatment for patients who have had a severe stroke and have an NIH
stroke score ≥ 8 when IV and/or intra-arterial thrombolysis has been ineffective; it must be done within 8 h
of symptom onset.
Mechanical thrombectomy may be part of standard of care in large stroke centers. It
should not be used outside of a stroke center and should not be used instead of IV recombinant tPA within
4.5 h of onset of symptoms in eligible patients with acute ischemic stroke. Devices used to remove thrombi
are being improved, and recent models reestablish perfusion in 90 to 100% of patients. It is unclear
whether clinical outcomes are better after successful mechanical reperfusion than after treatment with IV

8
tPA; evidence suggests that the earlier reperfusion is achieved, the better the outcome regardless how it is
achieved.
In some stroke centers, IV tPA, thrombolysis in situ, and/or mechanical thrombectomy are sometimes done
based on imaging criteria (tissue-based criteria) rather than on time after symptom onset (time-based
criteria). Tissue-based criteria can be used when time of symptom onset cannot be established (eg, if a
patient awakens with stroke symptoms after sleeping several hours or if a patient has aphasia and cannot
provide a time frame). To determine eligibility, clinicians use imaging to identify potentially salvageable
brain tissue (also called penumbral tissue). The volume of infarcted tissue identified by diffusion-weighted
MRI is compared with the volume of at-risk underperfused tissue identified by perfusion-weighted MRI or
CT. A sizeable mismatch between the volumes identified by diffusion-weighted and perfusion-weighted
imaging suggests that substantial penumbral tissue may still be rescued, and thus thrombolysis and/or
thrombectomy is indicated. However, time-based criteria are still used in clinical practice; studies to
determine whether outcomes are better using tissue- or time-based criteria are ongoing.
Anticoagulation with heparin
r low molecular weight heparin is used for stroke caused by cerebral venous
thrombosis and is sometimes used for emboli due to atrial fibrillation and for stroke due to presumed
progressive thrombosis if it continues to evolve despite use of antiplatelet drugs and cannot be treated any
other way (eg, with tPA or invasive methods). Warfarin is begun simultaneously with heparin. Before
anticoagulation, hemorrhage must be excluded by CT.
Long term:
Supportive care is continued during convalescence. Controlling hyperglycemia and fever can limit brain
damage after stroke, leading to better functional outcomes.Long-term management also focuses on
prevention of recurrent stroke (secondary prevention). Modifiable risk factors (eg, hypertension, diabetes,
smoking, alcoholism, dyslipidemia, obesity) are treated. Reducing systolic BP may be more effective when
the target BP is < 120 mm Hg rather than the typical level (< 140 mm Hg).
Extracranial carotid endarterectomy or stenting
is indicated for patients with recent nondisabling,
submaximal stroke attributed to an ipsilateral carotid obstruction of 70 to 99% of the arterial lumen or to an
ulcerated plaque if life expectancy is at least 5 yr. In other symptomatic patients (eg, patients with TIAs),
endarterectomy or stenting with antiplatelet therapy is indicated for carotid obstruction of
≥60% with or
without ulceration if life expectancy is at least 5 yr. These procedures should be done by surgeons and
interventionists who have a successful record with the procedure (ie, morbidity and mortality rate of < 3%)
in the hospital where it will be done. If carotid stenosis is asymptomatic, endarterectomy or stenting is
beneficial only when done by very experienced surgeons or interventionists, and that benefit is likely to be
small.
Extracranial vertebral angioplasty and/or stenting
can be used in certain patients with recurrent
symptoms of vertebrobasilar ischemia despite optimal medical treatment and a vertebral artery obstruction
of 50 to 99%.
Intracranial major artery angioplasty and/or
stenting is considered investigational for patients with
recurrent stroke or TIA symptoms despite optimal medical treatment and a 50 to 99% obstruction of a major
intracranial artery.

9
Oral antiplatelet drugs are used to prevent subsequent noncardioembolic (atherothrombotic, lacunar,
cryptogenic) strokes (secondary prevention). Aspirin
81 or 325 mg once/day, clopidogrel
75 mg once/day,
or the combination product aspirin
25 mg/extended-release dipyridamole
200 mg bid may be used.
If patients have had a TIA or minor stroke, clopidogrel
plus aspirin
given within 24 h of symptom onset
appears more effective than aspirin alone for reducing risk of stroke in the first 90 days and does not
increase risk of hemorrhage. However, prolonged (eg, > 6 mo) use of clopidogrel plus aspirin is avoided
because it has no advantage over aspirin alone in long-term secondary stroke prevention and results in
more bleeding complications. if patients cannot tolerate clopidogrel, ticlopidine
250 mg bid can be
substituted.
Oral anticoagulants are indicated for secondary prevention of cardioembolic strokes (as well as primary
prevention). Adjusted-dose warfarin
(a vitamin K antagonist) with a target INR of 2 to 3 is used for certain patients with nonvalvular or valvular
atrial fibrillation. A target INR of 2.5 to 3.5 is used if patients have a mechanical prosthetic cardiac valve.
Efficacious alternatives to warfarin for patients with nonvalvular atrial fibrillation include the following new
anticoagulants:
Dabigatran (a direct thrombin inhibitor) 150 mg bid in patients without severe renal failure (creatinine
clearance < 15 mL/min) and/or liver failure (elevated INR)
Apixaban (
a direct factor Xa inhibitor) 5 mg bid in patients ≥ 80 yr, in patients with serum creatinine ≥ 1.5
mg/dL and creatinine clearance ≥ 25 mL/min, or as an alternative to aspirin
In patients who cannot takewarfarin
Rivaroxaban (a direct factor Xa inhibitor) 20 mg once/day for patients without severe renal failure
(creatinine clearance < 15 mL/min)
The main advantage of these new anticoagulants is ease of use (eg, no need to check anticoagulation level
with a blood test after the initial dose Their main disadvantage is lack of an antidote to reverse
anticoagulation in case a hemorrhagic complication occurs. Efficacy and safety of combining any of these
new anticoagulants with an antiplatelet drug have not been established.
Statins are used to prevent recurrent strokes; lipid levels must be decreased by substantial
amounts.Atorvastatin 80 mg once/day is recommended for patients with evidence of atherosclerotic stroke
and LDL (low-
density lipoprotein) cholesterol ≥ 100 mg/dL. A reasonable LDL cholesterol target is a 50%
reduction or a level of < 70 mg/dL. Other statins (eg, simvastatin, pravastatin) may be also used.
Key Points
Differentiate ischemic stroke from hypoglycemia, postictal paralysis, hemorrhagic stroke, and migraine.
Although clinical differentiation is imprecise, some clues to help differentiate between common types of
stroke include symptom progression (maximal deficits within minutes of onset with embolic vs
sometimes stepwise or slow onset with thrombotic), time of onset (day with embolic vs night with
thrombotic), and type of deficits (eg, specific syndromes and absence of cortical signs with lacunar
infarcts).
Test patients for cardiac causes (including atrial fibrillation) and arterial stenosis, and do blood tests (eg,
for thrombotic, rheumatic, and other disorders as indicated).
In general, do not aggressively reduce BP soon after acute ischemic stroke.

11
To determine eligibility for tPA, use a checklist and, when available consult an expert, either in person or
via telemedicine.
To prevent future ischemic strokes, control modifiable risk factors and treat, when appropriate, with
antiplatelet therapy, statin therapy, and/or endarterectomy or stenting.
Transient Ischemic Attack (TIA)
A transient ischemic attack is focal brain ischemia that causes sudden, transient neurologic deficits and is
not accompanied by permanent brain infarction (eg, negative results on diffusion-weighted MRI).
TIA is similar to ischemic stroke except that symptoms usually last < 1 h; most TIAs last < 5 min. Infarction is
very unlikely if deficits resolve within 1 h. As shown by diffusion-weighted MRI and other studies, deficits
that resolve spontaneously within 1 to 24 h are often accompanied by infarction and are thus no longer
considered TIAs. TIAs are most common among the middle-aged and elderly. TIAs markedly increase risk of
stroke, beginning in the first 24 h.
Etiology
Risk factors for TIA are the same as those for ischemic stroke.
Most TIAs are caused by emboli, usually from carotid or vertebral arteries, although most of the causes of
ischemic stroke can also result in TIAs.
.
In subclavian steal syndrome, a subclavian artery stenosed proximal to the origin of the vertebral artery
“steals” blood from the vertebral artery (in which blood flow reverses) to supply the arm during exertion,
causing signs of vertebrobasilar ischemia.
Symptoms and Signs
Neurologic deficits are similar to those of strokes. Transient monocular blindness (amaurosis fugax), which
usually lasts < 5 min, may occur when the ophthalmic artery is affected. Symptoms begin suddenly, usually
last 2 to 30 min, then resolve completely. Patients may have several TIAs daily or only 2 or 3 over several
years. Symptoms are usually similar in successive carotid attacks but vary somewhat in successive
vertebrobasilar attacks.
Diagnosis
Resolution of strokelike symptoms within 1 h
Neuroimaging
Evaluation to identify the cause
Diagnosis is made retrospectively when sudden neurologic deficits referable to ischemia in an arterial
territory resolve within 1 h.
Isolated peripheral facial nerve palsy, loss of consciousness, or impaired consciousness does not suggest
TIA.

11
TIAs must be distinguished from other causes of similar symptoms (eg, hypoglycemia, migraine aura,
postictal [Todd] paralysis.
)
Because an infarct, a small hemorrhage, and even a mass lesion cannot be excluded clinically,
neuroimaging is required. Usually, CT is the study most likely to be immediately available. However, CT
may not identify infarcts for > 24 h. MRI usually detects evolving infarction within hours. Diffusion-
weighted MRI is the most accurate imaging test to rule out an infarct in patients with presumed TIA but is
not always available.
The cause of a TIA is sought as for that of ischemic strokes; evaluation includes tests for carotid stenosis,
cardiac sources of emboli, atrial fibrillation, and hematologic abnormalities and screening for stroke risk
factors.
Treatment
Prevention of strokes
Treatment is aimed at preventing strokes; antiplatelet drugs and statins are used. Carotid endarterectomy
or arterial angioplasty plus stenting can be useful for some patients, particularly those who have no
neurologic deficits but who are at high risk of stroke. Anticoagulation is indicated if cardiac sources of
emboli are present.
Modifying stroke risk factors, when possible, may prevent stroke.
