Carbohydrates-IStructure and Digestion
Faculty of Dentistry1
1. Definition and classification of sugars
2. Stereoisomers3. Glucose, mannose and galactose
4. Fructose
5. Reactions of monosaccharides
6. Glycosides
7. Amino sugars, deoxy sugars, pentoses
8. Sucrose, lactose and maltose
9. Starch, glycogen and cellulose
10. Heteroglycans, mucopolysaccharides
11. Absorption of glucose; glucose transporters
2
Introduction to carbohydrates
Carbohydrates are the most abundant organic molecules in nature.They have a wide range of functions, such as providing a significant part of the dietary calories for most organisms,
acting as a storage form of energy in the body,
and serving as cell membrane components that mediate some forms of intercellular communication.
3
Introduction to carbohydrates
Carbohydrates are compounds that contain at least three carbon atoms, a number of hydroxyl groups, and usually an aldehyde or ketone group.
They may contain phosphate, amino, or sulfate groups.
thus its name is “hydrate of carbon.”
Cn(H2O)n
4
Aldose - Ketose
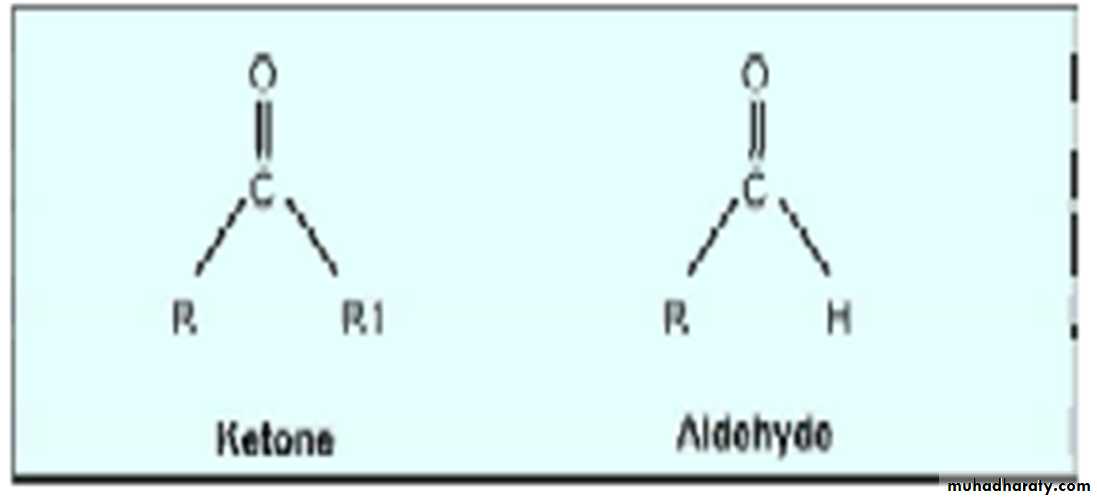
Carbohydrates with an aldehyde as their most oxidized functional group are called aldoses, whereas those with a keto as their most oxidized functional group are called ketoses.For example, glyceraldehyde is an aldose, whereas dihydroxy acetone is a ketose.
5
Aldose and ketose
They are called aldoses or ketoses,depending on whether their most oxidized functional group is an aldehyde or a ketone.
Monosaccharides
Monosaccharides (simple sugars) can be classified according to the number of carbon atoms they contain.
Monosaccharides are joined by glycosidic bonds to form disaccharides, oligosaccharides, and polysaccharides.
Monosaccharides form rings that usually contain five (furanose) or six (pyranoses) members.
7
A. Monosaccharides 1. Nomenclature
a. The simplest monosaccharides have the formula (CH2O)n.Monosaccarides that has
three carbons are called trioses;
Four carbons are called tetroses;
Five carbons are called pentoses;
and six carbons are called hexoses.
Disaccharides contain two monosaccharide units,
oligo saccharides contain from 3-10 monosaccharide units,polysaccharides contain more than 10 monosaccharide units, and can be hundreds of sugar units in length.
9
Functions of Carbohydrates
1. Carbohydrates are the main sources of energy in the body.
Brain cells and red blood cells are almost fully dependent on carbohydrates as the energy source.
Energy production from carbohydrates will be 4 kcal/g.
2. Storage form of energy such as starch and glycogen.
3. Excess carbohydrate is converted to fat.
4. Glycoproteins and glycolipids are components
of cell membranes and receptors.
10
Functions of Carbohydrates
5. Structural unit of many organisms: Cellulose of plants; cell wall of microorganisms.6. The general molecular formula of carbohydrates is Cn(H2O)n.
For example, glucose has the molecular formula C6H12O6.
Carbohydrates are polyhydroxy aldehydes or ketones or compounds which yield these on hydrolysis.
11
Polysaccharides having only one type of monosaccharide units are called homopolysaccharides and
those having different monosaccharide units are called heteropolysaccharides.
6. Sugars having aldehyde group are called aldoses and sugars with keto group are ketoses.
12
vii. the monosaccharides are named asDepending on the number of carbon atoms,
triose (C3),
tetrose (C4),
pentose (C5),
hexose (C6),
heptose (C7) …….
13
Examples of monosaccharides found in humans, classified according to the number of carbons they contain.
Generic Names
Example
3 Carbons: trioses
Glyceraldehyde
4 Carbons: tetroses
Erythrose
5 Carbons: pentoses
Ribose
6 Carbons: hexoses
Glucose
7 Carbons: heptoses
Sedoheptulose
9 Carbons: nonoses
Neuraminic acid
14
Hexoses of physiological importance
SugarImportance
D-Glucose
• Blood sugar. Main source of energy
in body.
D-Fructose.
Constituent of sucrose, the common sugar
D-Galactose• Constituent of lactose, glycolipids and glycoproteins.
D-Mannose
• Constituent of globulins, mucoproteins and glycoproteins15
STEREOISOMERS
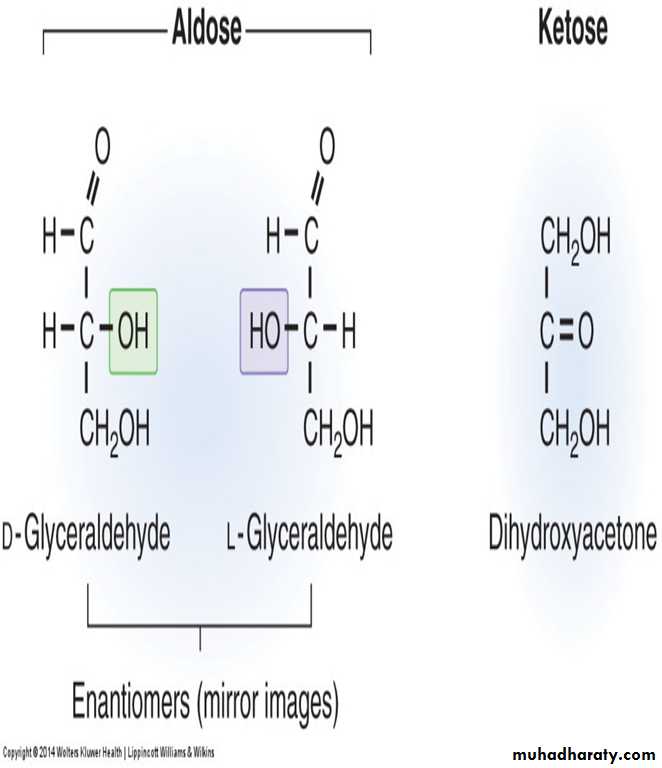
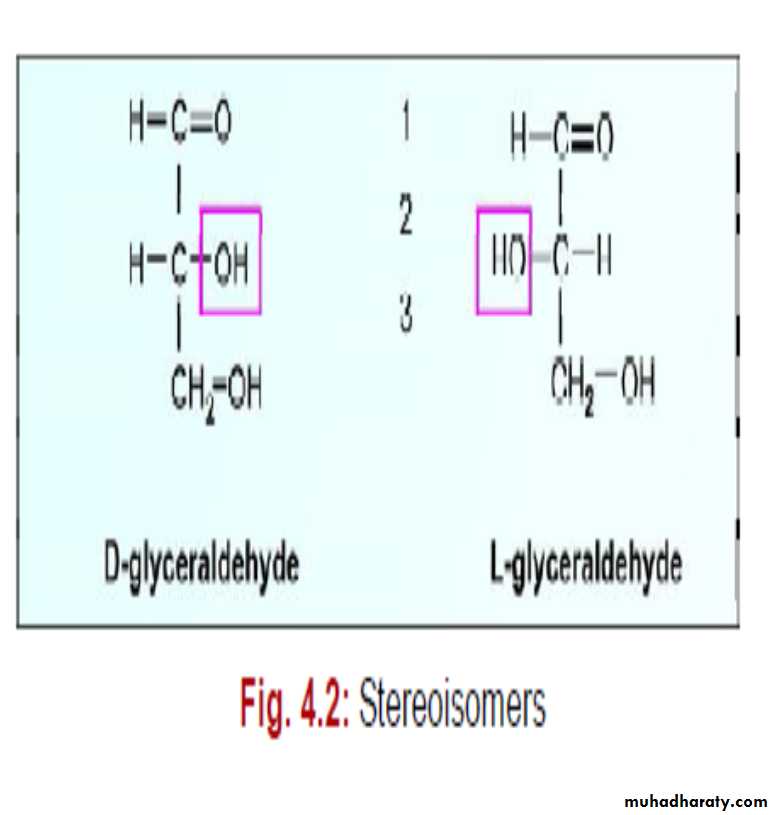
Compounds having same structural formula, but differ in spatial configuration are known as stereoisomers.While writing the molecular formula of monosaccharides, the spatial arrangements of H and OH groups are important, since they contain
asymmetric carbon atoms.
The reference molecule is glyceraldehyde which has a single asymmetric carbon atom.
16
D and L Isomerism of Glucose
All monosaccharides can be considered as molecules derived from glyceraldehyde.
Depending on the configuration of H and OH around the reference carbon atom, the two mirror forms are designated as L and D forms.
17
In the case of monosaccharides having more than 3 carbon atoms, the penultimate carbon atom (C5 in the case of glucose) is considered as the reference carbon atom.
It may be noted that in D and L varieties, the groups in 2nd, 3rd, 4th and 5th carbon atoms are totally reversed, so as to produce the mirror images.
Since enzymes are stereospecific, only D sugars are metabolized by the human body.
All naturally occurring sugars are D sugars.
18
Optical Activity
The presence of asymmetrical carbon atom causes optical activity.When a beam of plane-polarised light is passed through a solution of carbohydrates, it will rotate the light either to right or to left.
Please note that the D- and L-notation has no bearing with the optical activity.
Depending on the rotation, molecules are called dextrorotatory (+) (d) or levorotatory (–) (l).
Thus D glucose is dextrorotatory but D-fructose is levorotatory.
Equimolecular mixture of optical isomers has no net rotation (racemic mixture).
19
Glucose, Mannose and Galactose
• They are the common aldohexoses. Glucose is the sugar in human blood. It is the major source of energy.
• Mannose is a constituent of many glycoproteins.
3. Galactose is a constituent of lactose (milk sugar) and glycoproteins.
20
Glucose-fructose interconvention
21Comparison of different represantations of D Glucose.
22Epimerism
i. When sugars are different from one another, only in configuration with regard to a single carbon atom (other than the reference carbon atom), they are called epimers.23
Fructose:
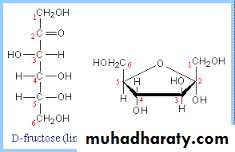
Fructose has the same molecular formula as glucose, but differs in structural formula.So glucose and fructose are functional group (aldose-ketose) isomers.
Fructose is a major constituent of honey.
24
Phosphorylated sugars
Sugar phosphates are very important for the body.Metabolism of sugars in body starts with phosphorilation.
25
10. Deoxy Sugars
Oxygen of the hydroxyl group may be removed to form deoxy sugars.Deoxyribose is an important part of nucleic acid.
11. Pentoses
They are sugars containing 5 carbon atoms.Ribose is a constituent of RNA while deoxyribose is seen in DNA
Ribose is also seen in coenzymes such as ATP and NAD.
26
DISACCHARIDES
When two monosaccharides are combined together by glycosidic linkage, a disaccharide is formed.
The important disaccharides are :
sucrose,
maltose,
isomaltose
lactose.
27
1. Sucrose
1. It is present in sugarcane and various fruits.Glucose + fructose = sucrose
2. The enzyme producing hydrolysis of sucrose is called sucrase.
28
2. Lactose
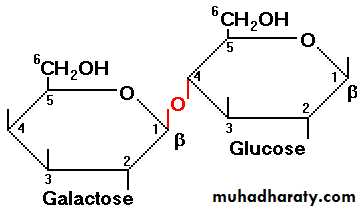
1. It is the sugar present in milk.2. Glucose + galactose = Lactose
Beta glycosidic linkage is present in lactose.
3. In lactose, the galactose residue is attached to the glucose through beta-1,4 glycosidic linkage.
29
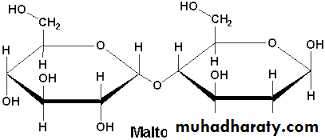
3. Maltose
It is another reducing disaccharide.Glucose + glucose = maltose .
Maltose contains two glucose residues with alpha- 1,4 linkage.
Maltose is a product of the hydrolysis of starch.
30
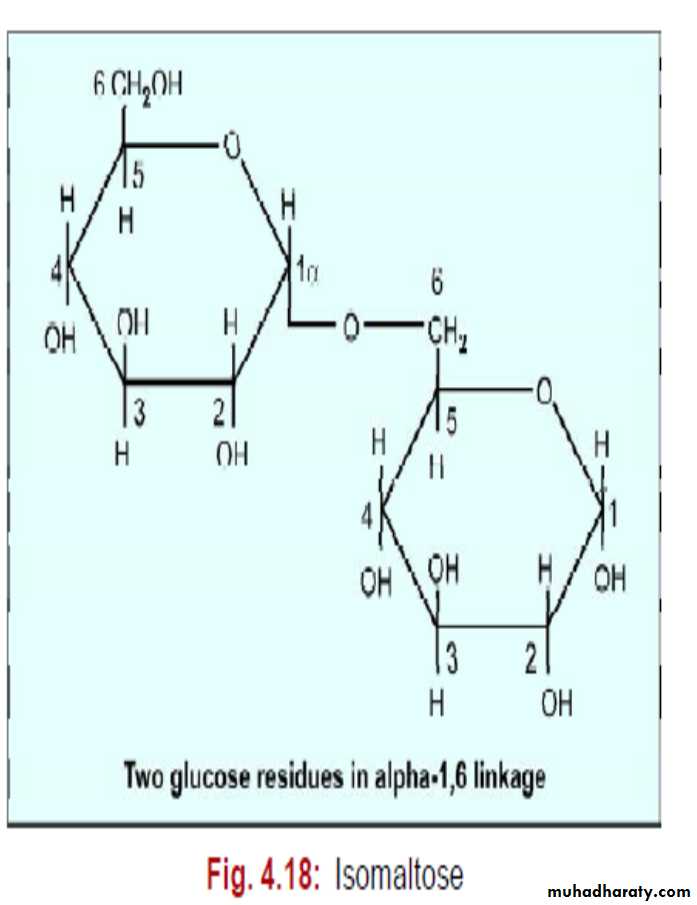
4. Isomaltose
It is also a reducing sugar.Glucose + glucose = isomaltose
It contains 2 glucose units combined in alpha -1, 6 linkage.
Partial hydrolysis of glycogen and starch produces isomaltose.
31
Disaccharides
Glucose + Galactose = Lactose (reducing)Glucose + Glucose = Maltose (reducing)
Glucose + Fructose = Sucrose (nonreducing)
32
POLYSACCHARIDES
These are polymerised products of many monosaccharide units.
They may be homoglycans composed of single kind of monosaccharides,
e.g. starch, glycogen and cellulose. Heteroglycans are composed of two or more different monosaccharides.
33
1. Starch
1. It is the reserve carbohydrate of plants and present in potatoes, rice, wheat and other food grains.When starch is treated with boiling water, 10-20% is solubilized; this part is called amylose.
The insoluble part is called amylopectin.
34
Starch
4. Hydrolysis of Starch:Starch will form a blue colored complex with iodine; this color disappears on heating and reappears when cooled.
This is a sensitive test for starch.
Starch is nonreducing because the free sugar groups are negligible in number. When starch is hydrolysed by mild acid, smaller and smaller fragments are produced.
Finally maltose units are produced.
35
Starch
5. Action of alpha-amylases on Starch:
Salivary amylase and pancreatic amylase are alpha amylases, which act at random on alpha-1,4 glycosidic bonds to split starch into smaller units (dextrins), and finally to alpha-maltose.
36
2. Glycogen
1. It is the reserve carbohydrate in animals.It is stored in liver and muscle.
About 5% of weight of liver is made up by glycogen.
2. Glycogen is composed of glucose units joined by alpha-1,4 and alpha-1,6 glycosidic linkages.
Glycogen is more branched and more compact than amylopectin.
3. The branching points are made by alpha-1, 6 linkages.
37
Glycogen structure
383. Cellulose
• It is the chief carbohydrate in plants. Cellulose constitutes 99% of cotton, 50% of wood and 40% of straw, and is the most abundant organic material in nature.2. It is made up of glucose units combined with cellobiose bridges or beta -1,4 linkages. It has a straightline structure, with no branching points.
3. Cellobiose bridges are hydrolysed by the enzyme cellobiase. But this enzyme is absent in animal and human digestive system, and hence cellulose cannot be digested in human.
39
HETEROGLYCANS
These are polysaccharides containing more than one type of sugar residues.A few examples are given below.
• Agar
• Mucopolysaccarides
• Hyaluronic Acid
• Heparin
• Keratan Sulphate
40
3. Hyaluronic Acid
It is present in connective tissues, tendons, synovial fluid and vitreous humor.It contains glucosamine and glucuronic acid.
41
4. Heparin
It is an anticoagulant widely used when taking blood in vitro for clinical studies.It is also used in vivo in suspected thromboembolic conditions to prevent intravascular coagulation.
It contains sulphated glucosamine.
42
GLYCOPROTEINS AND MUCOPROTEINS
When the carbohydrate chains are attached to a polypeptide chain it is called a proteoglycan.
If the carbohydrate content is less than 10%, it is generally named as a glycoprotein.
If the carbohydrate content is more than 10% it is a mucoprotein.
43
Glycoproteins are seen in almost all tissues and cell membranes.
The oligosaccharide chains of glycoproteins are composed of varying numbers of the following carbohydrate residue:Glucose (Glu);
mannose (Man);
galactose (Gal);
N-acetyl glucosamine (GluNAc);
N-acetyl galactosamine (GalNAc); etc.
44
DIGESTION OF CARBOHYDRATES
• Cooking helps in breaking of glycosidic linkages in polysaccharides and thus makes the digestion process easier.• In the diet carbohydrates are available as polysaccharides (starch, glycogen), and to a minor extent, as disaccharides (sucrose and lactose).
These complex carbohydrates are hydrolyzed to monosaccharide units in the gastrointestinal tract.
45
Digestion of carbohydrates
• This process of digestion starts in mouth by the salivary alpha-amylase.
However, the time available for digestion in the mouth is limited.
The gastric hydrochloric acid will inhibit the action of salivary amylase.
• Pancreas secretes pancreatic alpha-amylase which will hydrolyse the alpha-1,4 glycosidic linkages, so produce smaller subunits like maltose, isomaltose, dextrins and oligosaccharides.
46
Digestion of carbohydrates
• The intestinal juice and brush border of intestinal cells contain enzymes, which will hydrolyse disaccharides into component monosaccharides.These enzymes are:
sucrase,
maltase,
isomaltase
and lactase.
The monosaccharides are then absorbed.
47
Carbohydrate digestion
48Absorption of carbohydrates
Only monosaccharides are absorbed by the intestine.Absorption rate of galactose is more than glucose; while fructose is absorbed at a lesser rate than glucose.
Glucose is absorbed with the help of specific transporters called glucose tranporters.
These are transmembrane proteins spanning the width of the membrane.
49
1. Co-transport from Lumen to Intestinal Cell
i. Absorption from intestinal lumen into intestinal cell is by co-transport mechanism (secondary active transport).
This mechanism is also called Sodium Dependent Glucose Transporter (SGluT).
ii. Here a membrane bound carrier protein is involved, which carries glucose, along with sodium ion.
Glucose is transported from a lower concentration to a higher concentration.
50
Sodium Dependent Glucose Transport
iii. This is coupled with the movement of sodium from a higher concentration to lower concentration.This sodium is later expelled by the sodium pump (sodium-potassium ATPase) with utilization of energy. So energy is needed indirectly.
51
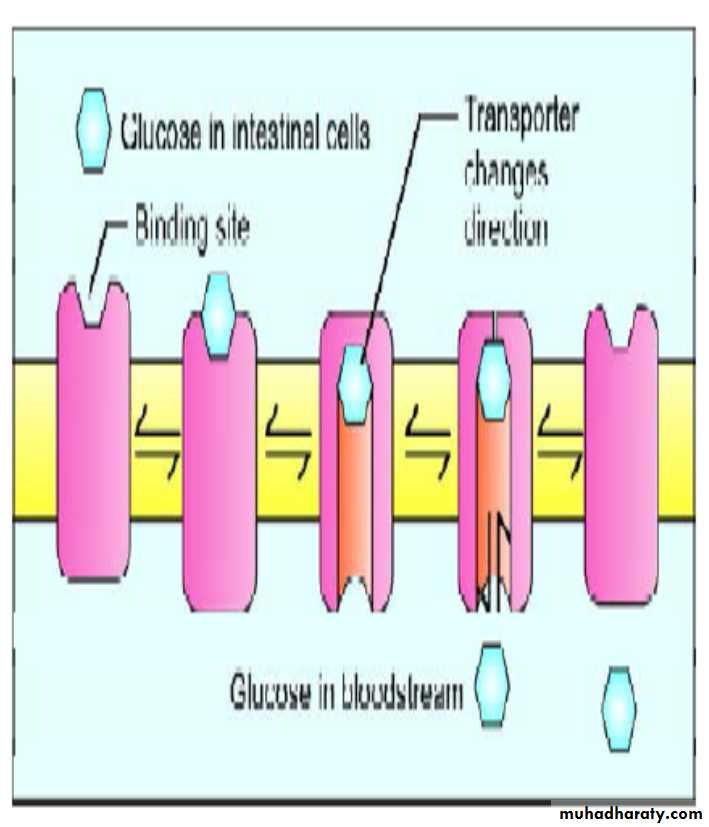
2. Release of Glucose into Blood
i. The same intestinal epithelial cells have a different transport mechanism on the membrane facing capillaries.Intestinal cells release glucose into blood stream by the carrier mechanism called Glucose Transporter Type 2 (GluT2).
This transporter is not dependent on sodium, but it is a uniport, facilitated diffusion system.
52
Glucose absorption
ii. The GluT2 first opens up on the outer side and takes the glucose molecule.When fixed, the complex changes configuration, and opens out at the inner side, releasing the glucose. The transporter behaves as a gated pore, and the process is called ping-pong mechanism.
53
3. GluT2 in Other Tissues
In other tissues GluT2 is involved in absorption of glucose from blood stream.GluT2 is present in :
intestinal epithelial cells
liver cells
beta cells of pancreas
kidney.
54
4. GluT4 in Muscle and Adipose Tissue
1. GluT4 is the major glucose transporter in skeletal muscle, heart muscle and adipocytes.The GluT4 is under control of insulin.
55
4. GluT4 in Muscle and Adipose Tissue
Insulin increases the number of transporter molecules, and thus glucose uptake is enhanced.ii. In Type 2 diabetes mellitus, insulin resistance is seen in muscle and fat cells.
In diabetes, entry of glucose into muscle is only half of normal cells.
This is because membrane bound GluT4 is reduced in insulin deficiency, due to defective recycling.
56
Glucose transporters
Transporter
• Present in
• Properties
GluT1
RBC, brain, kidneyGlucose uptake
in most of cells
GluT2
• Serosal surface of intestinal cells, beta cell pancreas
• Glucose uptake in liver; Glucose sensor in beta cells
GluT3
• Neurons, brain
• Glucose into brain
GluT4
• Skeletal, heart muscle adipose tissue
• Insulin mediated, glucose uptake
57Summary
Carbohydrates are polyhydroxy aldehydes or ketones or compounds.
• Carbohydrates are classified into Monosaccharides, Disaccharides and Polysaccharides, based on the number of sugar/saccharide units they possess.
• Common examples of monosaccharides include Glucose, fructose, galactose, and mannose.
• Important examples of disaccharides are Sucrose, Lactose and Maltose.
58
Summary
• Monosaccharides exhibit stereoisomerism, optical isomerism.• The stereoisomers are prefixed as ‘D’ or ‘L’. D sugars are naturally occurring.
59
Summary
Anomers of monosaccharides are produced by the spatial configuration with reference to the first carbon atom in aldoses and the second carbon atom in ketoses.• Two anomers of Glucose are alpha-D glucose and beta-D glucose.
• Mutarotation is the result of anomerism.
60
Summary
Sucrose is formed from glucose and fructose.• Lactose is formed from galactose and glucose.
• Maltose is formed from two glucose molecules
• Mucopolysaccharides or Glycosaminoglycans
(GAG’s) such as Hyaluronic acid, Chondroitin
sulfate and Keratan sulfate are associated with
connective tissue.
61
Summary
• Special glucose transporters perform the function of absorption of glucose in various cells.
Absorption of glucose from intestinal lumen into intestinal cell is by Sodium dependent glucose transporter (SGluT).
• Intestinal cells release glucose into blood stream
by the carrier mechanism called Glucose
transporter type 2 (GluT2).
• The GluT4 has been implicated in Type 2 diabetes
mellitus.
62