
first stage
Medical Physics
Lec-6
4/1/2016
د.تيماء
The Physics of the Lungs and Breathing
The human “ machine “ really consists of billions of very small “engines “ the
living cells of the body .Each of these a miniature engines must be provided with
fuel ,O2 ,and a method of getting rid of the products .
The blood and its vessels (cardiovascular system ) serve as the transport for these
engines .The lungs (pulmonary system )serve as the supplier of O2 and disposes
of the main by product - CO2 .The blood takes the O2 to the tissues and
removes the CO2 from the tissues ; it must come in close contact with the air in
the lungs in order to exchange its load of CO2 for a fresh load of O2 .
The function of the Lungs
1. Exchanging of O2 ands CO2 .
2. Keeping the PH (acidity ) of the blood constant .
3. Heat exchange .
4. Keeping the fluid of the body balance by warming and moisturizing
the air we breath .
5 . Controlled the flow of air for talking , coughing ,sneezing , sighing ,
laughing , and sniffing .
6 . Voice production .
Breathing
We breath about 6 liters of air per minute .Men breath about 12 times per
minute at rest ,Women breath about 20 times per minute ,Infants breath about 60
times per minute .The air we inspired is about 80% N2 and
20% O2 .Expired air is about 80% N2 ,16% O2 and 4% CO2 .We breath about
10 Kg of air each day .Of this the lungs absorbs 400 liters of O2
(0.5 Kg) and release a slightly smaller amount of CO2 .Each time we breath ,
about 10²² molecules of air enter our lungs .Each liters of air contain about
6 x10²³molecules (Avogadro’s number ).
2
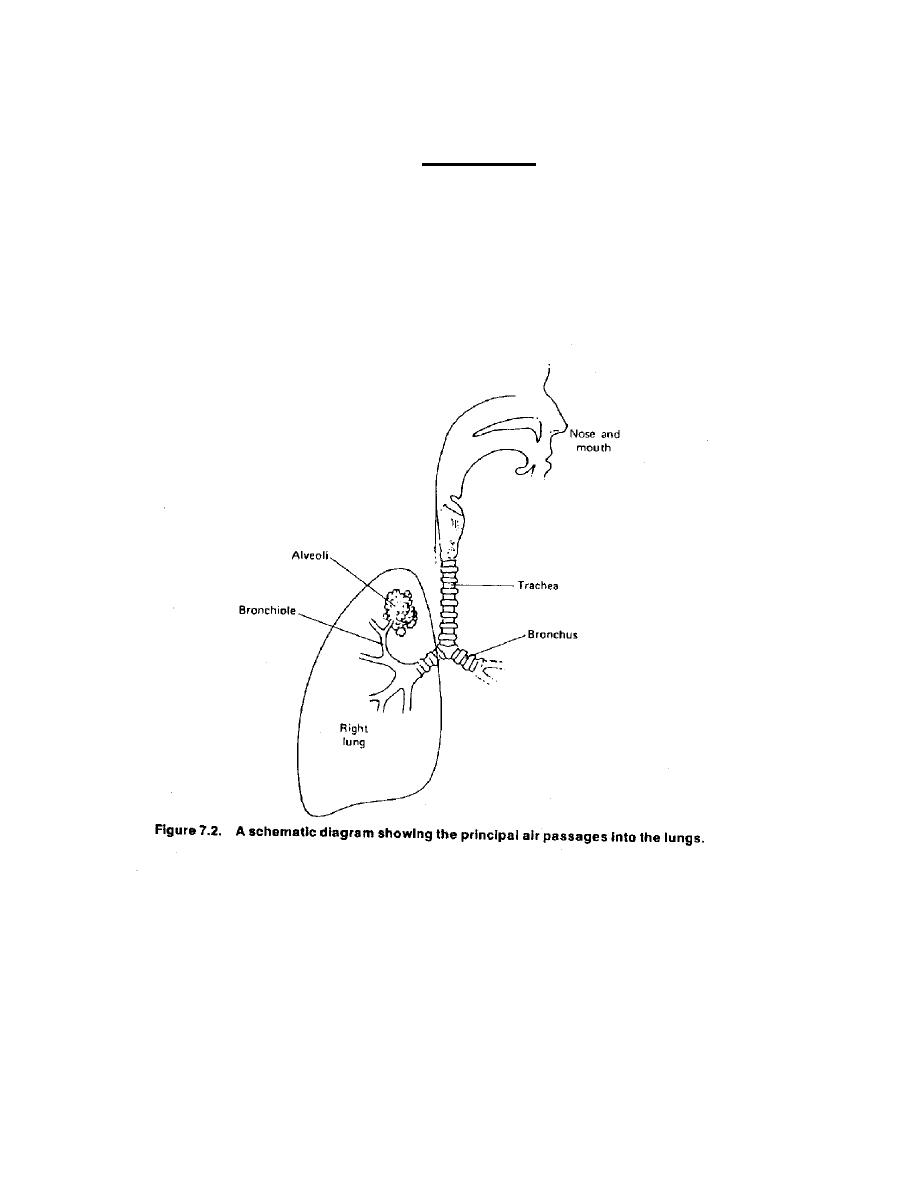
Air ways
The principal air passage into the lungs are shown in figure ( 1) . Air normally
enters the body through the nose where it is warmed , filtered and
moisturized .The air then passes through trachea .The trachea divides into two to
furnish air to each lung through the bronchi . Each bronchus divides and
re divides about 15 times , the resulting terminal bronchioles supply air to
millions of small sacs called alveoli .
figure 1
The alveoli is small interconnected bubbles are about 0.2 mm in diameter and
have walls only 0.4 μm thick (figure 2) .They expand and contract during
breathing ; they are in the exchanging of O2 and CO2 .Each alveolus is
surrounded by blood so that O2 can diffuse from the blood into the air in the
alveolus .
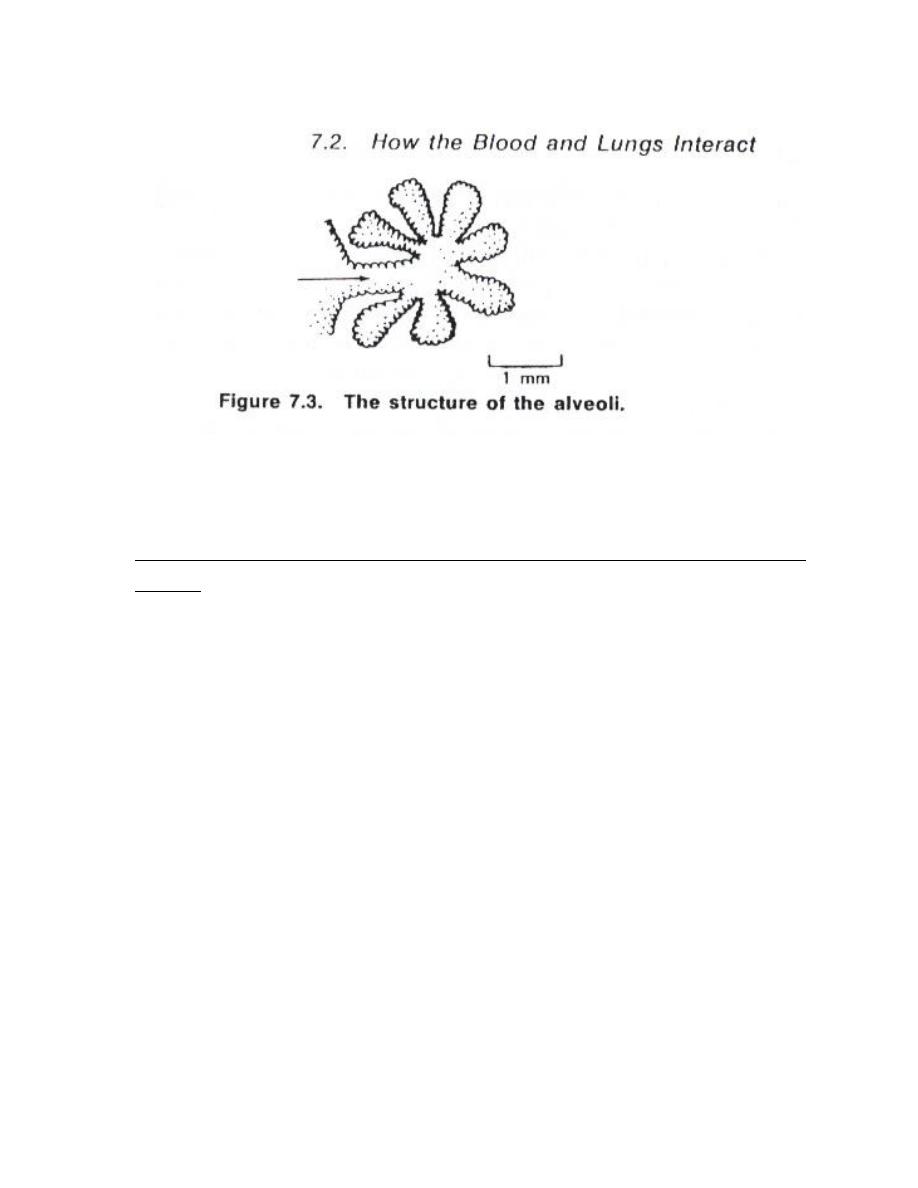
3
Figure (2 )
The Physics of Exchanging of Gas between the Lungs and the
Blood
The transfer of O2 and CO2 into and out of the blood is controlled by the
physical law of diffusion .All molecules are continually in motion. In gases and
liquids , and to certain extent even in solids , the molecules do not remain in one
direction .Molecules of a particular type diffuse from region of higher
concentration to a region of lower concentration until the concentration is
uniform In the lungs we are concerned with diffusion in both gas and liquids .In
the O2 and CO2 exchange in the tissues we are concerned only with diffusion in
liquids .The molecules in a gas at room temperature move at about the speed of
sound . Each molecule collides about 10¹º times each second with neighboring
molecules .

4
The distance D of molecule will travel from its origin after N collisions is
D= λ√ N
Where λ is the mean free path.
λ is defined as the average distance between collision .
in air λ =10 ‾ 7 m
in tissue λ = 10 ‾ ¹¹ m
Example (1)
What is the typical value of D in air and in tissue for an O2 molecule after 1 sec
if N= 10¹º in air and in tissue N = 10¹² ?
In air D = 10‾ 7 (10¹º)½ =10‾² m
- 5
In tissue D = 10‾¹¹ ( 10¹² )½ = 10m
Diffusion depends on the speed of the molecules , the speed of molecules
increases with temperature .
Since N is proportional to
the diffusion time Δ t .
N α Δt
D α √ Δt
Δt α D ²
In the lungs the distance to be traveled in air usually a small fraction of a
millimeter ,and diffusion takes place in a fraction of a second .The diffusion of
O2 and CO2 in tissue is about 10,000 times slower than it is in air , but the tissue
thickness of the molecules must diffuse through in the lungs is very small (0.4μm)
and diffusion through the alveolar wall takes place in much less than 1 sec .

5
To understand the behavior of gases in lungs it is necessary to know Dalton’ s
law of partial pressures .Dalton’s law state that if you have a mixture of several
gases , each gas make its own contribution to the total pressure as though it were
all alone .
The pressure exerted by any one of the gases is known as the partial pressure of
that gas , and the total pressure of these gases is the sum of the partial pressures
of the mixture gases .
p = p + p + p -----
total t 1 t2 t3
where p is a partial pressure
6
Henry s Law of Solubility of Gases
Henry s law states that the quantity of a gas going into simple solution at constant
temperature is proportional to the pressure .if we have a closed container of blood and
CO2 ,It found that some of O2 molecules collide with blood and are dissolved .After a
while the number of O2 molecules that are escaping from the blood each second is the
same as the number that are entering it .The blood then has a PO2 equal to that of the
O2 in contact with it .If PO2 in the gas phase is doubled , the amount of O2 dissolved
in the blood will also double . This proportionality is Henry s law of solubility of
gases .The amount of gas dissolved in blood varies greatly from one gas to
another .Oxygen is not very soluble in blood or water .The different solubility of O2
and CO2 in tissue affect the transport of these gases across the alveolar wall. A
molecule of O2 diffuse faster than a molecule of CO2 because of its smaller mass .
However , because of greater number of CO2 molecules in solution , the transport of
CO2 is more efficient than the transport of O2. The mixture of gases in the alveoli is
not the same as the mixture of gases in ordinary air .The lungs are not emptied during
expiration .During normal breathing the lungs retain about 30 % of their volume at the
end of each expiration . This is called the functional residual volume (FRC).At each
breath 500 cm³ of fresh air ( PO2 of 150 mm Hg ) mixes with 2000 cm³ of stale air in
the lungs to result in alveolar air with a PO2 100 mm Hg .The PCO2 in the alveoli is
about 40 mm Hg .Expired air includes about 150 cm of relatively fresh air from the
trachea that are not in contact with alveolar surface , so expired air has a slightly
higher PO2 and lower PCO2 than alveolar air .
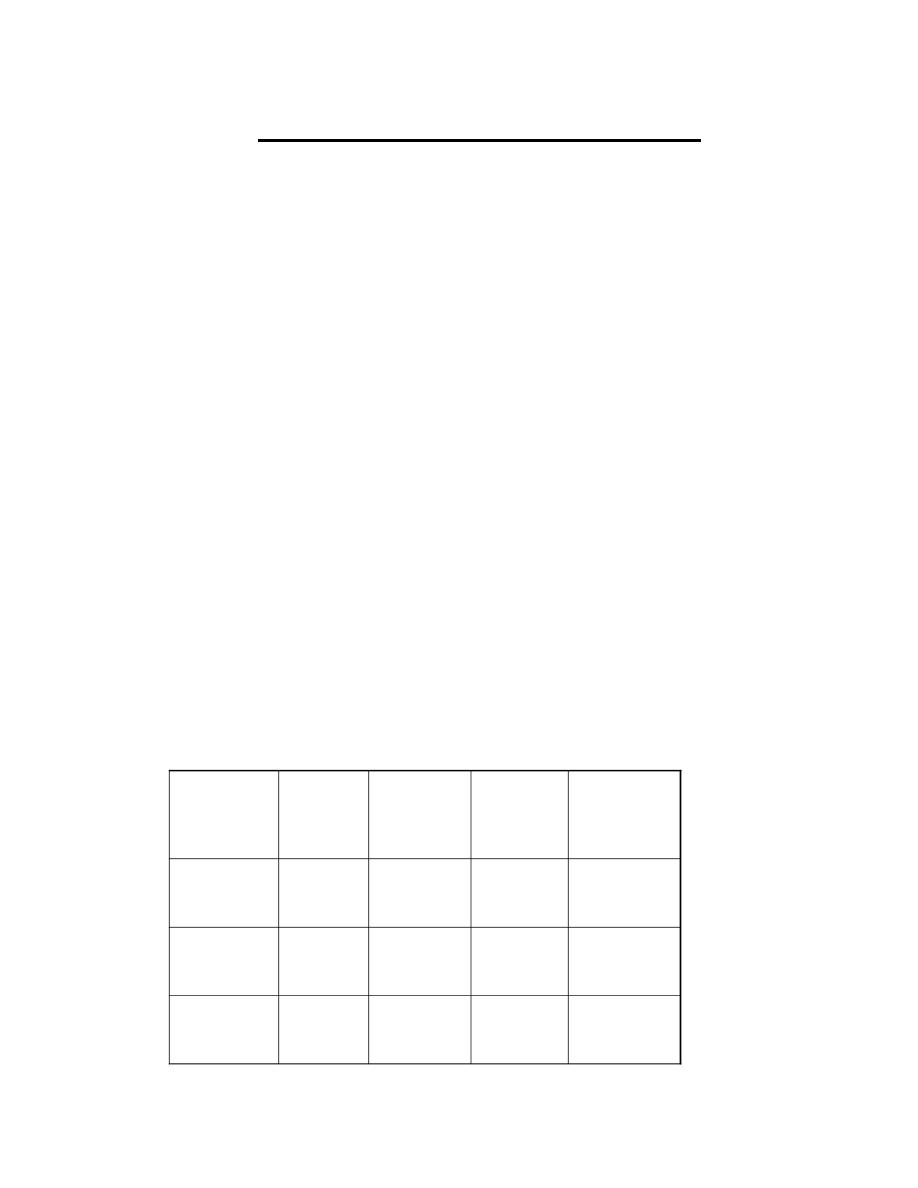
The percentages and partial pressures of O
2
and CO
2
in
inspired ,alveolar and Expired .
32
4.5
116
16.3
Expired
air
40
5.6
100
14.0
Alveolar
air
0.3
0.04
150
20.9
Inspired
air
PCO
2
(mmHg )
% CO
2
PO
2
(mmHg)
% O
2

7
