
1
ANTIPSYCHOTIC AGENTS
Schizophrenia (a severe mental disorder characterized by a disintegration of
the thinking process, or contact with reality and with emotional
responsiveness).
These agents produce a calming effect in agitated individuals and they
induce a lessening of reactivity to emotional stimuli without affecting
consciousness (which implies a different mechanism than other CNS
depressants).
Many of the conditions which the antipsychotic drugs are used to treat are
believed to be due to imbalances in the normal pattern of synthesis,
distribution, and metabolism of amino neurotransmitters such as Dopamine,
Norepinephrine, and Seratonin in the brain.
These imbalances lead to mood and behavioral alterations. For example in
Schizophrenia there is an excessive amount of dopamine activity in the
limbic system (responsible for the expression of instinct and mood).
Much evidence suggests that the antipsychotic agents function by blocking
Dopamine at the post-synaptic D-2 receptors. This is how the phenothiazines
and butyrophenones work. Many of these receptors are located in the limbic
and mesolimbic system (which has to do with emotion and motivation).
PHENOTHIAZINES
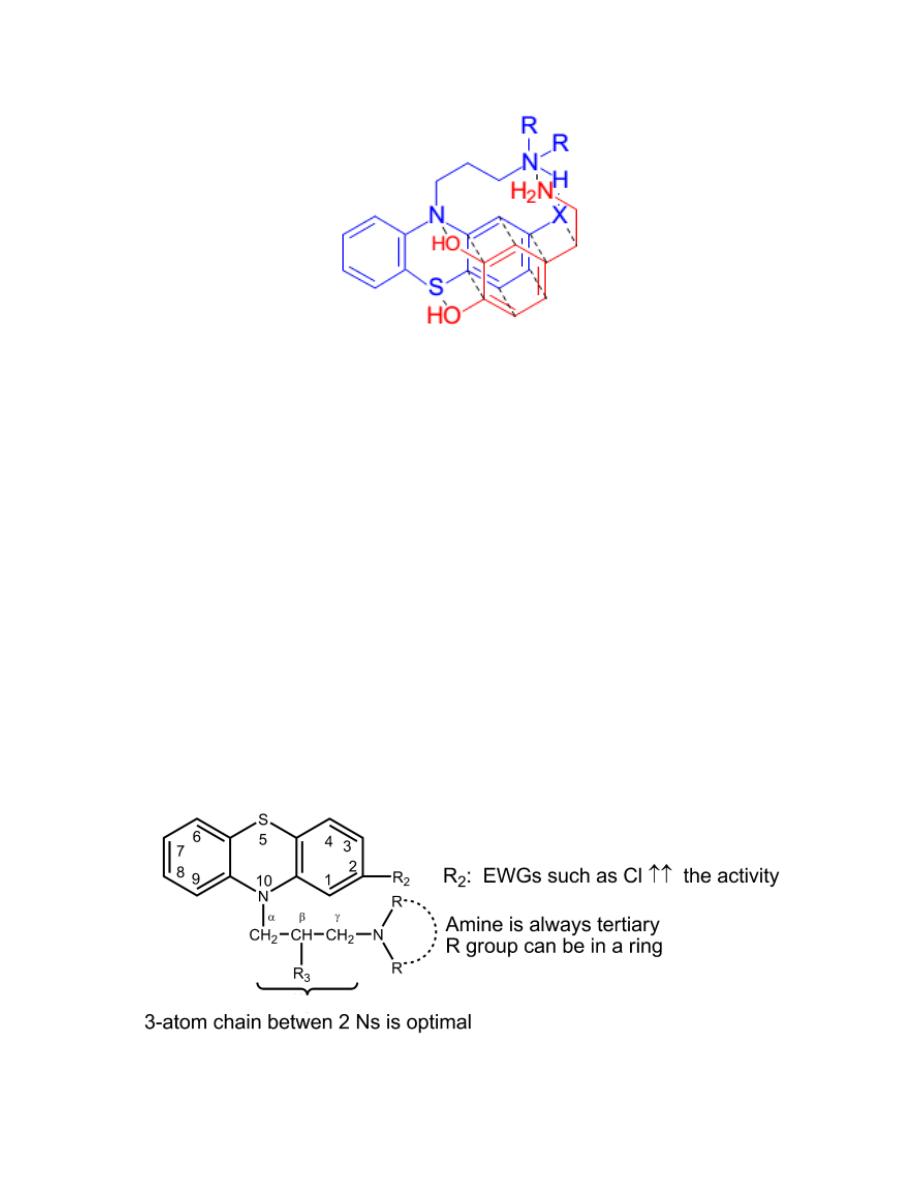
Phenothiazines are thought to bear a structural resemblance to Dopamine
when the side-chain amine is protonated the proton can form bond with the
substituent at the 2-position. In this conformation the aromatic ring and
sulfur of phenothiazine correlates with the structure of dopamine (S with p-
OH of dopamine
2
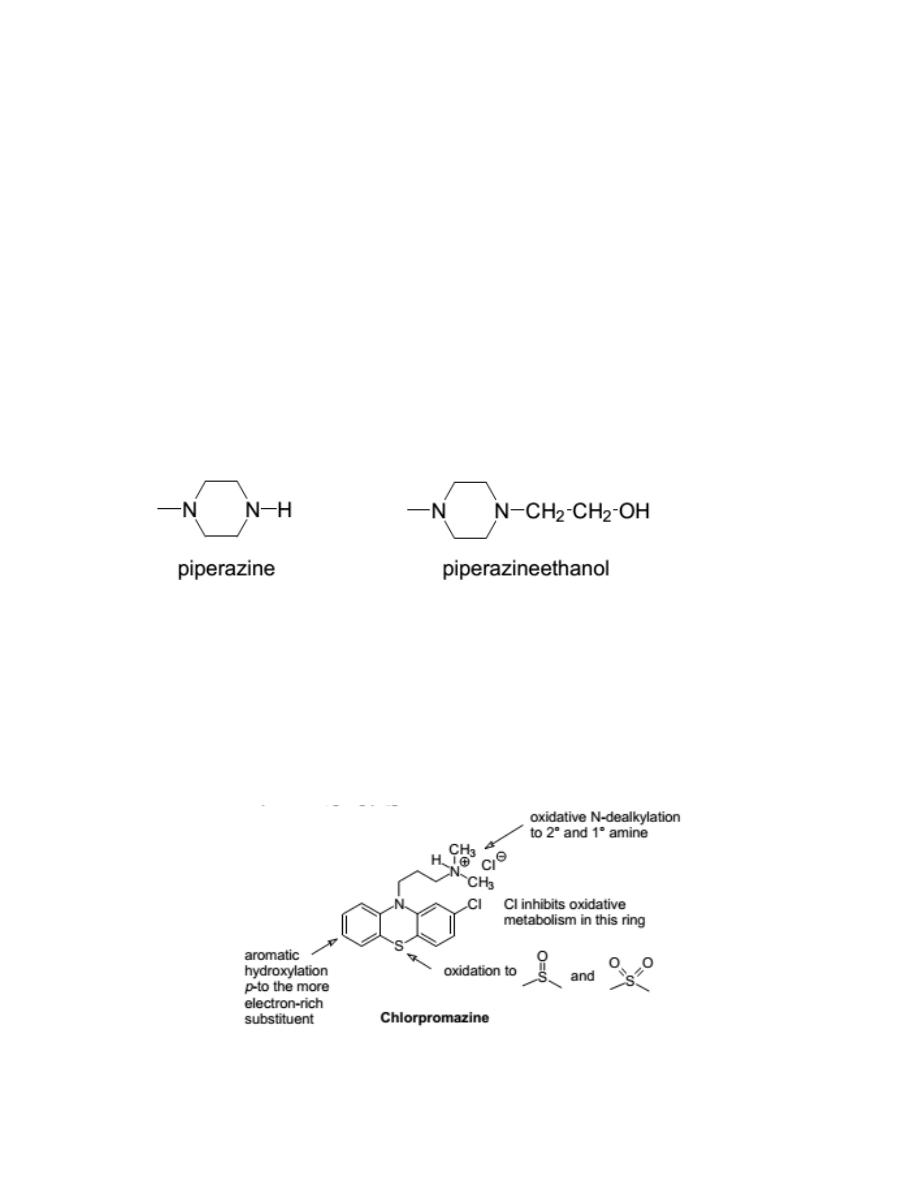
Structure-Activity Relationships
Phenothiazines have a tricyclic structure (6-6-6 system) in which two
benzene rings are linked by sulfur and nitrogen atoms.
The best position for substitution is the 2-position.
Substitution at the 1-position sterically hinders the ability of the side chain
to approach ring A. On the other hand, a substituent at the 3-position will be
too far from the side chain to provide attractions. A substituent at position 4
might interfere with receptor binding by the sulfur atom.
3
The maximum neuroleptic activity is observed with a 3-carbon chain
separating the two nitrogens.
The three-atom chain length may be necessary
to bring the protonated amino nitrogen in proximity with the 2-substituent.
The side-chain amine should be substituted with dimethyl or other small
alkyl groups.
A piperazine or piperazineethanol group as the side-chain amine gives
enhanced activity.
The relative order is: piperazineethanol > piperazine > dimethyl
Chlorpromazine HCl
It was the first phenothiazine compound introduced into therapy. It is still
useful as an antipsychotic. Other uses are in nausea, vomiting, and hiccough.
4
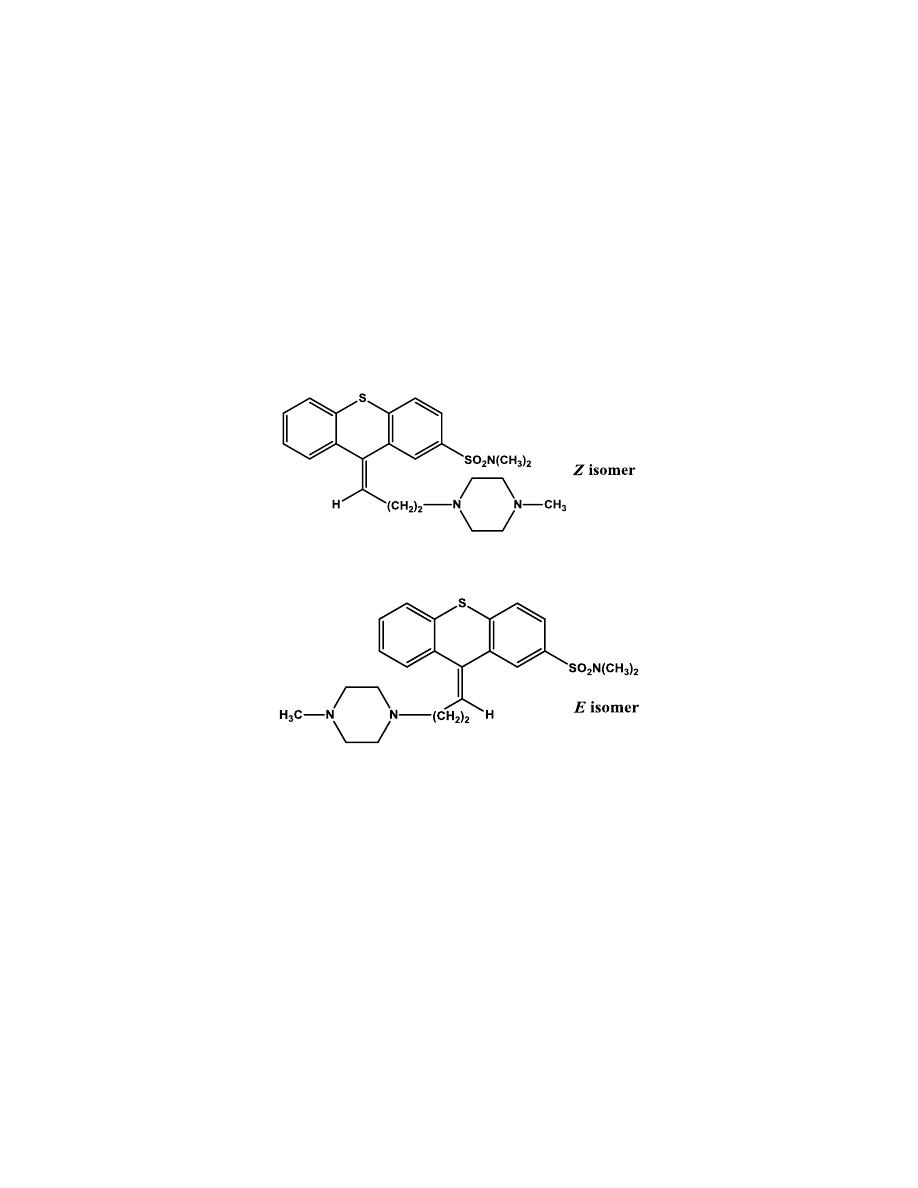
Thiothixene
The thioxanthene system differs from the phenothiazine system by
replacement of the N-H moiety with a carbon atom doubly bonded to the
propylidene side chain. With the substituent in the 2-position, Z and E-
isomers are produced. The useful antipsychotics can be superimposed on
DA, thus Z-isomers are the more active antipsychotic isomers.
Ring Analogs of Phenothiazines
These ring analogs of phenothiazines are structural relatives of the
phenothiazine antipsychotics; therefore, most of them share many clinical
properties with the phenothiazines. However, they have some important
differences, notably low production of EPS and reduction of negative
symptoms.
These benzazepines and other atypical antipsychotics including risperidone,
ziprasidone, and aripiprazole block both D
2
and 5-HT
2A
receptor, other DA
5
and serotonin receptor subtypes, adrenergic, histamine, and muscarinic
receptors.
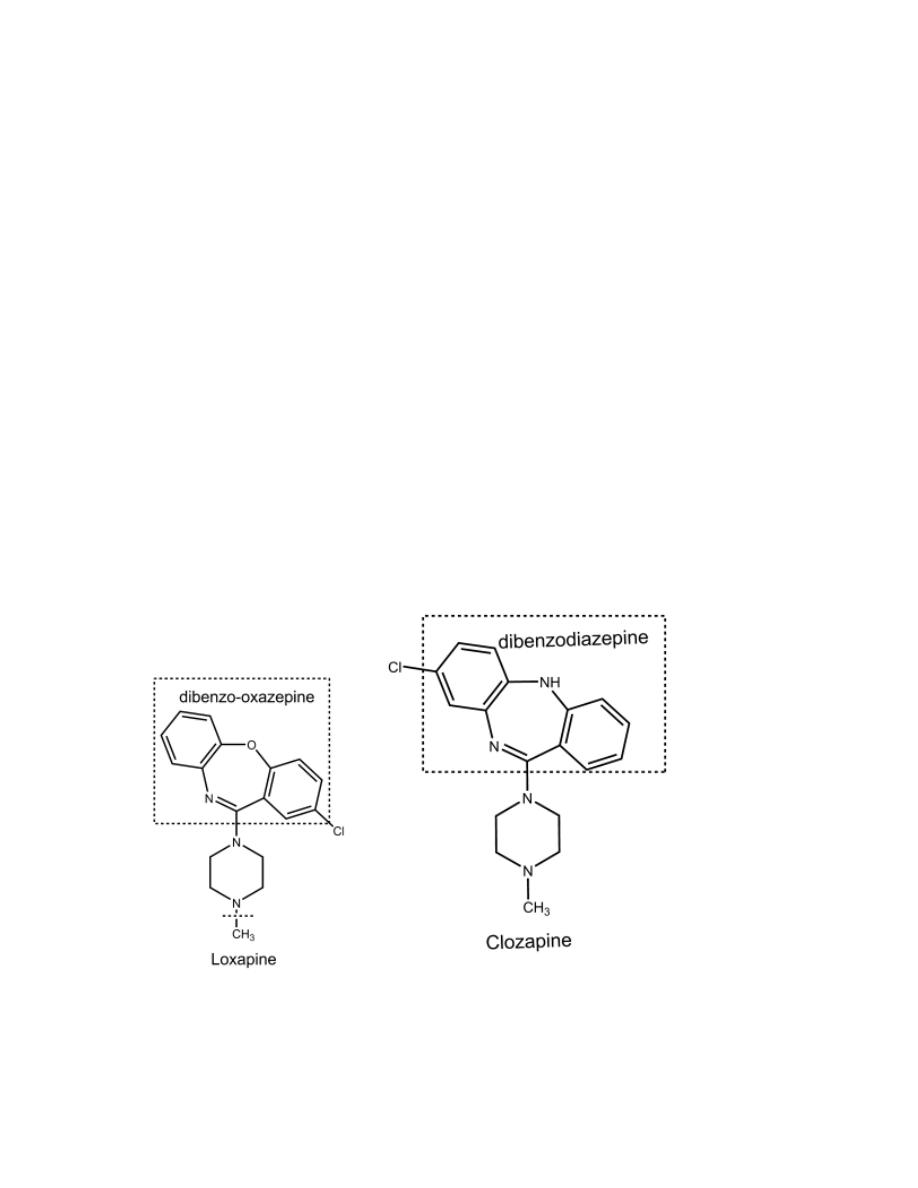
Loxapine
It is a dibenzoxazepine derivative.
Clozapine
It is a dibenzodiazepine derivative.
Olanzapine
It is a thienobenzodiazepines derivative.
Quetapine
It is a dibenzothiazepines derivative.
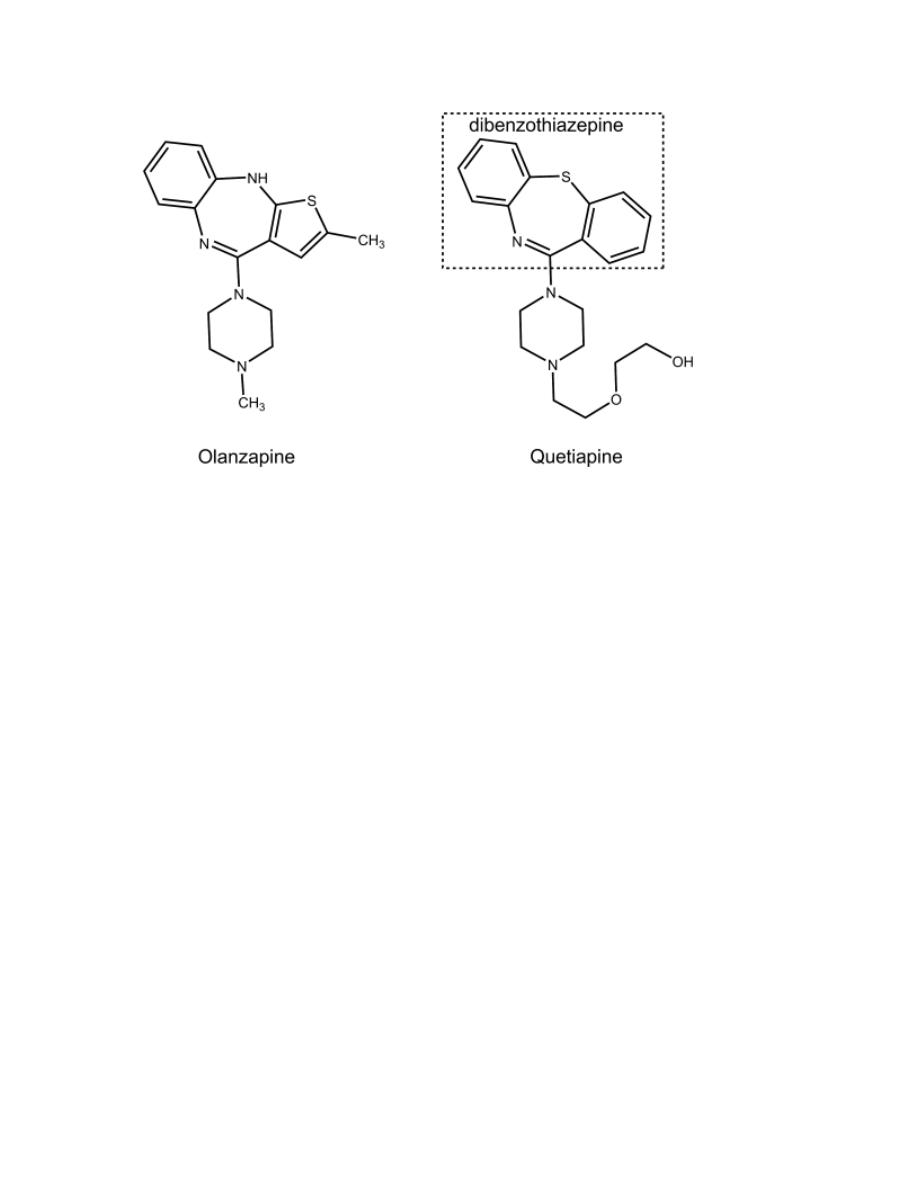
6
Fluorobutyrophenones
The fluorobutyrophenones belong to a much-studied group of compounds,
many of which possess high antipsychotic activity.
Attachment of a tertiary amino group to the fourth carbon of the
butyrophenone skeleton is essential for neuroleptic activity; lengthening,
shortening, or branching of the three carbon propyl chain decreases
neuroleptic potency.
This aliphatic amino nitrogen is required, and highest activity is seen when it
is incorporated into a cyclic form.
The C=O group gives optimal activity, although other groups, C(H)OH and
C(H) aryl, also give good activity.
The Y group can vary and assist activity, and an example is the hydroxyl
group of haloperidol.
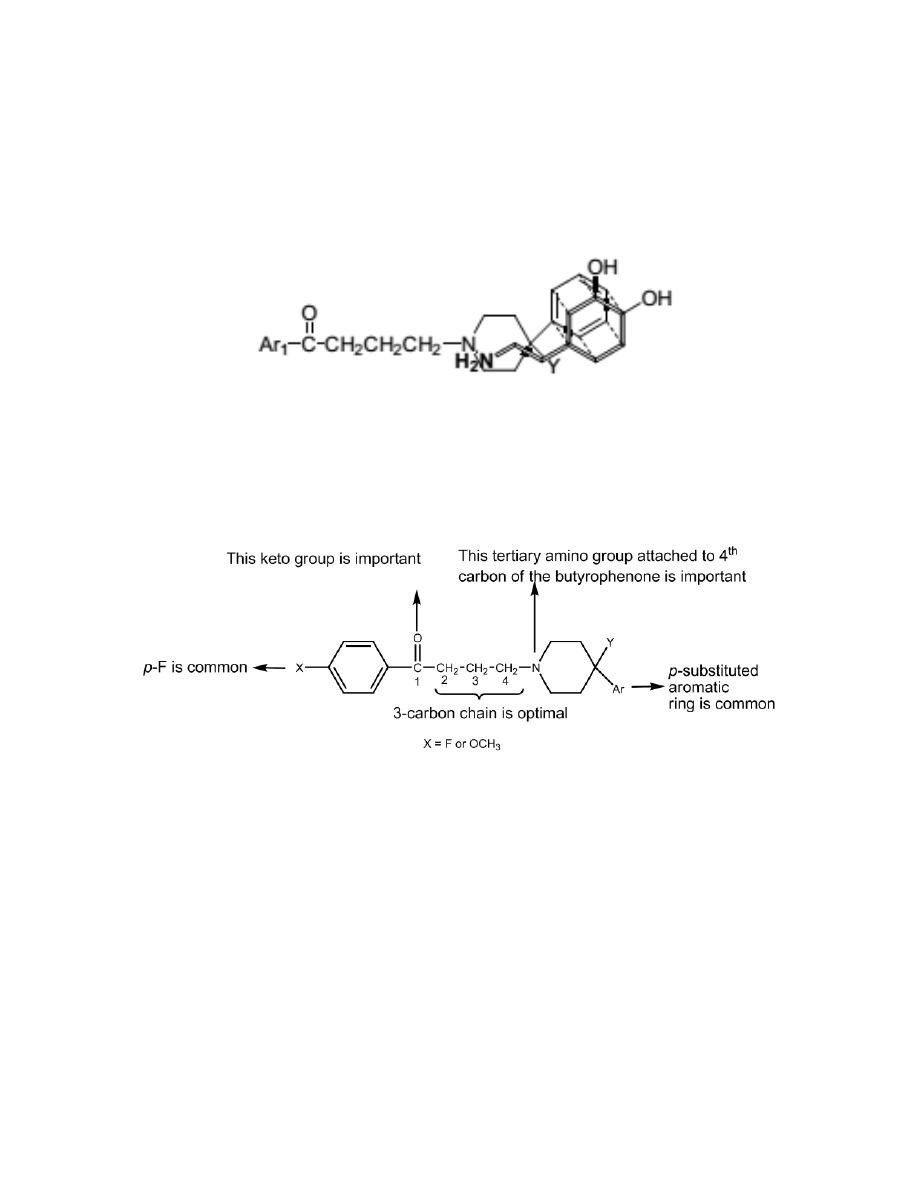
7
The SARs suggest that the 4-aryl piperidino moiety is superimposable on the
2-phenylethylamino moiety of DA and, accordingly, could promote affinity
for D
2
and D
3
receptors.
Haloperidol
It is typical potent antipsychotic useful in schizophrenia and in psychoses
associated with brain damage. It is frequently chosen as the agent to
terminate mania.
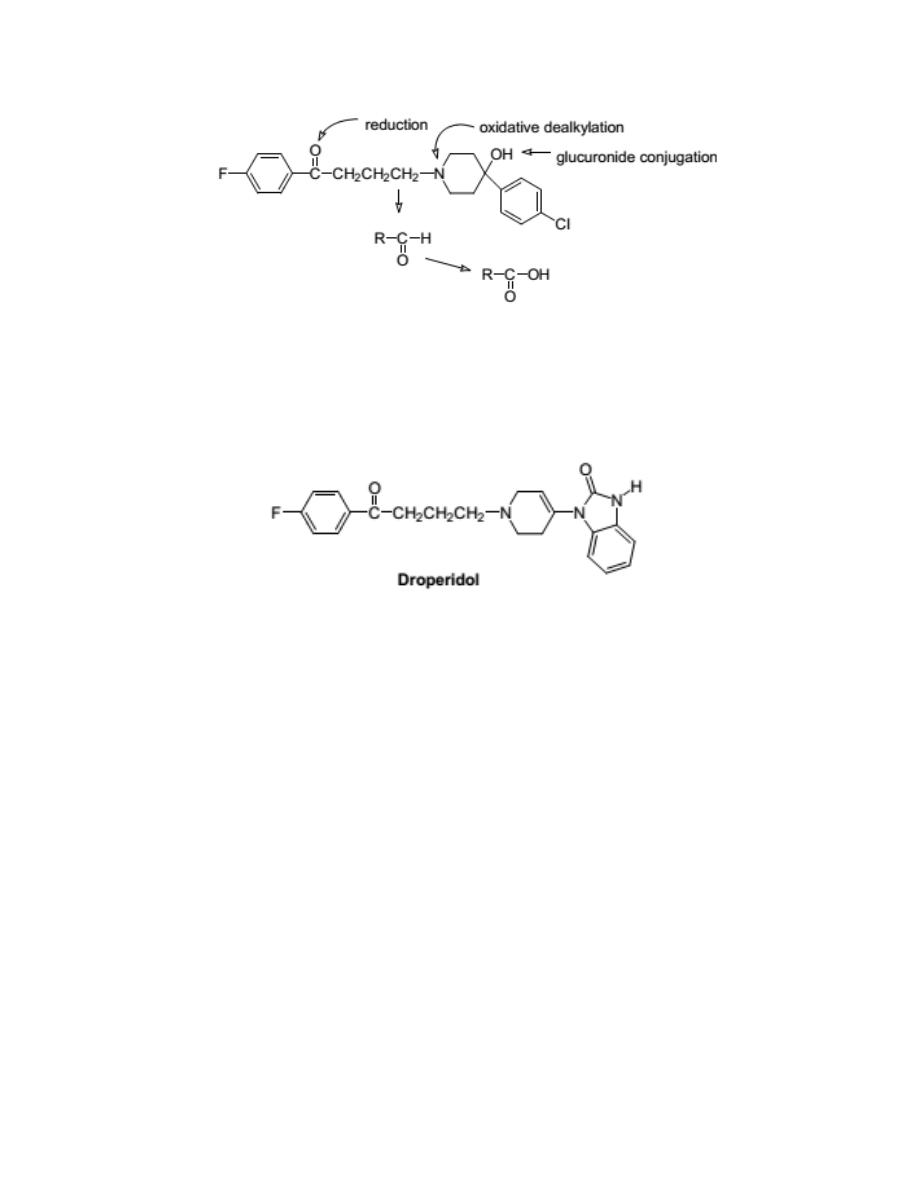
8
Droperidol
Risperidone
It has the structural features of a hybrid molecule between a butyrophenone
antipsychotic and a trazodone-like antidepressant. It decreases the negative
as well as the positive symptoms of schizophrenia due to 5-HT
2
–D
2
receptor
antagonistic properties.
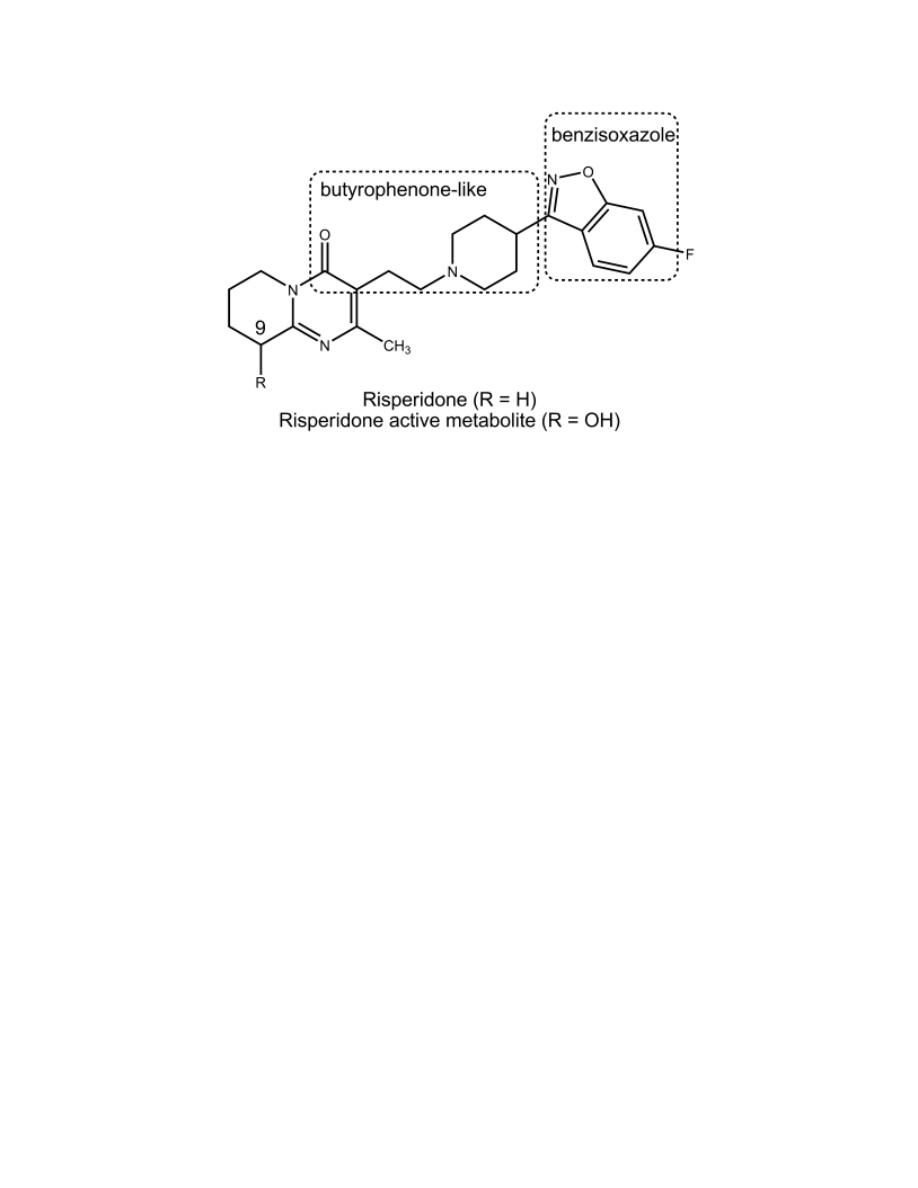
9
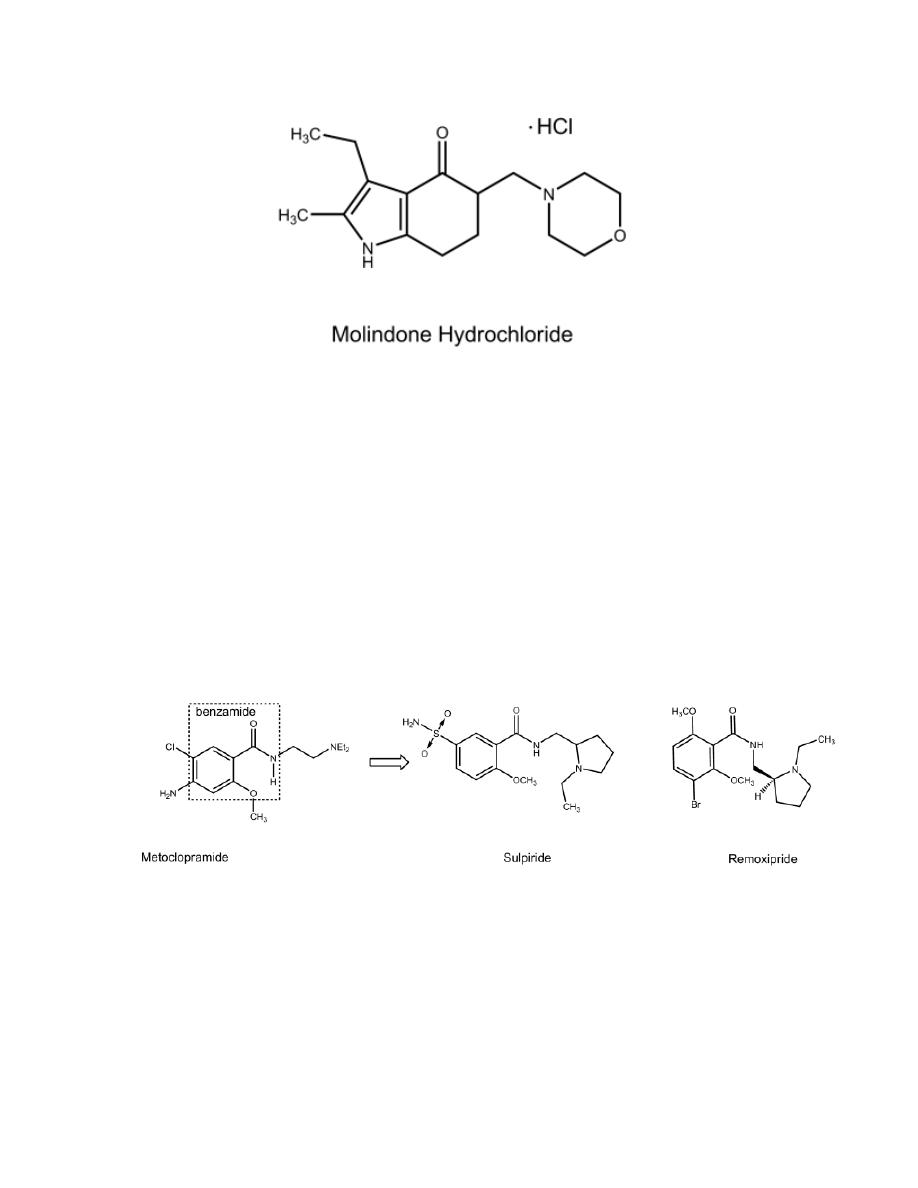
β-AMINOKETONES
Several β-aminoketones have been examined as antipsychotics. The overall
structural features associated with activity can be seen in the structure of
molindone. In addition to the β -aminoketone group, there must be an aryl
group positioned as in molindone. It might be that the proton on the
protonated amino group in these compounds can form bonds with the
electrons of the carbonyl oxygen atom and an aryl group that could be
superimposed on the analogous features of protonated DA.
Molindone Hydrochloride
It is about as potent an antipsychotic as trifluoperazine. Side effects
resemble those of the phenothiazines.
10
BENZAMIDES
The benzamides evolved from observations that the antiemetic agent,
metoclopramide, has antipsychotic activity related to D
2
receptor block. It
was hoped that the group might yield compounds with diminished EPS
liability. This expectation has been met. An H-bond between the amido H
and the unshared electrons of the methoxyl group to generate a pseudo ring
is considered important for antipsychotic activity in these compounds.
Remoxipride
It is a D
2
receptor blocker. It is as effective as haloperidol with fewer EPS.

11
Antimanic Agents
LITHIUM SALTS
The lithium salts used in the United States are the carbonate and the citrate.
Lithium chloride is not used because of its hygroscopic nature and because it
is more irritating than the carbonate or citrate to the GI tract.
The active species is the lithium ion. The classic explanation for its
antimanic activity is that it resembles the sodium ion (as well as potassium,
magnesium, and calcium ions) and can occupy the sodium pump. Unlike the
sodium ion, it cannot maintain membrane potentials. Accordingly, it might
prevent excessive release of NTs (e.g., DA) that characterize the manic state.
