1
Benzodiazepines and Related Compounds
GABA is the most common and major inhibitory neurotransmitter in the
brain and it exerts its rapid inhibitory action mostly through GABA
receptors.
GABA
A
receptor is the target for many anxiolytics and sedative–hypnotic
agents including benzodiazepines, barbiturates, zolpidem, zaleplon,
eszopiclone, steroids, anticonvulsive agents, and many other drugs that bind
to different binding sites of the GABA
A
receptors in neuronal membranes in
the CNS.
GABA
A
receptor is a ligand-gated chloride ion channel. Upon activation, Cl
-
influx is increased and the membrane becomes hyperpolarized, resulting in
neuronal inhibition.
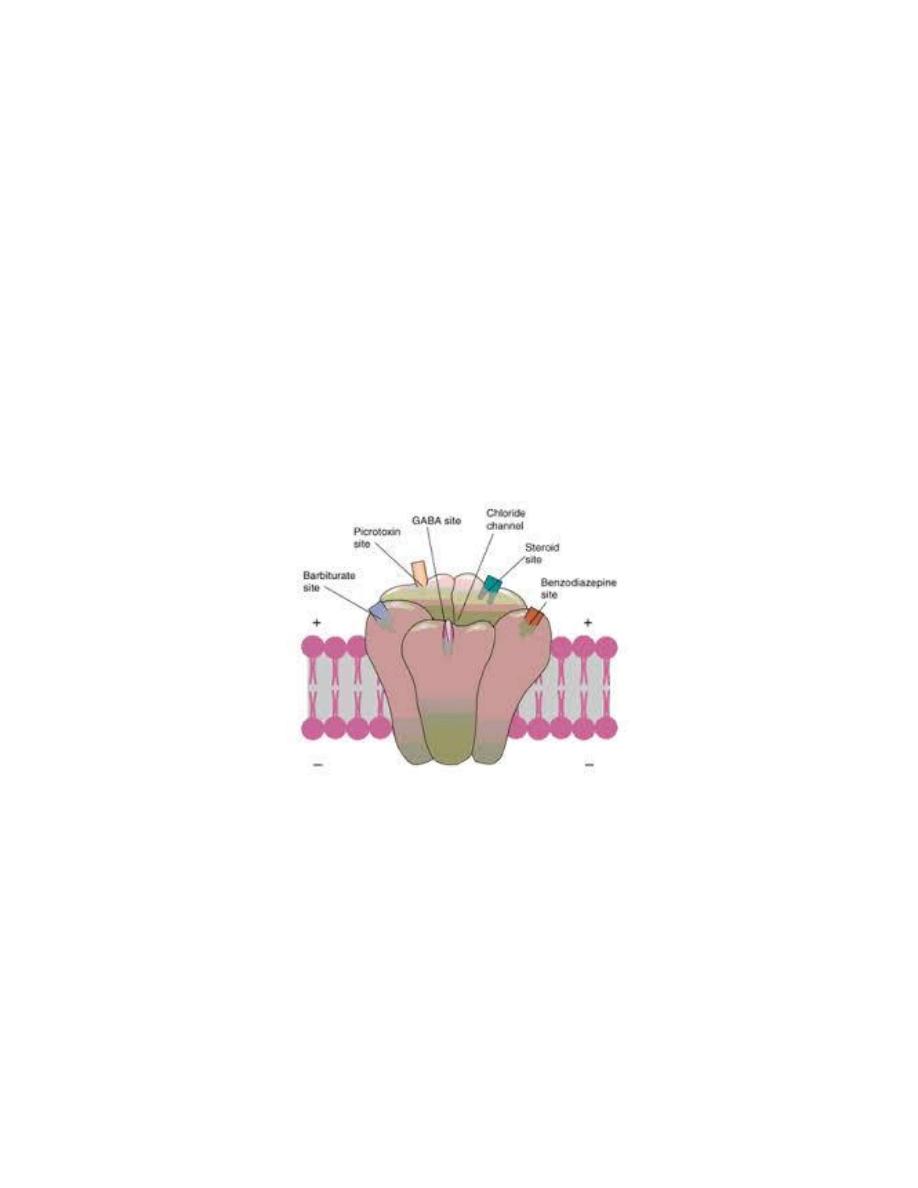
When benzodiazepines bind to a benzodiazepine recognition site (one of the
allosteric sites) they induce conformational changes in the GABA-binding
site, resulting in increasing the affinity of the receptor for GABA. As a
result, the frequency of Cl
-
channel openings is increased and the cell is
further hyperpolarized, yielding a more pronounced decrease in cellular
excitability.
Benzodiazepines and related compounds can act as agonists, antagonists, or
inverse agonists at the benzodiazepine-binding site on GABA
A
receptor.
2
The compounds that occupy benzodiazepine modulatory sites without
affecting chloride flux are called as antagonists, zero modulators. One such
compound, flumazenil, is used clinically to counteract the sedative effect of
benzodiazepines and benzodiazepine overdose.
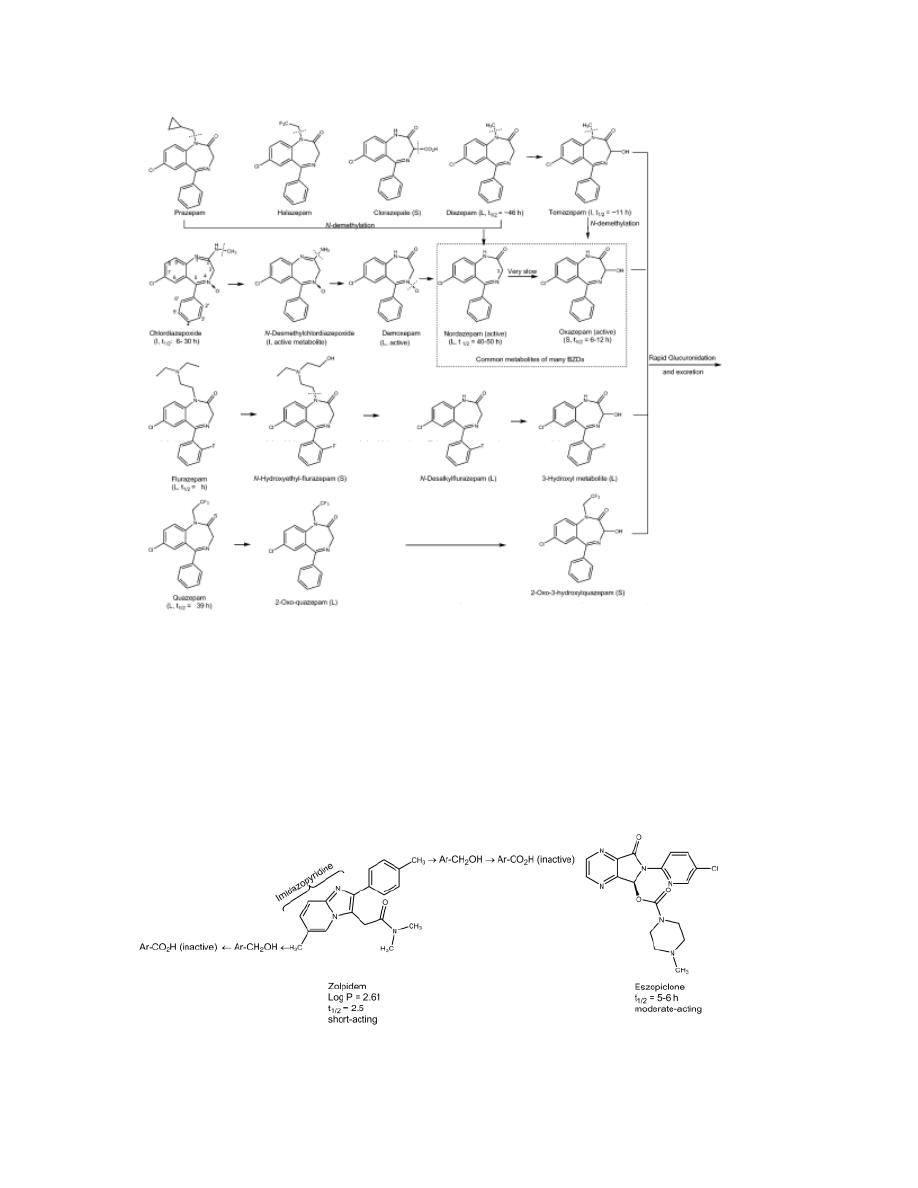
SAR
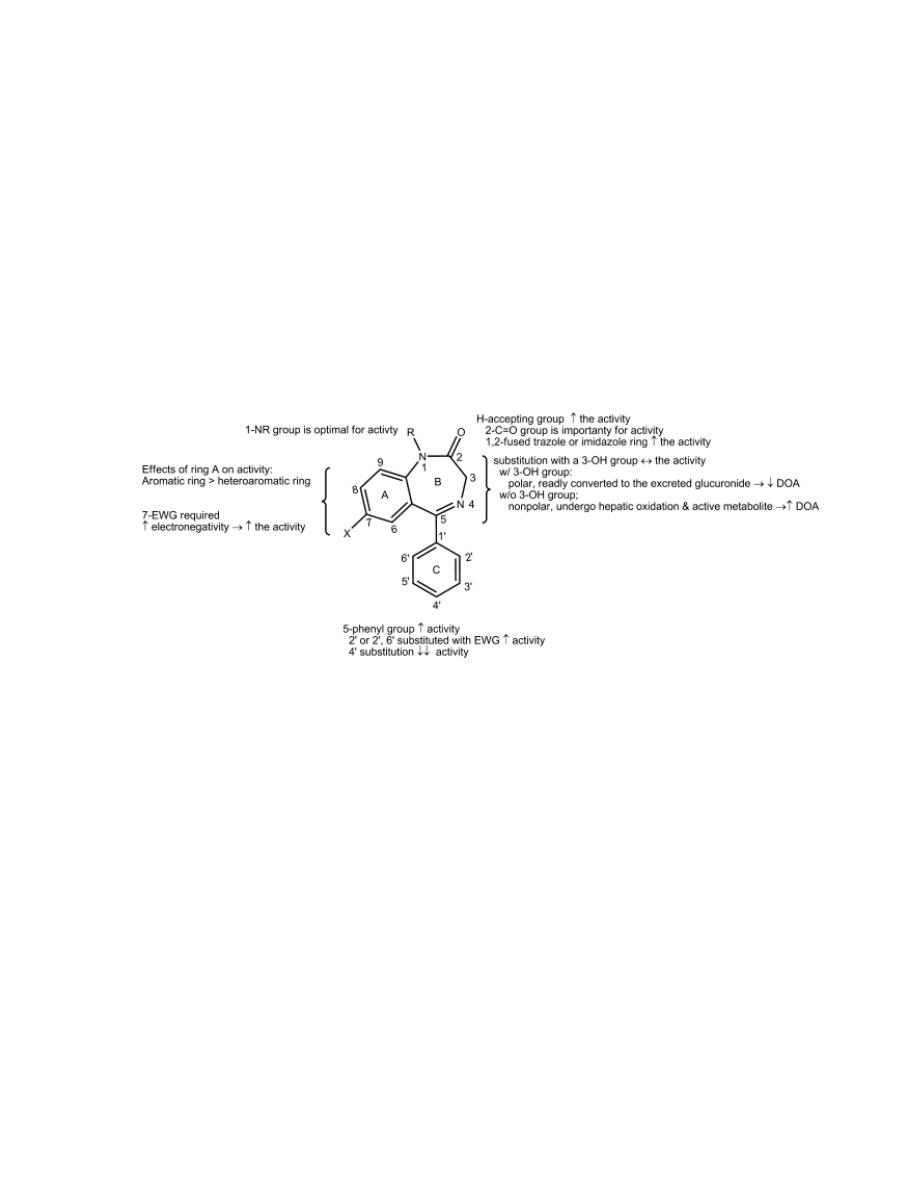
1- Most benzodiazepines are 5-aryl-1,4-benzodiazepines.
2- Aromatic or heteroaromatic ring A is required for the activity that
may participate in π- π interaction with aromatic amino acid residues
of the receptor.
3- An electronegative substituent at position 7 is required for activity,
and the more electronegative it is, the higher the activity.
4- Positions 6, 8, and 9 should not be substituted.

3
5- A phenyl ring C at position 5 promotes activity. If this phenyl group
is ortho(2´) or diortho (2´,6´) substituted with electron-withdrawing
groups, activity is increased. On the other hand, parasubstitution
decreases activity greatly.
6- In diazepine ring B, saturation of the 4,5-double bond or a shift of it
to the 3,4-position decreases activity.
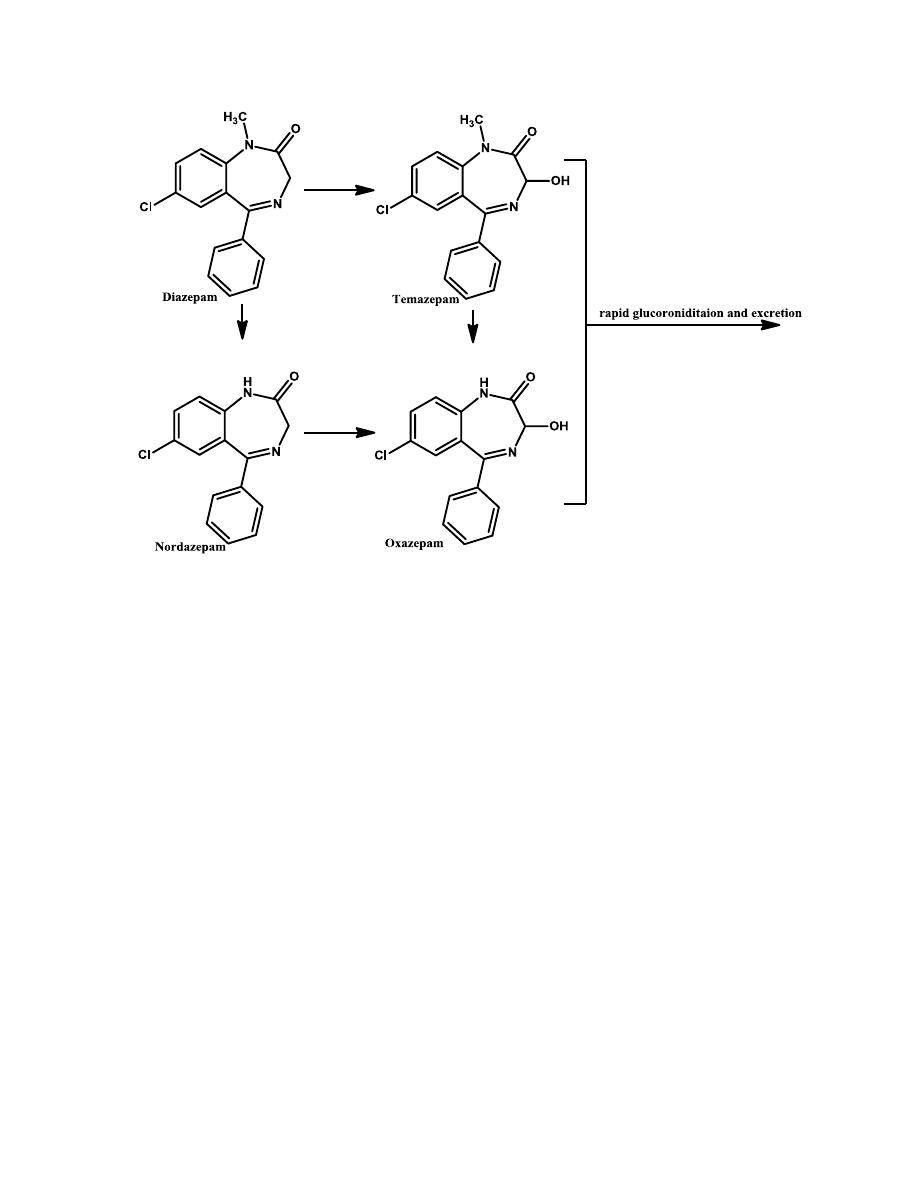
7- The presence or absence of the 3-hydroxyl group is important
pharmacokinetically.
Compounds without the 3-hydroxyl group are nonpolar, 3-hydroxylated in
liver slowly to active 3-hydroxyl metabolites, and have long overall half-
lives. In contrast, 3-hydroxyl compounds are much more polar, rapidly
converted to inactive 3-glucuronides, which are excreted in urine and thus
are short-lived.
8- The 2-carbonyl function is important for activity. A proton-accepting
group at C2 is required and may interact with the proton donor in
benzodiazepine binding site of GABA
A
receptor. Other triazole or
imidazole rings (which are able to form H-bond) can be fused on
positions 1 and 2 and increase the activity.
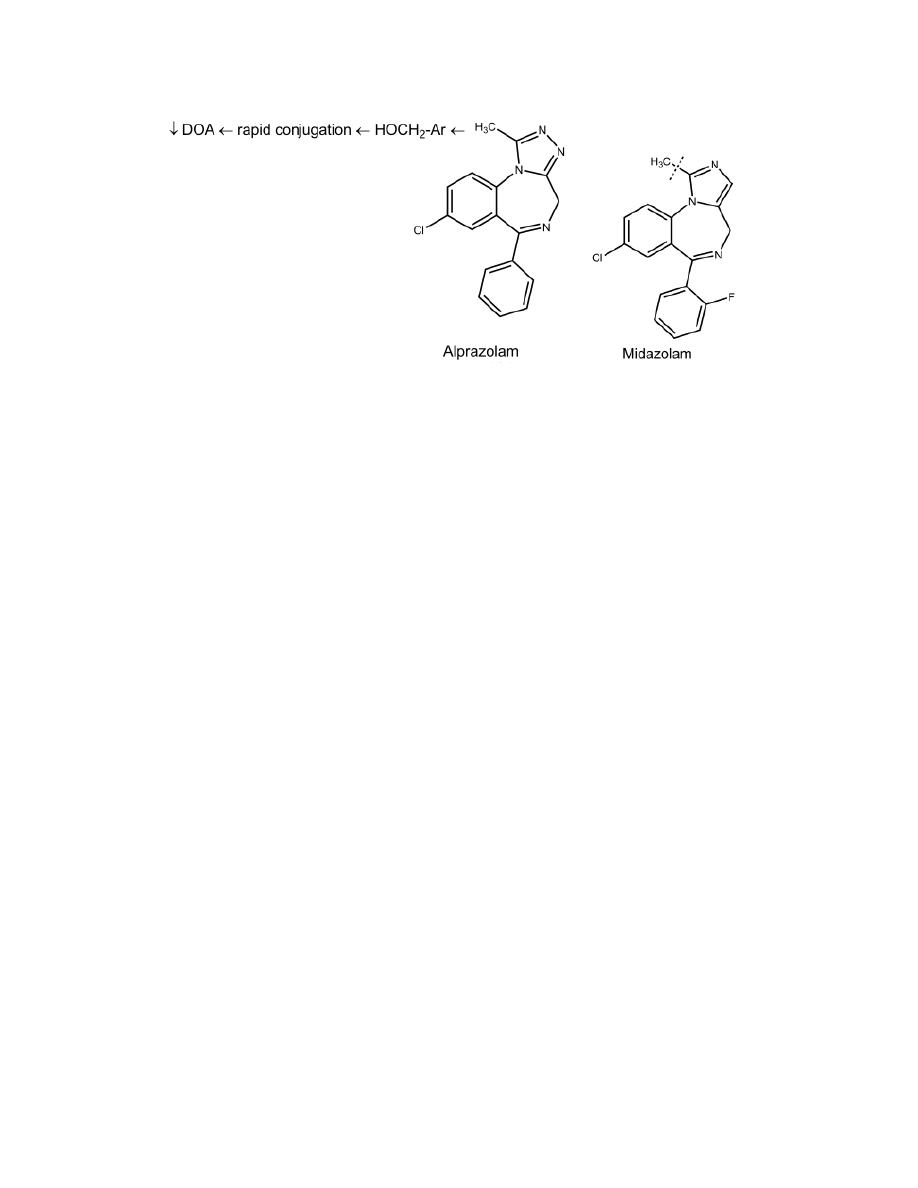
4
Compounds with a fused triazolo ring, (alprazolam) and compounds with a
fused imidazolo ring (midazolam) are short acting because they are
metabolized rapidly by α-hydroxylation of the methyl substituent on the
triazolo or imidazolo ring (analogs to benzylic oxidation). The resulting
active α -hydroxylated metabolite is quickly inactivated by glucuronidation.
Metabolites of some benzodiazepines are not only active but also have long
half-lives, thus these drugs are long acting.
5
6
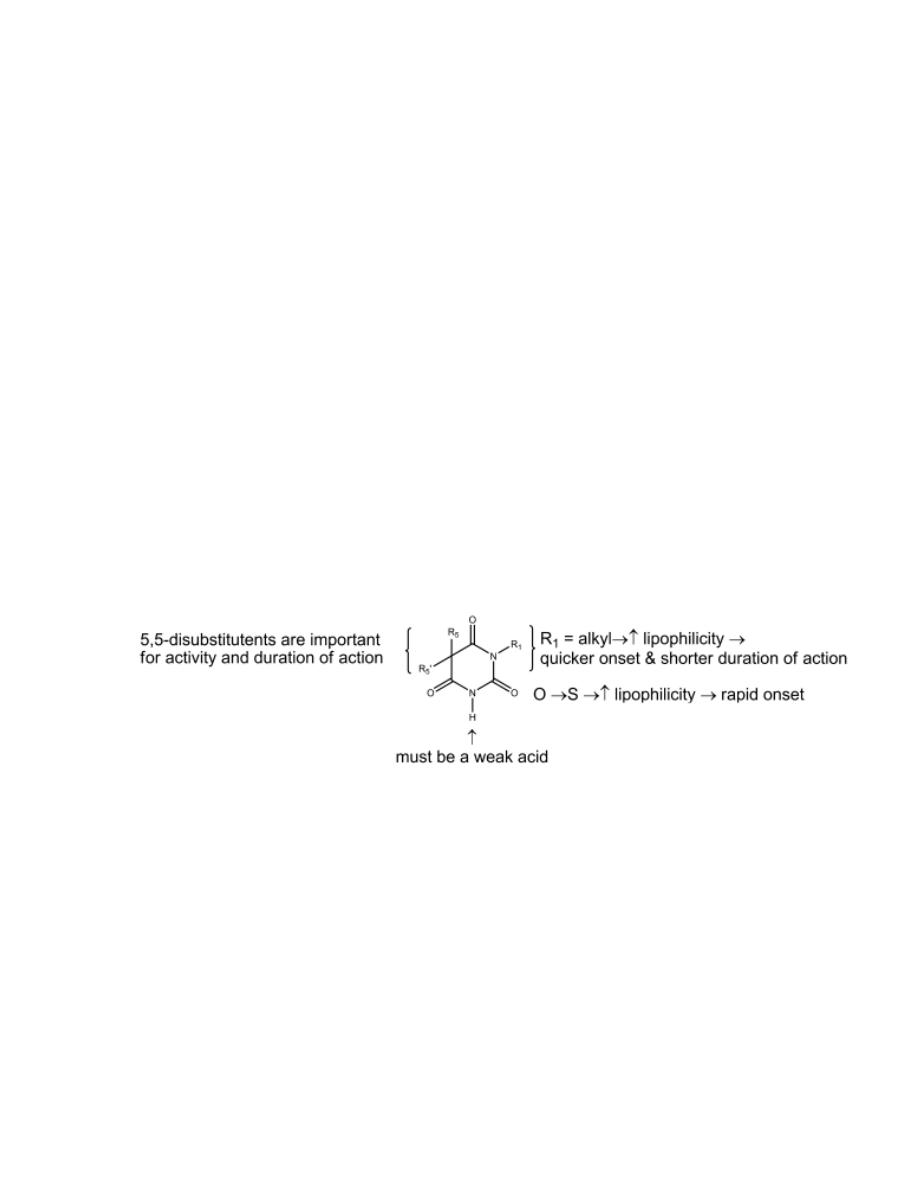
Nonbenzodiazepines BzRAs
Zolpidem and eszopiclone
They are nonbenzodiazepines and have been introduced as short- and
moderate-acting hypnotics, respectively.
7
Barbiturates
The barbiturates were used extensively as sedative–hypnotic drugs. They
have been replaced largely by the much safer benzodiazepine except for a
few specialized uses. Barbiturates exert most of their CNS effects mainly by
binding to an allosteric recognition site on GABA
A
receptors that positively
modulates the effect of the GABA
A
receptor—GABA binding. Unlike
benzodiazepines, they bind at different binding sites and appear to increase
the duration of the GABA-gated chloride channel openings. The
barbiturates
can also increase chloride ion flux without GABA attaching to its receptor
site on GABA
A
.
Sodium salts of the barbiturates are readily prepared and are water soluble.
Their aqueous solutions generate an alkaline pH. A classic incompatibility is
the addition of an agent with an acidic pH in solution, which results in
formation and precipitation of the free water-insoluble disubstituted
barbituric acid.
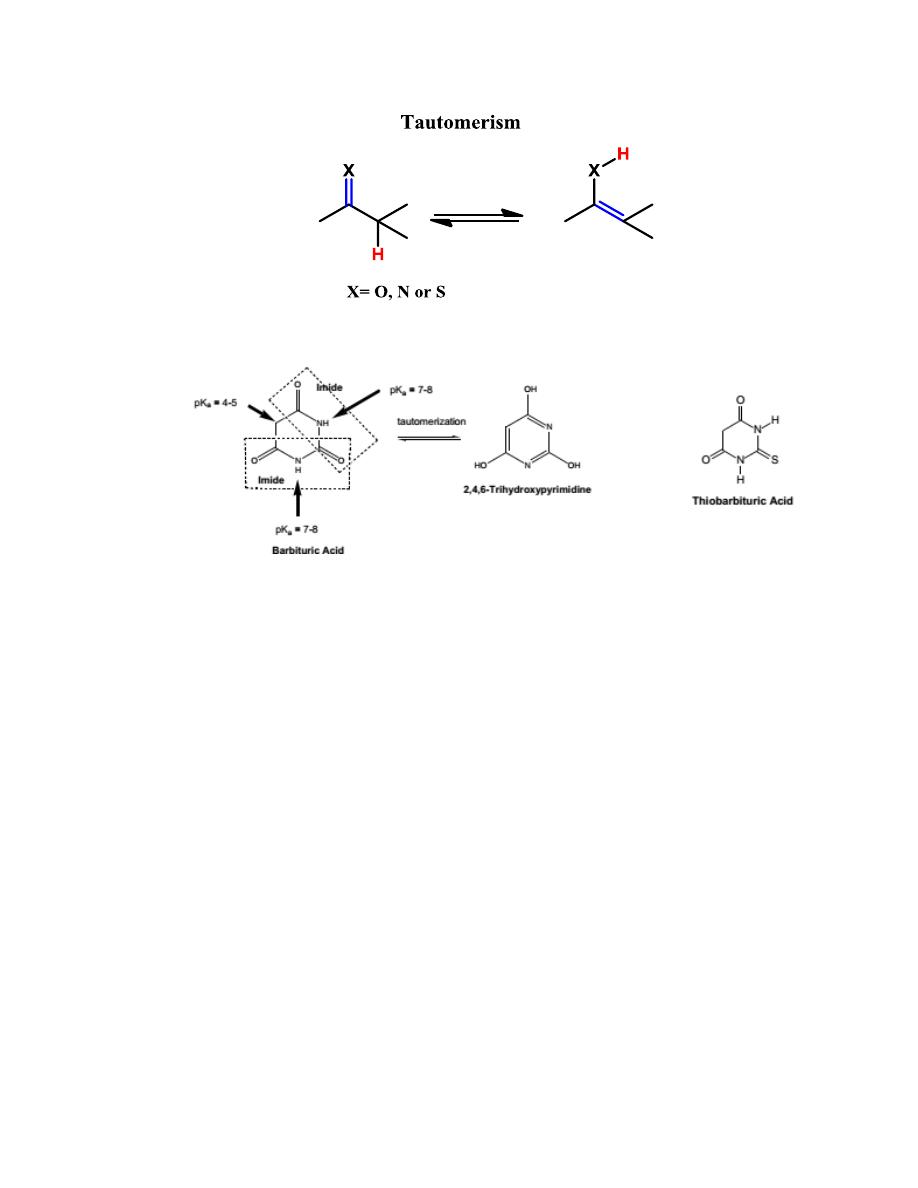
SAR
1- Both hydrogen atoms at the 5-position of barbituric acid must be
replaced. This may be because if one hydrogen is available at position
5, tautomerization to a highly acidic trihydroxypyrimidine (pK
a
4)
can occur. Consequently, the compound is largely in the anionic form
at physiological pH, with little nonionic lipid-soluble compound
available to cross the blood-brain barrier.
8
2- Increasing lipophilicity increases hypnotic potency and the onset of
action and decreases the duration of action. Why?
Barbiturates with Long Duration of Action
(More Than 6 Hours)
Phenobarbital (Luminal)
It is a long-acting sedative and hypnotic. It is also a valuable anticonvulsant.
Barbiturates with Intermediate Duration of Action
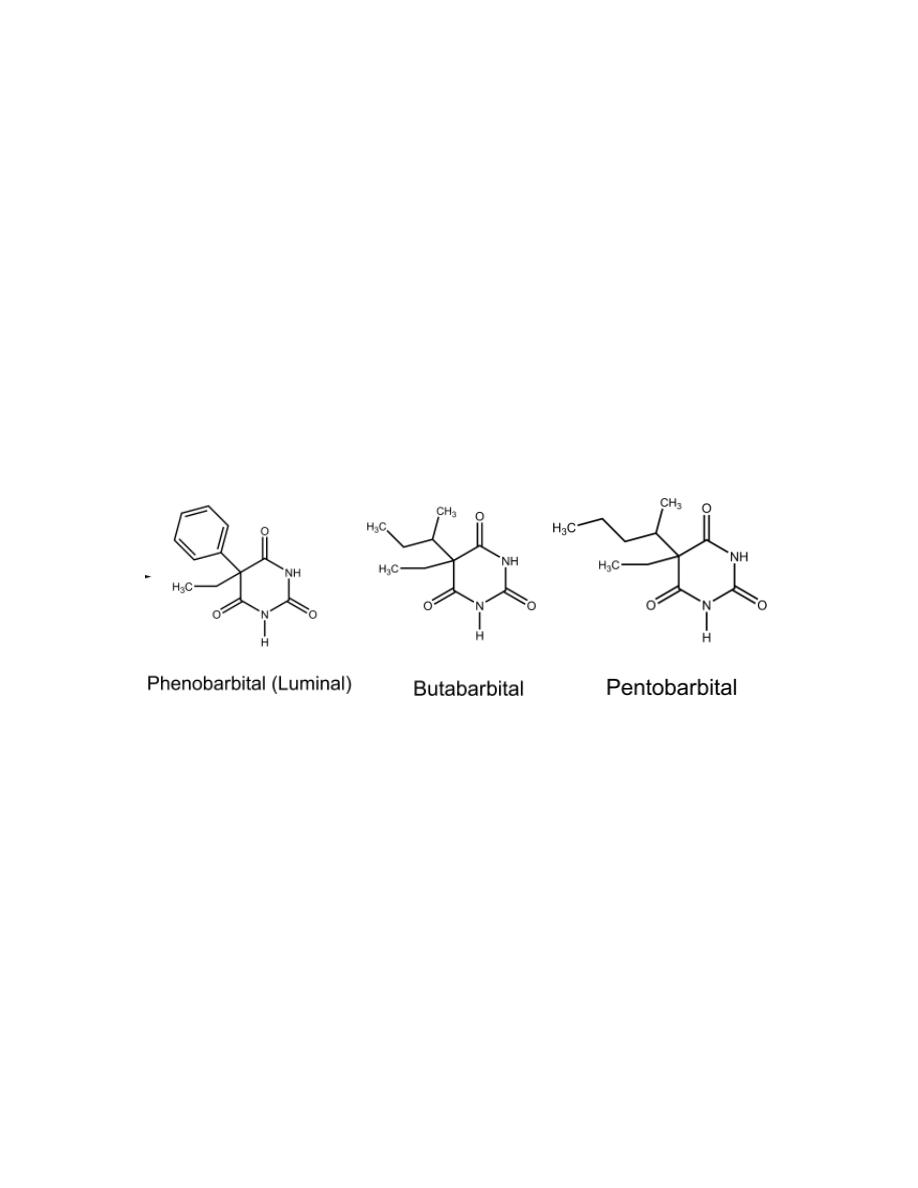
9
Duration of Action (3–6 Hours)
Barbiturates with an intermediate duration of action are used principally as
sedative–hypnotics. This class includes butabarbital sodium.
Barbiturates with Short Duration of Action
(Less Than 3 Hours)
Barbiturates that have substituents in the 5-position promoting more rapid
metabolism (e.g., by increasing the lipophilicity) than the intermediate
group. This class includes pentobarbital sodium.
