Cardiovascular System
CVS System AssessmentHISTORY
The focus of the cardiovascular history depends on patient's age and is directed by the chief complaint.
Prenatal history:
Maternal intake of medication, or alcohol use or excessive smoking during pregnancy may contribute to cardiac and other systemic findings.
Maternal infection early in pregnancy (possibly teratogenic) or later in pregnancy (causing myocarditis or myocardial dysfunction in infants).
Postnatal history:
The birth weight, Infants with HF grow poorly, with weight being more significantly affected than height and OFC.
Newborn with HF may present with fatigue or diaphoresis with feeds or fussiness. Feeding may be difficult and prolonged because of tachypnea.
Older children: HF presented with easy fatigability, SOB on exertion, and sometimes orthopnea & exercise intolerance.
Other symptoms of cardiac disease: include cyanosis, palpitations, chest pain, syncope.
Review of systems: assesses for possible systemic diseases or congenital malformation syndromes that may cause cardiac abnormalities.
History of drug use.
Family history: should be reviewed for hereditary diseases, early atherosclerotic heart disease, congenital heart disease, sudden unexplained deaths, thrombophilia, rheumatic fever, hypertension, and hypercholesterolemia.
PHYSICAL EXAMINATION
Vital signs: heart rate, respiratory rate, blood pressure, pulse pressure.Growth parameters: Wt, Ht & OFC.
Inspection:
General appearance:
Nutritional status & FTT.
Dysmorphic features.
Skin color must be assessed for cyanosis and pallor.
Clubbing of the fingernails and toenails.
Local Inspection of the chest: may reveal asymmetry or a prominent left precordium suggesting chronic cardiac enlargement.
Palpation:
Palpation of pulses in all four extremities (assessed for rate, regularity, intensity, symmetry, and timing between upper and lower extremities)
The precordial activity (assessed for apical impulse, point of maximum impulse, hyperactivity, and presence of a thrill)
The abdomen (assesses liver and spleen size. The liver size provides an assessment of intravascular volume and is enlarged with systemic venous congestion. The spleen may be enlarged with infective endocarditis).
Auscultation:
Systematic listening in a quiet room allows assessment of each portion of the cardiac cycle.
HR & regularity.
Heart sounds.
Clicks.
Murmurs
All need to be timed and characterized.
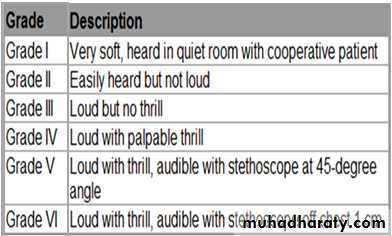
Heart Murmur Intensity
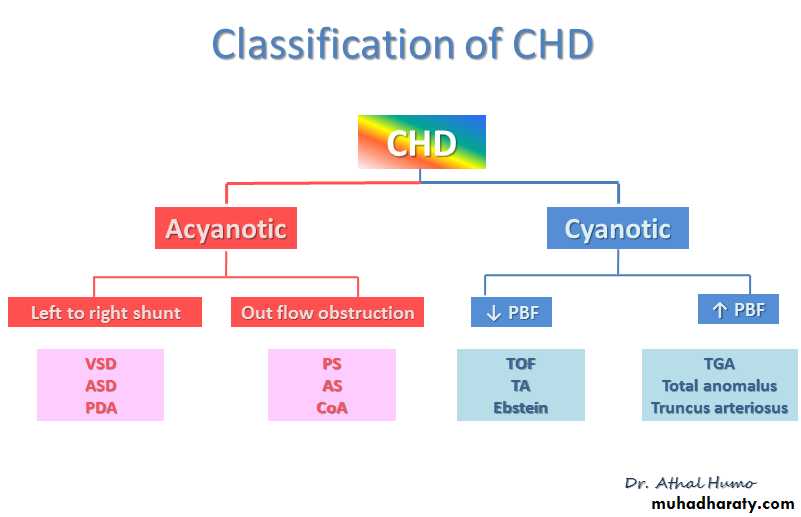
Heart Murmur IntensityAcyanotic Congenital Heart Disease
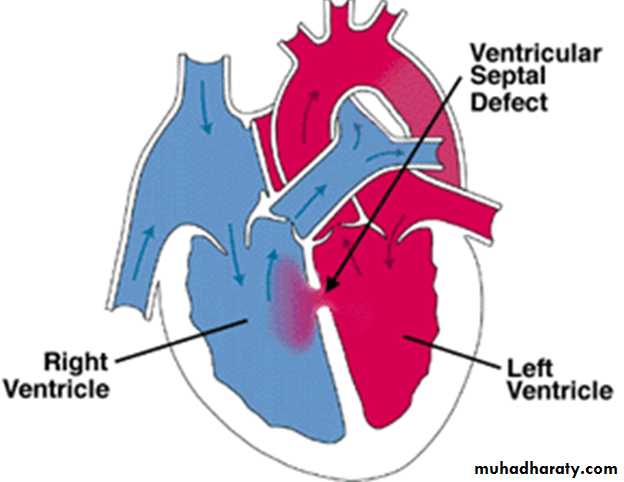
Ventricular Septal Defect
VSD is the most common cardiac malformation and accounts for 25% of congenital heart disease.
TYPES OF VSD
Defects may occur in any portion of the ventricular septum:
Membranous: is below the aortic valve and is relatively small, it is the most common of all VSDs .
Muscular: may be single or multiple (Swiss cheese septum).
The inlet : comprises endocardial cushion tissue.
supracristal septum: are less common; they are found just beneath the pulmonary valve and may impinge on an aortic sinus and cause aortic insufficiency.
Clinical Manifestations
The size of the VSD affects the clinical presentation:
Small VSDs: are often asymptomatic and the cardiac lesion is usually found during routine physical examination. Characteristically, a loud, harsh, or blowing holosystolic murmur is present and heard best over the lower left sternal border, and it is frequently accompanied by a thrill.
Moderate to large VSDs: result in pulmonary over circulation and heart failure, presenting as fatigue, sweating with feedings difficulties, recurrent pulmonary infections, and poor growth. Cyanosis is usually absent, but duskiness is sometimes noted during infections or crying.
On examination:
Prominence of the left precordium is common, as are a palpable parasternal lift.
laterally displaced apical impulse and a systolic thrill.
The holosystolic murmur of a large VSD is generally less harsh than that of a small VSD.
Investigation:
Electrocardiogram (ECG) and chest x-ray findings depend on the size of the VSD.
Small VSDs usually have normal studies.
Larger VSDs cause volume overload to the left side of the heart, resulting in:
ECG findings of left atrial and ventricular enlargement and hypertrophy.
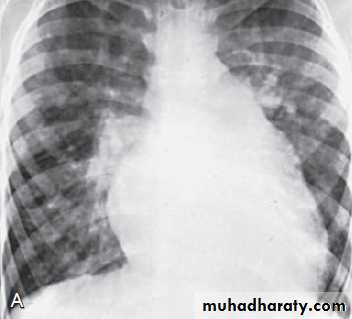
CXR may reveal cardiomegaly, enlargement of the left ventricle, an increase in the pulmonary artery silhouette, and increased pulmonary blood flow. Pulmonary hypertension due to either increased flow or increased pulmonary vascular resistance may lead to right ventricular enlargement and hypertrophy.
CXR in patient with VSD shows cardiomegaly & pulmonary overcirculation
CXR in patient with VSD shows cardiomegaly & pulmonary overcirculation
Echocardiogram shows the position and size of the VSD.
Treatment
Small VSD: no surgical intervention, no physical restrictions, just reassurance & periodic follow-up and endocarditis prophylaxis.Moderate to large VSD:
Management has 2 aims:
To get the symptoms of heart failure under control
Prevent the development of pulmonary vascular disease.
Initial treatment includes diuretics, digoxin and afterload reduction.
Indications for surgical closure of a VSD include:
Large VSD in whom clinical symptoms and FTT cannot be controlled medically.
Age 6 - 12 mo with large VSD with pulmonary hypertension, even if the symptoms are controlled by medication.
Patients >24 mo with a Qp : Qs > 2 : 1.
Supracristal VSD of any size because of the high risk for aortic valve regurgitation.
Complication:
Large defects lead to:
Heart failure.
Failure to thrive.
Endocarditis.
Pulmonary hypertension.
ATRIAL SEPTAL DEFECT
ASDs represent approximately 10% of all congenital heart defects.
Types of ASD:
Secundum ASD, with the hole in the region of the foramen ovale, is the most common ASD.
Primum ASD, located near the endocardial cushions, may be part of a complete atrioventricular canal defect.
Sinus Venosus ASD, high in the atrial septum, associated with anomalous venous return & the least common.
Clinical Manifestations
ASD is most often asymptomatic; the lesion is often discovered inadvertently during physical examination.
However, on closer evaluation, in younger children, subtle FTT may be present; in older children varying degrees of exercise intolerance may be noted.
Examination:
Mild left precordial bulge, prominent right ventricular impulse & right ventricular systolic lift at LLSB often can be palpated.
A soft (grade I or II) systolic ejection murmur in the region of the right ventricular outflow tract and a fixed split S2.
A larger shunt may result in a mid-diastolic murmur at LLSB as a result of the increased volume passing across the tricuspid valve.
Investigation
ECG: RAD and right ventricular enlargement.
CXR: varying degrees of enlargement of the right ventricle and atrium, pulmonary artery is enlarged, and pulmonary vascularity is increased.
Echocardiogram: definitive dx.
TREATMENT
Transcatheter or surgical closure is advised for all symptomatic patients and also for asymptomatic patients with Qp:Qs ratio >2:1.
The timing for elective closure is usually between 2 & 5 yrs to avoid late complications.
Mortality rate in surgical closure <1%.
Infective endocarditis is extremely rare.
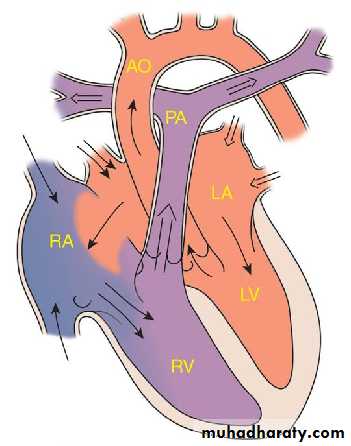
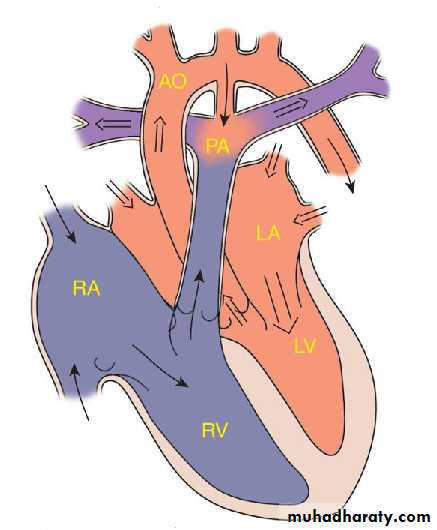
PATENT DUCTUS ARTERIOSUS
During fetal life, most of the pulmonary arterial blood is shunted right to-left through the ductus arteriosus into the aorta.Functional closure of the ductus normally occurs soon after birth.
If the ductus remains patent when pulmonary vascular resistance falls, aortic blood then is shunted left-to-right into the pulmonary artery.
So PDA: is persistence of the normal fetal vessel that joins the PA to the aorta.
Normally closes in the 1st wk of life.
Female : Male ratio of 2:1
Accounts for 10% of all CHD, seen in 10% of other congenital heart lesions and can often play a critical role in some lesions.
Often associated with coarctation & VSD.
Can be caused by congenital Rubella.
Clinical Manifestations
Symptoms depend on the amount of pulmonary blood flow which depends on the size of the PDA and the pulmonary vascular resistance.
Small PDAs: are asymptomatic.
Moderate to large PDA can produce:
Symptoms of HF as the pulmonary vascular resistance decreases.
Retardation of physical growth.
Bounding peripheral arterial pulses.
wide pulse pressure.
Enlarged heart, prominent apical impulse.
A thrill, maximal in the 2nd left interspace, is often present and may radiate toward the left clavicle, down the left sternal border, or toward the apex.
Classic continuous machinary murmur (It begins soon after onset of the 1st sound, reaches maximal intensity at the end of systole, and wanes in late diastole. It may be localized to the 2nd left intercostal space or radiate down the left sternal border or to the left clavicle).
Mid-diastolic murmur at the apex (as a result of the increased volume of blood flow across the mitral valve).
Investigation:
Small PDA: normal.
Moderate to large PDA:
CXR: cardiomegally, full pulmonary artery silhouette and increased pulmonary vascularity.
ECG: vary from normal to evidence of LVH. If pulmonary hypertension is present, there is also RVH.
Treatment:
Irrespective of age, patients with PDA require catheter or surgical closure.
Small PDA: the rationale for closure is prevention of bacterial endocarditis or other late complications.
Moderate to large PDA: closure is accomplished to treat heart failure or prevent the development of pulmonary vascular disease, or both.
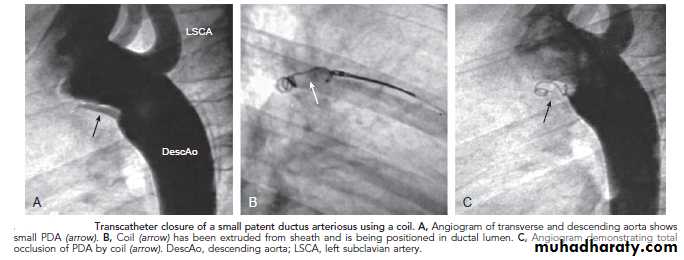
PDA requires surgical or catheter closure. Most PDAs can be closed in the catheterization laboratory by either coil embolization or a PDA closure device.
Indomethacin, inhibitor of prostaglandin synthesis can be used in premature infants.
Prophylaxis against infective endocarditis
Transcatheter closure of a small PDA