1
NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDs)
Inflammation which is a normal protective response to tissue injury caused
by physical trauma, noxious chemicals, or microbial agents releases certain
mediators including prostaglandins and leukotrienes that cause edema and
pain and can also raise body temperature.
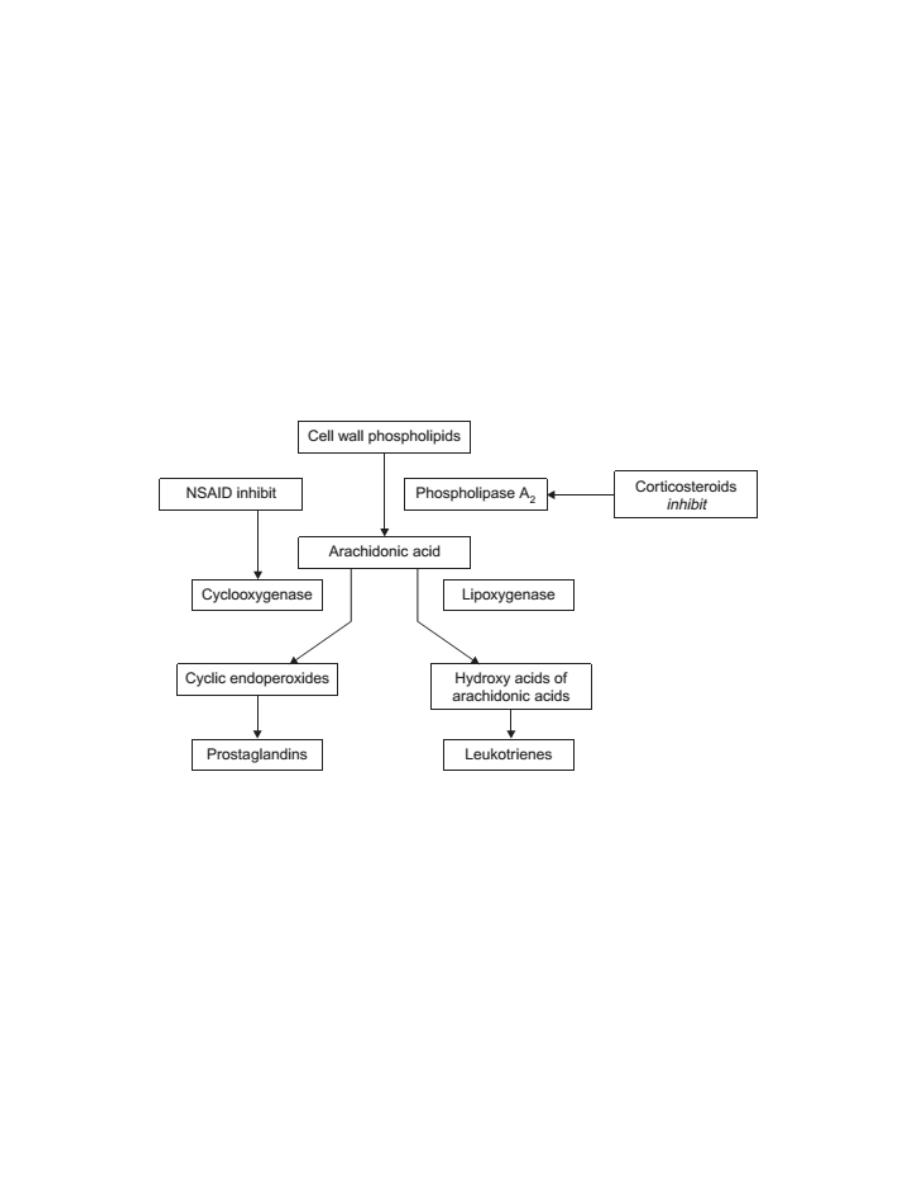
These inflammatory mediators are formed in the body from arachidonic acid
which is esterified to the phospholipids of cell membranes and released as
needed from phospholipids by the action of phospholipase A
2
.
Phospholipase A
2
, which is present in cell membranes, is stimulated or
activated by microbial products or physical trauma. The release of
arachidonic acid from the phospholipid initiates a cascade of reactions
catalyzed by cyclooxygenase(COX) or 5-lipoxygenase which lead ultimately
to the production of prostaglandins and leukotrienes, as well as other
products.
2
One of the pharmacological effects of the corticosteroidsis their ability to
inhibit the activation of phospholipase A
2
and thereby prevent the release of
arachidonic acid. Such steroids are therefore anti-inflammatory agents.
In this section however, the focus will be on non-steroidal agents which have
anti-inflammatory properties. These are referred to as non-steroidal anti-
inflammatory drugs or NSAIDs.
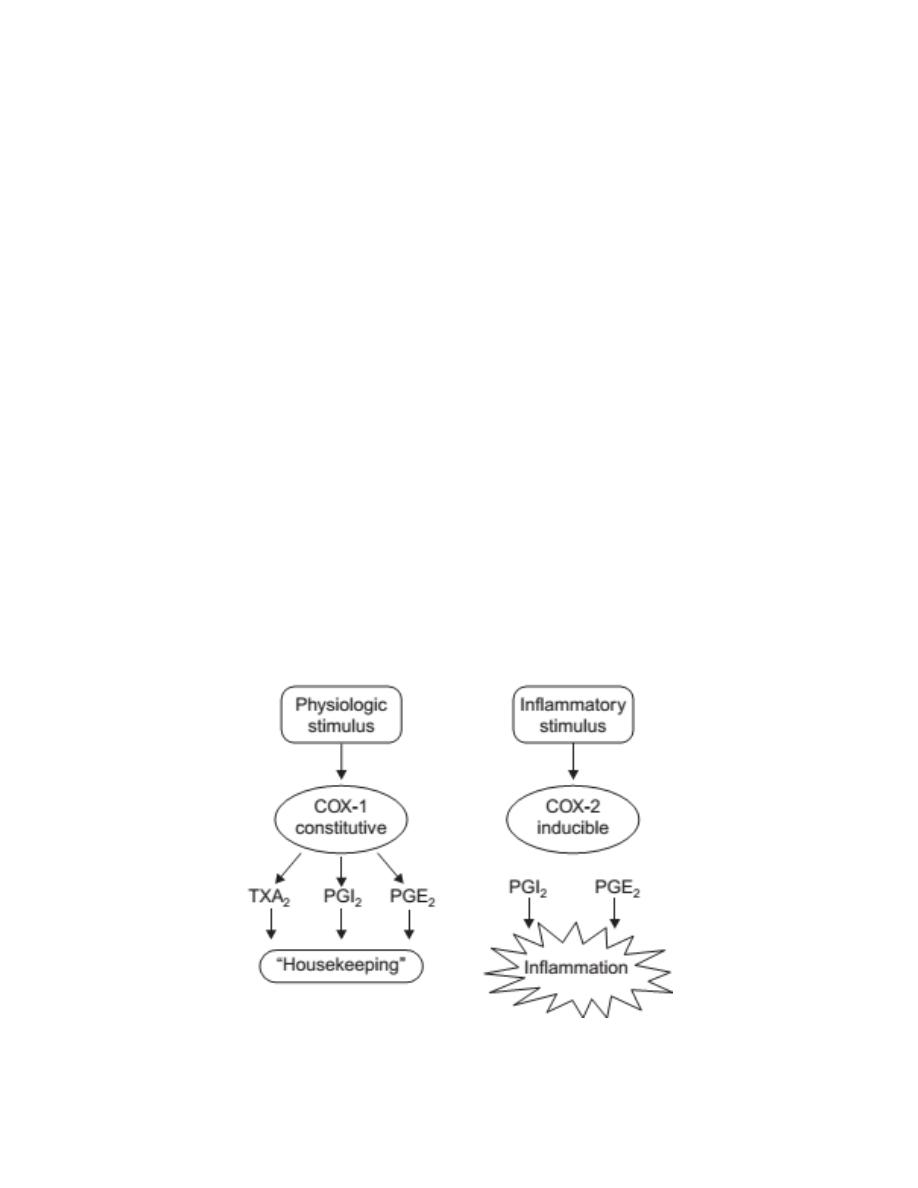
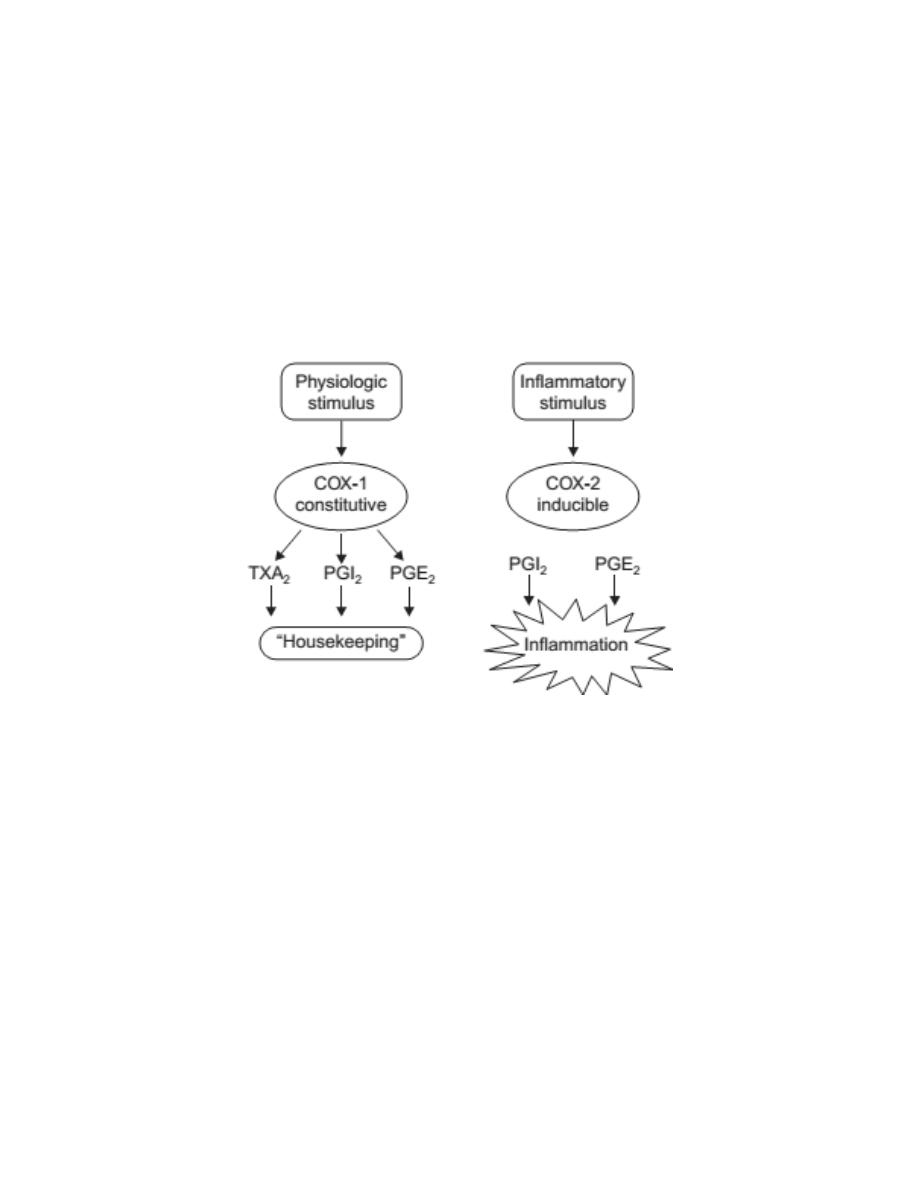
Cyclooxygenases
Two cyclooxygenase enzymes have been identified in humans. A third,
COX-3, is present in dogs but not in humans.
Cyclooxygenase-1 (COX-1) is responsible for the physiologic production of
prostanoids.
It is a constitutive enzyme that regulates normal cellular
processes, such as gastric cytoprotection, vascular homeostasis, platelet
aggregation, and kidney functions.
Cyclooxygenase-2 (COX-2) is an inducible enzyme that its expression is
induced by inflammatory mediators like t
umor necrosis factor
(TNF-α) and
Interleukin-1 (IL-1) and causes the elevated production of prostanoids that
occurs in sites of chronic disease and inflammation.

3
Therapeutic uses of NSAIDs
The NSAIDs are a group of compounds which generally have three main
activities:
•
Antipyretic Activity
•
Analgesic Activity
•
Anti-inflammatory Activity (except for acetaminophen)
Adverse events of NSAIDs
Because of the associated adverse events below, it is preferable to use
NSAIDs at the lowest effective dose for the shortest duration possible.
a. Gastrointestinal:
1- Direct irritation because all the NSAIDs are relatively strong organic
acids (mostly carboxylic acids).
2- Indirectly through the inhibition of physiological prostanoids
synthesis resulting in increased gastric acid secretion, diminished
mucus protection.
3-
b. Actions on the kidney:
NSAIDs prevent the synthesis of prostanoids that are responsible for
maintaining renal blood flow resulting in retention of sodium and water and
may cause edema in some patients.
c. Increased risk of bleeding (antiplatelet effect):
TXA
2
enhances platelet aggregation, whereas PGI
2
decreases it. Aspirin
irreversibly inhibits COX-1–mediated TXA
2
formation, while other NSAIDs
reversibly inhibit the production of TXA
2
. Because platelets lack nuclei,
they cannot synthesize new enzyme when inhibited by aspirin, and the lack
of thromboxane persists for the lifetime of the platelet (3 to 7 days).
4
d. Other side effects:
NSAIDs are inhibitors of cyclooxygenases and, therefore, inhibit the
synthesis of prostanoids but not of leukotrienes. For this reason, NSAIDs
should be used with caution in patients with asthma, as inhibition of
prostanoids synthesis can cause a shift toward leukotriene production and,
therefore, increase the risk of exacerbations of asthma.
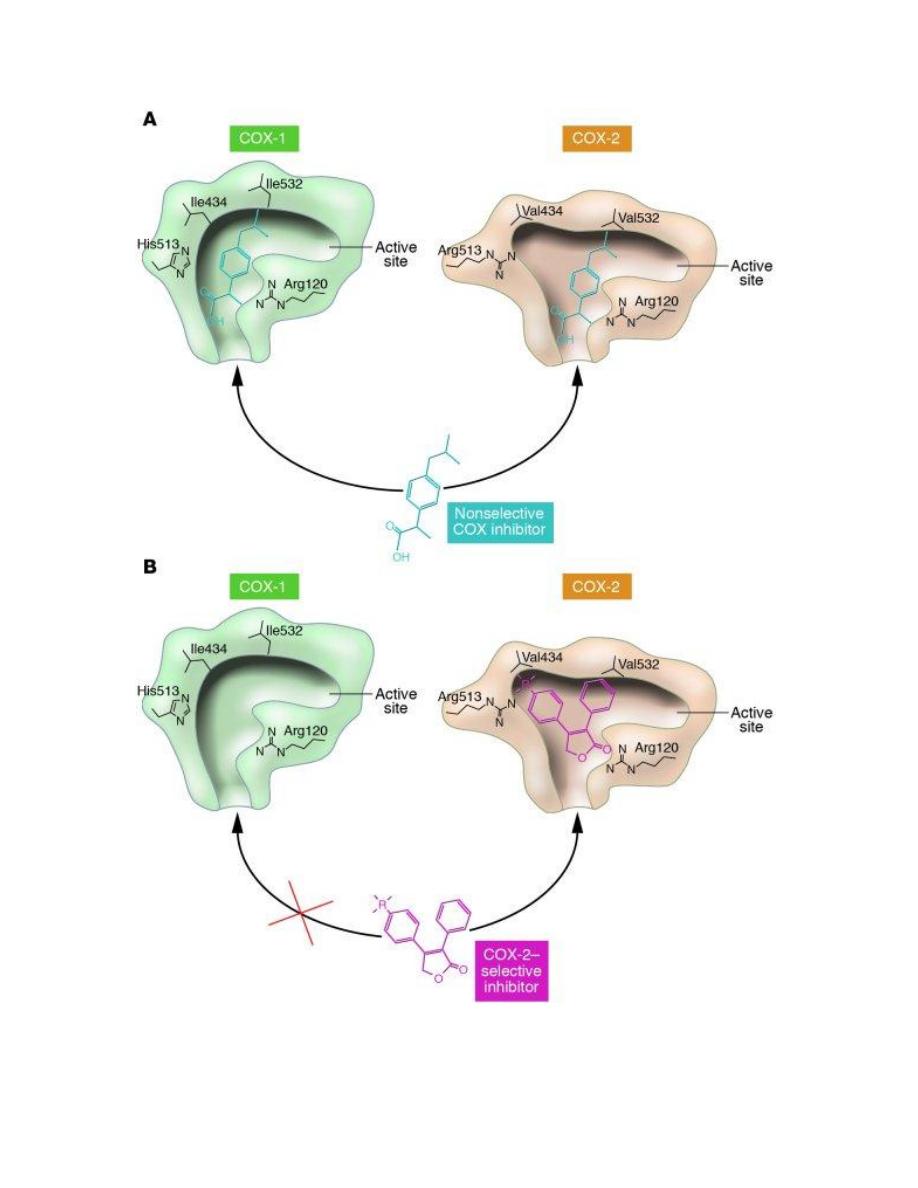
Enzymetic Structure of Cyclooxygenases
Cyclooxygenases (COX-1 and COX-2) are membrane-bound proteins that
share a high degree of sequence identity and also have very similar active
site.
Thus, despite their similarity, the active site for COX-2 is approximately
20% larger than the COX-1 binding site because of the replacement of Ile-
523 in COX-1 with a smaller Val-509 in COX-2. Differences in binding site
shape have permitted the development of selective COX-2 inhibitors.
5

6
General structure and properties of NSAIDs
In general, NSAIDs structurally consist of an acidic moiety (carboxylic acid,
enols) attached to a planar, aromatic functionality. Some analgesics also
contain a polar linking group, which attaches the planar moiety to an
additional lipophilic group. The NSAIDs are characterized by the following
chemical/ pharmacologic properties:
1. All are relatively strong organic acids with pKa in the 3.0–5.0 range.
Most, but not all, are carboxylic acids. The acidic group is essential for COX
inhibitory activity.
2. The NSAIDs differ in their lipophilicities based on the lipophilic character
of their aryl groups and additional lipophilic moieties.
3. The acidic group in these compounds serves a major binding group with
plasma proteins. They are also highly bound to plasma proteins, a major
source of potential drug interactions with other medications.
4. The acidic group also serves as a major site of metabolism by conjugation.
Thus a major pathway of clearance for many NSAIDs is glucuronidation
(and inactivation) followed by renal elimination.
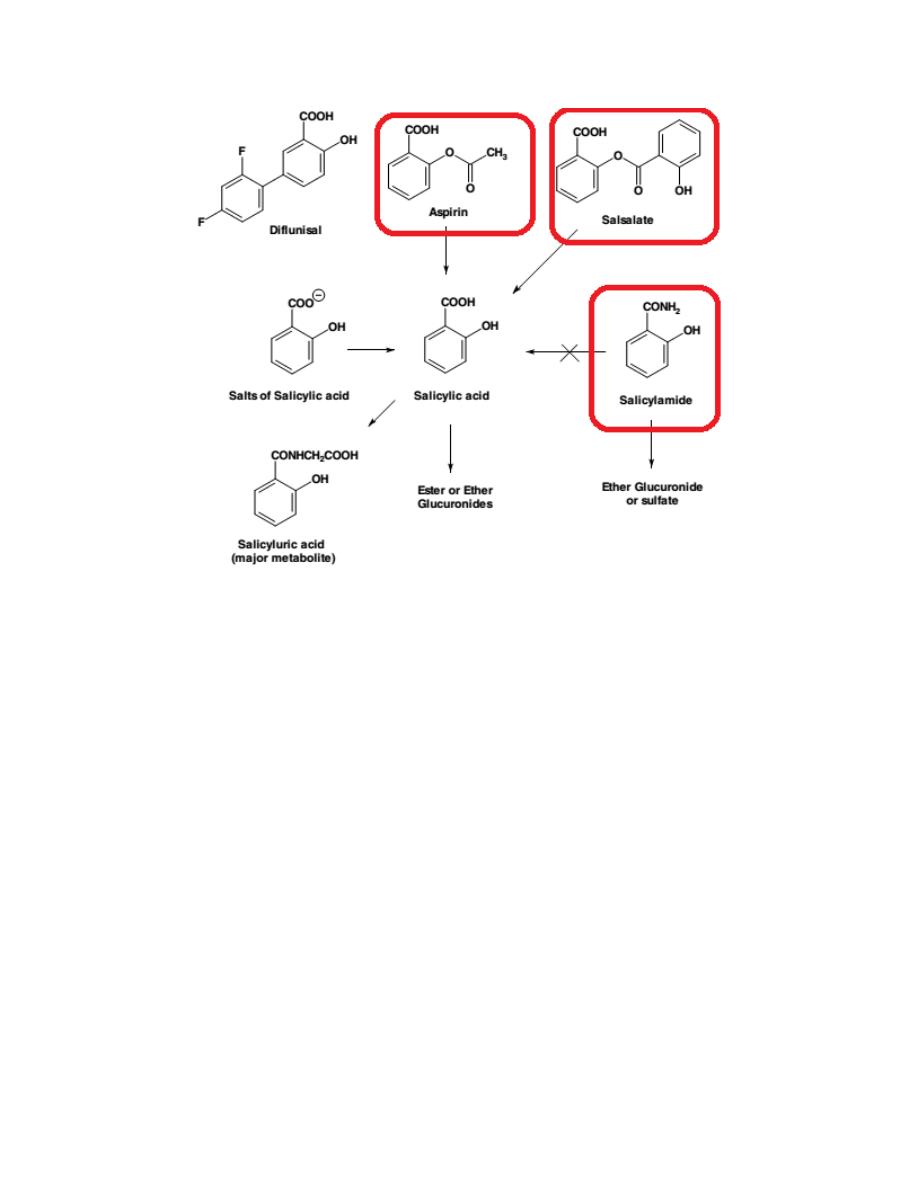
Salicylates
Most of the salicylic acid drugs (commonly referred to as the salicylates) are
either marketed as salts of salicylic acid or as ester or amide derivatives
(aspirin, salsalate, salicylamide).
Children, between the ages of 3 and 12, who are recovering from flu or
chicken pox, should not be taking aspirin or any salicylates because of the
perceived risks of a rare disease known as Reye syndrome.
7
Aspirin
Aspirin occurs as white crystals or as a white crystalline powder and must be
kept under dry conditions, because aspirin is slowly decomposed into acetic
and salicylic acids in the presence of heat and moisture.
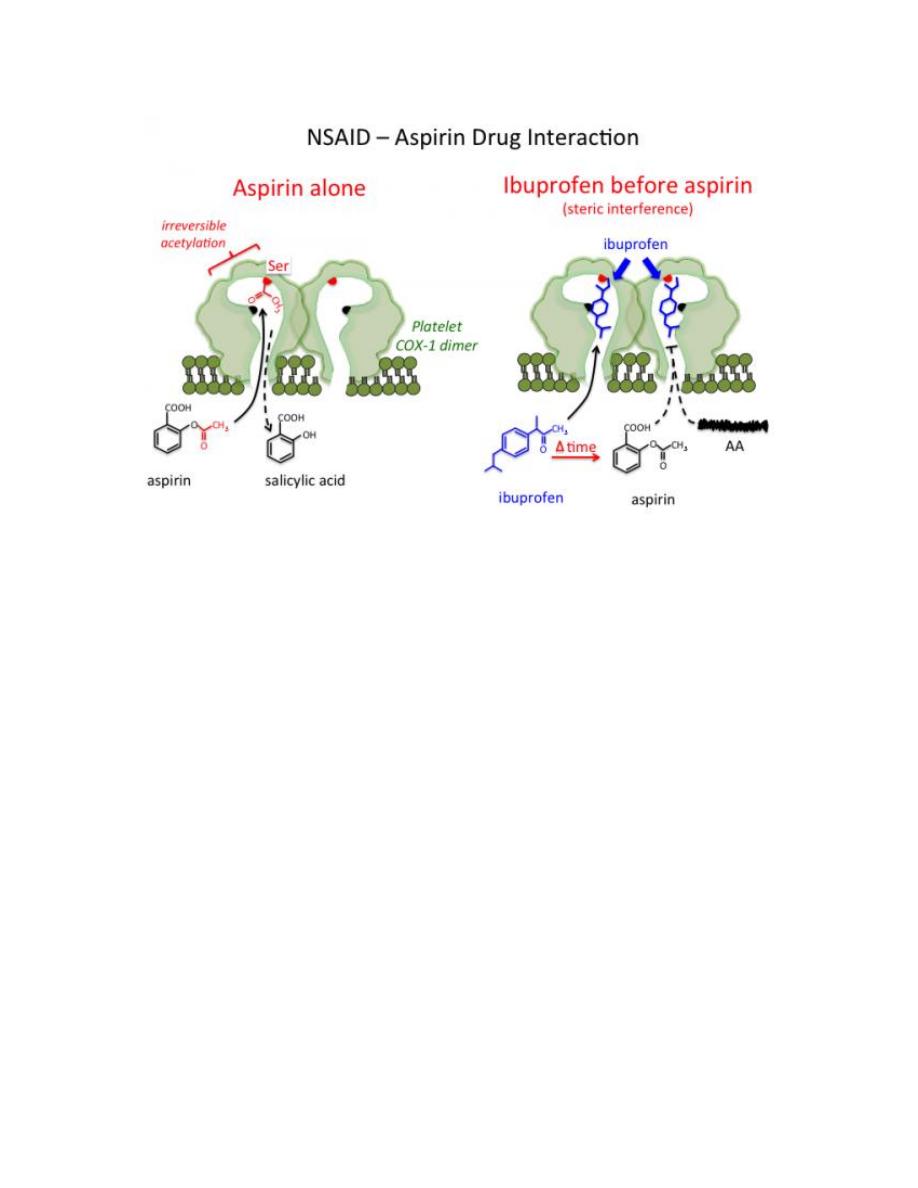
Aspirin and its COX-1 selectivity
Aspirin covalently modifies COX-1 and COX-2 isozymes by acetylating the
OH group of Ser in COX isozymes. Even though both COX isozymes are
irreversibly acetylated by aspirin, acetylation of Ser in COX-1 totally blocks
the accessibility of substrate AA from entering into the active site, whereas
an acetylated COX-2 is still able to form a significant amount of PGG2.
Thus, aspirin, among all conventional NSAIDs,
exhibits the highest

8
selectivity toward the COX-1 isozyme, especially the COX-1 isozyme
present in the platelets.
A low daily dose of aspirin (75–100 mg or one tablet of baby aspirin) is
sufficient to completely block platelet TXA
2
production and its ability to
induce platelet aggregation, thereby preventing the risk of a cardiovascular
event, including myocardial infarction and ischemic stroke.
Salsalate
Salsalate, salicylsalicylic acid is the ester formed between two salicylic acid
molecules. It is rapidly hydrolyzed to salicylic acid following its absorption.
Salicylamide
Salicylamide, is a derivative of salicylic acid.
The Conventional Nonselective Cyclooxygenase Inhibitors
For the purpose of comparing their SAR, toxicity, and metabolic
biotransformations, the conventional NSAIDs are further divided into
several chemical classes:
1- Aryl- and heteroarylacetic acid
As a group, they show high analgesic potency and potent antiinflammatory
activity, needed for treating inflammatory diseases. This class includes
ketorolac, indomethacin, sulindac and nabumetone.
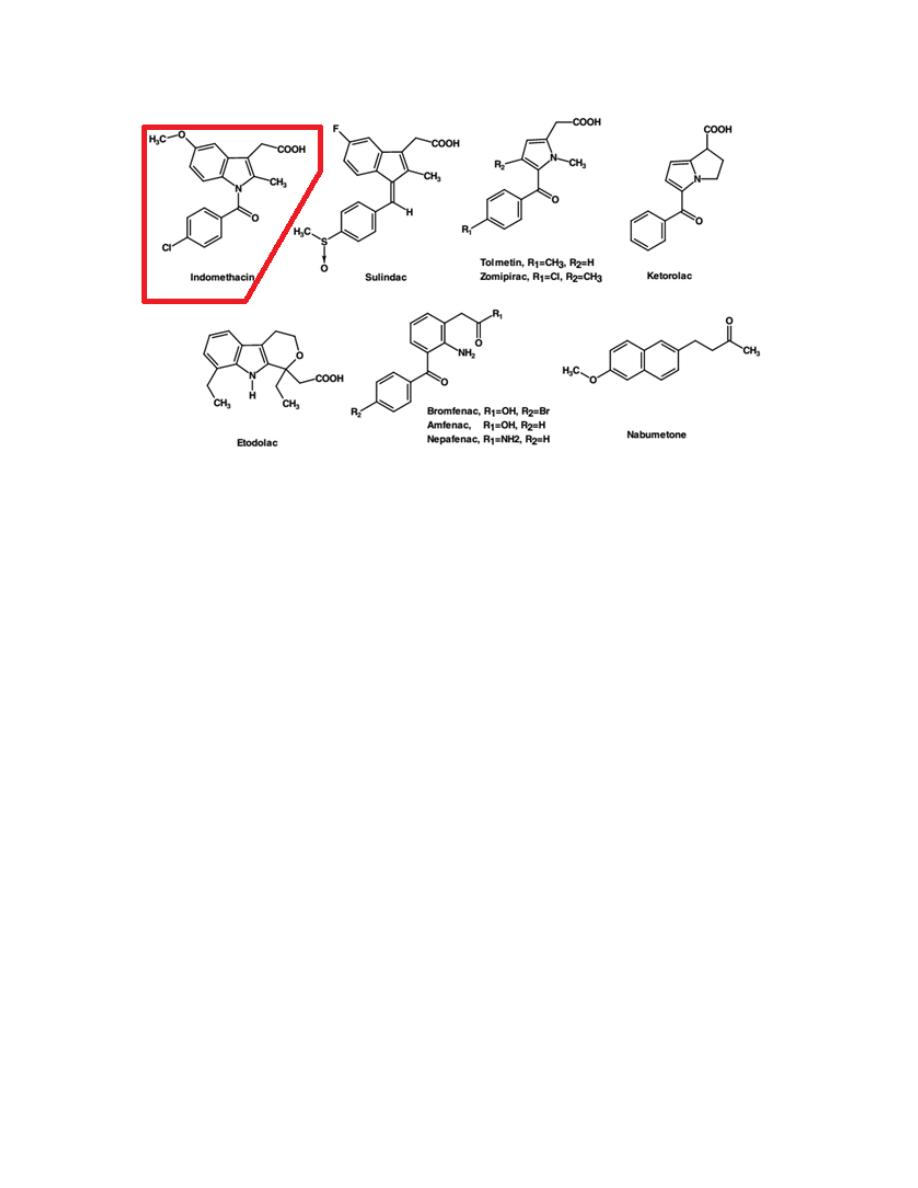
9
Indomethacin
Although both its analgesic and anti-inflammatory activities are well
established, its use is often limited because of frequent GI distress and
potential drug interactions.
2- Aryl- and heteroarylpropanoic acid
All of the members of this class (except oxaprozin) contain a chiral carbon
in the -position of the acetic acid side chain. Even though most are marketed
as racemates, only the (S)-enantiomer was found to have any inhibitory
activity against the COX isozymes. Furthermore, in most cases, the inactive
(R)-enantiomer is epimerized in vivo, via the 2-arylpropionyl coenzyme-A
epimerase to its active (S)-enantiomer. This class includes Ibuprofen,
Ketoprofen, Naproxen and Oxaprozin.
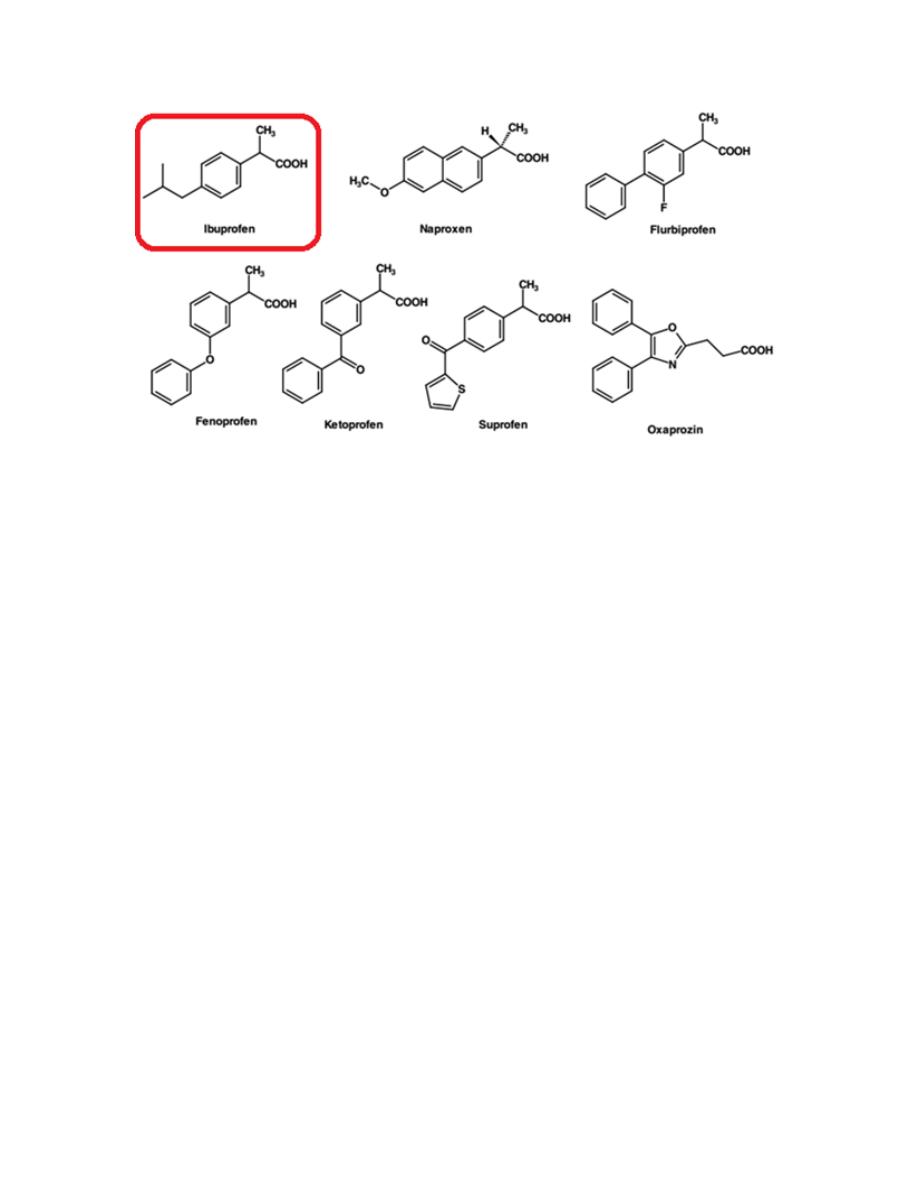
10
Ibuprofen
Ibuprofen appears to have comparable efficacy to aspirin in the treatment of
RA, but with a lower incidence of side effects.
A recent study indicates that concurrent use of ibuprofen and aspirin may
actually interfere with the cardioprotective effects of aspirin, at least in
patients with established cardiovascular disease. This is because ibuprofen
can reversibly bind to the platelet COX-1 isozymes, thereby blocking
aspirin’s ability to inhibit TXA
2
synthesis in platelets.
11
3- N-Arylanthranilic acid (fenamates) and structurally related
analogs
Unlike other classes discussed earlier, the second aromatic ring in this class
is connected to the main aromatic carboxylic acid containing ring through a
secondary amine linkage (rather than carbonyl group or other nonbasic
linker) and at the ortho position rather than at the meta or para position. As a
result of this structural feature, this class of NSAIDs appears to have a lower
risk of causing GI irritation.
This class includes Mefenamic acid (Ponstan) and Diclofenac (Voltaren).
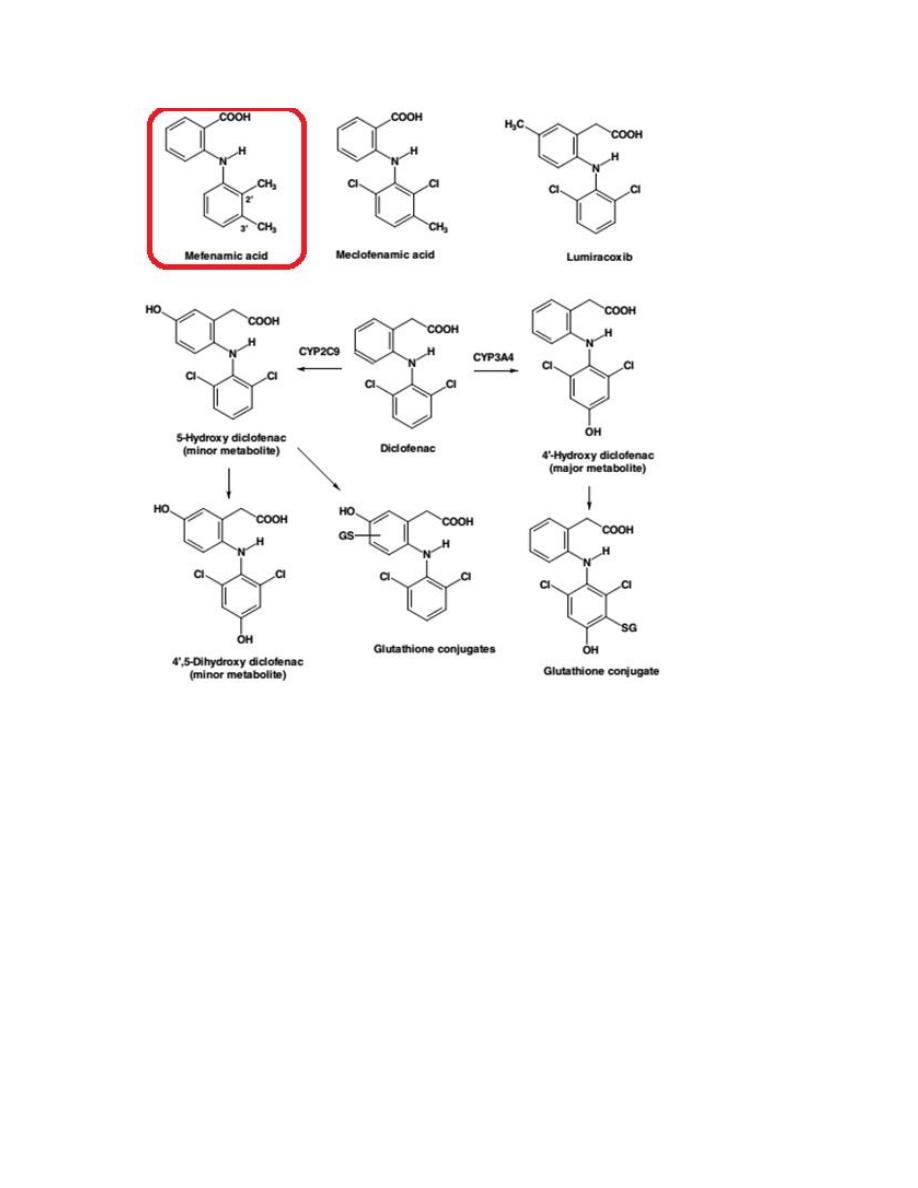
12
Mefenamic acid
Mefenamic acid (Ponstan) is one of the oldest NSAIDs, introduced into the
market for mild to moderate pain.The possibility of blood disorders has also
prompted limitation of its administration to 7 days.
13
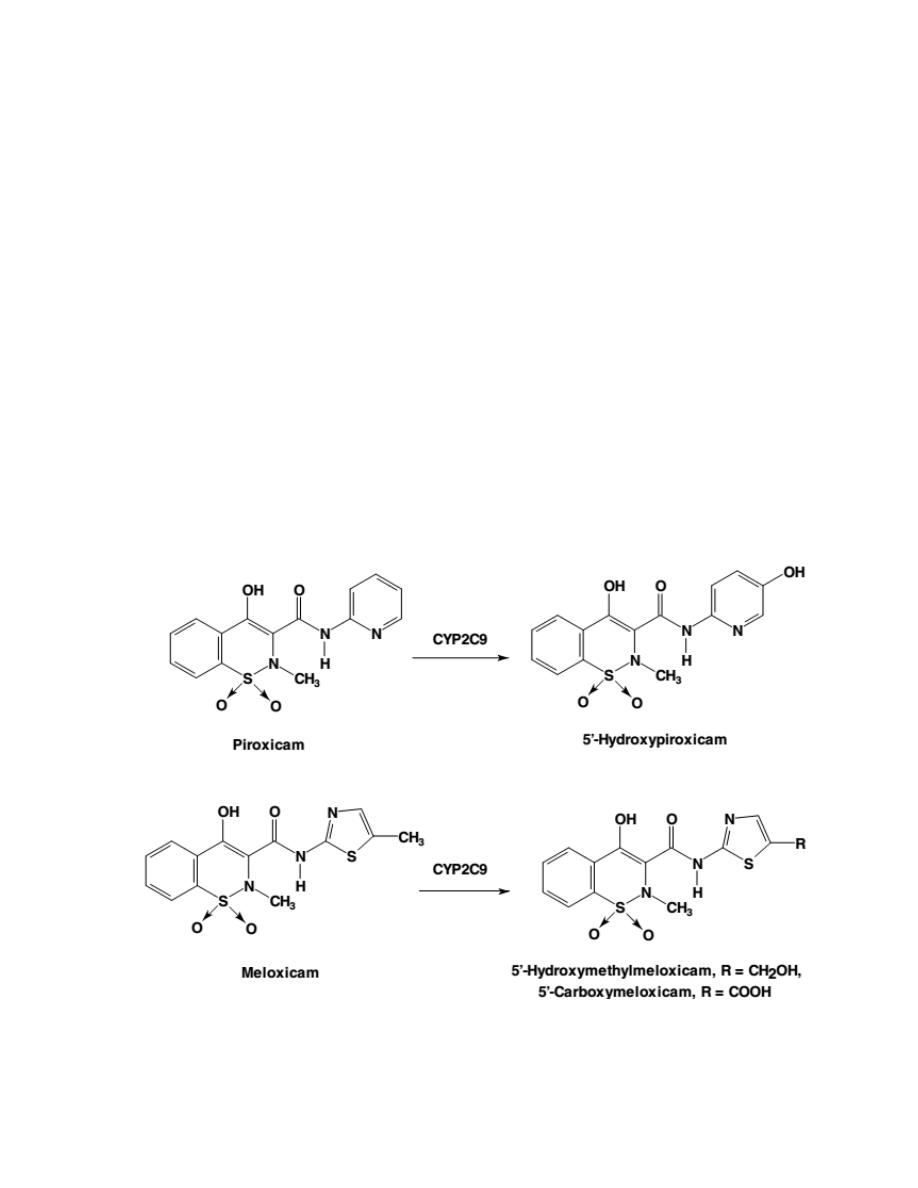
4- Oxicams
Oxicams, are first-generation NSAIDs that lack a free carboxylic acid side
chain but with an acidic enolic 1,2-benzothiazine carboxamide ring.
Piroxicam and meloxicam (the most common Oxicams) have nearly
identical structural features but also have at least a ninefold difference in
selectivity for meloxicam to COX-2 isozyme.
A closer comparison of their
structure reveals no apparent reason for these differences, either in size,
lipophilicity, or electronic properties, between the piroxicam and the
meloxicam that may alter their ability to bind COX isozymes.
However, these drastic differences in their COX selectivity may be
attributed to the differences in COX selectivity of their metabolite.
Piroxicam is metabolized to 5´-hydroxypiroxicam while meloxicam is
metabolized to 5´-hydroxy-methylmeloxicam and 5´-carboxymeloxicam
(which has high affinity for COX-2).
14
Meloxicam
Meloxicam is a selective COX-2 inhibitor among oxicams and has a much
lower rate of serious GI side effects and a lower than average risk of
nephropathy when compared with other conventional NSAIDs.
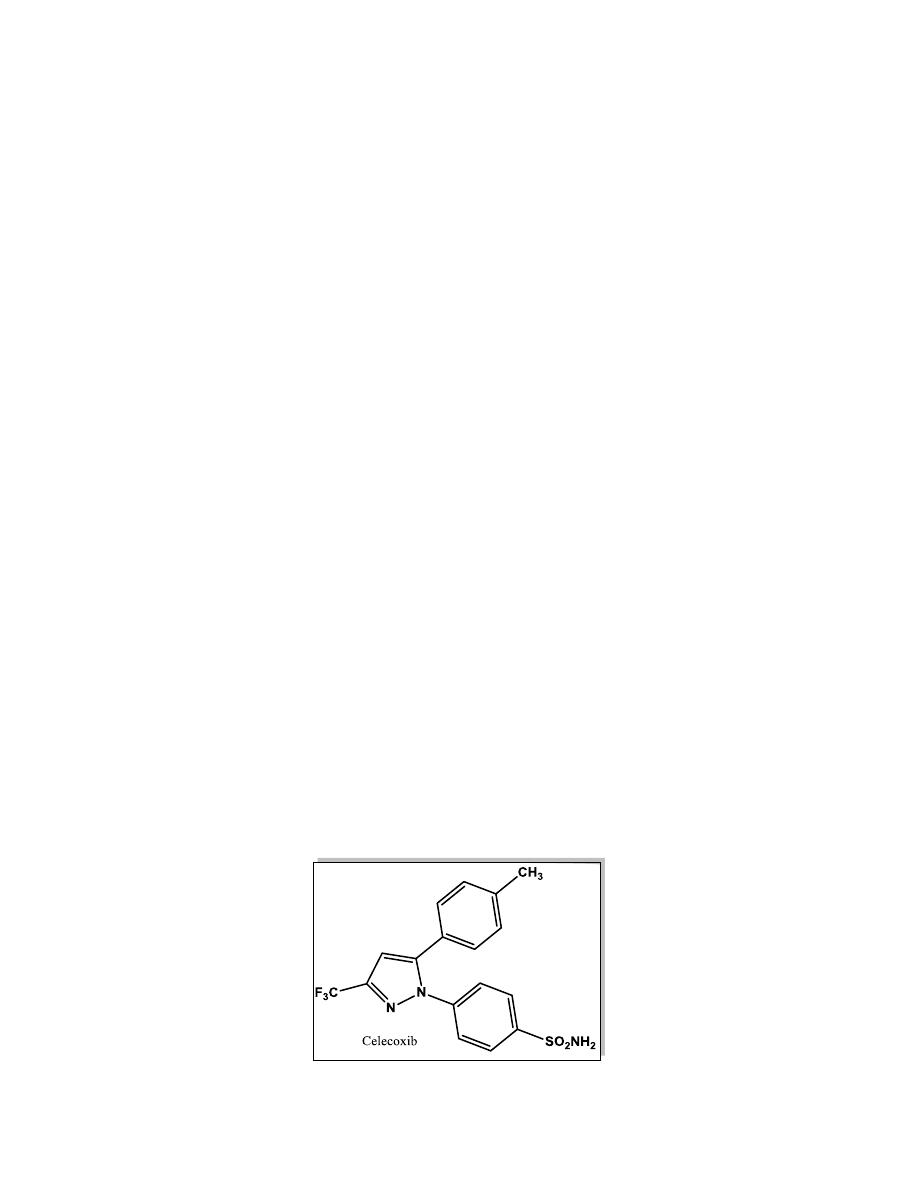
The Selective COX-2 Inhibitors
COX-2 inhibitors are the “new-generation” NSAIDs that may selectively
block the COX-2 without affecting COX-1 function. This may result in
control of pain and inflammation with a lower rate of adverse effects
compared with older nonselective NSAIDs.
All of COX-2 inhibitors except celecoxib, have been removed from the
worldwide market due to their cardiovascular effects. The chief mechanism
proposed to explain the cardiotoxicity of selective COX-2 inhibitors is the
suppression of prostacyclin (PGI
2
), an anti-clotting agent.
An overexpression of COX-2 was found in multiple cancer types, especially
in colorectal cancer, thus future roles of the selective COX-2 inhibitors may
be realized in the chemoprevention of cancers and other inflammatory
degenerative diseases.
Celecoxib
Celecoxib was the first selective COX-2 inhibitor drug introduced into the
market. The real benefit is that it has caused fewer GI complications when
compared with other conventional NSAIDs.

15
Acetaminophen
Acetaminophen (also known as paracetamol) has similar analgesic and
antipyretic efficacies to the conventional NSAIDs such as aspirin, ibuprofen,
or diclofenac. However, it lacks the antiplatelet effects of aspirin or the GI
side effects associated with NSAIDs. Acetaminophen also has little or no
anti-inflammatory properties. Although it has been in use for nearly a
century, the mechanism of action of acetaminophen and related analgesic
antipyretics remains unknown, but it is generally assumed acetaminophen
does not compete with AA for the binding site on the COX enzyme. Its
mechanism of action is via inhibiting the peroxidase activity of the COX
enzyme.
TOXICITY IN ACETAMINOPHEN
In healthy individuals, acetaminophen is primarily eliminated as its O-
sulfates and O-glucuronides, with only a small amount of the N-
hydroxylated metabolite, which can be sulfated or glucuronidated. Small
amounts of these O-sulfates, if accumulated in liver or renal tubules, can
slowly rearrange to form the reactive N-acetyliminoquinone metabolites.
However, these reactive metabolites, once formed, are immediately
deactivated by glutathione, the body’s defense mechanism for detoxifying
reactive metabolites.
Acetaminophen-induced toxicity can be greatly increased in alcoholic
individuals. This is because of enzymes induction by alcohol consumption.
N-acetylcysteine is an antidote to treat possible acetaminophen poisoning, it
is similar to glutathione. It deactivates the N-acetyliminoquinone metabolite
before it changes to covalently bind cellular proteins.
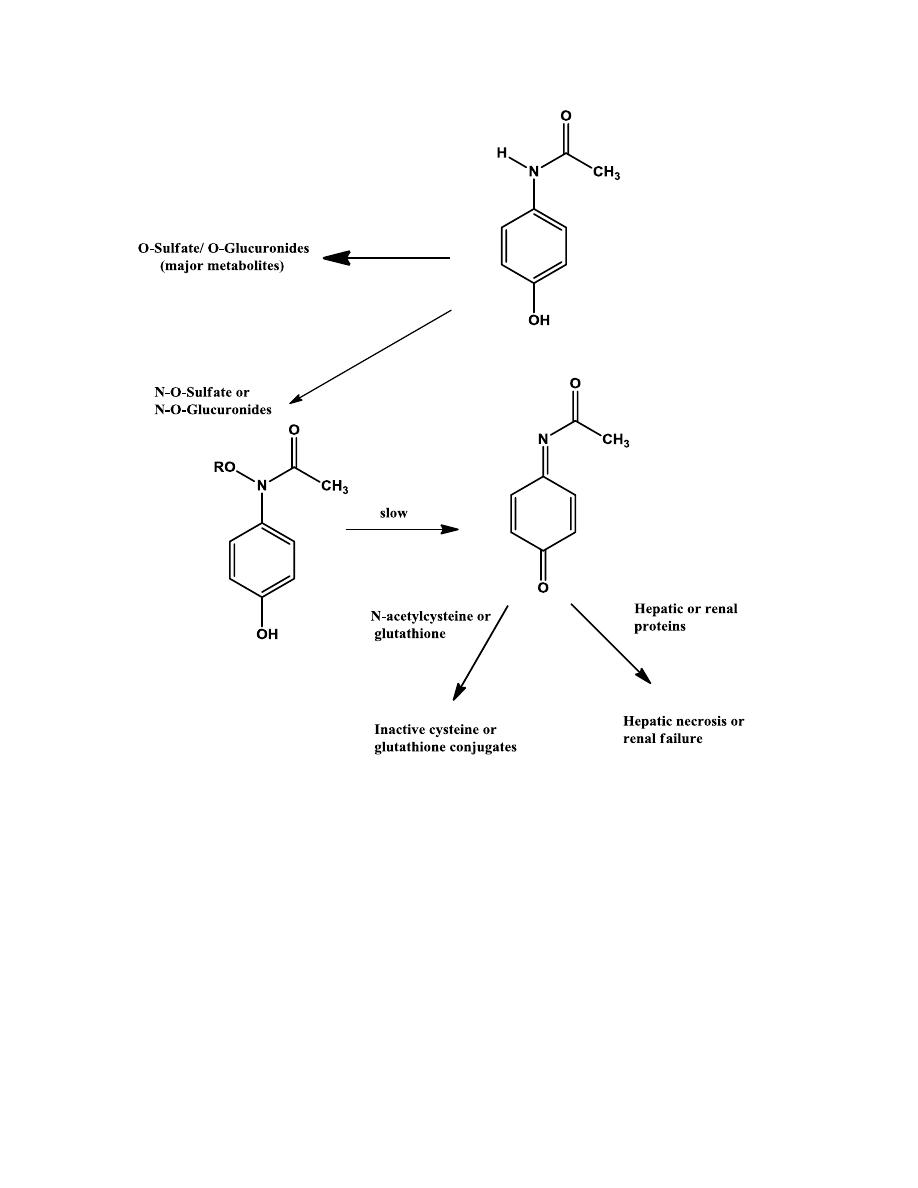
16
