
Introduction to Histology
and Basic Histological
Techniques
1st lecture
October 22, 2015

Introduction
What’
s histology?
II. Why do we study histology ?
III. How to study histology ?-Histological methods.

• Histology is the study of the tissues of the body and how
these tissues are arranged to constitute organs.
• The Greek root histo can be translated as either "tissue" or
"web" and both translations are appropriate because most
tissues are webs of interwoven filaments and fibers, both
cellular and noncellular, with membranous linings.
• Histology involves all aspects of tissue biology, with the
focus on how cells' structure and arrangement optimize
functions specific to each organ.

Cell: smallest unit of structure and function
of body
↓
tissue: group of cell+extracellular ground
substance
four basic tissue:
---epithelium
↓ ---connective tissue
---muscular tissue
---nervous tissue
organ: made up of tissue, have special shape,
structure and function
↓
system: organs Which have related function
get together.

Why we study histology?
n
It is the bases of other subject in medicine.
It
intertwines the disciplines of cell biology,
biochemistry, physiology, and as appropriate,
pathology. Students will recognize the importance
of this subject as they refer to the text later in your
careers.

How to study histology - histological methods
---Development of histology depends on the
development of technique.
---Histology studies the microstructures. So,
we should have the aid of microscope to
study. Several types of microscopes are
available.

According to the light source used, microscopes can be basally
classified as:
1- light microscopy (LM) including several type,
such as
• bright-field microscopy
• Confocal and polarizing microscopy
• Phase-contrast microscopy
2- Eloctron microscopy (EM) that includes
• Transmission EM
• Scanning EM
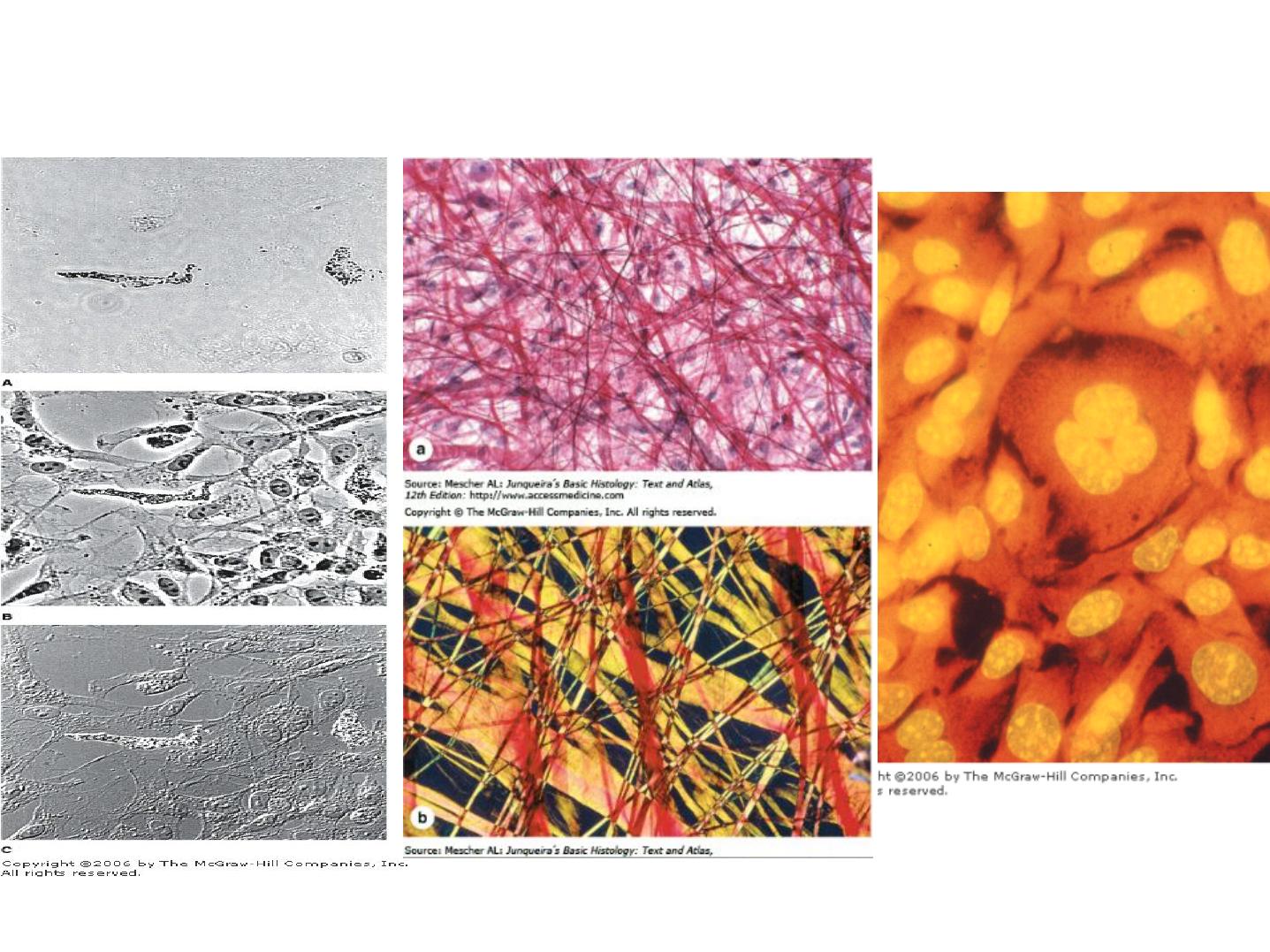
Phase-Contrast Microscopy
a-Bright field & b-Polarizing Microscopy
Fluorescence Microscopy
Types of light microscopy
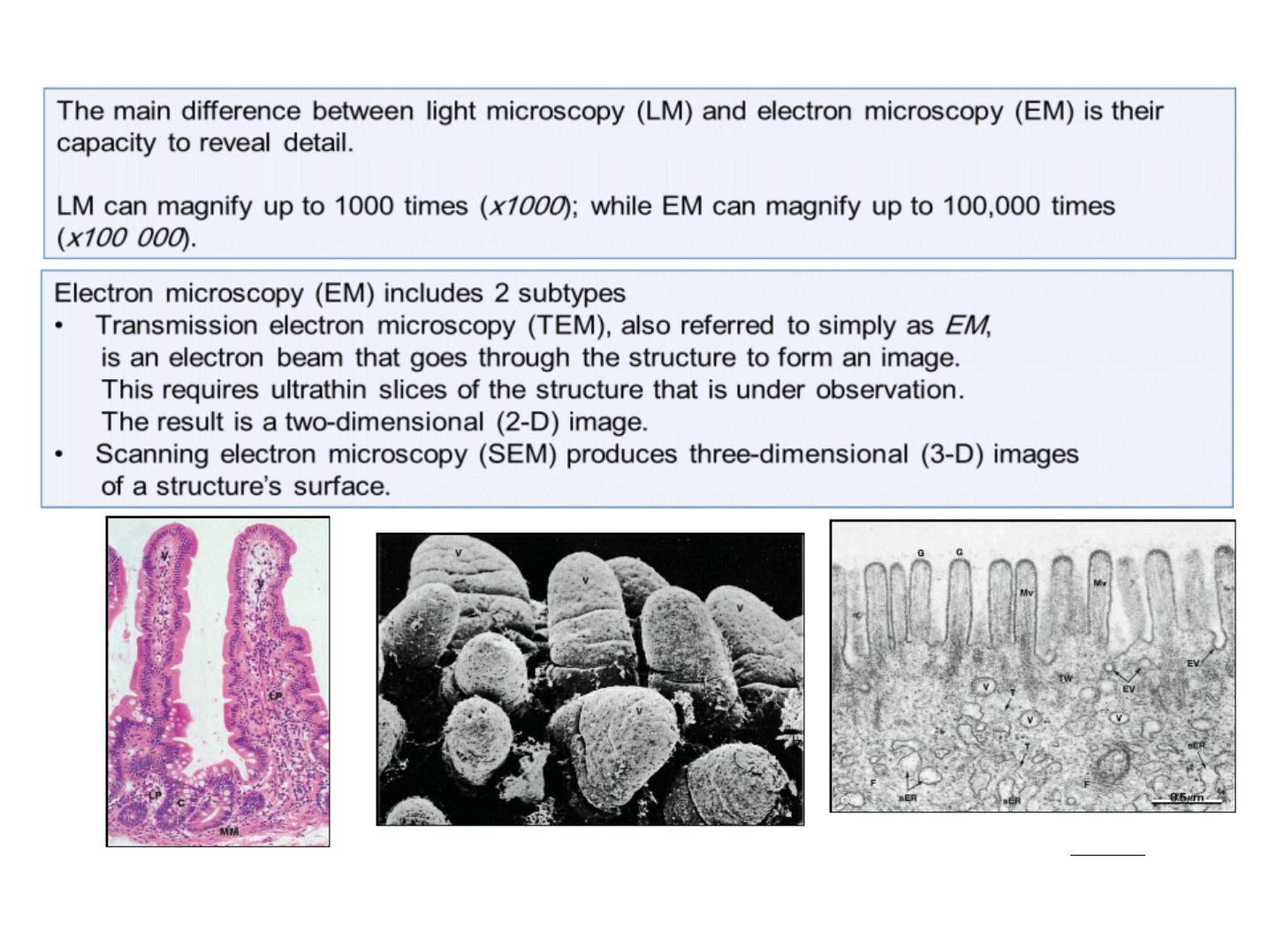
LM image of 2 villi in the intestinal
wall (x150).
SEM image of villi in the intestinal wall (x100).
TEM image of the surface of a villus
in the intestinal wall (x56 000).

• Light Microscopes
• Compound microscopes are composed of a specific
arrangement of lenses that permit a high magnification and
good resolution of the tissues being viewed.
• The present-day light microscope uses a specific arrangement
of groups of lenses to magnify an image (Fig. 1).
• Because this instrument uses more than just a single lens, it is
known as a compound microscope.
• The light source is an electric bulb with a tungsten filament
whose light is gathered into a focused beam by the condenser
lens.
• The light beam is located below and is focused on the
speccimen

• Light passing through the specimen enters one of the objective
lenses; these lenses sit on a movable turret located just above
the specimen.
• Usually four objective lenses are available on a single turret,
providing low, medium, high, and oil magnifications.
Generally, in most microscopes the first three lenses magnify 4,
10, and 40 times, respectively, and are used without oil; the oil
lens magnifies the image 100 times. The image from the
objective lens is gathered and further magnified by the ocular
lens of the eyepiece.
• This lens usually magnifies the image by a factor of 10— for
total magnifications of 40, 100, 400, and 1000—and focuses
the resulting image on the retina of the eye.
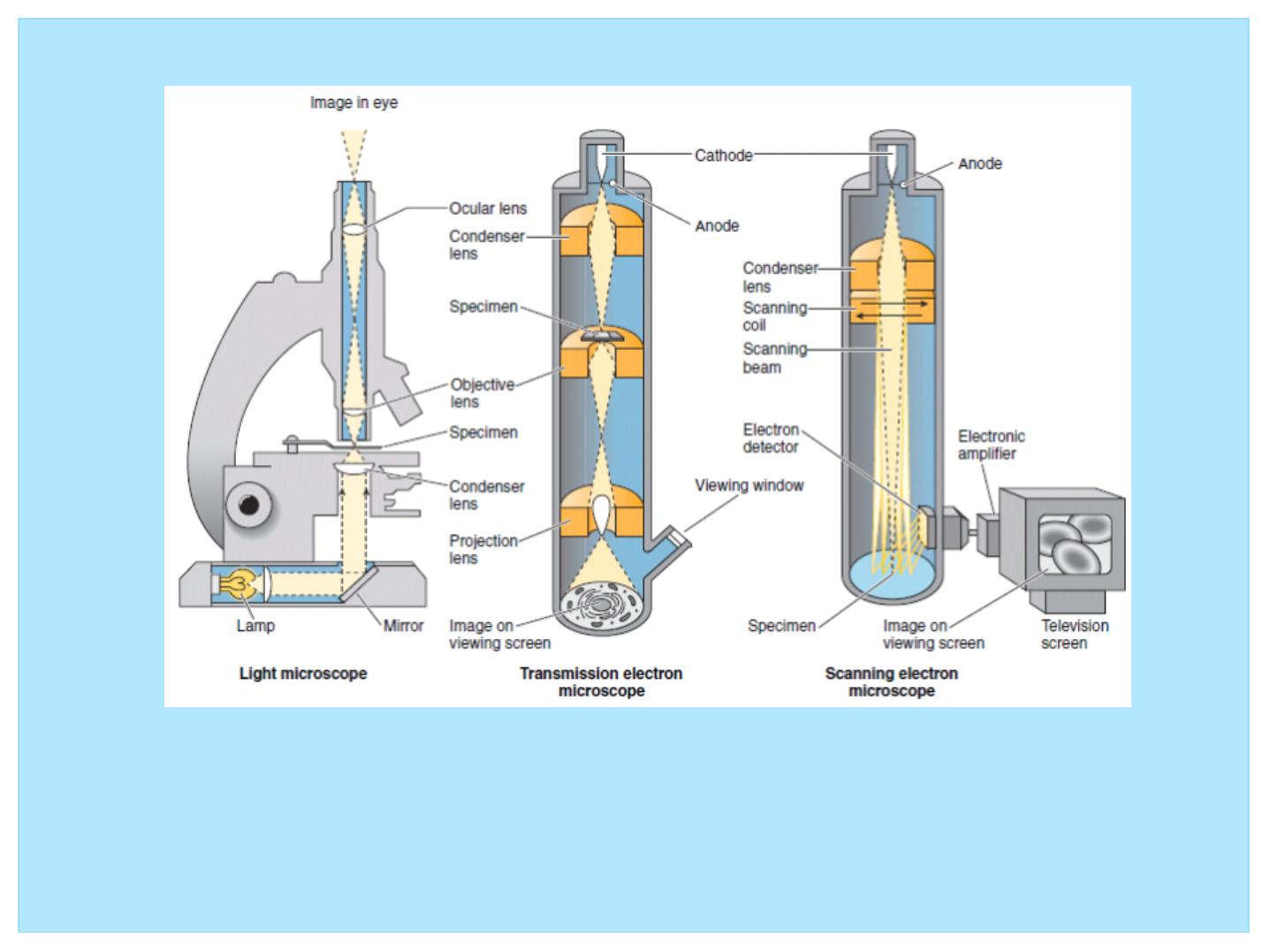
Figure 1: Comparison of light, transmission electron, and
scanning electron microscopes

• There are several of the more common methods used to
study cells and tissues.
• Because tissues and organs are usually too thick for light to
pass through them, they must be sectioned to obtain thin,
translucent sections and then attached to glass slides before
they can be examined.

• Steps required in preparing tissues for light microscopy
include
• (1) fixation
• (2) dehydration and clearing
• (3) embedding
• (4) sectioning
• (5) mounting and staining the sections.

• Fixation refers to treatment of the tissue with chemical agents
that not only retard the alterations of tissue subsequent to
death (or after removal from the body) but also maintain its
normal architecture.
• The most common fixative agents used in light microscopy are
neutral buffered formalin and Bouin’
s fluid.

• Dehydration and Clearing
• Because a large fraction of the tissue is composed of water, a
graded series of alcohol baths, beginning with 50% alcohol
and progressing in graded steps to 100% alcohol, are used to
remove the water (dehydration).
• The tissue is then treated with xylene, a chemical that is
miscible with melted paraffin. This process is known as
clearing, because the tissue becomes transparent in xylene.
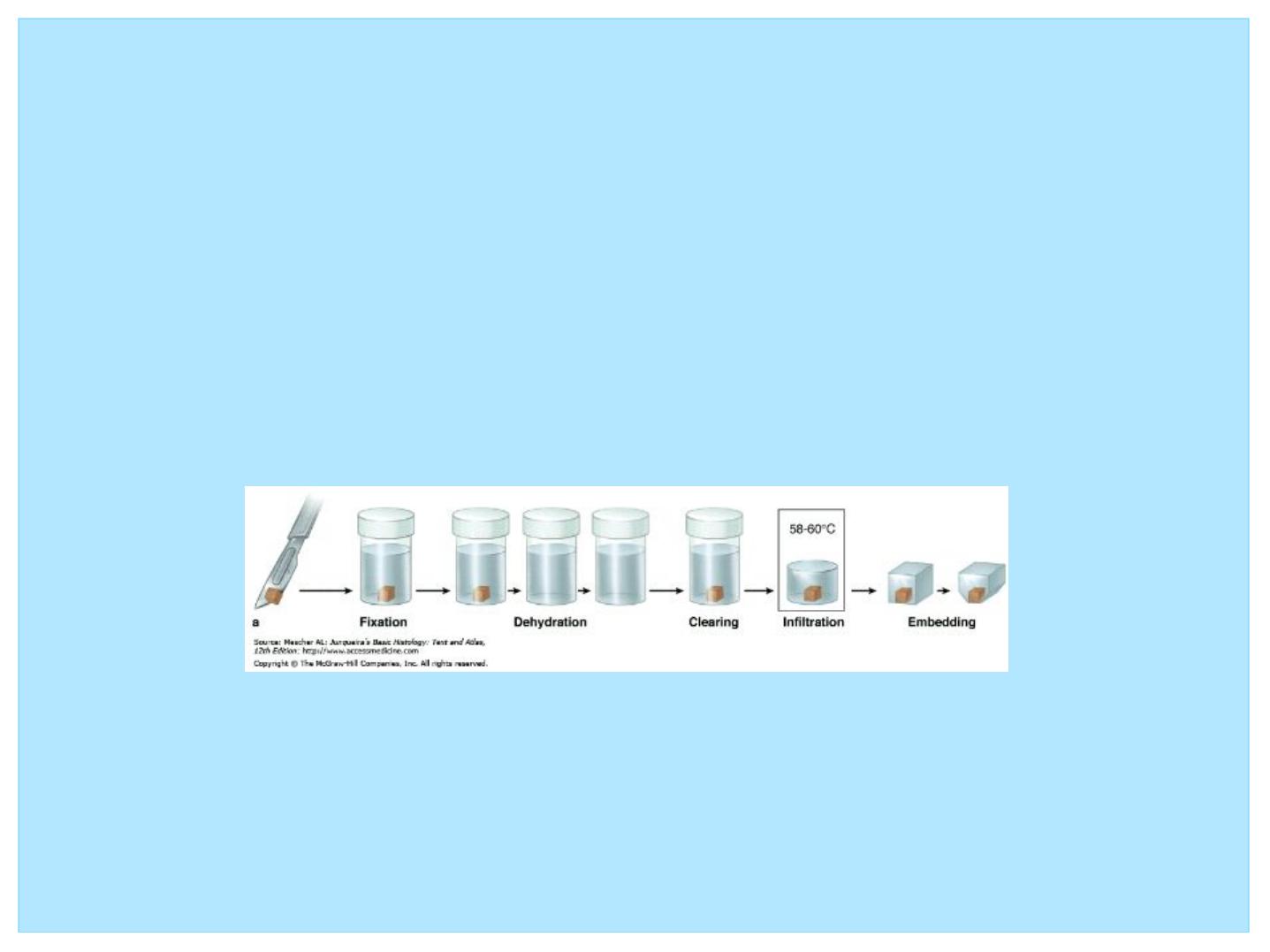
• Embedding
• In order to distinguish the overlapping cells in a tissue and the
extracellular matrix from one another, the histologist must
embed the tissues in a proper medium and then slice them into
thin sections.
• For light microscopy, the usual embedding medium is paraffin.

• Sectioning
• After the blocks of tissue are trimmed of excess embedding
material, they are mounted for sectioning. This task is
performed using a microtome, a machine equipped with a
blade and an arm that advances the tissue block in specific
equal increments. For light microscopy, the thickness of each
section is about 5 to 10 μ
m.

• Mounting and Staining
• Paraffin sections are mounted (placed) on glass slides and then
stained by water-soluble stains that permit differentiation of
the various cellular components.
• Because many tissue constituents have approximately the
same optical densities, they must be stained for light
microscopy, usually with water soluble stains.
• Therefore, the paraffin must first be removed from the section,
after which the tissue is rehydrated and stained.
• After staining, the section is again dehydrated so that the
coverslip may be permanently affixed by the use of a suitable
mounting medium. The coverslip not only protects the tissue
from damage but also is necessary for viewing the section with
the microscope.

• Various types of stains have been developed for
visualization of the many components of cells and tissues;
• The most commonly used stains in histology are hematoxylin
and eosin (H&E).
• Hematoxylin is a base that preferentially colors the acidic
components of the cell a bluish tint. Because the most acidic
components are deoxyribonucleic acid (DNA) and ribonucleic
acid (RNA), the nucleus and regions of the cytoplasm rich in
ribosomes stain dark blue; these components are referred to as
basophilic.
• Eosin is an acid that dyes the basic components of the cell a
pinkish color. Because many cytoplasmic constituents have a
basic pH, regions of the cytoplasm stain pink; these elements
are said to be acidophilic.
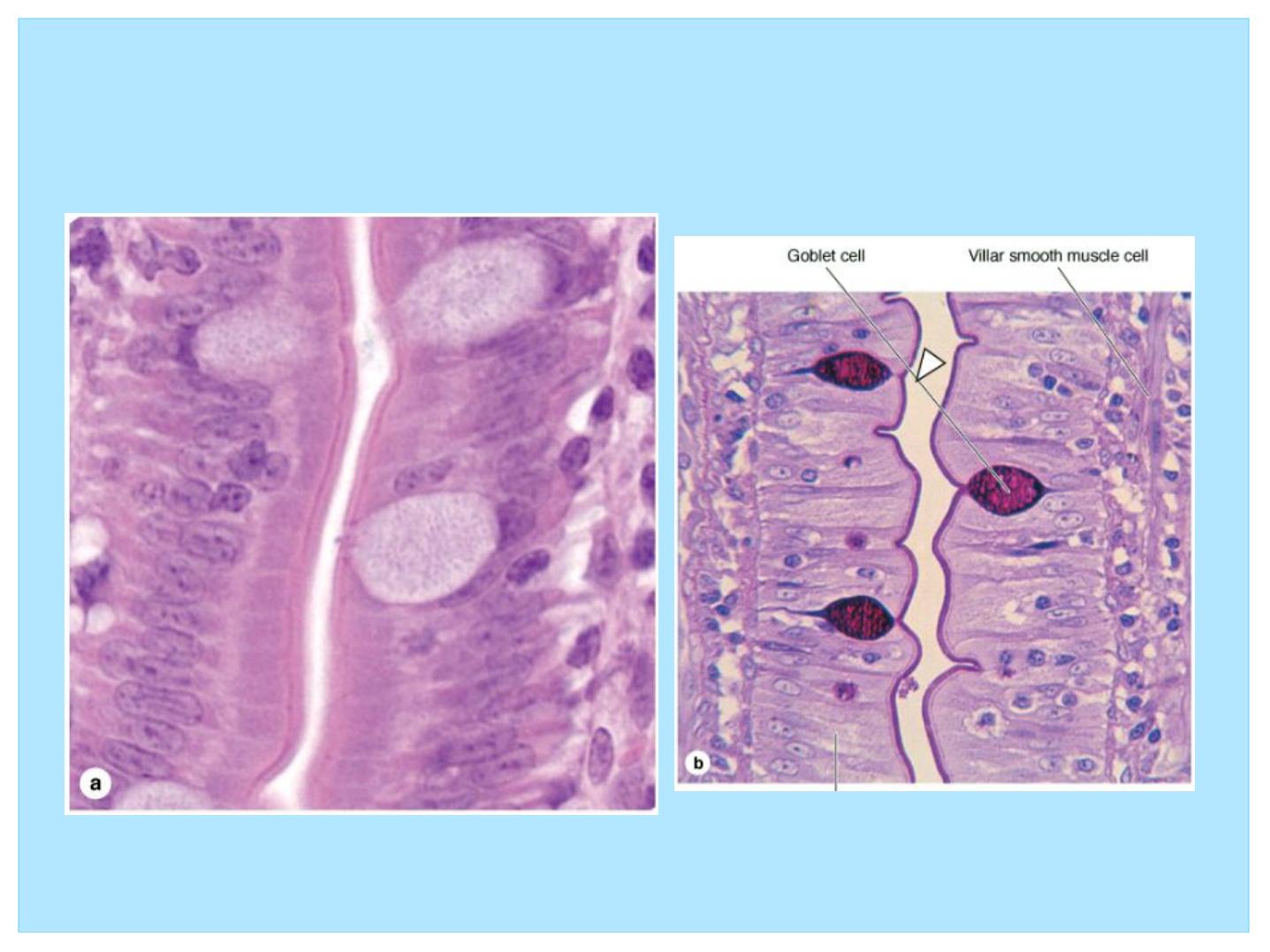
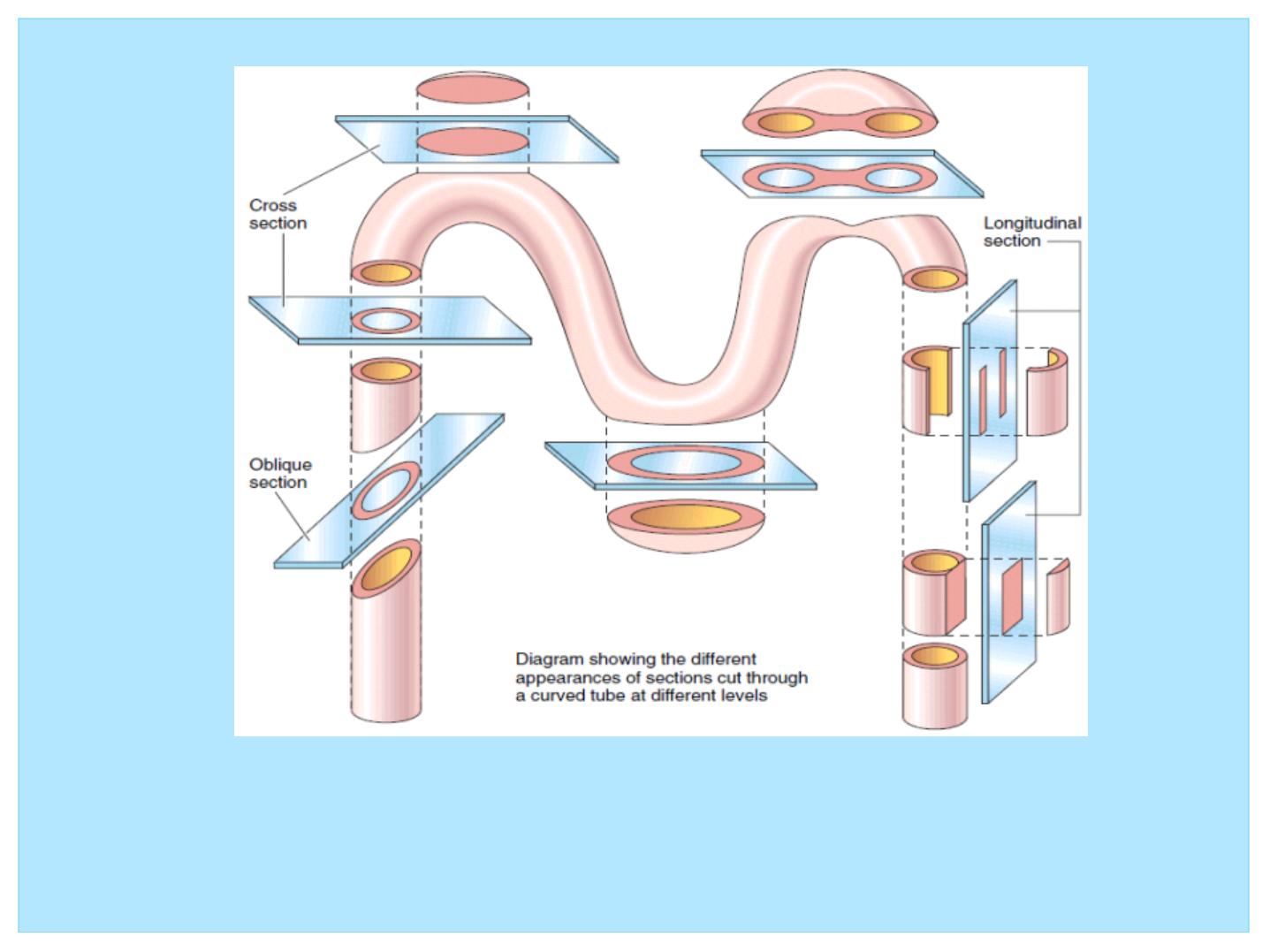
Figure 2: Histology requires a mental reconstruction of two dimensional
images into the three dimensional solid from which they were sectioned. Here,
a curved tube is sectioned in various planes to illustrate the relationship
between a series of two-dimensional sections and their three-dimensional
structure.
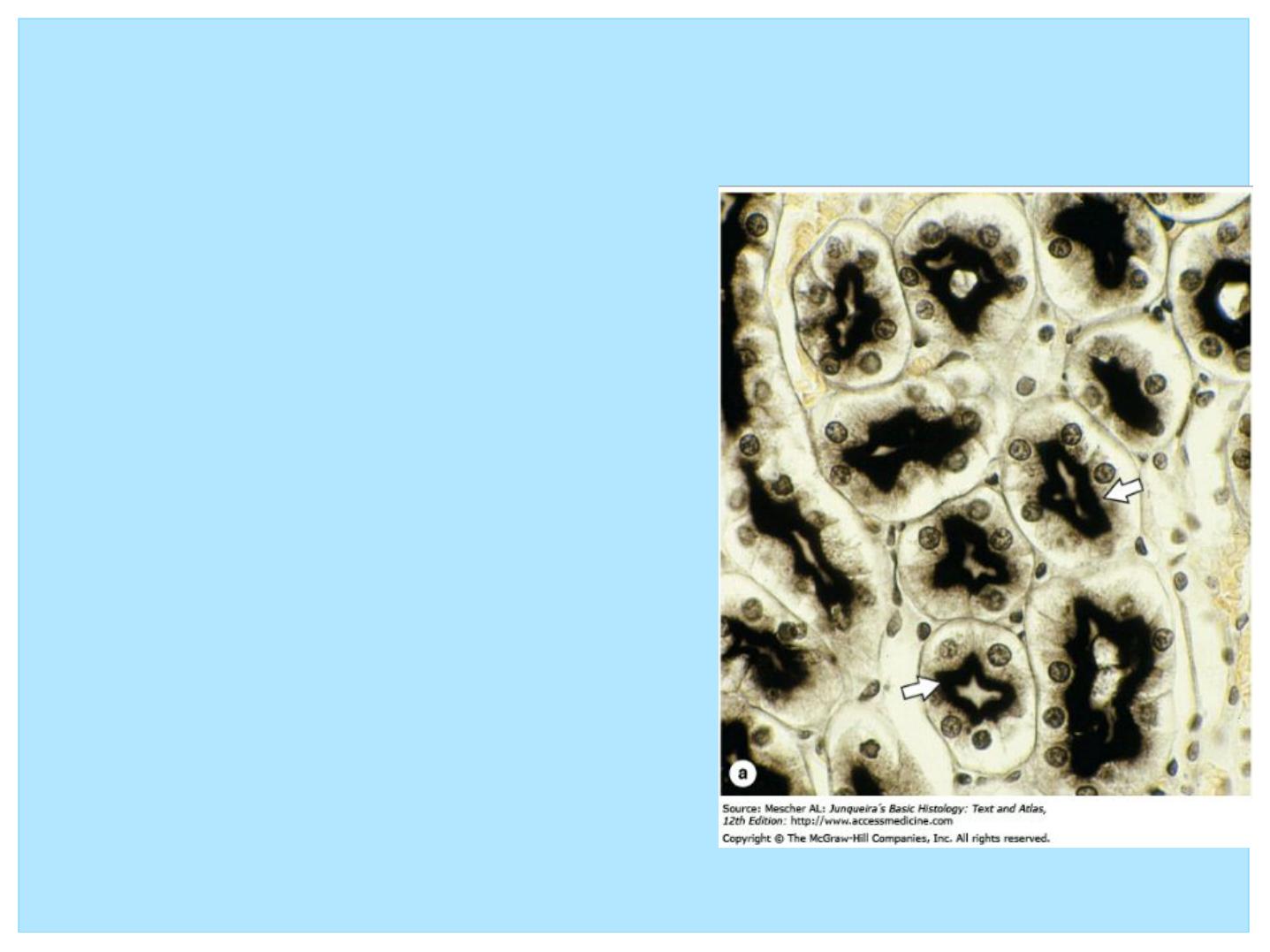
Histochemistry & Cytochemistry
• The terms histochemistry and
cytochemistry indicate methods
for localizing cellular structures
in tissue sections using unique
enzymatic activity present in
those structures.
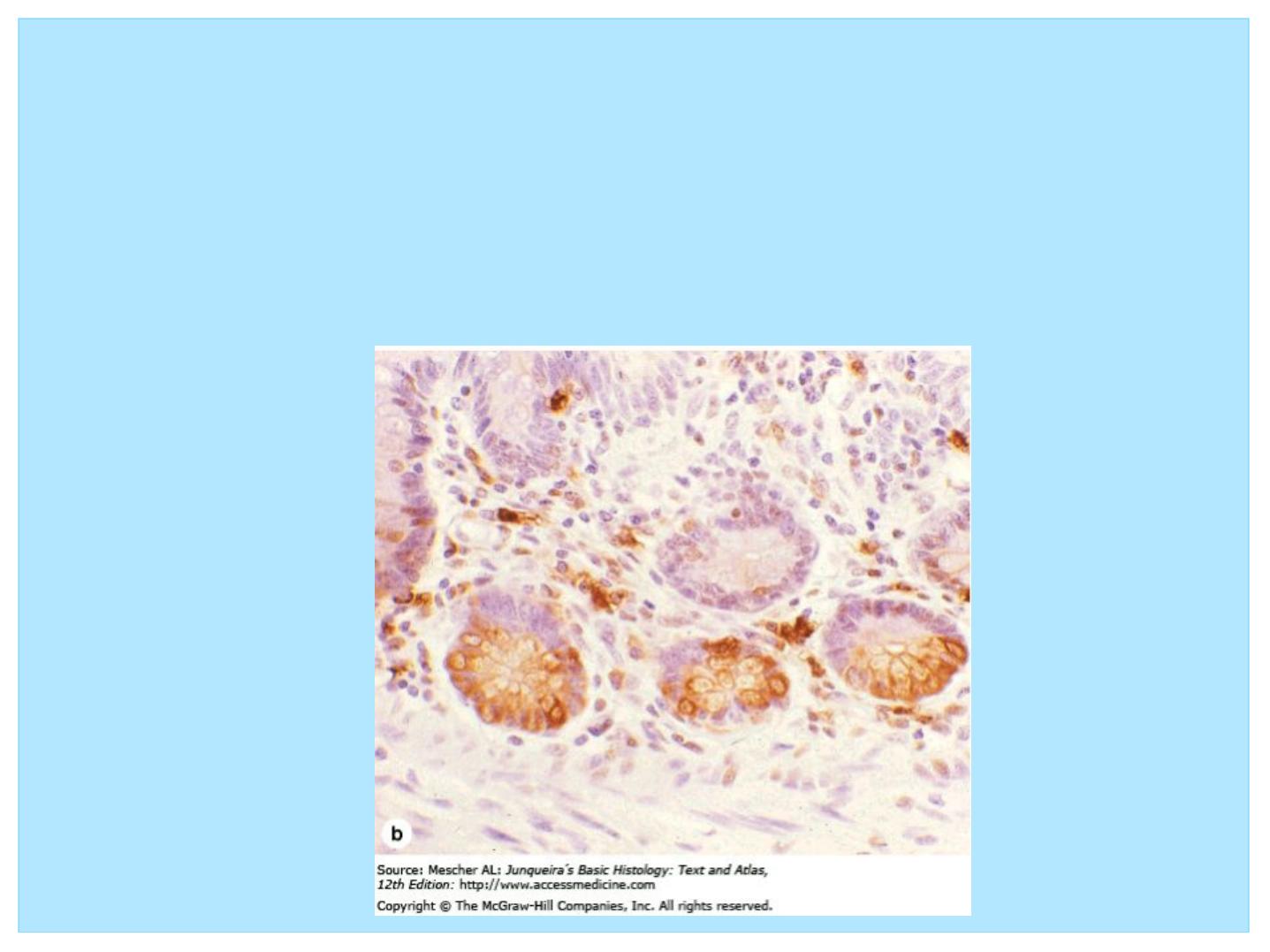
Immunohistochemistry
• A highly specific interaction between molecules is that between an antigen
and its antibody. For this reason, methods using labeled antibodies have
become extremely useful in identifying and localizing many specific
proteins, not just those with enzymatic activity that can be demonstrated by
histochemistry.

At the end of this lecture, students should be
familiar with the following aspects
• 1- What is histology, tissue and body organization?
• 2- Having knowledge about types of microscopy
particularly LM
• 3- Having knowledge about the steps required for tissue
processing and could be able to answer the following
questions:
• 1- what is the importance of each steps?
• 2- what is the common chemical agents used for each
steps usually?

Thanks
for the next lecture
we will discuss about types of tissue
