ISHIK UNIVERSITYFACULTY OF DENTISTRY
PharmacologyFall 2015
Dr. Esra Tariq
Pharmacist (M.Sc.)1
Pharmacology
The science of drugs Greek: Pharmacos (drug) + logos (study)
The study of substances that interact with living system through chemical processes, especially by binding to regulatory molecules and activating or inhibiting normal body processes.
2
Pharmacotherapeutics: is to achieve a desired beneficial effect with minimal adverse effects. When a medicine has been selected for a patient, the clinician must determine the dose that most closely achieves this goal.
Clinical Pharmacology : Is carried out for evaluation of efficacy and safety of drugs, and provides data for optimum use of drugs in human. The science that deals with materials and drugs used to prevent, diagnosis and treat diseases.
Pharmacognosy: Identification and preparation of drugs from natural sources.
3
Chemotherapy : Deals with treatment of systemic infection, malignancy with spesific drugs. Usually, these drugs have selective toxicity for the infecting organisms, malignant cell with no minimal effects on the host cells.
Toxicology : Is the branch of pharmacology that deals with the undesirable effects of chemicals on living systems, from individual cells to humans to complex ecosystems
4
Biotechnology: this was the production of drugs or other useful products by biological means (antibiotic production from microorganisms or production of monoclonal antibodies).
Pharmacogenetics: Is the study of genetic influences on responses to drugs.
It is focused on drug reactions, where affected individuals show an abnormal response to a class of drug .
5
Pharmacogenomics : It describes the use of genetic information to guide the choice of drug therapy on an individual basis.
Pharmacoeconomics : Is branch of health economics aims to quantify in economic terms the cost and benefit of drugs used therapeutically.
6
Pharmacovigilance : The pharmacological science that deals with detection, assessment , understanding and prevention of adverse effects particularly long term and short term side effects of medicine.
7
Drug
A chemical substance of known structure, other than a nutrient or an essential dietary ingredient, when administered to a living organism, produces a biological effect.8
Drug------dried herbs
They are chemical substances which modify the activity of living system orThey are compounds that used to diagnos , prevent and treat a disease.
9
Sources of Drugs
Animal: Insulin, thyroid, heparin, gonadotrophins..Mineral: zinc, Aluminium hydroxide..
Microorganisms: Important sources of antibacterials (penicillins and other antibiotics)
Synthetic : Paracetamol..
Drugs produced by genetic engineering: HGH, HI
Plant : Oil, alkaloids..
10
Side effects of drug: Production of other effects rather than original effects.
Adverse effect: Undesired effect of drug.Contraindication: Is a situation I which application of a particular drug not advisable because it may increase the risk on the patient.
11
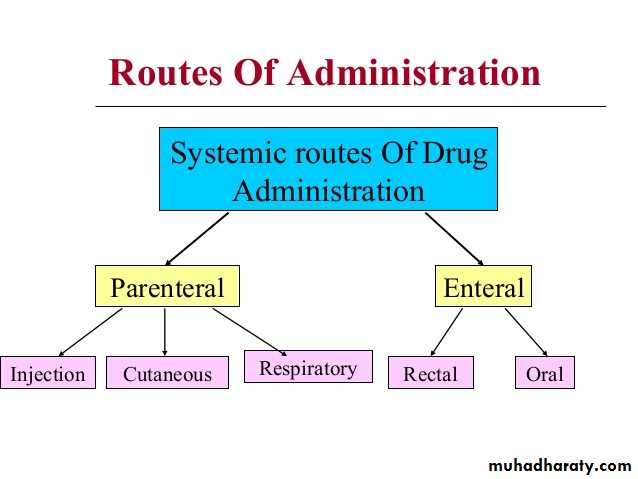
Routes of Drug Administration
Routes of drug administration depends on the site of effect that we want to get from the drug :Local Drug Administration : When a superfacial effect is needed. To increase the effect of the drug, absorption should be avoided.
Systemic Drug Administration: When a wide effect is desired, or when local effect could not be achieved and is not possible,
Drug absorption should be fast and complete
Undesired effects of the drug beside its main effect
12
13
A) Enteral
1. Oral Administration :Most drugs are taken by mouth and swallowed. Little absorption occurs until the drug enters the small intestine.
2. Sublingual : Placement under the tongue allows a drug to diffuse into the capillary network and, therefore, to enter the systemic circulation directly.
Administration of an agent, sublingually, has several advantages including rapid absorption, convenience of administration, low incidence of infection, avoidance of the harsh GI environment, and avoidance of first-pass metabolism.
14
3. Rectal: Fifty percent of the drainage of the rectal region bypasses the portal circulation; thus, the biotransformation of drugs by the liver is minimized. Like the sublingual route of administration, the rectal route of administration has the additional advantage of preventing the destruction of the drug by intestinal enzymes or by low pH in the stomach.
15
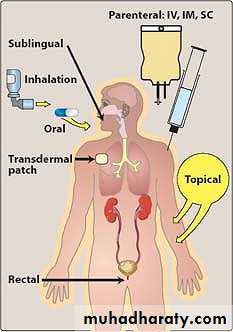
B) Parenteral
The parenteral route introduces drugs directly across the body's barrier defenses into the systemic circulation or other vascular tissue. Parenteral administration is used for drugs that are poorly absorbed from the GI tract (for example heparin) and for agents that are unstable in the GI tract (for example, insulin).16
Parenteral
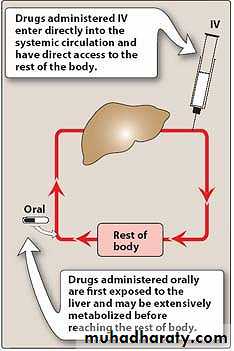
1. Intravenous (IV): Injection is the most common parenteral route. For drugs that are not absorbed orally, such as the neuromuscular blocker atracurium, there is often no other choice. With IV administration, the drug avoids the GI tract and therefore, first-pass metabolism by the liver.Intravenous delivery permits a rapid effect and a maximal degree of control over the circulating levels of the drug.
17
2. Intramuscular (IM): Drugs administered IM can be aqueous solutions or specialized depot preparations.
3. Subcutaneous (SC): This route of administration, like that of IM injection, requires absorption and is somehow slower than the IV route. Subcutaneous injection minimizes the risks associated with intravascular injection.
18
Other routes of administration
Intranasal
Topical
Transdermal
Buccal (inside mouth)
Intravaginal
Intraplevral
Intracardiac
19
20
Disadvantages of Oral Administration compared to Parenterals
Inactivation after the absorption (First pass effect)
Incomplete absortion of the drugs from the GIS (digestion enzymes, stomach acid)
The drug-food interaction that affects drug absorption
Patient cooperation
21
22
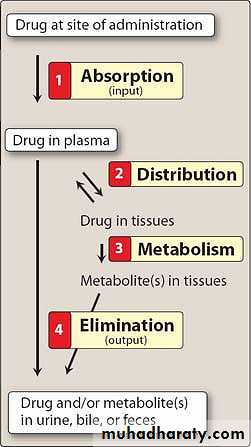
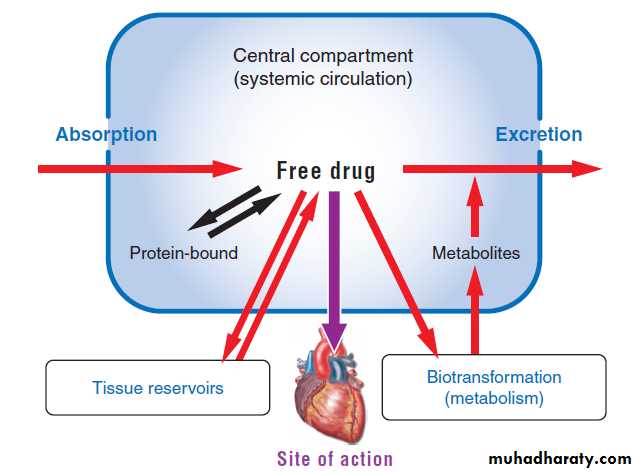
PharmacokineticThe pharmacokinetic processes of absorption, distribution, and elimination determine how rapidly and for how long the drug will appear at the target organ. The pharmacodynamic concepts of maximum response and sensitivity determine the magnitude of the effect at a particular concentration.
23
Pharmacokinetics can be defined as the measurement and formal interpretation of changes with time of drug concentrations in one or more different regions of the body in relation to dosing ('what the body does to the drug'). This distinguishes it from pharmacodynamics ('what the drug does to the body
24
Pharmacodynamics deals with physiological and biochemical effects of drugs and their meachanism of action at macromolecular , subcellular, organ, system level.
25
Drug Absorption
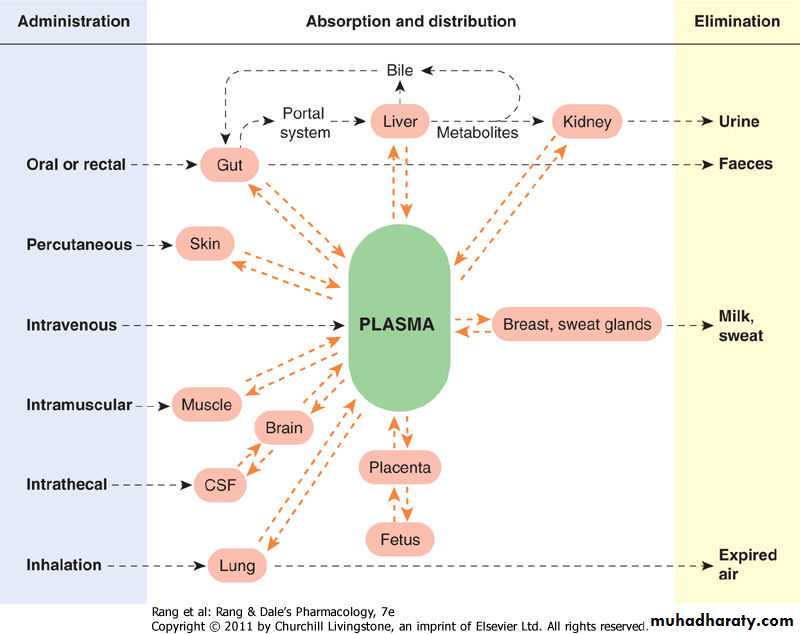
Absorption is the transfer of a drug from its site of administration to the bloodstream.The rate and efficiency of absorption depend on the route of administration.
26
For IV delivery, absorption is complete; that is, the total dose of drug reaches the systemic circulation.
Drug delivery by other routes may result in only partial absorption and, thus, lower bioavailability.
For example, the oral route requires that a drug dissolve in the GI fluid and then penetrate the epithelial cells of the intestinal mucosa, yet disease states or the presence of food may affect this process.
27
Factors Affecting Absorption Rate
• Drug: type, physicochemical characters, dissolution, concentration, and pharmacological function.• Route of administration and the biological effect: blood flow rate , and the permeability and width of the surface that the drug is absorbed.
28
1. Drug Factor
The physicochemical properties of the drugMolecular weight : The absorption rate increases with decreasing the MW of the drug.
Lipophilicity: The ratio of concentration of the drug dissolved in lipophilic phase to the hydrophilic phase lipid/water partision coefficient
The physical properties of the pharmaceutical form of the drug and its dissolver (suspension, strong acid, tablet)
29
The concentration of the drug: The same amount of the drug absorption can be increased by increasing the concentration.
Pharmacological properties of the drug.
302. Route of administration and the biological effect:
Blood flow rate at the site of the drug that applied to the body surface or tissue.The permeability and width of the area of administration.
31
32
Transport of a drug from the GI tract
Depending on their chemical properties, drugs may be absorbed from the GI tract by either passive diffusion oractive transport:1)Passive diffusion: The driving force for passive absorption of a drug is the concentration gradient across a membrane separating two body compartments; that is, the drug moves from a region of high concentration to one of lower concentration
Lipid-soluble drugs readily move across most biologic membranes due to their solubility in the membrane bilayers. Water-soluble drugs penetrate the cell membrane through aqueous channels or pores
33
2)Active transport: This mode of drug entry also involves specific carrier proteins that span the membrane. A few drugs that closely resemble the structure of naturally occurring metabolites are actively transported across cell membranes using these specific carrier proteins.
Active transport is energy-dependent and is driven by the hydrolysis of adenosine triphosphate .
It is capable of moving drugs against a concentration gradient that is, from a region of low drug concentration to one of higher drug concentration.
34
4) Endocytosis and exocytosis: This type of drug delivery transports drugs of exceptionally large size across the cell membrane.
Example: Transport of B12 across the gut wall by endocytosis.
Exocytosis is the reverse of endocytosis and is used by cells to secrete many substances by a similar vesicle formation process
Norepinephrine is stored in membrane-bound vesicles in the nerve terminal and are released by exocytosis.
35
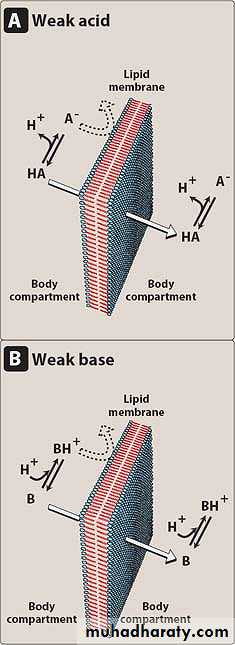
Most drugs are either weak acids or weak bases. Acidic drugs (HA) release an H+ causing a charged anion (A-) to form
HA ↔ H⁺ + A⁻
Weak bases (BH+) can also release an H+. However, the protonated form of basic drugs is usually charged, and loss of a proton produces the uncharged base (B):
BH ↔ B + H⁺
36
A drug passes through membranes more readily if it is uncharged .
Thus, for a weak acid, the uncharged HA can permeate through membranes, and A⁻ cannot.For a weak base, the uncharged form, B, penetrates through the cell membrane, but BH+ does not.
Therefore, the effective concentration of the permeable form of each drug at its absorption site is determined by the relative concentrations of the charged and uncharged forms.
37
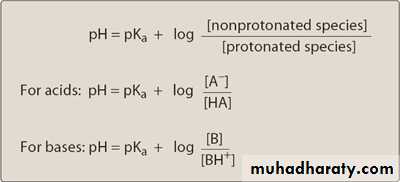
The ratio between the two forms is, in turn,
determined by the pH at the site of absorption and by the strength of the weak acid or base, which is represented by the pKa.
The pKa is a measure of the strength of the interaction of a compound with a proton. The lower the pKa of a drug, the more acidic it is.
Conversely, the higher the pKa, the more basic is the drug.
Highly lipid-soluble drugs rapidly cross membranes and often enter tissues at a rate determined by blood flow
38
The relationship of pKa and the ratio of acid-base concentrations to pH is expressed by the Henderson-Hasselbalch equation:
stomach (pH 1.0-1.5) , and blood plasma (pH 7.4).
39
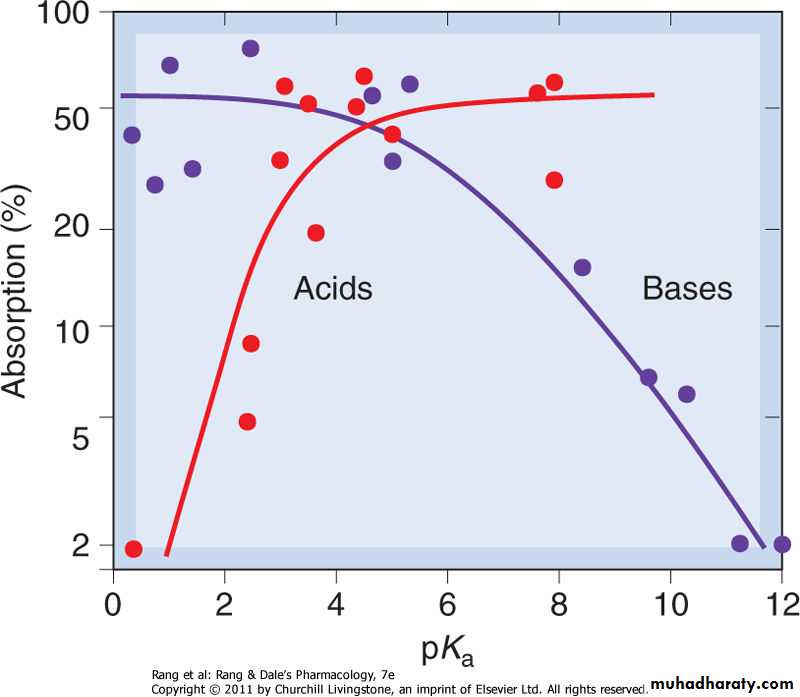
Absorption of drugs from the intestine, as a function of pKa, for acids and bases. Weak acids and bases are well absorbed; strong acids and bases are poorly absorbed.
40
Bioavailibility
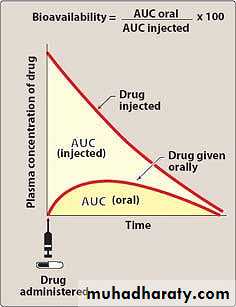
Bioavailability is the fraction of administered drug that reaches the systemic circulation.Bioavailability is expressed as the fraction of administered drug that gains access to the systemic circulation in a chemically unchanged form.
For example, if 100 mg of a drug is administered orally and 70 mg of this drug are absorbed unchanged, the bioavailability is 70%.
41
Bioavailability relates to the total proportion of the drug that reaches the systemic circulation and neglects the rate of absorption
42
Bioequivalence
Two related drugs are bioequivalent if they show comparable bioavailability and similar times to achieve peak blood concentrations.Two related drugs with a significant difference in bioavailability are said to be bioinequivalent.
Therapeutic equivalence
Two similar drugs are therapeutically equivalent if they have comparable efficacy and safety.
43
Factors that effecting bioavailability
1. First-pass hepatic metabolism: When a drug is absorbed across the GI tract, it enters the portal circulation before entering the systemic circulation. If the drug is rapidly metabolized by the liver, the amount of unchanged drug that gains access to the systemic circulation is decreased. Many drugs, such as propranolol or lidocaine, undergo significant biotransformation during a single passage through the liver.2. Solubility of the drug: Very hydrophilic drugs are poorly absorbed because of their inability to cross the lipid-rich cell membranes.
Drugs that are extremely hydrophobic are also poorly absorbed, because they are totally insoluble in aqueous body fluids and, therefore, cannot gain access to the surface of cells.
44
Factors that effecting bioavailability
3. Chemical instability: Some drugs, such as penicillin G, are unstable in the pH of the gastric contents. Others, such as insulin, are destroyed in the GI tract by degradative enzymes.4. Nature of the drug formulation: Drug absorption may be altered by factors unrelated to the chemistry of the drug. For example, particle size, salt form, crystal polymorphism, enteric coatings and the presence of excipients (such as binders and dispersing agents) can influence the ease of dissolution and, therefore, alter the rate of absorption.
45